Abstract
A major goal in cardiovascular biology is to repair diseased or damaged hearts with newly generated myocardial tissue. Stem cells offer a potential source of replacement myocytes for restoring cardiac function. Yet little is known about the nature of the cells that are able to generate myocardium and the conditions they require to form heart tissue. A source of information that may be pertinent to addressing these issues is the study of how the myocardium arises from progenitor cells in the early vertebrate embryo. Accordingly, this review will examine the initial events of cardiac developmental biology for insights into the identity and characteristics of the stem cells that can be used to generate myocardial tissue for therapeutic purposes.
Keywords: stem cells, cardiac, myocardium, heart development
The principal tissue that makes up the heart is the cardiac muscle: the myocardium. Until recently, it was thought that the adult myocardium is a postmitotic tissue. It is now known that cardiac myocytes are regenerated in the adult heart (Beltrami et al., 2001; Quaini et al., 2002). Yet the myocardium is still unable to restore itself in response to severe injury. For example, following a myocardial infarction, when large areas of cardiac myocytes die, the affected tissue is not repaired into functional cardiac muscle. One of the great hopes for stem cell research is to provide the means to repair diseased or damaged hearts. Two questions that are fundamental to realizing the potential use of stem cells for myocardial repair concern: 1) the nature of the stem cells that regenerate adult cardiac myocytes and 2) the conditions necessary for allowing precardiac cells to knit together into a functional myocardial tissue. It is reasonable to believe that an examination of heart formation in the early embryo may offer clues for answering these questions. Accordingly, we will discuss the initial stages in the development of the vertebrate heart, when cardiac tissue is first formed in the embryo and gives rise to the primary contractile heart tube. In addition, these events will be examined through the prism of stem cell biology to assess whether this developmental information provides insights for manipulating stem cells for repair of the adult heart.
CARDIAC TISSUE FORMATION IN THE VERTEBRATE EMBRYO—A SYNOPSIS
Soon after fertilization, vertebrate embryos grow very rapidly. Thus, very early in gestation a sizeable yet underdeveloped organism requires circulating blood. This need dictates the early appearance of a contractile heart, which is the first functional organ in both the bird and mammalian embryo. Our current understanding of cardiac tissue formation has been derived by experimentation from multiple animal species. Historically, the favorite animal model for examining the formation of the vertebrate heart has been the embryonic chick, due to the relative ease in obtaining and manipulating early-stage avian embryos, compared to the mouse. Moreover, the structure of both the bird and mammalian heart and the events that underlie its formation are very similar. The embryonic mouse has only gained status as an important animal model for studying early cardiogenesis with the advent of transgenic and gene-targeted mice. The embryonic frog and fish have also provided valuable experimental models for elucidating the mechanisms that promote cardiac tissue formation. Because each of theses animal models has various strengths and weaknesses for revealing how the heart forms, a full picture of early cardiogenesis requires a cross-reference of information compiled from these different species. However, our discussion will be dominated by avian development for the sake of both simplicity and substance, as the initial events of cardiogenesis have been best characterized in the embryonic chick. To facilitate an understanding of these early events in cardiogenesis, a description of relevant terminology in chick development is provided in Table 1.
TABLE 1.
Definitions in early chick development
| Primitive streak: The first three-dimensional structure of the avian embryo, whose orientation indicates the anterior-posterior axis. The appearance of this structure defines gastrulation, as cells of the epiblast ingress through the streak to generate the mesoderm and endoderm layers. |
| Hensen’s node: The cellular region at the anterior end of the primitive streak in avian embryos, which controls the development of the body plan during gastrulation. The homologous structures in mammals and amphibians are referred to as the Node and Organizer, respectively. |
| Rostral: The anterior end of the embryo (i.e., towards the head). |
| Caudal: The posterior end of the embryo (i.e., towards the tail). |
| Lateral mesoderm: Regions of the mesodermal layer that are bilaterally situated on both sides of the embryonic anterior-posterior midline. At the onset of gastrulation, this term is used to refer generally to the mesoderm that flanks the primitive streak. However, the mesoderm rapidly organizes into morphologically distinct regions, and therefore the definition of lateral mesoderm becomes more specific. At the time just prior to somite formation, the mesoderm that flanks the midline becomes subdivided into paraxial, intermediate, and lateral regions with the latter region giving rise to the heart. |
| Splanchnic mesoderm: The ventral layer of the lateral mesoderm, which lays adjacent to the endoderm. This tissue layer forms when lateral mesoderm separates into distinct dorsal and ventral layers: the somatic and splanchnic mesoderm. It is the splanchnic mesoderm that will give rise to the heart. |
| Hamburger and Hamilton (HH) staging: The commonly accepted system for staging avian embryogenesis, based on the sequence of morphological changes that occur during development. |
| HH stage 2: Beginning of gastrulation as the primitive streak begins to form at the posterior end. |
| HH stage 3: Early gastrulation with the primitive streak extending to the center of the embryo. |
| HH stage 4: The primitive streak is now extended to its most anterior position. Increased numbers of ingressing cells through the primitive streak leads to the formation of definitive mesoderm and endoderm layers. |
| HH stage 5: The anterior apex of the primitive streak has begun to recede in a posterior direction. The notochord is now visible as a rod of mesoderm extending above Hensen’s node. |
| HH stage 6: The anterior end of the embryo has folded to form the head fold. Lateral mesoderm begins to separate into dorsal and ventral layers. |
| HH stage 7: One somite stage. Splanchnic and somatic mesoderm are now distinct layers. First expression of sarcomeric myosin in the cardiogenic mesoderm. |
| HH stage 8: Four somite stage. |
| HH stage 9: Seven somite stage. |
| HH stage 10: Ten somite stage. The first display of beating by the primitive heart. |
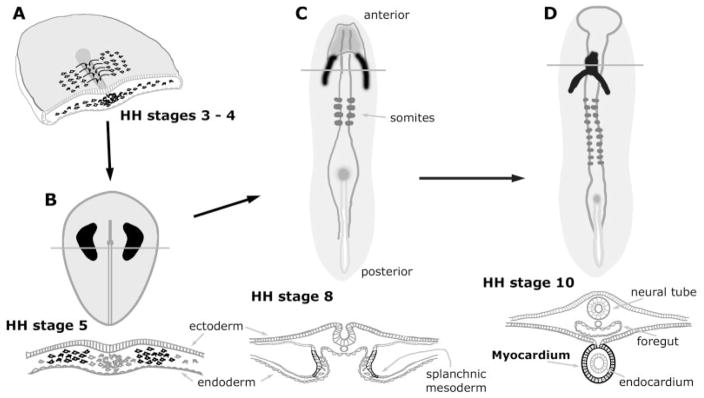
Cardiogenesis begins during the onset of gastrulation, which in the chick corresponds to Hamburger-Hamilton (HH) (Hamburger and Hamilton, 1951) stages 3–5. At these stages, the embryo is a relatively simple structure consisting of the three primary germ layers. Cells fated to become heart tissue localize to paired regions of mesoderm within the anterior half of the embryo (Stalsberg and DeHaan, 1969; Keller, 1976; Stainier et al., 1993; Redkar et al., 2001). As development proceeds, the lateral mesoderm splits into distinct somatic and splanchnic layers (HH stages 6–7 in the chick), with the precardiac cells sorting to the splanchnic mesoderm (DeHaan, 1963, 1965; Linask, 1992). Subsequently, the bilateral heart-forming fields move toward the ventral midline and fuse to give rise to the primary heart tube (DeHaan, 1965; Lohr and Yost, 2000; Eisenberg and Eisenberg, 2002). Concurrent with these morphological events, the cells within these heart fields begin to show evidence of a cardiac phenotype. By the time the fusion of the heart fields is initiated, several contractile proteins are exhibited in a prestriated pattern by the cardiogenic cells (Tokuyasu and Maher, 1987a, b; Ruzicka and Schwartz, 1988; de Jong et al., 1990; Bisaha and Bader, 1991; Han et al., 1992; Colas et al., 2000). Concomitant with the first appearance of striated muscle by the 10 somite stage (HH stage 10 in the chick) is the first display of beating by the primitive heart. The formation of the primary heart tube (summarized in Fig. 1) is completed by embryonic days (EDs) 2 and 8 of the chick and mouse embryo, respectively.
Fig. 1.
Schematic diagram depicting the initial morphological events in the development of the avian heart. Precardiac and definitive myocardial tissue are illustrated in black, while the lines overlaying the cardiac areas denote the plane of the transverse sections shown immediately below the embryos. A: Three-dimensional view of a cross-sectioned chick embryo, which is in transition between HH stages 3 and 4. Precardiac cells undergo gastrulation through the primitive streak and move laterally to reside in lateral mesoderm within the anterior half of the embryo. B: During HH stage 5, the myocardial progenitors coalesce into morphologically distinct heart-forming fields, which are distributed bilaterally to the primitive streak. C: At HH stage 8, the two heart-forming areas have sorted to splanchnic mesoderm and begun to merge at the embryonic midline. By this stage, these cells have become definitive cardiac myocytes, as they will exhibit a number of muscle proteins in a prestriated pattern. D: By HH stage 10 a fully contractile heart has developed. This primitive heart tube consists of an outer sheet of myocardium, surrounding an inner endocardial layer.
