Abstract
In an effort to improve biological activities and to examine antimycobacterial-lipophilicity relationships of 2-[(1E)-alkenyl)]-4-(1H)-quinolones, we have synthesized a series of 30 quinolones by introducing several alkyl groups, an alkenyl and an alkynyl group at N-1. All synthetic compounds were first tested in vitro against Mycobacterium smegmatis and the most active compounds (MIC values ∼3.0–7.0 μM) were further examined against three other rapidly growing strains of mycobacteria using a microtiter broth dilution assay. The Clog P values of the synthetic compounds were calculated to provide an estimate of their lipophilicity. Compounds 18e, 19a and 19b displayed the most potent inhibitory effect against M. smegmatis mc2155 with an MIC value of ∼1.5 μM, which was twenty fold and thirteen fold more potent than isoniazid and ethambutol, respectively. On the other hand, compounds 17e, 18e and 19a were most active against Mycobacterium fortuitum and Mycobacterium phlei with an MIC value of ∼3.0 μM. In the human diploid embryonic lung cell line MRC-5 cytotoxicity assay, the derivatives showed moderate to strong cytotoxic activity. Although the antimycobacterial activity of our synthetic compounds could not be correlated with the calculated log P values, an increase in lipophilicity enhances the antimycobacterial activity and C13–C15 total chain length at positions 1 and 2 is required to achieve optimal inhibitory effect against the test strains.
Keywords: Antimycobacterial, Lipophilicity, N-Substituted-2-[(1E)-alkenyl]-4-(1H)-quinolone, Structure-activity relationship
Graphical abstract
Thirty new N-substituted 2-[(1E)-alkenyl]-4-(1H)-quinolones were prepared and examined for their in vitro antimycobacterial activities against four rapidly growing strains of mycobacteria.
Highlights
► Synthesis of 30 new N-substituted-2-[(1E)-alkenyl]-4-(1H)-quinolones. ► MIC till 0.5 mg/L against one of the fast growing Mycobacteria in vitro. ► Substituents at N-1 influence the antimycobacterial activity. ► Best activity with 13–15 C-atoms overall in both side chains.
1. Introduction
Since the discovery of nalidixic acid in the early 1960s, quinolones have been the subject of continuous academic interest and various structural modifications have resulted in second, third and fourth-generation quinolone antibiotics, which are currently in the market. Continuous modifications in the basic structure of quinolones have increased their antibacterial spectrum and potency, making quinolones useful for the treatment of urinary, systemic and respiratory tract infections. Furthermore, along with other antibacterial agents, quinolones have been used extensively in veterinary practice, either as antibacterial or as growth promoters. Various studies blame their wide spread overuses and misuses for emergency of resistance to quinolones [1]. Ciprofloxacin, for example, is the most consumed antibacterial quinolone worldwide [2], and consequently most exposed to resistance. In the last decade there is a dramatic increase in the number of quinolone antimycobacterial agents under development, which is mainly driven by the problem of multi-drug resistance. Multi drug resistant tuberculosis (MDR-TB) is treated with second-line drugs, where treatment takes two years or longer and is expensive. Some of the fluoroquinolones used to treat MDR-TB are too toxic as they were not originally developed specifically for tuberculosis. The emergency of extensively drug resistant tuberculosis (XDR-TB) further complicated the treatment and management of TB. Therefore, new drugs with new mode of action are required to cope with this disturbing trend of drug resistance.
Although a series of fluoroquinolone derivatives containing a carboxyl group at C-3 have been synthesized and are commercially available for the treatment of many types of infectious diseases including tuberculosis, no attempt has been made to evaluate the antimycobacterial potential of quinolones with an aliphatic substituent at position 2. In our previous studies we disclosed the antimycobacterial properties of 1-methyl-2-alkenyl-4-(1H)-quinolones isolated from the fruits of Euodia rutaecarpa Hook f. & Thomson (Rutaceae) [3], [4]. Very recently, we have reported the structural requirements for potent antimycobacterial activities of 1-methyl-2-alkenyl-4-(1H)-quinolones derivatives [5]. Results of our studies revealed that lipophilicity is essential for growth inhibition and compounds having C11–C14 alkenyl groups at C-2 displayed potent inhibitory effects compared to the antibiotics ethambutol and isoniazid. In an effort to improve the antimycobacterial activities and further explore the structure-activity relationships at position 1, in this paper, we report synthesis and antimycobacterial activity evaluation of 2-alkenyl-4-(1H)-quinolones possessing ethyl, propyl, butyl or pentyl group at N-1. To further assess whether unsaturated groups at N-1 has any impact on the activity of compounds of this type, 2-propenyl and 2-propynyl groups were introduced to position 1.
2. Results and discussion
2.1. Chemistry
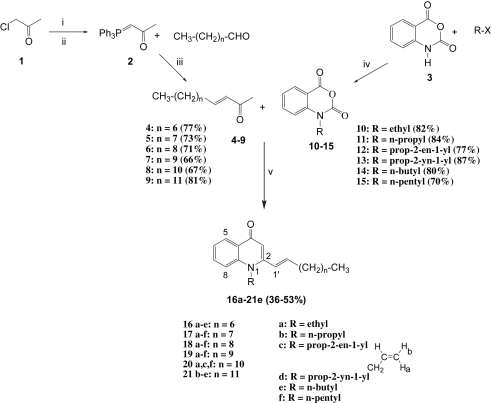
In order to identify more potent antimycobacterial agents and to examine the lipophilicity-antimycobacterial relationship, thirty derivatives were synthesized and evaluated. The synthesis route to N-substituted 2-[(1E)-alkenyl]-4-(1H)-quinolones 16a–21e is depicted in Scheme 1, and the structures of the derivatives are shown in Table 1. Methylcarbonylmethylenephosphorane (2) required for Wittig condensation was prepared in high yield according to previously described methods [6]. Subsequent treatment of the ylide 2 with the corresponding aldehydes afforded α,ß-unsaturated methyl ketones (4–9) with trans configuration in moderate yields. Isatoic acid anhydride (3) was converted to the corresponding N-substituted isatoic acid anhydrides 10–15 by the reaction with alkyl halides in the presence of NaH [7]. Finally, the desired substances (16a–21e) were obtained by the reaction between unsaturated methyl ketones (4–9) and N-substituted isatoic anhydrides (10–15) using the procedure of Coppola [8] with slight modification. The identity of the quinolones and their corresponding intermediates was confirmed by analysis of 1- and 2D NMR spectroscopy and LC-ESI-MS data.
Scheme 1.
Reagents and conditions: i) PPh3, MeCN, reflux, 24 h; ii) 1 N NaOH (pH = 7–8), iii) THF, reflux, 48 h; iv) NaH, DMF, 24 h; v) LDA, THF, −78 °C.
Table 1.
| Compound | R | R′ | Clog P | MIC (μM) |
MIC (μg/mL) |
Metabolic active cells (%)b |
||||
|---|---|---|---|---|---|---|---|---|---|---|
| A | A | B | C | D | 100 μM | 30 μM | ||||
| 16a | ethyl | n-heptyl | 5.3 | 26.9 | 8 | ND | ND | ND | 52.61 ± 8.51 | 96.64 ± 1.28 |
| 16b | n-propyl | n-heptyl | 5.8 | 12.9 | 4 | ND | ND | ND | 7.40 ± 5.52 | 32.94 ± 7.07 |
| 16c | 2-propenyl | n-heptyl | 5.5 | 25.9 | 8 | ND | ND | ND | 0.53 ± 0.04 | 10.05 ± 0.15 |
| 16d | 2-propynyl | n-heptyl | 4.9 | 6.5 | 2 | 2 | 32 | 4 | 63.86 ± 2.82 | 71.08 ± 1.40 |
| 16e | n-butyl | n-heptyl | 6.4 | 3.1 | 1 | 2 | 2 | 2 | 0.04 ± 0.05 | 42.92 ± 3.73 |
| 17a | ethyl | n-octyl | 5.8 | 12.9 | 4 | ND | ND | ND | 0.53 ± 0.06 | 39.16 ± 8.49 |
| 17b | n-propyl | n-octyl | 6.4 | 6.2 | 2 | 2 | 2 | 2 | 0.50 ± 0.06 | 56.09 ± 4.18 |
| 17c | 2-propenyl | n-octyl | 6.1 | 6.2 | 2 | 1 | 4 | 2 | 0.63 ± 0.05 | 32.96 ± 8.00 |
| 17d | 2-propynyl | n-octyl | 5.4 | 49.8 | 16 | ND | ND | ND | 0.40 ± 0.05 | 43.57 ± 9.94 |
| 17e | n-butyl | n-octyl | 6.9 | 2.9 | 1 | 1 | 1 | 1 | 0.55 ± 0.05 | 55.14 ± 3.69 |
| 17f | n-pentyl | n-octyl | 7.4 | 5.7 | 2 | 1 | 4 | 4 | 0.46 ± 0.05 | 61.71 ± 3.84 |
| 18a | ethyl | n-nonyl | 6.4 | 6.2 | 2 | 1 | 2 | 2 | 0.50 ± 0.08 | 91.31 ± 1.86 |
| 18b | n-propyl | n-nonyl | 6.9 | 2.9 | 1 | 1 | 4 | 2 | 0.71 ± 0.07 | 3.64 ± 2.02 |
| 18c | 2-propenyl | n-nonyl | 6.6 | 3.0 | 1 | 2 | 2 | 2 | 0.19 ± 0.23 | 75.05 ± 0.86 |
| 18d | 2-propynyl | n-nonyl | 5.9 | 11.9 | 4 | ND | ND | ND | 26.34 ± 6.30 | 92.31 ± 1.26 |
| 18e | n-butyl | n-nonyl | 7.4 | 2.8 | 1 | 0.5 | 1 | 1 | 0.44 ± 0.07 | 15.11 ± 6.13 |
| 18f | n-pentyl | n-nonyl | 7.9 | 10.9 | 4 | ND | ND | ND | 0.38 ± 0.04 | 41.60 ± 5.69 |
| 19a | ethyl | n-decyl | 6.9 | 2.9 | 1 | 0.5 | 1 | 1 | 0.69 ± 0.04 | 97.86 ± 2.43 |
| 19b | n-propyl | n-decyl | 7.4 | 2.8 | 1 | 0.5 | 2 | 2 | 0.65 ± 0.03 | 4.20 ± 1.87 |
| 19c | 2-propenyl | n-decyl | 7.1 | 2.8 | 1 | 1 | 2 | 2 | 1.06 ± 0.08 | 19.75 ± 5.91 |
| 19d | 2-propynyl | n-decyl | 6.5 | 183.4 | 64 | ND | ND | ND | 0.64 ± 0.15 | 40.71 ± 7.08 |
| 19e | n-butyl | n-decyl | 7.9 | 10.9 | 4 | ND | ND | ND | 0.45 ± 0.07 | 42.40 ± 8.98 |
| 19f | n-pentyl | n-decyl | 8.5 | 21.0 | 8 | ND | ND | ND | 0.25 ± 0.06 | 31.6 ± 0.32 |
| 20a | ethyl | n-undecyl | 7.4 | 2.8 | 1 | 1 | 4 | 2 | 0.60 ± 0.05 | 100.00 ± 2.16 |
| 20c | 2-propenyl | n-undecyl | 7.7 | 10.9 | 4 | ND | ND | ND | 1.06 ± 0.03 | 17.92 ± 4.46 |
| 20f | n-pentyl | n-undecyl | 9.0 | 324.1 | 128 | ND | ND | ND | 0.25 ± 0.04 | 11.74 ± 0.06 |
| 21b | n-propyl | n-dodecyl | 8.5 | 42.0 | 16 | ND | ND | ND | 0.72 ± 0.16 | 42.60 ± 6.02 |
| 21c | 2-propenyl | n-dodecyl | 8.2 | 42.2 | 16 | ND | ND | ND | 0.70 ± 0.07 | 98.16 ± 1.42 |
| 21d | 2-propynyl | n-dodecyl | 7.5 | 339.5 | 128 | ND | ND | ND | 10.00 ± 5.51 | 100.21 ± 1.53 |
| 21e | n-butyl | n-dodecyl | 9.0 | 324.1 | 128 | ND | ND | ND | 0.51 ± 0.18 | 10.93 ± 7.10 |
| Isoniazid | ND | 29.2 | 4 | 4 | 1 | 16 | ND | ND | ||
| Ethambutol | ND | 9.8 | 2 | 4 | 16 | 4 | ND | ND | ||
| Ciprofloxacin | ND | 0.4 | 0.125 | ND | ND | ND | ND | ND | ||
| Vinblastin at 0.12 μM | ND | ND | ND | ND | ND | ND | 60.06 ± 4.11 | |||
A is M. smegmatis (ATCC 14468); B is M. smegmatis mc2155; C is M. fortuitum (ATCC 6841); D is M. phlei (ATCC 11758); Clog P values were determined using ChemDraw Ultra Version 6.1.
ND- not determined.
Cytotoxicity against human diploid embryonic lung cell line MRC-5 compared to control cells (treated with 0.1% EtOH), 72 h incubation time, mean ± SEM, n = 6.
2.2. In vitro antimycobacterial and cytotoxic activities
All synthetic derivatives were first tested against Mycobacterium smegmatis in our mycobacterial susceptibility assay and the most active compounds which gave MIC values of 1 and 2 mg/L, were further evaluated against Mycobacterium fortuitum, Mycobacterium phlei and M. smegmatis mc2155 (Table 1). Against M. smegmatis compounds 16e, 17e, 18b, 18c, 18e, 19a–c and 20a (Clog P = 6.4–7.4), bearing N-alkyl groups, displayed the most potent inhibitory effect with an MIC value of 1 mg/L that was twofold and fourfold more potent than ethambutol and isoniazid, respectively, but eightfold less potent than ciprofloxacin. In contrast, among N-alkyl bearing derivatives, compounds 20f and 21e (Clog P value of 9.0) were found to be least active with MIC value of 128 mg/L. Against M. smegmatis mc2155, which is often used as a model organism to study efflux pump-mediated multi-drug resistance in mycobacteria [9], compounds 18e, 19a and 19b (Clog P values of 6.9 and 7.4) exhibited the most potent growth inhibition with an MIC value of 0.5 mg/L, being eightfold more potent than isoniazid and ethambutol. The other tested substances also displayed significant inhibitory effects with MIC values of 1–2 mg/L. On the other hand, compounds 17e, 18e and 19a were most active against M. fortuitum and M. phlei with an MIC value of 1 mg/L. Interestingly, compounds 17e and 17b possessing a 1-decenyl moiety, but having N-butyl and N-propyl, respectively, showed similar inhibitory effects against all strains, with MIC values of 1 and 2 mg/L, respectively.
