Abstract
Cells build plasma membrane protrusions supported by parallel bundles of F-actin to enable a wide variety of biological functions, ranging from motility to host defense. Filopodia, microvilli and stereocilia are three such protrusions that have been the focus of intense biological and biophysical investigation in recent years. While it is evident that actin dynamics play a significant role in the formation of these organelles, members of the myosin superfamily have also been implicated as key players in the maintenance of protrusion architecture and function. Based on a simple analysis of the physical forces that control protrusion formation and morphology, as well as our review of available data, we propose that myosins play two general roles within these structures: (1) as cargo transporters to move critical regulatory components toward distal tips and (2) as mediators of membrane-cytoskeleton adhesion.
Keywords: Microvilli, Stereocilia, Filopodia, Brush border, Tension, Transport
Introduction
Surface protrusions are prominent features of many prokaryotic and eukaryotic cells. Protrusions provide cells with a means to harvest nutrients, move through the environment, or sense and respond to external physical or chemical cues. For the purposes of this review, we define a protrusion as a region of membrane that extends from the cell surface and is supported by an actin or microtubule-based cytoskeletal feature. The underlying cytoskeleton not only provides a protrusion with its characteristic shape and mechanical stability, but also dictates in large part its specific biological role. Given the central importance of these organelles to cell function and survival, a major goal of cell biologists is to develop an understanding of the molecular machinery and mechanisms involved in the formation, maintenance and function of these organelles. This review will highlight recent developments in our understanding of actin-based membrane protrusions, with a specific focus on the physical challenges that cells face in building and maintaining these structures, and how myosin motors are used to overcome some of these challenges.
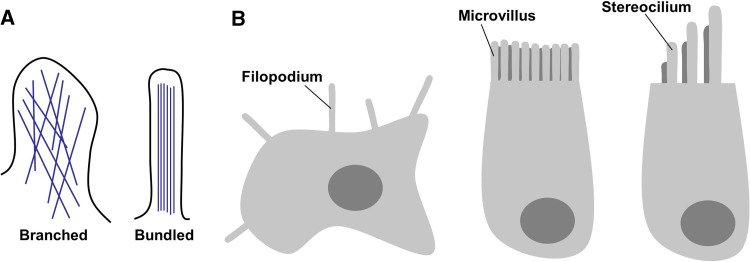
Eukaryotic cells produce a variety of actin-based structures that protrude from the cell. These features can be divided into two general categories, referred to here as branched or bundled arrays (Fig. 1a). Branched arrays of actin include the dense meshwork of filaments underlying the lamella of spreading/crawling cells or the invadopodia found on cancer cells [1, 2]. These arise from Arp2/3 based nucleation, with new daughter filaments growing from the sides of mother filaments at fixed 70° angles, toward the leading edge of the cell [3]. Given that branched actin array formation has been investigated and discussed extensively in recent years, we refer the reader to other excellent reviews for more details on this architecture [1, 2, 4]. Bundled arrays of actin, however, provide the structural support for cylindrical protrusions such as filopodia, microvilli and stereocilia (Fig. 1b). In these cases, ensembles (tens to hundreds) of actin filaments are bundled together by small crosslinking proteins (e.g., espin, villin, fimbrin or fascin) [5, 6]. Crosslinking proteins also have the ability to orient actin filaments during bundle formation [7]. This enables the production of “parallel” actin bundles (PABs) and allows motor proteins to exploit these structures as polarized tracks for directed movement and transport of cargoes. Filopodia, microvilli and stereocilia are all membrane protrusions that are supported by PABs [8]. These structures are of great interest to cytoskeletal biologists, not only because they serve a variety of important functional roles in distinct cell types, but also because they provide a unique opportunity to investigate the directed movement of myosin motors in a region of the cell with a well-organized and uniformly oriented actin cytoskeleton. As such, PAB-supported protrusions will serve as the focus of our discussion from this point forward.
Fig. 1.
Actin-based membrane protrusions. a A cartoon depiction of branched and bundled arrays of actin protruding from a cell surface. b Three forms of PAB-supported protrusions (PSPs) depicted in their cellular context. Filopodia extend several microns from the plasma membrane of motile cells in small numbers. Microvilli extend only a couple of microns, but in large numbers, from the apical surface of polarized cells such as those found in the intestinal or kidney epithelium. Stereocilia are depicted emerging from the apical surface of a mechanosensory hair cell in rows of graded height forming a staircase pattern; stereocilia also exceed microvilli in length and diameter
Actin bundles protrude from the cell in three forms
PABs support three basic types of membrane protrusions: filopodia, microvilli and stereocilia. Herein we will refer to these structures as PAB-supported protrusions (PSPs).
Filopodia
Filopodia extend from the surface of motile cells. They function in probing the physical and chemical properties of the external environment during migration and establishing cell-cell contacts (Fig. 1b) [9]. A filopodium is a slender (~200 nm in diameter) membrane protrusion supported by a parallel bundle of 10–20 actin filaments crosslinked together by the protein fascin [6, 9]. Filopodia are derived from a reorganization of the branched lamellipodial actin network in a process that is positively regulated by the ena/VASP complex and negatively regulated by capping protein [10–13]. As dynamic structures that can grow to be several microns long, the supporting PAB within a filopodium also demonstrates “retrograde flow,” where actin monomers are continuously incorporated at filament plus-ends, treadmill through the bundle lattice and are then lost from the base of the bundle via depolymerization. Substantial retrograde flow rates can be observed despite small changes in overall length (~1.2 μm/min) [14]. Recent studies have also demonstrated that motor proteins such as myosin-10 [14, 15] and myosin-15 [16] actively traffic within these structures. One distinguishing feature of filopodia relative to other forms of PSPs (microvilli and stereocilia) is that these structures extend from the cell surface at a relatively low density (number of protrusions per μm2), e.g., <100 may be distributed over the entire surface of a fibroblast. We will return to this point below as it has important implications for understanding differences in the molecular architecture of the three forms of PSPs.
Microvilli
Microvilli are cylindrical membrane protrusions typically found on the apical surface of epithelial cells lining hollow organs including the intestine, the kidney and the ventricles of the brain. The most well-characterized example is provided by the “brush border” of small intestinal epithelial cells. In the brush border, up to ~1,000 microvilli protrude from an apical surface that may only be ~100 μm2. To accommodate the large number of protrusions, brush border microvilli are tightly packed in an exquisite, well-ordered hexagonal pattern [17]. Microvilli are generally short (~1–5 μm long) and are supported by a PAB composed of ~10–30 filaments. For kidney and intestinal microvilli, the principle actin-bundling proteins are fimbrin, villin and espin [18–20]. Microvillar PABs also demonstrate retrograde flow, but rates are much slower than those observed for filopodia (~0.2 μm/min) [7, 21]. Similar to other PSPs, the actin-rich environment within the microvillus attracts a variety of unconventional myosins [22]. Among the most abundant is myosin-1a, a class I membrane-binding motor that forms a spiraling array of lateral bridges linking apical membrane to the underlying PAB [17, 23]. Other proteins that may play a role in linking membrane to cytoskeleton include members of the ezrin, radixin, moesin (ERM) superfamily [24–26]. In the gut, brush border microvilli increase the surface area of a cell exposed to the lumen, allowing a greater capacity for processing and absorbing nutrients. More recent studies have revealed that microvilli release vesicles from their distal tips [27, 28]. These vesicles are enriched in intestinal alkaline phosphatase and accumulate in the lumen. Given that intestinal alkaline phosphatase has recently been implicated in gut host defense [29, 30], vesicles release from the tips of microvilli might represent an important component of the intestinal barrier. In the context of kidney proximal tubule epithelial cells, microvilli have also been implicated in fluid flow sensing and mechano-transduction [31–33].
Stereocilia
Our remarkable ability to hear sounds with frequencies from 20 Hz to 20 kHz and to detect changes in head position and velocity (i.e., balance) are enabled by specialized microvilli called stereocilia, which extend from the apical surface of sensory “hair cells” in the cochlea and vestibular apparatus. At the apex of a single cochlear hair cell, 50–100 stereocilia are organized into a “hair bundle” consisting of three rows of graded height (5–10 μm long) (Fig. 1b); these rows are arranged in a distinctive V-shape with the tallest stereocilia lining the outside of the V [34]. Each stereocilium is composed of an overlying membrane supported by a parallel bundle of at least 200 actin filaments that are extensively crosslinked by fimbrin and espin [35–37]. Although stereocilia do demonstrate retrograde actin flow similar to filopodia and microvilli [34], observed rates are very slow (~7 nm/min), and these structures are relatively static in their dimensions once assembled by the cell [38]. In addition, a number of unconventional myosins are known to localize to and traffic within these PSPs [34]. The diameter of the stereocilium actin core is large (up to ~900 nm) for most of its length, but tapers to only ~100 nm at its base where it inserts into the cuticular plate [39]. A functional consequence of this geometry is that the bundle is much stiffer along its length; thus, forces applied to the tip of the protrusion do not cause the stereocilium to flex, but rather to pivot at its base [40]. Similar to microvilli, stereocilia extend from the cell surface as a tightly packed ensemble of protrusions. Stereocilia are also connected to their neighbors with a series of extracellular links, including a link at the tip that is at least partially composed of cadherin-23 and protocadherin-15 [41]. This “tip link” is believed to be physically connected to a unique transduction channel that resides in the apical membrane. During a positive mechanical stimulation, provided by sound or head movement, stereocilia are deflected toward the tallest row; these deflections pull on tip links, which in turn raise the probability of the opening of connected transduction channels. The resulting influx of cations is proportional to the size of the mechanical deflection; subsequent depolarization of the cell ultimately leads to neurotransmitter release, i.e., output in the form of an electrical impulse. This direct link between mechanical input and electrical output provides the molecular basis for hearing [42, 43].
Forming protrusions: actin takes action
During extension of a membrane protrusion from the cell surface, cells face a physical challenge and must expend energy to overcome the significant forces that oppose bending of membrane. Previous experimental and theoretical studies have established that the protrusion length (L μm), protrusion radius (R nm), membrane surface (in plane) tension (σ pN/nm), membrane-cytoskeleton adhesion energy (γ pN nm/nm2) and membrane bending stiffness (κ pN nm) all impact the energy required to create a protrusion [44–46]. For the relatively long membrane structures discussed here, the energy stored in the protrusion (E P) is:
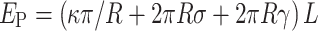 |
1 |
A relationship of this form was first derived by Helfrich [47] while exploring the physical basis of bilayer elasticity. The first term of this equation represents the work done by the cell in overcoming the resistance of the plasma membrane to bending. The next two terms represent respectively the work done by the cell against the cell surface tension (σ) and the membrane-cytoskeleton adhesion energy (γ), while attempting to increase the surface area of the cell by 2πRL (the surface area of a protrusion of radius R and length L). Because E P is linear with respect to L, calculating this energy also provides access to the restoring force exerted by the plasma membrane (F R), i.e., the force that a cell must overcome during the formation of a protrusion:
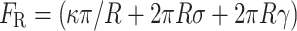 |
2 |
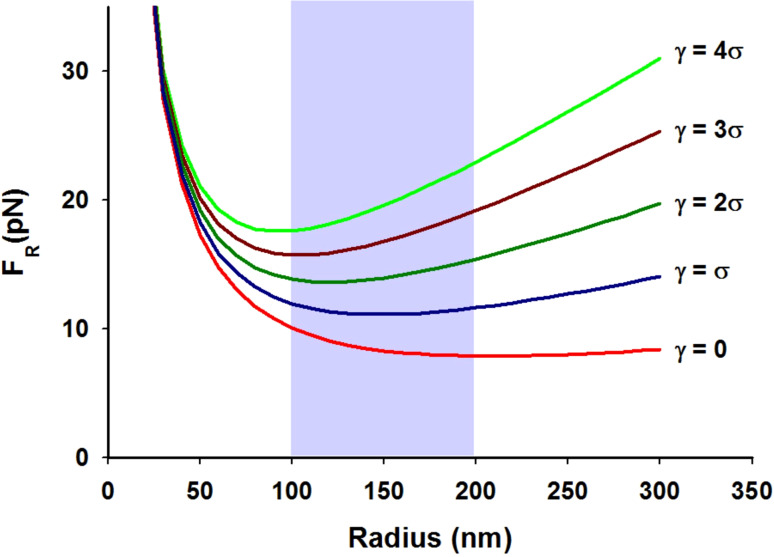
Thus, forces that oppose protrusion formation are provided by the mechanical properties of the plasma membrane (κ and σ), adhesion to the cytoskeleton (γ) and dimensions of the protrusion (R). To obtain information regarding membrane mechanical properties, “tether force” assays have emerged as a popular experimental approach [48]. Tether force assays measure the force required to form and hold (F R) a thin tubule of plasma membrane using a nano- or micro-scale force transducer such as a micropipette or optical tweezer. Based on assays performed with neuronal growth cones (a popular model system for the study of filopodia [49]), we find that for a protrusion with a radius of 200 nm, membrane bending stiffness of ~270 pN nm and surface tension of ~0.003 pN/nm, in the absence of any membrane-cytoskeleton adhesion, the membrane exerts a maximum restoring force of ~8 pN at zero protrusion growth velocity [48] (Fig. 2). This value can increase significantly with bonding between the plasma membrane and cytoskeleton. In order to minimize the energy of the membrane cylinder formed by the protrusion, surface tension and membrane-cytoskeleton adhesion work to reduce the radius, while membrane bending stiffness has the opposite effect. These modeled data reveal that for the different values of membrane-cytoskeleton adhesion, the range of radii over which the protrusion energy is minimum (assuming a constant length) falls between 100 and 200 nm, a range comparable to the radii of the PSPs reviewed here. The values for restoring force calculated here are also comparable in magnitude to values obtained by others using similar analyses [50, 51].