THE LOCATION OF PRECARDIAC STEM CELLS
The preceding synopsis of the early events in vertebrate cardiogenesis describes a process whereby a functional heart is formed from primary nondifferentiated mesoderm. The mesodermal region that will give rise to myocardial tissue is often referred to as the precardiac mesoderm or heart-forming fields. In essence, the precardiac mesoderm comprises a collection of myocardial stem cells. As an initial step in describing these myocardial stem cells, we will first consider how they were identified in the early embryo.
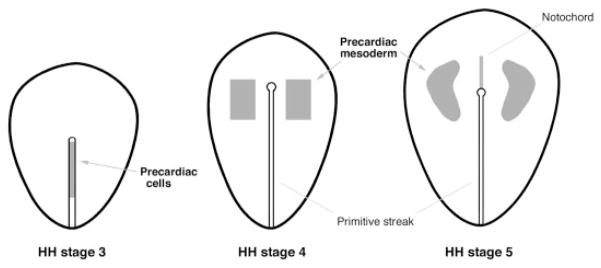
The study of cardiac progenitor cells commenced in the 1930s, a period when several laboratories set out to examine the organ-forming capabilities of early embryonic tissue (Rawles, 1936). It was through the efforts of Mary Rawles (1943) that the heart-forming areas were initially described for precontractile chick embryos. This map was delineated by culturing tissue fragments isolated from gastrulating chick embryos that displayed the head process—a developmental stage now referred as HH stage 5—and examining these explants for subsequent development of contractile tissue. She was able to show that heart-forming potential was possessed by two large embryonic regions, lateral to Hensen’s node. In reporting these findings, Rawles speculated that the actual precardiac fields were probably narrower than the map she defined for cardiac potency of embryonic tissue. This was borne out by the investigations of Stalsberg and DeHaan (1969), who radiolabeled small tissue fragments consisting of the mesoderm and endoderm layers (mesendoderm) from HH stage 5 embryos, which were subsequently transplanted into a second identically staged embryo, at the exact same position. Following their further development, these embryos were then sectioned and analyzed for the radioactive label. Thus, by examining whether a particular region of the early embryo gave rise to heart tissue, they formulated a map for presumptive precardiac tissue (Fig. 2). More recently, Redkar et al. (2001) described a cardiac fate map that was generated by the direct labeling of mesodermal cells within individual chick embryos. Their experimentation consisted of microinjecting the fluorescent label DiI into precisely localized regions within the mesodermal layer of early gastrula-stage embryos. After allowing the embryos to develop to tubular heart stages, the fates of individual fluorescently tagged mesoderm cells were compiled, with regard to whether heart or nonheart tissue was subsequently labeled. Despite great differences in the methodology, the cardiac fate maps of Stalsberg and DeHaan (1969) and Redkar et al. (2001) are almost identical. Within the HH stage 5 chick embryo, the medial border of the precardiac mesoderm is located ~0.3 mm lateral to the primitive groove. The anterior border of the precardiac mesoderm resides just above Hensen’s node, with the posterior border extending one-fourth of the distance down the primitive streak.
Fig. 2.
Location of precardiac cells for HH stages 3–5, as determined by the fate map studies of Stalsberg and DeHaan (1969), Garcia-Martinez and Schoenwolf (1993), and Redkar et al. (2001). The precardiac cells are exhibited within the caudal half of the primitive streak in HH stage 3 avian embryo. At HH stages 4 and 5, precardiac cells become distributed bilaterally and reside within the anterior lateral mesoderm. Precardiac areas are shown in gray.
At HH stage 5, the precardiac mesoderm can be observed with the scanning electron microscope as morphologically distinct cellular fields (Drake and Jacobson, 1988). Yet, even prior to this stage, precardiac stem cells can be definitively mapped in the embryo (Fig. 2). In the early gastrula stage avian embryo (HH stage 3), cells fated to become myocardium are localized in the rostral half of the primitive streak (Garcia-Martinez and Schoenwolf, 1993). Localization of myocardial progenitors to the mesodermal layer occurs by HH stage 4, as these stem cells spread anteriorly and laterally from the streak to assume the classic distribution of the heart-forming fields (Redkar et al., 2001). Cardiac fate maps have also been derived for the frog and zebrafish and show great similarity to those described for the embryonic chick (Keller, 1976; Stainier et al., 1993). For example, in the early frog gastrula, precardiac stem cells localize to bilateral mesodermal fields that flank Spemann’s organizer, which is the embryonic structure homologous to Hensen’s node. Thus, the early morphological events that lead up to the formation of the heart appear to be highly conserved among vertebrate species.
THE MOLECULAR PHENOTYPE OF PRECARDIAC STEM CELLS
The fate map data have unambiguously determined where precardiac stem cells are located. The next obvious question would be: What are the characteristics of these cells? Here, the answer must be an equivocal one. To date, there has not been a molecule described whose expression at HH stages 4 and 5 delineates the heart-forming fields. This situation changes dramatically by HH stages 6 and 7, when the precardiac cells sort to the splanchnic mesoderm. During these latter stages, a large number of transcription factors become expressed in the cardiogenic mesoderm in a pattern that demarcates this merging tissue. Among the genes expressed in the early cardiogenic mesoderm of the vertebrate embryo are Nkx.2.5, Nkx.2.6, Nkx.2.8, GATA4, GATA5, GATA6, Tbx2, Tbx3, Tbx5, MEF2a, MEF2c, serum response factor (SRF), the homeodomain molecule HOP, and the histone deacetylase-dependent transcriptional repressor Bop (Edmondson et al., 1994; Schultheiss et al., 1995; Croissant et al., 1996; Brand et al., 1997; Reecy et al., 1997; Biben et al., 1998; Jiang et al., 1998; Buchberger and Arnold, 1999; Yamada et al., 2000; Chen et al., 2002; Gottlieb et al., 2002; Shin et al., 2002). Two points need to be kept in mind when understanding the function of these molecules for cardiac differentiation. First, these transcription factors are cardiac associated, but not cardiac specific. This is true even for Nkx2.5 (Kasahara et al., 1998), which is often thought of as the primary determinant of the cardiac phenotype (more on this molecule below). Nonetheless, these transcription factors serve as markers of the cardiac phenotype because their combined expression is unique to the myocardium. For example, GATA4 expression is exhibited by myocardial, endothelial, and endodermal cells, while MEF2c is exhibited by both cardiac and skeletal muscle (Edmondson et al., 1994; Laverriere et al., 1994; Narita et al., 1997). However, cardiac muscle is the only tissue that expresses both transcription factors. Second, the timing of the expression of these transcription factors either just predates or is simultaneous with the expression of sarcomeric gene expression within the cardiogenic fields. As a case in point, expression of the first muscle isoforms of actin are exhibited within the cardiogenic fields by HH stage 6 (Colas et al., 2000), while sarcomeric myosin expression comes on a few hours later by HH stage 7 (Bisaha and Bader, 1991). Much molecular data have shown that the transcriptional proteins associated with the cardiogenic mesoderm are very important for regulating the structural gene expression of the myocardium. Yet the developmental stages when these transcriptional factors are first expressed suggest that these molecules are exhibited by cells that are already cardiogenic and not pre-cardiac. Moreover, ectopic expression of these transcriptional proteins has never been shown to convert a nondifferentiated cell to a cardiomyocyte, nor has targeted deletions of these genes in mice suppressed the development of cardiomyocytes in the embryo. While targeted disruption of these individual genes (e.g., Nkx2.5, GATA4, MEF2a, MEF2c, Bop, etc.) in knockout mice dramatically affected structural development of the heart, it did not prevent the formation of myocardial tissue (Lyons et al., 1995; Lin et al., 1997; Narita et al., 1997; Gottlieb et al., 2002; Naya et al., 2002). Since a great amount of development occurs between the stages when the precardiac stem cells are localized at HH stage 3 and the first appearance of these cardiac-associated genes at HH stage 6, a fair conclusion would be that expression of these transcription factors defines an early myocardial phenotype and not that of a precardiac stem cell. Accordingly, the molecular phenotype that characterizes a precardiac stem cell either is unknown or may not be distinct from the total mesoderm population of the early gastrula.