Among compounds 18a–f (Clog P = 6.4–7.9) and 19a–f (Clog P = 6.9–8.5), bearing 1-undecenyl and 1-dodecenyl moieties at C-2, respectively the antimycobacterial activity decreases with increase in lipophilicity at position 1. However, among compounds 16a–16e (Clog P = 5.3–6.4), containing 1-nonenyl moiety, the activity increases with increase in lipophilicity at N-1. Among compounds 20a–20f and 21b–21e a dramatic loss in activity was observed with further increase in lipophilicity. Among the N-ethyl derivatives, 16a, 17a, 18a, and 19a, interesting structural-activity relationship is observed. Starting from 16a to 19a each additional methylene group in the aliphatic side chain at position 2 causes a twofold enhancement of the antimycobacterial activity against M. smegmatis.
Compared to the other strains M. fortuitum was found to be less susceptible towards this group of synthetic quinolones. Among the compounds tested against M. fortuitum and M. phlei, compound 17e bearing butyl and 1-decenyl groups, compound 18e bearing butyl and undecenyl groups and compound 19a having an ethyl and a dodecenyl group at N-1 and C-2 positions, respectively, showed the highest activity with MIC value of 1 mg/L.
The antimycobacterial activities of compounds 16e, 17e and 18e, all bearing an N-butyl residue but differing in the length of the aliphatic chain at C-2, against M. smegmatis are similar, suggesting that the increase in lipophilicity appear to have no effect upon antimycobacterial activity. However, among compounds 17f, 18f and 19f, all bearing N-pentyl but containing decenyl, undecenyl and dodecenyl group, respectively, a twofold decrease in activity was observed for chain elongation by a methylene group at C-2. Elongation of the alkenyl chain to 14 carbons with introduction of various alkyl groups at N-1 led to compounds with lower activity. These results reinforce our previous observations that a certain level of lipophilicity is the basic requirement for our quinolones activity against the less pathogenic mycobacterial strains employed in our study.
Several reports also discussed the structure-activity relationship between alkyl substituent at N-1 and the antimycobacterial activities of fluoroquinolones [10], [11], [12], [13].
In order to understand the influence of further structural requirements at position 1 of the quinolones for antimycobacterial activity, we have introduced various alkyls, an alkenyl and an akynyl group at position 1. Various studies have revealed that the presence of a hydrophobic substituent at N-1 of quinolones has an effect on pharmacokinetics and is essential to control antibacterial potency [14]. Introduction of a t-butyl group was also reported to enhance antibacterial potency of fluoroquinolones [15], [16].
To compare the antimycobacterial activity with toxicity to mammalian cells, the cytotoxic activity of the synthesized quinolones was evaluated using the human diploid embryonic lung cell line MRC-5, which can be used for further antimycobacterial investigations like both antimycobacterial and host-cell toxicity studies [17]. Percentage of metabolic viable cells at 30 and 100 μM as given in Table 1 indicated the degree of the cytotoxicity of our compounds.
At 100 μM most of our compounds showed high cytotoxic activity, but among the compounds with the highest activity against M. smegmatis (ATCC 14468), of which the IC50 values were determined (see Table 2), 17e and 18a showed selectivity to the mycobacterial cells as indicated by selectivity index (SI) values of 13 and 14, respectively. In addition, compounds 18c, 19a and 20a displayed low cytotoxicity and good antimycobacterial selectivity with SI values ranging from 19 to 27. In contrast to the antimycobacterial-lipophilicity relationship, where mycobacterial growth inhibition decreases with increase in Clog P values, the most lipophilic compounds 20f and 21e were found to be toxic to the human diploid embryonic lung cell line MRC-5.
Table 2.
Selectivity index against M. smegmatis (ATCC 14468) for the most antimycobacterial compounds.
| Compound | MIC (μM) | IC50 (μM) | SI |
|---|---|---|---|
| 16e | 3.1 | 26 ± 3.5 | 8.4 |
| 17e | 2.9 | 36.6 ± 3.6 | 12.6 |
| 18a | 6.1 | 82.7 ± 2.3 | 13.6 |
| 18b | 2.9 | 12.7 ± 2.5 | 4.4 |
| 18c | 3.0 | 57.1 ± 1.0 | 19.0 |
| 18e | 2.8 | 6.3 ± 2.5 | 2.3 |
| 19a | 2.9 | 79.3 ± 2.6 | 27.3 |
| 19b | 2.8 | 13 ± 2.4 | 4.6 |
| 19c | 2.8 | 17.3 ± 2.2 | 6.2 |
| 20a | 2.8 | 52.9 ± 2.6 | 18.9 |
IC50 values of MRC-5 cells were determined in three concentrations, each in at least six independent experiments using SigmaPlot program package employing the 4-parameter logistic regression model. Results are given as mean ± SEM. SI calculated as IC50/MIC.
In our recent investigation, we have disclosed the importance of the double bond in the aliphatic side chain at C-2 for mycobacterial growth inhibition [5]. To further assess the effect of unsaturated lipophilic groups on the antimycobacterial activities, we introduced 2-propenyl and 2-propynyl groups at position 1. Comparing the activities of the compounds 16c and 16d, 17c and 17d, 18c and 18d, 19c and 19d, 21c and 21d bearing N-2-propenyl and N-2-propynyl groups the increase in degree of unsaturation seems to be of less important than the lipophilic property, since except compound 16d (Clog P = 4.9; MIC = 2 mg/L), the N-2-propynyl derivatives were found to be 4–64 fold less active than the corresponding N-2-propenyl derivatives. It is therefore reasonable to suggest that the triple bond in the 2-propynyl group, which results in lower Clog P values compared to the more saturated analogues, is less significant for mycobacterial growth inhibition than the double bond in the 2-propenyl moiety.
3. Conclusion
To improve antimycobacterial potency and explore the structure-activity relationships, we synthesized a series of 30 new N-substituted-2-[(1E)-alkenyl]-4-(1H)-quinolones having different lipophilic groups at position 1 and 2. Compounds bearing C13–C15 aliphatic groups in the entire molecule, position 1 and 2, appeared to exert the highest potency against all four mycobacterial test strains. The results of this study are consistent with our previous findings that 1-methyl-2-alkenyl-4-(1H)-quinolones with C12–C15 unsaturated aliphatic groups at position 2 are endowed with a potent antimycobacterial activity. The various substituents at position 1 also exert additional contribution to the antimycobacterial activity.
4. Experimental
4.1. Materials
Strains of M. smegmatis (ATCC 14468), M. smegmatis mc2 155, M. fortuitum (ATCC 6841) and M. phlei (ATCC 11758) were obtained from American Type Culture Collection or German Collection of Microorganisms and Cell Culture (DSMZ).
All chemicals were purchased from Sigma–Aldrich, Germany. THF was distilled from sodium. DMF was distilled from CaH2. Melting points were determined with KOFLER microscope and are uncorrected. IR spectra obtained on a Perkin–Elmer 281 B spectrometer, were recorded in KBr. 1H and 13C NMR spectra were recorded on a Varian 400 MHz spectrometer (400 and 100 MHz, respectively) using deuterated chloroform as solvent with TMS as internal standard. Mass spectra were obtained by LC-ESI-MS analysis on positive mode on a Thermo Finnigan LCQ Deca XP Plus mass spectrometer connected to a Surveyor LC-system (Thermo-Finnigan). Precoated Si gel 60 F254 plates (Merck, Darmstadt) were used to monitor the progress of the reactions and column fractions. Spots were detected by UV/254 nm and spraying with molybdophosphoric acid and subsequent heating. Compounds were purified by column chromatography on silica gel 60 (0.063–0.200 mm) using cyclohexane/ethyl acetate mixtures as eluent.
4.2. Chemistry
4.2.1. Synthesis of α,ß-unsaturated ketones (4–9)
The synthesis was conducted analogous to previously reported procedures [5] from methylcarbonylmethylenephosphorane (2) (1 equiv.) and the corresponding aldehyde (0.75 equiv.) in THF.
4.2.1.1. (E)-3-Undecen-2-one (4)
Prepared from methylcarbonylmethylenephosphorane (2) (15.0 g, 47.2 mmol) and octanal (4.5 g, 35.4 mmol) in THF (350 mL) as a colourless oil (6.1 g, 77%); and its spectral data agree with the literature [18].
4.2.1.2. (E)-3-Dodecen-2-one (5)
Prepared from methylcarbonylmethylenephosphorane (2) (15.0 g, 47.2 mmol) and nonanal (5.0 g. 35.4 mmol) in THF (350 mL). 5 was obtained as a colourless oil (6.3 g, 73%).1H NMR: δ 6.78 (dt, J = 16.1, 6.7 Hz, 1H, H-4), 6.05 (bd J = 16.1 Hz, 1H, H-3), 2.21 (s, 3H, H-1), 2.19 (m, 2H, H-5), 1.44 (m, 2H, H-6), 1.23–1.32 (m, 10H, H-7–11), 0.86 (t, J = 6.7 Hz, 3H, H-12). 13C NMR: δ 198.6 (C-2), 148.5 (C-4), 131.2 (C-3), 32.4 (C-5), 31.8 (C-10), 29.3, 29.1, 29.1, 28.0, 26.8 (C-1), 22.6 (C-11), 14.0 (C-12).
4.2.1.3. (E)-3-Tridecen-2-one (6)
Prepared from methylcarbonylmethylenephosphorane (2) (20.0 g, 62.9 mmol) and decanal (7.4 g, 47.2 mmol) in THF (400 mL) as colourless oil (8.8 g, 71%); and its spectral properties are recently reported [5].
4.2.1.4. (E)-3-Tetradecen-2-one (7)
Prepared from methylcarbonylmethylenephosphorane (2) (20.0 g, 62.9 mmol) and undecanal (8.0 g, 47.2 mmol) in THF (400 mL). 7 was formed as a colourless oil (8.7 g, 66%). 1H NMR: δ 6.75 (dt, J = 16.1, 6.6 Hz, 1H, H-4), 6.02 (d, J = 16.1 Hz, 1H, H-3), 2.18 (s, 3H, H-1), 2.15 (m, 2H, H-5), 1.43 (m, 2H, H-6), 1.22–1.31 (m, 14H, H-7–13), 0.84 (t, J = 6.6 Hz, 3H, H-14). 13C NMR: δ 198.6 (C-2), 148.5 (C-4), 131.2 (C-3), 32.5 (C-5), 31.8 (C-12), 29.4, 29.3, 29.2, 29.1, 29.1, 28.0, 26.7 (C-1), 22.6 (C-13), 14.0 (C-14).
4.2.1.5. (E)-3-Pentadecen-2-one (8)
Prepared from methylcarbonylmethylenephosphorane (2) (20.0 g, 62.9 mmol) and dodecanal (8.7 g, 47.2 mmol) in THF (400 mL). 8 was obtained as a colourless oil (9.4 g, 67%). 1H NMR: δ 6.78 (dt, J = 16.1, 6.7 Hz, 1H, H-4), 6.05 (bd, J = 16.1 Hz, 1H, H-3), 2.20 (s, 3H, H-1), 2.17 (m, 2H, H-5), 1.44 (m, 2H, H-6), 1.21–1.33 (m, 16H, H-7–14), 0.86 (t, J = 6.7 Hz, 3H, H-15). 13C NMR: δ 198.4 (C-2), 148.3 (C-4), 131.2 (C-3), 32.4 (C-5), 31.8 (C-13), 29.5, 29.4, 29.3, 29.2, 29.1, 29.1, 28.0, 26.8 (C-1), 22.6 (C-14), 14.0 (C-15).
4.2.1.6. (E)-3-Hexadecen-2-one (9)
Prepared from methylcarbonylmethylenephosphorane (2) (15.0 g, 47.2 mmol) and tridecanal (8.4 g, 35.4 mmol) in THF (350 mL). 9 was obtained as a colourless oil (9.1 g, 81%). 1H NMR: δ 6.77 (dt, J = 16.1, 6.7 Hz, 1H, H-4), 6.03 (bd, J = 16.1 Hz, 1H, H-3), 2.19 (s, 3H, H-1), 2.16 (m, 2H, H-5), 1.44 (m, 2H, H-6), 1.21–1.33 (m, 18H, H-7–15), 0.85 (t, J = 6.7 Hz, 3H, H-16). 13C NMR: δ 198.5 (C-2), 148.4 (C-4), 131.2 (C-3), 32.6 (C-5), 31.8 (C-14), 29.4, 29.5, 29.4, 29.3, 29.2, 29.1, 29.1, 28.0, 26.8 (C-1), 22.6 (C-15), 14.0 (C-16).
4.2.2. General procedure for the synthesis of N-substituted isatoic acid anhydrides (10–15)
Sodium hydride (60% dispersion in mineral oil) was washed with hexane and 1.0 equiv of the dry NaH was added slowly in portions to a stirring solution of isatoic acid anhydride (1.0 equiv) in absolute DMF. After 1 h of stirring alkyl halide (1.5 equiv) was added and stirring continued for 24 h. DMF was removed under reduced pressure and the residue was poured into ice water and extracted with ether. The crude product was purified by column chromatography eluting with cyclohexane/ethyl acetate (9:1) to afford the corresponding N-substituted isatoic acid anhydride.
4.2.2.1. N-Ethyl-isatoic acid anhydride (10)
Starting from isatoic acid anhydride (3) (16.3 g, 0.1 mol) in DMF (150 mL), NaH 60% dispersion in mineral oil (4.1 g, 0.1 mol) and ethyl iodide (23.4 g, 0.15 mol). 10 was obtained as light yellow solid (15.6 g, 82%), m.p. 115–117 °C. 1H NMR: δ 8.18 (d, J = 8.0 Hz, 1H, H-5), 7.77 (t, J = 8.0 Hz, 1H, H-7), 7.28 (t, J = 8.0 Hz, 1H, H-6), 7.19 (d, J = 8.4 Hz, 1H, H-8), 4.14 (q, J = 7.2 Hz, 2H, N–CH2–CH3), 1.49 (t, J = 6.8 Hz, 3H, N–CH2–CH3). 13C NMR: δ 158.6 (C-4), 147.7 (C-2), 141.2 (C-8a), 137.3 (C-7), 130.8 (C-5), 123.7 (C-6), 114.6 (C-8), 111.5 (C-4a), 40.3 (N–CH2–CH3), 14.1 (N–CH2–CH3).
