Abstract
Activation of NF-κB in airway epithelium is observed in allergic asthma and is induced by inhalation of numerous infectious and reactive substances. Many of the substances that activate NF-κB in the airway epithelium are also capable of acting as adjuvants to elicit antigen-specific sensitization to concomitantly inhaled protein, thereby circumventing the inherent bias of the lung to promote tolerance to innocuous antigens. We have used a transgenic mouse inducibly expressing a constitutively active mutant of the inhibitor of nuclear factor κB (IκB) kinase β (CAIKKβ) that activates NF-κB only in nonciliated airway epithelial cells to test whether activation of this intracellular signaling pathway in this specific cell type is sufficient to establish a pulmonary environment permissive to the development of allergic sensitization to inhaled protein. When airway epithelial CAIKKβ was transiently expressed in antigen-naive mice only during initial inhalation of ovalbumin, the mice became allergically sensitized to the antigen. As a consequence, subsequent inhalation of ovalbumin alone led to an allergic asthma–like response that included airway hyperresponsiveness to methacholine, eosinophilia, mucus expression, elevated serum levels of antigen-specific IgE and IgG1, and splenic CD4+ T cells that secreted T helper type 2 and type 17 cytokines in response to in vitro antigen restimulation. Furthermore, CD11c+ cells in the mediastinal lymph nodes (MLN) of CAIKKβ-expressing mice displayed significantly elevated levels of activation markers. These data implicate airway epithelial NF-κB activation as a critical modulator of the adaptive immune response to inhaled antigens via the secretion of soluble mediators that affect the capacity of CD11c+ cells to undergo maturation and promote antigen-specific allergic responses.
Keywords: epithelial cell, antigen-presenting cell, NF-κB, allergy, asthma
CLINICAL RELEVANCE.
These studies demonstrate that airway epithelial NF-κB activation, an event common to several inhalational stimuli, is sufficient to promote allergic sensitization to innocuous inhaled antigens. Activation of this pathway in this cell type may represent a therapeutic target for the prevention or treatment of allergic asthma.
Allergic asthma affects approximately 300 million people worldwide, with the incidence continuing to increase steadily in industrialized and developing countries (1). A multifaceted syndrome, allergic asthma is defined by chronic inflammation, bronchoconstriction, mucus metaplasia, airway hyperresponsiveness, T helper (Th) 2 phenotype skewing, and elevated levels of serum IgE (2, 3). Individuals with severe asthma also suffer from increases in airway smooth muscle proliferation and a predisposition to airway remodeling, leading to further permanent long-term damage (4, 5). Current treatment relies on inhaled corticosteroids to control the disorder, which do not prevent asthmatic attacks and do not work effectively for certain patients, such as those with neutrophilic (rather than eosinophilic) infiltrates, those with pre-exisiting inflammatory conditions, and those with certain glucocorticoid receptor polymorphisms (3, 6). Recent studies also suggest that activated Th17-skewed cells are contributors to steroid-resistant and severe allergic asthma (reviewed in Ref. 7).
Allergic asthma is widely recognized as an inflammatory disorder, and recent studies have implicated the transcription factor, NF-κB, considered to be a critical modulator of inflammation in the pathogenesis of lung disease (8–10). Indeed, prolonged and robust airway epithelial NF-κB activation is observed in patients with asthma (11). In previous studies, we have demonstrated that lack of airway epithelial NF-κB activation in a mouse model of allergic asthma results in a diminished inflammatory phenotype; specifically, abrogation of cellular influx, mucus production, production of Th2 cytokines, IL-5 and IL-13, and antigen-specific serum antibodies (12). Th2 cells, which secrete primarily IL-4, -5, and -13, play a key role in allergic asthma by promoting class switching to IgE by B cells, and supporting the accumulation and survival of eosinophils and mast cells, in addition to inducing goblet cell hyperplasia and altering airway smooth muscle constriction and hyperresponsiveness (13, 14).
The airway epithelium, in addition to being the first line of defense against inhaled pathogens, has recently been shown to play an immunomodulatory role in the lung (11). In addition to robust NF-κB activity and the release of proinflammatory cytokines and chemokines, the epithelium also secretes factors that directly influence dendritic cells, the major antigen-presenting cell (APC) in the immune system (13, 15, 16). Epithelial cells recruit dendritic cells to the lung via secretion of the chemokine, CCL20 (macrophage inflammatory protein [MIP]-3α), which is the only cytokine known to interact with the CCR6 receptor expressed on immature dendritic cells (11). Furthermore, airway epithelial cells can also secrete thymic stromal lymphopoeitin (TSLP), granulocyte/macrophage colony–stimulating factor (GM-CSF), and IL-6 in response to antigen, factors that influence the maturation of dendritic cells and polarize CD4+ T cells toward a Th2 phenotype (11, 13, 17, 18).
Under normal circumstances, inhalation of an innocuous antigen, such as an environmental allergen, is perceived and responded to by the immune system in a manner that promotes antigen-specific immunologic tolerance. In experimental models, this immunologic tolerance is prevented by the use of adjuvants (e.g., aluminum hydroxide, cytokines, microbial products, cholera toxin, etc.), which prime the immune system to respond to an otherwise innocuous antigen. We have previously demonstrated that the oxidant gas, nitrogen dioxide, can promote the sensitization of mice to an innocuous inhaled antigen, and that this sensitization occurs in conjunction with activation of airway epithelial NF-κB (19).