Among the cardiac-associated transcriptional proteins, Nkx2.5 has been best characterized for its critical importance for heart development. When Nkx2.5 expression in the chick embryo was first described, it was suggested that this molecule is first exhibited by precardiac cells as early as HH stage 5. However, it now appears that the Nkx2.5-positive cells within the HH stage 5 embryo are not cells that will contribute to the heart, as the recent cardiac fate map generated by Redkar et al. (2001) clearly indicates that the Nkx2.5 domain does not coincide with the heart-forming fields until HH stage 6 (Eisenberg, 2002). Instead, it is likely that the Nkx2.5-expressing cells at HH stage 5 are either endoderm or ectoderm, which would be consistent with the demonstration that Nkx2.5 is expressed in all three germ layers within an HH stage 6 embryo (Schultheiss et al., 1995). The implication of these observations is that Nkx2.5 is not expressed any earlier than a multitude of other cardiac-associated transcription factors (i.e., at HH stage 6) and therefore does not have a higher hierarchical value within a cardiogenic pathway than GATA4, GATA5, Tbx5, SRF, MEF2c, and other transcriptional factors of the precontractile heart. Like these other cardiac regulatory genes, the function of Nkx2.5 is to regulate the expression of various molecular components of the myocardium (e.g., sarcomeric proteins, ion channels, etc.), and it does so in combination with these other transcriptional factors (Chen and Schwartz, 1996; Black and Olson, 1998; Lee et al., 1998; Chen et al., 2002; Sepulveda et al., 2002). In other words, Nkx2.5 helps regulate the functional properties of the myocardium, but does not determine the properties of cardiac stem cells.
COMMITMENT OF PRECARDIAC STEM CELLS
Fate mapping is an essential tool for understanding organ formation during embryogenesis. Yet it is important to appreciate the limitations of this technique. Fate maps will provide the history of a cell’s location within a developing embryo but cannot reveal a cell’s potential and capabilities. Although the embryonic area corresponding to the precardiac mesoderm has been determined definitively (Stalsberg and DeHaan, 1969; Redkar et al., 2001), that fate map information does not tell us whether those stem cells are fully committed to become cardiomyocytes, and therefore no longer able to give rise to other lineages. In other words, fate mapping cannot determine whether the stem cells residing in the precardiac mesoderm are unipotential or multipotential. One method to address this question involves the explantation of precardiac mesoderm tissue. These tissue explants could be either cultured under diverse conditions or inserted into various regions of a second embryo, and then assayed for various cell phenotypes. Explants of precardiac mesoderm placed in culture inevitably give rise to cardiac tissue (Lough et al., 1996; Ladd et al., 1998; Eisenberg and Eisenberg, 1999), although the presence of the adjacent endoderm is required for the formation of a full contractile phenotype (Antin et al., 1994; Sugi and Lough, 1994). The apparent unavoidability of cardiac differentiation by cultured precardiac mesoderm does not necessarily imply that these cells are fully committed to become myocardium. A limitation of tissue explants for studying phenotypic commitment is that the extracellular components of the tissue may contain instructive information that determines the phenotypic outcome. Thus, the breadth of the phenotypic potential of the cells contained in the explant may be obscured by the presence of the native extracellular environment (i.e., cytokines, growth factors, matrix molecules, etc.).
A way to diminish presumptive cardiogenic signals from precardiac mesoderm is to dissociate the tissue by trypsinization prior to culture. Single-cell suspensions of HH stage 4 and stage 5 precardiac mesoderm have been shown to be able to undergo limited cardiac differentiation without the addition of specific inductive signals, although high concentrations of serum and/or chick embryo extract is required to obtain this outcome (González-Sánchez and Bader, 1990; Litvin et al., 1992). Do these results indicate that the precardiac cells are committed to the cardiac lineage? If yes, then precardiac mesoderm cells should not respond to instructive signals for other lineages. To examine whether stem cells from the heart-forming regions are multipotential, we isolated and dissociated small areas of HH stage 5 precardiac mesoderm and placed these cells under hematopoietic-promoting conditions. Great care was taken in ensuring that only mesoderm was present and that the harvested tissue was obtained from areas clearly within the precardiac mesoderm. In response to these conditions, cells from the heart-forming regions, which normally never give rise to hematopoietic cells, produced differentiated blood cells (Fig. 3A and B)—indicating that the cells of the precardiac mesoderm are multipotential.
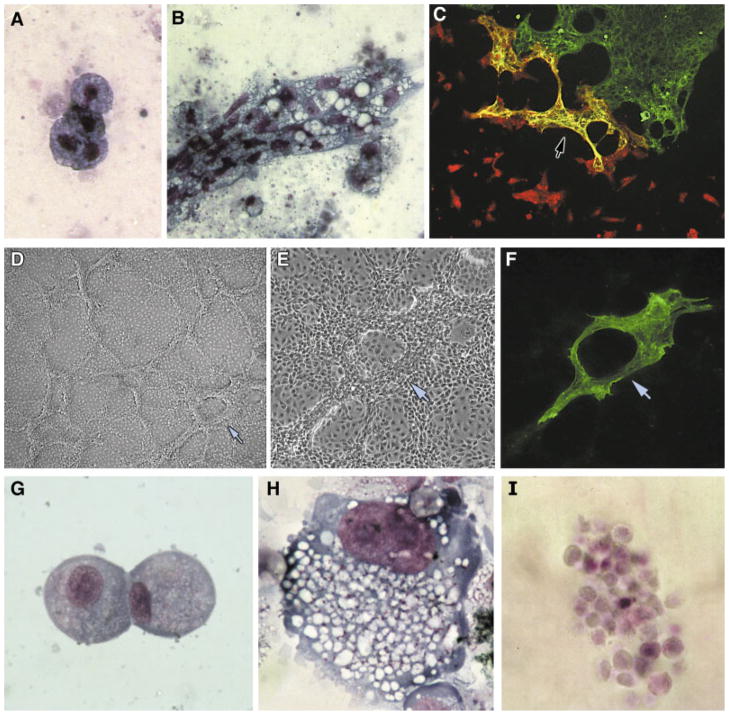
Fig. 3.
Multipotentiality of precardiac mesoderm. A and B: Precardiac mesoderm possesses hematopoietic potential. Precardiac mesoderm from HH stage 5 (A) and HH stage 6 (B) mesodermal cells obtained from precardiac regions were dissociated and cultured for 4 days in fibrin gels under conditions that promote hematopoiesis (Brandon et al., 2000). The presence of blood cells was verified by cytological examination with May-Grünwald-Giemsa stain. Panels A and B display clusters of monocytes and macrophages, respectively. C–I: Multipotentiality of the precardiac mesoderm-derived cell line QCE6. C: QCE6 cells undergo myocardial differentiation when co-cultured with HH stage 15 embryonic chick heart tissue. After 48 hr of incubation, the cultures were dual stained for both sarcomeric myosin (green) and β-galactosidase (a marker of the virally labeled QCE6 cells, red). The green staining on the right is sarcomeric myosin-positive ventricular tissue, while in the center of the field are QCE6 cells that incorporated into the heart tissue and thus subsequently exhibited a cardiac phenotype. This is indicated by the dual reactivity with both antibodies, which produced the yellow staining (arrow). D–F: Endothelial differentiation of QCE6 cells as indicated by branching morphogenesis and von Willebrand factor (vWF) expression. Cells were treated for 48 hr, immunostained for vWF, and imaged by brightfield (D), phase (E), and fluorescent microscopy (F). Low- (D) and high-magnification (E) views show extensive branching morphogenesis. F: Moreover, these cultures produced high levels of vWF protein. Arrow in panels D–F corresponds to the identical position within the culture. G–I: Hematopoiesis of QCE6 cells. Cells were cultured as described previously (Brandon et al., 2000) and stained with May-Grünwald-Giemsa dye to identify specific blood cell phenotypes. Here are shown QCE6-derived monocytes (G), macrophages (H), and red blood cells (I).
An objection could be raised about the preceding data that the precardiac areas may contain multiple stem cell populations, with the cells exhibiting hematopoietic potential possibly being distinct from the cells that give rise to the myocardium. Since markers for these stem cells have not been described, the only way to confront this objection would be to plate individual cells at clonal density and look at their differentiated progeny. To study the properties of myocardial progenitors in the embryo, the clonal stem cell line QCE6 was derived from precardiac mesoderm of HH stage 4 quail embryos (Eisenberg and Bader, 1995, 1996). As may be expected considering their precardiac mesoderm origin, QCE6 cells are able to give rise to cardiomyocytes (Fig. 3C) (Eisenberg and Bader, 1996; Eisenberg and Markwald, 1997). Yet, they also possess the ability to differentiate into endothelial cells (Fig. 3D–F) and generate a broad range of blood cell phenotypes (Fig. 3G–I) (Eisenberg and Bader, 1996; Eisenberg and Markwald, 1997; Brandon et al., 2000). No claim will be made that information derived from a cell line is definitive in regards to the properties of cells endogenous to the embryo. Yet in combination with data showing that precardiac mesodermal cells can give rise to blood (Fig. 3A and B), these studies with QCE6 cells lend credence to the proposition that the precardiac mesoderm consists of multipotential stem cells.