4.2.2.2. N-Propyl isatoic acid anhydride (11)
Starting from isatoic acid anhydride (3) (16.3 g, 0.1 mol) in DMF (150 mL), NaH 60% dispersion in mineral oil (4.1 g, 0.1 mol) and 1-iodopropane (25.5 g, 0.15 mol). 11 was obtained as light yellow solid (17.2 g, 84%), m.p. 93–95 °C. 1H NMR: δ 8.09 (dd, J = 8.0, 1.6 Hz, 1H, H-5), 7.75 (td, J = 8.0, 1.6 Hz, 1H, H-7), 7.26 (t, J = 8.0 Hz, 1H, H-6), 7.17 (d, J = 8.4 Hz, 1H, H-8), 4.00 (t, J = 8.0 Hz, 2H, N–CH2–CH2–CH3), 1.76 (sext, J = 7.6 Hz, 2H, N–CH2–CH2–CH3), 1.01 (t, J = 6.8 Hz, 3H, N–(CH2)2–CH3). 13C NMR: δ 158.5 (C-4), 147.6 (C-2), 141.2 (C-8a), 137.2 (C-7), 130.7 (C-5), 123.8 (C-6), 113.9 (C-8), 111.5 (C-4a), 46.3 (N–CH2–CH2–CH3), 20.1 (N–CH2–CH2–CH3), 10.9 (N–(CH2)2–CH3).
4.2.2.3. N-2-Propenyl isatoic acid anhydride (12)
Starting from isatoic acid anhydride (3) (8.3 g, 0.05 mol) in DMF (90 mL), NaH 60% dispersion in mineral oil (2.1 g, 0.05 mol) and allyl bromide (9.1 g, 0.075 mol). 12 was obtained as yellowish solid (7.8 g, 77%), m.p. 104–106 °C. 1H NMR: δ 8.09 (d, J = 8.0 Hz, 1H, H-5), 7.73 (t, J = 8.0 Hz, 1H, H-7), 7.27 (t, J = 7.6 Hz, 1H, H-6), 7.16 (d, J = 8.4 Hz, 1H, H-8), 5.91 (ddt, J = 17.2, 10.0, 4.0 Hz, 1H, N–CH2–CH CH2), 5.30 (d, J = 10.0 Hz, 1H, N–CH2–CH CHHb), 5.26 (d, J = 17.2 Hz, 1H, N–CH2–CH CHaH), 4.68 (d, J = 1.6 Hz, 2H, N–CH2–CH CH2). 13C NMR: δ 158.3 (C-4), 147.6 (C-2), 141.2 (C-8a), 137.2 (C-7), 130.5 (C-5), 129.9 (N–CH2–CH CH2), 123.9 (C6), 118.4 (N–CH2–CH CH2), 114.5 (C-8), 111.4 (C-4a), 46.9 (N–CH2–CH CH2).
4.2.2.4. N-2-Propynyl isatoic acid anhydride (13)
Prepared from isatoic acid anhydride (3) (8.3 g, 0.05 mol) in DMF (90 mL), NaH 60% dispersion in mineral oil (2.1 g, 0.05 mol) and propargyl bromide (8.9 g, 0.075 mol). 13 was obtained as yellowish solid (8.7 g, 87% yield), m.p. 131–133 °C. 1H NMR: δ 8.14 (d, J = 8.0 Hz, 1H, H-5), 7.81 (t, J = 8.0 Hz, 1H, H-7), 7.36 (d, J = 7.6 Hz, 1H, H-8), 7.34 (t, J = 7.6 Hz, 1H, H-6), 4.86 (s, 2H, N–CH2–C≡CH), 2.37 (s, 1H, N–CH2–C≡CH). 13C NMR: δ 157.9 (C-4), 147.9 (C-2), 140.3 (C-8a), 137.3 (C-7), 130.8 (C-5), 124.4 (C-6), 114.4 (C-8), 111.7 (C-4a), 75.9 (N–CH2–C≡CH), 74.3 (N–CH2–C≡CH), 34.5 (N–CH2–C≡CH).
4.2.2.5. N-Butyl isatoic acid anhydride (14)
Prepared from isatoic acid anhydride (3) (8.3 g, 0.05 mol) in DMF (90 mL), NaH 60% dispersion in mineral oil (2.1 g, 0.05 mol) and 1-iodobutane (13.8 g, 0.075 mol). 14 was obtained as yellowish solid (8.8 g, 80%), m.p. 58–60 °C. 1H NMR: δ 8.07 (d, J = 8.0 Hz, 1H, H-5), 7.75 (t, J = 8.0 Hz, 1H, H-7), 7.24 (t, J = 8.0 Hz, 1H, H-6), 7.17 (d, J = 8.4 Hz, 1H, H-8), 4.02 (t, J = 7.6 Hz, 2H, N–CH2–(CH2)2–CH3), 1.70 (quint, J = 6.8 Hz, 2H, N–CH2–CH2–CH2–CH3), 1.42 (m, 2H, N–(CH2)2–CH2–CH3), 0.95 (t, J = 6.8 Hz, 3H, N–(CH2)3–CH3). 13C NMR: δ 158.4 (C-4), 147.5 (C-2), 141.1 (C-8a), 137.1 (C-7), 130.5 (C-5), 123.7 (C-6), 113.9 (C-8), 111.5 (C-4a), 44.6 (N–CH2–(CH2)2–CH3), 28.7 (N–CH2–CH2–CH2–CH3), 18.7 (N–(CH2)2–CH2–CH3), 13.5 (N–(CH2)3–CH3).
4.2.2.6. N-Pentyl isatoic acid anhydride (15)
Prepared from isatoic acid anhydride (3) (16.3 g, 0.1 mol) in DMF (150 mL), NaH 60% dispersion in mineral oil (4.1 g, 0.1 mol) and 1-bromopentane (22.7 g, 0.15 mol). 15 was obtained as yellowish solid (16.4 g, 70%), m.p. 55–57 °C. 1H NMR: δ 8.15 (d, J = 8.0 Hz, 1H, H-5), 7.75 (t, J = 8.0 Hz, 1H, H-7), 7.28 (t, J = 8.0 Hz, 1H, H-6), 7.15 (d, J = 8.4 Hz, 1H, H-8), 4.04 (t, J = 8.0 Hz, 2H, N–CH2–(CH2)3–CH3), 1.70 (quint, J = 6.8 Hz, 2H, N–CH2–CH2–(CH2)2–CH3), 1.40 (m, 4H, N–(CH2)2–CH2–CH2–CH3), 0.94 (t, J = 6.8 Hz, 3H, N–(CH2)4–CH3). 13C NMR: δ 158.5 (C-4), 147.7 (C-2), 141.3 (C-8a), 137.1 (C-7), 130.9 (C-5), 123.8 (C-6), 113.8 (C-8), 111.9 (C-4a), 44.9 (N–CH2–(CH2)3–CH3), 29.3 (N–(CH2)3–CH2–CH3), 28.9 (N–CH2–CH2–(CH2)2–CH3), 22.4 (N–(CH2)3–CH2–CH3), 13.9 (N–(CH2)4–CH3).
4.2.3. Synthesis of N-substituted 2-(1E-alkenyl)-4-(1H)-quinolones (16a–21e)
These substances were prepared from α,ß-unsaturated ketones (4–9) and N-substituted isatoic acid anhydrides (10–15) in the presence of LDA as previously described method [5].
4.2.3.1. 1-Ethyl-2-[(1E)-nonenyl]-4-(1H)-quinolone (16a)
Prepared from (E)-3-undecen-2-one (4) (1.0 g, 5.9 mmol) in THF (20 mL), LDA (3.0 mL, 5.9 mmol) and N-ethyl isatoic acid anhydride (10) (0.85 g, 4.5 mmol) in THF (20 mL). 16a was formed as yellow semisolid (0.62 g, 46%). IR (KBr, νmax, cm−1): 3443, 2926, 2854, 1624, 1597, 1486, 1436, 1186, 1119, 722, 540. 1H NMR: δ 8.38 (dd, J = 8.4, 1.6 Hz, 1H, H-5), 7.59 (td, J = 8.4, 1.6 Hz, 1H, H-7), 7.43 (d, J = 8.0 Hz, 1H, H-8), 7.27 (t, J = 7.2 Hz, 1H, H-6), 6.38 (d, J = 15.6 Hz, 1H, H-1′), 6.34 (s, 1H, H-3), 6.30 (dt, J = 15.6, 6.8 Hz, 1H, H-2′), 4.17 (q, J = 6.8 Hz, 2H, N–CH2–CH3), 2.22 (q, J = 7.2 Hz, 2H, H-3′), 1.45 (quint, J = 6.8 Hz, 2H, H-4′), 1.38 (t, J = 7.2 Hz, 3H, N–CH2–CH3), 1.31–1.22 (m, 8H, H-5′–8′), 0.85 (t, J = 6.8 Hz, 3H, H-9′). 13C NMR: δ 177.5 (C-4), 151.6 (C-2), 141.8 (C-2′), 140.1 (C-8a), 131.9 (C-7), 126.8 (C-4a), 126.6 (C-5), 123.2 (C-1′), 123.0 (C-6), 115.2 (C-8), 109.5 (C-3), 42.3 (N–CH2–CH3), 32.9 (C-3′), 31.6(C-7′), 28.9 (C-6′), 28.9 (C-5′), 28.5 (C-4′), 22.5 (C-8′), 13.9 (C-9′), 13.8 (N–CH2–CH3). ESI-MS m/z (%): [M + 1]+ 298 (100), 268 [M + 2–C2H5]+, 254, 226, 212, 198, 172, 159, 120.
4.2.3.2. 1-Propyl-2-[(1E)-nonenyl]-4-(1H)-quinolone (16b)
Prepared from (E)-3-undecen-2-one (4) (1.0 g, 5.9 mmol) in THF (20 mL), LDA (3.0 mL, 5.9 mmol) and N-propyl isatoic acid anhydride (11) (0.92 g, 4.5 mmol) in THF (20 mL). 16b was formed as light yellow oil (0.58 g, 41%). IR (KBr, νmax cm−1): 3422, 2926, 2854, 1624, 1598, 1486, 1422, 759, 542. 1H NMR: δ 8.41 (dd, J = 8.4, 1.6 Hz, 1H, H-5), 7.61 (td, J = 8.4, 1.6 Hz, 1H, H-7), 7.44 (d, J = 8.4 Hz, 1H, H-8), 7.31 (t, J = 7.6 Hz, 1H, H-6), 6.41 (d, J = 15.2 Hz, 1H, H-1′), 6.37 (s, 1H, H-3), 6.33 (dt, J = 15.2, 6.8 Hz, 1H, H-2′), 4.07 (t, J = 8.0 Hz, 2H, N–CH2–CH2–CH3), 2.25 (q, J = 6.8 Hz, 2H, H-3′), 1.81 (m, 2H, N–CH2–CH2–CH3), 1.48 (quint, J = 7.2 Hz, 2H, H-4′), 1.33–1.22 (m, 8H, H-5′–8′), 1.01 (t, J = 7.2 Hz, 3H, N–(CH2)2–CH3), 0.87 (t, J = 6.8 Hz, 3H, H-9′). 13C NMR: δ 177.5 (C-4), 151.9 (C-2), 141.9 (C-2′), 140.4 (C-8a), 132.0 (C-7), 126.9 (C-4a), 126.7 (C-5), 123.5 (C-1′), 123.1 (C-6), 115.5 (C-8), 109.6 (C-3), 48.9 (N–CH2–CH2–CH3), 33.0 (C-3′), 31.7 (C-7′), 29.0 (C-6′), 29.0 (C-5′), 28.5 (C-4′), 22.5 (C-8′), 21.9 (N–CH2–CH2–CH3), 13.9 (C-9′), 10.9 (N–(CH2)2–CH3). ESI-MS m/z (%): [M + 1]+ 312 (100), 270 [M + 2–C3H7]+, 242, 228, 212, 198, 172, 159, 130, 115.
4.2.3.3. 1-(2-Propenyl)-2-[(1E)-nonenyl]-4-(1H)-quinolone (16c)
Prepared from (E)-3-undecen-2-one (4) (1.0 g, 5.9 mmol) in THF (20 mL), LDA (3.0 mL, 5.9 mmol) and N-2-propenyl isatoic acid anhydride (12) (0.91 g, 4.5 mmol) in THF (20 mL). 16c was formed as yellow oil (0.68 g, 49%). IR (KBr, νmax cm−1): 3422, 2926, 2854, 1623, 1598, 1486, 1422, 760, 542. 1H NMR: δ 8.39 (dd, J = 8.0, 1.6 Hz, 1H, H-5), 7.55 (td, J = 8.0, 1.6 Hz, 1H, H-7), 7.37 (d, J = 8.4 Hz, 1H, H-8), 7.28 (t, J = 7.2 Hz, 1H, H-6), 6.38 (s, 1H, H-3), 6.34 (dt, J = 16.0, 6.4 Hz, 1H, H-2′), 6.28 (d, J = 16.0 Hz, 1H, H-1′), 6.00 (ddt, J = 17.2, 10.4, 4.0 Hz, 1H, N–CH2–CH CH2), 5.26 (d, J = 10.4 Hz, 1H, N–CH2–CH CHHb), 4.97 (d, J = 17.2 Hz, 1H, N–CH2–CH CHaH), 4.72 (dt, J = 4.0, 1.6 Hz, 2H, N–CH2–CH CH2), 2.21 (q, J = 6.8 Hz, 2H, H-3′), 1.45 (quint, J = 6.8 Hz, 2H, H-4′), 1.34–1.23 (m, 8H, H-5′–8′), 0.85 (t, J = 6.8 Hz, 3H, H-9′). 13C NMR: δ 177.8 (C-4), 152.1 (C-2), 142.1 (C-2′), 140.8 (C-8a), 132.0 (C-7), 131.1 (N–CH2–CH CH2), 126.6 (C-4a), 126.4 (C-5), 123.2 (C-1′), 123.2 (C-6), 117.4 (N–CH2–CH CH2), 115.9 (C-8), 109.2 (C-3), 49.9 (N–CH2–CH CH2), 32.9 (C-3′), 31.6 (C-7′), 29.0 (C-6′), 28.9 (C-5′), 28.5 (C-4′), 22.5 (C-8′), 13.9 (C-9′). ESI-MS m/z (%): [M + 1]+ 310 (100), 268, 254, 240, 224, 210, 198, 184, 159, 109.