Using a transgenic mouse model in which mice express a doxycycline (Dox)-inducible, constitutively active form of the inhibitor of NF-κB (IκB) kinase β (CAIKKβ) specifically in the airway epithelium, we have previously published that airway epithelial activation of NF-κB during antigen challenge exacerbates a conventional alum/ovalbumin (OVA) model of allergic airways disease. This exacerbation promotes neutrophilia, as well as increasing the magnitude of eosinophilia, enhancing lung heterogeneity in response to methacholine, and augmenting bronchoalveolar lavage (BAL) cytokine levels of IL-17, IL-4, keratinocyte-derived chemokine (KC), and MIP-1β (10). Given these results, we sought to determine the effects of airway epithelial NF-κB activation alone during the antigen sensitization phase.
We therefore investigated whether activation of airway epithelial NF-κB is sufficient to promote allergic sensitization to an innocuous inhaled antigen, independent of an exogenously administered adjuvant, and, if so, whether the effect is at least partially due to the activation of pulmonary CD11c+ APCs. Our results demonstrate that transient activation of NF-κB in the airway epithelium is sufficient to initiate events that promote allergic sensitization to an inhaled antigen. These data implicate airway epithelial NF-κB activity as an important facilitator of allergic sensitization that may be a common mediator involved in the capacity of several infectious and environmental agents to predispose susceptible individuals to developing atopic responses to inhaled antigens.
MATERIALS AND METHODS
Mice
CC10-rtTA × TetOP-CAIKKβ bitransgenic mice (10, 20) on the C57BL/6 background express CAIKKβ in bronchiolar epithelium after administration of 6 g/kg Dox in chow (TestDiet, Richmond, IN). Wild-type mice were age- and sex-matched transgene-negative littermates. Mice were housed in an Association for Assessment and Accreditation of Laboratory Animal Care-approved facility, maintained on a 12-hour light/dark cycle, and were provided food and water ad libitum. All animal studies were approved by the University of Vermont Institutional Animal Care and Use committee.
Model of Airway Epithelial NF-κB–Promoted Allergic Sensitization
Mice received Dox chow for 48 hours before 30 min inhalation of 1% OVA (grade V; Sigma-Aldrich, St. Louis, MO) on Day 2, after which they returned to normal food. On Day 7, mice were provided Dox chow for another 48-hour period, followed by a second 1% OVA inhalation on Day 9, after which they were again returned to normal food, on which they were maintained for the remainder of the study. After 1 week, on Days 14, 15, and 16, mice inhaled 1% OVA for 30 minutes each day. Mice were analyzed on Day 18, 48 hours after the last antigen challenge inhalation.
Pulmonary Function Assessment to Measure Airway Hyperresponsiveness
Mice were anesthetized and mechanically ventilated for the assessment of pulmonary function using the forced oscillation technique, as previously described (12, 21). Airway resistance (RN), tissue damping (G), and tissue stiffness (H) (22) were calculated at baseline and after challenge with 25 mg/ml aerosolized methacholine (Sigma-Aldrich) in saline, an appropriate concentration for submucosal responses based on our previously published dose response studies (19, 20). The percentage change from baseline (ΔRN, ΔG, and ΔH), and the peak change in each parameter is reported.
Bronchoalveolar Lavage and Lung Processing
Lungs were lavaged with a single instillation and recovery of 1 ml ice-cold PBS containing protease inhibitor cocktail (Sigma-Aldrich) and processed for cytospin counting as previously described (19). Right lung lobes were flash frozen for RNA and protein analysis. Left lung lobes were processed for hematoxylin and eosin staining as previously described (19). Photomicrographs of airways with a length-to-width ratio of 0.5:2.0 were provided for assessment by two independent individuals blinded to the identity of the specimens, and representative pictures were selected for presentation.
Immunoblotting
Protein (25 μg) from lung homogenates prepared in 1× PBS were run on a 4–20% Tris-Glycine gel and transferred to a nitrocellulose membrane. Lysates were probed for IKKβ (Cell Signaling Technology, Danvers, MA) and reprobed with β-actin as a loading control as previously described (10).
Cytokine Profiling, Protein Assessment, and Lactate Dehydrogenase Measurement from BAL Fluid
Cytokine levels were assessed using a Bioplex 23-plex panel (Bio-Rad, Hercules, CA), as previously described (23). Total protein levels were measured using the Bradford Assay (Bio-Rad), and lactate hehydrogenase (LDH) activity was measured using the LDH Detection Assay Kit (Promega, Madison, WI).
Serum Collection and Ig Analysis
After mice were killed, blood was collected via cardiac puncture into serum separator tubes (Becton Dickinson, Franklin Lanes, NJ), centrifuged, and serum was kept frozen at −80°C. Antigen-specific and total Ig ELISAs were performed as previously described (19).
Quantitative RT-PCR
Total RNA was isolated from lungs using the PrepEase RNA Isolation Kit (USB Corp., Cleveland, OH) and reverse transcribed into cDNA using the iScript cDNA Synthesis Kit (Bio-Rad). Quantitative RT-PCR was performed using SYBR Green PCR Supermix (Bio-Rad) and intron-spanning primers designed for mouse GM-CSF, IL-12p19 (IL-23), serum amyloid A (SAA)3, TSLP, IL-6, IL-23, and IL-25. IQ Supermix (Bio-Rad) and TaqMan AODs (Applied Biosystems Inc, Foster City, CA) were used for GAPDH, CCL20, ZO-1, E-cadherin, and Muc5AC. The level of gene expression was normalized to GAPDH levels and relative gene expression was calculated using the ΔΔCt method, as previously described (19).
Preparation, Stimulation, and Analysis of Single-Cell Mediastinal Lymph Node and CD4+ Lymphocyte Suspensions
Splenic tissue was processed for the isolation of CD4+ T cells from experimental mice and APCs from naive C57BL/6 mice, as previously described (24). CD4+ T cells (4 × 106 cells/ml) were activated with 100 μg/ml OVA in the presence of syngeneic APCs (4 × 106 cells/ml in 48-well plates). After 96 hours of stimulation, supernatants were collected and analyzed by Bio-Plex (Bio-Rad).