SPECIFICATION OF PRECARDIAC MESODERM CELLS
In the preceding section, we argued that the cells comprising the precardiac mesoderm are multipotential and therefore are not fully committed to their eventual cardiac cell fate. However, a distinct and important issue is whether precardiac mesoderm cells are uniquely specified to become heart. Ever since Mary Rawles’s (1943) land-mark studies in the 1940s examining the heart-forming capabilities of early embryonic tissue, it has been widely accepted that only explants containing anterior lateral mesoderm (i.e., the precardiac mesoderm) will generate cardiac tissue in culture. In our own studies using explants from HH stage 4–6 avian embryos (Eisenberg and Eisenberg, 1999), we were surprised to find that this was not absolutely true. As expected, explants containing the precardiac mesoderm from each of these stages readily gave rise to contractile tissue in culture. Unexpectedly, explants of posterior lateral mesoderm from HH stage 4 embryos also readily developed cardiac tissue (Fig. 4) (Eisenberg and Eisenberg, 1999), even though fate mapping studies demonstrated that this region does not contribute any cells to the future heart (Redkar et al., 2001). The heart-forming capacity of noncardiac posterior tissue is lost in subsequent stages, as explants from similar regions of HH stage 5 or stage 6 embryos did not exhibit the same ability to generate contractile cardiac tissue in culture (Fig. 4).
Fig. 4.
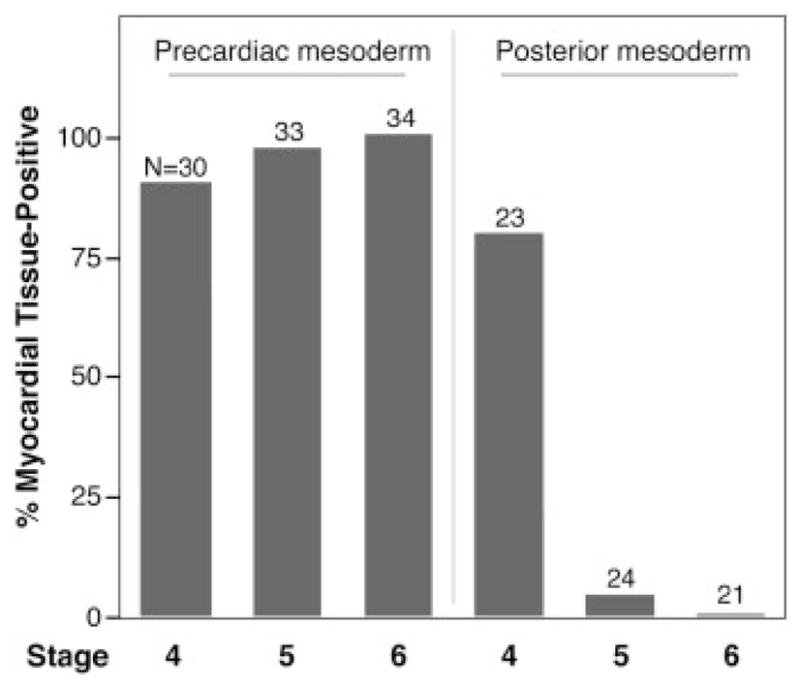
Myocardial potential of early avian mesoderm. Explants containing lateral mesoderm and the underlying endoderm were microdissected from HH stage 4, 5, and 6 avian embryos and cultured in minimal media. Subsequently, the presence of cardiac tissue was verified by immunostaining cultures for sarcomeric myosin. As previously described (Eisenberg and Eisenberg, 1999), explants containing precardiac mesoderm from each of the stages readily developed into myocardial tissue. In contrast, tissue from posterior noncardiac regions of HH stage 5 or stage 6 did not undergo cardiac differentiation in culture. Surprisingly, posterior lateral mesoderm from HH stage 4 embryos, which does not contribute to the heart in the embryo, generated cardiac tissue in culture. The total number of explants examined for each group is listed above each bar.
Why HH stage 4 posterior lateral mesoderm forms cardiac tissue when placed in culture is not understood. Of possible relevance is the sparse cellular density exhibited by HH stage 4 mesoderm that resides lateral to the most posterior portion of the primitive streak. Unlike mesoderm obtained from the anterior portion of the embryo, HH stage 4 posterior mesoderm consists of small, low-density clusters of cells. Perhaps, the sparseness of HH stage 4 posterior mesoderm allows it to develop in culture in a distinct way than it would within the embryo. By HH stage 5, the posterior mesoderm contains much greater numbers of cells, which associate into a cellular sheet. The increased cell-cell interactions that are established in the posterior mesoderm by HH stage 5 may limit its ability to undergo tissue diversification, unless exposed to exogenous inductive signals.
By HH stages 5 and 6, the distinct developmental fates of anterior and posterior mesoderm are more faithfully recapitulated in culture (Fig. 4). However, despite the noncardiac cell fate of HH stage 5 and 6 posterior lateral mesoderm in the developing embryo, this tissue is still cardiac competent. The cardiogenic differentiation of this presumptive noncardiac tissue can be promoted by a variety of extracellular signaling proteins (Fig. 5). These cardiogenic enhancers include several members of the BMP and FGF growth factor families (Lough et al., 1996; Ladd et al., 1998; Alsan and Schultheiss, 2002) and three distinct modulators of WNT signal transduction: WNT11, Dkk1, and crescent (Eisenberg and Eisenberg, 1999; Marvin et al., 2001; Schneider and Mercola, 2001; Pandur et al., 2002). The cardiac-promoting activities of these molecules appear to correlate with their involvement in normal cardiogenesis in the embryo. In situ hybridization data in the developing chick, frog, and/or mouse has shown that BMP2, BMP4, FGF4, FGF8, WNT11, Dkk1, and crescent are expressed at the right time and place to act as primary stimulators of heart formation in the developing embryo (Kispert et al., 1996; Eisenberg et al., 1997; Schultheiss et al., 1997; Shibata et al., 2000; Niehrs et al., 2001; Alsan and Schultheiss, 2002). Moreover, specific inhibition of the expression and/or activity of WNT11 or BMP can significantly diminish the formation of cardiac tissue in the vertebrate embryo (Schultheiss et al., 1997; Schlange et al., 2000; Pandur et al., 2002).
Fig. 5.
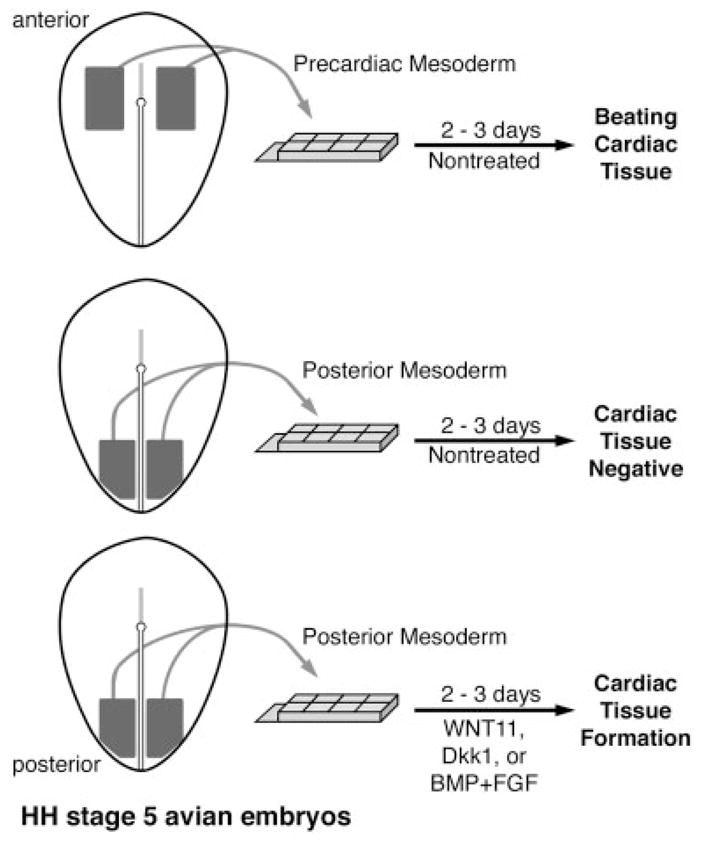
The formation of myocardial tissue from embryonic avian explants. As depicted in this schematic diagram, tissue containing lateral mesoderm and the underlying endoderm (mesendoderm) from either anterior precardiac or posterior noncardiac regions was removed from HH stage 5 embryos and cultured for cultured 2–3 days. Tissue obtained from anterior precardiac areas will form beating tissue within the first 30 hr of culture and display large regions of myocardium. In contrast, explants of noncardiac posterior mesendoderm will not generate cardiac tissue. However, treatment of noncardiac posterior mesendoderm with WNT11 (Eisenberg and Eisenberg, 1999), Dkk1 (Marvin et al., 2001), or BMP4 + FGF2 (Lough et al., 1996; Ladd et al., 1998) will promote the development of cardiac tissue, as exhibited by clustered fields of cells expressing cardiac proteins. Thus, despite the posterior origin of these latter explants, which correspond to regions of the embryo that do not contribute to the heart in situ, this tissue is still able to form myocardial tissue in response to changes in the extracellular environment.
