Abstract
Acute lung injury (ALI) and its severe form, acute respiratory distress syndrome (ARDS), are major causes of acute respiratory failure with high rates of morbidity and mortality. Although surfactant protein (SP)-D plays a critical role in pulmonary innate immunity and several clinical studies suggest that this protein may be implicated in the pathophysiology of ARDS, little is known regarding the function of SP-D in ARDS. In the present study, we induced indirect lung injury by intraperitoneal injection of LPS and direct lung injury by intratracheal injection of LPS in wild-type and Sftpd−/− mice to elucidate the role of SP-D during ALI/ARDS. Results indicate that pulmonary levels of IL-6 and TNF-α were higher in Sftpd−/− mice when compared with wild-type mice. However, the magnitude of this difference was 10-fold greater after indirect lung injury compared with direct lung injury. After indirect lung injury, there was a 2-fold increase in the number of pulmonary monocyte/macrophages in the Sftpd−/− mice when compared with wild-type mice, whereas pulmonary neutrophils were not increased. After indirect injury, the concentration of granulocyte-macrophage colony stimulating factor (GM-CSF) was approximately 5-fold greater in Sftpd−/− mice than wild-type mice. In contrast, after direct injury, the concentration of GM-CSF was 20-fold less in Sftpd−/− mice than wild-type mice. Despite increased inflammatory cells and markers of inflammation, survival in Sftpd−/− mice after indirect lung injury was paradoxically increased. In conclusion, these results suggest that SP-D inhibits pulmonary inflammation and migration of peripheral monocyte/macrophages into the lung through GM-CSF–dependent pathways during indirect lung injury.
Keywords: surfactant protein D, acute respiratory distress syndrome, macrophage, GM-CSF
Clinical Relevance.
During indirect lung injury surfactant protein (SP)-D inhibits pulmonary inflammation and migration of peripheral monocyte/macrophages into the lung through granulocyte/macrophage colony–stimulating factor–dependent pathways. However, despite the decreased pulmonary inflammation, SP-D was not associated with decreased mortality in mice. This study raises the importance of SP-D during acute lung injury/acute respiratory distress syndrome induced by indirect lung injury and suggests a potential therapeutic intervention in neonatal and adult patients with this syndrome.
Acute lung injury (ALI), and its severe form, acute respiratory distress syndrome (ARDS), are characterized by acute pulmonary inflammation and pulmonary edema. The inflammatory response in ARDS originates from the loss of vascular endothelial cell integrity, resulting in alveolar capillary leak, development of protein-rich pulmonary edema, surfactant dysfunction, pulmonary inflammation, damage to the lung parenchyma and pulmonary epithelium, and respiratory insufficiency (1). The outcome of this self-perpetuating process is excessive lung injury and, in many cases, multiple organ dysfunction syndrome, organ failure, and death.
ALI/ARDS develops from a variety of clinical disorders that can be differentiated into those associated with direct lung injury (e.g., pneumonia, aspiration) and those causing indirect lung injury (e.g., sepsis, shock). Although the resulting pulmonary inflammation and damage to the lung parenchyma are similar, there is a growing body of evidence that suggests that the two modes of injury have unique underlying pathophysiological mechanisms (2).
Analysis of bronchoalveolar lavage fluid (BALF) samples from adult patients with ARDS has demonstrated that, in addition to inflammation, alterations in lung surfactant function and composition are present. Whereas pulmonary levels of surfactant proteins A, B, and C decrease during ARDS, levels of SP-D increase (3). Moreover, patients with ARDS who have relatively higher levels of pulmonary SP-D have increased survival rates, suggesting a vital role for SP-D in ARDS.
SP-D is a member of the collectin family of innate defense proteins that binds inflammatory molecules such as LPS as well as a variety of viral, bacterial, and fungal pathogens (4). SP-D binding facilitates the uptake and clearance of pathogens from the lung by alveolar macrophages and neutrophils (5). Although binding infectious microbes is a critical feature of SP-D function, animal models of SP-D deficiency indicate that SP-D also regulates pulmonary immune cells and decreases inflammation during direct pulmonary infection (6, 7). SP-D is also required for normal surfactant structure and for uptake and recycling of surfactant by alveolar type II cells (8, 9). Taken collectively, the changes of SP-D expression during ARDS and the critical role of SP-D in regulating immune cells and maintaining normal surfactant homeostasis suggest that SP-D plays a critical role in the body's response to ARDS.
Therefore, we hypothesize that SP-D attenuates pulmonary immune cell activation during ALI/ARDS and as a result reduces pulmonary inflammation and limits lung injury. To determine if SP-D decreased inflammation during ALI/ARDS, we induced lung injury in wild-type mice and mice lacking SP-D (Sftpd−/−) by intraperitoneal or intratracheal injection of LPS. Whereas SP-D deficiency resulted in increased macrophage recruitment and a marked increase in pulmonary inflammation in mice after indirect lung injury, survival was improved in SP-D–deficient mice.
MATERIALS AND METHODS
Animals
Studies were performed on 6- to 10-week-old Sftpd−/− and age-matched Sftpd+/+ litter mate wild-type control mice (10). Mice were housed in conditions approved by the Institutional Animal Care and Use Committee at Cincinnati Children's Hospital Medical Center and maintained in the vivarium under barrier containment facilities. Evidence of common murine pathogens was not detected in sentinel mice in the colony.