Flow Cytometric Analysis of Mediastinal Lymph Node Cells
Mediastinal lymph node (MLN) cells were dissociated by mechanical disruption, filtered through a 40-μm nylon mesh membrane and stained with the following antibodies: CD45-PO, CD11c-PETR, F4/80-Alexa 647, CD86-Alexa 647 (all from Caltag, Carlsbad, CA); I-A/I-E-PerCP/Cy5.5 (BioLegend, San Diego, CA); and CD11b-APCcy7 and glucocorticoid receptor–1–PE (BD Pharmingen, San Diego, CA). Cells (1 × 106) were first blocked with Fc block (anti-CD16/CD32) (2.5 μg/ml; BD Pharmingen) for 30 minutes at 4°C, washed in FACS buffer (Dulbecco's phosphate buffered saline [DPBS]; CellGro, Manassas, VA) DPBS with 5% FBS (Invitrogen, Carlsbad, CA), and then stained for 30 minutes at 4°C in 100 μl of antibody solution at the optimal concentration. After staining, all cells were washed and fixed in DPBS with 5% FBS and 1% paraformaldhehyde. Cells were analyzed on a Becton Dickinson LSR II FACS flow cytometer equipped to distinguish as many as seven fluorophores 1–3 days after staining. Dead cells were excluded from analysis by forward scatter (FSC) and side scatter (SSC) gating, and CD45+, F4/80neg, FITClow cells were gated for further analysis of major histocompatability complex class II (MHCII), CD11b, and CD11c staining.
Statistical Analysis
Data were analyzed by two-tailed unpaired t test using GraphPad Prism 4 for Windows (GraphPad Software, Inc., La Jolla, CA). A P value less than 0.05 was considered statistically significant.
RESULTS
Mice Allergically Sensitized to OVA via Airway Epithelial NF-κB Activation and Antigen Inhalation Exhibit Hyperresponsiveness to Inhaled Methacholine after Antigen Challenge
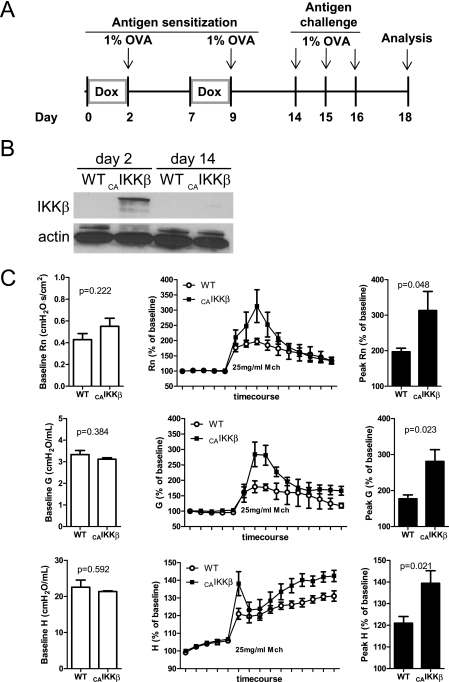
Transgenic (CAIKKβ) and wild-type littermate mice received Dox for two 48-hour periods, each of which were followed by a 30-minute exposure to 1% aerosolized OVA. A week later, mice were challenged with aerosolized 1% OVA on 3 consecutive days and analyzed 48 hours after the final exposure (Figure 1A). Western blot analysis shows that IKKβ can be detected at the protein level after 48 hours of Dox chow. However, when analyzed just before inhaled antigen challenge on Day 14, the IKKβ protein is undetectable, illustrating that, after 5 days on normal chow, transgene activation returns to the undetectable levels observed before Dox administration (Figure 1B). All mice exhibited normal pulmonary physiology at baseline, as illustrated in the first five points of the time course before the inhalation of methacholine, but the antigen-challenged CAIKKβ mice were hyperresponsive to methacholine in comparison with their wild-type counterparts (Figure 1C). Methacholine responsiveness, as measured by the parameters RN, G, and H, were significantly increased in CAIKKβ mice as a consequence of antigen challenge (Figure 1C, insets). Because methacholine hyperresponsiveness is indicative of an allergic asthma-like phenotype, we further characterized inflammatory and immunologic consequences in the CAIKKβ mice.
Figure 1.
Airway epithelial NF-κB activation promoted antigen sensitization and airway hyperresponsiveness to methacholine. (A) A timeline of exposure regimens to promote antigen sensitization for constitutively active inhibitor of NF-κB (IκB) kinase β (CAIKKβ) transgenic and wild-type (WT) littermate mice. All 1% ovalbumin (OVA) inhalations were 30 minutes long. (B) Western blot of lung homogenates for expression of the CAIKKβ transgene at Days 2 and 14. Note that endogenous IKKβ is below the detection limit of these Western blot procedures. (C) Respiratory mechanics assessment of methacholine responsiveness using the forced oscillation technique in mice on Day 18 at baseline before administration of methacholine (left), during the methacholine challenge regimen (middle), and at the peak response to 25 mg/ml methacholine (right) (n = 5 mice/group).
