Abstract
The central melanocortin system, consisting of melanocortin peptides, agouti gene related peptide and their receptors plays a critical role in the homeostatic control of energy balance. Loss of function mutations in the genes encoding proopiomelanocortin or melanocortin MC4 receptors cause profound obesity and hyperphagia. However, little is known about the functional relationship of melanocortin neurocircuits to the temporal organization of meal-taking behavior. We used an operant paradigm that combined lever pressing for food pellet deliveries with free water intake monitored by lickometers to quantify meal patterns in mutant mice that selectively lack proopiomelanocortin expression in hypothalamic neurons (nPOMCKO). Compared to wildtype siblings, nPOMCKO mice consumed 50% more food and water daily and exhibited a more stereotyped feeding pattern characterized by reduced inter-meal and inter-mouse variations. Average meals were larger in size but shorter in duration, with no change in meal number. Consequently, intermeal intervals were prolonged in nPOMCKO mice. Similar patterns were observed in pre-obese juvenile and frankly obese adult mice suggesting that neither age nor degree of obesity was responsible for the altered phenotypes. Spontaneous locomotion and wheel running were decreased in nPOMCKO mice, but circadian variations in locomotor and feeding activity were conserved. These data show that hyperphagia in male nPOMCKO mice is due to increased meal size but not meal number, and this pattern is established by age 5 wk. The combination of larger, more rapidly consumed meals and prolonged intermeal intervals suggests that proopiomelanocortin peptides are necessary for normal meal termination, but not the maintenance of satiety.
Keywords: Operant behavior, Obesity, Satiety, Hyperphagia, Circadian, Locomotor activity
1. Introduction
The central melanocortin system has been investigated intensively for its roles in regulating energy homeostasis (Cone, 2005; Ellacott and Cone, 2006; Tolle and Low, 2008). Melanocortin agonists such as alpha-melanocyte stimulating hormone are derived by posttranslational cleavage of proopiomelanocortin (POMC), a propeptide expressed in the arcuate nucleus and the nucleus tractus solitarius. Melanocortins inhibit feeding and stimulate energy expenditure by activation of melanocortin MC3 and MC4 receptors (Adan et al., 2006; Cone, 2005). The opposite effect is produced by agouti-related peptide, an endogenous melanocortin MC3 and MC4 receptor antagonist/inverse agonist expressed in the hypothalamus, which stimulates feeding (Adan et al., 2006; Haskell-Luevano et al., 1999; Tolle and Low, 2008). Disruption of central melanocortin signaling by genetic lesions of either the melanocortin MC4 receptor or POMC results in hyperphagia and early onset obesity (Challis et al., 2004; Huszar et al., 1997; Yaswen et al., 1999). In contrast, cachexia that develops with chronic diseases including cancer and renal failure is associated with increased melanocortin peptide tone in the brain (DeBoer and Marks, 2006).
Despite these advances, little is known about how the central melanocortin system regulates the timing of feeding episodes that underlie increased or decreased food intake. Yet most, if not all, mammals eat in discrete episodes or meals (Collier, 1980). Total food intake is determined by the product of two variables, meal size and meal number (Meguid et al., 1998), both reflected in the temporal organization of an animal’s feeding behavior. Meal pattern analysis allows one to ascertain when feeding behavior is taking place across time, the quantities ingested, and rate of eating during each feeding episode.
Investigations into meal pattern have suffered from lack of unanimity over the best method for defining meals. A recent study using rats exhaustively compared the most frequently used meal definition methods with a “drinking-explicit” model (Zorrilla et al., 2005). Unlike previous models, which only include feeding events in the definition of meal boundaries, the drinking-explicit model includes both feeding and drinking events. This model satisfies two predictions of meal pattern that many previous meal definitions do not; first, the probability of meal initiation increases with time as expected by the concept of satiety. Second, rats are much more likely to exhibit the behavioral satiety sequence associated with meal termination (Halford et al., 1998). Thus, this model more accurately characterizes meal taking in rats and we recently validated a similar paradigm in wildtype C57BL/6 mice (Richard, 2008).
Here, we employed meal pattern analysis to investigate the role that central POMC peptide signaling plays in the initiation, maintenance and termination of meals in a strain of neural-specific POMC knockout (nPOMCKO) mice generated previously in our lab (Smart et al., 2006). nPOMCKO mice are hyperphagic and develop an early onset obesity phenotype that is more severe than that of either global POMCKO or melanocortin MC4 receptor knockout mice. Meal patterns were compared between groups of adult obese and juvenile pre-obese nPOMCKO mice to determine whether age or the development of obesity per se influence the behavioral patterns underlying hyperphagia in the mutant mice.
2. Materials and methods
2.1. Subjects
nPOMCKO mice and wild-type littermates (nPOMCWT) were generated by crossing Pomc−/− mice (Yaswen et al., 1999) with mice carrying a Pomc ‘rescue’ transgene as described previously (Smart et al., 2006). The transgene contained a functional Pomc gene downstream of the pituitary-specific Pomc promoter and restored Pomc expression, and thus ACTH production, in the pituitary glands of mice globally deficient in POMC. nPOMCKO and nPOMCWT mice were hemizygous for the Pomc ‘rescue’ transgene and had a hybrid genetic background of approximately 80% C57BL/6, 10% DBA/2, and 10% 129X1;129S6. Mice were kept on a 12h:12h light-dark cycle (lights on at 07:00) and provided free access to water and chow containing 28.0 kcal% protein, 12.1 kcal% fat, and 59.8 kcal% carbohydrate (Rodent chow diet no. 5001; PMI Feeds Inc., St. Louis, MO). Mice were group housed after weaning and then individually housed in home cages for at least one wk prior to the start of locomotor activity or meal pattern experiments. All procedures were approved by the Institutional Animal Care and Use Committee and followed the Public Health Service guidelines for the humane care and use of experimental animals.
2.2. Measurement of locomotor activity in open fields
2.2.1. Equipment and procedure
The 43.18 × 43.18 cm open field chambers (Med-Associates, St. Albans, VT) were enclosed in ventilated and sound-attenuating boxes that were contained in a separate room from the colony. Horizontal distance was measured by the sequential breaking of infrared beams, 2.54 cm on center, in the horizontal plane of the x- and y-axes. Software (Med-Associates Activity Monitor v3.26) analyzed the distance traveled during the testing sessions. Initiation of movement was incremented each time a break in ambulatory activity occurred for >1 sec. Ambulatory time was incremented when a mouse was active for >1 sec. Rearing movements were counted each time an animal passed above and then below the level of a sensor in the z-axis vertical plane (the mouse must have remained below the level of a sensor for at least 1 sec before it could score again). Mice were acclimated to the procedure and testing environment by placing them in open field chambers during the middle of the light cycle (between 12.00–14:00) for two consecutive days. The day of experiment, subjects were introduced to the activity chambers in the middle of the light cycle (between 12.00–14:00) for a 23h-recording period. Mice had ad libitum access to food placed in a Petri dish inside the chamber and water was available from a bottle equipped with a lixit.