Treatment with Lipopolysaccharide
Animals were anesthetized with isoflurane. LPS, serotype Escherichia coli O111:B4, dissolved in PBS in doses of 20 and 40 mg/kg was injected into the intraperitoneal space. For direct lung injury, PBS or 20 μg/kg LPS was administered by tracheal aspiration.
Assessment of Pulmonary Injury
Mice were killed with intraperitoneal injection of pentobarbital. BAL was performed by intratracheal instillion and recovery of a 1 ml aliquot of PBS. The fluid was stored at −20°C until analysis or −80°C for antioxidant evaluation.
Blood samples were obtained by cardiac puncture at the time of death and stored at −20°C until cytokine levels were determined.
IL-6 was quantified in BALF and plasma with a murine enzyme-linked immunosorbent assay using a commercially available kit (R&D Systems, Minneapolis, MN). Granulocyte-macrophage colony stimulating factor (GM-CSF), IL-10, IL-12p70, IL1b, IL-2, and TNF-α were quantified with a multiplex bead-based assay (Bio-Rad, Hercules, CA). Chromogenic limulus amebocyte lysate test was used to quantify endotoxin in BALF (Cambrex BioScience, Walkersville, MD). A BCA Protein Assay kit (Pierce, Rockford, IL) was used to determine protein concentration in BALF. Antioxidant activity was quantified by measuring copper-reducing equivalents using a commercially available assay (Oxford Biomedical Research, Oxford, MI).
Cell counts were determined in BALF samples. BALF cells were centrifuged and resuspended in 500 μL of PBS. Cell counts were performed on cytospin preparations of the cell pellet suspension after staining with hematoxylin and eosin.
For histology, lungs were inflation fixed at 25 cm H2O. Immunohistochemistry for MAC-3 was performed at dilutions of 1:200 by using rabbit polyclonal antibody. Immune complexes were detected using an avidin-biotin-peroxidase technique.
Assessment of Mortality
For the general assessment of mortality, wild-type and Sftpd−/− mice were treated with 40 mg/kg of LPS injected into the intraperitoneal space and followed hourly for 24 hours.
Mice containing the doxycylcine-inducible Sftpd gene (CCSPrtTA+ (tetO)7-rSP-D+, mSP-D−/− mice) were used to investigate the role of surfactant lipid levels on survival in Sftpd−/− mice. The high-surfactant lipid group was kept on a doxycycline-free diet until the time of the mortality study. The low-surfactant lipid group was kept on doxycycline from the time of delivery until 4 weeks of age and then transitioned to a doxycycline-free diet. At 7 weeks of age, each group was treated with 40 mg/kg of LPS injected into the intraperitoneal space and followed hourly for 24 hours.
Statistical Analysis
All results are given as the mean ± SEM. The groups were compared using two-tailed Student's t test. Differences of P < 0.05 were considered significant.
RESULTS
Previous results from both clinical studies in patients with ARDS and from mouse models of SP-D deficiency led us to the hypothesis that SP-D decreases pulmonary inflammation and limits lung injury during ALI. Because ALI/ARDS can be caused by indirect and direct lung injury, this hypothesis was tested by intraperitoneal injection of LPS to induce indirect lung injury and intratracheal aspiration of LPS to induce direct lung injury in wild-type and Sftpd−/− mice.
Sftpd−/− Mice Have Increased Inflammation after Indirect Lung Injury
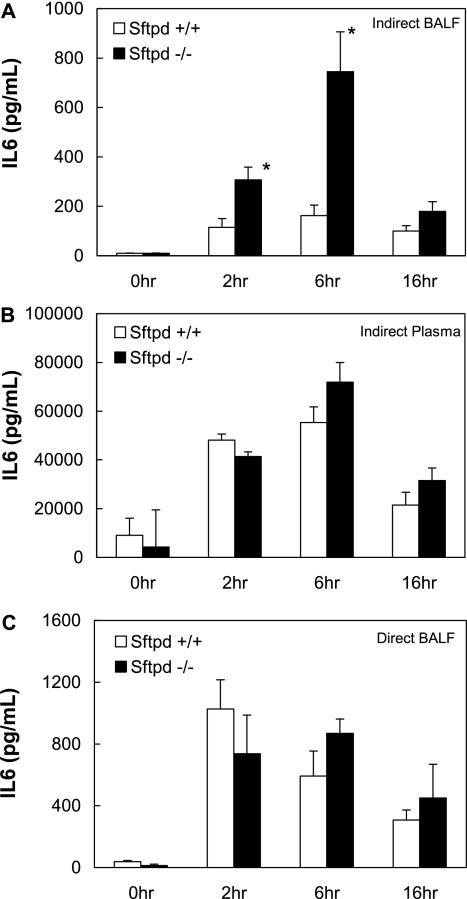
To evaluate the role of SP-D on inflammation during ALI, pulmonary IL-6 concentrations were assessed after indirect lung injury with intraperitoneal LPS. BALF from Sftpd−/− had 4-fold greater concentrations of IL-6 compared with wild-type mice 6 hours after treatment (P < 0.05) (Figure 1A). These results suggest that SP-D limits pulmonary inflammation during indirect lung injury; however, SP-D is present in serum during sepsis, raising the possibility that the increase in pulmonary inflammation seen in Sftpd−/− mice may result from elevated systemic inflammation. Therefore, markers of systemic inflammation were also assessed. Similar to BALF findings, plasma IL-6 concentrations were increased after intraperitoneal LPS injection in Sftpd−/− mice when compared with wild-type mice; however, the increase was an order of magnitude less than the 4-fold difference observed in pulmonary samples (Figure 1B). Taken together, these results indicate that SP-D inhibits pulmonary inflammation during indirect lung injury.
