Abstract
The pre-engraftment syndrome (PES) after cord blood (CB) transplantation (CBT) is poorly characterized. Therefore, we reviewed 52 consecutive double unit CBT recipients treated for high-risk hematological malignancies. PES was defined as unexplained fever >38.3°C (101F) not associated with infection and unresponsive to antimicrobials, and/or unexplained rash occurring before or at neutrophil recovery. CBT recipients (median 38 years, range 3-66) received either myeloablative (n=36) or non-myeloablative (n=16) conditioning. Sixteen patients (31%) fulfilled PES criteria: 15 with fever [onset median 39°C (102.2F)] with 13 also having rash, and one with rash alone. The median onset was 9 days (range 5-12) post-transplant (a median of 14 days before neutrophil recovery). Sixteen patients received IV methylprednisolone (MP) (14 PES, 2 with infection and possible PES; median dose 1 mg/kg, median duration 3 days) with 15/16 (94%) having fever resolution in ≤24 hours. Recurrent PES (n=3) resolved with retreatment. There was no association between the development of PES and the likelihood of sustained donor engraftment, the speed of neutrophil recovery, grade II-IV acute graft-versus-host disease (aGVHD), day 180 transplant-related mortality, or survival. PES is common after CBT, precedes neutrophil recovery, is distinct from and does not predict for aGVHD, and responds promptly to short course corticosteroids.
Keywords: HSC transplantation, cord blood transplantation, engraftment, GVHD
Introduction
Engraftment syndrome, a clinical entity of unknown pathogenesis, has been described in patients receiving both autologous1-3 and allogeneic4,5 hematopoietic transplantation. While a uniform definition is lacking, one suggested by Spitzer et al is a clinical syndrome after hematopoietic stem cell transplantation characterized by non-infectious fever, erythematous skin rash, and pulmonary infiltrates occurring immediately prior to or at neutrophil engraftment6. Kishi et al were the first to report a pre-engraftment immune reaction that occurred in 35/45 (78%) of adult recipients of reduced intensity cord blood (CB) transplantation (CBT). This was associated with various manifestations including fever, rash, diarrhea, jaundice, or weight gain (greater than 10% from the baseline) occurring before neutrophil engraftment and not explained by infection or adverse drug reactions7. The authors suggested this pre-engraftment syndrome (PES) differed from engraftment syndrome or acute graft-versus-host disease (aGVHD)7. PES remains poorly characterized, however, and the prognosis and appropriate management are unclear. Therefore, we conducted a retrospective review of 52 consecutive CBT recipients treated for high-risk hematological malignancies to determine the incidence, manifestations, and outcomes of PES. Our hypothesis was that PES is distinct from and does not predict aGVHD.
Materials and methods
Patient and graft characteristics
This was a retrospective review of consecutive CBT recipients transplanted at Memorial Sloan-Kettering Cancer Center who were recipients of first allograft and collection of transplant complications and outcome data was sanctioned by the MSKCC IRB. Survivors had at least 100 days of follow-up post-transplant. Patients (n=52) had a median age of 38 years (range 3-66), a median weight of 70 kg (range 13-102), and all had high-risk hematologic malignancies: acute myelogenous leukemia (n=12), acute lymphoblastic leukemia (n=10), acute biphenotypic leukemia (n=2), Non-Hodgkin lymphoma (n=15), Hodgkin lymphoma (n=9), chronic lymphocytic leukemia (n=3), and prolymphocytic leukemia (n=1). Patients were transplanted with either myeloablative (n=36) or non-myeloablative (n=16) conditioning according to age, extent of prior therapy, co-morbidities, and diagnosis. Cyclosporine-A and mycophenolate mofetil were used as GVHD prophylaxis, and all patients received post-transplant granulocyte colony-stimulating factor. All patients received double unit grafts to augment engraftment8,9 with a median infused total nucleated cell (TNC) dose of 2.5 × 107/kg (range 1.42-7.30) in the larger unit and 1.9 × 107/kg (range 0.91-5.26) in the smaller unit. Units were 6/6 (n=5), 5/6 (n=51), and 4/6 (n=48) human leucocyte antigen (HLA)-A,-B antigen and -DRB1 allele matched to the recipient. Donor-recipient and unit-unit HLA-matching was also determined at high resolution for HLA-A,-B,-C,-DRB1,-DQ alleles.