Fig. 2.
Forces involved in deforming plasma membrane. The restoring force (F R) exerted by the plasma membrane is plotted as a function of protrusion radius (R), using the relationship shown in Eq. 2, for different levels of membrane-cytoskeleton adhesion. Here, F R is calculated assuming a membrane bending stiffness, κ = 270 pN nm, and a surface tension, σ = 0.003 pN/nm. The region shaded in light blue represents the range of protrusion radii over which the energy of the protrusion (the product of F R and protrusion length) is minimized for the different levels of adhesion. The range obtained here is in good agreement with actual values of protrusion radii observed in PSPs discussed here
How then do cells generate forces that exceed F R in order to form filopodia, microvilli or stereocilia? Recent cell biological and biophysical studies suggest that most of the forces that favor protrusion arise from the polymerization of actin filaments and the assembly of these filaments into bundles [1].
Actin filament polymerization
The mechanics of actin filament force production is a well-studied cell biological problem. One of the most long-standing and well-supported models is based on a Brownian or thermal ratchet mechanism. First introduced by Richard Feynman [52], the Brownian ratchet is essentially a rectifier that takes advantage of the thermal fluctuations inherent to matter to produce directed force and motion. The original application to actin polymerization was made by Peskin and colleagues [50], who postulated that actin filaments polymerizing near one side of a microscopic particle could take advantage of the thermal fluctuations of the particle to “push” it in a unidirectional manner. Here, backward fluctuations of the particle (toward the F-actin array) are prevented by the ends of polymerized actin filaments. Particle fluctuations in the forward direction would give rise to transient gaps, allowing the intercalation of new G-actin monomers onto the ends of existing filaments. The incremental polymerization of actin into these transient gaps produces a force that propels the particle forward. Indeed, biophysical studies over a decade ago first established that actin polymerization exerts such a protrusive force on liposome membranes [53]. Other investigations with the microbial pathogens Listeria and Shigella demonstrated that these microbes propel themselves through the cytosol of host cells using force derived from actin polymerization [54]. However, this early model implied that the velocity with which the particle moves forward is proportional to its own diffusion coefficient, as the thermal fluctuations of the particle are critical to allow growth of the propulsive actin array. Some in vitro experiments provide support for this model by demonstrating that fluctuations of an actin-propelled microsphere determine its velocity in a load-sensitive manner [55]. Other data point to a different mechanism; for example, Listeria and Shigella are different sizes, yet they move at roughly the same velocity [56]. Thus, more recent iterations of the Brownian Ratchet model incorporate thermal fluctuations of polymerizing actin filaments as well as the interactions between filaments and the object under propulsion [57, 58]. In the context of PAB-supported protrusion formation, fluctuations of both the plasma membrane and polymerizing actin filaments are both likely to be important in the production of force at the membrane interface. Regardless of the source of thermal fluctuations, the current model suggests that at a given G-actin concentration (C), the force produced by polymerization is related to the critical concentration at the plus-end of the actin filament (C Critical ~0.1 μM, the concentration above which polymerization takes place) and the distance that an object is moved with the incorporation of a single actin monomer (δ ~ 2.7 nm) [59]:
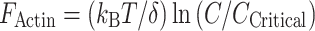 |
3 |
Using this model and parameter estimates from the literature, one can calculate the maximum force produced by a single actin filament as ~9 pN [60]. While connecting theoretical models with biological observations has not been easy, several recent studies provide absolute measurements of mechanical performance of actin polymerization in the context of in vitro assays. Kovar and colleagues deduced polymerization forces by observing filaments with both ends tethered to a glass surface polymerize until curvature induced a stall. This approach yielded a stall force (F Actin) of ~1.3 pN for a single filament [61]. More recent optical trapping studies directly measured forces at the plus-end and showed that small bundles of F-actin can exert up to ~1 pN during polymerization [62].
Actin filament bundling
While actin filaments clearly produce force, their ability to transmit force to the propelled object (e.g., the plasma membrane) may be limited by factors such as filament bending or buckling. Individual actin filaments are very flexible; although the bending fluctuations of actin filaments can contribute to polymerization forces as described above, the low flexural rigidity of actin (~0.06 pN μm2) [63] prevents single filaments from bearing large loads before buckling. Cells work around this mechanical limitation by bundling actin filaments together in cases where polymerization derived forces must overcome substantial loads. As discussed above, filopodia, stereocilia and microvilli each possess a unique complement of actin bundling proteins to achieve this architecture (e.g., fascin, espin and villin, respectively). In the special case of filopodia, myosin-X may also play a role in bringing filaments together to facilitate bundling at the actin-membrane interface [64]. Interestingly, recent in vitro studies have also shown that the membrane itself may play a role in organizing branched actin filaments into bundles [65]. Bundling actin filaments tends to increase their persistence length L P [66, 67] in the same way that stabilization by phalloidin does [68]. Because the bending stiffness of an actin filament is equal to the product of thermal energy and the persistence length (L P k B T), flexural rigidity increases as filaments get bundled together. Indeed, bundled actin filaments can be up to ~100 times stiffer than a comparable array of unlinked filaments [69]. As a result, an actin bundle can exert a substantial force at the plus-end during polymerization, and at the same time effectively transmit this force to the object being pushed (e.g., the plasma membrane). Recent modeling of filopodial protrusion suggests that at least ten filaments are needed to create a bundle with sufficient stiffness to overcome the restoring force presented by the membrane [51]. Intriguingly, this value is comparable to the minimum number of actin filaments found in filopodia and microvilli.
Maintaining protrusions: send in the myosins
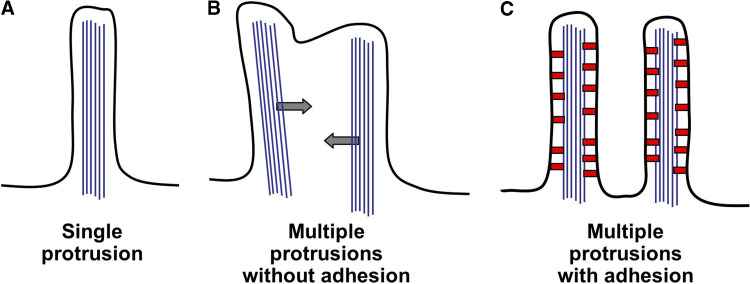
Once a polymerizing actin bundle has deformed the plasma membrane to create a nascent protrusion, cells employ additional mechanisms to maintain the protrusion and extend its lifetime on the cell surface. Because the interior of a protrusion is crowded by the core actin bundle, diffusive movement of macromolecules throughout this microenvironment will be hindered relative to “open” cytosol found in the cell body. Moreover, the protrusion may extend several microns away from the cell body. Both of these factors conspire to reduce the effectiveness of diffusion in supplying and distributing new components to the distal end of a protrusion. Additional complications arise when high densities of cell surface protrusions are required, as is the case with the enterocyte brush border or hair cell hair bundle. In both cases, membrane surface tension will tend to promote the coalescence of these closely spaced PSPs (Fig. 4). To counter this effect, the cell must furnish these structures with high levels of adhesion energy to keep the membrane tightly bound to the underlying actin core bundle and prevent coalescence. Fortunately, myosin motors are well equipped to help cells overcome both of these obstacles in the maintenance of PSPs. We argue that published studies suggest two primary roles for myosin motors in maintaining PSPs: (1) powering the directed transport of protrusion components within PSPs and (2) creating adhesion between the inner leaflet of the plasma membrane and the supporting actin bundle. Both of these roles are discussed in more detail below.
Fig. 4.
Membrane protrusion coalescence. a In the case of a single protrusion, membrane tension functions to keep the plasma membrane closely associated with the underlying actin bundle. b If a second protrusion forms too close to the first, tension will force the actin bundles to coalesce into a single protrusion. c Two adjacent protrusions are stabilized by the presence of high levels of membrane-cytoskeleton adhesion (provided by red linker molecules), which serves to counter coalescence
Directed transport in PSPs
Theoretical and experimental studies have established the presence of a diffusion barrier in the context of microvilli that extend from the apical surface of kidney tubule epithelial cells [70]. Moreover, recent proteomic analyses have shown that stereocilia contain their own complement of metabolic enzymes (e.g., creatine kinase), which are required for proper mechano-transduction [71]. These results suggest that diffusion from the cell body cytosol is insufficient for maintaining adequate levels of high-energy compounds (e.g., ATP) in these distal structures. This limitation is probably exacerbated in the case of larger macromolecules or proteins, which will diffuse even slower. By limiting the supply of important architectural, regulatory or signaling proteins, a diffusion barrier would have an adverse impact on the maintenance of PSPs. As discussed above, actin polymerization at the plus-end of bundled filaments is responsible for overcoming the membrane restoring force during protrusion formation. Thus, the distal tip of the PSP is likely to be a critical site for governing protrusion architecture (i.e., size and shape). Indeed, each of the protrusions highlighted in this review are known to assemble an electron dense ‘tip complex,’ believed to contain regulatory components that function to control actin dynamics or mediate binding to the overlying membrane [12, 23, 38]. The tip complex may also contain signaling molecules that enable protrusions to transduce signals from the external environment into the cell [72]. Given the functional importance of components that localize to the tip, cells must provide a mechanism ensuring directed transport and proper localization of these molecules to the distal end of the protrusion.
One of the first examples of directed transport within a cellular protrusion was observed in the flagella that extend from the surface of the biflagellate green alga Chlamydomonas reinhardtii [73]. In these studies, investigators from the Rosenbaum laboratory recorded time-lapse movies revealing the movement of small particles along the length of the flagellum, just beneath the plasma membrane. This new form of motility was termed intra-flagellar transport (IFT) and has been observed in all cilia and flagella studied to date [74]. IFT is evolutionarily conserved from algae through vertebrates, and its significance is evident in the wide range of human diseases (collectively referred to as ‘ciliopathies’) caused by mutations in IFT proteins (reviewed in [75]). Both anterograde (toward the tip) and retrograde (toward the cell body) transports are critical for maintaining functional cilia/flagella of proper length [76]. Investigations of IFT have provided critical insight into how cells deal with the unique challenges posed by maintaining functional protrusions.