2.2.2 Data analyses
Activity readings recorded each minute were summed over 30-min intervals to obtain a circadian pattern of activity during the light/dark cycle. Data were then analyzed according to the following time intervals: 23 hr activity and three equal 4 hr nocturnal activity periods (19:00–23:00, 23:00–03:00, and 03:00–07:00). Parameters analyzed were the following: ambulatory time, distance traveled, vertical counts (rearing), and ambulatory speed (total distance/total ambulatory time).
2.3. Measurement of locomotor and feeding activities in running wheel cages
2.3.1. Equipment and procedure
Mice were acclimated for at least two days before data collection by housing them in custom cages equipped with running wheels to measure locomotor activity and specialized food containers to monitor feeding activities (Mini Mitter, Bend, OR). Consumption of powdered chow from the containers was measured daily to assure that 24h food intake was not affected. Feeding frequency and feeding duration were measured continuously over the 24h test period using an automated food intake system (Mini Mitter) (Harkin et al., 2002). A monitor attached to the feed cup generated an infrared light beam that was broken each time the mouse placed its head into the feeding container. Feeding frequency was reported as the number of times an animal broke the photobeam and feeding duration was reported as the length of time the photobeam was interrupted. Data were collected at 1 min intervals using the VitalView data-acquisition and software system (Mini Mitter). Running wheel activity was continuously measured over the 24h test period and the number of wheel revolutions recorded every 1 min.
2.3.2 Data analyses
Activity readings recorded each minute (wheel counts, feeding counts, feeding duration) were summed over 30 min intervals to obtain a circadian pattern of activity during the light/dark cycle. Based on these observations, data were analyzed according to the following time intervals: total 24h activity and three equal 4 hr nocturnal activity periods (18:00–22:00, 22:00–02:00, and 02:00–06:00).
2.4. Measurement of feeding and drinking behavior in operant chambers
2.4.1. Equipment
Four 16×14×13 cm and four 22×18×13 cm instrumental conditioning chambers with acrylic walls and floors consisting of 2.5 mm stainless steel rods spaced 1 cm on center were used in meal pattern studies (Med-Associates). The chambers were outfitted with a food lever to the right of the food magazine and a retracted dummy lever to the left. Water was supplied from sipper tubes, located on the wall opposite to the food magazine and recessed 0.5 cm to prevent false licks, and equipped with lick-o-meters to record drinking events. A house light (100 mA) was situated above the access opening for the sipper tube, and a 7.9 mm LED stimulus light above the food magazine. A 4.4 cm2 acrylic platform was secured to the grid floor in the center of each chamber to provide the mice with a solid resting surface. Each chamber was enclosed in a light- and sound-attenuating, ventilated cabinet. Control of the apparatus and records of lever presses, pellet deliveries and lick events were made through a computer interface and MedPC for Windows software (Med-Associates). Numerical data records for individual mice were graphically rendered into cumulative records for visual inspection using Med-Associates SoftCR 4.0 for Windows.
2.4.2. General Procedures and Experimental Design
Mice lived in operant conditioning chambers continuously for 14 days while their spontaneous responses to obtain food and water were recorded over 23 hr sessions that started between 16:00–17:00. At the beginning of each session, a response lever and sipper tube in each chamber were extended. Fixed-ratio (FR) reinforcement schedules were employed to set the “cost” per food pellet and performance of a fixed number of lever presses resulted in the delivery of a single nutritionally complete 20 mg food pellet (FO163; Bio-Serve, Frenchtown, NJ). The macronutrient composition of the food pellets was similar to standard chow with 23.9 kcal% protein, 10.3 kcal% fat, and 65.7 kcal% carbohydrate. House lights within each chamber were programmed to turn off at 19:00 and turn back on at 07:00 in synchrony with the room lighting. On training day 1, delivery of each food pellet was accompanied by a 1 sec illumination of the stimulus light above the food magazine. On subsequent days, the stimulus lights were disabled but the levers were programmed to retract for 10 sec immediately following completion of the FR schedule criterion. This was done to prevent perseverative lever pressing identified in pilot studies, and to encourage pellet consumption immediately after delivery. At session termination, levers were retracted but house lights remained on to maintain diurnal illumination conditions. The mice were weighed and placed in their home-cages without food or water for 1h. During this time, sipper tubes were weighed to measure water consumption, and bedding trays were removed to count uneaten pellets, washed thoroughly and replaced in their respective chambers. Mice whose body weights fell below 80% of starting weight during the initial training sessions were removed from the experiment and excluded from subsequent data analyses (adult nPOMCKO, n = 2).
nPOMCWT mice started at FR10 on training day 1 and reached FR30 by day 2. Once FR30 was achieved the schedule did not change for the remainder of the experiment. The protocol was modified slightly for nPOMCKO mice after pilot studies revealed that the mutant animals failed to lever press for food if the FR schedule increased too rapidly during acclimitation to the operant chambers. Consequently, during the first five days the FR schedule for food pellets was increased at the beginning of each new session, from FR1, to FR5, FR10, and FR20 until the nPOMCKO mice were working for food under a FR30 schedule. FR30 was chosen for the meal pattern measurement period because this schedule simultaneously minimized pellet waste/loss while reproducing the free feeding intake reported previously for both genotypes. Only feeding and drinking event data from experimental days 6–14, when body weights and daily food intake were stabilized, were used to calculate meal patterns.
2.4.3. Meal pattern analysis
We developed custom software, written in Visual Basic for Applications (VBA) and run on Excel spreadsheets (Microsoft Corp., Redwood, WA), to automate each processing step in meal pattern analysis. Raw data were structured as temporally consecutive intervals, in seconds, between lever press, pellet delivery and/or lick events representing the ingestive activity of an individual mouse while living in an operant conditioning chamber. Data were transferred from MED-PC files into Excel using MED2XL (Med-Associates). Validation of the operant procedure to measure meal patterns and the algorithm we created for meal definitions, based on the “drinking-explicit” model developed by Zorrilla and colleagues (Zorrilla et al., 2005), are described in detail elsewhere (Richard, 2008). Meal parameter values were calculated based on the identified threshold intermeal interval and minimal meal criterion.
2.5. Statistics
Data were analyzed by repeated measures ANOVAS, multifactor ANOVAs, paired T-tests, or Mann Whitney U tests for nonparametric data sets as appropriate for the design of each experiment with group, genotype, time and/or meal order as independent variables using Stat View Power PC for Macintosh version 5.0.1 (SAS Institute Inc.) or Graphpad PRISM v.4.03 for Windows. One factor ANOVAs were used to follow up significant main effects. For ANOVAs, Bonferroni’s multiple comparisons post hoc tests were conducted when appropriate and P < 0.05 was considered significant.