Figure 1.
Bronchoalveolar lavage fluid (BALF) and plasma IL-6 levels after lung injury. Sftpd−/− and wild-type mice were treated with PBS or LPS via intraperitoneal (40 mg/kg LPS) or intratracheal (20 μg/kg LPS) injection (n = 5 for each group). BALF or blood was harvested 2, 6, and 16 hours after injection, and IL-6 levels were quantified by ELISA. (A) At 6 hours, BALF IL-6 levels were 4-fold greater in Sftpd−/− mice than in wild-type mice after intraperitoneal LPS treatment (P ≤ 0.01). (B) Plasma IL-6 levels were increased in Sftpd−/− mice compared with wild-type mice after intraperitoneal LPS treatment; however, this 30% increase (P = 0.12) was not as dramatic as the differences seen in BALF samples. (C) After intratracheal LPS treatment, BALF IL-6 levels were similar between wild-type and Sftpd−/− mice, suggesting that SP-D does not significantly protect the lung from inflammation 6 hours after direct lung injury.
Considering the primary location of SP-D is in the lung and the known role of SP-D in binding inhaled pathogens, one would expect that SP-D would have a greater impact on inflammation in direct lung injury (i.e., intratracheal administration of LPS) than indirect lung injury (i.e., intraperitoneal injection of LPS). Previous studies have examined the role of SP-D after direct lung injury and, in marked contrast to our findings, observed only a 50% increase in pulmonary IL-6 levels in Sftpd−/− mice compared with wild-type mice (11). To confirm that the difference in pulmonary inflammation in Sftpd−/− compared with wild-type mice after indirect and direct lung injury was due to the route of lung injury and not to a variation in experimental conditions, we induced direct lung injury in Sftpd−/− and wild-type mice by intratracheal aspiration of LPS. To ensure that we were inducing similar levels of lung injury, we used a dose of intratracheal LPS that achieved similar levels of pulmonary inflammation as that obtained after intraperitoneal LPS in wild-type mice. BALF IL-6 concentrations were increased above baseline in Sftpd−/− and wild-type mice after intratracheal LPS; however, the relative increase in BALF IL-6 concentrations in Sftpd−/− mice was only 50% higher than wild-type mice 6 and 16 hours after treatment (Figure 1C). Our results with direct lung injury are similar to results obtained by Ikegami and colleagues and support the concept that SP-D has a larger role in protecting the lungs from indirect lung injury compared with direct lung injury.
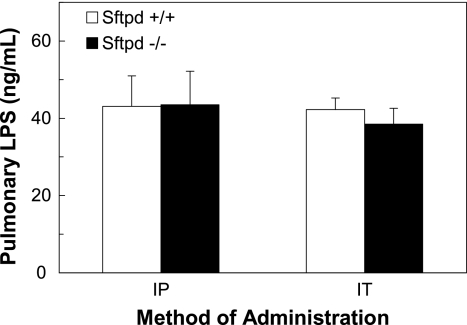
Distinct abnormalities in lung structure and alveolar cell morphology in SP-D–deficient animals have been well described (10, 12). Consequently, the increase in pulmonary inflammation observed after an indirect injury may be the result of increased capillary-alveolar leakage of systemic LPS inducing an exaggerated local inflammatory response in the lung. To assess if the pulmonary inflammation was the result of inherently incompetent tissue structure and subsequent increased capillary-alveolar leakage of systemic LPS into the lung, BALF LPS was quantified after indirect and direct lung injury. There were no differences in intrapulmonary BALF LPS concentrations between the Sftpd−/− and wild-type mice after either method of lung injury, indicating that the elevated pulmonary inflammation in Sftpd−/− mice after indirect lung injury is not due to increased LPS leakage into the lung (Figure 2).
Figure 2.
LPS levels in BALF after indirect and direct lung injury. The amount of LPS in BALF samples (n = 5 for each group) was quantified by Limulus assay after mice were treated with intraperitoneal (IP) or intratracheal (IT) LPS to assess if the increased pulmonary inflammation observed in Sftpd−/− mice was due to increased capillary-alveolar leakage of LPS. The levels of LPS were similar between the wild-type and Sftpd−/− mice after either route of administration.