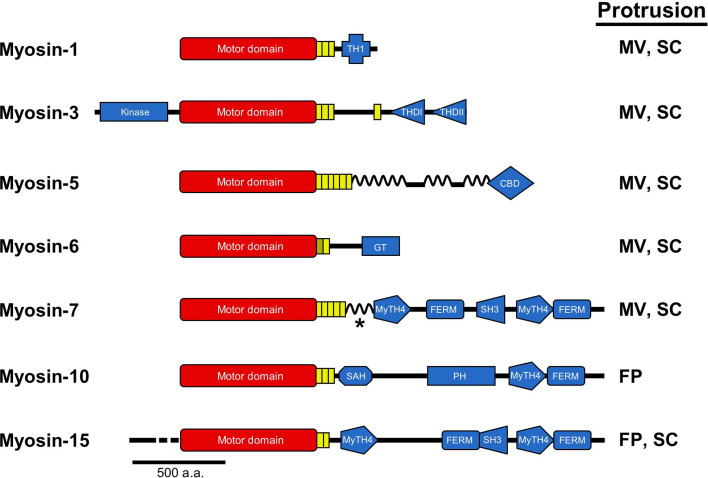
While microtubule-based IFT motors are clearly not useful in the case of PSPs, these structures have their own complement of motor proteins that function in a similar manner: myosin superfamily members. Myosins generally consist of a heavy chain that contains three domains [77]: an N-terminal motor domain that contains the actin-binding and ATPase activities [78]; a neck domain, which is the site of light chain binding and acts as a mechanical lever arm [79, 80]; and a C-terminal tail domain that mediates dimerization in some cases, as well as interactions with other proteins or membrane lipids [81] (Fig. 3). Although highly conserved, subtle sequence differences in the motor domain have resulted in a wide range of kinetic and mechanical properties that are utilized for specific functions inside cells. Numerous myosin motors are known to reside in PAB-supported protrusions; the exact complement of myosins varies depending on the cellular context. Although directed transport has only been observed directly in filopodia of live cells, studies with microvilli and stereocilia allow us to infer that a similar process occurs in these structures as well.
Fig. 3.
Myosins that reside in PAB-supported protrusions. Bar diagrams representing the primary structures of the seven different myosins discussed in this review are drawn along with an indication of the protrusion(s) that each motor occupies (FP filopodia, MV microvilli, SC stereocilia). All myosins possess N-terminal motor domains (red) coupled to structurally diverse C-terminal tail domains (blue). Myosin-1 contains a tail homology domain I (THI), which directs binding to membrane lipids. Myosin-3 is unique among myosins in that it possesses an N-terminal kinase domain. Tail homology domains I (THDI) and II (THDII) are also shown; the former binds to espin 1, while the latter may mediate direct binding to actin. Myosin-5 is a dimeric and processive motor with coiled-coil forming regions throughout its tail and a cargo-binding domain at the C-terminus. Myosin-6, the sole minus-end directed motor, is depicted with a cargo-binding globular tail domain (GT), which also mediates dimerization. Myosins-7, 10 and 15 are MyTH4-FERM domain myosins containing at least one MyTH4-FERM domain in their tails. These domains are known to mediate binding to a number of important proteins and lipids: the FERM domain of myosin-7a binds the transmembrane protein vezatin and thus links it to the cadherin/α-catenin complex; the MyTH4 domain of myosin-10 binds to microtubules, whereas the FERM domain binds to the cytoplasmic NPXY domain of β-integrins; the FERM domain of myosin-15 binds to the third PDZ domain of Whirlin, its primary cargo. In the case of myosin-7, the asterisk represents a coiled-coil domain that may facilitate dimerization in myosin-7a. Myosin-10 also has a non-dimerizing stable alpha helix (SAH) and PH domain that mediates interactions with PIP3
Transport in filopodia
Filopodia have proven to be a powerful model system for investigating directed transport within PSPs. The most well-characterized case of directed transport within a PSP is powered by myosin-10 in filopodia [14, 15] (Figs. 3, 5). Myosin-10 is expressed in a variety of tissues, but only in higher eukaryotes, where it is highly enriched in brain [82]. Early cell biological studies with this motor revealed prominent localization at the tips of filopodia. Later live cell imaging experiments demonstrated bidirectional movements of myosin-10 puncta in filopodia extending from the surface of HeLa cells, with the most prominent puncta exhibiting retrograde movement back toward the cell [15]. This seems counterintuitive at first as myosin-10 is believed to be plus-end directed, and actin filament plus-ends are oriented away from the cell into the distal tip of the filopodium. However, recent total internal reflectance fluorescence (TIRF) imaging experiments have revealed that single molecules of myosin-10 move anterograde, toward filopodial tips. After sufficient accumulation at the distal end, large puncta/ensembles of myosin-10 release from the tip and stream back to the cell at a rate dictated by retrograde flow of F-actin [14, 15].
Fig. 5.
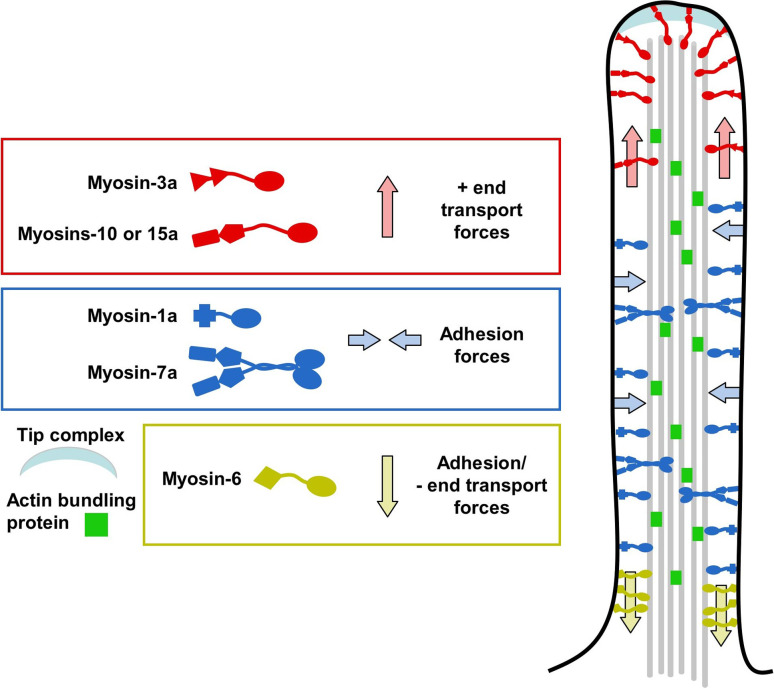
Roles for myosin motor proteins in membrane protrusions. A cartoon of a generic membrane protrusion (i.e., a composite of filopodia, microvilli and stereocilia), depicting general features and possible roles for many of the myosins discussed in this review. Motors colored in red are those myosins (myosins-3a, -10 and -15a) that are likely to function in plus-end directed transport within PSPs. Myosins colored blue are plus-end directed motors (myosins-1a and -7a) that are likely contributing to membrane-cytoskeleton adhesion along the length of core actin bundles. Colored yellow is myosin-6, the sole minus-ended directed motor that may play roles in both membrane-cytoskeleton adhesion and transport of components toward, or retention at, the base of PSPs
For a motor protein to function in directed transport along a cytoskeletal track, it must be physically and/or kinetically equipped to do so. For example, all myosin motors are ATPases, which cycle between actin-bound and detached states. Thus, mechanisms must exist to keep the motor associated with the track during periods of detachment and prevent motor and cargo from diffusing away. While this may not be major concern in the tight confines of the filopodium, myosin-10 is kinetically equipped for transport with a high duty ratio, i.e., spending the majority (~70%) of its ATPase cycle time bound to actin [83]. Another feature that would facilitate transport is the ability for myosin heavy chains to dimerize. Although myosin-10 was originally predicted to exist as a dimer [84], more recent studies have revealed that the putative coiled-coil domain (that would be responsible for dimerization) is an atypical stable α-helical (SAH) [85]. The SAH functions to lengthen the neck domain of myosin-10, but does not dimerize due to the presence of charged residues at sites where hydrophobicity is required for coiled-coil formation. As many recent studies on myosin-10 have been carried out with constructs that “force” the dimerization with the presence of a stable coiled-coil domain (e.g., the GCN4 leucine zipper) [64, 86], the biological significance of dimer formation in myosin-10 function is still unclear.
Does myosin-10 transport critical structural or regulatory components along PABs to filopodial tips? Overexpression of myosin-10 gives rise to cells with higher densities of filopodia, suggesting that this motor may transport cargoes that are important for forming and/or stabilizing filopodia [87, 88]. The C-terminal tail of myosin-10 contains several pleckstrin homology (PH) domains that bind to PIP3 [84], a myosin tail homology 4 (MyTH4) domain that interacts with microtubules [89] and a “band 4.1, ezrin, radixin, moesin” (FERM) domain known to interact with β-integrin [90]. The FERM domain interaction is intriguing as integrins are an essential component of the adhesion complex that physically links the tip of the filopodium to extracellular matrix. Thus, myosin-10 could play a role in supplying integrin subunits to the tip, thereby stabilizing filopodial adhesions. Biochemical studies have also established that myosin-10 can interact with the anti-capping protein, ena/VASP, although the sub-domain of the tail responsible for this interaction has not been mapped [91]. Live cell imaging data presented in the same paper also showed that myosin-10 puncta move in parallel with ena/VASP puncta along filopodia, implicating this critical regulator of actin dynamics as another potential cargo of myosin-10. Despite the compelling data presented in all of these studies, more recent experiments show that myosin-10 can induce and stabilize filopodia in the absence of integrin-rich adhesions or ena/VASP transport to the filopodial tip [87]. This suggests that the transport of these particular cargoes is not a significant function of myosin-10 in filopodia. Interestingly, these experiments also established that deletion of the myosin-X MyTH4/FERM domain impaired filopodial induction, suggesting that other potential unidentified cargoes (e.g., formins?) are likely playing a role. However, as noted above, studies utilizing a dimer-induction technique have shown that two-headed myosin-10 can induce filopodia in the absence of the cargo-binding tail [64]. Further studies will be needed before a consensus is reached on the exact molecular mechanism by which myosin-10 functions in filopodial formation and maintenance.
Transport in microvilli
Microvilli are also highly enriched in myosin motors [22]. One of the most abundant motors in the microvillus is the class I motor, myosin-1a [17] (Figs. 3, 5). Class I myosins are small monomeric motors that are known to interact with acidic phospholipids in the inner leaflet of the microvillar membrane through a highly basic C-terminal tail domain (Fig. 3) [92]. By considering the high concentration of myosin-1a [23] and the dimensions of the supporting PAB, one can gain an appreciation for the enormous force-generating potential localized to this structure. Here, the total force produced by the microvillar population of myosin-1a can be calculated as the product of the number of binding sites accessible along the length of a single filament in the bundle (2,000 nm length/38 nm inter-binding site distance = ~53 sites), the number of filaments in the core bundle that are accessible to myosin-1a (~15), the unitary force generated by a single myosin-1a molecule (~3 pN) and the duty ratio (~0.1 or higher, see below). Using these conservative estimates, the population of myosin-1a in the microvillus may produce a minimum ~240 pN. This is an enormous force for a structure on the micron scale. By comparison, a single kinesin motor generates ~4–8 pN and is capable of propelling the movement of small organelles through the cytosol [59]. Interestingly, recent studies do suggest that plus-end directed force may be important for powering directed transport along microvillar PABs. The most direct evidence comes from experiments with isolated brush borders [28]; when brush border microvilli are exposed to ATP, apical membrane actively slides towards the plus-ends of microvillar actin bundles, which ultimately leads to the release of small vesicles from microvillar tips. Further experiments revealed that this activity is in fact powered by myosin-1a. More recent biochemical experiments clearly demonstrate that enterocyte microvilli release vesicles from their distal tips into the intestinal lumen [27]. Interestingly, specific components of the microvillar membrane (e.g., intestinal alkaline phosphatase) appear to be enriched at the tips of microvilli and in vesicles released into the lumen. Together, the enrichment of proteins at the tip and the release of vesicles into the lumen strongly suggest the presence of directed transport in microvilli, although the precise contribution of myosin-1a to this process in native tissues remains to be elucidated.