3. Results
3.1. Spontaneous locomotor activity
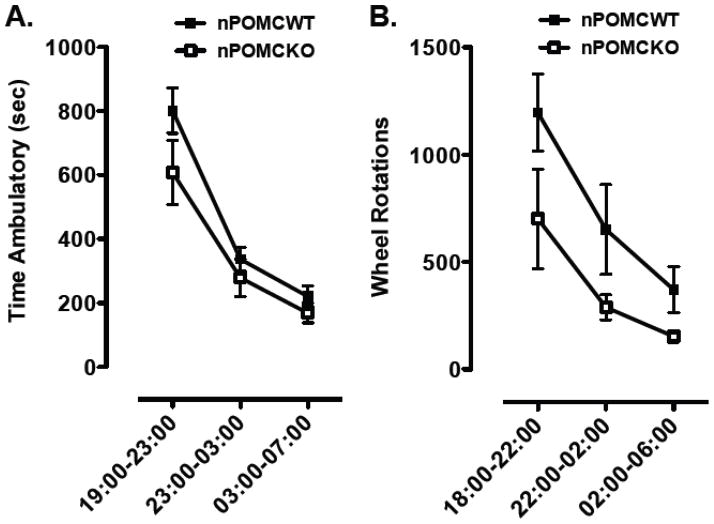
Locomotor activity of 9 wk old male nPOMCWT and nPOMCKO mice was measured in open field chambers. nPOMCWT mice were significantly more active than nPOMCKO mice across the 23 hr recording period. Total ambulatory time was 2625 ± 284 sec for nPOMCWT compared to 1708 ± 226 sec for nPOMCKO mice (P < 0.05). Rearing activity, measured by vertical counts, was 4984 ± 528 for nPOMCWT compared to 2911 ± 558 for nPOMCKO mice (P < 0.05). However, both genotypes exhibited a similar temporal pattern of decreasing activity levels from a peak in the first nocturnal interval to a nadir in the third nocturnal interval (F2,22 = 52.8, P < 0.0001, main effect of interval; F2,22 = 1.2, no significant interaction between interval and genotype) (Fig. 1A). The horizontal speed of nPOMCKO mice (13.0 ± 0.3 cm/sec) was only marginally less than that of nPOMCWT mice (13.8 ± 0.1 cm/sec; P = 0.05). Therefore, the ~40% reduction in total distance traveled over 23h by nPOMCKO (22,146 ± 3069 cm) compared to nPOMCWT (36,363 ± 4060 cm; P < 0.05) mice in the open fields was likely due to a combination of decreased movement initiation and shorter individual movement bouts.
Fig. 1.
Nocturnal locomotor activity of neuron-specific POMC knockout (nPOMCKO) mice and wild-type littermates (nPOMCWT). (A) Ambulatory time in an open field recorded over 4h intervals between 19:00–07:00 from nPOMCKO (n = 7, open squares) and nPOMCWT (n = 6, filled squares) male mice. (B) Running wheel revolutions recorded over 4h intervals between 18:00–06:00 from nPOMCKO (n = 7, open squares) and nPOMCWT (n = 5, filled squares) male mice. Data are means ± SEM.
Running wheel activity of the same two groups of mice was measured at age 10–12 wk. The total number of wheel revolutions over 24h in nPOMCWT mice (2289 ± 405) was almost twice that of nPOMCKO mice (1234 ± 237; P < 0.05). Because nPOMCKO and nPOMCWT mice exhibited an identical, abrupt onset of running wheel activity during the last 1h of the light period that anticipated the onset of darkness at 19:00 (data not shown), 18:00 was used instead of 19:00 to mark the start of the nocturnal activity measurements. The total number of nocturnal wheel revolutions was greater in nPOMCWT compared to nPOMCKO mice (F1,10 = 5.8, P < 0.05, main effect of genotype) but both genotypes exhibited a similar profile of decreased running over the course of the dark period (F2,20 = 13.0, P < 0.0005, main effect of interval; F2,20 = 0.5, no significant interaction between genotype and interval) (Fig. 1B). Taken together, data from the open field chambers and running wheels demonstrate that the absence of neuronal POMC expression and/or resulting obesity resulted in a significant attenuation of locomotor activity across the nocturnal and diurnal periods, but did not alter the normal circadian pattern of activity that peaks in the first third of the dark period (19:00–23:00) and then decreases to a nadir during the light-time hours of 07:00–18:00.
3.2. Feeding activity in running wheel cages
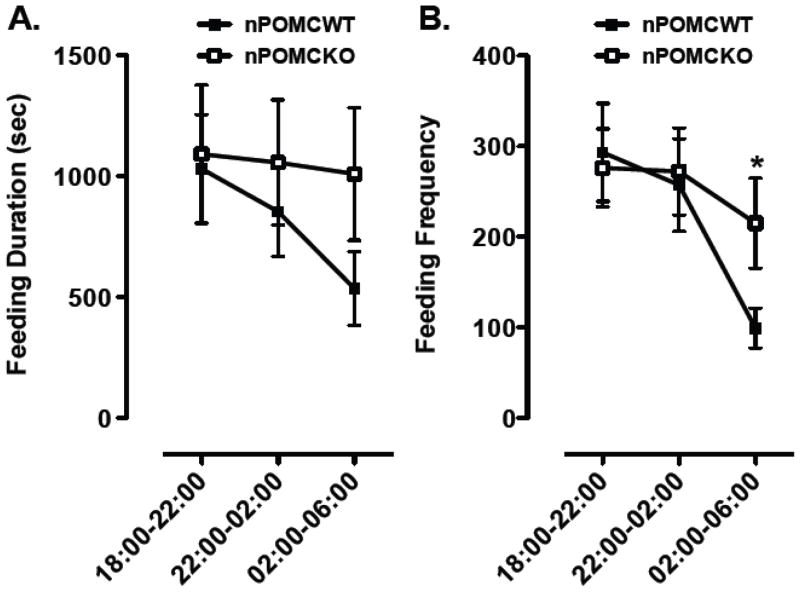
The cages containing running wheels were also equipped with specialized feeding containers to monitor the frequency and duration of head pokes of mice to obtain food in the form of powdered chow. These food containers were designed so that mice could only consume food while their head or body blocked the infrared beam at the containers’s throat. Male nPOMCKO and nPOMCWT mice exhibited almost identical mean feeding durations (Fig. 2A) and feeding frequencies (Fig. 2B) during the first third of the nocturnal period (18:00–22:00). This similar level of feeding activity between genotypes was in contrast to the decreased locomotor and running wheel activity of nPOMCKO mice at all time intervals (see section 3.1.). By the third nocturnal interval (02:00–06:00) the two measures of feeding activity were decreased in both genotypes of mice (Feeding duration: F2,26 = 3.2, P = 0.06, nonsignificant trend for interval; F2,26 = 1.6, no significant interaction between genotype and interval; Feeding frequency: F2,26 = 4.8, P = 0.02, main effect of interval; F2,26 = 1.2, no significant interaction between genotype and interval). However, feeding frequency declined significantly less for the nPOMCKO compared to nPOMCWT mice during the third nocturnal interval from 02:00–06:00 (P < 0.05).
Fig. 2.