Sftpd−/− Mice Have Increased Morbidity but Decreased Mortality
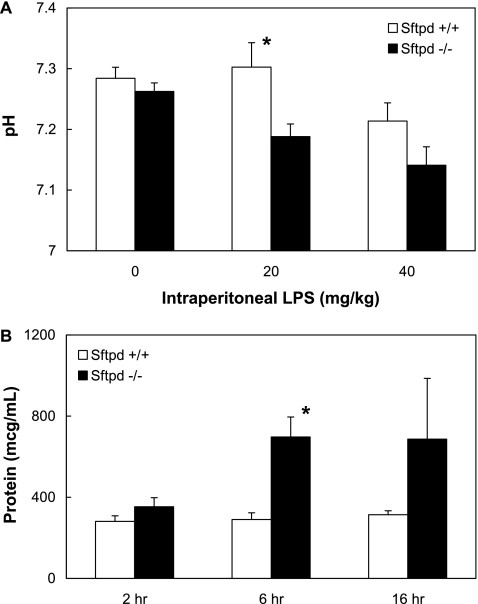
Although there is quantitative evidence of increased pulmonary inflammation in Sftpd−/− mice during indirect lung injury, markers of inflammation may not correlate with clinical morbidity. Subjectively, LPS-treated mice were lethargic and exhibited hunched backs, ruffled fur, and ocular discharge; these characteristics were more severe in Sftpd−/− mice than in wild-type mice. Blood gas analysis and alveolar protein levels were assessed to objectively measure the clinical effect of SP-D during indirect lung injury. In support of the subjective observations, Sftpd−/− mice were significantly more acidotic and had higher alveolar protein levels when compared with wild-type mice after LPS-induced indirect lung injury (Figure 3).
Figure 3.
pH and alveolar protein values after indirect lung injury. Blood was collected for blood gas analysis 6 hours after intraperitoneal injection of PBS or LPS, and BALF was collected for protein analysis 2, 6, or 16 hours after injection. Sftpd−/− mice were more acidotic than wild-type mice (P ≤ 0.02, 20 mg/kg; P = 0.01, 40 mg/kg; n = 5 in all groups) and trended to have more alveolar protein (P ≤ 0.02 at 6 h).
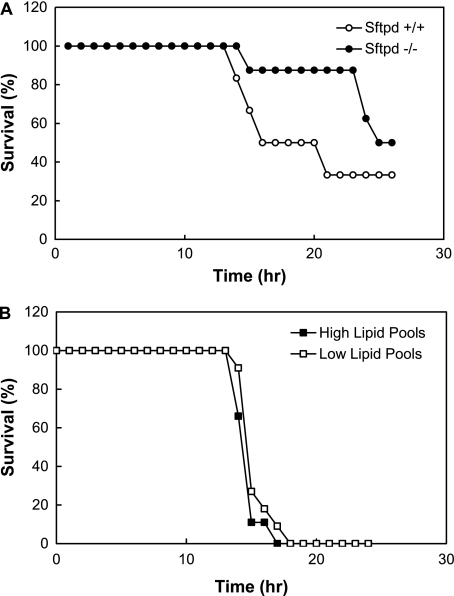
Severity of morbidity often predicts risk of mortality; however, previous studies examining hyperoxia-induced lung injury in Sftpd−/− mice demonstrated increased pulmonary inflammation with a paradoxical decrease in mortality in Sftpd−/− mice when compared with wild-type control mice. We found similar results after indirect lung injury. Twenty-four hours after injection of LPS, survival was 63% in Sftpd−/− mice and only 33% in wild-type mice, indicating that although pulmonary inflammation and morbidity are increased in Sftpd−/− mice, overall mortality is decreased (Figure 4A).
Figure 4.
Survival after indirect lung injury. (A) Sftpd−/− and wild-type mice were treated with intraperitoneal injection of LPS. (B) CCSPrtTA+ (tetO)7-rSP-D+, Sftpd−/− mice were kept on a doxycycline-free diet (Sftpd−/− high-surfactant-lipid group) or CCSPrtTA+ (tetO)7-rSP-D+. Sftpd−/− mice remained on doxycycline from the time of delivery until 4 weeks of age, at which time they were transitioned to a doxycycline-free diet (i.e., Sftpd−/− low-surfactant-lipid group). Survival for each group (n = 10 for each group) was recorded throughout a 24-hour observation period and plotted as a Kaplan-Meier survival analysis. Comparison at 24 hours revealed enhanced survival in the Sftpd−/− mice compared with wild-type mice but no difference in Sftpd−/− mice with low or high surfactant lipid levels.
Several previous studies have established that alveolar surfactant lipid pools are increased in Sftpd−/− mice. Therefore, to determine if the paradoxical decrease in mortality in Sftpd−/− mice after indirect lung injury was due to elevated surfactant lipid pool sizes, mice containing the doxycycline-inducible Sftpd gene (CCSPrtTA+ (tetO)7-rSP-D+, Sftpd−/−) were used. Zhang and colleagues demonstrated that when these mice were placed on a doxycycline diet during the first 2 weeks of life, lipid pool sizes decreased to wild-type levels and remained at wild-type levels after removal of doxycycline for up to 4 weeks (13). We took advantage of this finding to generate Sftpd−/− mice with high- and low-surfactant lipid pool sizes. The high-surfactant-lipid group was kept on a doxycycline-free diet (i.e., Sftpd−/− phenotype with high-surfactant lipids), whereas the low-surfactant lipid-group remained on doxycycline from the time of delivery until 4 weeks of age, at which time they were transitioned to a doxycycline-free diet (i.e., Sftpd−/− phenotype with low-surfactant lipids). After intraperitoneal LPS injection, survival was similar in these two groups, suggesting that surfactant lipid pool size does not affect mortality after indirect lung injury in Sftpd−/− mice (Figure 4B).