Do other microvillar myosin motors carry out transport along the core actin bundle? Immuno-localization studies have revealed that myosins-1d, -7b and -5a (Fig. 3) are enriched in the distal half, or specifically at the tips, of microvilli in intestinal or kidney epithelial cells (Tyska laboratory, unpublished data) [93, 94]. Although steady-state localization is not direct evidence for a role in transport, accumulation at distal tips does imply that plus-end directed motors are moving in their preferred direction along microvillar PABs. While the duty ratio of myosin-1d has not been characterized, myosins-7b and -5a are both considered high duty ratio motors [95, 96]. Moreover, myosin-5 is well characterized as a dimeric transporter that moves processively along actin filaments [97]. As of the writing of this review, no cargoes have been identified for any of these motors in the context of brush border microvilli.
In contrast to active plus-end directed transport, other myosin motors may play an important role in preventing the accumulation of components in microvilli or at microvillar tips. A good example is provided by the minus-end directed motor, myosin-6 (Figs. 3, 5). Although native myosin-6 is monomeric, this unique motor can dimerize when bound to actin filaments [98] or specific cargo molecules [99, 100]. Kinetic and single molecule biophysical studies have established that dimeric myosin-6 has a high duty ratio and is processive [101, 102], suggesting that this motor is well adapted for a role in transport. Indeed, in kidney epithelial cells, acute hypertension leads to redistribution in myosin-6 immunoreactivity from the tips of microvilli down to the base [103]. These data complement the fact that myosin-6 physically interacts with Dab2 and other proteins that play important roles in receptor-mediated endocytosis [104], an activity that is localized to the base of the brush border [105, 106]. Moreover, recent TIRF imaging studies of kidney epithelial cell brush borders suggest that myosin-6 functions in the parathyroid hormone induced, minus-end directed movement of the sodium phosphate cotransporter [107].
Transport in stereocilia
Stereocilia are similar to microvilli in that they extend from the apical surface of polarized epithelial cells. However, these PSPs are highly specialized structures and can grow much larger than microvilli in terms of bundle length and the number of filaments that comprise each bundle [34]. Given the critical roles for stereocilia in the process of mechanotransduction and hearing, it comes as no surprise that a number of myosins and myosin-associated molecules that localize to stereocilia, have been linked to non-syndromic (inherited) deafness [108, 109].
With regard to transport potential in the stereocilium, one of the most highly characterized motors is myosin-15a, a MyTH4/FERM domain containing myosin that localizes to striking puncta at the tips of stereocilia [38, 110] (Figs. 3, 5). Although the mechanochemical properties of myosin-15a have yet to be characterized, cell biological studies have shown that when expressed exogenously in cells that form filopodia, this motor is capable of moving in a manner similar to that observed for myosin-10 [111]. Myosin-15a also interacts with proteins that localize to the stereocilia tip complex and are critical for stereocilia length control, including the multi-PDZ domain protein, whirlin [111]. Myosin-15a binds to whirlin via a PDZ binding ligand located C-terminal to the MyTH4/FERM domain [111]. Given the presence of multiple protein interacting domains, whirlin is believed to serve as a scaffold for other proteins that must be targeted to the stereocilia tip to control actin dynamics, including p55/MAGUK [112]. Indeed, mice with mutations in either myosin-15a or whirlin demonstrate abnormal stereocilia lengthening during development, leading to hair bundles with short, non-functional stereocilia [152, 153]. Interestingly, when EGFP-myosin-15a and DsRed-whirlin are co-expressed in native hair cells, the amount of whirlin that localizes to the tip compartment is directly proportional to the level of myosin-15a found in these structures [111]. This strongly suggests that myosin-15a is specifically transporting whirlin and functions to maintain a high whirlin concentration at the tips of stereocilia.
Direct evidence implicating myosins in the transport of proteins that regulate stereocilia actin dynamics comes from recent studies with myosin-3a and myosin-7a [113, 114] (Figs. 3, 5). Myosin-3a is unique among myosin family members in that it possesses an active N-terminal kinase domain [115]. Kinase activity may be involved in regulating myosin-3a movement along actin as removal of this region alters myosin-3a ATPase activity and localization in stereocilia [113, 116, 117]. In native hair cells, myosin-3a localizes to the distal tips of stereocilia, forming a “cap” of signal that corresponds to the distal ~500 nm of the structure [113]. Recent studies from the Kachar laboratory used filopodial induction and lengthening in COS-7 cells as an assay to study interactions between myosin-3a and the actin bundling protein, espin-1 [118]. The results clearly show that when these proteins are co-expressed, they function together to stimulate a striking increase in filopodial length. Moreover, in a manner similar to myosin-15a and whirlin, the fluorescence intensity distributions of myosin-3a and espin-1 are nearly identical at the tips of filopodia, further suggesting that myosin-3a is actively enriching espin-1 at the distal ends. Indeed, careful examination of image data revealed that these proteins colocalize in small puncta along the length of filopodia. The model that emerges suggests that myosin-3a is specifically transporting espin-1 to the tips of stereocilia, where it can incorporate into the plus-end of the PAB and promote bundle lengthening [113]. In contrast, recent experiments with the MyTH4/FERM domain containing motor, myosin-7a [114] suggest that this motor functions in the tip-ward transport of twinfilin-2, which may play a role in inhibiting actin polymerization and limiting stereocilia growth.
In contrast to myosins-15a, -3a and -7a, which facilitate the distal tip accumulation of critical cargo molecules, myosin-6 has been shown to prevent the movement of specific components into the stereocilium, restricting these cargoes to the base. In particular, myosin-6 interacts with PTPRQ, a fibronectin domain-containing protein that localizes specifically to the base of stereocilia and contributes to the specialized membrane architecture in this region [119] (Figs. 3, 5). Studies in Snell’s Waltzer mice, which are a functional null for myosin-6, show that the normally restricted PTPRQ localization is perturbed. Instead, a uniform distribution of PTPRQ along the full length of stereocilia is observed. These studies indicate that myosin-6 and presumably its minus-end directed motor activity play a role in concentrating specific proteins at the base of stereocilia and preventing their diffusion into the stereocilium [119].
Membrane-cytoskeleton adhesion in PSPs
An additional function for myosin motors in PSPs becomes evident when we consider cells exhibiting high densities of adjacent protrusions on their surface (here density is defined as the number of PSPs per unit membrane area). As discussed above, one of the dominant forces controlling PSP architecture is membrane surface tension (σ). Surface tension can be thought of as an “in plane” stiffness, which tends to minimize membrane surface area and oppose membrane deformation. When a single actin bundle protrudes from the cell membrane, as in the case of a filopodium, surface tension functions to keep the plasma membrane wrapped tightly around the protruding actin bundle, minimizing the area of deformed membrane. However, when a second bundle is forced to protrude immediately adjacent to the first, surface tension functions to promote the coalescence of the two bundles into a single protrusion (Fig. 4). Therefore, if cellular function requires a high surface density of PSPs, as in the case of enterocyte microvilli or hair cell stereocilia, there must be a mechanism in place to stabilize individual structures and prevent coalescence.
To stabilize a high surface density of PSPs, cells provide the plasma membrane with a mechanism for adhering to the underlying actin cytoskeleton. Indeed, the ability of cells to adopt complex non-spherical shapes can be attributed to extensive physical bonding with the cytoskeleton, referred to herein as membrane-cytoskeleton adhesion. Previous biophysical studies of membrane mechanics have shown that membrane-cytoskeleton adhesion (γ pN nm/nm2) sums with the membrane surface tension (σ N/m) to create an “apparent” membrane tension (T App N/m) [120]. The fraction of T App occupied by γ is substantial, ~50% in cultured epithelial cells [121, 122].
Connections to the underlying actin cytoskeleton are known to be important for the control of T App, yet few studies have explored the direct impact of specific molecules on membrane-cytoskeleton adhesion, and thus the mechanical properties of the cell membrane. The most obvious candidates for contributing to adhesion are those proteins that can simultaneously bind to membrane and F-actin, e.g., ezrin/radixin/moesin (ERM) family proteins [25]. While the contribution of ERM proteins to membrane mechanics has yet to be confirmed with biophysical studies, cell biological data do suggest that ezrin plays a role in the stability of the microvillar morphology in retina [24] and intestine [26]. As several members of the myosin superfamily are known to interact either directly or indirectly with cellular membranes [81], these actin-based motor proteins are also prime candidates for controlling interactions between the plasma membrane and actin cytoskeleton. Below we consider the function of myosin motors in two biological contexts where maintaining high levels of membrane-cytoskeleton adhesion is essential: enterocyte microvilli and hair cell stereocilia.
Adhesion in microvilli
In the context of microvilli, recent studies implicate the monomeric motor, myosin-1a, in the control of membrane mechanics. As discussed above, myosin-1a is one of the most abundant components of the enterocyte microvillus and was first observed in ultrastructural studies, forming lateral bridges that linked apical membrane to underlying actin core bundles [23] (Figs. 3, 5). As a monomeric membrane-binding motor that binds to acidic phospholipids [92], myosin-1a is ideally suited to contribute to adhesion in this cellular context. Analysis of the myosin-1a knockout mouse provided some of the first data to highlight the significance of membrane-cytoskeleton adhesion in the elaborate architecture of enterocyte brush border [17]. Ultrastructural studies of knockout brush borders showed that the absence of myosin-1a leads to the formation of large herniations, i.e., regions of apical membrane that have detached from underlying actin core bundles. The observed herniations are likely driven off the brush border by the “outward” hydrostatic pressure exerted by the cytosol, in the absence of adhesion normally provided by myosin-1a.
More recent biophysical studies have sought to test this hypothesis directly [122]. Using an optical trap, Nambiar and colleagues directly measured the amount of force required to deform the plasma membrane in brush borders isolated from myosin-1a knockout mice. The results of a simple force-extension assay clearly show that the effective stiffness of the membrane-cytoskeleton interaction in brush borders lacking myosin-1a is ~tenfold lower relative to wild-type samples. These investigators also carried out measurements in live epithelial cells, showing that levels of membrane-cytoskeleton adhesion could be manipulated by expressing a dominant negative myosin-1a (consisting of only the C-terminal lipid binding domain) to reduce apparent membrane tension, or over-expressing full-length myosin-1a, which increased tension. These results were extended to include other class I myosins and non-epithelial cell types, suggesting that the control of membrane-cytoskeleton adhesion may be a general class-wide function for these membrane and actin linking motors.
As mentioned previously, myosin-1a has also been shown to function in the release of vesicles from the tips of enterocyte microvilli [28]. How might myosin-1a simultaneously function in microvillar membrane shedding and membrane-cytoskeleton adhesion? These two activities appear to have very distinct kinetic requirements. In the case of membrane movement and the enrichment of specific components at the tips of microvilli (see above), a dynamic population of motors is required. However, a role in adhesion would benefit from a more static ensemble of cross-linker molecules. Interestingly, recent single molecule studies with the class I motor, myosin-1b, provide direct evidence that class I myosins may be able to switch between low and high duty ratio states in response to even small opposing loads, ~2 pNs [123]. In the context of myosin-1a function, a low duty ratio state would be more appropriate for the repeated ATPase cycling required to move apical membrane along core actin bundles. However, myosin-1a in a high duty ratio state would be better suited for contributing to membrane-cytoskeleton adhesion. Does myosin-1a switch between these two states to fulfill all of its responsibilities in the microvillus? Does the interaction between myosin-1a and the apical membrane even provide enough “resistance” to increase the duty ratio of myosin-1a in a load-dependent manner? More detailed studies of the molecular mechanism underlying myosin-1a/membrane interactions will be needed before we can answer these questions.