All patients or their parents signed informed consent prior to transplantation. Patients were hospitalized in high efficiency particulate air (HEPA) filtered single protective environment rooms and received prophylaxis for fungal infections (including mold), Herpes simplex, and Pneumocystis jiroveci, as well as bacterial infections during neutropenia. Neutropenic fever was treated with broad-spectrum intravenous (IV) antibiotics.
Definition of PES
Medical records were reviewed for clinical features suggestive of PES and the associated laboratory and radiological findings. PES was defined as: 1) unexplained fever greater than 38.3° C (101° F) not associated with documented infection and unresponsive to antimicrobial manipulations; and/or 2) unexplained erythematous skin rash resembling that of aGVHD, with either the fever or the rash occurring prior to or at neutrophil recovery. Specifically, fever attributed to PES was not associated with any clinical evidence of infection with patients having both negative infectious disease work-up and continued lack of response to broad-spectrum antimicrobial agents. All patients with fever underwent extensive infectious disease work-up that included serial blood cultures (all ports), urine cultures, stool studies if diarrhea was present, relevant viral polymerase chain reactions (PCRs), and relevant radiological studies including CT scan of the lungs. Further, the erythematous skin rash attributed to PES was not associated with any clinical suspicion of drug allergy.
Weight gain was calculated as the percentage change in weight between transplant day and the day of PES onset. For comparison, the weight gain in patients without any evidence of PES was calculated as the percentage change in weight between transplant day and day 9 (the median day of onset of PES). Non-infectious diarrhea was defined as liquid stools more than twice a day for at least three consecutive days with stool studies negative for any infectious etiology.
Statistical analysis
As there were no early deaths prior to day 28 there were no competing risks in the calculation of the incidence of PES. Time to neutrophil recovery was defined as the first of three consecutive days with absolute neutrophil count (ANC) >0.5 ×109/l after the first post-transplant nadir. Sustained donor engraftment was defined as sustained donor-derived count recovery with donor chimerism of at least 90% donor (both units combined). Overall staging of aGVHD was based on International Bone Marrow Transplant Registry criteria10. Transplant-related mortality (TRM) was defined as any death not due to relapse or persistence of malignancy. Neutrophil engraftment, acute GVHD, and transplant-related mortality (TRM) were computed using the cumulative incidence function. For neutrophil engraftment the competing risks were autologous recovery, infusion of a back-up graft, or death. Graft failure or death was the competing event for acute GVHD, whereas relapse was the competing event for TRM. Survival was calculated using Kaplan-Meier methodology. The relationship between PES outcome and binary, ordinal, and continuous factors was determined using Fisher’s exact test, the Wilcoxon rank sum test, and the t-test, respectively. The difference in survival rates based on PES classification was determined using the log-rank test; the difference in the cumulative incidence curves was based on Gray’s test.
Results
Incidence and manifestation of PES
Of the 52 patients eligible for analysis, 16 (31%) fulfilled the diagnostic criteria of PES (12 recipients of myeloablative and 4 of non-myeloablative conditioning). Of these 16 patients, 15 (94%) had unexplained fever with 13 also having rash. The median temperature at onset was 39°C (102.2F) (range 38.4-39.4) and had a spiking pattern. One patient, a recipient of non-myeloablative conditioning, had rash alone as the only PES manifestation. Although we originally defined PES as potentially occurring prior to or at neutrophil recovery, we found the median day of onset of PES was early at 9 days (range 5-12) post-transplant. This was a median of 14 days before neutrophil recovery overall. The median total white cell count at PES onset was 0 × 109/l (range 0-0.9) with a median ANC of 0 × 109/l (range 0-0.5). The day of onset and appearance of symptoms were similar regardless of conditioning
The remaining 36 patients who did not fulfill strict PES criteria included: 5 patients without post-transplant fever or rash; 26 with fever secondary to either documented infection or febrile neutropenia responsive to anti-microbials; and 5 with fever thought by the treating physician to be due to infection and possible PES.