Nocturnal feeding duration (A) and feeding frequency (B) of neuron-specific POMC knockout (nPOMCKO) mice and wild-type littermates (nPOMCWT) under free-feeding conditions in running wheel cages. Both measures of feeding activity were recorded over 4h intervals between 18:00–06:00 from nPOMCKO (n = 7, open squares) and nPOMCWT (n = 8, filled squares) male mice. Data are means ± SEM. *P < 0.05, between genotypes
3.3. Meal pattern analysis of male mice from operant chambers
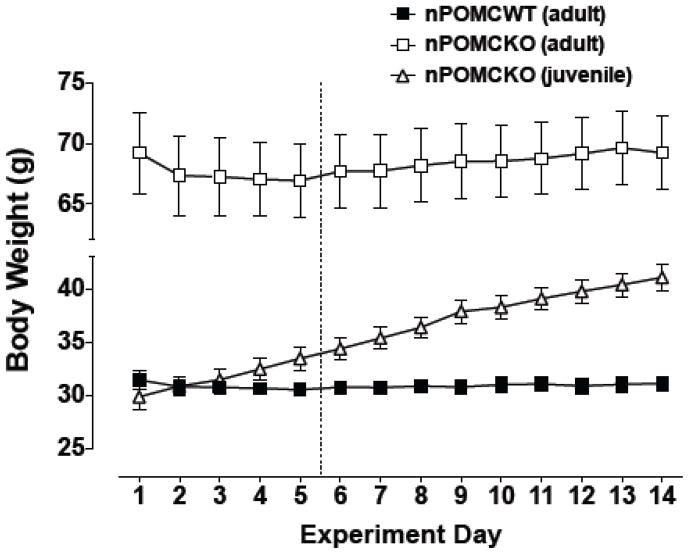
Because the food monitor system utilized in the experiment described in section 3.2 could not provide a direct measure of individual meal sizes, we employed an alternative operant method based on food pellet delivery in response to lever pressing to more precisely quantify meal size and number. Three new groups of male mice were studied with the indicated ages at the start of operant training: adult nPOMCWT (18 – 23 wk old), adult nPOMCKO (18 – 23 wk old), and juvenile nPOMCKO (5 – 6 wk old). Body weights of the juvenile nPOMCKO mice at the start of the experiment were indistinguishable from those of the adult nPOMCWT mice (Fig. 3). Body weights of adult mice from both genotypes were stable throughout the study following a slight initial drop when moved into the operant conditioning chambers. In contrast, body weights of juvenile nPOMCKO mice increased continuously from 29.9 ± 1.2g to 41.1 ± 1.2 g (P < 0.0001) during the two weeks of the experiment, indicating that the operant conditions did not alter their developmental trajectory of genetically predetermined obesity.
Fig. 3.
Daily body weights of male neuron-specific POMC knockout (nPOMCKO) mice and wild-type littermates (nPOMCWT) while living continuously in operant chambers. The dotted line indicates the end of the 5d training period, after which all mice responded for food pellets on an FR30 schedule. Adult nPOMCKO (n = 10), open squares; juvenile nPOMCKO (n = 6), open triangles; and adult nPOMCWT (n = 11), filled squares. Data are means ± SEM.
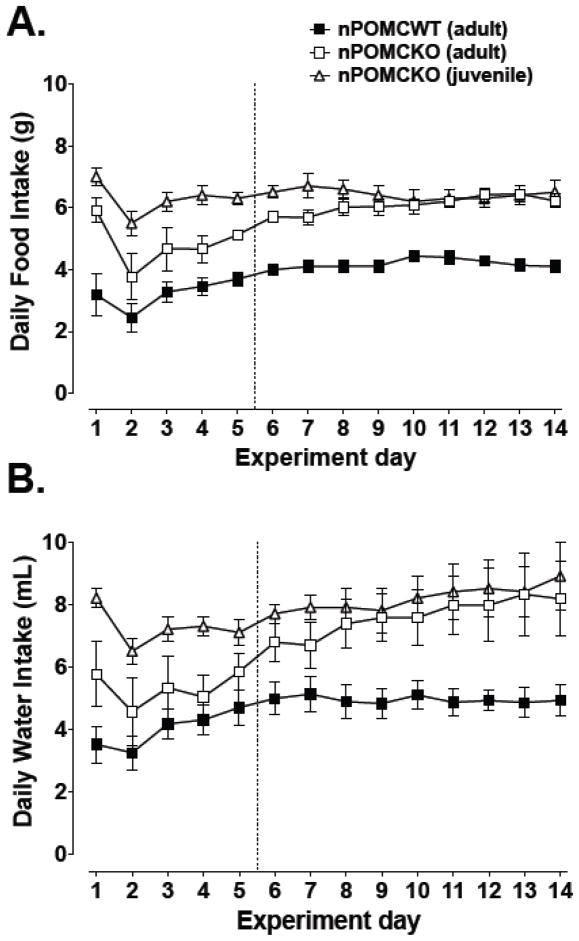
Food intake was most variable during the first training days when the reinforcement schedule was adjusted (Fig. 4A). Average food intake decreased for all groups after the first schedule change from FR1 to FR5 (nPOMCKO mice) or FR10 to FR30 (nPOMCWT mice) on day 2, then gradually recovered despite further increases in the reinforcement schedule. Daily food intake stabilized on an FR30 schedule after day five for all mice, with nPOMCKO mice consuming approximately 6g of food regardless of age and nPOMCWT mice eating approximately 4g. Daily water intake by the three groups followed similar trends as those for food intake (Fig. 4B). Overall, nPOMCKO mice drank proportionately more water than nPOMCWT mice, although adult nPOMCKO mice consumed intermediate quantities of water during the first five days when their daily food intake was lower.
Fig. 4.
Daily food (A) and water (B) intake of male neuron-specific POMC knockout (nPOMCKO) mice and wild-type littermates (nPOMCWT) while living continuously in operant chambers. The dotted line indicates the end of the 5d training period, after which all mice responded for food pellets on an FR30 schedule. Adult nPOMCKO (n = 10), open squares; juvenile nPOMCKO (n = 6), open triangles; and adult nPOMCWT (n = 11), filled squares. Data are means ± SEM.