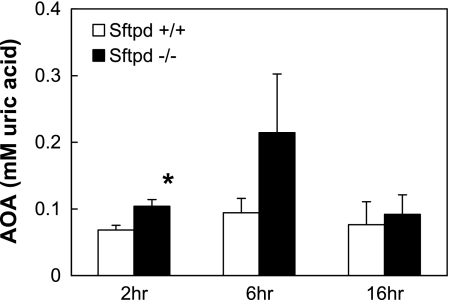
Because similar unanticipated mortality results have been demonstrated in a hyperoxia model, another plausible rationale for improved survival in these animal models may be attributable to increased levels of antioxidant enzymes. Sftpd−/− mice demonstrated a 1.5- to 2- fold increase in antioxidant activity in BALF when compared with wild-type control mice after indirect lung injury (Figure 5). This result may provide some insight into the survival mechanism involved in Sftpd−/− mice subjected to indirect lung injury.
Figure 5.
Antioxidant activity after indirect lung injury. Antioxidant activity in BALF samples from Sftpd−/− and wild-type mice was quantified after treatment with intraperitoneal LPS (n = 5 for each group). Sftpd−/− mice demonstrated an increase in antioxidant activity in BALF when compared with wild-type control mice after indirect lung injury (P ≤ 0.02 at 2 h).
Macrophages Implicated in Inflammation
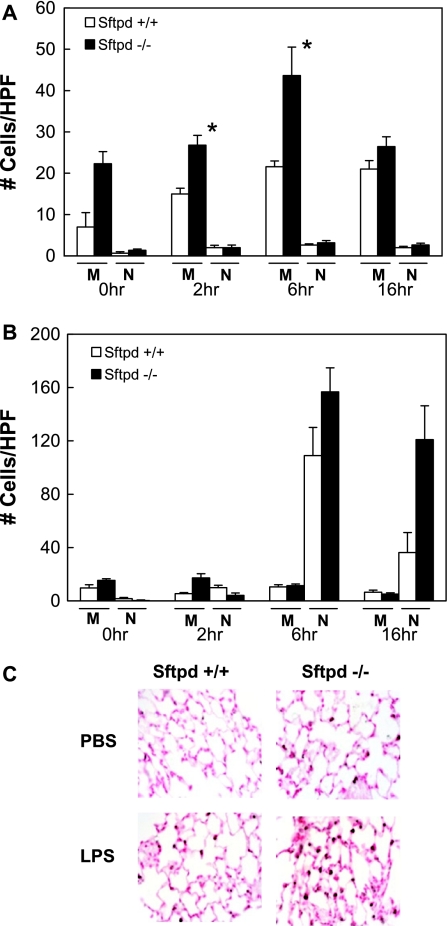
Previous studies demonstrated that Sftpd−/− mice accumulate enlarged foamy alveolar macrophages, and several studies have implicated SP-D in macrophage regulation. However, results by Ikegami and colleagues suggested that an influx of pulmonary neutrophils was responsible for the increased inflammation observed in Sftpd−/− mice after direct lung injury (11). Upon examination of BALF after indirect lung injury, we discovered that, although pulmonary neutrophils were not increased, there was a 2-fold increase in the number of monocyte/macrophages in the Sftpd−/− mice compared with wild-type mice (Figure 6A). In contrast, after direct lung injury, Sftpd−/− and wild-type mice had a marked increase in pulmonary neutrophils but no increase in the number of monocyte/macrophages (Figure 6B). These results suggest that SP-D inhibits the influx of circulating monocytes into the lung or that SP-D inhibits the migration of macrophages from the pulmonary interstitium to the alveoli (and subsequently BALF) during indirect lung injury. To differentiate between these two mechanisms of monocyte/macrophage movement, mouse lungs were immunostained with MAC-3, a macrophage-specific antibody. During indirect lung injury, the number of MAC-3–positive cells increased approximately 2-fold in Sftpd−/− mice, whereas there was no increase in MAC-3–positive cells in wild-type mice (Figure 6C). These results indicate that there is an increase in the total number of pulmonary monocyte/macrophages in the absence of SP-D and suggest that SP-D inhibits migration of monocyte/macrophages into the lung after indirect lung injury.
Figure 6.
Cell count after acute lung injury. BALF samples from Sftpd −/− and wild-type mice after treatment with PBS or LPS were stained, and cell counts were quantified (n = 5 for each group). (A) After indirect lung injury, there was a 2-fold increase in the number of monocytes/macrophages (M) in the Sftpd−/− mice compared with wild-type mice, whereas pulmonary neutrophils (N) were not increased. (B) In contrast, after direct lung injury, Sftpd−/− and wild-type mice had a 5-fold increase in pulmonary neutrophils but no increase in the number of monocyte/macrophages. (C) Mouse lungs were immunostained with MAC-3, a macrophage-specific antibody. MAC-3–positive cells increased in Sftpd−/− mice after LPS treatment, whereas there was no increase in MAC-3–positive cells in wild-type mice.