Adhesion in stereocilia
The PSPs produced and maintained by cochlear hair cells are subject to many of the same physical demands as brush border microvilli. Hair cells maintain a large number of closely spaced stereocilia and must employ specific mechanisms to prevent the coalescence of protruding actin bundles. Interestingly, a large-scale genetic screen in Italy revealed that a subset of sensorineural deafness is associated with eight distinct point mutations in myosin-1a [124]. Although myosin-1a mRNA has been detected in cochlear samples, there is currently no information available on the localization or function(s) of this motor in the ear. However, myosin-1a is only one of eight class I myosins expressed in vertebrates; is it possible that other myosin-I isoforms are expressed in hair cells and fulfill a role similar to that observed for myosin-1a in enterocyte microvilli? Indeed, myosin-1c is highly expressed in cochlear and vestibular hair cells and is known to localize to stereocilia [125]. Most of the functional studies on myosin-1c have focused on elucidating its role in the mechanotransduction process. Physiological studies suggest that the kinetics of myosin-1c binding to actin dictate the time course of the adaptation process that allows hair cells to control the sensitivity of mechanotransduction [126]. Given that this topic has been discussed in great depth in other recent reviews, we refer the reader to these discussions for more information on this aspect of myosin-1c function [127]. Although transduction machinery (e.g., tip links, transduction channels) localizes to the tips of stereocilia and numerous studies certainly suggest that myosin-1c plays a role in this process [126–128], the steady-state localization of this motor implies that it may have additional responsibilities in the hair bundle. Immunofluorescence labeling [129, 130] and the expression of EGFP-tagged myosin-1c [113] both reveal that this motor is distributed along the entire length of stereocilia in a manner similar to myosin-1a in microvilli. Myosin-1c binds tightly and specifically to the highly charged phosphoinositide, PIP2, which has been shown to mediate membrane-cytoskeleton adhesion in other cell types [131, 132]. This important lipid is found throughout the stereocilium, yet it becomes highly enriched toward the distal tip, and its presence is required for the proper function of the transduction apparatus [133]. Myosin-1c also binds to the PH domain containing protein, PHR1; this protein could provide an additional mechanism for indirectly binding to the membrane or simply increase the affinity of this motor for membrane lipids [134]. If myosin-1c shares the load-dependent kinetics demonstrated by myosin-1b [123] and membrane attachment provides mechanical resistance, the result would be a high duty ratio membrane-cytoskeleton linker for the stereocilium.
Other myosins that may play a role in the maintenance of membrane-cytoskeleton adhesion in the context of stereocilia are myosin-7a, and the minus-end directed motor, myosin-6 (Figs. 3, 5). Myosin-7a is a high duty ratio motor [135] enriched at the base of stereocilia, where supporting actin bundles appear to taper as they enter the apical surface of the hair cell [136, 137]. Mutations in this motor have been linked to deafness in Shaker mice and humans with Usher syndrome [138, 139]. Shaker mice produce fused stereocilia and disorganized hair bundles during cochlear development [140]. Because stereocilia formation is still initiated in the absence of functional myosin-7a, this motor is clearly playing a role in the later processes, such as the maintenance of these PSPs. Other studies have revealed that myosin-7a interacts with a number of proteins that are critical for stereocilia structure and function. These include cadherin-23 [141], protocadherin-15 [142] and vezatin [143]; myosin-7a can either bind directly to these proteins or indirectly via the multi-PDZ domain containing protein, harmonin [141, 144]. Both cadherin isoforms and vezatin are transmembrane proteins that may enable myosin-7a to link plasma membrane to underlying cytoskeleton. These interactions may also be important for creating adhesion between adjacent stereocilia and thus the maintenance of hair bundle organization [145]. These data suggest that myosin-7a may play an important role not only in creating adhesion between plasma membrane and cytoskeleton, but also between plasma membrane and the external substrates. This model is reminiscent of myosin-7 function in the slime mold Dictyostelium; here this motor clearly plays a role in adhesion dynamics at the cell/substrate interface via a physical interaction with talin [146, 147].
Snell’s waltzer mice, which are functionally null for myosin-6, are unable to hear and demonstrate major defects in balance and coordination [148, 149]. Cell biological studies have also revealed gross morphological perturbations in cochlear hair bundles [150]. Indeed, striking scanning electron micrographs clearly show that, in the absence of myosin-6, the apical membrane between stereocilia is poorly anchored. This results in a high incidence of stereocilia coalescence and defective mechano-transduction. Although the molecular details of how myosin-6 interacts with membrane lipids or proteins in stereocilia and contributes to membrane-cytoskeleton adhesion are unknown, recent studies indicate that dimer formation and mechanical coordination between myosin-VI motor domains are critical for these functions [151].
Conclusions
In summary, we have provided a comprehensive review of the factors that regulate the architecture of PAB-supported membrane protrusions, with a specific focus on the myosin motors that are known to enrich in these structures. In the case of protrusion formation, force production by actin polymerization and structural support provided by actin crosslinkers are critical factors that enable cells to deform the plasma membrane. However, cells rely on myosin motors to overcome the physical barriers that are inherent to the unique geometry of these structures. Indeed, mounting evidence suggests that plus-end directed myosins play roles in the transport of critical regulatory components to the tips of PSPs (Fig. 5, red); in contrast, the minus-end directed motor myosin-6 is needed to capture specific molecules and prevent them from traveling out into these protrusions (Fig. 5, yellow). Additional complications arise in cases where large numbers of protrusions are required to extend from the same cell surface (e.g., microvilli and stereocilia); here, myosins play a role in linking membrane to the actin cytoskeleton and thus preventing coalescence of adjacent protrusions (Fig. 5, blue).
Although the field has made great progress toward elucidating the molecular mechanisms underlying protrusion architecture in recent years, several outstanding questions remain. For example, cargo molecules have been discovered for several myosins, but in general the list of myosin interacting components is short, and many myosins have no known binding partners. Therefore, biochemical studies aimed at defining the components that specific PSP myosins interact with should be high on the priority list of experiments. A second unanswered question relates to the interplay between myosin dynamics and actin dynamics. Actin polymerization at the tips of PSPs is believed to exert force against the plasma membrane. One consequence of this force is that core PABs are constantly being pushed back toward the cell, i.e., undergoing retrograde flow. Do myosins “feel” and respond to retrograde flow? Do myosin-derived forces contribute to retrograde movement of PABs? While available data have suggested interesting links between actin dynamics and function of myosins that are found in PSPs [34], the nature of this interplay and its importance in the control of protrusion architecture remains unclear. Finally, we propose that future studies should take advantage of the unique architecture of PSPs to image myosin molecules carrying out their functional roles in living cells, and perhaps even at the resolution of single molecules. While this work has already begun in filopodia, live cell trafficking observations have been difficult to obtain in microvilli and stereocilia. This is undoubtedly based on the fact that both of these PSPs extend from the cell surface into space, without adhering to an extracellular substrate. To circumnavigate this problem, cell biologists will need to develop methods for immobilizing these protrusions to enable imaging studies with high temporal and spatial resolution. Combined with the recent development of specialized live cell imaging hardware, including high sensitivity, cooled EM-CCDs, these experiments will provide unprecedented insight into the mechanisms underlying myosin-based movement and adhesion in these unique structures.
Acknowledgments
The authors thank members of the Tyska Laboratory for helpful suggestions and advice. This work was supported in part by grants from the National Institutes of Health (R01-DK075555, MJT) and the American Heart Association (09GRNT2310188, MJT; Pre-doctoral Fellowship, REM; Post-doctoral Fellowship, RN).
References
- 1.Pollard TD, Borisy GG. Cellular motility driven by assembly and disassembly of actin filaments. Cell. 2003;112:453–465. doi: 10.1016/s0092-8674(03)00120-x. [DOI] [PubMed] [Google Scholar]
- 2.Weaver AM. Invadopodia. Curr Biol. 2008;18:R362–R364. doi: 10.1016/j.cub.2008.02.028. [DOI] [PubMed] [Google Scholar]
- 3.Svitkina TM, Borisy GG. Arp2/3 complex and actin depolymerizing factor/cofilin in dendritic organization and treadmilling of actin filament array in lamellipodia. J Cell Biol. 1999;145:1009–1026. doi: 10.1083/jcb.145.5.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Small JV, Stradal T, Vignal E, Rottner K. The lamellipodium: where motility begins. Trends Cell Biol. 2002;12:112–120. doi: 10.1016/s0962-8924(01)02237-1. [DOI] [PubMed] [Google Scholar]
- 5.Bretscher A. Purification of the intestinal microvillus cytoskeletal proteins villin, fimbrin, and ezrin. Methods Enzymol. 1986;134:24–37. doi: 10.1016/0076-6879(86)34072-2. [DOI] [PubMed] [Google Scholar]
- 6.Vignjevic D, Kojima S, Aratyn Y, Danciu O, Svitkina T, Borisy GG. Role of fascin in filopodial protrusion. J Cell Biol. 2006;174:863–875. doi: 10.1083/jcb.200603013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Loomis PA, Zheng L, Sekerkova G, Changyaleket B, Mugnaini E, Bartles JR. Espin cross-links cause the elongation of microvillus-type parallel actin bundles in vivo. J Cell Biol. 2003;163:1045–1055. doi: 10.1083/jcb.200309093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Revenu C, Athman R, Robine S, Louvard D. The co-workers of actin filaments: from cell structures to signals. Nat Rev Mol Cell Biol. 2004;5:635–646. doi: 10.1038/nrm1437. [DOI] [PubMed] [Google Scholar]
- 9.Mattila PK, Lappalainen P. Filopodia: molecular architecture and cellular functions. Nat Rev Mol Cell Biol. 2008;9:446–454. doi: 10.1038/nrm2406. [DOI] [PubMed] [Google Scholar]