Airway Epithelial NF-κB Activation in Combination with OVA Inhalation Promotes an Allergic Pattern of Pulmonary Inflammation, Mucus Metaplasia, and a Th2-Skewed Phenotype in Response to Challenge with Inhaled OVA
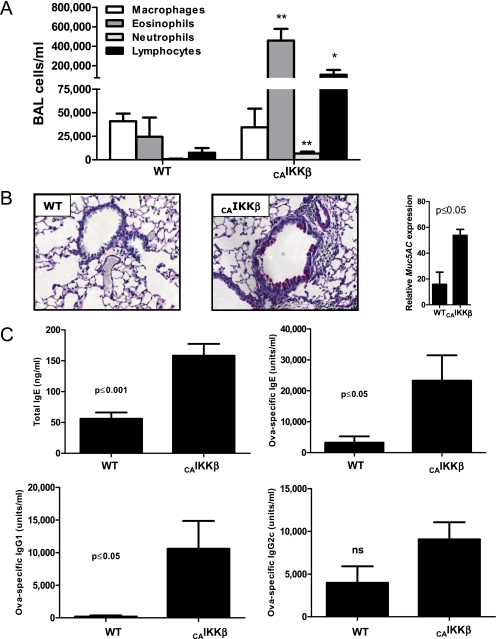
Lungs were lavaged at harvest and the BAL fluid was examined for cellular infiltration. Whereas wild-type mice subjected to the OVA inhalation regimen described in Figure 1A exhibited normal levels of macrophages and a small number of eosinophils, CAIKKβ mice displayed substantial and significant increase in eosinophils and lymphocytes (Figure 2A), as is observed in conventional allergic asthma models in which alum is used as an adjuvant. In addition, we also measured significant increases in airway neutrophils after allergen challenge (Figure 2A). Airway epithelial NF-κB activation also promoted mucus metaplasia in response to inhaled antigen, as well as augmented gene expression of Muc5ac, a product of mucus-producing cells (Figure 2B). Furthermore, when serum from the mice was examined by ELISA, CAIKKβ mice displayed significant increases in Th2 cytokine–promoted total and antigen-specific IgE, as well as antigen-specific IgG1, with no significant changes in IgG2c, a Th1-driven Ig (Figure 2C).
Figure 2.
Airway epithelial activation at the time of first encounter with antigen induces an influx of inflammatory cells subsequent to inhaled allergen challenge. CAIKKβ and WT littermate mice were exposed as depticed in Figure 1A. (A) On Day 18, differential cell counts were assessed from the bronchoalveolar lavage (BAL). Periodic acid–Schiff reactivity was assessed from histologic lung specimens visualized at 200× (B), and expression of the mucus gene Muc5AC was measured by quantitative RT-PCR and displayed relative to the levels in naive mice. (C) Serum Ig levels were measured by ELISA. Standard curves for OVA-specific IgE and IgG1 were generated from alum/OVA-sensitized BALB/cJ mouse serum, whereas nitrogen dioxide/OVA-sensitized (19) C57BL/6J serum was used to generate standards for OVA-specific IgG2c. For comparisons, values for the most concentrated standards were set to 10,000 U/ml (n = 6 mice/group). Data are representative of studies performed twice. *P ≤0.05; **P ≤0.01.
CD4+ T Cells from Transgenic Mice Restimulated with Antigen Secrete Th2 Cytokines and IL-17
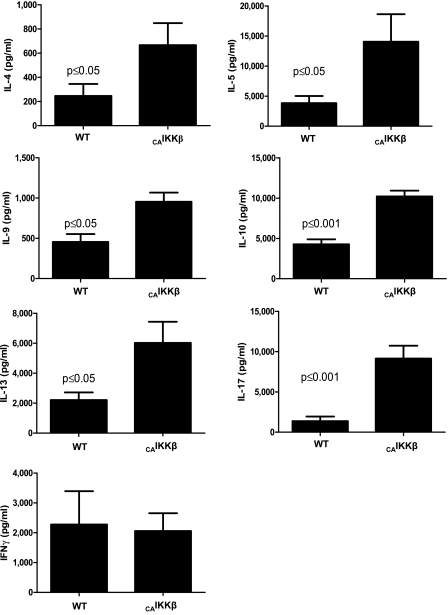
CD4+ T cells purified from the spleen at Day 18 were stimulated with OVA by APCs from naive C57BL/6J mice. Cell supernatants analyzed by Bio-Plex at 96 hours displayed a distinctive Th2 profile, with significant increases in IL-4, -5, -9, -10, and -13 (Figure 3), and no significant changes in the Th1-driven cytokine IFN-γ. Interestingly, CAIKKβ transgenic mice also showed substantial increases in IL-17, a cytokine not abundantly produced by CD4+ T cells in alum/OVA-sensitized mice.
Figure 3.
CAIKKβ transgene expression promotes an antigen-specific T helper (Th) 2– and Th17-skewed CD4+ T-cell phenotype. Splenic CD4+ T cells were incubated with antigen-presenting cells (APCs) and OVA for 96 hours, after which cytokines in cell culture supernatants were measured by Bio-Plex (n = 6 mice/group). Data are representative of studies performed twice.
Airway Epithelial NF-κB Expression Promotes the Activation of Pulmonary Dendritic Cells
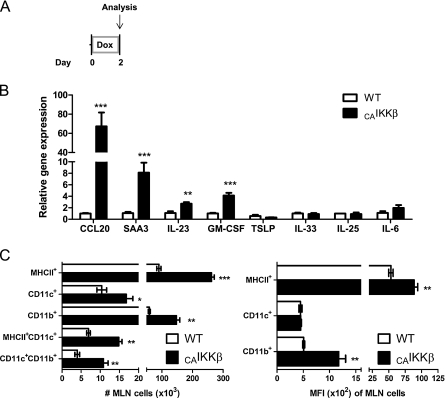
As a potential mechanism to explain the capacity of airway epithelial NF-κB activation to promote allergic sensitization to an innocuous inhaled antigen, we measured several parameters related to pulmonary dendritic cell activation. RNA from whole-lung homogenates was analyzed by quantitative RT-PCR from CAIKKβ and wild-type littermate mice after 48 hours of Dox chow (Figure 4A). Expression of several genes implicated in dendritic cell recruitment and differentiation, including Ccl20, Gmcsf, Il12p19 (IL-23), and Saa3 were significantly increased in CAIKKβ mice on Dox compared with wild-type littermates (Figure 4B). However, several other Th2- or Th17-promoting genes, which are capable of being expressed by epithelium, including IL-6, -33, -25, and TSLP (25), were not elevated in the lungs of CAIKKβ mice on Dox (Figure 4B). Furthermore, mediastinal lymph nodes from these mice contained significantly more cells with a surface phenotype indicative of mature dendritic cells, along with increases in cell surface expression of MHCII and CD11b. as analyzed by flow cytometry (Figure 4C).