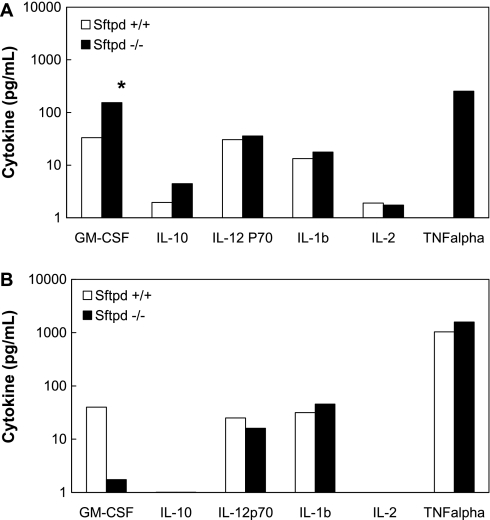
Macrophages respond to a variety of innate and adaptive stimuli after injury or infection. To identify the mechanism for the observed influx of macrophages seen in Sftpd−/− mice after indirect lung injury, a multiplex cytokine assay (which allows for the identification and quantification of several cytokines/chemokines simultaneously) was performed on BALF samples after indirect or direct lung injury. After indirect injury, the concentration of GM-CSF was approximately 5-fold greater in Sftpd−/− mice than in wild-type mice (Figure 7A). In contrast, after direct injury, the concentration of GM-CSF was 20-fold less in Sftpd−/− mice than in wild-type mice (Figure 7B). Although not statistically significant because of sample variability, TNF-α was also greater in Sftpd−/− mice than in wild-type mice after indirect injury, yet there was no difference between the two groups in direct injury. There were no differences in IL-10, IL-12p70, IL-1β, or IL-2 between the two groups after either type of lung injury. Because GM-CSF is known to increase myeloid adhesion, proliferation, and differentiation, these results raise the possibility that SP-D regulates the migration of monocytes into the lung by inhibiting GM-CSF secretion during indirect lung injury. In addition, the suppression of GM-CSF may lead to a decrease in TNF-α and other inflammatory cytokines, further altering macrophage activation and regulation.
Figure 7.
BALF cytokines after indirect and direct lung injury. A multiplex cytokine assay was performed on BALF samples after (A) intraperitoneal or (B) intratracheal LPS treatment (n = 5 in each group). Granulocyte/macrophage colony–stimulating factor was dramatically higher in the Sftpd−/− mice than in wild-type mice after indirect injury, yet this difference was not seen after direct injury (P ≤ 0.02). TNF-α levels were also higher in the Sftpd−/− mice than in wild-type mice after indirect injury.
DISCUSSION
ARDS develops in patients of all ages from a variety of insults. Although SP-D plays a critical role in pulmonary innate immunity and several clinical studies suggest that this protein may be implicated in the pathophysiology of ARDS, little is known regarding the function of SP-D in ALI/ARDS (14). In the present study, we induced lung injury in wild-type and Sftpd−/− mice to elucidate the role of SP-D during endotoxemia-induced ALI/ARDS.
Based on inflammatory cytokine levels, pulmonary inflammation was significantly higher in Sftpd−/− mice when compared with wild-type mice with ALI after indirect lung injury. Considering the primary role and location of SP-D is in the lung, it is counterintuitive that the difference in pulmonary inflammation between Sftpd−/− and wild-type mice was greater after indirect lung injury than after direct lung injury. The simplest explanation for this discrepancy is increased systemic LPS leakage into the lung through damaged alveolar capillary membranes in Sftpd−/− mice. However, the quantity of LPS in the lung after indirect or direct lung injury was similar, which contradicts this hypothesis and raises the possibility that SP-D plays a more complex role in regulating the interchange between systemic and pulmonary inflammation during ALI/ARDS. BALF cell counts in Sftpd−/− and wild-type mice after lung injury suggest that the mechanistic details of this role entail monocyte/macrophage recruitment and activation. Whereas clinical studies have shown that there is a predominant increase in neutrophils in BALF obtained from patients with ARDS and animal studies demonstrate significant elevation of neutrophils in the pulmonary microcirculation after endotoxin treatment, our results demonstrate that after indirect lung injury SP-D deficiency increases extravasation of peripheral blood macrophages into the alveolar spaces without a change in neutrophil influx (15, 16). Conversely, BALF cell counts suggest that, after direct lung injury, SP-D deficiency does not alter monocyte/macrophage migration (11). Analysis of inflammatory cytokine profiles suggests that SP-D may control inflammatory cell migration by inhibiting GM-CSF secretion and consequently altering TNF-α and other inflammatory cytokine expression, but the specifics of this signaling cascade require clarification by further experiments. Together, these changes in cellular and cytokine response demonstrate the importance of SP-D during indirect lung injury. Finally, although several studies have suggested that SP-D regulates pulmonary host defense cells and that SP-D preserves the integrity of the alveolar-capillary interface by maintaining balance in the pulmonary inflammatory cascade, this is the first study to suggest that SP-D protects this interface from the effects of the systemic inflammatory cascade (6, 17).