Although the importance of BMP, FGF, and WNT signal modulators for formation of the vertebrate heart has been established, the actual roles of these various molecules in this process are still ill defined. The mechanism of action of extracellular regulators of embryogenesis is often portrayed to involve the direct stimulation of cell phenotypic specification. Yet it should be noted that the candidate primary regulatory events that initiate heart formation—BMP, FGF, and WNT signal transduction—seem to be overburdened in their requirements for the formation of many other tissues (Hogan, 1996; Brandon et al., 2000; Kuure et al., 2000; Huelsken and Birchmeier, 2001; Faure et al., 2002; Marie et al., 2002; Niswander, 2002). Since none of these other tissues contains cardiomyocytes, how can it be reconciled that these molecules appear to be primary signals for promoting the formation of the myocardium? To put it another way, how can molecules expressed everywhere in the embryo have a primary importance for expression of individual cell phenotypes? An explanation may be that these molecules do not promote cell phenotype per se, but promote morphological events that are required for tissue formation and organogenesis. For example, we have suggested that the role of WNT11 in promoting heart formation may not involve the direct induction of a lineage-specific gene expression program, but facilitate the coalescing of newly gastrulated mesoderm to form a tissue (Eisenberg and Eisenberg, 1999). Subsequent differentiation into myocardium would follow or coincide with this initial event in cardiac organogenesis.
In addition to providing a description on the makeup of the extracellular environment that stimulates the formation of heart tissue in the early mesoderm, this molecular information also reveals the overall cardiac competence of the early mesoderm. The growth factor data indicate that many cells (if not all) throughout the mesoderm are cardiac competent, as various extracellular signals can promote cardiogenesis of cells that normally give rise to other tissue types. Since, as discussed in the preceding section, precardiac mesoderm may be competent to give rise to multiple cell phenotypes, a fair conclusion would be that the mesoderm of the gastrula stage embryo is comprised of multipotential stem cells. If so, are there any differences among the stem cells that make up the precardiac areas vs. the rest of the mesoderm? Well, differences have not been shown when the cells are taken out of the embryo. Thus, the differences in cell fate appear to be related to the localized signals within the embryo that steer equipotential stem cells to distinct tissue fates.
DOES THE PRECARDIAC MESODERM UNIQUELY CONTRIBUTE TO THE MYOCARDIUM?
The heart is a complex organ with many tissue types arising from various embryonic sources throughout development (e.g., neural crest, dorsal mesocardium, and epicardial organ). Yet until recently it was thought that the myocardium was solely derived from the primary bilateral heart-forming fields. It now appears that at least one other cellular source contributes cells to the myocardium (Mjaatvedt et al., 2001; Waldo et al., 2001). Immediately anterior to the heart tube at HH stages 12–16 is a mesodermal field that provides cells for the outflow tract myocardium. Apparently, this anterior heart field is the sole supplier of cardiomyocytes to the conus and truncus of the cardiac outlet, while the precardiac mesoderm gives rise to the ventricular, atrial, and atrioventricular canal regions of the myocardium. More recently, it has been proposed that the formation of the caval and pulmonary myocardia may also be generated from noncardiac mesoderm, by recruitment of the mesenchymal cells lining the veins into the myocardial lineage (Kruithof et al., 2003).
Studies in the adult suggest the intriguing possibility that another cellular source may contribute to the development of the myocardium. Circulating stem cells in the adult have been shown to help replenish various tissues, such as blood vessels, the liver, and heart (Harraz et al., 2001; Anversa and Nadal-Ginard, 2002; Krause, 2002). Among higher vertebrates, blood circulation is established early during embryogenesis. Since phenotypic differentiation is a more dynamic process within the developing embryo than within the adult, it may be predicted that circulating cells would incorporate more readily into embryonic tissues. Thus, it would not be surprising if circulating cells provide at least a minor cellular contribution to the development of various tissues. To investigate that possibility, we examined whether labeled cells introduced into the circulation of early embryos would incorporate into the heart and undergo cardiac differentiation. For these studies, we used the mesodermal stem cell line QCE6, which in previous studies was shown to possess properties of a hematopoietic progenitor cell, as they will give rise to a broad range of differentiated hematopoietic cells when placed under conditions that promote blood differentiation (Eisenberg and Markwald, 1997; Brandon et al., 2000). In these more recent experiments, labeled QCE6 cells were injected into the yolk sac vessel leading to the sinus venous of ED 9 whole mouse embryos and allowed to develop for 24 hr ex ovo, according to previously established procedures (Sturm and Tam, 1993; Eto and Osumi-Yamashita, 1995). Subsequent analysis of these embryos showed individual labeled cells that integrated into the myocardium and expressed cardiac proteins (Fig. 6). Although the number of injected cells that incorporated into the embryonic tissue was low, it is worth considering that the embryos were only provided with a single dose of labeled circulating cells over a 24-hr assay period. Since blood cells circulate through the heart continuously throughout embryogenesis, it is possible that our experimental data greatly underestimate the numbers of circulating cells that actually give rise to functional cardiac cells upon integration into the heart during fetal development.
Fig. 6.
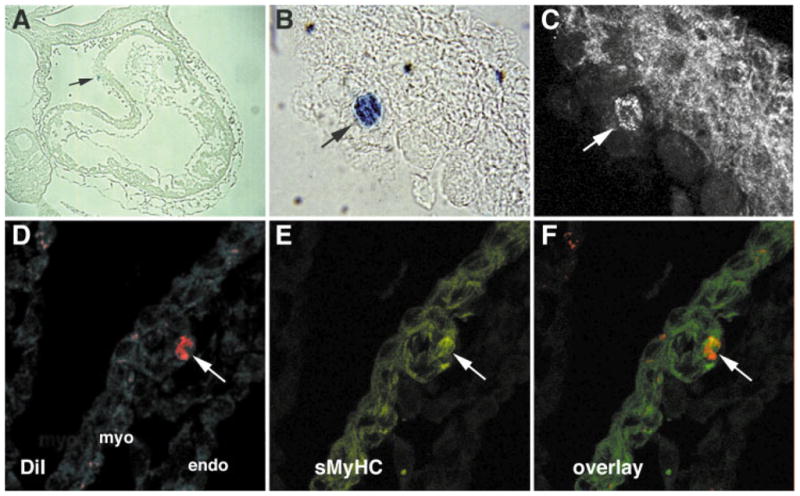
Incorporation and differentiation of circulating cells into the embryonic myocardium. ED9 mouse embryos were dissected from the uterus with fetal placenta and yolk sac intact and then injected into the yolk sac vessel leading to the sinus venous with QCE6 cells labeled with either a β-galactosidase-positive retrovirus (Eisenberg and Markwald, 1997) (A–C) or the fluorescent cell marker DiI (1,1′-dioctadecyl-3, 3,3′, 3′-tetramethyl-indocarbocyanine perchlorate) (D–F). The injected embryos were allowed to develop for 24 hr ex ovo, according to established procedures (Sturm and Tam, 1993; Eto and Osumi-Yamashita, 1995). A–C: QCE6 cells in the outer myocardial wall overlying the AV canal. X-gal staining for β-galactosidase (blue) indicates the presence of QCE6 cells (arrow) at low(A) and high (B) magnification. C: A sister section stained with the MF20 antibody, which recognizes sarcomeric myosin heavy chain (sMyHC). The arrow indicates a sMyHC-positive QCE6 cell and thus displays a myocardial phenotype. D–F: A DiI-labeled QCE6 cell in the myocardium of the outflow tract. An individual heart section is exhibited for DiI-labeling only (red) (D), sMyHC immunostaining only (green) (E), or both DiI labeling and sMyHC staining (F). The juxtaposition of these panels demonstrates that the DiI-labeled QCE6 cell (arrow) is sMyHC positive. The myocardial (myo) and endocardial (endo) layers of the heart tissue are indicated in panel D.
STEM CELL PLASTICITY—IMPLICATIONS FOR HEART FORMATION IN THE EARLY EMBRYO
In subsequent articles of this special issue, many of the recent findings in cardiovascular stem cell biology will be presented. For this review, we examined the initial events of cardiac developmental biology with the stated goal that this information would be valuable for understanding how to use stem cells to produce myocardial tissue for therapeutic purposes. Yet research in stem cell biology may also have great impact on cardiovascular developmental biology. For example, studies of adult stem cells have indicated that stem cells may exhibit a much higher degree of plasticity than had been previously realized (Bjornson et al., 1999; Orlic et al., 2001; Zuk et al., 2001; Krause, 2002). Accordingly, the cells of the early embryo may possess a much broader potential than is often conceded.