4.2.3.4. 1-(2-Propynyl)-2-[(1E)-nonenyl]-4-(1H)-quinolone (16d)
Prepared from (E)-3-undecen-2-one (4) (1.0 g, 5.9 mmol) in THF (20 mL), LDA (3.0 mL, 5.9 mmol) and N-2-propynyl isatoic acid anhydride (13) (0.90 g, 4.5 mmol) in THF (20 mL). 16d was formed as a light yellow solid (0.73 g, 53%), m.p. 118–120 °C. IR (KBr, νmax cm−1): 3423, 3219, 2925, 2853, 2116, 1620, 1596, 1487, 1422, 761. 1H NMR: δ 8.37 (dd, J = 8.0, 1.6 Hz, 1H, H-5), 7.64 (td, J = 8.0, 1.6 Hz, 1H, H-7), 7.56 (d, J = 8.4 Hz, 1H, H-8), 7.33 (t, J = 7.6 Hz, 1H, H-6), 6.47 (d, J = 15.6 Hz, 1H, H-1′), 6.39 (dt, J = 15.6, 6.8, Hz, 1H, H-2′), 6.34 (s, 1H, H-3), 4.81 (d, 4J = 2.4 Hz, 2H, N–CH2–C≡CH), 2.47 (t, 4J = 2.4 Hz, 1H, N–CH2–C≡CH), 2.27 (q, J = 7.2 Hz, 2H, H-3′), 1.49 (quint, J = 7.2 Hz, 2H, H-4′), 1.32–1.24 (m, 8H, H-5′–8′), 0.87 (t, J = 6.8 Hz, 3H, H-9′). 13C NMR: δ 177.9 (C-4), 151.5 (C-2), 142.8 (C-2′), 140.5 (C-8a), 132.3 (C-7), 126.8 (C-4a), 126.6 (C-5), 123.5 (C-1′), 122.9 (C-6), 115.5 (C-8), 109.6 (C-3), 76.9 (N–CH2–C≡CH), 74.8 (N–CH2–C≡CH), 38.0 (N–CH2–C≡CH), 33.1 (C-3′), 31.7 (C-7′), 29.0 (C-6′), 29.0 (C-5′), 28.5 (C-4′), 22.5 (C-8′), 13.9 (C-9′). ESI-MS m/z (%): [M + 1]+ 308 (100), 280 [M + 2–C2H5]+, 266, 236, 224, 210, 198, 184, 160, 107, 93.
4.2.3.5. 1-Butyl-2-[(1E)-nonenyl]-4-(1H)-quinolinone (16e)
Prepared from (E)-3-undecen-2-one (4) (1.0 g, 5.9 mmol) in THF (20 mL), LDA (3.0 mL, 5.9 mmol) and N-butyl isatoic acid anhydride (14) (0.99 g, 4.5 mmol) in THF (20 mL). 16e was formed as light yellow oil (0.77 g, 53%). IR (KBr, νmax cm−1): 3421, 2927, 2855, 1623, 1598, 1486, 1423, 759. 1H NMR: δ 8.41 (dd, J = 8.0, 1.6 Hz, 1H, H-5), 7.61 (td, J = 8.0, 1.6 Hz, 1H, H-7), 7.43 (d, J = 8.4 Hz, 1H, H-8), 7.31 (t, J = 7.6 Hz, 1H, H-6), 6.41 (d, J = 15.6 Hz, 1H, H-1′), 6.37 (s, 1H, H-3), 6.33 (dt, J = 15.6, 6.8 Hz, 1H, H-2′), 4.11 (t, J = 8.4 Hz, 2H, N–CH2–(CH2)2–CH3), 2.25 (q, J = 7.2 Hz, 2H, H-3′), 1.77 (quint, J = 7.6 Hz, 2H, N–CH2–CH2–CH2–CH3), 1.45 (quint, J = 6.8 Hz, 2H, H-4′), 1.41 (m, 2H, N–(CH2)2–CH2–CH3), 1.30–1.21 (m, 8H, H-5′–8′), 0.99 (t, J = 7.2 Hz, 3H, N–(CH2)3–CH3), 0.87 (t, J = 6.8 Hz, 3H, H-9′). 13C NMR: δ 177.5 (C-4), 151.9 (C-2), 141.9 (C-2′), 140.4 (C-8a), 131.9 (C-7), 126.8 (C-4a), 126.7 (C-5), 123.5 (C-1′), 123.1 (C-6), 115.4 (C-8), 109.6 (C-3), 47.3 (N–CH2–(CH2)2–CH3), 33.0 (C-3′), 31.7 (C-7′), 30.6 (N–CH2–CH2–CH2–CH3), 29.0 (C-6′), 29.0 (C5′), 28.5 (C-4′), 22.5 (C-8′), 19.8 (N–(CH2)2–CH2–CH3), 13.9 (C-9′), 13.6 (N–(CH2)3–CH3). ESI-MS m/z (%): [M + 1]+ 326 (100), 270 [M + 2–C4H9]+, 242, 226, 198, 172, 159, 130, 117, 107.
4.2.3.6. 1-Ethyl-2-[(1E)-decenyl]-4-(1H)-quinolone (17a)
Prepared from (E)-3-dodecen-2-one (4) (1.0 g, 5.5 mmol) in THF (20 mL), LDA (2.7 mL, 5.5 mmol) and N-ethyl isatoic anhydride (10) (0.79 g, 4.1 mmol) in THF (20 mL). 17a was formed as a light yellow semisolid (0.52 g, 41%). IR (KBr, νmax cm−1): 3423, 2926, 2854, 1624, 1597, 1487, 1424, 1189, 1120, 722, 542. 1H NMR: δ 8.43 (d, J = 7.6 Hz, 1H, H-5), 7.64 (t, J = 7.6 Hz, 1H, H-7), 7.44 (d, J = 8.0 Hz, 1H, H-8), 7.31 (t, J = 7.6 Hz, 1H, H-6), 6.42 (d, J = 15.6 Hz, 1H, H-1′), 6.37 (dt, J = 15.6, 6.8 Hz, 1H, H-2′), 6.36 (s, 1H, H-3), 4.20 (q, J = 6.2 Hz, 2H, N–CH2–CH3), 2.26 (q, J = 6.8 Hz, 2H, H-3′), 1.49 (quint, J = 6.8 Hz, 2H, H-4′), 1.41 (t, J = 7.2 Hz, 3H, N–CH2–CH3), 1.32–1.22 (m, 10H, H-5′–9′), 0.86 (t, J = 6.8 Hz, 3H, H-10′). 13C NMR: δ 177.8 (C-4), 151.6 (C-2), 141.8 (C-2′), 140.2 (C-8a), 132.0 (C-7), 127.0 (C-4a), 126.8 (C-5), 123.3 (C-1′), 123.0 (C-6), 115.2 (C-8), 109.7 (C-3), 42.3 (N–CH2–CH3), 33.1 (C-3′), 31.8 (C-8′), 29.3 (C-7′), 29.2 (C-6′), 29.1 (C-5′), 28.6 (C-4′), 22.6 (C-9′), 14.0 (C-10′), 13.9 (N–CH2–CH3). ESI-MS m/z (%): [M + 1]+ 312 (100), 284 [M + 2–C2H5]+, 270, 254, 226, 212, 198, 186, 172, 159, 146, 120, 93.
4.2.3.7. 1-Propyl-2-[(1E)-decenyl]-4-(1H)-quinolone (17b)
Prepared from (E)-3-dodecen-2-one (5) (1.0 g, 5.5 mmol) in THF (20 mL), LDA (2.7 mL, 5.5 mmol) and N-propyl isatoic acid anhydride (11) (0.84 g, 4.1 mmol) in THF (20 mL). 17b was formed as yellow oil (0.60 g, 45%). IR (KBr, νmax cm−1): 3422, 2926, 2854, 1624, 1598, 1486, 1422, 759, 542. 1H NMR: δ 8.43 (dd, J = 8.0, 1.6 Hz, 1H, H-5), 7.61 (td, J = 7.6, 1.6 Hz, 1H, H-7), 7.43 (d, J = 8.4 Hz, 1H, H-8), 7.32 (t, J = 7.6 Hz, 1H, H-6), 6.42 (d, J = 15.2 Hz, 1H, H-1′), 6.35 (s, 1H, H-3), 6.33 (dt, J = 15.2, 6.8 Hz, 1H, H-2′), 4.07 (t, J = 8.0 Hz, 2H, N–CH2–CH2–CH3), 2.26 (q, J = 6.8 Hz, 2H, H-3′), 1.82 (m, 2H, N–CH2–CH2–CH3), 1.49 (quint, J = 7.2 Hz, 2H, H-4′), 1.31–1.22 (m, 10H, H-5′–9′), 1.01 (t, J = 7.2 Hz, 3H, N–(CH2)2–CH3), 0.88 (t, J = 6.8 Hz, 3H, H-10′). 13C NMR: δ 177.8 (C-4), 151.9 (C-2), 141.7 (C-2′), 140.5 (C-8a), 131.9 (C-7), 127.0 (C-4a), 126.8 (C-5), 123.6 (C-1′), 123.0 (C-6), 115.4 (C-8), 109.8 (C-3), 48.9 (N–CH2–CH2–CH3), 33.1 (C-3′), 31.8 (C-8′), 29.3 (C-7′), 29.2 (C-6′), 29.1 (C-5′), 28.6 (C-4′), 22.6 (C-9′), 21.9 (N–CH2–CH2–CH3), 14.0 (C-10′), 10.9 (N–(CH2)2–CH3). ESI-MS m/z (%): [M + 1]+ 326 (100), 284 [M + 2–C3H7]+, 270, 254, 242, 212, 186, 172, 159, 130, 106.
4.2.3.8. 1-(2-Propenyl)-2-[(1E)-decenyl]-4-(1H)-quinolone (17c)
Prepared from (E)-3-dodecen-2-one (5) (1.0 g, 5.5 mmol) in THF (20 mL), LDA (2.7 mL, 5.5 mmol) and N-2-propenyl isatoic acid anhydride (12) (0.83 g, 4.1 mmol) in THF (20 mL). 17c was formed as light yellow oil (0.55 g, 42%). IR (KBr, νmax cm−1): 3420, 2925, 2854, 1623, 1598, 1486, 1422, 760. 1H NMR: δ 8.39 (d, J = 8.0 Hz, 1H, H-5), 7.56 (t, J = 7.2 Hz, 1H, H-7), 7.33 (d, J = 8.4 Hz, 1H, H-8), 7.29 (t, J = 7.6 Hz, 1H, H-6), 6.38 (s, 1H, H-3), 6.35 (dt, J = 15.6, 6.8 Hz, 1H, H-2′), 6.26 (d, J = 15.6 Hz, 1H, H-1′), 6.00 (ddt, J = 17.2, 10.0, 4.0 Hz, 1H, N–CH2–CH CH2), 5.27 (d, J = 10.0 Hz, 1H, N–CH2–CH CHHb), 5.00 (d, J = 17.2 Hz, 1H, N–CH2–CH CHaH), 4.73 (d, J = 2.0 Hz, 2H, N–CH2–CH CH2), 2.22 (q, J = 6.8 Hz, 2H, H-3′), 1.46 (quint, J = 6.8 Hz, 2H, H-4′), 1.31–1.21 (m, 10H, H-5′–9′), 0.86 (t, J = 6.8 Hz, 3H, H-10′). 13C NMR: δ 177.9 (C-4), 152.1 (C-2), 142.0 (C-2′), 140.8 (C-8a), 132.0 (C-7), 131.1 (N–CH2–CH CH2), 126.7 (C-4a), 126.4 (C-5), 123.3 (C-1′), 123.1 (C-6), 117.4 (N–CH2–CH CH2), 115.9 (C-8), 109.3 (C-3), 50.0 (N–CH2–CH CH2), 33.0 (C-3′), 31.8 (C-8′), 29.3 (C-7′), 29.1 (C-6′), 29.0 (C-5′), 28.5 (C-4′), 22.6 (C-9′), 14.0 (C-10′). ESI-MS m/z (%): [M + 1]+ 324 (100), 282 [M + 2–C3H5]+, 254, 240, 224, 210, 198, 184, 172, 159, 146, 109, 95.
4.2.3.9. 1-(2-Propynyl)-2-[(1E)-decenyl]-4-(1H)-quinolone (17d)
Prepared from (E)-3-dodecen-2-one (5) (1.0 g, 5.5 mmol) in THF (20 mL), LDA (2.7 mL, 5.5 mmol) and N-2-propynyl isatoic acid anhydride (13) (0.82 g, 4.1 mmol) in THF (20 mL). 17d was formed as a light yellow solid (0.60 g, 46%), m.p. 79–91 °C. IR (KBr, νmax cm−1): 3445, 3188, 2925, 2854, 2115, 1617, 1593, 1562, 1485, 1422, 1272, 759. 1H NMR: δ 8.39 (dd, J = 8.0, 1.6 Hz, 1H, H-5), 7.67 (td, J = 8.0, 1.6 Hz, 1H, H-7), 7.58 (d, J = 8.4 Hz, 1H, H-8), 7.35 (t, J = 7.2 Hz, 1H, H-6), 6.49 (d, J = 15.2 Hz, 1H, H-1′), 6.40 (dt, J = 15.2, 6.8 Hz, 1H, H-2′), 6.36 (s, 1H, H-3), 4.82 (d, 4J = 2.4 Hz, 2H, N–CH2–C≡CH), 2.48 (t, 4J = 2.4 Hz, 1H, N–CH2–C≡CH), 2.29 (q, J = 6.8 Hz, 2H, H-3′), 1.50 (quint, J = 7.2 Hz, 2H, H-4′), 1.34–1.22 (m, 10H, H-5′–9′), 0.88 (t, J = 6.8 Hz, 3H, H-10′). 13C NMR: δ 178.0 (C-4), 151.6 (C-2), 142.8 (C-2′), 140.5 (C-8a), 132.3 (C-7), 126.7 (C-4a), 126.6 (C-5), 123.5 (C-1′), 123.0 (C-6), 115.5 (C-8), 109.7 (C-3), 76.9 (N–CH2–C≡CH), 74.8 (N–CH2–C≡CH), 38.0 (N–CH2–C≡CH), 33.1 (C3′), 31.8 (C-8′), 29.3 (C-7′), 29.2 (C-6′), 29.1 (C-5′), 28.6 (C-4′), 22.6 (C-9′), 14.0 (C-10′). ESI-MS m/z (%): [M + 1]+ 322 (100), 282 [M + 2–C3H3]+, 268, 230, 224, 210, 198, 184, 160, 107, 95.