We found that, despite increased inflammatory cells and markers of inflammation, Sftpd−/− mice demonstrated a paradoxical increase in survival. The explanation for this is unclear. This same phenomenon was seen in another study in mice exposed to hyperoxia. Jain and colleagues suggested that the improvement in survival in Sftpd−/− mice exposed to hyperoxia may be due to increased surfactant pool sizes, preserved surfactant function, or an adaptive increase in antioxidant components (18). Our results with CCSPrtTA+ (tetO)7-rSP-D+, Sftpd−/− mice indicate that increased surfactant pool sizes do not improve survival after lung injury in Sftpd−/− mice. In contrast, the discovery of increased antioxidant activity in BALF of Sftpd−/− mice after indirect lung injury suggests one possible adaptive process that may improve survival in these mice. Given that antioxidants increase only 2-fold in Sftpd−/− mice, it is plausible that additional factors may influence survival in Sftpd−/− mice subjected to lung injury (10, 12, 19).
Although the majority of clinical research on ARDS has focused on adult patients, premature neonates represent a clinically important population. Every year, 12.9 million babies are born premature (< 37 wk gestation) worldwide, representing a prevalence of preterm birth of 9.6% (20). Unfortunately, this rate continues to increase. Premature infants are known to have immature lung structure as well as quantitative and functional deficiencies in surfactant. Infants born premature also have higher rates of respiratory illness and septicemia than infants born at term (21). Knowing that these are all risk factors for ALI/ARDS, the cumulative effect is that ALI/ARDS is a leading cause of morbidity and mortality in premature infants. SP-D is first detected in the lung, but at low levels, at 16 to 18 weeks gestation, with levels rising moderately from 32 weeks to term (22). Thus, it is important to reveal the SP-D–dependent mechanisms of ALI/ARDS after endotoxemia to improve the prognosis in SP-D–deficient states, such as premature birth.
Due to its success in the treatment of premature infants with respiratory distress syndrome, surfactant replacement therapy is under investigation as a potential therapy for ALI/ARDS in pediatric and adult patients (23, 24). Initial studies using surfactant have not been encouraging; however, these studies used an aerosolized form of surfactant or protein-free phospholipid preparations of surfactant that did not contain SP-D (25–27). With the findings of this study and further investigation, it is plausible that the instillation of surfactant that contains SP-D may reverse or impede the pulmonary inflammation and injury that develops in patients of all ages with ALI/ARDS caused by indirect lung injury. However, this possibility is highly dependent on whether the paradoxical decreased mortality rate observed in Sftpd−/− mice is also observed in human SP-D–deficient states.
In summary, during indirect lung injury, SP-D inhibits pulmonary inflammation and migration of peripheral monocytes/macrophages into the lung through GM-CSF–dependent pathways. However, despite the decreased pulmonary inflammation, SP-D was not associated with decreased mortality in mice. This study raises the importance of SP-D during ALI/ARDS induced by indirect lung injury and suggests a potential therapeutic intervention in neonatal and adult patients with this syndrome.
Acknowledgments
The authors thank Dr. Jeffrey Whitsett for supplying Sftpd−/− mice and critical reading of the manuscript.
Supported by National Institutes of Health grant HL089505 (P.S.K.) and Ikaria Advancing Newborn Medicine Grant for Fellows in Neonatology (B.A.K.).
Originally Published in Press as DOI: 10.1165/rcmb.2009-0436OC on July 16, 2010
Author Disclosure: None of the authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Ware LB. Pathophysiology of acute lung injury and the acute respiratory distress syndrome. Semin Respir Crit Care Med 2006;27:337–349. [DOI] [PubMed] [Google Scholar]
- 2.Matthay MA, Zimmerman GA, Esmon C, Bhattacharya J, Coller B, Doerschuk CM, Floros J, Gimbrone MA Jr, Hoffman E, Hubmayr RD, et al. Future research directions in acute lung injury: summary of a national heart, lung, and blood institute working group. Am J Respir Crit Care Med 2003;167:1027–1035. [DOI] [PubMed] [Google Scholar]
- 3.Greene KE, Wright JR, Steinberg KP, Ruzinski JT, Caldwell E, Wong WB, Hull W, Whitsett JA, Akino T, Kuroki Y, et al. Serial changes in surfactant-associated proteins in lung and serum before and after onset of ards. Am J Respir Crit Care Med 1999;160:1843–1850. [DOI] [PubMed] [Google Scholar]
- 4.Crouch E, Wright JR. Surfactant proteins a and d and pulmonary host defense. Annu Rev Physiol 2001;63:521–554. [DOI] [PubMed] [Google Scholar]
- 5.van de Wetering JK, van Golde LM, Batenburg JJ. Collectins: players of the innate immune system. Eur J Biochem 2004;271:1229–1249. [DOI] [PubMed] [Google Scholar]