The mean weight gain at PES onset was 3% in PES and non-PES patients at the same time-point post-transplant (p = 0.60). In addition, almost half of the patients in each group had non-infectious diarrhea (p = 1.00). There was also no significant difference in mean peak bilirubin levels between the two groups at days 0-7 (p = 0.32), 8-15 (p = 0.29), 16-21 (p = 0.76) and 22-28 (p = 0.54) post-transplant. Notably, 11/16 (69%) PES patients developed hypoxia and/or pulmonary infiltrates at a median of 12 days (range 7-15) post-transplant as compared with 16/36 (44%) of non-PES patients at a median of 12 days post-transplant (range 5-32). This difference was not statistically significant (p = 0.14), however.
Human herpes virus 6 (HHV-6) reactivation can be associated with fever and rash and is well documented after CBT 11. Therefore, HHV-6 viremia was examined as a potential factor that could have accounted for the manifestations of PES. Fifteen of the 16 PES patients had serial assays for HHV-6 virus reactivation using quantitative PCR of the serum and all were positive. However, of the 26 patients without PES whose sera were also evaluated, 24 (92%) of these also had HHV-6 reactivation. In patients with PES, the mean time HHV-6 viremia (>100 copies/ml) was detected for the first time was 24 days (range 10-37 days) post-transplant, notably later than the onset of PES. This was no different from the 21 day mean onset of detection in patients without PES (range 10-40 days) post-transplant (p = 0.25). The mean peak HHV-6 was 22,900 copies for those with PES (range 200-116,000 copies) and 17,100 copies in those without PES (100-128,000 copies) (p = 0.58). Thus, there was no evidence that the manifestations of PES could be accounted for by HHV-6.
Response to corticosteroids
A total of 16 patients received IV methylprednisolone (MP) for PES treatment: 14 with PES and 2 with infection and possible PES. All treated patients had high fevers for a median of 5.5 days (range 3-11) prior to corticosteroid treatment and received a median dose of 1 mg/kg (range 0.5-2). All patients treated with MP responded as evidenced by fever resolution within 48 hours combined with resolution of rash. Thirteen out of 16 (81%) had fever resolution within <12 hours, two within 13-24 hours, and one within 25-48 hours of the first dose of MP. Two remaining PES patients did not receive MP. One patient had rash alone and had spontaneous resolution over seven days. Another was not treated due to concerns about the infection risk of corticosteroid therapy and remained febrile for 33 days. Corticosteroid treatment was continued for a median of 3 days (range 2-44). Of the 16 treated patients, three had recurrent fever attributed to PES and it resolved with corticosteroid re-treatment (median duration 10 days, range 1-27).
Patient demographics and graft characteristics and the development of PES
Table 1 outlines the patient demographics and graft characteristics of the 16 PES patients as compared with those of the 36 patients who did not meet PES criteria. There were no significant differences between these two groups according to age, gender, weight, underlying malignancy, or conditioning regimen. There were also no differences in the total infused TNC dose, the donor-recipient HLA-match of each CB unit, the unit-unit HLA-match, the infused TNC dose of the engrafting unit, or the donor-recipient HLA-match of the engrafting unit (at low or high resolution) in patients with and without PES.
Table 1.
Comparison of patient and graft characteristics in patients with and without PES.