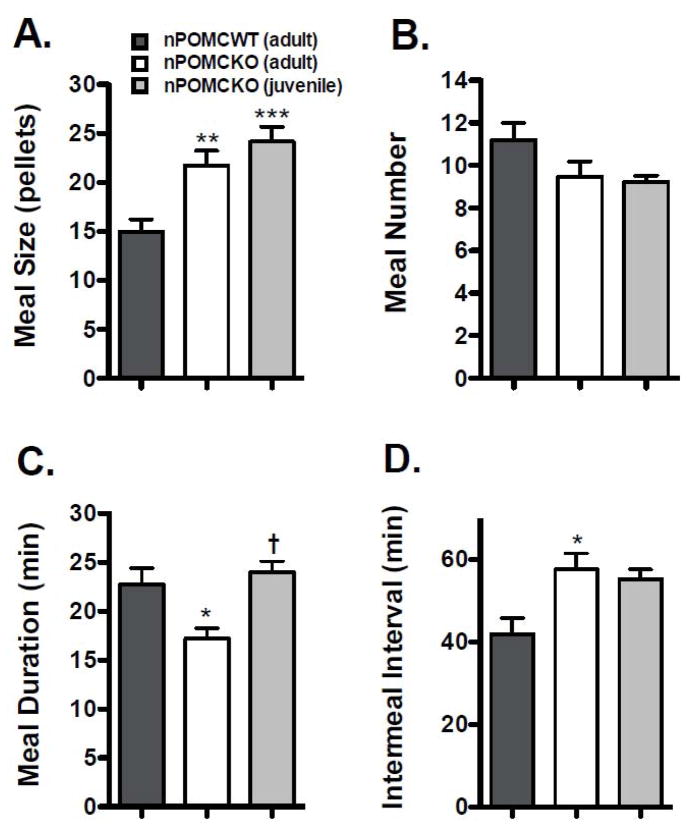
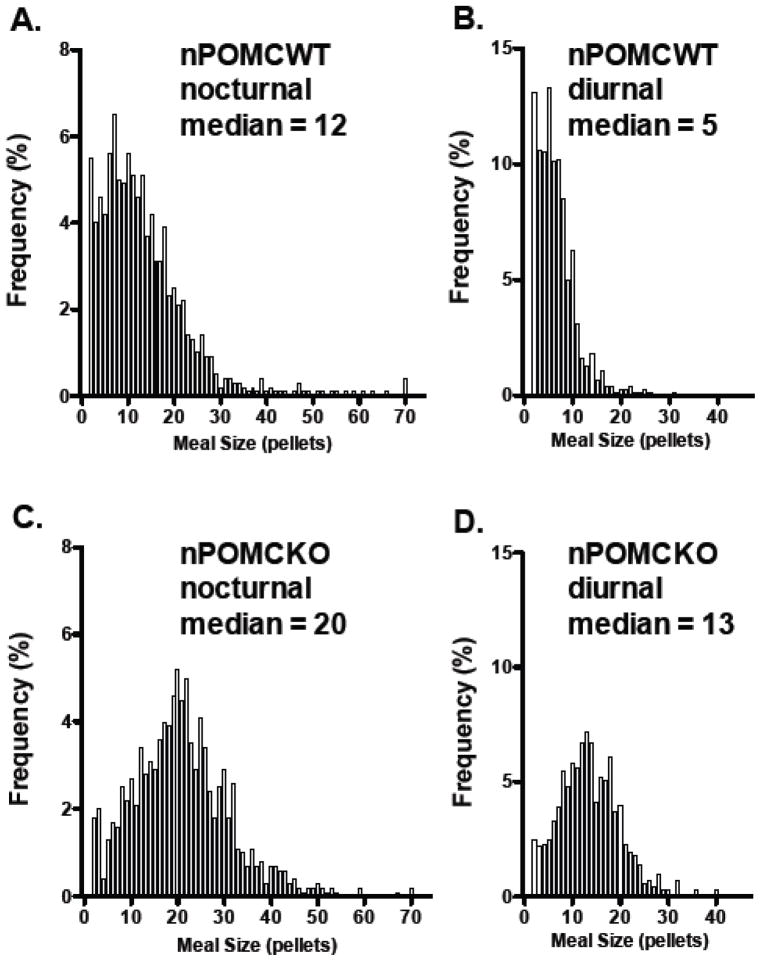
Meal pattern analyses for the male mice were performed using a threshold intermeal interval of 9 min and a minimum meal size of 2 pellets (40 mg). The hyperphagic phenotype of nPOMCKO mice was primarily attributable to increased meal size (Fig. 5). nPOMCKO mice ate larger meals than nPOMCWT mice regardless of age (F2,24 = 10.9, P < 0.0005), but no group differences in meal number were found (Fig. 5A and B). The larger meals of nPOMCKO mice during both nocturnal and diurnal feeding periods are further illustrated by the shift of median values and meal size distributions to the right in frequency histograms of all meals consumed during the 9d data collection period (Mann Whitney U = 215,100, P < 0.0001) (Fig. 6). Adult but not juvenile nPOMCKO mice had significantly longer intermeal interval durations when compared to nPOMCWT controls (F2,24 = 5.4, P < 0.05) although there was a trend for longer intermeal intervals in juvenile nPOMCKO mice (Fig. 5D). Prolonged intermeal intervals would be expected following large meals if the mechanisms regulating satiety, i.e. that suppress initiation of new meals, were intact; this is in contrast to satiation, which refers to the processes responsible for meal termination. The only significant difference found between juvenile and adult nPOMCKO mice was in their average nocturnal meal durations (Fig. 5C). Adult nPOMCKO mice had shorter meals than either young nPOMCKO or adult nPOMCWT mice (F2,24 = 6.1, P < 0.01).
Fig. 5.
Average nocturnal meal values of male neuron-specific POMC knockout (nPOMCKO) mice and wild-type littermates (nPOMCWT). (A) Meal size. (B) Meal number. (C) Meal duration. (D) Intermeal duration. Adult nPOMCWT (n = 11), dark gray columns; adult nPOMCKO (n = 10), white columns; and juvenile nPOMCKO (n = 6), light gray columns. Data are means + SEM. * P < 0.05, ** P < 0.01, *** P < 0.001 compared to nPOMCWT; † compared to adult nPOMCKO.
Fig. 6.
Frequency histograms for all individual meal sizes calculated from nine consecutive nocturnal (A, C) and diurnal periods (B, D). Adult male nPOMCWT mice (n = 11) (A, B). Adult male nPOMCKO mice (n = 10) (C, D). Bin size increments are based on 20 mg food pellets.
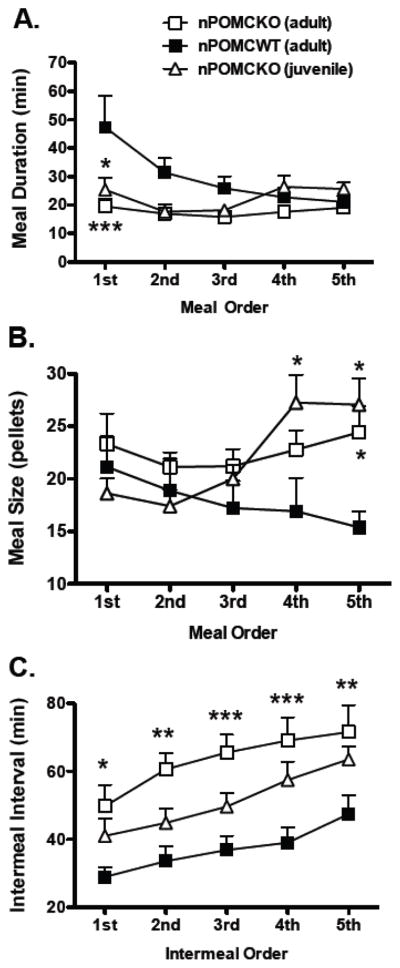
An analysis of sequential meal durations revealed significant main effects of group (F2,96 = 5.6, P < 0.05) and nocturnal meal order (F4,96 = 2.9, P < 0.05) and an interaction between the two factors (F8,96 = 2.5, P < 0.05) (Fig. 7A). The duration of the first nocturnal meal for nPOMCWT mice was longer than for either adult nPOMCKO mice (t19 = 4.6, P < 0.001) or juvenile nPOMCKO mice (t15 = 3.2, P < 0.05). When sequential meal sizes were analyzed, a significant interaction between meal order and group was also found (F8,96 = 2.8, P < 0.01). This effect was due to larger fifth meals in both adult (P < 0.05) and juvenile nPOMCKO mice (P < 0.05) compared to nPOMCWT mice (Fig. 7B). Fourth meals were also larger for juvenile nPOMCKO compared to nPOMCWT mice (P < 0.05). Sequential intermeal intervals analysis showed significant main effects of group (F2,96 = 8.4, P < 0.01) and intermeal interval sequence (F4,96 = 25.7, P < 0.0001), but no interaction between the two variables (F8,96 = 0.9, not significant). All five intermeal intervals were significantly longer for adult nPOMCKO mice than nPOMCWT mice (Fig. 7C). Intermeal intervals for juvenile nPOMCKO mice were intermediate between the other two groups.