- 10.Mejillano MR, Kojima S, Applewhite DA, Gertler FB, Svitkina TM, Borisy GG. Lamellipodial versus filopodial mode of the actin nanomachinery: pivotal role of the filament barbed end. Cell. 2004;118:363–373. doi: 10.1016/j.cell.2004.07.019. [DOI] [PubMed] [Google Scholar]
- 11.Bear JE, Svitkina TM, Krause M, Schafer DA, Loureiro JJ, Strasser GA, Maly IV, Chaga OY, Cooper JA, Borisy GG, Gertler FB. Antagonism between Ena/VASP proteins and actin filament capping regulates fibroblast motility. Cell. 2002;109:509–521. doi: 10.1016/s0092-8674(02)00731-6. [DOI] [PubMed] [Google Scholar]
- 12.Svitkina TM, Bulanova EA, Chaga OY, Vignjevic DM, Kojima S, Vasiliev JM, Borisy GG. Mechanism of filopodia initiation by reorganization of a dendritic network. J Cell Biol. 2003;160:409–421. doi: 10.1083/jcb.200210174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Applewhite DA, Barzik M, Kojima S, Svitkina TM, Gertler FB, Borisy GG. Ena/VASP proteins have an anti-capping independent function in filopodia formation. Mol Biol Cell. 2007;18:2579–2591. doi: 10.1091/mbc.E06-11-0990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kerber ML, Jacobs DT, Campagnola L, Dunn BD, Yin T, Sousa AD, Quintero OA, Cheney RE. A novel form of motility in filopodia revealed by imaging myosin-X at the single-molecule level. Curr Biol. 2009;19:967–973. doi: 10.1016/j.cub.2009.03.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Berg JS, Cheney RE. Myosin-X is an unconventional myosin that undergoes intrafilopodial motility. Nat Cell Biol. 2002;4:246–250. doi: 10.1038/ncb762. [DOI] [PubMed] [Google Scholar]
- 16.Liu R, Woolner S, Johndrow JE, Metzger D, Flores A, Parkhurst SM. Sisyphus, the Drosophila myosin XV homolog, traffics within filopodia transporting key sensory and adhesion cargos. Development. 2008;135:53–63. doi: 10.1242/dev.011437. [DOI] [PubMed] [Google Scholar]
- 17.Tyska MJ, Mackey AT, Huang JD, Copeland NG, Jenkins NA, Mooseker MS. Myosin-1a is critical for normal brush border structure and composition. Mol Biol Cell. 2005;16:2443–2457. doi: 10.1091/mbc.E04-12-1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bartles JR, Zheng L, Li A, Wierda A, Chen B. Small espin: a third actin-bundling protein and potential forked protein ortholog in brush border microvilli. J Cell Biol. 1998;143:107–119. doi: 10.1083/jcb.143.1.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bretscher A, Weber K. Villin: the major microfilament-associated protein of the intestinal microvillus. Proc Natl Acad Sci USA. 1979;76:2321–2325. doi: 10.1073/pnas.76.5.2321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bretscher A, Weber K. Fimbrin, a new microfilament-associated protein present in microvilli and other cell surface structures. J Cell Biol. 1980;86:335–340. doi: 10.1083/jcb.86.1.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tyska MJ, Mooseker MS. MYO1A (brush border myosin I) dynamics in the brush border of LLC-PK1-CL4 cells. Biophys J. 2002;82:1869–1883. doi: 10.1016/S0006-3495(02)75537-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Heintzelman M, Hasson T, Mooseker M. Multiple unconventional myosin domains of the intestinal brush border cytoskeleton. J Cell Sci. 1994;107:3535–3543. doi: 10.1242/jcs.107.12.3535. [DOI] [PubMed] [Google Scholar]
- 23.Mooseker MS, Tilney LG. Organization of an actin filament-membrane complex. Filament polarity and membrane attachment in the microvilli of intestinal epithelial cells. J Cell Biol. 1975;67:725–743. doi: 10.1083/jcb.67.3.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bonilha VL, Rayborn ME, Saotome I, McClatchey AI, Hollyfield JG. Microvilli defects in retinas of ezrin knockout mice. Exp Eye Res. 2006;82:720–729. doi: 10.1016/j.exer.2005.09.013. [DOI] [PubMed] [Google Scholar]
- 25.Bretscher A, Edwards K, Fehon RG. ERM proteins and merlin: integrators at the cell cortex. Nat Rev Mol Cell Biol. 2002;3:586–599. doi: 10.1038/nrm882. [DOI] [PubMed] [Google Scholar]
- 26.Saotome I, Curto M, McClatchey AI. Ezrin is essential for epithelial organization and villus morphogenesis in the developing intestine. Dev Cell. 2004;6:855–864. doi: 10.1016/j.devcel.2004.05.007. [DOI] [PubMed] [Google Scholar]
- 27.McConnell RE, Higginbotham JN, Shifrin DA, Jr, Tabb DL, Coffey RJ, Tyska MJ. The enterocyte microvillus is a vesicle-generating organelle. J Cell Biol. 2009;185:1285–1298. doi: 10.1083/jcb.200902147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McConnell RE, Tyska MJ. Myosin-1a powers the sliding of apical membrane along microvillar actin bundles. J Cell Biol. 2007;177:671–681. doi: 10.1083/jcb.200701144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bates JM, Akerlund J, Mittge E, Guillemin K. Intestinal alkaline phosphatase detoxifies lipopolysaccharide and prevents inflammation in zebrafish in response to the gut microbiota. Cell Host Microbe. 2007;2:371–382. doi: 10.1016/j.chom.2007.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Goldberg RF, Austen WG, Jr, Zhang X, Munene G, Mostafa G, Biswas S, McCormack M, Eberlin KR, Nguyen JT, Tatlidede HS, Warren HS, Narisawa S, Millan JL, Hodin RA. Intestinal alkaline phosphatase is a gut mucosal defense factor maintained by enteral nutrition. Proc Natl Acad Sci USA. 2008;105:3551–3556. doi: 10.1073/pnas.0712140105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Guo P, Weinstein AM, Weinbaum S. A hydrodynamic mechanosensory hypothesis for brush border microvilli. Am J Physiol Renal Physiol. 2000;279:F698–F712. doi: 10.1152/ajprenal.2000.279.4.F698. [DOI] [PubMed] [Google Scholar]
- 32.Du Z, Duan Y, Yan Q, Weinstein AM, Weinbaum S, Wang T. Mechanosensory function of microvilli of the kidney proximal tubule. Proc Natl Acad Sci USA. 2004;101:13068–13073. doi: 10.1073/pnas.0405179101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang T. Flow-activated transport events along the nephron. Curr Opin Nephrol Hypertens. 2006;15:530–536. doi: 10.1097/01.mnh.0000242180.46362.c4. [DOI] [PubMed] [Google Scholar]
- 34.Manor U, Kachar B. Dynamic length regulation of sensory stereocilia. Semin Cell Dev Biol. 2008;19:502–510. doi: 10.1016/j.semcdb.2008.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Flock A, Bretscher A, Weber K. Immunohistochemical localization of several cytoskeletal proteins in inner ear sensory and supporting cells. Hear Res. 1982;7:75–89. doi: 10.1016/0378-5955(82)90082-x. [DOI] [PubMed] [Google Scholar]
- 36.Zheng L, Sekerkova G, Vranich K, Tilney LG, Mugnaini E, Bartles JR. The deaf jerker mouse has a mutation in the gene encoding the espin actin-bundling proteins of hair cell stereocilia and lacks espins. Cell. 2000;102:377–385. doi: 10.1016/s0092-8674(00)00042-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hirokawa N. Cytoskeletal architecture of the chicken hair cells revealed with the quick-freeze, deep-etch technique. Hear Res. 1986;22:41–54. doi: 10.1016/0378-5955(86)90076-6. [DOI] [PubMed] [Google Scholar]
- 38.Rzadzinska AK, Schneider ME, Davies C, Riordan GP, Kachar B. An actin molecular treadmill and myosins maintain stereocilia functional architecture and self-renewal. J Cell Biol. 2004;164:887–897. doi: 10.1083/jcb.200310055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tilney LG, Derosier DJ, Mulroy MJ. The organization of actin filaments in the stereocilia of cochlear hair cells. J Cell Biol. 1980;86:244–259. doi: 10.1083/jcb.86.1.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hudspeth AJ. How the ear’s works work. Nature. 1989;341:397–404. doi: 10.1038/341397a0. [DOI] [PubMed] [Google Scholar]
- 41.Sakaguchi H, Tokita J, Muller U, Kachar B. Tip links in hair cells: molecular composition and role in hearing loss. Curr Opin Otolaryngol Head Neck Surg. 2009;17:388–393. doi: 10.1097/MOO.0b013e3283303472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Corey DP, Hudspeth AJ. Response latency of vertebrate hair cells. Biophys J. 1979;26:499–506. doi: 10.1016/S0006-3495(79)85267-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Howard J, Hudspeth AJ. Compliance of the hair bundle associated with gating of mechanoelectrical transduction channels in the bullfrog’s saccular hair cell. Neuron. 1988;1:189–199. doi: 10.1016/0896-6273(88)90139-0. [DOI] [PubMed] [Google Scholar]
- 44.Atilgan E, Wirtz D, Sun SX. Mechanics and dynamics of actin-driven thin membrane protrusions. Biophys J. 2006;90:65–76. doi: 10.1529/biophysj.105.071480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pronk S, Geissler PL, Fletcher DA. Limits of filopodium stability. Phys Rev Lett. 2008;100:258102. doi: 10.1103/PhysRevLett.100.258102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Derenyi I, Julicher F, Prost J. Formation and interaction of membrane tubes. Phys Rev Lett. 2002;88:238101. doi: 10.1103/PhysRevLett.88.238101. [DOI] [PubMed] [Google Scholar]
- 47.Helfrich W. Elastic properties of lipid bilayers—theory and possible experiments. Z Naturforsch C. 1973;28:693–703. doi: 10.1515/znc-1973-11-1209. [DOI] [PubMed] [Google Scholar]
- 48.Hochmuth FM, Shao JY, Dai J, Sheetz MP. Deformation and flow of membrane into tethers extracted from neuronal growth cones. Biophys J. 1996;70:358–369. doi: 10.1016/S0006-3495(96)79577-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Davenport RW, Dou P, Rehder V, Kater SB. A sensory role for neuronal growth cone filopodia. Nature. 1993;361:721–724. doi: 10.1038/361721a0. [DOI] [PubMed] [Google Scholar]
- 50.Peskin CS, Odell GM, Oster GF. Cellular motions and thermal fluctuations: the Brownian ratchet. Biophys J. 1993;65:316–324. doi: 10.1016/S0006-3495(93)81035-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mogilner A, Rubinstein B. The physics of filopodial protrusion. Biophys J. 2005;89:782–795. doi: 10.1529/biophysj.104.056515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Feynman RP. The Feynman lectures on physics. Massachussetts: Addison-Wesley; 1963. [Google Scholar]
- 53.Miyata H, Nishiyama S, Akashi K, Kinosita K., Jr Protrusive growth from giant liposomes driven by actin polymerization. Proc Natl Acad Sci USA. 1999;96:2048–2053. doi: 10.1073/pnas.96.5.2048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Theriot JA. The cell biology of infection by intracellular bacterial pathogens. Annu Rev Cell Dev Biol. 1995;11:213–239. doi: 10.1146/annurev.cb.11.110195.001241. [DOI] [PubMed] [Google Scholar]
- 55.Shaevitz JW, Fletcher DA. Load fluctuations drive actin network growth. Proc Natl Acad Sci USA. 2007;104:15688–15692. doi: 10.1073/pnas.0702601104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Goldberg MB, Theriot JA. Shigella flexneri surface protein IcsA is sufficient to direct actin-based motility. Proc Natl Acad Sci USA. 1995;92:6572–6576. doi: 10.1073/pnas.92.14.6572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mogilner A, Oster G. Cell motility driven by actin polymerization. Biophys J. 1996;71:3030–3045. doi: 10.1016/S0006-3495(96)79496-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mogilner A, Oster G. Force generation by actin polymerization II: the elastic ratchet and tethered filaments. Biophys J. 2003;84:1591–1605. doi: 10.1016/S0006-3495(03)74969-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Howard J. Mechanics of motor proteins and the cytoskeleton. New York: Sinauer Associates; 2001. [Google Scholar]