| PES (n=16) | No PES (n=36) | P-value | |
|---|---|---|---|
| Age (years) | |||
| Mean (range) | 31 (3-65) | 41 (7-63) | 0.08 |
| Gender (n) | |||
| Male | 8 | 20 | 0.77 |
| Female | 8 | 16 | |
| Weight (kilogram) | |||
| Median (range) | 66 (13-108) | 71 (22-109) | 0.44 |
| Diagnosis (n) | |||
| Lymphoid malignancy | 4 | 10 | 1.00 |
| Myeloid malignancy | 12 | 26 | |
| Preparative Regimen (n) | |||
| Myeloablative | 12 | 24 | 0.75 |
| Non-myeloablative | 4 | 12 | |
|
| |||
| Infused Total TNC × 107/kg | |||
| Mean (range) | 4.5 (2.7-12.6) | 4.8 (2.6-9.6) | 0.53 |
| Donor-Recipient HLA-match* | |||
| Median match of better matched unit (range) | 6/10 (4-8/10) | 6/10 (3-9/10) | 0.46 |
| Median match of lesser matched unit (range) | 5/10 (4-8/10) | 5/10 (2-9/10) | 0.42 |
| Unit-Unit HLA-match * | |||
| Median (range) | 6/10 (3-10/10) | 5/10 (2-9/10) | 0.57 |
| Engrafting Unit TNC × 107/kg ** | |||
| Mean (range) | 2.3 (1.3-5.3) | 2.5 (1.4-5.1) | 0.51 |
|
Donor-Recipient HLA-match of Engrafting unit*/ ** |
|||
| Median (range) | 6/10 (4-8/10) | 6/10 (2-8/10) | 0.90 |
10 allele HLA-A,-B,-C,-DRB1,-DQ match
Excludes 2 patients with graft failure in each group and the single patient who was in the “No-PES” group who engrafted with both units.
Abbreviations: PES pre-engraftment syndrome; TNC total nucleated cell; HLA human leucocyte antigen.
PES and transplant outcome
Overall, three patients had primary and one patient had secondary graft failure. Three were recipients of myeloablative and one received non-myeloablative conditioning. Thus, for the entire study group the cumulative incidence of sustained donor engraftment was 92% (95%CI: 84-100) with neutrophil recovery occurring at a median of 25 days in myeloablated (range 13-43) and 11 days (range 7-36) in non-myeloablated recipients. Consistent with prior reports 8,9 engraftment was accounted for by a single unit except for one patient who had sustained engraftment of both units.
There was no difference in sustained donor engraftment between patients with and without PES with 2 PES and 2 non-PES patients having graft failure (p = 0.58). Excluding the four patients with failure of sustained donor engraftment, the day to neutrophil recovery in PES patients receiving myeloablative conditioning was a median of 25 days (range 13-43) compared to 25 days (range 14-33) in patients without PES (p = 0.83); in non-myeloablative recipients it was 22 days (range 7-36) in PES and 11 days (range 7-22) in non-PES patients (p = 0.35).
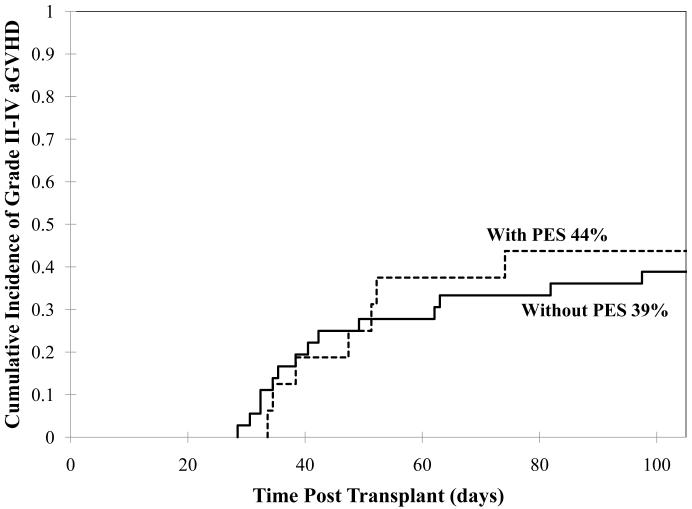
For the entire group the cumulative incidence of day 100 grades II-IV aGVHD was 40% (95%CI: 27-53). There was no difference between aGVHD incidence in PES patients and non-PES patients (44% versus 39%, p = 0.79, Figure 1) with a median day of onset of 50 days (range 34-70) in PES and 41 days (range 29-99) in non-PES patients, respectively.
Fig 1.
Cumulative incidence of grades II-IV aGVHD by day 100 in patients with or without PES.