Figure 4.
Airway epithelial NF-κB expression modulates pulmonary CD11c+ APCs. (A) CAIKKβ and WT littermates fed doxycycline (Dox) chow for 48 hours and harvested immediately were (B) assessed by quantitative RT-PCR from whole lung (n = 4 mice/group). (C) Mediastinal lymph node cells were stained and analyzed by flow cytometry for APC markers. Total cell numbers in each subset were calculated (left) and median fluorescence intensity (MFI) was calculated for each of the cell markers used (right) (n = 3 mice/group). *P ≤ 0.05; **P ≤ 0.01; ***P ≤ 0.001.
Airway Epithelial NF-κB Activation Does Not Compromise Epithelial Barrier Integrity
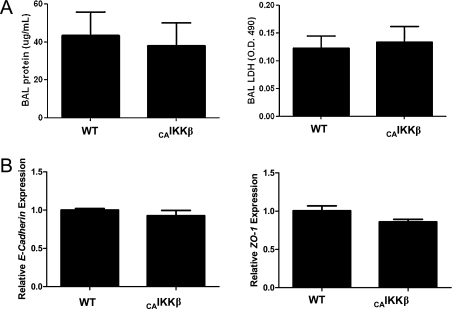
Recent studies have suggested that dendritic cells can be primed by antigen alone if there is sufficient disruption of the epithelial barrier comprised of the adherens junction complex (26). Therefore, we measured BAL total protein and lactate dehydrogenase (Figure 5A), and found no significant differences between CAIKKβ mice and wild-type control animals, both of which were fed Dox for 48 hours. In addition, we measured gene expression of zona occludens (ZO)–1 and E-cadherin, two constituents of epithelial adherens junctions, and found no reduction in expression in CAIKKβ mice compared with wild-type control mice (Figure 5B). Together, these data indicate that the epithelial barrier remains intact subsequent to airway epithelial NF-κB activation.
Figure 5.
Airway epithelial NF-κB expression does not affect epithelial barrier integrity. CAIKKβ and WT littermates fed Dox chow for 48 hours and harvested immediately. (A) BAL fluid was collected and total protein by Bradford assay (left) and lactate dehydrogenase activity (right) was recorded. (B) Whole-lung homogenates were assessed by quantitative RT-PCR for epithelial adherens junction markers zona occludens (ZO)–1 (left) and E-cadherin (right) (n = 4 mice per group). Data are representative of studies performed twice.
DISCUSSION
Once considered to primarily serve a barrier function in the lung, airway epithelium is now widely recognized for its immunomodulatory capabilities. This has allowed for a better understanding of how allergic sensitization in the lung-draining lymph node may occur subsequent to inhalation of an antigen in the context of an appropriate immunostimulatory milieu. Indeed, the data presented herein are the first to demonstrate that airway epithelial intracellular signaling through the NF-κB pathway is sufficient to promote allergic sensitization to an innocuous inhaled antigen. Many immunostimulatory factors are expressed by airway epithelial cells subsequent to inhalational exposure to microbes or environmental antigens (11). Several of these immunostimulatory factors (8–10) are transcriptionally regulated by NF-κB and were measured in our model of inducible airway epithelial NF-κB activation. The CAIKKβ mice expressed a robust Th2 and Th17 cytokine profile as a consequence of antigen inhalation, creating the proper environment for dendritic cells to drive a mixed Th2 and Th17 effector response instead of developing inhalational tolerance. It is possible that viral and bacterial infections can act in the same manner: as an “NF-κB adjuvant,” creating an inflammatory environment that allows for otherwise harmless inhaled antigens to become immunogenic. Similarly, several agents that are potent activators of NF-κB are also capable of promoting allergic sensitization to innocuous inhaled proteins. These include both microbial products and environmental agents, such as endotoxin (27), silica (28), residual oil fly ash (29), diesel exhaust particles (30), ultrafine particles (31), cigarette smoke (32), ozone (33), and nitrogen dioxide (19), suggesting that airway epithelial NF-κB activation may be a common mechanism through which several agents promote antigen-specific allergic sensitization.
The presence of a mixed Th2 and Th17 response is not new; recent studies have revealed that inhalational methods of allergic sensitization (as opposed to the traditional intraperitoneal alum/OVA model of sensitization) generate a modest Th2 profile and a more robust Th17 response (34, 35). Our model of sensitization also relies on inflammatory events that originate in the lung; therefore, it is not surprising that we have seen similar mixed T-cell phenotypes, with a Th2 profile that is modest compared with that seen in the traditional alum/OVA model. It is important to note that recent studies have implicated inhalational models of allergic sensitization to be more physiologically relevant than the traditional intraperitoneal alum/OVA model. For instance, the alum/OVA model can induce eosinophilia so severe that it makes up 80% of the total BAL cells, whereas, in patients with asthma, eosinophils make up 5% at most (34). Our previous alum/OVA sensitization regimen achieved eosinophilia on the order of 80–90% of total BAL cells (10), in contrast to the more modest 60% seen in our current model. Likewise, inhalational models, such as LPS/OVA achieve a similar moderate eosinophilia (27, 31, 36).