Reexamining early cardiac development through the prism of stem cell biology puts a different light on how the development of the vertebrate heart may be interpreted. Heart development is usually presented as a series of progressive and unilateral steps as cells become specified, committed, and then fully differentiated. However, as discussed above, stem cells of the precardiac mesoderm are neither uniquely specified to become heart nor fully committed to a myocardial cell fate. At the stages immediately prior to the formation of the heart, mesodermal cells within precardiac and noncardiogenic regions possess equivalent potential to generate cardiomyocytes. Cells within the mesodermal layer of the early embryo can readily be shifted to or from a cardiac phenotype by changing their extracellular environment. Thus, despite the precise fate mapping of the heart-forming cells within the early mesoderm, the cells that comprise the entirety of that embryonic layer may differ very little in their phenotypic potential.
An additional observation from research on adult stem cells that may shed light on the formation of the myocardium in the embryo is that noncardiac stem cells can differentiate into cardiomyocytes if introduced into the adult heart (Jackson et al., 2001; Malouf et al., 2001; Orlic et al., 2001; Anversa and Nadal-Ginard, 2002). That is to say, what provoked these noncardiac stem cells to become cardiomyocytes is their association with the myocardial environment. Likewise, in the embryo, what may distinguish the embryonic progenitors that give rise to the myocardium is where they have located. In other words, it may be the region of the embryo that becomes committed to producing heart, rather than the stem cells per se. Presumably, what allows these stem cells within the precardiac area to exclusively give rise to the heart is the unique extracellular environment that is set up within the precardiac regions, which despite the identification of a few key extracellular signals (e.g., BMPs, FGF, and WNT modulators) is still rather undefined.
In light of these observations, maybe the concept of a precardiac stem cell needs to be thought of differently. It appears that cardiac tissue formation in the embryo involves the defining of the molecular environment that allows multipotential cells to assemble into the heart. The implication for both cardiac embryology and stem cell biology is that multiple stem cell populations may be cardiac competent. Thus, attempts to define small subsets of stem cells that can uniquely generate differentiated cardiac tissue may not be relevant to understanding how to repair diseased or damaged myocardium in the adult. Of greater clinical relevance would be research emphasizing the development of conditions that will allow an easily obtainable and plentiful source of cells to generate new cardiac tissue.
Acknowledgments
Grant sponsor: American Heart Association; Grant number: 0355766U; Grant sponsor: National Institutes of Health; Grant number: R21-HL66055; Grant sponsor: NASA EPSCoR; Grant number: HEDS6
LITERATURE CITED
- Alsan BH, Schultheiss TM. Regulation of avian cardiogenesis by Fgf8 signaling. Development. 2002;129:1935–1943. doi: 10.1242/dev.129.8.1935. [DOI] [PubMed] [Google Scholar]
- Antin PB, Taylor RG, Yatskievych T. Precardiac mesoderm is specified during gastrulation in quail. Dev Dyn. 1994;200:144–154. doi: 10.1002/aja.1002000206. [DOI] [PubMed] [Google Scholar]
- Anversa P, Nadal-Ginard B. Myocyte renewal and ventricular remodelling. Nature. 2002;415:240–243. doi: 10.1038/415240a. [DOI] [PubMed] [Google Scholar]
- Beltrami AP, Urbanek K, Kajstura J, Yan S-M, Finato N, Bussani R, Nadal-Ginard B, Silvestri F, Leri A, Beltrami CA, Anversa P. Evidence that human cardiac myocytes divide after myocardial infarction. N Engl J Med. 2001;344:1750–1757. doi: 10.1056/NEJM200106073442303. [DOI] [PubMed] [Google Scholar]
- Biben C, Hatzistavrou T, Harvey RP. Expression of NK-2 class homeobox gene Nkx2-6 in foregut endoderm and heart. Mech Dev. 1998;73:125–127. doi: 10.1016/s0925-4773(98)00037-9. [DOI] [PubMed] [Google Scholar]
- Bisaha JG, Bader D. Molecular analysis of myogenic differentiation: isolation of a cardiac-specific myosin heavy chain. Dev Biol. 1991;148:335–364. doi: 10.1016/0012-1606(91)90343-2. [DOI] [PubMed] [Google Scholar]
- Bjornson CRR, Rietze RL, Reynolds BA, Magli MC, Vescovi AL. Turning brain into blood: a hematopoietic fate adopted by adult neural stem cells in vivo. Science. 1999;283:534–537. doi: 10.1126/science.283.5401.534. [DOI] [PubMed] [Google Scholar]
- Black BL, Olson EN. Transcriptional control of muscle development by myocyte enhancer factor-2 (MEF2) proteins. Annu Rev Cell Dev Biol. 1998;14:167–196. doi: 10.1146/annurev.cellbio.14.1.167. [DOI] [PubMed] [Google Scholar]
- Brand T, Andree B, Schneider A, Buchberger A, Arnold HH. Chicken Nkx2-8, a novel homeobox gene expressed during early heart and foregut development. Mech Dev. 1997;64:53–59. doi: 10.1016/s0925-4773(97)00044-0. [DOI] [PubMed] [Google Scholar]
- Brandon C, Eisenberg LM, Eisenberg CA. WNT signaling modulates the diversification of hematopoietic cells. Blood. 2000;96:4132–4141. [PubMed] [Google Scholar]
- Buchberger A, Arnold HH. The MADS domain containing transcription factor cMef2a is expressed in heart and skeletal muscle during embryonic chick development. Dev Genes Evol. 1999;209:376–381. doi: 10.1007/s004270050267. [DOI] [PubMed] [Google Scholar]
- Chen CY, Schwartz RJ. Recruitment of the tinman homolog Nkx-2.5 by serum response factor activates cardiac alpha-actin gene transcription. Mol Cell Biol. 1996;16:6372–6384. doi: 10.1128/mcb.16.11.6372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen F, Kook H, Milewski R, Gitler AD, Lu MM, Li J, Nazarian R, Schnepp R, Jen K, Biben C, Runke G, Mackay JP, Novotny J, Schwartz RJ, Harvey RP, Mullins MC, Epstein JA. Hop is an unusual homeobox gene that modulates cardiac development. Cell. 2002;110:713–723. doi: 10.1016/s0092-8674(02)00932-7. [DOI] [PubMed] [Google Scholar]
- Colas J-F, Lawson A, Schoenwolf GC. Evidence that translation of smooth muscle alpha-actin mRNA is delayed in the chick pro-myocardium until fusion of the bilateral heart-forming regions. Dev Dyn. 2000;218:316–330. doi: 10.1002/(SICI)1097-0177(200006)218:2<316::AID-DVDY6>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Croissant JD, Kim JH, Eichele G, Goering L, Lough J, Prywes R, Schwartz RJ. Avian serum response factor expression restricted primarily to muscle cell lineages is required for alpha-actin gene transcription. Dev Biol. 1996;177:250–264. doi: 10.1006/dbio.1996.0160. [DOI] [PubMed] [Google Scholar]
- DeHaan RL. Migration patterns of the precardiac mesoderm in the early chick embryo. Exp Cell Res. 1963;29:544–560. doi: 10.1016/s0014-4827(63)80016-6. [DOI] [PubMed] [Google Scholar]
- DeHaan RL. Morphogenesis of the vertebrate heart. In: DeHaan RL, Ursprung H, editors. Organogenesis. New York: Holt, Rinehart and Winston; 1965. pp. 377–419. [Google Scholar]
- de Jong F, Geerts WJ, Lamers WH, Los JA, Moorman AF. Isomyosin expression during pattern formation of the tubular heart: a three-dimensional immunohistochemical analysis. Anat Rec. 1990;226:213–227. doi: 10.1002/ar.1092260211. [DOI] [PubMed] [Google Scholar]
- Drake CJ, Jacobson AG. A survey by scanning electron microscopy of the extracellular matrix and endothelial components of the primordial chick heart. Anat Rec. 1988;222:391–400. doi: 10.1002/ar.1092220411. [DOI] [PubMed] [Google Scholar]
- Edmondson DG, Lyons GE, Martin JF, Olson EN. Mef2 gene expression marks the cardiac and skeletal muscle lineages during mouse embryogenesis. Development. 1994;120:1251–1263. doi: 10.1242/dev.120.5.1251. [DOI] [PubMed] [Google Scholar]