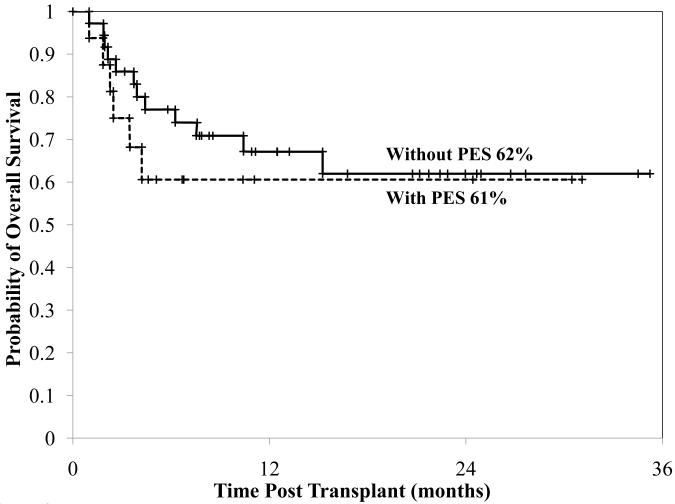
For the entire group the TRM at day 180 was 24% (95%CI: 13-35). Four patients with PES and four without PES died from transplant-related causes by day 180 (p = 0.23). With a median follow-up of 12 months (range 1-36), the one year overall survival is 64% (95%CI: 52-80). There was no difference between the survival of PES patients and non-PES patients (61% versus 62%, p = 0.43, Figure 2).
Fig 2.
Cumulative incidence of overall survival in patients with or without PES.
Discussion
Engraftment syndrome (ES) has been well described after autologous transplantation although the reported incidence is highly variable according to the definition used6. ES is less well understood after allogeneic transplantation with some investigators attributing the syndrome to hyperacute aGVHD6. Interestingly, a number of early reports of CBT described an onset of aGVHD well in advance of neutrophil engraftment. For example, Sanz et al described a median time to onset of aGVHD of 9 days (range 4-14) despite the median time to neutrophil recovery being 22 days (range 13-52)12. Further, while Wagner et al reported a median day of onset of aGVHD of 35 days the lower limit of the range was 8 days post-transplant13. It is likely that some patients with this “early aGVHD” may have had PES. Kishi et al defined a pre-engraftment immune reaction in reduced intensity single unit CBT recipients which included the presence of fever, skin eruption, diarrhea, jaundice, or weight gain greater than 10% of baseline that could not be attributed to infection or adverse effects of medications7. These broad criteria likely accounted for the high incidence of 78% of PES reported in their study. Using a stricter definition of unexplained non-infectious fever and/or unexplained skin rash, we found that 31% of our CBT patients fulfilled PES criteria. This indicates that this syndrome, as with allogeneic transplant using other stem cell sources6, is relatively common. On the other hand, our strict definition may have led to an under-estimation of this syndrome. We excluded five patients as they were thought by the treating transplant physician to be infected. However, they could have also had PES especially as two of these patients were treated with and responded to corticosteroids.
The onset of PES in our CBT series was identical to that described by Kishi et al and was a median of two weeks before neutrophil recovery clearly justifying the term “pre-engraftment”7. Interestingly, we did not find our PES patients suffered weight gain, hyperbilirubinemia, or non-infectious diarrhea more frequently than those without PES. The difference between PES and non-PES patients in terms of hypoxia and/or pulmonary infiltrates was also not significant. The temporal correlation with the development of pulmonary manifestations following the onset of unexplained fever in patients with PES nevertheless warrants further study. Such investigation may be hampered, however, by lack of any understanding of the etiology or predisposing factors to the syndrome.
The development of ES in autologous and allogeneic bone marrow transplant recipients has been associated with a wide variety of risk factors. In studies of autologous transplantation predisposing factors included specific diagnoses3,14, less extensive prior therapy14, busulfan-based conditioning2, a higher number of infused hematopoietic cells2,15, use of G-CSF1, and early and steep neutrophil recovery2. In the allogeneic setting, Gorak et al described older age, female sex, and the use of amphotericin formulations as predisposing factors for engraftment syndrome after non-myeloablative conditioning4. Schmid et al also reported treatment with amphotericin, use of G-CSF, and grafts with higher cell doses as risk factors for engraftment syndrome in pediatric allograft recipients5. In contrast, we found no significant differences between patients with and without PES according to age, gender, conditioning regimen, infused cell dose, or HLA-match. We also found that this syndrome could not be attributed to HHV6 virus reactivation. Thus, the mechanism and predisposing factors for this relatively common syndrome after CBT remain unknown.