Recent studies have shown that airway epithelial cells exert control over dendritic cells, the primary APC instructing adaptive immune responses, which are therefore a key player in allergic disease (11, 17). Under normal conditions, dendritic cells that encounter a harmless antigen do not up-regulate the expression of costimulatory molecules and do not initiate an inflammatory T-cell effector response; instead, they promote a tolerogenic response accompanied by the activation of T regulatory cells (2). This tolerance is assisted by incompletely matured dendritic cells, including plasmacytoid dendritic cells, which suppress T cell effector generation. In the case of encounters with microbial products or environmental agents, airway epithelial cells secrete TSLP, which acts directly upon dendritic cells to up-regulate the costimulatory molecules, OX40L, CD40, and CD80, which facilitate Th2 polarization (17). In addition to TSLP, it has been reported that epithelial cells are capable of promoting Th2 polarization by secretion of IL-33, -25, and -6 (18, 25, 37). Furthermore, airway epithelial cells secrete factors that induce the differentiation of monocytes into dendritic cells and promote their maturation, namely, GM-CSF (11). GM-CSF has been shown to demonstrate adjuvant-like properties, and concomitant exposure of GM-CSF and OVA (38) or GM-CSF and Derp1 (39), can sensitize mice to a Th2 allergic response. Although our mouse model did not demonstrate any significant differences in TSLP expression (data not shown), CAIKKβ mice did transcriptionally up-regulate GM-CSF. Further studies focused on neutralization of GM-CSF in our sensitization model may further elucidate the critical role of this cytokine in polarizing pulmonary CD11c+ cells, but were beyond the scope of the current study. In this study, we have demonstrated that airway epithelial NF-κB is sufficient to promote an inflammatory milieu that favors allergic sensitization and modulation of pulmonary dendritic cell activation.
It is important to note, however, that, although our model of inducible airway epithelial NF-κB activation induces cytokine production and exerts an effect upon other surrounding cells, transgene activation is transient and resolved before antigen challenge. Recently, there has been an emergence of reports supporting a role for antigens in directly effecting dendritic cells (26). Herbert and colleagues (40) demonstrated that house dust mite challenge causes a disruption in epithelial tight junctions, allowing access to dendritic cells within the airway wall. It has likewise been shown that the lungs of patients with asthma demonstrate poorly developed tight junctions, as evidenced by poor staining of ZO-1 (41). We examined epithelial integrity by measuring BAL protein and LDH that would suggest epithelial leakage or airway cell death. However, we observed no changes in either after 48 hours of Dox administration. In addition, gene expression of ZO-1 and E-cadherin remained unchanged. This leads us to suspect that the changes in dendritic cell maturity found in the MLN of CAIKKβ mice are not due to increased interaction with environmental antigen as a consequence of compromised epithelial barrier integrity. Certainly, if mice were provided Dox chow ad libitum for a more protracted period of time than we employed, and epithelial NF-κB activation was sustained, we would likely have revealed substantial compromise of the epithelial barrier integrity. However, at the time points relevant to our study, airway epithelial NF-κB activation itself was not sufficient to induce fluid leak or alter expression of tight junction–expressing genes. Instead, airway epithelial NF-κB activation did itself promote the expression of cytokines implicated in dendritic cell and Th cell recruitment and activation.
The analysis of gene induction in our transgenic mice after 48 hours of Dox chow revealed an increase in both CCL20, a potent dendritic cell chemokine, and GM-CSF, which differentiates monocytes into a dendritic cell phenotype. CAIKKβ mice also expressed increased levels of the IL-12p19 subunit, which helps to form the active IL-23 heterodimer, a cytokine that recent studies have implicated as a precursor to the Th17-skewed T-cell response (42, 43). SAA3, an acute-phase protein expressed outside the liver, the gene expression of which is up-regulated in the CAIKKβ mice, has been shown to cause blood monocytes to preferentially secrete IL-23 instead of IL-12p40 (42), thus possibly contributing to the generation of a Th17-involving antigen-specific allergic response. In addition, SAA3 signals through Toll-like receptor 2 (44, 45), a pattern recognition receptor that we have demonstrated to be essential for nitrogen dioxide–promoted allergic sensitization (19).
In a healthy lung, tolerance is the prevailing response after initial exposure to innocuous inhaled antigen (46), and requires the collaboration of several cell types and soluble mediators. However, when the signals for APC activation and Th cell polarization are already present as a consequence of lung inflammation, an otherwise harmless antigen can initiate an allergic response, mimicking the manner in which an adjuvant allows for immune sensitization. Although we have used inducible airway epithelial NF-κB activation as an effective model to illustrate this point, it is clear that other signaling pathways and cell types could mediate this effect as well. Therefore, airway epithelial NF-κB activity appears to be a central mediator in the inflammatory and immune response to infections and environmental insults that modulates allergic sensitization and the severity of subsequent allergic airway disease.
This work was supported by National Institutes of Health grants R01 HL089177 (M.E.P.), R01 HL060014 (Y.M.W.J.-H.), and by National Center for Research Resources Center of Biomedical Research Excellence P20RR15557.