Fig. 7.
Comparisons of sequential nocturnal meal pattern values of male neuron-specific POMC knockout (nPOMCKO) mice and wild-type littermates (nPOMCWT). (A) Sequential meal durations. (B) Sequential meal sizes. (C) Sequential intermeal intervals. Adult nPOMCKO (n = 10), open squares; juvenile nPOMCKO (n = 6), open triangles; and adult nPOMCWT (n = 11), filled squares. Data are means + SEM. * P < 0.05, ** P < 0.01, *** P < 0.001 compared to nPOMCWT.
4. Discussion
Lean nPOMCWT and obese nPOMCKO mice were both capable of adapting to a novel caging environment and acquiring operant lever pressing behavior for the delivery of food pellets without prior food deprivation. These pellets were the animals sole food source for a period of two weeks. After the initial five days in operant chambers, all adult mice exhibited stable body weights and total daily food intakes that were essentially identical to those measured previously under home cage free-feeding conditions (Smart et al., 2006), suggesting that energy homeostasis was maintained. In fact, the pre-obese juvenile nPOMCKO mice gained body weight at the same rate in the operant chambers as under home cage conditions. Notably, adult and juvenile nPOMCKO mice required additional training days and a more shallow escalation in their bar pressing schedule compared to wildtype controls to stably acquire the final FR30 contingency. This acquisition difference may simply be secondary to the decreased locomotor and exploratory behavior of the mutant mice or alternatively reflect an independent learning deficit. Regardless of the cause, nPOMCKO mice eventually emitted 50% more bar presses per day than nPOMCWT controls to obtain food and did so with an increased rate of bar pressing, decreased duration of work bouts, and decreased post-reinforcement pauses or the time elapsed between successive work bouts when food pellets were consumed (data not shown). Characterization of the meal patterns of hyperphagic adult nPOMCKO mice showed that they consumed excess food during each meal throughout the circadian cycle. Moreover, the absence of a genotype difference in the meal number indicates that their increased food intake resulted exclusively from increased meal size. If anything, average meal number for the nPOMCKO mice tended to be lower than nPOMCWT mice. Two other prominent features of nPOMCKO mouse nocturnal meal patterns were the differences in meal and intermeal interval durations. The amount of time nPOMCKO mice took to complete meals was significantly shorter than nPOMCWT controls indicating that nPOMCKO mice ate at a faster rate. nPOMCKO mice compensated for their larger meals with significantly longer intermeal interval durations. Overall, juvenile nPOMCKO mice exhibited similar meal patterns to the cohort of older, substantially more obese adult nPOMCKO mice. Juvenile nPOMCKO mice not only ate larger meals than controls, their meal sizes were comparable to adult nPOMCKO meal sizes. As observed in adult nPOMCKO mice, there were no differences in meal number from nPOMCWT mice. The similarities extended into the pattern of sequential nocturnal meals. Regardless of age, the first meal of the night was shorter in nPOMCKO than in nPOMCWT mice. Average nPOMCKO meal sizes diverged from nPOMCWT mice by the fifth meal, and by the fourth meal in the young nPOMCKO mice. These differences resulted from a gradual decline in meal size over the nocturnal period by nPOMCWT mice in contrast to the more consistent, larger meal sizes of nPOMCKO mice. It is interesting that the rapidly growing juvenile and stably obese nPOMCKO mice ate the same amount of food pellets per day. Apparently feed efficiency increases in the older mutant mice possibly due to a combination of decreasing locomotor activity and metabolic rate.
An additional striking feature of the feeding behavior exhibited by the nPOMCKO mice was the reduced inter- and intra-mouse variation across sequential meals on single nights or between separate nights. This stereotypy was particularly evident in the tighter distribution of meal sizes around a median and the shorter meal duration of adult nPOMCKO mice due to reduced pauses between consecutive pellet deliveries compared to nPOMCWT mice. In fact, the more inflexible pattern of meal taking across the nocturnal period in nPOMCKO mice while their general locomotor activity decreased was first suggested in our analysis of feeding behavior of mice in the wheel running cages. The mechanism responsible for this feature of meal patterns and its relationship to the loss of central POMC peptide signaling is unknown.
Mice with other monogenic mutations leading to an obese phenotype have been shown to express similar meal patterns to the nPOMCKO mice. The closest parallel may be mice that are globally deficient in both melanocortin MC3 and MC4 receptors, which, like nPOMCKO mice, are significantly heavier than mice with deletions of the individual receptor subtypes and exhibit hyperphagia due predominantly to increased meal size (Atalayer et al., 2010). However, the phenotypic expression of hyperphagia and increased meal size by single mutant melanocortin MC4 receptor-deficient mice in operant chambers is highly dependent on the type of reinforcement schedule and unit cost for food. Progressive ratio schedules and relatively low unit costs favored hyperphagia and large meals (Atalayer et al., 2010; Vaughan et al., 2006) while the addition of separate foraging costs or higher fixed ratio unit costs blocked hyperphagia (Vaughan et al., 2005). These effects of melanocortins on meal size could be due to actions at melanocortin MC4 receptors in various brain locations including the paraventricular nucleus of the hypothalamus, the ventromedial hypothalamus, lateral hypothalamus, and brainstem nuclei (Adan et al., 2006; de Backer et al., 2010).
Mice homozygous for the ob gene variant are unable to express functional leptin (Campfield et al., 1995; Pelleymounter et al., 1995). Meal pattern analysis of ob/ob mice showed a similar nocturnal meal number to lean controls but a significantly increased meal size (Ho and Chin, 1988). The meal parameter values relied on a threshold intermeal interval of 12 minutes and a ‘complete meal’ definition, which similar to our study included both eating and drinking events. An independent meal pattern study in ob/ob mice that validated the use of a threshold intermeal interval on the inclusion of the behavioral satiety sequence also concluded that the increased food intake of the obese mice was due to their eating larger, but less frequent meals (Strohmayer and Smith, 1987). Similarly, Vaughan and Rowland demonstrated that ob/ob mice continue to eat larger meals than lean wild-type mice in an operant protocol that simulates foraging by the inclusion of a procurement lever in addition to a fixed ratio consummatory lever (Vaughan and Rowland, 2003).
The similarities in the respective meal patterns of ob/ob mice and nPOMCKO mice suggest the possibility that the primary causal factor behind both is a disruption of central POMC neuron signaling. Leptin receptors are expressed on POMC neurons in the hypothalamic arcuate nucleus and the nucleus tractus solitarius in the brainstem (Ellacott et al., 2006) and leptin increases action potentials in POMC neurons (Cowley et al., 2001). Dysfunction of POMC signaling should decrease the sensitivity of these mice to peripheral signals responsible for meal termination. Consistent with this model, the selective expression of leptin receptors from an adenoviral vector in the arcuate nucleus of fak/fak leptin-receptor deficient rats effectively reduced meal size and enhanced CCK-induced satiety (Morton et al., 2005). However, the compensatory increase in the time between meals exhibited by nPOMCKO mice suggests that intermeal satiety was intact and not regulated by POMC.