- Eisenberg CA, Bader DM. QCE6: a clonal cell line with cardiac myogenic and endothelial cell potentials. Dev Biol. 1995;167:469–481. doi: 10.1006/dbio.1995.1042. [DOI] [PubMed] [Google Scholar]
- Eisenberg CA, Bader DM. The establishment of the mesodermal cell line QCE6: a model system for cardiac cell differentiation. Circ Res. 1996;78:205–216. doi: 10.1161/01.res.78.2.205. [DOI] [PubMed] [Google Scholar]
- Eisenberg CA, Eisenberg LM. WNT11 promotes cardiac tissue formation of early mesoderm. Dev Dyn. 1999;216:45–58. doi: 10.1002/(SICI)1097-0177(199909)216:1<45::AID-DVDY7>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Eisenberg CA, Markwald RR. Mixed cultures of avian blastoderm cells and the quail mesoderm cell line QCE6 provide evidence for the pluripotentiality of early mesoderm. Dev Biol. 1997;191:167–181. doi: 10.1006/dbio.1997.8718. [DOI] [PubMed] [Google Scholar]
- Eisenberg CA, Gourdie RG, Eisenberg LM. Wnt11 is expressed in early avian mesoderm and required for the differentiation of the quail mesoderm cell line QCE6. Development. 1997;124:525–536. doi: 10.1242/dev.124.2.525. [DOI] [PubMed] [Google Scholar]
- Eisenberg LM. Belief versus scientific observation: the curious story of the precardiac mesoderm. Anat Rec. 2002;266:194–197. doi: 10.1002/ar.10066. [DOI] [PubMed] [Google Scholar]
- Eisenberg LM, Eisenberg CA. Onset of a cardiac phenotype in the early embryo. In: Dube DK, editor. Cardiovascular molecular morphogenesis: myofibrillogenesis. New York: Springer Verlag; 2002. pp. 181–205. [Google Scholar]
- Eto K, Osumi-Yamashita N. Whole embryo culture and the study of postimplantation mammalian development. Dev Growth Differ. 1995;37:123–132. doi: 10.1046/j.1440-169X.1995.t01-1-00001.x. [DOI] [PubMed] [Google Scholar]
- Faure S, de Santa Barbara P, Roberts DJ, Whitman M. Endogenous patterns of BMP signaling during early chick development. Dev Biol. 2002;244:44–65. doi: 10.1006/dbio.2002.0579. [DOI] [PubMed] [Google Scholar]
- Garcia-Martinez V, Schoenwolf GC. Primitive-streak origin of the cardiovascular system in avian embryos. Dev Biol. 1993;159:706–719. doi: 10.1006/dbio.1993.1276. [DOI] [PubMed] [Google Scholar]
- González-Sánchez A, Bader D. In vitro analysis of cardiac progenitor cell differentiation. Dev Biol. 1990;139:197–209. doi: 10.1016/0012-1606(90)90288-t. [DOI] [PubMed] [Google Scholar]
- Gottlieb PD, Pierce SA, Sims RJ, Yamagishi H, Weihe EK, Harriss JV, Maika SD, Kuziel WA, King HL, Olson EN, Nakagawa O, Srivastava D. Bop encodes a muscle-restricted protein containing MYND and SET domains and is essential for cardiac differentiation and morphogenesis. Nat Genet. 2002;31:25–32. doi: 10.1038/ng866. [DOI] [PubMed] [Google Scholar]
- Hamburger V, Hamilton HL. A series of normal stages in the development of the chick embryo. J Morphol. 1951;88:49–92. [PubMed] [Google Scholar]
- Han Y, Dennis JE, Cohen-Gould L, Bader DM, Fischman DA. Expression of sarcomeric myosin in the presumptive myocardium of chicken embryos occurs within six hours of myocyte commitment. Dev Dyn. 1992;193:257–265. doi: 10.1002/aja.1001930306. [DOI] [PubMed] [Google Scholar]
- Harraz M, Jiao C, Hanlon HD, Hartley RS, Schatteman GC. CD34(–) blood-derived human endothelial cell progenitors. Stem Cells. 2001;19:304–312. doi: 10.1634/stemcells.19-4-304. [DOI] [PubMed] [Google Scholar]
- Hogan BLM. Bone morphogenetic proteins: multifunctional regulators of vertebrate development. Genes Dev. 1996;10:1580–1594. doi: 10.1101/gad.10.13.1580. [DOI] [PubMed] [Google Scholar]
- Huelsken J, Birchmeier W. New aspects of Wnt signaling pathways in higher vertebrates. Curr Opin Genet Dev. 2001;11:547–553. doi: 10.1016/s0959-437x(00)00231-8. [DOI] [PubMed] [Google Scholar]
- Jackson KA, Majka SM, Wang H, Pocius J, Hartley CJ, Majesky MW, Entman ML, Michael LH, Hirschi KK, Goodell MA. Regeneration of ischemic cardiac muscle and vascular endothelium by adult stem cells. J Clin Invest. 2001;107:1395–1402. doi: 10.1172/JCI12150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Y, Tarzami S, Burch JB, Evans T. Common role for each of the cGATA-4/5/6 genes in the regulation of cardiac morphogenesis. Dev Genet. 1998;22:263–277. doi: 10.1002/(SICI)1520-6408(1998)22:3<263::AID-DVG8>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Kasahara H, Bartunkova S, Schinke M, Tanaka M, Izumo S. Cardiac and extracardiac expression of Csx/Nkx2.5 homeodomain protein. Circ Res. 1998;82:936–946. doi: 10.1161/01.res.82.9.936. [DOI] [PubMed] [Google Scholar]
- Keller RE. Vital dye mapping of the gastrula and neurula of Xenopus laevis. II. Prospective areas and morphogenetic movements of the deep layer. Dev Biol. 1976;51:118–137. doi: 10.1016/0012-1606(76)90127-5. [DOI] [PubMed] [Google Scholar]
- Kispert A, Vainio S, Shen L, Rowitch DH, McMahon AP. Proteoglycans are required for maintenance of Wnt-11 expression in the ureter tips. Development. 1996;122:3627–3637. doi: 10.1242/dev.122.11.3627. [DOI] [PubMed] [Google Scholar]
- Krause DS. Plasticity of marrow-derived stem cells. Gene Therapy. 2002;9:754–758. doi: 10.1038/sj.gt.3301760. [DOI] [PubMed] [Google Scholar]
- Kruithof BPT, Hoff MJBVd, Tesink-Taekema S, Moorman AFM. Recruitment of intra- and extracardiac cells into the myocardial lineage during mouse development. Anat Rec. 2003;271A:303–314. doi: 10.1002/ar.a.10033. [DOI] [PubMed] [Google Scholar]
- Kuure S, Vuolteenaho R, Vainio S. Kidney morphogenesis: cellular and molecular regulation. Mech Dev. 2000;92:31–45. doi: 10.1016/s0925-4773(99)00323-8. [DOI] [PubMed] [Google Scholar]
- Ladd AN, Yatskievych TA, Antin PB. Regulation of avian cardiac myogenesis by activin/TGF beta and bone morphogenetic proteins. Dev Biol. 1998;204:407–419. doi: 10.1006/dbio.1998.9094. [DOI] [PubMed] [Google Scholar]
- Laverriere AC, MacNeill C, Mueller C, Poelmann RE, Burch JB, Evans T. GATA-4/5/6, a subfamily of three transcription factors transcribed in developing heart and gut. J Biol Chem. 1994;269:23177–23184. [PubMed] [Google Scholar]
- Lee Y, Shioi T, Kasahara H, Jobe SM, Wiese RJ, Markham BE, Izumo S. The cardiac tissue-restricted homeobox protein Csx/Nkx2.5 physically associates with the zinc finger protein GATA4 and cooperatively activates atrial natriuretic factor gene expression. Mol Cell Biol. 1998;18:3120–3129. doi: 10.1128/mcb.18.6.3120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Q, Schwarz J, Bucana C, Olson EN. Control of mouse cardiac morphogenesis and myogenesis by transcription factor MEF2C. Science. 1997;276:1404–1407. doi: 10.1126/science.276.5317.1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linask KK. N-cadherin localization in early heart development and polar expression of Na+,K+-ATPase, and integrin during pericardial coelom formation and epithelization of the differentiating myocardium. Dev Biol. 1992;151:213–224. doi: 10.1016/0012-1606(92)90228-9. [DOI] [PubMed] [Google Scholar]
- Litvin J, Montgomery M, Arlene Gonzalez-Sanchez JGB, Bader D. Commitment and differentiation of cardiac myocytes. Trends Cardiovasc Med. 1992;2:27–32. doi: 10.1016/1050-1738(92)90041-P. [DOI] [PubMed] [Google Scholar]
- Lohr JL, Yost HJ. Vertebrate model systems in the study of early heart development: Xenopus and zebrafish. Am J Med Genet. 2000;97:248–257. doi: 10.1002/1096-8628(200024)97:4<248::aid-ajmg1275>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- Lough J, Barron M, Brogley M, Sugi Y, Bolender DL, Zhu X. Combined BMP-2 and FGF-4, but neither factor alone, induces cardiogenesis in non-precardiac embryonic mesoderm. Dev Biol. 1996;178:198–202. doi: 10.1006/dbio.1996.0211. [DOI] [PubMed] [Google Scholar]