- 6.Gardai SJ, Xiao YQ, Dickinson M, Nick JA, Voelker DR, Greene KE, Henson PM. By binding sirpalpha or calreticulin/cd91, lung collectins act as dual function surveillance molecules to suppress or enhance inflammation. Cell 2003;115:13–23. [DOI] [PubMed] [Google Scholar]
- 7.Yoshida M, Korfhagen TR, Whitsett JA. Surfactant protein d regulates nf-kappa b and matrix metalloproteinase production in alveolar macrophages via oxidant-sensitive pathways. J Immunol 2001;166:7514–7519. [DOI] [PubMed] [Google Scholar]
- 8.Ikegami M, Hull WM, Yoshida M, Wert SE, Whitsett JA. Sp-d and gm-csf regulate surfactant homeostasis via distinct mechanisms. Am J Physiol Lung Cell Mol Physiol 2001;281:L697–L703. [DOI] [PubMed] [Google Scholar]
- 9.Ikegami M, Na CL, Korfhagen TR, Whitsett JA. Surfactant protein d influences surfactant ultrastructure and uptake by alveolar type ii cells. Am J Physiol Lung Cell Mol Physiol 2005;288:L552–L561. [DOI] [PubMed] [Google Scholar]
- 10.Korfhagen TR, Sheftelyevich V, Burhans MS, Bruno MD, Ross GF, Wert SE, Stahlman MT, Jobe AH, Ikegami M, Whitsett JA, et al. Surfactant protein-d regulates surfactant phospholipid homeostasis in vivo. J Biol Chem 1998;273:28438–28443. [DOI] [PubMed] [Google Scholar]
- 11.Ikegami M, Scoville EA, Grant S, Korfhagen T, Brondyk W, Scheule RK, Whitsett JA. Surfactant protein-d and surfactant inhibit endotoxin-induced pulmonary inflammation. Chest 2007;132:1447–1454. [DOI] [PubMed] [Google Scholar]
- 12.Wert S, Jones T, Korfhagen T, Fisher J, Whitsett J. Spontaneous emphysema in surfactant protein d gene-targeted mice. Chest 2000;117(Suppl 1):248S. [DOI] [PubMed] [Google Scholar]
- 13.Zhang L, Ikegami M, Dey CR, Korfhagen TR, Whitsett JA. Reversibility of pulmonary abnormalities by conditional replacement of surfactant protein d (sp-d) in vivo. J Biol Chem 2002;277:38709–38713. [DOI] [PubMed] [Google Scholar]
- 14.Eisner MD, Parsons P, Matthay MA, Ware L, Greene K. Plasma surfactant protein levels and clinical outcomes in patients with acute lung injury. Thorax 2003;58:983–988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Weiland JE, Davis WB, Holter JF, Mohammed JR, Dorinsky PM, Gadek JE. Lung neutrophils in the adult respiratory distress syndrome: clinical and pathophysiologic significance. Am Rev Respir Dis 1986;133:218–225. [DOI] [PubMed] [Google Scholar]
- 16.Kinoshita M, Mochizuki H, Ono S. Pulmonary neutrophil accumulation following human endotoxemia. Chest 1999;116:1709–1715. [DOI] [PubMed] [Google Scholar]
- 17.LeVine AM, Whitsett JA, Gwozdz JA, Richardson TR, Fisher JH, Burhans MS, Korfhagen TR. Distinct effects of surfactant protein a or d deficiency during bacterial infection on the lung. J Immunol 2000;165:3934–3940. [DOI] [PubMed] [Google Scholar]
- 18.Jain D, Atochina-Vasserman E, Kadire H, Tomer Y, Inch A, Scott P, Savani RC, Gow AJ, Beers MF. Sp-d-deficient mice are resistant to hyperoxia. Am J Physiol Lung Cell Mol Physiol 2007;292:L861–L871. [DOI] [PubMed] [Google Scholar]
- 19.Hawgood S, Poulain FR. The pulmonary collectins and surfactant metabolism. Annu Rev Physiol 2001;63:495–519. [DOI] [PubMed] [Google Scholar]
- 20.Howson CP, Zhong N, Padilla C, Yunis K, Giugliani R. The march of dimes global network for maternal and infant health: Harnessing the power of experts in lower-income countries to improve the health of women, mothers, newborns and babies. J. Peking Univ. 2009;41:392–394. [PubMed] [Google Scholar]
- 21.Lawn JE, Wilczynska-Ketende K, Cousens SN. Estimating the causes of 4 million neonatal deaths in the year 2000. Int J Epidemiol 2006;35:706–718. [DOI] [PubMed] [Google Scholar]
- 22.Stahlman MT, Gray ME, Hull WM, Whitsett JA. Immunolocalization of surfactant protein-d (sp-d) in human fetal, newborn, and adult tissues. J Histochem Cytochem 2002;50:651–660. [DOI] [PubMed] [Google Scholar]
- 23.Ramanathan R. Surfactant therapy in preterm infants with respiratory distress syndrome and in near-term or term newborns with acute rds. J Perinatol 2006;26(Suppl 1):S51–S56; discussion, S63–S54. [DOI] [PMC free article] [PubMed]
- 24.Wirbelauer J, Speer CP. The role of surfactant treatment in preterm infants and term newborns with acute respiratory distress syndrome. J Perinatol 2009;29:S18–S22. [DOI] [PubMed] [Google Scholar]
- 25.Sun Y, Yang R, Zhong JG, Fang F, Jiang JJ, Liu MY, Lu J. Aerosolised surfactant generated by a novel noninvasive apparatus reduced acute lung injury in rats. Crit Care 2009;13:R31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Maruscak A, Lewis JF. Exogenous surfactant therapy for ARDS. Expert Opin Investig Drugs 2006;15:47–58. [DOI] [PubMed] [Google Scholar]
- 27.Adhikari N, Burns KE, Meade MO. Pharmacologic treatments for acute respiratory distress syndrome and acute lung injury: systematic review and meta-analysis. Treat Respir Med 2004;3:307–328. [DOI] [PubMed] [Google Scholar]