- 60.Theriot JA. The polymerization motor. Traffic. 2000;1:19–28. doi: 10.1034/j.1600-0854.2000.010104.x. [DOI] [PubMed] [Google Scholar]
- 61.Kovar DR, Pollard TD. Progressing actin: formin as a processive elongation machine. Nat Cell Biol. 2004;6:1158–1159. doi: 10.1038/ncb1204-1158. [DOI] [PubMed] [Google Scholar]
- 62.Footer MJ, Kerssemakers JW, Theriot JA, Dogterom M. Direct measurement of force generation by actin filament polymerization using an optical trap. Proc Natl Acad Sci USA. 2007;104:2181–2186. doi: 10.1073/pnas.0607052104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yasuda R, Miyata H, Kinosita K., Jr Direct measurement of the torsional rigidity of single actin filaments. J Mol Biol. 1996;263:227–236. doi: 10.1006/jmbi.1996.0571. [DOI] [PubMed] [Google Scholar]
- 64.Tokuo H, Mabuchi K, Ikebe M. The motor activity of myosin-X promotes actin fiber convergence at the cell periphery to initiate filopodia formation. J Cell Biol. 2007;179:229–238. doi: 10.1083/jcb.200703178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Liu AP, Richmond DL, Maibaum L, Pronk S, Geissler PL, Fletcher DA. Membrane-induced bundling of actin filaments. Nat Phys. 2008;4:789–793. doi: 10.1038/nphys1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Claessens MM, Bathe M, Frey E, Bausch AR. Actin-binding proteins sensitively mediate F-actin bundle stiffness. Nat Mater. 2006;5:748–753. doi: 10.1038/nmat1718. [DOI] [PubMed] [Google Scholar]
- 67.Bathe M, Heussinger C, Claessens MM, Bausch AR, Frey E. Cytoskeletal bundle mechanics. Biophys J. 2008;94:2955–2964. doi: 10.1529/biophysj.107.119743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Isambert H, Venier P, Maggs AC, Fattoum A, Kassab R, Pantaloni D, Carlier MF. Flexibility of actin filaments derived from thermal fluctuations. Effect of bound nucleotide, phalloidin, and muscle regulatory proteins. J Biol Chem. 1995;270:11437–11444. doi: 10.1074/jbc.270.19.11437. [DOI] [PubMed] [Google Scholar]
- 69.Shin JH, Mahadevan L, So PT, Matsudaira P. Bending stiffness of a crystalline actin bundle. J Mol Biol. 2004;337:255–261. doi: 10.1016/j.jmb.2004.01.028. [DOI] [PubMed] [Google Scholar]
- 70.Lange K. Microvillar Ca++ signaling: a new view of an old problem. J Cell Physiol. 1999;180:19–34. doi: 10.1002/(SICI)1097-4652(199907)180:1<19::AID-JCP3>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 71.Shin JB, Streijger F, Beynon A, Peters T, Gadzala L, McMillen D, Bystrom C, Van der Zee CE, Wallimann T, Gillespie PG. Hair bundles are specialized for ATP delivery via creatine kinase. Neuron. 2007;53:371–386. doi: 10.1016/j.neuron.2006.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Drees F, Gertler FB. Ena/VASP: proteins at the tip of the nervous system. Curr Opin Neurobiol. 2008;18:53–59. doi: 10.1016/j.conb.2008.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kozminski KG, Johnson KA, Forscher P, Rosenbaum JL. A motility in the eukaryotic flagellum unrelated to flagellar beating. Proc Natl Acad Sci USA. 1993;90:5519–5523. doi: 10.1073/pnas.90.12.5519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Silverman MA, Leroux MR. Intraflagellar transport and the generation of dynamic, structurally and functionally diverse cilia. Trends Cell Biol. 2009;19:306–316. doi: 10.1016/j.tcb.2009.04.002. [DOI] [PubMed] [Google Scholar]
- 75.Pedersen LB, Rosenbaum JL. Intraflagellar transport (IFT) role in ciliary assembly, resorption and signalling. Curr Top Dev Biol. 2008;85:23–61. doi: 10.1016/S0070-2153(08)00802-8. [DOI] [PubMed] [Google Scholar]
- 76.Qin H, Diener DR, Geimer S, Cole DG, Rosenbaum JL. Intraflagellar transport (IFT) cargo: IFT transports flagellar precursors to the tip and turnover products to the cell body. J Cell Biol. 2004;164:255–266. doi: 10.1083/jcb.200308132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Berg JS, Powell BC, Cheney RE. A millennial myosin census. Mol Biol Cell. 2001;12:780–794. doi: 10.1091/mbc.12.4.780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Tyska MJ, Warshaw DM. The myosin power stroke. Cell Motil Cytoskeleton. 2002;51:1–15. doi: 10.1002/cm.10014. [DOI] [PubMed] [Google Scholar]
- 79.Uyeda TQ, Abramson PD, Spudich JA. The neck region of the myosin motor domain acts as a lever arm to generate movement. Proc Natl Acad Sci USA. 1996;93:4459–4464. doi: 10.1073/pnas.93.9.4459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Warshaw DM, Guilford WH, Freyzon Y, Krementsova E, Palmiter KA, Tyska MJ, Baker JE, Trybus KM. The light chain binding domain of expressed smooth muscle heavy meromyosin acts as a mechanical lever. J Biol Chem. 2000;275:37167–37172. doi: 10.1074/jbc.M006438200. [DOI] [PubMed] [Google Scholar]
- 81.Krendel M, Mooseker MS. Myosins: tails (and heads) of functional diversity. Physiology (Bethesda) 2005;20:239–251. doi: 10.1152/physiol.00014.2005. [DOI] [PubMed] [Google Scholar]
- 82.Sousa AD, Berg JS, Robertson BW, Meeker RB, Cheney RE. Myo10 in brain: developmental regulation, identification of a headless isoform and dynamics in neurons. J Cell Sci. 2006;119:184–194. doi: 10.1242/jcs.02726. [DOI] [PubMed] [Google Scholar]
- 83.Homma K, Ikebe M. Myosin X is a high duty ratio motor. J Biol Chem. 2005;280:29381–29391. doi: 10.1074/jbc.M504779200. [DOI] [PubMed] [Google Scholar]
- 84.Berg JS, Derfler BH, Pennisi CM, Corey DP, Cheney RE. Myosin-X, a novel myosin with pleckstrin homology domains, associates with regions of dynamic actin. J Cell Sci. 2000;113(Pt 19):3439–3451. doi: 10.1242/jcs.113.19.3439. [DOI] [PubMed] [Google Scholar]
- 85.Knight PJ, Thirumurugan K, Xu Y, Wang F, Kalverda AP, Stafford WF, 3rd, Sellers JR, Peckham M. The predicted coiled-coil domain of myosin 10 forms a novel elongated domain that lengthens the head. J Biol Chem. 2005;280:34702–34708. doi: 10.1074/jbc.M504887200. [DOI] [PubMed] [Google Scholar]
- 86.Nagy S, Ricca BL, Norstrom MF, Courson DS, Brawley CM, Smithback PA, Rock RS. A myosin motor that selects bundled actin for motility. Proc Natl Acad Sci USA. 2008;105:9616–9620. doi: 10.1073/pnas.0802592105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Bohil AB, Robertson BW, Cheney RE. Myosin-X is a molecular motor that functions in filopodia formation. Proc Natl Acad Sci USA. 2006;103:12411–12416. doi: 10.1073/pnas.0602443103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Pi X, Ren R, Kelley R, Zhang C, Moser M, Bohil AB, Divito M, Cheney RE, Patterson C. Sequential roles for myosin-X in BMP6-dependent filopodial extension, migration, and activation of BMP receptors. J Cell Biol. 2007;179:1569–1582. doi: 10.1083/jcb.200704010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Weber KL, Sokac AM, Berg JS, Cheney RE, Bement WM. A microtubule-binding myosin required for nuclear anchoring and spindle assembly. Nature. 2004;431:325–329. doi: 10.1038/nature02834. [DOI] [PubMed] [Google Scholar]
- 90.Zhang H, Berg JS, Li Z, Wang Y, Lang P, Sousa AD, Bhaskar A, Cheney RE, Stromblad S. Myosin-X provides a motor-based link between integrins and the cytoskeleton. Nat Cell Biol. 2004;6:523–531. doi: 10.1038/ncb1136. [DOI] [PubMed] [Google Scholar]
- 91.Tokuo H, Ikebe M. Myosin X transports Mena/VASP to the tip of filopodia. Biochem Biophys Res Commun. 2004;319:214–220. doi: 10.1016/j.bbrc.2004.04.167. [DOI] [PubMed] [Google Scholar]
- 92.Hayden SM, Wolenski JS, Mooseker MS. Binding of brush border myosin I to phospholipid vesicles. J Cell Biol. 1990;111:443–451. doi: 10.1083/jcb.111.2.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Chen ZY, Hasson T, Zhang DS, Schwender BJ, Derfler BH, Mooseker MS, Corey DP. Myosin-VIIb, a novel unconventional myosin, is a constituent of microvilli in transporting epithelia. Genomics. 2001;72:285–296. doi: 10.1006/geno.2000.6456. [DOI] [PubMed] [Google Scholar]
- 94.Heintzelman MB, Hasson T, Mooseker MS. Multiple unconventional myosin domains of the intestinal brush border cytoskeleton. J Cell Sci. 1994;107(Pt 12):3535–3543. doi: 10.1242/jcs.107.12.3535. [DOI] [PubMed] [Google Scholar]
- 95.Henn A, De La Cruz EM. Vertebrate myosin VIIb is a high duty ratio motor adapted for generating and maintaining tension. J Biol Chem. 2005;280:39665–39676. doi: 10.1074/jbc.M507667200. [DOI] [PubMed] [Google Scholar]
- 96.Yang Y, Kovacs M, Xu Q, Anderson JB, Sellers JR. Myosin VIIB from Drosophila is a high duty ratio motor. J Biol Chem. 2005;280:32061–32068. doi: 10.1074/jbc.M506765200. [DOI] [PubMed] [Google Scholar]
- 97.Trybus KM. Myosin V from head to tail. Cell Mol Life Sci. 2008;65:1378–1389. doi: 10.1007/s00018-008-7507-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Park H, Ramamurthy B, Travaglia M, Safer D, Chen LQ, Franzini-Armstrong C, Selvin PR, Sweeney HL. Full-length myosin VI dimerizes and moves processively along actin filaments upon monomer clustering. Mol Cell. 2006;21:331–336. doi: 10.1016/j.molcel.2005.12.015. [DOI] [PubMed] [Google Scholar]
- 99.Yu C, Feng W, Wei Z, Miyanoiri Y, Wen W, Zhao Y, Zhang M. Myosin VI undergoes cargo-mediated dimerization. Cell. 2009;138:537–548. doi: 10.1016/j.cell.2009.05.030. [DOI] [PubMed] [Google Scholar]
- 100.Phichith D, Travaglia M, Yang Z, Liu X, Zong AB, Safer D, Sweeney HL. Cargo binding induces dimerization of myosin VI. Proc Natl Acad Sci USA. 2009;106:17320–17324. doi: 10.1073/pnas.0909748106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Altman D, Sweeney HL, Spudich JA. The mechanism of myosin VI translocation and its load-induced anchoring. Cell. 2004;116:737–749. doi: 10.1016/s0092-8674(04)00211-9. [DOI] [PubMed] [Google Scholar]
- 102.De La Cruz EM, Ostap EM, Sweeney HL. Kinetic mechanism and regulation of myosin VI. J Biol Chem. 2001;276:32373–32381. doi: 10.1074/jbc.M104136200. [DOI] [PubMed] [Google Scholar]
- 103.Yang LE, Maunsbach AB, Leong PK, McDonough AA. Redistribution of myosin VI from top to base of proximal tubule microvilli during acute hypertension. J Am Soc Nephrol. 2005;16:2890–2896. doi: 10.1681/ASN.2005040366. [DOI] [PubMed] [Google Scholar]
- 104.Hasson T. Myosin VI: two distinct roles in endocytosis. J Cell Sci. 2003;116:3453–3461. doi: 10.1242/jcs.00669. [DOI] [PubMed] [Google Scholar]
- 105.Biemesderfer D, Mentone SA, Mooseker M, Hasson T. Expression of myosin VI within the early endocytic pathway in adult and developing proximal tubules. Am J Physiol Renal Physiol. 2002;282:F785–F794. doi: 10.1152/ajprenal.00287.2001. [DOI] [PubMed] [Google Scholar]
- 106.Ameen N, Apodaca G. Defective CFTR apical endocytosis and enterocyte brush border in myosin VI-deficient mice. Traffic. 2007;8:998–1006. doi: 10.1111/j.1600-0854.2007.00587.x. [DOI] [PubMed] [Google Scholar]
- 107.Blaine J, Okamura K, Arnal H, Breusegem S, Caldas Y, Millard A, Barry N, Levi M. PTH-induced internalization of apical membrane NaPi2a: role of actin and myosin VI. Am J Physiol Cell Physiol. 2009;297:C1339–C1346. doi: 10.1152/ajpcell.00260.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Redowicz MJ. Myosins and deafness. J Muscle Res Cell Motil. 1999;20:241–248. doi: 10.1023/a:1005403725521. [DOI] [PubMed] [Google Scholar]
- 109.Redowicz MJ. Myosins and pathology: genetics and biology. Acta Biochim Pol. 2002;49:789–804. [PubMed] [Google Scholar]
