Abstract
The PI3K/Akt/mTOR pathway mediates multiple myeloma (MM) cell proliferation, survival, and development of drug resistance, underscoring the role of mTOR inhibitors such as rapamycin with potential anti-MM activity. However, recent data demonstrate a positive feedback loop from mTOR/S6K1 to Akt, whereby Akt activation confers resistance to mTOR inhibitors. We confirmed that suppression of mTOR signaling in MM cells by rapamycin was associated with upregulation of Akt phosphorylation. We hypothesized that inhibiting this positive feedback by a potent Akt inhibitor perifosine would augment rapamycin-induced cytotoxicity in MM cells. Perifosine inhibited rapamycin-induced p-Akt, resulting in enhanced cytotoxicity in MM.1S cells even in the presence of IL-6, IGF-1 or bone marrow stromal cells. Moreover, rapamycin induced autophagy in MM.1S MM cells as evidenced by electron microscopy and immunocytochemistry, was augmented by perifosine. Combination therapy increased apoptosis detected by Annexin/PI analysis and caspase/PARP cleavage. Importantly, in vivo antitumor activity and prolongation of survival in a MM mouse xenograft model after treatment was enhanced with combination of nab-rapamycin and perifosine. Utilizing the in silico predictive analysis we confirmed our experimental findings of this drug combination on PI3K, Akt, mTOR kinases, and the caspases. Our data suggests that mutual suppression of the PI3K/Akt/mTOR pathway by rapamycin and perifosine combination induces synergistic MM cell cytotoxicity, providing the rationale for clinical trials in patients with relapsed / refractory MM.
Keywords: myeloma, Akt, mTOR, apoptosis, autophagy
INTRODUCTION
Multiple myeloma (MM) is a bone marrow (BM) cancer driven by the interaction between clonal plasma cells and the BM microenvironment (1, 2). Among the major pathways mediating cytokine-induced MM cell growth and survival, PI3K/Akt/mTOR kinase cascade plays a cardinal role in cell proliferation, survival and development of drug resistance (3-5). Cytokine-induced activation of Akt results in various down-stream anti-apoptotic effects via BAD and forkhead transcription factor (FKHR) phosphorylation and inhibition of the catalytic subunit of caspase-9. Besides its direct anti-apoptotic effects, p-Akt promotes growth and survival via phosphorylation of glycogen synthase kinase (GSK)-3β and mammalian target of rapamycin (mTOR). Moreover, Akt-induced activation of mTOR, enables mRNA translation through the activation of P70S6 kinase and the inhibition of 4E-BP1, a translational repressor of mRNAs. Consequently Akt which is constitutively activated in MM patient cells and correlates with advanced stage and poor prognosis (6), represents a rational target for novel therapeutics.
Identifying mTOR as a key kinase downstream of Akt led to the prediction that rapamycin, a universal inhibitor of mTORC1-dependent S6K1 phosphorylation may be useful in the treatment of MM (7-9). In vitro and in vivo preclinical studies have demonstrated anti-MM activity of rapamycin and its analogs (CCI-779 and RAD001) (10-14). First-generation mTOR inhibitors when used as single agents have demonstrated only modest efficacy in clinical trials (15-17), resulting in attempts to define mechanisms underlying rapamycin resistance. A growing body of evidence supports the hypothesis that resistance to rapamycin results from a strong positive feedback loop from mTOR/S6K1 to Akt, resulting in Akt activation (18-20). Indeed immunohistochemical analysis of paired tissue biopsies, before and after treatment with rapamycin-derivatives, revealed that non-responders frequently develop increased p-Akt, supporting the view that increased intra-tumoral phosphorylation of Akt mediates rapamycin resistance (21, 22).
The low response rate observed in many tumor types to rapamycin-derivatives led to two strategies to overcome rapamycin resistance. First, the implementation of nano-particle albumin-bound (nab) technology to augment rapamycin delivery to tumor tissue (23, 24). Second, combination strategies such as rapamycin with lenalidomide with the ability to overcome the protective effects of growth factors in the tumor milieu are in use (10).