4.2.3.10. 1-Butyl-2-[(1E)-decenyl]-4-(1H)-quinolone (17e)
Prepared from (E)-3-dodecen-2-one (5) (1.0 g, 5.5 mmol) in THF (20 mL), LDA (2.7 mL, 5.5 mmol) and N-butyl isatoic acid anhydride (14) (0.90 g, 4.1 mmol) in THF (20 mL). 17e was formed as yellow oil (0.52 g, 37%). IR (KBr, νmax cm−1): 3422, 2926, 2854, 1623, 1598, 1485, 1422, 759. 1H NMR: δ 8.42 (dd, J = 8.0, 1.6 Hz, 1H, H-5), 7.61 (td, J = 8.0, 1.6 Hz, 1H, H-7), 7.43 (d, J = 8.4 Hz, 1H, H-8), 7.31 (t, J = 7.2 Hz, 1H, H-6), 6.41 (d, J = 15.6 Hz, 1H, H-1′), 6.34 (s, 1H, H-3), 6.33 (dt, J = 15.6, 6.8 Hz, 1H, H-2′), 4.10 (t, J = 8.0 Hz, 2H, N–CH2–(CH2)2–CH3), 2.25 (q, J = 6.8 Hz, 2H, H-3′), 1.77 (quint, J = 7.6 Hz, 2H, N–CH2–CH2–CH2–CH3), 1.49–1.40 (m, 4H, H-4′, N–(CH2)2–CH2–CH3), 1.31–1.21 (m, 10H, H-5′–9′), 1.00 (t, J = 7.2 Hz, 3H, N–(CH2)3–CH3), 0.87 (t, J = 6.8 Hz, 3H, H-10′). 13C NMR: δ 177.7 (C-4), 151.8 (C-2), 141.7 (C-2′), 140.4 (C-8a), 131.9 (C-7), 127.0 (C-4a), 126.8 (C-5), 123.6 (C-1′), 123.0 (C-6), 115.4 (C-8), 109.7 (C-3), 47.3 (N–CH2–(CH2)2–CH3), 33.0 (C-3′), 31.8 (C-8′), 30.6 (N–CH2–CH2–CH2–CH3), 29.3 (C-7′), 29.2 (C-6′), 29.1 (C-5′), 28.6 (C-4′), 22.6 (C-9′), 19.9 (N–(CH2)2–CH2–CH3), 14.0 (C-10′), 13.6 (N–(CH2)3–CH3). ESI-MS m/z (%): [M + 1]+ 340 (100), 284 [M + 2–C4H9]+, 256, 242, 226, 198, 172, 159, 117, 105.
4.2.3.11. 1-Pentyl-2-[(1E)-decenyl]-4-(1H)-quinolone (17f)
Prepared from (E)-3-dodecen-2-one (5) (1.0 g, 5.5 mmol) in THF (20 mL), LDA (2.7 mL, 5.5 mmol) and N-pentyl isatoic acid anhydride (15) (0.96 g, 4.1 mmol) in THF (20 mL). 17f was formed as light yellow oil (0.65 g, 45%). IR (KBr, νmax cm−1): 3423, 2926, 2855, 1623, 1598, 1487, 1424, 759. 1H NMR: δ 8.44 (dd, J = 8.0, 1.6 Hz, 1H, H-5), 7.62 (td, J = 8.0, 1.6 Hz, 1H, H-7), 7.44 (d, J = 8.4 Hz, 1H, H-8), 7.33 (t, J = 7.2 Hz, 1H, H-6), 6.42 (d, J = 15.6 Hz, 1H, H-1′), 6.36 (s, 1H, H-3), 6.34 (dt, J = 15.6, 6.8 Hz, 1H, H-2′), 4.11 (t, J = 8.0 Hz, 2H, N–CH2–(CH2)3–CH3), 2.27 (q, J = 6.8 Hz, 2H, H-3′), 1.80 (m, 2H, N–CH2–CH2–(CH2)2–CH3), 1.48 (quint, J = 7.2 Hz, 2H, H-4′), 1.41–1.39 (m, 4H, N–(CH2)2–CH2–CH2–CH3), 1.32–1.22 (m, 10H, H-5′–9′), 0.94 (t, J = 6.8 Hz, 3H, N–(CH2)4–CH3), 0.87 (t, J = 6.8 Hz, 3H, H-10′). 13C NMR: δ 177.8 (C-4), 151.8 (C-2), 141.7 (C-2′), 140.5 (C-8a), 132.0 (C-7), 127.1 (C-4a), 126.9 (C-5), 123.6 (C-1′), 123.1 (C-6), 115.4 (C-8), 109.8 (C-3), 47.5 (N–CH2–(CH2)3–CH3), 33.1 (C-3′), 31.8 (C-8′), 29.4 (C-7′), 29.2 (C-6′), 29.1 (C-5′), 28.8 (N–CH2–CH2–(CH2)2–CH3), 28.6 (C-4′), 28.3 (N–(CH2)2–CH2–CH2–CH3), 22.6 (C-9′), 22.3 (N–(CH2)3–CH2–CH3), 14.0 (C-10′), 13.9 (N–(CH2)4–CH3). ESI-MS m/z (%): [M + 1]+ 354 (100), 284 [M + 2–C5H11]+, 256, 240, 214, 198, 184, 172, 159.
4.2.3.12. 1-Ethyl-2-[(1E)-undecenyl]-4-(1H)-quinolone (18a)
Prepared from (E)-3-tridecen-2-one (6) (1.3 g, 6.6 mmol) in THF (25 mL), LDA (3.3 mL, 6.6 mmol) and N-ethyl isatoic acid anhydride (10) (0.96 g, 5.0 mmol) in THF (20 mL). 18a was formed as a yellow semisolid (0.59 g, 36%). IR (KBr, νmax cm−1): 3421, 2925, 2853, 1625, 1597, 1486, 1424, 1189, 1119, 759, 722, 543. 1H NMR: δ 8.45 (d, J = 8.0 Hz, 1H, H-5), 7.65 (t, J = 8.0 Hz, 1H, H-7), 7.51 (d, J = 8.0 Hz, 1H, H-8), 7.36 (t, J = 6.8 Hz, 1H, H-6), 6.48 (d, J = 15.2 Hz, 1H, H-1′), 6.43 (s, 1H, H-3), 6.40 (dt, J = 15.2, 6.8 Hz, 1H, H-2′), 4.25 (q, J = 6.8 Hz, 2H, N–CH2–CH3), 2.28 (q, J = 6.8 Hz, 2H, H-3′), 1.50 (m, 2H, H-4′), 1.45 (t, J = 6.8 Hz, 3H, N–CH2–CH3), 1.37–1.22 (m, 12H, H-5′–10′), 0.88 (t, J = 6.8 Hz, 3H, H-11′). 13C NMR: δ 177.4 (C-4), 151.9 (C-2), 142.4 (C-2′), 140.2 (C-8a), 132.2 (C-7), 126.9 (C-4a), 126.8 (C-5), 123.3 (C-1′), 123.1 (C-6), 115.3 (C-8), 109.5 (C-3), 42.5 (N–CH2–CH3), 33.2 (C-3′), 31.8 (C-9′), 29.5 (C-8′), 29.4 (C-7′), 29.3 (C-6′), 29.1 (C-5′), 28.6 (C-4′), 22.6 (C-10′), 14.1 (C-11′), 13.9 (N–CH2–CH3). ESI-MS m/z (%): [M + 1]+ 326 (100), 298, 254, 240, 212, 198, 186, 172, 159, 118, 104, 93.
4.2.3.13. 1-Propyl-2-[(1E)-undecenyl]-4-(1H)-quinolone (18b)
Prepared from (E)-3-tridecen-2-one (6) (1.3 g, 6.6 mmol) in THF (25 mL), LDA (3.3 mL, 6.6 mmol) and N-propyl isatoic acid anhydride (11) (1.02 g, 5.0 mmol) in THF (20 mL). 18b was formed as light yellow oil (0.68 g, 40%). IR (KBr, νmax cm−1): 3421, 2925, 2854, 1624, 1599, 1486, 1422, 759. 1H NMR: δ 8.44 (d, J = 8.0 Hz, 1H, H-5), 7.64 (t, J = 8.0 Hz, 1H, H-7), 7.45 (d, J = 8.4 Hz, 1H, H-8), 7.35 (t, J = 7.6 Hz, 1H, H-6), 6.43 (s, 1H, H-3), 6.42 (d, J = 14.8 Hz, 1H, H-1′), 6.37 (dt, J = 14.8, 6.4 Hz, 1H, H-2′), 4.10 (t, J = 8.0 Hz, 2H, N–CH2–CH2–CH3), 2.27 (q, J = 6.8 Hz, 2H, H-3′), 1.84 (m, 2H, N–CH2–CH2–CH3), 1.50 (m, 2H, H-4′), 1.37–1.21 (m, 12H, H-5′–10′), 1.04 (t, J = 7.2 Hz, 3H, N–(CH2)2–CH3), 0.88 (t, J = 7.2 Hz, 3H, H-11′). 13C NMR: δ 177.4 (C-4), 152.1 (C-2), 142.2 (C-2′), 140.4 (C-8a), 132.1 (C-7), 126.8 (C-4a), 126.7 (C-5), 123.5 (C-1′), 123.3 (C-6), 115.5 (C-8), 109.6 (C-3), 49.1 (N–CH2–CH2–CH3), 33.1 (C-3′), 31.8 (C-9′), 29.5 (C-8′), 29.4 (C-7′), 29.2 (C-6′), 29.1 (C-5′), 28.6 (C-4′), 22.6 (C-10′), 22.0 (N–CH2–CH2–CH3), 14.0 (C-11′), 11.0 (N–(CH2)2–CH3). ESI-MS m/z (%): [M + 1]+ 340 (100), 298 [M + 2–C3H7]+, 254, 228, 198, 172, 159, 117, 103.
4.2.3.14. 1-(2-Propenyl)-2-[(1E)-undecenyl]-4-(1H)-quinolone (18c)
Prepared from (E)-3-tridecen-2-one (6) (1.3 g, 6.6 mmol) in THF (25 mL), LDA (3.3 mL, 6.6 mmol) and N-2-propenyl isatoic acid anhydride (12) (1.01 g, 5.0 mmol) in THF (20 mL). 18c was formed as yellow oil (0.71 g, 42%). IR (KBr, νmax cm−1): 3444, 2925, 2853, 1624, 1599, 1487, 1421, 760. 1H NMR: δ 8.40 (dd, J = 8.0, 1.6 Hz, 1H, H-5), 7.58 (td, J = 8.0, 1.6 Hz, 1H, H-7), 7.36 (d, J = 8.4 Hz, 1H, H-8), 7.31 (t, J = 7.6 Hz, 1H, H-6), 6.42 (s, 1H, H-3), 6.39 (dt, J = 15.2, 6.8 Hz, 1H, H-2′), 6.31 (d, J = 15.2 Hz, 1H, H-1′), 6.03 (ddt, J = 17.2, 10.8, 4.0 Hz, 1H, N–CH2–CH CH2), 5.30 (d, J = 10.8 Hz, 1H, N–CH2–CH CHHb), 4.99 (d, J = 17.2 Hz, 1H, N–CH2–CH CHaH), 4.75 (dt, J = 4.0, 1.6 Hz, 2H, N–CH2–CH CH2), 2.23 (q, J = 6.8 Hz, 2H, H-3′), 1.47 (quint, J = 7.2 Hz, 2H, H-4′), 1.31–1.23 (m, 12H, H-5′–10′), 0.87 (t, J = 6.8 Hz, 3H, H-11′). 13C NMR: δ 177.8 (C-4), 152.2 (C-2), 142.2 (C-2′), 140.8 (C-8a), 132.1 (C-7), 131.1 (N–CH2–CH CH2), 126.6 (C-4a), 126.5 (C-5), 123.2 (C-1′), 123.2 (C-6), 117.5 (N–CH2–CH CH2), 115.9 (C-8), 109.3 (C-3), 50.0 (N–CH2–CH CH2), 33.0 (C-3′), 31.8 (C-9′), 29.5 (C-8′), 29.4 (C-7′), 29.2 (C-6′), 29.1 (C-5′), 28.6 (C-4′), 22.6 (C-10′), 14.0 (C-11′). ESI-MS m/z (%): [M + 1]+ 338 (100), 296, 240, 224, 210, 198, 184, 172, 159, 109, 95.
4.2.3.15. 1-(2-Propynyl)-2-[(1E)-undecenyl]-4-(1H)-quinolone (18d)
Prepared from (E)-3-tridecen-2-one (6) (1.3 g, 6.6 mmol) in THF (25 mL), LDA (3.3 mL, 6.6 mmol) and N-2-propynyl isatoic acid anhydride (13) (1.0 g, 5.0 mmol) in THF (20 mL). 18d was formed as a light yellow solid (0.67 g, 40%), m.p. 115–117 °C. IR (KBr, νmax cm−1): 3445, 3224, 2922, 2849, 2118, 1620, 1597, 1489, 1422, 764, 711. 1H NMR: δ 8.41 (d, J = 8.0 Hz, 1H, H-5), 7.69 (t, J = 8.0 Hz, 1H, H-7), 7.60 (d, J = 8.4 Hz, 1H, H-8), 7.38 (t, J = 7.6 Hz, 1H, H-6), 6.51 (d, J = 15.6 Hz, 1H, H-1′), 6.44 (dt, J = 15.6, 6.4 Hz, 1H, H-2′), 6.43 (s, 1H, H-3), 4.85 (d, 4J = 2.4 Hz, 2H, N–CH2–C≡CH), 2.48 (bs, 1H, N–CH2–C≡CH), 2.30 (q, J = 6.8 Hz, 2H, H-3′), 1.52 (quint, J = 7.2 Hz, 2H, H-4′), 1.32–1.23 (m, 12H, H-5′–10′), 0.88 (t, J = 6.8 Hz, 3H, H-11′). 13C NMR: δ 177.9 (C-4), 151.7 (C-2), 143.1 (C-2′), 140.5 (C-8a), 132.4 (C-7), 126.7 (C-4a), 126.5 (C-5), 123.7 (C-1′), 122.9 (C-6), 115.5 (C-8), 109.6 (C-3), 76.8 (N–CH2–C≡CH), 74.9 (N–CH2–C≡CH), 38.1 (N–CH2–C≡CH), 33.1 (C-3′), 31.8 (C-9′), 29.5 (C-8′), 29.4 (C-7′), 29.3 (C-6′), 29.1 (C-5′), 28.5 (C-4′), 22.6 (C-11′), 14.0 (C-11′). ESI-MS m/z (%): [M + 1]+ 336 (100), 294, 236, 224, 210, 196, 182, 160, 107, 95.