Originally Published in Press as DOI: 10.1165/rcmb.2010-0106OC on June 25, 2010
Author Disclosure: J.L.A. received a sponsored grant from Sepracor for $50,001–$100,000 and from the National Institutes of Health (NIH) for more than $100,001; Y.M.W.J.-H. received sponsored grants from NIH for $10,001–$50,000 and for more than $100,001; M.E.P. received lecture fees for $1,001–$5,000 and a sponsored grant for $50,001–$100,000 from Sepracor, and a sponsored grant from NIH for more than $100,001; S.R.H. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Braman SS. The global burden of asthma. Chest 2006;130(1 Suppl):4S–12S. [DOI] [PubMed] [Google Scholar]
- 2.Willart MA, Lambrecht BN. The danger within: endogenous danger signals, atopy and asthma. Clin Exp Allergy 2009;39:12–19. [DOI] [PubMed] [Google Scholar]
- 3.Fanta CH. Asthma. N Engl J Med 2009;360:1002–1014. [DOI] [PubMed] [Google Scholar]
- 4.Shore SA. Airway smooth muscle in asthma—not just more of the same. N Engl J Med 2004;351:531–532. [DOI] [PubMed] [Google Scholar]
- 5.Borger P, Tamm M, Black JL, Roth M. Asthma: is it due to an abnormal airway smooth muscle cell? Am J Respir Crit Care Med 2006;174:367–372. [DOI] [PubMed] [Google Scholar]
- 6.Adcock IM, Barnes PJ. Molecular mechanisms of corticosteroid resistance. Chest 2008;134:394–401. [DOI] [PubMed] [Google Scholar]
- 7.Alcorn JF, Crowe CR, Kolls JK. Th17 cells in asthma and COPD. Annu Rev Physiol 2010;72:495–516. [DOI] [PubMed] [Google Scholar]
- 8.Wright JG, Christman JW. The role of nuclear factor kappa B in the pathogenesis of pulmonary diseases: implications for therapy. Am J Respir Med 2003;2:211–219. [DOI] [PubMed] [Google Scholar]
- 9.Hayden MS, West AP, Ghosh S. Nf-kappaB and the immune response. Oncogene 2006;25:6758–6780. [DOI] [PubMed] [Google Scholar]
- 10.Pantano C, Ather JL, Alcorn JF, Poynter ME, Brown AL, Guala AS, Beuschel SL, Allen GB, Whittaker LA, Bevelander M, et al. Nuclear factor-κB activation in airway epithelium induces inflammation and hyperresponsiveness. Am J Respir Crit Care Med 2008;177:959–969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kato A, Schleimer RP. Beyond inflammation: airway epithelial cells are at the interface of innate and adaptive immunity. Curr Opin Immunol 2007;19:711–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Poynter ME, Cloots R, van Woerkom T, Butnor KJ, Vacek P, Taatjes DJ, Irvin CG, Janssen-Heininger YM. Nf-kappa B activation in airways modulates allergic inflammation but not hyperresponsiveness. J Immunol 2004;173:7003–7009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hammad H, Lambrecht BN. Dendritic cells and epithelial cells: linking innate and adaptive immunity in asthma. Natl Rev 2008;8:193–204. [DOI] [PubMed] [Google Scholar]
- 14.Shore SA. Direct effects of Th2 cytokines on airway smooth muscle. Curr Opin Pharmacol 2004;4:235–240. [DOI] [PubMed] [Google Scholar]
- 15.Stumbles PA, Strickland DH, Pimm CL, Proksch SF, Marsh AM, McWilliam AS, Bosco A, Tobagus I, Thomas JA, Napoli S, et al. Regulation of dendritic cell recruitment into resting and inflamed airway epithelium: use of alternative chemokine receptors as a function of inducing stimulus. J Immunol 2001;167:228–234. [DOI] [PubMed] [Google Scholar]
- 16.Bleck B, Tse DB, Jaspers I, Curotto de Lafaille MA, Reibman J. Diesel exhaust particle–exposed human bronchial epithelial cells induce dendritic cell maturation. J Immunol 2006;176:7431–7437. [DOI] [PubMed] [Google Scholar]
- 17.Wang Y, Bai C, Li K, Adler KB, Wang X. Role of airway epithelial cells in development of asthma and allergic rhinitis. Respir Med 2008;102:949–955. [DOI] [PubMed] [Google Scholar]
- 18.Neveu WA, Allard JB, Dienz O, Wargo MJ, Ciliberto G, Whittaker LA, Rincon M. IL-6 is required for airway mucus production induced by inhaled fungal allergens. J Immunol 2009;183:1732–1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bevelander M, Mayette J, Whittaker LA, Paveglio SA, Jones CC, Robbins J, Hemenway D, Akira S, Uematsu S, Poynter ME. Nitrogen dioxide promotes allergic sensitization to inhaled antigen. J Immunol 2007;179:3680–3688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ather JL, Alcorn JF, Brown AL, Guala AS, Suratt BT, Janssen-Heininger YM, Poynter ME. Distinct functions of airway epithelial nuclear factor–kB activity regulate nitrogen dioxide–induced acute lung injury. Am J Respir Cell Mol Biol 2010;43:443–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tomioka S, Bates JH, Irvin CG. Airway and tissue mechanics in a murine model of asthma: alveolar capsule vs. forced oscillations. J Appl Physiol 2002;93:263–270. [DOI] [PubMed] [Google Scholar]
- 22.Lundblad LK, Thompson-Figueroa J, Leclair T, Sullivan MJ, Poynter ME, Irvin CG, Bates JH. Tumor necrosis factor–α overexpression in lung disease: a single cause behind a complex phenotype. Am J Respir Crit Care Med 2005;171:1363–1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Paveglio SA, Allard J, Mayette J, Whittaker LA, Juncadella I, Anguita J, Poynter ME. The tick salivary protein, Salp15, inhibits the development of experimental asthma. J Immunol 2007;178:7064–7071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Paveglio SA, Allard J, Foster Hodgkins SR, Ather J, Bevelander M, Mayette Campbell J, Whittaker Leclair LA, McCarthy SM, van der Vliet A, Suratt BT, et al. Airway epithelial indoleamine 2,3-dioxygenase inhibits CD4+ T cells during Aspergillus fumigatus antigen exposure. Am J Respir Cell Mol Biol 2010 Jan 29; Epub ahead of print. [DOI] [PMC free article] [PubMed]