Alternatively, and/or concomitantly, POMC deficiency may increase appetitive motivation. POMC is known to be centrally involved in a number of appetitive behaviors including grooming, sex and feeding (Spruijt et al., 1992; Van der Ploeg et al., 2002). Several studies have implicated POMC in drug reward (Alvaro et al., 1997; Alvaro et al., 1996; Alvaro et al., 2003; Hsu et al., 2005). Furthermore, melanocortin receptors are expressed in several brain regions important to natural and drug reward (Adan and Gispen, 1997; Alvaro et al., 1996) and as noted above melanocortin MC4 receptor-deficient mice reached higher break-points under a progressive ratio schedule to obtain food pellets, suggesting the mutant mice had greater motivation to eat (Vaughan et al., 2006).
POMC is also the precursor for the endogenous opioid peptide beta-endorphin. Beta-endorphin 1–31 is a potent agonist at mu and delta opioid receptor subtypes and its pharmacological administration increases feeding, particularly of highly palatable foods (Glass et al., 1999). However, additional posttranslational processing of beta-endorphin 1–31 to N-acetylated beta-endorphin 1–27 and 1–26 negates the opioid stimulatory action on food intake (Plum et al., 2009). Interestingly, mice that express a mutant allele of Pomc encoding a selective carboxyl truncation of POMC that blocks the production of all forms of beta-endorphin peptides causes a mild obesity syndrome despite normal translation of melanocortin peptides (Appleyard et al., 2003). Therefore the extreme hyperphagia and altered meal patterns of nPOMCKO mice may be secondary to the loss of both melanocortin and opioid peptides and their interaction with reward and homeostatic brain circuits.
In conclusion, the results of this study provide new details about the effects of neuronal POMC deficiency on the development of meal pattern phenotype. The primary findings were that adult nPOMCKO mice ate significantly more food in less time than controls, exhibiting compensatory increases in the duration of their inter-meal interval durations. Juvenile nPOMCKO mice shared elements of the aberrant meal pattern phenotype seen in adults by eating significantly larger meals with a trend towards longer inter-meal interval durations, while having average nocturnal meal durations comparable in length to nPOMCWT mice. It remains to be determined whether the aberrant meal pattern phenotype of nPOMCKO mice is primarily the result of desensitization to food satiation signals, an enhancement of appetitive and consumatory drive, or some combination of mechanisms.
Acknowledgments
This work was supported by National Institutes of Health grants T32 NS007466 (C.D.R) and R01 DK066604 (M.J.L.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The authors thank Dr. Eric Zorrilla for useful discussions and insights regarding the application of a drinking-explicit model of meal definition to meal pattern analysis in mice and Dr. Michael Hayward for his practical assistance and suggestions to implement the final operant paradigm.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adan RA, Gispen WH. Brain melanocortin receptors: from cloning to function. Peptides. 1997;18:1279–1287. doi: 10.1016/s0196-9781(97)00078-8. [DOI] [PubMed] [Google Scholar]
- Adan RA, Tiesjema B, Hillebrand JJ, la Fleur SE, Kas MJ, de Krom M. The MC4 receptor and control of appetite. Br J Pharmacol. 2006;149:815–827. doi: 10.1038/sj.bjp.0706929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvaro JD, Tatro JB, Duman RS. Melanocortins and opiate addiction. Life Sci. 1997;61:1–9. doi: 10.1016/s0024-3205(97)00029-5. [DOI] [PubMed] [Google Scholar]
- Alvaro JD, Tatro JB, Quillan JM, Fogliano M, Eisenhard M, Lerner MR, Nestler EJ, Duman RS. Morphine down-regulates melanocortin-4 receptor expression in brain regions that mediate opiate addiction. Mol Pharmacol. 1996;50:583–591. [PubMed] [Google Scholar]
- Alvaro JD, Taylor JR, Duman RS. Molecular and behavioral interactions between central melanocortins and cocaine. J Pharmacol Exp Ther. 2003;304:391–399. doi: 10.1124/jpet.102.040311. [DOI] [PubMed] [Google Scholar]
- Appleyard SM, Hayward M, Young JI, Butler AA, Cone RD, Rubinstein M, Low MJ. A role for the endogenous opioid beta-endorphin in energy homeostasis. Endocrinology. 2003;144:1753–1760. doi: 10.1210/en.2002-221096. [DOI] [PubMed] [Google Scholar]
- Atalayer D, Robertson KL, Haskell-Luevano C, Andreasen A, Rowland NE. Food demand and meal size in mice with single or combined disruption of melanocortin type 3 and 4 receptors. Am J Physiol Regul Integr Comp Physiol. 2010;298:R1667–1674. doi: 10.1152/ajpregu.00562.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campfield LA, Smith FJ, Guisez Y, Devos R, Burn P. Recombinant mouse OB protein: evidence for a peripheral signal linking adiposity and central neural networks. Science. 1995;269:546–549. doi: 10.1126/science.7624778. [DOI] [PubMed] [Google Scholar]
- Challis BG, Coll AP, Yeo GS, Pinnock SB, Dickson SL, Thresher RR, Dixon J, Zahn D, Rochford JJ, White A, Oliver RL, Millington G, Aparicio SA, Colledge WH, Russ AP, Carlton MB, O’Rahilly S. Mice lacking pro-opiomelanocortin are sensitive to high-fat feeding but respond normally to the acute anorectic effects of peptide-YY(3–36) Proc Natl Acad Sci U S A. 2004;101:4695–4700. doi: 10.1073/pnas.0306931101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collier G. An ecological analysis of motivation. Academic Press; New York; London: 1980. [Google Scholar]
- Cone RD. Anatomy and regulation of the central melanocortin system. Nat Neurosci. 2005;8:571–578. doi: 10.1038/nn1455. [DOI] [PubMed] [Google Scholar]
- Cowley MA, Smart JL, Rubinstein M, Cerdan MG, Diano S, Horvath TL, Cone RD, Low MJ. Leptin activates anorexigenic POMC neurons through a neural network in the arcuate nucleus. Nature. 2001;411:480–484. doi: 10.1038/35078085. [DOI] [PubMed] [Google Scholar]
- de Backer MW, la Fleur SE, Brans MA, van Rozen AJ, Luijendijk MC, Merkestein M, Garner KM, van der Zwaal EM, Adan RA. Melanocortin receptor-mediated effects on obesity are distributed over specific hypothalamic regions. Int J Obes (Lond) 2010 doi: 10.1038/ijo.2010.169. [DOI] [PubMed] [Google Scholar]