- Lyons I, Parsons LM, Hartley L, Li R, Andrews JE, Robb L, Harvey RP. Myogenic and morphogenetic defects in the heart tubes of murine embryos lacking the homeo box gene Nkx2-5. Genes Dev. 1995;9:1654–1666. doi: 10.1101/gad.9.13.1654. [DOI] [PubMed] [Google Scholar]
- Malouf NN, Coleman WB, Grisham JW, Lininger RA, Madden VJ, Sproul M, Anderson PAW. Adult-derived stem cells from the liver become myocytes in the heart in vivo. Am J Pathol. 2001;158:1929–1935. doi: 10.1016/S0002-9440(10)64661-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marie PJ, Debiais F, Hay E. Regulation of human cranial osteoblast phenotype by FGF-2, FGFR-2 and BMP-2 signaling. Histol Histopath. 2002;17:877–885. doi: 10.14670/HH-17.877. [DOI] [PubMed] [Google Scholar]
- Marvin MJ, Di Rocco G, Gardiner A, Bush SM, Lassar AB. Inhibition of Wnt activity induces heart formation from posterior mesoderm. Genes Dev. 2001;15:316–327. doi: 10.1101/gad.855501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mjaatvedt CH, Nakaoka T, Moreno-Rodriguez R, Norris RA, Kern MJ, Eisenberg CA, Turner D, Markwald RR. The outflow tract of the heart is recruited from a novel heart-forming field. Dev Biol. 2001;238:97–109. doi: 10.1006/dbio.2001.0409. [DOI] [PubMed] [Google Scholar]
- Narita N, Bielinska M, Wilson DB. Wild-type endoderm abrogates the ventral developmental defects associated with GATA-4 deficiency in the mouse. Dev Biol. 1997;189:270–274. doi: 10.1006/dbio.1997.8684. [DOI] [PubMed] [Google Scholar]
- Naya FJ, Black BL, Wu H, Bassel-Duby R, Richardson JA, Hill JA, Olson EN. Mitochondrial deficiency and cardiac sudden death in mice lacking the MEF2A transcription factor. Nat Med. 2002;8:1303–1309. doi: 10.1038/nm789. [DOI] [PubMed] [Google Scholar]
- Niehrs C, Kazanskaya O, Wu W, Glinka A. Dickkopf1 and the Spemann-Mangold head organizer. Int J Dev Biol. 2001;45:237–240. [PubMed] [Google Scholar]
- Niswander L. Interplay between the molecular signals that control vertebrate limb development. Int J Dev Biol. 2002;46:877–881. [PubMed] [Google Scholar]
- Orlic D, Kajstura J, Chimenti S, Jakoniuk I, Anderson SM, Li B, Pickel J, McKay R, Nadal-Ginard B, Bodine DM, Leri A, Anversa P. Bone marrow cells regenerate infarcted myocardium. Nature. 2001;410:701–705. doi: 10.1038/35070587. [DOI] [PubMed] [Google Scholar]
- Pandur P, Läsche M, Eisenberg LM, Kühl M. Wnt-11 activation of a non-canonical Wnt signaling pathway is required for cardiogenesis. Nature. 2002;418:636–641. doi: 10.1038/nature00921. [DOI] [PubMed] [Google Scholar]
- Quaini F, Urbanek K, Beltrami AP, Finato N, Beltrami CA, Nadal-Ginard B, Kajstura J, Leri A, Anversa P. Chimerism of the transplanted heart. N Engl J Med. 2002;346:5–15. doi: 10.1056/NEJMoa012081. [DOI] [PubMed] [Google Scholar]
- Rawles ME. A study in the localization of organ-forming areas in the chick blastoderm of the head process stage. J Exp Zool. 1936;72:271. [Google Scholar]
- Rawles ME. The heart-forming areas of the early chick blastoderm. Physiol Zool. 1943;16:22–42. [Google Scholar]
- Redkar A, Montgomery M, Litvin J. Fate map of early avian cardiac progenitor cells. Development. 2001;128:2269–2279. doi: 10.1242/dev.128.12.2269. [DOI] [PubMed] [Google Scholar]
- Reecy JM, Yamada M, Cummings K, Sosic D, Chen CY, Eichele G, Olson EN, Schwartz RJ. Chicken Nkx-2.8 —a novel homeobox gene expressed in early heart progenitor cells and pharyngeal pouch-2 and -3 endoderm. Dev Biol. 1997;188:295–311. doi: 10.1006/dbio.1997.8641. [DOI] [PubMed] [Google Scholar]
- Ruzicka DL, Schwartz RJ. Sequential activation of alpha-actin genes during avian cardiogenesis: vascular smooth muscle alpha-actin gene transcripts mark the onset of cardiomyocyte differentiation. J Cell Biol. 1988;107:2575–2586. doi: 10.1083/jcb.107.6.2575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlange T, Andree B, Arnold HH, Brand T. BMP2 is required for early heart development during a distinct time period. Mech Dev. 2000;91:259–270. doi: 10.1016/s0925-4773(99)00311-1. [DOI] [PubMed] [Google Scholar]
- Schneider VA, Mercola M. Wnt antagonism initiates cardiogenesis in Xenopus laevis. Genes Dev. 2001;15:304–315. doi: 10.1101/gad.855601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultheiss TM, Xydas S, Lassar AB. Induction of avian cardiac myogenesis by anterior endoderm. Development. 1995;121:4203–4214. doi: 10.1242/dev.121.12.4203. [DOI] [PubMed] [Google Scholar]
- Schultheiss TM, Burch JB, Lassar AB. A role for bone morphogenetic proteins in the induction of cardiac myogenesis. Genes Dev. 1997;11:451–462. doi: 10.1101/gad.11.4.451. [DOI] [PubMed] [Google Scholar]
- Sepulveda JL, Vlahopoulos S, Iyer D, Belaguli N, Schwartz RJ. Combinatorial expression of GATA4, Nkx2-5, and serum response factor directs early cardiac gene activity. J Biol Chem. 2002;277:25775–25782. doi: 10.1074/jbc.M203122200. [DOI] [PubMed] [Google Scholar]
- Shibata M, Ono H, Hikasa H, Shinga J, Taira M. Xenopus crescent encoding a Frizzled-like domain is expressed in the Spemann organizer and pronephros. Mech Dev. 2000;96:243–246. doi: 10.1016/s0925-4773(00)00399-3. [DOI] [PubMed] [Google Scholar]
- Shin CH, Liu ZP, Passier R, Zhang CL, Wang DZ, Harris TM, Yamagishi H, Richardson JA, Childs G, Olson EN. Modulation of cardiac growth and development by HOP, an unusual homeodomain protein. Cell. 2002;110:725–735. doi: 10.1016/s0092-8674(02)00933-9. [DOI] [PubMed] [Google Scholar]
- Stainier DYR, Lee RK, Fishman MC. Cardiovascular development in the zebrafish. I. Myocardial fate map and heart field formation. Development. 1993;119:31–40. doi: 10.1242/dev.119.1.31. [DOI] [PubMed] [Google Scholar]
- Stalsberg H, DeHaan RL. The precardiac areas and formation of the tubular heart in the chick embryo. Dev Biol. 1969;19:128–159. doi: 10.1016/0012-1606(69)90052-9. [DOI] [PubMed] [Google Scholar]
- Sturm K, Tam PPL. Isolation and culture of whole postimplantation embryos and germ layer derivatives. Methods Enzymol. 1993;225:164–190. doi: 10.1016/0076-6879(93)25013-r. [DOI] [PubMed] [Google Scholar]
- Sugi Y, Lough J. Anterior endoderm is a specific effector of terminal cardiac myocyte differentiation in cells from the embryonic heart forming region. Dev Dyn. 1994;200:155–162. doi: 10.1002/aja.1002000207. [DOI] [PubMed] [Google Scholar]
- Tokuyasu KT, Maher PA. Immunocytochemical studies of cardiac myofibrillogenesis in early chick embryos. I. Presence of immunofluorescent titin spots in premyofibril stages. J Cell Biol. 1987a;105:2781–2793. doi: 10.1083/jcb.105.6.2781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokuyasu KT, Maher PA. Immunocytochemical studies of cardiac myofibrillogenesis in early chick embryos. II. Generation of alpha-actinin dots within titin spots at the time of the first myofibril formation. J Cell Biol. 1987b;105:2795–2801. doi: 10.1083/jcb.105.6.2795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldo KL, Kumiski DL, Wallis KT, Stadt HA, Hutson MR, Platt DH, Kirby ML. Conotruncal myocardium arises from a secondary heart field. Development. 2001;128:3179–3188. doi: 10.1242/dev.128.16.3179. [DOI] [PubMed] [Google Scholar]
- Yamada M, Revelli JP, Eichele G, Barron M, Schwartz RJ. Expression of chick Tbx-2, Tbx-3, and Tbx-5 genes during early heart development: evidence for BMP2 induction of Tbx2. Dev Biol. 2000;228:95–105. doi: 10.1006/dbio.2000.9927. [DOI] [PubMed] [Google Scholar]
- Zuk PA, Zhu M, Mizuno H, Huang J, Futrell W, Katz AJ, Benhaim P, Lorenz HP, Hedrick MH. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng. 2001;7:211–228. doi: 10.1089/107632701300062859. [DOI] [PubMed] [Google Scholar]