While corticosteroids have been used to treat ES after autologous1-3,14 or allogeneic hematopoietic stem cell transplantation4,5 as well as pre-engraftment immune reactions in CBT recipients7,16, there is no agreement as to the correct dose or treatment duration. All PES patients in this study treated with corticosteroids responded rapidly with fever resolving within less than 12 hours in the majority. It is interesting to postulate that PES may have become more frequent since corticosteroids have been abandoned as GVHD prophylaxis by many centers. This does not warrant the use of either corticosteroids (and their associated infection risk) or methotrexate (and its associated risk of delayed engraftment)16 as preventative therapy of PES after CBT, however, given that this syndrome is profoundly steroid sensitive. Further, while the majority of patients did not have PES recurrence, the three patients who did responded promptly to re-treatment. More importantly, there was no association between PES and the subsequent development of acute GVHD with the median day of onset of aGVHD onset being 50 days post-transplant in PES patients. Clearly, a very detailed workup to exclude infection is mandatory in CBT recipients being considered for PES. However, for patients who fulfill PES criteria (pre-engraftment, without documented infection, no response to broad-antimicrobial coverage, and no other features such as progressive gut or liver pathology to suggest aGVHD) it is reasonable to diagnose PES and treat with short course corticosteroids and not diagnose early aGVHD predating engraftment. While the optimal PES therapy is unknown and no definitive recommendations can be made based on a relatively small series, we are now investigating 1 mg/kg/day of intravenous methylprednisolone for three days with no taper and close monitoring for patient well-being after corticosteroid cessation.
One further aspect of PES after CBT deserves emphasis. While we did not find increased mortality in PES patients, PES is associated with significant morbidity. Failure to recognize this syndrome in CBT recipients risks unnecessary complications of high fevers with possible pulmonary complications, as have been seen in autologous transplant recipients with engraftment syndrome. Prompt recognition of PES and short course corticosteroids also avoids unnecessarily long, empiric courses of treatment that could promote opportunistic infections. Further validation of our findings in a prospective investigation of a larger series of CBT recipients is therefore warranted. This should include studying the incidence of PES to ascertain whether there are differences between recipients of single versus double unit CBT, characterizing associated end-organ toxicities (especially possible pulmonary manifestations), and, most importantly, searching for biomarkers that may provide clues to etiology17.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lee CK, Gingrich RD, Hohl RJ, Ajram KA. Engraftment syndrome in autologous bone marrow and peripheral stem cell transplantation. Bone Marrow Transplant. 1995;16:175–182. [PubMed] [Google Scholar]
- 2.Ravoet C, Feremans W, Husson B, et al. Clinical evidence for an engraftment syndrome associated with early and steep neutrophil recovery after autologous blood stem cell transplantation. Bone Marrow Transplant. 1996;18:943–947. [PubMed] [Google Scholar]
- 3.Maiolino A, Biasoli I, Lima J, Portugal AC, Pulcheri W, Nucci M. Engraftment syndrome following autologous hematopoietic stem cell transplantation: definition of diagnostic criteria. Bone Marrow Transplant. 2003;31:393–397. doi: 10.1038/sj.bmt.1703855. [DOI] [PubMed] [Google Scholar]