4.2.3.16. 1-Butyl-2-[(1E)-undecenyl]-4-(1H)-quinolone (18e)
Prepared from (E)-3-tridecen-2-one (6) (1.3 g, 6.6 mmol) in THF (25 mL), LDA (3.3 mL, 6.6 mmol) and N-butyl isatoic acid anhydride (14) (1.09 g, 5.0 mmol) in THF (20 mL). 18e was formed as yellow oil (0.74 g, 42%). IR (KBr, νmax cm−1): 3424, 2926, 2854, 1624, 1598, 1486, 1423, 1177, 759. 1H NMR: δ 8.43 (d, J = 8.4 Hz, 1H, H-5), 7.63 (t, J = 8.4 Hz, 1H, H-7), 7.45 (d, J = 8.4 Hz, 1H, H-8), 7.33 (t, J = 7.6 Hz, 1H, H-6), 6.42 (d, J = 15.6 Hz, 1H, H-1′), 6.41 (s, 1H, H-3), 6.35 (dt, J = 15.6, 6.0 Hz, 1H, H-2′), 4.13 (t, J = 8.0 Hz, 2H, N–CH2–(CH2)2–CH3), 2.27 (q, J = 7.6 Hz, 2H, H-3′), 1.79 (quint, J = 7.6 Hz, 2H, N–CH2–CH2–CH2–CH3), 1.48 (quint, J = 7.2 Hz, 2H, H-4′), 1.44 (m, 2H, N–(CH2)2–CH2–CH3), 1.31–1.22 (m, 12H, H-5′–10′), 1.01 (t, J = 7.2 Hz, 3H, N–(CH2)3–CH3), 0.87 (t, J = 6.8 Hz, 3H, H-11′). 13C NMR: δ 177.5 (C-4), 152.0 (C-2), 142.0 (C-2′), 140.4 (C-8a), 132.0 (C-7), 126.9 (C-4a), 126.8 (C-5), 123.5 (C-1′), 123.2 (C-6), 115.4 (C-8), 109.7 (C-3), 47.4 (N–CH2–(CH2)2–CH3), 33.1 (C-3′), 31.8 (C-9′), 30.6 (N–CH2–CH2–CH2–CH3), 29.5 (C-8′), 29.4 (C-7′), 29.3 (C-6′), 29.1 (C-5′), 28.6 (C-4′), 22.6 (C-10′), 19.9 (N–(CH2)2–CH2–CH3), 14.0 (C-14′), 13.7 (N–(CH2)3–CH3). ESI-MS m/z (%): [M + 1]+ 354 (100), 298 [M + 2–C4H9]+, 256, 242, 228, 198, 172, 159, 130, 106.
4.2.3.17. 1-Pentyl-2-[(1E)-undecenyl]-4-(1H)-quinolone (18f)
Prepared from (E)-3-tridecen-2-one (6) (1.3 g, 6.6 mmol) in THF (25 mL), LDA (3.3 mL, 6.6 mmol) and N-pentyl isatoic acid anhydride (15) (1.16 g, 5.0 mmol) in THF (20 mL). 18f was formed as light yellow oil (0.70 g, 38%). IR (KBr, νmax cm−1): 3422, 2926, 2854, 1624, 1598, 1487, 1423, 759. 1H NMR: δ 8.45 (dd, J = 8.0, 1.6 Hz, 1H, H-5), 7.66 (td, J = 8.0, 1.6 Hz, 1H, H-7), 7.48 (d, J = 8.4 Hz, 1H, H-8), 7.37 (t, J = 7.2 Hz, 1H, H-6), 6.51 (s, 1H, H-3), 6.45 (d, J = 15.2 Hz, 1H, H-1′), 6.40 (dt, J = 15.2, 6.8 Hz, 1H, H-2′), 4.15 (t, J = 8.4 Hz, 2H, N–CH2–(CH2)3–CH3), 2.29 (q, J = 7.2 Hz, 2H, H-3′), 1.83 (m, 2H, N–CH2–CH2–(CH2)2–CH3), 1.51 (quint, J = 6.8 Hz, 2H, H-4′), 1.44–1.41 (m, 4H, N–(CH2)2–CH2–CH2–CH3), 1.33–1.22 (m, 12H, H-5′-10′), 0.95 (t, J = 6.8 Hz, 3H, N–(CH2)4–CH3), 0.88 (t, J = 6.8 Hz, 3H, H-11′). 13C NMR: δ 177.6 (C-4), 152.2 (C-2), 142.4 (C-2′), 140.4 (C-8a), 132.2 (C-7), 126.9 (C-4a), 126.8 (C-5), 123.4 (C-1′), 123.2 (C-6), 115.5 (C-8), 109.5 (C-3), 47.7 (N–CH2–(CH2)3–CH3), 33.2 (C-3′), 31.9 (C-9′), 29.5 (C-8′), 29.4 (C-7′), 29.3 (C-6′), 29.2 (C-5′), 28.8 (N–CH2–CH2–(CH2)2–CH3), 28.6 (C-4′), 28.3 (N–(CH2)2–CH2–CH2–CH3), 22.6 (C-10′), 22.3 (N–(CH2)3–CH2–CH3), 14.1 (C-11′), 13.9 (N–(CH2)4–CH3). ESI-MS m/z (%): [M + 1]+ 368 (100), 296 [M + 2–C5H11]+, 281, 268, 256, 228, 198, 184, 172, 159.
4.2.3.18. 1-Ethyl-2-[(1E)-dodecenyl]-4-(1H)-quinolone (19a)
Prepared from (E)-3-tetradecen-2-one (7) (1.3 g, 6.2 mmol) in THF (25 mL), LDA (3.1 mL, 6.1 mmol) and N-ethyl isatoic acid anhydride (10) (0.89 g, 4.6 mmol) in THF (20 mL). 19a was formed as a light yellow semisolid (0.68 g, 44%). IR (KBr, νmax cm−1): 3420, 2925, 2853, 1623, 1597, 1488, 1424, 759. 1H NMR: δ 8.43 (d, J = 8.0 Hz, 1H, H-5), 7.63 (t, J = 8.0 Hz, 1H, H-7), 7.48 (d, J = 7.2 Hz, 1H, H-8), 7.32 (t, J = 7.2 Hz, 1H, H-6), 6.42 (d, J = 15.2 Hz, 1H, H-1′), 6.40 (s, 1H, H-3), 6.35 (dt, J = 15.2, 6.4 Hz, 1H, H-2′), 4.21 (q, J = 6.4 Hz, 2H, N–CH2–CH3), 2.26 (q, J = 6.4 Hz, 2H, H-3′), 1.49 (m, 2H, H-4′), 1.42 (t, J = 6.4 Hz, 3H, N–CH2–CH3), 1.38–1.22 (m, 14H, H-5′–11′), 0.86 (t, J = 6.4 Hz, 3H, H-12′). 13C NMR: δ 177.6 (C-4), 151.7 (C-2), 142.1 (C-2′), 140.2 (C-8a), 132.1 (C-7), 126.9 (C-4a), 126.8 (C-5), 123.2 (C-1′), 123.1 (C-6), 115.3 (C-8), 109.6 (C-3), 42.4 (N–CH2–CH3), 33.1 (C-3′), 31.8 (C-10′), 29.5 (C-9′), 29.5 (C-8′), 29.4 (C-7′), 29.2 (C-6′), 29.1 (C-5′), 28.6 (C-4′), 22.6 (C-11′), 14.0 (C-12′), 13.9 (N–CH2–CH3). ESI-MS m/z (%): [M + 1]+ 340 (100), 312 [M + 2–C2H5]+, 268, 254, 240, 214, 198, 186, 172, 159, 117.
4.2.3.19. 1-Propyl-2-[(1E)-dodecenyl]-4-(1H)-quinolone (19b)
Prepared from (E)-3-tetradecen-2-one (7) (1.3 g, 6.2 mmol) in THF (25 mL), LDA (3.1 mL, 6.1 mmol) and N-propyl isatoic acid anhydride (11) (0.95 g, 4.6 mmol) in THF (20 mL). 19b was formed as light yellow oil (0.76 g, 47%). IR (KBr, νmax cm−1): 3421, 2925, 2853, 1624, 1598, 1486, 1422, 759. 1H NMR: δ 8.45 (dd, J = 8.0, 1.6 Hz, 1H, H-5), 7.64 (td, J = 8.0, 1.6 Hz, 1H, H-7), 7.46 (d, J = 8.4 Hz, 1H, H-8), 7.35 (t, J = 7.2 Hz, 1H, H-6), 6.44 (d, J = 15.2 Hz, 1H, H-1′), 6.43 (s, 1H, H-3), 6.37 (dt, J = 15.2, 6.4 Hz, 1H, H-2′), 4.11 (t, J = 8.0 Hz, 2H, N–CH2–CH2–CH3), 2.28 (q, J = 6.8 Hz, 2H, H-3′), 1.85 (m, 2H, N–CH2–CH2–CH3), 1.50 (quint, J = 6.8 Hz, 2H, H-4′), 1.32–1.22 (m, 14H, H-5′–11′), 1.05 (t, J = 7.2 Hz, 3H, N–(CH2)2–CH3), 0.88 (t, J = 6.8 Hz, 3H, H-12′). 13C NMR: δ 177.6 (C-4), 152.1 (C-2), 142.1 (C-2′), 140.5 (C-8a), 132.1 (C-7), 126.9 (C-4a), 126.8 (C-5), 123.5 (C-1′), 123.2 (C-6), 115.5 (C-8), 109.7 (C-3), 49.1 (N–CH2–CH2–CH3), 33.1 (C-3′), 31.9 (C-10′), 29.6 (C-9′), 29.6 (C-8′), 29.4 (C-7′), 29.3 (C-6′), 29.1 (C-5′), 28.6 (C-4′), 22.6 (C-11′), 22.0 (N–CH2–CH2–CH3), 14.0 (C-12′), 10.9 (N–(CH2)2–CH3). ESI-MS m/z (%): [M + 1]+ 354 (100), 312 [M + 2–C3H7]+, 298, 254, 186, 172, 107.
4.2.3.20. 1-(2-Propenyl)-2-[(1E)-dodecenyl]-4-(1H)-quinolone (19c)
Prepared from (E)-3-tetradecen-2-one (7) (1.3 g, 6.2 mmol) in THF (25 mL), LDA (3.1 mL, 6.2 mmol) and N-2-propenyl isatoic acid anhydride (12) (0.94 g, 4.6 mmol) in THF (20 mL). 19c was formed as a light yellow semisolid (0.66 g, 41%). IR (KBr, νmax cm−1): 3419, 2925, 2853, 1625, 1599, 1486, 1422, 760. 1H NMR: δ 8.41 (dd, J = 8.0, 1.6 Hz, 1H, H-5), 7.59 (td, J = 8.0, 1.6 Hz, 1H, H-7), 7.36 (d, J = 8.4 Hz, 1H, H-8), 7.32 (t, J = 7.6 Hz, 1H, H-6), 6.44 (s, 1H, H-3), 6.40 (dt, J = 15.6, 6.4 Hz, 1H, H-2′), 6.31 (d, J = 15.6 Hz, 1H, H-1′), 6.02 (ddt, J = 17.2, 10.4, 4.0 Hz, 1H, N–CH2–CH CH2), 5.30 (d, J = 10.4 Hz, 1H, N–CH2–CH CHHb), 5.01 (d, J = 17.2 Hz, 1H, N–CH2–CH CHaH), 4.76 (dt, J = 4.0, 1.6 Hz, 2H, N–CH2–CH CH2), 2.24 (q, J = 6.8 Hz, 2H, H-3′), 1.47 (quint, J = 7.2 Hz, 2H, H-4′), 1.36–1.22 (m, 14H, H-5′–11′), 0.86 (t, J = 6.8 Hz, 3H, H-12′). 13C NMR: δ 177.8 (C-4), 152.2 (C-2), 142.3 (C-2′), 140.9 (C-8a), 132.1 (C-7), 131.1 (N–CH2–CH CH2), 126.6 (C-4a), 126.5 (C-5), 123.3 (C-1′), 123.2 (C-6), 117.5 (N–CH2–CH CH2), 116.0 (C-8), 109.3 (C-3), 50.1 (N–CH2–CH CH2), 33.1 (C-3′), 31.8 (C-10′), 29.5 (C-9′), 29.5 (C-8′), 29.4 (C-7′), 29.2 (C-6′), 29.1 (C-5′), 28.6 (C-4′), 22.6 (C-11′), 14.0 (C-12′). ESI-MS m/z (%): [M + 1]+ 352 (100), 310 [M + 2–C3H5]+, 224, 210, 198, 184, 172, 146, 107.
4.2.3.21. 1-(2-Propynyl)-2-[(1E)-dodecenyl]-4-(1H)-quinolone (19d)
Prepared from (E)-3-tetradecen-2-one (7) (1.3 g, 6.2 mmol) in THF (25 mL), LDA (3.1 mL, 6.2 mmol) and N-2-propynyl isatoic acid anhydride (13) (0.93 g, 4.6 mmol) in THF (20 mL). 19d was formed as a light yellow semisolid (0.70 g, 44%). IR (KBr, νmax cm−1): 3445, 3182, 2922, 2847, 2112, 1623, 1598, 1487, 1418, 764. 1H NMR: δ 8.39 (dd, J = 8.0, 1.6 Hz, 1H, H-5), 7.66 (td, J = 8.0, 1.6 Hz, 1H, H-7), 7.58 (d, J = 8.4 Hz, 1H, H-8), 7.35 (t, J = 7.6 Hz, 1H, H-6), 6.49 (d, J = 14.6 Hz, 1H, H-1′), 6.40 (dt, J = 14.6, 6.8 Hz, 1H, H-2′), 6.37 (s, 1H, H-3), 4.82 (d, 4J = 2.4 Hz, 2H, N–CH2–C≡CH), 2.48 (t, 4J = 2.4 Hz, 1H, N–CH2–C≡CH), 2.29 (q, J = 6.8 Hz, 2H, H-3′), 1.50 (quint, J = 7.2 Hz, 2H, H-4′), 1.36–1.22 (m, 14H, H-5′–11′), 0.87 (t, J = 6.8 Hz, 3H, H-12′) 13C NMR: δ 177.9 (C-4), 151.6 (C-2), 142.8 (C-2′), 140.5 (C-8a), 132.3 (C-7), 126.6 (C-4a), 126.6 (C-5), 123.6 (C-1′), 122.9 (C-6), 115.9 (C-8), 109.7 (C-3), 76.9 (N–CH2–C≡CH), 74.8 (N–CH2–C≡CH), 38.1 (N–CH2–C≡CH), 33.1 (C-3′), 31.8 (C-10′), 29.5 (C-9′), 29.5 (C-8′), 29.4 (C-7′), 29.2 (C-6′), 29.1 (C-5′), 28.6 (C-4′), 22.6 (C-11′), 14.0 (C-12′). ESI-MS m/z (%): [M + 1]+ 350 (100), 310 [M + 2–C3H3]+, 294, 280, 236, 224, 210, 198, 184, 160, 107.