- 110.Belyantseva IA, Boger ET, Friedman TB. Myosin XVa localizes to the tips of inner ear sensory cell stereocilia and is essential for staircase formation of the hair bundle. Proc Natl Acad Sci USA. 2003;100:13958–13963. doi: 10.1073/pnas.2334417100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Belyantseva IA, Boger ET, Naz S, Frolenkov GI, Sellers JR, Ahmed ZM, Griffith AJ, Friedman TB. Myosin-XVa is required for tip localization of whirlin and differential elongation of hair-cell stereocilia. Nat Cell Biol. 2005;7:148–156. doi: 10.1038/ncb1219. [DOI] [PubMed] [Google Scholar]
- 112.Mburu P, Kikkawa Y, Townsend S, Romero R, Yonekawa H, Brown SD. Whirlin complexes with p55 at the stereocilia tip during hair cell development. Proc Natl Acad Sci USA. 2006;103:10973–10978. doi: 10.1073/pnas.0600923103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Schneider ME, Dose AC, Salles FT, Chang W, Erickson FL, Burnside B, Kachar B. A new compartment at stereocilia tips defined by spatial and temporal patterns of myosin IIIa expression. J Neurosci. 2006;26:10243–10252. doi: 10.1523/JNEUROSCI.2812-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Rzadzinska AK, Nevalainen EM, Prosser HM, Lappalainen P, Steel KP. MyosinVIIa interacts with Twinfilin-2 at the tips of mechanosensory stereocilia in the inner ear. PLoS One. 2009;4:e7097. doi: 10.1371/journal.pone.0007097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Dose AC, Burnside B. Cloning and chromosomal localization of a human class III myosin. Genomics. 2000;67:333–342. doi: 10.1006/geno.2000.6256. [DOI] [PubMed] [Google Scholar]
- 116.Dose AC, Ananthanarayanan S, Moore JE, Corsa AC, Burnside B, Yengo CM. The kinase domain alters the kinetic properties of the myosin IIIA motor. Biochemistry. 2008;47:2485–2496. doi: 10.1021/bi7021574. [DOI] [PubMed] [Google Scholar]
- 117.Dose AC, Ananthanarayanan S, Moore JE, Burnside B, Yengo CM. Kinetic mechanism of human myosin IIIA. J Biol Chem. 2007;282:216–231. doi: 10.1074/jbc.M605964200. [DOI] [PubMed] [Google Scholar]
- 118.Salles FT, Merritt RC, Jr, Manor U, Dougherty GW, Sousa AD, Moore JE, Yengo CM, Dose AC, Kachar B. Myosin IIIa boosts elongation of stereocilia by transporting espin 1 to the plus ends of actin filaments. Nat Cell Biol. 2009;11:443–450. doi: 10.1038/ncb1851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Sakaguchi H, Tokita J, Naoz M, Bowen-Pope D, Gov NS, Kachar B. Dynamic compartmentalization of protein tyrosine phosphatase receptor Q at the proximal end of stereocilia: implication of myosin VI-based transport. Cell Motil Cytoskeleton. 2008;65:528–538. doi: 10.1002/cm.20275. [DOI] [PubMed] [Google Scholar]
- 120.Sheetz MP. Cell control by membrane-cytoskeleton adhesion. Nat Rev Mol Cell Biol. 2001;2:392–396. doi: 10.1038/35073095. [DOI] [PubMed] [Google Scholar]
- 121.Dai J, Sheetz MP. Membrane tether formation from blebbing cells. Biophys J. 1999;77:3363–3370. doi: 10.1016/S0006-3495(99)77168-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Nambiar R, McConnell RE, Tyska MJ. Control of cell membrane tension by myosin-I. Proc Natl Acad Sci USA. 2009;106:11972–11977. doi: 10.1073/pnas.0901641106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Laakso JM, Lewis JH, Shuman H, Ostap EM. Myosin I can act as a molecular force sensor. Science. 2008;321:133–136. doi: 10.1126/science.1159419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Donaudy F, Ferrara A, Esposito L, Hertzano R, Ben-David O, Bell RE, Melchionda S, Zelante L, Avraham KB, Gasparini P. Multiple mutations of MYO1A, a cochlear-expressed gene, in sensorineural hearing loss. Am J Hum Genet. 2003;72:1571–1577. doi: 10.1086/375654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Gillespie PG, Wagner MC, Hudspeth AJ. Identification of a 120 kd hair-bundle myosin located near stereociliary tips. Neuron. 1993;11:581–594. doi: 10.1016/0896-6273(93)90071-x. [DOI] [PubMed] [Google Scholar]
- 126.Stauffer EA, Scarborough JD, Hirono M, Miller ED, Shah K, Mercer JA, Holt JR, Gillespie PG. Fast adaptation in vestibular hair cells requires myosin-1c activity. Neuron. 2005;47:541–553. doi: 10.1016/j.neuron.2005.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Gillespie PG, Cyr JL. Myosin-1c, the hair cell’s adaptation motor. Annu Rev Physiol. 2004;66:521–545. doi: 10.1146/annurev.physiol.66.032102.112842. [DOI] [PubMed] [Google Scholar]
- 128.Holt JR, Gillespie SK, Provance DW, Shah K, Shokat KM, Corey DP, Mercer JA, Gillespie PG. A chemical-genetic strategy implicates myosin-1c in adaptation by hair cells. Cell. 2002;108:371–381. doi: 10.1016/s0092-8674(02)00629-3. [DOI] [PubMed] [Google Scholar]
- 129.Garcia JA, Yee AG, Gillespie PG, Corey DP. Localization of myosin-Ibeta near both ends of tip links in frog saccular hair cells. J Neurosci. 1998;18:8637–8647. doi: 10.1523/JNEUROSCI.18-21-08637.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Dumont RA, Zhao YD, Holt JR, Bahler M, Gillespie PG. Myosin-I isozymes in neonatal rodent auditory and vestibular epithelia. J Assoc Res Otolaryngol. 2002;3:375–389. doi: 10.1007/s101620020049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Raucher D, Sheetz MP. Phospholipase C activation by anesthetics decreases membrane-cytoskeleton adhesion. J Cell Sci. 2001;114:3759–3766. doi: 10.1242/jcs.114.20.3759. [DOI] [PubMed] [Google Scholar]
- 132.Hokanson DE, Ostap EM. Myo1c binds tightly and specifically to phosphatidylinositol 4, 5-bisphosphate and inositol 1, 4, 5-trisphosphate. Proc Natl Acad Sci USA. 2006;103:3118–3123. doi: 10.1073/pnas.0505685103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Hirono M, Denis CS, Richardson GP, Gillespie PG. Hair cells require phosphatidylinositol 4, 5-bisphosphate for mechanical transduction and adaptation. Neuron. 2004;44:309–320. doi: 10.1016/j.neuron.2004.09.020. [DOI] [PubMed] [Google Scholar]
- 134.Etournay R, El-Amraoui A, Bahloul A, Blanchard S, Roux I, Pezeron G, Michalski N, Daviet L, Hardelin JP, Legrain P, Petit C. PHR1, an integral membrane protein of the inner ear sensory cells, directly interacts with myosin 1c and myosin VIIa. J Cell Sci. 2005;118:2891–2899. doi: 10.1242/jcs.02424. [DOI] [PubMed] [Google Scholar]
- 135.Inoue A, Ikebe M. Characterization of the motor activity of mammalian myosin VIIA. J Biol Chem. 2003;278:5478–5487. doi: 10.1074/jbc.M210489200. [DOI] [PubMed] [Google Scholar]
- 136.Hasson T, Walsh J, Cable J, Mooseker MS, Brown SD, Steel KP. Effects of shaker-1 mutations on myosin-VIIa protein and mRNA expression. Cell Motil Cytoskeleton. 1997;37:127–138. doi: 10.1002/(SICI)1097-0169(1997)37:2<127::AID-CM5>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 137.Hasson T, Gillespie PG, Garcia JA, MacDonald RB, Zhao Y, Yee AG, Mooseker MS, Corey DP. Unconventional myosins in inner-ear sensory epithelia. J Cell Biol. 1997;137:1287–1307. doi: 10.1083/jcb.137.6.1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Weil D, Blanchard S, Kaplan J, Guilford P, Gibson F, Walsh J, Mburu P, Varela A, Levilliers J, Weston MD, Kelley PM, Kimberling WJ, Wagenaar M, Levi-Acobas F, Larget-Piet D, Munnich A, Steel KP, Brown SDM, Petit C. Defective myosin VIIA gene responsible for Usher syndrome type 1B. Nature. 1995;374:60–61. doi: 10.1038/374060a0. [DOI] [PubMed] [Google Scholar]
- 139.Gibson F, Walsh J, Mburu P, Varela A, Brown KA, Antonio M, Beisel KW, Steel KP, Brown SD. A type VII myosin encoded by the mouse deafness gene shaker-1. Nature. 1995;374:62–64. doi: 10.1038/374062a0. [DOI] [PubMed] [Google Scholar]
- 140.Self T, Mahony M, Fleming J, Walsh J, Brown SD, Steel KP. Shaker-1 mutations reveal roles for myosin VIIA in both development and function of cochlear hair cells. Development. 1998;125:557–566. doi: 10.1242/dev.125.4.557. [DOI] [PubMed] [Google Scholar]
- 141.Boeda B, El-Amraoui A, Bahloul A, Goodyear R, Daviet L, Blanchard S, Perfettini I, Fath KR, Shorte S, Reiners J, Houdusse A, Legrain P, Wolfrum U, Richardson G, Petit C. Myosin VIIa, harmonin and cadherin 23, three Usher I gene products that cooperate to shape the sensory hair cell bundle. EMBO J. 2002;21:6689–6699. doi: 10.1093/emboj/cdf689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Senften M, Schwander M, Kazmierczak P, Lillo C, Shin JB, Hasson T, Geleoc GS, Gillespie PG, Williams D, Holt JR, Muller U. Physical and functional interaction between protocadherin 15 and myosin VIIa in mechanosensory hair cells. J Neurosci. 2006;26:2060–2071. doi: 10.1523/JNEUROSCI.4251-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Kussel-Andermann P, El-Amraoui A, Safieddine S, Nouaille S, Perfettini I, Lecuit M, Cossart P, Wolfrum U, Petit C. Vezatin, a novel transmembrane protein, bridges myosin VIIA to the cadherin–catenins complex. EMBO J. 2000;19:6020–6029. doi: 10.1093/emboj/19.22.6020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Siemens J, Kazmierczak P, Reynolds A, Sticker M, Littlewood-Evans A, Muller U. The Usher syndrome proteins cadherin 23 and harmonin form a complex by means of PDZ-domain interactions. Proc Natl Acad Sci USA. 2002;99:14946–14951. doi: 10.1073/pnas.232579599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Muller U. Cadherins and mechanotransduction by hair cells. Curr Opin Cell Biol. 2008;20:557–566. doi: 10.1016/j.ceb.2008.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Tuxworth RI, Stephens S, Ryan ZC, Titus MA. Identification of a myosin VII-talin complex. J Biol Chem. 2005;280:26557–26564. doi: 10.1074/jbc.M503699200. [DOI] [PubMed] [Google Scholar]
- 147.Tuxworth RI, Weber I, Wessels D, Addicks GC, Soll DR, Gerisch G, Titus MA. A role for myosin VII in dynamic cell adhesion. Curr Biol. 2001;11:318–329. doi: 10.1016/s0960-9822(01)00097-5. [DOI] [PubMed] [Google Scholar]
- 148.Deol MS, Green MC. Snell’s waltzer, a new mutation affecting behaviour and the inner ear in the mouse. Genet Res. 1966;8:339–345. doi: 10.1017/s0016672300010193. [DOI] [PubMed] [Google Scholar]
- 149.Avraham KB, Hasson T, Steel KP, Kingsley DM, Russell LB, Mooseker MS, Copeland NG, Jenkins NA. The mouse Snell’s waltzer deafness gene encodes an unconventional myosin required for structural integrity of inner ear hair cells. Nat Genet. 1995;11:369–375. doi: 10.1038/ng1295-369. [DOI] [PubMed] [Google Scholar]
- 150.Self T, Sobe T, Copeland NG, Jenkins NA, Avraham KB, Steel KP. Role of myosin VI in the differentiation of cochlear hair cells. Dev Biol. 1999;214:331–341. doi: 10.1006/dbio.1999.9424. [DOI] [PubMed] [Google Scholar]
- 151.Hertzano R, Shalit E, Rzadzinska AK, Dror AA, Song L, Ron U, Tan JT, Shitrit AS, Fuchs H, Hasson T, Ben-Tal N, Sweeney HL, de Angelis MH, Steel KP, Avraham KB. A Myo6 mutation destroys coordination between the myosin heads, revealing new functions of myosin VI in the stereocilia of mammalian inner ear hair cells. PLoS Genet. 2008;4:e1000207. doi: 10.1371/journal.pgen.1000207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Holme RH, Kiernan BW, Brown SD, Steel KP. Elongation of hair cell stereocilia is defective in the mouse mutant whirler. J Comp Neurol. 2002;450:94–102. doi: 10.1002/cne.10301. [DOI] [PubMed] [Google Scholar]
- 153.Probst FJ. Correction of deafness in shaker-2 mice by an unconventional myosin in a BAC transgene. Science. 1998;280:1444–1447. doi: 10.1126/science.280.5368.1444. [DOI] [PubMed] [Google Scholar]