- DeBoer MD, Marks DL. Therapy insight: Use of melanocortin antagonists in the treatment of cachexia in chronic disease. Nat Clin Pract Endocrinol Metab. 2006;2:459–466. doi: 10.1038/ncpendmet0221. [DOI] [PubMed] [Google Scholar]
- Ellacott KL, Cone RD. The role of the central melanocortin system in the regulation of food intake and energy homeostasis: lessons from mouse models. Philos Trans R Soc Lond B Biol Sci. 2006;361:1265–1274. doi: 10.1098/rstb.2006.1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellacott KL, Halatchev IG, Cone RD. Characterization of leptin-responsive neurons in the caudal brainstem. Endocrinology. 2006;147:3190–3195. doi: 10.1210/en.2005-0877. [DOI] [PubMed] [Google Scholar]
- Glass MJ, Billington CJ, Levine AS. Opioids and food intake: distributed functional neural pathways? Neuropeptides. 1999;33:360–368. doi: 10.1054/npep.1999.0050. [DOI] [PubMed] [Google Scholar]
- Halford JC, Wanninayake SC, Blundell JE. Behavioral satiety sequence (BSS) for the diagnosis of drug action on food intake. Pharmacol Biochem Behav. 1998;61:159–168. doi: 10.1016/s0091-3057(98)00032-x. [DOI] [PubMed] [Google Scholar]
- Harkin A, O’Donnell JM, Kelly JP. A study of VitalView for behavioural and physiological monitoring in laboratory rats. Physiol Behav. 2002;77:65–77. doi: 10.1016/s0031-9384(02)00810-7. [DOI] [PubMed] [Google Scholar]
- Haskell-Luevano C, Chen P, Li C, Chang K, Smith MS, Cameron JL, Cone RD. Characterization of the neuroanatomical distribution of agouti-related protein immunoreactivity in the rhesus monkey and the rat. Endocrinology. 1999;140:1408–1415. doi: 10.1210/endo.140.3.6544. [DOI] [PubMed] [Google Scholar]
- Ho A, Chin A. Circadian feeding and drinking patterns of genetically obese mice fed solid chow diet. Physiol Behav. 1988;43:651–656. doi: 10.1016/0031-9384(88)90221-1. [DOI] [PubMed] [Google Scholar]
- Hsu R, Taylor JR, Newton SS, Alvaro JD, Haile C, Han G, Hruby VJ, Nestler EJ, Duman RS. Blockade of melanocortin transmission inhibits cocaine reward. Eur J Neurosci. 2005;21:2233–2242. doi: 10.1111/j.1460-9568.2005.04038.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huszar D, Lynch CA, Fairchild-Huntress V, Dunmore JH, Fang Q, Berkemeier LR, Gu W, Kesterson RA, Boston BA, Cone RD, Smith FJ, Campfield LA, Burn P, Lee F. Targeted disruption of the melanocortin-4 receptor results in obesity in mice. Cell. 1997;88:131–141. doi: 10.1016/s0092-8674(00)81865-6. [DOI] [PubMed] [Google Scholar]
- Meguid MM, Laviano A, Rossi-Fanelli F. Food intake equals meal size times mean number. Appetite. 1998;31:404. doi: 10.1006/appe.1998.0209. [DOI] [PubMed] [Google Scholar]
- Morton GJ, Blevins JE, Williams DL, Niswender KD, Gelling RW, Rhodes CJ, Baskin DG, Schwartz MW. Leptin action in the forebrain regulates the hindbrain response to satiety signals. J Clin Invest. 2005;115:703–710. doi: 10.1172/JCI200522081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelleymounter MA, Cullen MJ, Baker MB, Hecht R, Winters D, Boone T, Collins F. Effects of the obese gene product on body weight regulation in ob/ob mice. Science. 1995;269:540–543. doi: 10.1126/science.7624776. [DOI] [PubMed] [Google Scholar]
- Plum L, Lin HV, Dutia R, Tanaka J, Aizawa KS, Matsumoto M, Kim AJ, Cawley NX, Paik JH, Loh YP, Depinho RA, Wardlaw SL, Accili D. The obesity susceptibility gene Cpe links FoxO1 signaling in hypothalamic pro-opiomelanocortin neurons with regulation of food intake. Nat Med. 2009;15:1195–1201. doi: 10.1038/nm.2026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richard CD. Behavioral Phenotyping Meal Patterns In Mus Musculus: Validation, Testing and Application Department of Behavioral Neuroscience. Oregon Health & Science University; Portland, OR: 2008. [Google Scholar]
- Smart JL, Tolle V, Low MJ. Glucocorticoids exacerbate obesity and insulin resistance in neuron-specific proopiomelanocortin-deficient mice. J Clin Invest. 2006;116:495–505. doi: 10.1172/JCI25243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spruijt BM, van Hooff JA, Gispen WH. Ethology and neurobiology of grooming behavior. Physiol Rev. 1992;72:825–852. doi: 10.1152/physrev.1992.72.3.825. [DOI] [PubMed] [Google Scholar]
- Strohmayer AJ, Smith GP. The meal pattern of genetically obese (ob/ob) mice. Appetite. 1987;8:111–123. doi: 10.1016/s0195-6663(87)80004-1. [DOI] [PubMed] [Google Scholar]
- Tolle V, Low MJ. Melanocortins and the control of body weight. Cambridge University Press; Cambridge: 2008. [Google Scholar]
- Van der Ploeg LH, Martin WJ, Howard AD, Nargund RP, Austin CP, Guan X, Drisko J, Cashen D, Sebhat I, Patchett AA, Figueroa DJ, DiLella AG, Connolly BM, Weinberg DH, Tan CP, Palyha OC, Pong SS, MacNeil T, Rosenblum C, Vongs A, Tang R, Yu H, Sailer AW, Fong TM, Huang C, Tota MR, Chang RS, Stearns R, Tamvakopoulos C, Christ G, Drazen DL, Spar BD, Nelson RJ, MacIntyre DE. A role for the melanocortin 4 receptor in sexual function. Proc Natl Acad Sci U S A. 2002;99:11381–11386. doi: 10.1073/pnas.172378699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughan C, Moore M, Haskell-Luevano C, Rowland NE. Food motivated behavior of melanocortin-4 receptor knockout mice under a progressive ratio schedule. Peptides. 2006;27:2829–2835. doi: 10.1016/j.peptides.2006.07.008. [DOI] [PubMed] [Google Scholar]
- Vaughan CH, Moore MC, Haskell-Luevano C, Rowland NE. Meal patterns and foraging in melanocortin receptor knockout mice. Physiol Behav. 2005;84:129–133. doi: 10.1016/j.physbeh.2004.10.016. [DOI] [PubMed] [Google Scholar]
- Vaughan CH, Rowland NE. Meal patterns of lean and leptin-deficient obese mice in a simulated foraging environment. Physiol Behav. 2003;79:275–279. doi: 10.1016/s0031-9384(03)00094-5. [DOI] [PubMed] [Google Scholar]
- Yaswen L, Diehl N, Brennan MB, Hochgeschwender U. Obesity in the mouse model of pro-opiomelanocortin deficiency responds to peripheral melanocortin. Nat Med. 1999;5:1066–1070. doi: 10.1038/12506. [DOI] [PubMed] [Google Scholar]
- Zorrilla EP, Inoue K, Fekete EM, Tabarin A, Valdez GR, Koob GF. Measuring meals: structure of prandial food and water intake of rats. Am J Physiol Regul Integr Comp Physiol. 2005;288:R1450–1467. doi: 10.1152/ajpregu.00175.2004. [DOI] [PubMed] [Google Scholar]