4.2.3.22. 1-Butyl-2-[(1E)-dodecenyl]-4-(1H)-quinolone (19e)
Prepared from (E)-3-tetradecen-2-one (7) (1.3 g, 6.2 mmol) in THF (25 mL), LDA (3.1 mL, 6.2 mmol) and N-butyl isatoic acid anhydride (14) (1.02 g, 4.6 mmol) in THF (20 mL). 19e was formed as light yellow oil (0.81 g, 48%). IR (KBr, νmax cm−1): 3424, 2925, 2854, 1623, 1598, 1486, 1423, 1176, 759. 1H NMR: δ 8.44 (d, J = 8.4 Hz, 1H, H-5), 7.64 (t, J = 8.4 Hz, 1H, H-7), 7.46 (d, J = 8.4 Hz, 1H, H-8), 7.34 (t, J = 7.6 Hz, 1H, H-6), 6.44 (s, 1H, H-3), 6.37 (d, J = 16.0 Hz, 1H, H-1′), 6.35 (dt, J = 16.0, 6.4 Hz, 1H, H-2′), 4.14 (t, J = 8.0 Hz, 2H, N–CH2–(CH2)–CH3), 2.27 (q, J = 7.2 Hz, 2H, H-3′), 1.80 (quint, J = 7.6 Hz, 2H, N–CH2–CH2–CH2–CH3), 1.49 (quint, J = 7.2 Hz, 2H, H->4′), 1.44 (m, 2H, N–(CH2)2–CH2–CH3), 1.37–1.22 (m, 14H, H-5′–11′), 1.01 (t, J = 7.6 Hz, 3H, N–(CH2)3–CH3), 0.87 (t, J = 6.8 Hz, 3H, H-12′). 13C NMR: δ 177.5 (C-4), 151.9 (C-2), 142.0 (C-2′), 140.4 (C-8a), 132.0 (C-7), 126.8 (C-4a), 126.8 (C-5), 123.5 (C-1′), 123.2 (C-6), 115.4 (C-8), 109.6 (C-3), 47.3 (N–CH2–(CH2)2–CH3), 33.1 (C-3′), 31.8 (C-10′), 30.6 (N–CH2–CH2–CH2–CH3), 29.5 (C-9′), 29.5 (C-8′), 29.4 (C-7′), 29.2 (C-6′), 29.1 (C-5′), 28.6 (C-4′), 22.6 (C-11), 19.9 (N–(CH2)2–CH2–CH3), 14.0 (C-14′), 13.7(N–(CH2)3–CH3). ESI-MS m/z (%): [M + 1]+ 368 (100), 312, 298, 284, 256, 242, 198, 172, 159, 117, 105.
4.2.3.23. 1-Pentyl-2-[(1E)-dodecenyl]-4-(1H)-quinolone (19f)
Prepared from (E)-3-tetradecen-2-one (7) (1.3 g, 6.2 mmol) in THF (25 mL), LDA (3.1 mL, 6.2 mmol) and N-pentyl isatoic acid anhydride (15) (1.09 g, 4.6 mmol) in THF (20 mL). 19f was formed as a light yellow semisolid (0.75 g, 43%). IR (KBr, νmax cm−1): 3423, 2925, 2854, 1624, 1598, 1486, 1422, 759. 1H NMR: δ 8.44 (dd, J = 8.0, 1.6 Hz, 1H, H-5), 7.64 (td, J = 8.0, 1.6 Hz, 1H, H-7), 7.45 (d, J = 8.4 Hz, 1H, H-8), 7.34 (t, J = 7.6 Hz, 1H, H-6), 6.42 (d, J = 15.6 Hz, 1H, H-1′), 6.39 (s, 1H, H-3), 6.36 (dt, J = 15.6, 6.8 Hz, 1H, H-2′), 4.11 (t, J = 8.0 Hz, 2H, N–CH2–(CH2)3–CH3), 2.27 (q, J = 6.8 Hz, 2H, H-3′), 1.81 (m, 2H, N–CH2–CH2–(CH2)2–CH3), 1.50 (quint, J = 6.8 Hz, 2H, H-4′), 1.44–1.39 (m, 4H, N–(CH2)2–CH2–CH2–CH3), 1.32–1.23 (m, 14H, H-5′–11′), 0.94 (t, J = 6.8 Hz, 3H, N–(CH2)4–CH3), 0.87 (t, J = 6.8 Hz, 3H, H-12′). 13C NMR: δ 177.6 (C-4), 151.9 (C-2), 142.0 (C-2′), 140.4 (C-8a), 132.1 (C-7), 126.9 (C-4a), 126.8 (C-5), 123.5 (C-1′), 123.2 (C-6), 115.4 (C-8), 109.7 (C-3), 47.6 (N–CH2–(CH2)3–CH3), 33.1 (C-3′), 31.9 (C-10′), 29.6 (C-9′), 29.6 (C-8′), 29.4 (C-7′), 29.3 (C-6′), 29.1 (C-5′), 28.8 (N–CH2–CH2–(CH2)2–CH3), 28.6 (C-4′), 28.3 (N–(CH2)2–CH2–CH2–CH3), 22.6 (C–11′), 22.3 (N–(CH2)3–CH2–CH3), 14.0 (C-12′), 13.9 (N–(CH2)4–CH3). ESI-MS m/z (%): [M + 1]+ 382 (100), 310 [M + 2–C5H11]+, 284, 256, 240, 198, 184, 159.
4.2.3.24. 1-Ethyl-2-[(1E)-tridecenyl]-4-(1H)-quinolone (20a)
Prepared from (E)-3-pentadecen-2-one (8) (1.5 g, 6.7 mmol) in THF (25 mL), LDA (3.4 mL, 6.7 mmol) and N-ethyl isatoic acid anhydride (10) (0.96 g, 5.0 mmol) in THF (20 mL). 20a was formed as a yellow semisolid (0.79 g, 45%). IR (KBr, νmax cm−1): 3422, 2925, 2853, 1626, 1597, 1487, 1423, 1177, 759. 1H NMR: δ 8.44 (dd, J = 8.0, 1.6 Hz, 1H, H-5), 7.65 (td, J = 8.0, 1.6 Hz, 1H, H-7), 7.50 (d, J = 8.4 Hz, 1H, H-8), 7.34 (t, J = 7.6 Hz, 1H, H-6), 6.45 (d, J = 15.2 Hz, 1H, H-1′), 6.42 (s, 1H, H-3), 6.37 (dt, J = 15.2, 6.4 Hz, 1H, H-2′), 4.25 (q, J = 6.4 Hz, 2H, N–CH2–CH3), 2.27 (q, J = 6.4 Hz, 2H, H-3′), 1.49 (m, 2H, H-4′), 1.44 (t, J = 6.4 Hz, 3H, N–CH2–CH3), 1.38–1.21 (m, 16H, H-5′–12′), 0.87 (t, J = 6.4 Hz, 3H, H-13′). 13C NMR: δ 177.5 (C-4), 151.8 (C-2), 142.3 (C-2′), 140.2 (C-8a), 132.2 (C-7), 126.9 (C-4a), 126.8 (C-5), 123.2 (C-1′), 123.2 (C-6), 115.3 (C-8), 109.6 (C-3), 42.5 (N–CH2–CH3), 33.1 (C-3′), 31.9(C-11′), 29.6 (C-10′), 29.6 (C-9′), 29.5 (C-8′), 29.4 (C-7′), 29.3 (C-6′), 29.1 (C-5′), 28.6 (C-4′), 22.6 (C-12′), 14.1 (C-13′), 13.9 (N–CH2–CH3). ESI-MS m/z (%): [M + 1]+ 354 (100), 326 [M + 2–C2H5]+, 312, 282, 268, 212, 198, 186, 172, 159, 117, 103.
4.2.3.25. 1-(2-Propenyl)-2-[(1E)-tridecenyl]-4-(1H)-quinolone (20c)
Prepared from (E)-3-pentadecen-2-one (8) (1.5 g, 6.7 mmol) in THF (25 mL), LDA (3.4 mL, 6.7 mmol) and N-2-propenyl isatoic acid anhydride (12) (1.02 g, 5.0 mmol) in THF (20 mL). 20c was formed as yellow oil (0.76 g, 42%). IR (KBr, νmax cm−1): 3443, 2924, 2853, 1624, 1599, 1486, 1422, 759. 1H NMR: δ 8.40 (dd, J = 8.0, 1.6 Hz, 1H, H-5), 7.58 (td, J = 8.0, 1.6 Hz, 1H, H-7), 7.35 (d, J = 8.4 Hz, 1H, H-8), 7.31 (t, J = 7.6 Hz, 1H, H-6), 6.41 (s, 1H, H-3), 6.37 (dt, J = 16.0, 6.4 Hz, 1H, H-2′), 6.31 (d, J = 16.0 Hz, 1H, H-1′), 6.02 (ddt, J = 17.2, 10.7, 4.0 Hz, 1H, N–CH2–CH CH2), 5.29 (d, J = 10.8 Hz, 1H, N–CH2–CH CHHb), 5.00 (d, J = 17.2 Hz, 1H, N–CH2–CH CHaH), 4.74 (d, J = 2.0 Hz, 2H, N–CH2–CH CH2), 2.23 (q, J = 6.8 Hz, 2H, H-3′), 1.47 (m, 2H, H-4′), 1.36–1.21 (m, 16H, H-5′–12′), 0.86 (t, J = 6.8 Hz, 3H, H-13′). 13C NMR: δ 177.9 (C-4), 152.2 (C-2), 142.2 (C-2′), 140.9 (C-8a), 132.1 (C-7), 131.1 (N–CH2–CH CH2), 126.6 (C-4a), 126.5 (C-5), 123.5 (C-1′), 123.3 (C-6), 117.5 (N–CH2–CH CH2), 115.9 (C8), 109.3 (C-3), 50.0 (N–CH2–CH CH2), 33.0 (C-3′), 31.8 (C-11′), 29.6 (C-10′), 29.5 (C-9′), 29.5 (C-8′), 29.4 (C-7′), 29.3 (C-6′), 29.1 (C-5′), 28.6 (C-4′), 22.6 (C-12′), 14.0 (C-13′). ESI-MS m/z (%): [M + 1]+ 366 (100), 324 [M + 2–C3H5]+, 306, 224, 210, 198, 184, 146, 107.
4.2.3.26. 1-Pentyl-2-[(1E)-tridecenyl]-4-(1H)-quinolone (20f)
Prepared from (E)-3-pentadecen-2-one (8) (1.5 g, 6.7 mmol) in THF (25 mL), LDA (3.4 mL, 6.7 mmol) and N-pentyl isatoic acid anhydride (15) (1.17 g, 5.0 mmol) in THF (20 mL). 20f was formed as a yellow semisolid (0.98 g, 50%). IR (KBr, νmax cm−1): 3424, 2925, 2854, 1624, 1598, 1486, 1422, 759. 1H NMR: δ 8.43 (dd, J = 8.0, 1.6 Hz, 1H, H-5), 7.62 (td, J = 8.0, 1.6 Hz, 1H, H-7), 7.44 (d, J = 8.4 Hz, 1H, H-8), 7.32 (t, J = 7.6 Hz, 1H, H-6), 6.41 (d, J = 15.2 Hz, 1H, H-1′), 6.38 (s, 1H, H-3), 6.34 (dt, J = 15.2, 6.8 Hz, 1H, H-2′), 4.11 (t, J = 8.4 Hz, 2H, N–CH2–(CH2)3–CH3), 2.26 (q, J = 6.8 Hz, 2H, H-3′), 1.80 (m, 2H, N–CH2–CH2–(CH2)2–CH3), 1.49 (quint, J = 7.2 Hz, 2H, H-4′), 1.41–1.37 (m, 4H, N–(CH2)2–CH2–CH2–CH3), 1.34–1.22 (m, 16H, H-5′–12′), 0.94 (t, J = 6.8 Hz, 3H, N–(CH2)4–CH3), 0.86 (t, J = 6.8 Hz, 3H, H-13′). 13C NMR: δ 177.6 (C-4), 151.9 (C-2), 141.9 (C-2′), 140.4 (C-8a), 132.0 (C-7), 126.8 (C-4a), 126.8 (C-5), 123.5 (C-1′), 123.1 (C-6), 115.4 (C-8), 109.7 (C-3), 47.5 (N–CH2–(CH2)3–CH3), 33.1 (C-3′), 31.9 (C-11′), 29.6 (C-10′), 29.6 (C-9′), 29.5 (C-8′), 29.4 (C-7′), 29.3 (C-6′), 29.1 (C-5′), 28.7 (N–CH2–CH2–(CH2)2–CH3), 28.6 (C-4′), 28.3 (N–(CH2)2–CH2–CH2–CH3), 22.6 (C-12′), 22.3 (N–(CH2)3–CH2–CH3), 14.0 (C-13′), 13.9 (N–(CH2)4–CH3). ESI-MS m/z (%): [M + 1]+ 396 (100), 324 [M + 2–C5H11]+, 310, 282, 256, 240, 198, 184, 159.
4.2.3.27. 1-Propyl-2-[(1E)-tetradecenyl]-4-(1H)-quinolone (21b)
Prepared from (E)-3-hexadecen-2-one (9) (1.5 g, 6.3 mmol) in THF (25 mL), LDA (3.2 mL, 6.3 mmol) and N-propyl isatoic acid anhydride (11) (0.96 g, 4.7 mmol) in THF (20 mL). 21b was formed as light yellow oil (0.87 g, 49%). IR (KBr, νmax cm−1): 3422, 2924, 2853, 1625, 1598, 1485, 1422, 759. 1H NMR: δ 8.44 (d, J = 8.0 Hz, 1H, H-5), 7.64 (t, J = 7.6 Hz, 1H, H-7), 7.45 (d, J = 8.0 Hz, 1H, H-8), 7.34 (t, J = 7.6 Hz, 1H, H-6), 6.42 (s, 1H, H-3), 6.26 (d, J = 14.8 Hz, 1H, H-1′), 6.36 (dt, J = 14.8, 6.4 Hz, 1H, H-2′), 4.10 (t, J = 8.0 Hz, 2H, N–CH2–CH2–CH3), 2.27 (q, J = 6.8 Hz, 2H, H-3′), 1.84 (m, 2H, N–CH2–CH2–CH3), 1.50 (m, 2H, H-4′), 1.38–1.22 (m, 18H, H-5′–13′), 1.04 (t, J = 7.2 Hz, 3H, N–(CH2)2–CH3), 0.87 (t, J = 7.2 Hz, 3H, H-14′). 13C NMR: δ 177.5 (C-4), 152.0 (C-2), 142.1 (C-2′), 140.4 (C-8a), 132.1 (C-7), 126.8 (C-4a), 126.8 (C-5), 123.5 (C-1′), 123.2 (C-6), 115.5 (C-8), 109.6 (C-3), 49.0 (N–CH2–CH2–CH3), 33.1 (C-3′), 31.9 (C-12′), 29-6 (C-11′), 29.6 (C-10′), 29.6 (C-9′), 29.6 (C-8′), 29.4 (C-7′), 29.3 (C-6′), 29.1 (C-5′), 28.6 (C-4′), 22.6 (C-13′), 22.0 (N–CH2–CH2–CH3), 14.1 (C-14′), 11.0 (N–(CH2)2–CH3). ESI-MS m/z (%): [M + 1]+ 382 (100), 340, 326, 312, 298, 254, 172, 107.