- 25.Saenz SA, Taylor BC, Artis D. Welcome to the neighborhood: epithelial cell–derived cytokines license innate and adaptive immune responses at mucosal sites. Immunol Rev 2008;226:172–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wills-Karp M, Nathan A, Page K, Karp CL. New insights into innate immune mechanisms underlying allergenicity. Mucosal Immunol 2010;3:104–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Eisenbarth SC, Piggott DA, Huleatt JW, Visintin I, Herrick CA, Bottomly K. Lipopolysaccharide-enhanced, Toll-like receptor 4–dependent T helper cell type 2 responses to inhaled antigen. J Exp Med 2002;196:1645–1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Arras M, Huaux F, Vink A, Delos M, Coutelier JP, Many MC, Barbarin V, Renauld JC, Lison D. Interleukin-9 reduces lung fibrosis and type 2 immune polarization induced by silica particles in a murine model. Am J Respir Cell Mol Biol 2001;24:368–375. [DOI] [PubMed] [Google Scholar]
- 29.Lambert AL, Dong W, Winsett DW, Selgrade MK, Gilmour MI. Residual oil fly ash exposure enhances allergic sensitization to house dust mite. Toxicol Appl Pharmacol 1999;158:269–277. [DOI] [PubMed] [Google Scholar]
- 30.Diaz-Sanchez D. The role of diesel exhaust particles and their associated polyaromatic hydrocarbons in the induction of allergic airway disease. Allergy 1997;52(38 Suppl):52–56; discussion, 57–58. [DOI] [PubMed] [Google Scholar]
- 31.de Haar C, Hassing I, Bol M, Bleumink R, Pieters R. Ultrafine but not fine particulate matter causes airway inflammation and allergic airway sensitization to co-administered antigen in mice. Clin Exp Allergy 2006;36:1469–1479. [DOI] [PubMed] [Google Scholar]
- 32.Trimble NJ, Botelho FM, Bauer CM, Fattouh R, Stampfli MR. Adjuvant and anti-inflammatory properties of cigarette smoke in murine allergic airway inflammation. Am J Respir Cell Mol Biol 2009;40:38–46. [DOI] [PubMed] [Google Scholar]
- 33.Koike E, Watanabe H, Kobayashi T. Exposure to ozone enhances antigen-presenting activity concentration dependently in rats. Toxicology 2004;197:37–46. [DOI] [PubMed] [Google Scholar]
- 34.Wilson RH, Whitehead GS, Nakano H, Free ME, Kolls JK, Cook DN. Allergic sensitization through the airway primes Th17-dependent neutrophilia and airway hyperresponsiveness. Am J Respir Crit Care Med 2009;180:720–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zygmunt BM, Rharbaoui F, Groebe L, Guzman CA. Intranasal immunization promotes Th17 immune responses. J Immunol 2009;183:6933–6938. [DOI] [PubMed] [Google Scholar]
- 36.Tan AM, Chen HC, Pochard P, Eisenbarth SC, Herrick CA, Bottomly HK. TLR4 signaling in stromal cells is critical for the initiation of allergic Th2 responses to inhaled antigen. J Immunol 2010;184:3535–3544. [DOI] [PubMed] [Google Scholar]
- 37.Paul WE, Zhu J. How are T(H)2-type immune responses initiated and amplified? Nat Rev Immunol 2010;10:225–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stampfli MR, Wiley RE, Neigh GS, Gajewska BU, Lei XF, Snider DP, Xing Z, Jordana M. GM-CSF transgene expression in the airway allows aerosolized ovalbumin to induce allergic sensitization in mice. J Clin Invest 1998;102:1704–1714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cates EC, Fattouh R, Wattie J, Inman MD, Goncharova S, Coyle AJ, Gutierrez-Ramos JC, Jordana M. Intranasal exposure of mice to house dust mite elicits allergic airway inflammation via a GM-CSF–mediated mechanism. J Immunol 2004;173:6384–6392. [DOI] [PubMed] [Google Scholar]
- 40.Herbert CA, King CM, Ring PC, Holgate ST, Stewart GA, Thompson PJ, Robinson C. Augmentation of permeability in the bronchial epithelium by the house dust mite allergen Der p1. Am J Respir Cell Mol Biol 1995;12:369–378. [DOI] [PubMed] [Google Scholar]
- 41.Holgate ST. Epithelium dysfunction in asthma. J Allergy Clin Immunol 2007;120:1233–1244; quiz 1245–1236. [DOI] [PubMed] [Google Scholar]
- 42.He R, Shepard LW, Chen J, Pan ZK, Ye RD. Serum amyloid a is an endogenous ligand that differentially induces IL-12 and IL-23. J Immunol 2006;177:4072–4079. [DOI] [PubMed] [Google Scholar]
- 43.Lewkowich IP, Lajoie S, Clark JR, Herman NS, Sproles AA, Wills-Karp M. Allergen uptake, activation, and IL-23 production by pulmonary myeloid dcs drives airway hyperresponsiveness in asthma-susceptible mice. PLoS ONE 2008;3:e3879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cheng N, He R, Tian J, Ye PP, Ye RD. Cutting edge: TLR2 is a functional receptor for acute-phase serum amyloid A. J Immunol 2008;181:22–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.He RL, Zhou J, Hanson CZ, Chen J, Cheng N, Ye RD. Serum amyloid a induces G-CSF expression and neutrophilia via Toll-like receptor 2. Blood 2009;113:429–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Holt PG, McMenamin C. Defence against allergic sensitization in the healthy lung: the role of inhalation tolerance. Clin Exp Allergy 1989;19:255–262. [DOI] [PubMed] [Google Scholar]