- 4.Gorak E, Geller N, Srinivasan R, et al. Engraftment syndrome after nonmyeloablative allogeneic hematopoietic stem cell transplantation: incidence and effects on survival. Biol Blood Marrow Transplant. 2005;11:542–550. doi: 10.1016/j.bbmt.2005.04.009. [DOI] [PubMed] [Google Scholar]
- 5.Schmid I, Stachel D, Pagel P, Albert MH. Incidence, predisposing factors, and outcome of engraftment syndrome in pediatric allogeneic stem cell transplant recipients. Biol Blood Marrow Transplant. 2008;14:438–444. doi: 10.1016/j.bbmt.2008.02.002. [DOI] [PubMed] [Google Scholar]
- 6.Spitzer TR. Engraftment syndrome following hematopoietic stem cell transplantation. Bone Marrow Transplant. 2001;27:893–898. doi: 10.1038/sj.bmt.1703015. [DOI] [PubMed] [Google Scholar]
- 7.Kishi Y, Kami M, Miyakoshi S, et al. Early immune reaction after reduced-intensity cord-blood transplantation for adult patients. Transplantation. 2005;80:34–40. doi: 10.1097/01.tp.0000163289.20406.86. [DOI] [PubMed] [Google Scholar]
- 8.Barker JN, Weisdorf DJ, DeFor TE, Blazar BR, Miller JS, Wagner JE. Rapid and complete donor chimerism in adult recipients of unrelated donor umbilical cord blood transplantation after reduced-intensity conditioning. Blood. 2003;102:1915–1919. doi: 10.1182/blood-2002-11-3337. [DOI] [PubMed] [Google Scholar]
- 9.Barker JN, Weisdorf DJ, DeFor TE, et al. Transplantation of Two Partially HLA-Matched Umbilical Cord Blood Units To Enhance Engraftment in Adults with Hematologic Malignancy. Blood. 2005;105:1343–1347. doi: 10.1182/blood-2004-07-2717. [DOI] [PubMed] [Google Scholar]
- 10.Rowlings PA, Przepiorka D, Klein JP, et al. IBMTR Severity Index for grading acute graft-versus-host disease: retrospective comparison with Glucksberg grade. British Journal of Haematology. 1997;97:855–864. doi: 10.1046/j.1365-2141.1997.1112925.x. [DOI] [PubMed] [Google Scholar]
- 11.Sashihara J, Tanaka-Taya K, Tanaka S, et al. High incidence of human herpesvirus 6 infection with a high viral load in cord blood stem cell transplant recipients. Blood. 2002;100:2005–2011. [PubMed] [Google Scholar]
- 12.Sanz GF, Saavedra S, Planelles D, et al. Standardized, unrelated donor cord blood transplantation in adults with hematologic malignancies. Blood. 2001;98:2332–2338. doi: 10.1182/blood.v98.8.2332. [DOI] [PubMed] [Google Scholar]
- 13.Wagner JE, Barker JN, DeFor TE, et al. Transplantation of unrelated donor umbilical cord blood in 102 patients with malignant and nonmalignant diseases: influence of CD34 cell dose and HLA disparity on treatment-related mortality and survival. Blood. 2002;100:1611–1618. doi: 10.1182/blood-2002-01-0294. [DOI] [PubMed] [Google Scholar]
- 14.Moreb JS, Kubilis PS, Mullins DL, Myers L, Youngblood M, Hutcheson C. Increased frequency of autoaggression syndrome associated with autologous stem cell transplantation in breast cancer patients. Bone Marrow Transplant. 1997;19:101–106. doi: 10.1038/sj.bmt.1700615. [DOI] [PubMed] [Google Scholar]
- 15.Kawano C, Muroi K, Kuribara R, et al. Engraftment syndrome after autologous peripheral blood stem cell transplantation with high numbers of peripheral blood stem cells followed by granulocyte colony-stimulating factor administration. Bone Marrow Transplant. 2000;25:228–229. doi: 10.1038/sj.bmt.1702110. [DOI] [PubMed] [Google Scholar]
- 16.Narimatsu H, Terakura S, Matsuo K, et al. Short-term methotrexate could reduce early immune reactions and improve outcomes in umbilical cord blood transplantation for adults. Bone Marrow Transplant. 2007;39:31–39. doi: 10.1038/sj.bmt.1705539. [DOI] [PubMed] [Google Scholar]
- 17.Schots R, Kaufman L, Van Riet I, et al. Proinflammatory cytokines and their role in the development of major transplant-related complications in the early phase after allogeneic bone marrow transplantation. Leukemia. 2003;17:1150–1156. doi: 10.1038/sj.leu.2402946. [DOI] [PubMed] [Google Scholar]