4.2.3.28. 1-(2-Propenyl)-2-[(1E)-tetradecenyl]-4-(1H)-quinolone (21c)
Prepared from (E)-3-hexadecen-2-one (9) (1.5 g, 6.3 mmol) in THF (25 mL), LDA (3.2 mL, 6.3 mmol) and N-2-propenyl isatoic acid anhydride (12) (0.95 g, 4.7 mmol) in THF (20 mL). 21c was formed as a light yellow semisolid (0.76 g, 43%). IR (KBr, νmax cm−1): 3445, 2919, 2849, 1622, 1599, 1488, 1425, 755. 1H NMR: δ 8.39 (dd, J = 8.4, 1.6 Hz, 1H, H-5), 7.56 (td, J = 8.4, 1.6 Hz, 1H, H-7), 7.34 (d, J = 7.6 Hz, 1H, H-8), 7.29 (t, J = 7.6 Hz, 1H, H-6), 6.39 (s, 1H, H-3), 6.35 (dt, J = 15.6, 6.8 Hz, 1H, H-2′), 6.30 (d, J = 15.6 Hz, 1H, H-1′), 6.00 (m, 1H, N–CH2–CH CH2), 5.27 (d, J = 10.4 Hz, 1H, N–CH2–CH CHHb), 4.98 (d, J = 17.2 Hz, 1H, N–CH2–CH CHaH), 4.73 (dt, J = 4.0, 1.6 Hz, 2H, N–CH2–CH CH2), 2.22 (q, J = 6.8 Hz, 2H, H-3′), 1.46 (m, 2H, H-4′), 1.33–1.20 (m, 18H, H-5′–13′), 0.85 (t, J = 6.8 Hz, 3H, H-14′). 13C NMR: δ 177.8 (C-4), 152.1 (C-2), 142.1 (C-2′), 140.8 (C-8a), 132.0 (C-7), 131.1 (N–CH2–CH CH2), 126.7 (C-4a), 126.5 (C-5), 123.2 (C-1′), 123.2 (C-6), 117.4 (N–CH2–CH CH2), 115.9 (C-8), 109.3 (C-3), 49.9 (N–CH2–CH CH2), 33.0 (C-3′), 31.8 (C-12′), 29.6 (C-11′), 29.5 (C-10′), 29.5 (C-9′), 29.5 (C-8′), 29.3 (C-7′), 29.2 (C-6′), 29.1 (C-5′), 28.5 (C-4′), 22.6 (C-13′), 14.0 (C-14′). ESI-MS m/z (%): [M + 1]+ 380 (100), 338 [M + 2–C3H5]+, 310, 224, 210, 198, 184, 172, 146.
4.2.3.29. 1-(2-Propynyl)-2-[(1E)-tetradecenyl]-4-(1H)-quinolone (21d)
Prepared from (E)-3-hexadecen-2-one (9) (1.5 g, 6.3 mmol) in THF (25 mL), LDA (3.2 mL, 6.3 mmol) and N-2-propynyl isatoic acid anhydride (13) (0.95 g, 4.7 mmol) in THF (20 mL). 21d was formed as a light yellow solid (0.79 g, 45%). IR (KBr, νmax cm−1): 3446, 3179, 2921, 2848, 2113, 1622, 1597, 1487, 1420, 763. 1H NMR: δ 8.40 (d, J = 8.0 Hz, 1H, H-5), 7.69 (t, J = 8.0 Hz, 1H, H-7), 7.59 (d, J = 8.4 Hz, 1H, H-8), 7.37 (t, J = 7.6 Hz, 1H, H-6), 6.50 (d, J = 16.0 Hz, 1H, H-1′), 6.43 (dt, J = 16.0, 6.4 Hz, 1H, H-2′), 6.41 (s, 1H, H-3), 4.84 (s, 2H, N–CH2–C≡CH), 2.48 (s, 1H, N–CH2–C≡CH), 2.30 (q, J = 6.8 Hz, 2H, H-3′), 1.51 (m, 2H, H-4′), 1.38–1.22 (m, 18H, H-5′–13′), 0.87 (t, J = 6.8 Hz, 3H, H-14′). 13C NMR: δ 177.9 (C-4), 151.7 (C-2), 143.0 (C-2′), 140.5 (C-8a), 132.4 (C-7), 126.7 (C-4a), 126.6 (C-5), 123.6 (C-1′), 122.9 (C-6), 115.5 (C-8), 109.6 (C-3), 76.8 (N–CH2–C≡CH), 74.9 (N–CH2–C≡CH), 38.1 (N–CH2–C≡CH), 33.1 (C-3′), 31.9 (C-12′), 29.6 (C-11′), 29.6, (C-10′), 29.6 (C-9′), 29.6 (C-8′), 29.4 (C-7′), 29.3 (C-6′), 29.1 (C-5′), 28.6 (C-4′), 22.6 (C-13′), 14.1 (C-14′). ESI-MS m/z (%): [M + 1]+ 378 (100), 338, 310, 210, 198, 184, 172, 146.
4.2.3.30. 1-Butyl-2-[(1E)-tetradecenyl]-4-(1H)-quinolone (21e)
Prepared from (E)-3-hexadecen-2-one (9) (1.5 g, 6.3 mmol) in THF (25 mL), LDA (3.2 mL, 6.3 mmol) and N-butyl isatoic acid anhydride (14) (1.03 g, 4.7 mmol) in THF (20 mL). 21e was formed as yellow oil (0.81 g, 44%). IR (KBr, νmax cm−1): 3422, 2925, 2853, 1625, 1598, 1485, 1423, 759. 1H NMR: δ 8.43 (dd, J = 8.0, 1.6 Hz, 1H, H-5), 7.63 (td, J = 8.0, 1.6 Hz, 1H, H-7), 7.46 (d, J = 8.4 Hz, 1H, H-8), 7.33 (t, J = 7.6 Hz, 1H, H-6), 6.43 (d, J = 15.6 Hz, 1H, H-1′), 6.42 (s, 1H, H-3), 6.36 (dt, J = 15.6, 6.0 Hz, 1H, H-2′), 4.12 (t, J = 7.6 Hz, 2H, N–CH2–(CH2)2–CH3), 2.27 (q, J = 6.8 Hz, 2H, H-3′), 1.79 (quint, J = 7.2 Hz, 2H, N–CH2–CH2–CH2–CH3), 1.48 (quint, J = 7.2 Hz, 2H, H-4′), 1.42 (m, 2H, N–(CH2)2–CH2–CH3), 1.32–1.22 (m, 18H, H-5′–13′), 1.01 (t, J = 7.6 Hz, 3H, N–(CH2)3–CH3), 0.86 (t, J = 6.8 Hz, 3H, H-14′). 13C NMR: δ 177.5 (C-4), 152.0 (C-2), 142.0 (C-2′), 140.4 (C-8a), 132.0 (C-7), 126.8 (C-4a), 126.8 (C-5), 123.5 (C-1′), 123.2 (C-6), 115.4 (C-8), 109.6 (C-3), 47.3 (N-CH2–(CH2)2–CH3), 33.1 (C-3′), 31.8 (C-12′), 30.6 (N–CH2–CH2–CH2–CH3), 29.6 (C-11′), 29.6 (C-10′), 29.6 (C-9′), 29.5 (C-8′), 29.4 (C-7′), 29.3 (C-6′), 29.1 (C-5′), 28.6 (C-4′), 22.6 (C-13′), 19.9 (N–(CH2)2–CH2–CH3), 14.0 (C-14′), 13.6 (N–(CH2)3–CH3). ESI-MS m/z (%): [M + 1]+ 396 (100), 338 [M + 2–C4H9]+, 296, 282, 242, 226, 198, 172, 159, 130, 107.
4.3. In vitro antimycobacterial assay
The synthetic compounds were evaluated for mycobacterial growth inhibitory effect as previously described method [19] on 96-wells using a microdilution assay. The mycobacterial strains, which were cultivated on Columbia blood agar supplemented with 7% defibrinated horse blood and prepared in 0.9% NaCl solution to get inoculum’s density of 5 × 105 cells/mL, were dispensed into each well, except the blank. 125 μL of each test material solution and the standard antibiotic, prepared by dissolving in DMSO and further dilution in MHB, were added to well 1 of the lane in duplicate followed by serial double dilutions to well 10 of the lane, so that the final concentration ranged from 128 mg/L at well 1 to 0.25 mg/L at well 10. The last two wells in the lane were used as a sterile and growth control respectively. Isoniazid was used as a positive control and tests were repeated three times. Growth inhibition was determined after 3 days of incubation at 37 °C and methanol solution of tetrazolium redox dye (MTT) was employed as mycobacterial growth indicator producing a dark violet colouration when there is growth.
4.4. Cytotoxicity assay
Cytotoxicity was assessed using human diploid embryonic lung cell line MRC-5 as previously described method [20] with some modifications. Growth inhibitory effect of respective samples on cells was measured by XTT proliferation assay.
The toxicity of compounds was determined by means of the XTT Cell Proliferation Kit II (Roche Diagnostics, Mannheim, Germany). Fresh stock solutions of each compound were prepared in Ethanol and afterwards diluted with buffer or water, respectively. Cells were seeded at a concentration of 5 × 104 cells/well and cultivated for 24 h in 96-well culture plates. Marginal wells were filled with 100 μL of pure medium (MEM) in order to minimize effects of evaporation. Besides, wells filled with medium were required to determine the background absorbance caused by non-metabolized XTT. A row of wells containing cells treated with ethanol (0.1%) served as solvent control. The other rows of wells containing cells were supplemented with different concentrations of the respective compound. Each concentration was tested in triplicate in at least two independent plates containing different batches of cells (n = 6).
After incubation for 72 h with respective compounds at 37 °C, 5% CO2 in humidified atmosphere, XTT reagent was freshly prepared and added to each well as specified by the manufacturer: XTT-labelling reagent and electron-coupling reagent were mixed in a ratio of 50:1 and 50 μL of this mixture were added to each well of the 96-well plate. The plates were incubated for additional 4 h at 37 °C, 5% CO2 in humidified atmosphere and read out afterwards. Quantification of cell viability was performed with a microplate reader at 490 nm with a reference wavelength of 655 nm. Absorbance values at both wavelengths were subtracted. The cytotoxic effect of the treatment was determined as percentage of viability compared to cells treated with solvent solely.
Acknowledgements
The Austrian Science Fund (FWF) project no. P21152-B18 is gratefully acknowledged for financial support. We thank Mr. Andreas Leitner, Department of Pharmaceutical Chemistry, for measurement of IR spectra.
References
- 1.Ruiz J. J. Antimicrob. Chemother. 2003;51:1109–1117. doi: 10.1093/jac/dkg222. [DOI] [PubMed] [Google Scholar]
- 2.Acar J.F., Goldstein F.W. Clin. Infect. Dis. 1997;24(Suppl. 1):S67–S73. doi: 10.1093/clinids/24.supplement_1.s67. [DOI] [PubMed] [Google Scholar]
- 3.Adams M., Wube A.A., Bucar F., Bauer R., Kunert O., E Haslinger Int. J. Antimicrob. Agents. 2005;26:262–264. doi: 10.1016/j.ijantimicag.2005.06.003. [DOI] [PubMed] [Google Scholar]
- 4.Adams M., Bauer R., Bucar F., Wube A.A. PCT Int Appl. 2006:18. WO 2006094327 A120060914. [Google Scholar]
- 5.Wube A.A., Hüfner A., Thomaschitz C., Blunder M., Kollroser M., Bauer R., Bucar F. Bioorg. Med. Chem. 2011;19:567–579. doi: 10.1016/j.bmc.2010.10.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kurado H., Hanaki E., Izawa H., Kano M., Itahashi H. Tetrahedron. 2004;60:1913–1920. [Google Scholar]
- 7.Hardtmann G.E., Koletar G., Pfister O.R. J. Heterocycl. Chem. 1975;12:565–572. [Google Scholar]
- 8.Coppola G.M. J. Heterocycl. Chem. 1985;22:491–494. [Google Scholar]
- 9.Xian-Zhi L., Zhangi L., Nikaido H. Antimicrob. Agents Chemother. 2004;48:2415–2423. doi: 10.1128/AAC.48.7.2415-2423.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Franzblau S.G., White K.E. Antimicrob. Agents Chemother. 1990;34:229–231. doi: 10.1128/aac.34.2.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Klopman G., Fercu D., Renau T.E., Jacobs M.R. Antimicrob. Agents Chemother. 1996;40:2637–2643. doi: 10.1128/aac.40.11.2637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Renau T.E., Sanchez J.P., Gage J.W., Dever J.A., Shapiro S.J., Grachek S.J., Domagala J.M. J. Med. Chem. 1996;39:729–735. doi: 10.1021/jm9507082. [DOI] [PubMed] [Google Scholar]
- 13.Renau T.E., Sanchez J.P., Shapiro M.A., Dever J.A., Grachek S.J., Domagala J.M. J. Med. Chem. 1995;38:2974–2977. doi: 10.1021/jm00015a021. [DOI] [PubMed] [Google Scholar]
- 14.Andersson M.I., MacGowan A.P. J. Antimicrob. Chemother. 2003;51(Suppl. S1):1–11. doi: 10.1093/jac/dkg212. [DOI] [PubMed] [Google Scholar]
- 15.Gootz T.D., Brighty K.E. Med. Res. Rev. 1996;16:433–486. doi: 10.1002/(SICI)1098-1128(199609)16:5<433::AID-MED3>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 16.Bouzard D., Di Cesare P., Essiz M., Jacquet J.P., Remuzon P., Weber A., Oki T., Masuyoshi M. J. Med. Chem. 1989;32:537–542. doi: 10.1021/jm00123a005. [DOI] [PubMed] [Google Scholar]
- 17.Takii T., Yamamoto Y., Chiba T., Abe C., Belisle J.T., Brennan P.J., Onozaki K. Antimicrob. Agents Chemother. 2002;46:2533–2539. doi: 10.1128/AAC.46.8.2533-2539.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wu J., Zhang D., Wei S. Synth. Commun. 2005;35:223–227. [Google Scholar]
- 19.Wube A.A., Bucar F., Gibbons S., Asres K. Phytochemistry. 2005;66:2309–2315. doi: 10.1016/j.phytochem.2005.07.018. [DOI] [PubMed] [Google Scholar]
- 20.Konkimalla V.B., Blunder M., Korn B., Soomro S.A., Jansen H., Chang W., Posner G.H., Bauer R., Efferth T. Nitric Oxide. 2008;19:184–191. doi: 10.1016/j.niox.2008.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]