Given that mTOR inhibitors induce PI3K/Akt activity in MM cells (25), we have examined the utility of adding an Akt inhibitor to overcome mTOR resistance and have also taken the advantage of nano-particle technology with nab-rapamycin. To date, the best-characterized and most developed clinical inhibitor of Akt is the novel alkylphospholipid, perifosine (26, 27). We first confirmed that suppression of mTOR signaling by rapamycin was associated with upregulation of Akt activation. We therefore inquired whether perifosine could: (i) inhibit rapamycin-induced p-Akt; (ii) augment rapamycin-induced cytotoxicity in vitro; and (iii) translate into enhanced in vivo anti-tumor activity when used with the nab-based rapamycin (ABI-009). Our data suggests that rapamycin-induced cytotoxicity was predominantly triggered as a consequence of autophagy in MM cells. The combination of rapamycin and perifosine resulted in 2 cell death-inducing events: autophagy and apoptosis. Furthermore, the combination of nab-rapamycin and perifosine resulted in significant antitumor activity in an in vivo human MM cell xenograft murine model. Finally, utilizing the in silico predictive analysis based on a systems biology approach (28, 29) we confirmed our experimental findings regarding the biological effects of this drug combination. These studies therefore provide the preclinical rationale for combination clinical trials in patients with MM.
MATERIALS AND METHODS
Cell culture and reagents
MM derived cell lines
Dexamethasone (Dex) –sensitive (MM.1S) MM cell line was provided by Dr. Steven Rosen (Northwestern University, Chicago, IL). The INA-6 cell line was kindly provided by Dr Martin Gramatzki (University of Erlangen-Nuernberg, Erlangen, Germany). OPM1 cell line was provided by Dr P. Leif Bergsagel (Mayo Clinic, Scottsdale, AZ). All MM cell lines were cultured in RPMI-1640 containing 10% fetal bovine serum (FBS; Sigma Chemical, St Louis, MO), 2 M L-glutamine, 100 U/mL penicillin, and 100 g/mL streptomycin (GIBCO, Grand Island, NY). Generation of bone marrow stroma cells (BMSCs) from BM specimens from MM patients obtained after appropriate IRB approved informed consent has been previously described (10). Once confluent, the cells were trypsinized and passaged as needed. BMSC were incubated in 96 well culture plates (approximately 5000 to 10,000 BMSC/well) for 24 hours, MM.1S cells were then added to the wells (2 × 10,000 cells/well) and incubated with media alone, rapamycin, perifosine, or combination for 48 hours at 37°C at the specified concentrations.
Rapamycin
Rapamycin was obtained from Calbiochem (San Diego, CA).
Perifosine
Perifosine (NSC 639966), a synthetic substituted heterocyclic alkylphospholipid, was provided by Keryx Biopharmaceuticals (New York, NY).
nab-rapamycin
nab-rapamycin (ABI-009) was provided by Abraxis Bioscience LLC (Los Angeles, CA).
Akti-½
Akti-½ was procured from Calbiochem (San Diego, CA).
Cell viability and proliferation assays
Colorimetric assay
Colorimetric assays were performed to assay drug activity. Forty eight hour cultures were pulsed with 10 μL of 5 mg/mL 3- (4,5-dimethylthiazol-2-yl) -2,5-diphenyl tetrasodium bromide (MTT; Chemicon International Inc, Temecula, CA) to each well, followed by incubation at 37°C for 4 hours, and addition of 100 μL isopropanol with 0.04 HCl. Absorbance readings at a wavelength of 570 nm (with correction using readings at 630 nm) were taken on a spectrophotometer (Molecular Devices Corp., Sunnyvale, CA).
Proliferation assay
DNA synthesis was measured by tritiated thymidine uptake [3H-TdR] (Perkin Elmer, Boston, MA) as previously described (10). Briefly, MM.1S cells (2-3 × 10.000 cells/well) were incubated in 96-well culture plates alone or in co-culture with BMSCs, recombinant IL-6 (10 ng/mL) or IGF-1 (50 ng/mL) in the presence of media or varying concentrations of rapamycin, perifosine, or combination for 48 hours at 37°C.
Immunoblotting
MM cells were harvested and whole-cell lysates were subjected to sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to nitrocellulose membrane (Bio-Rad Laboratories, Hercules, CA), as described previously (10). The antibodies used for immunoblotting included: anti–phospho (p)-Akt (Ser473), anti-Akt, anti–phospho (p)-P70S6K, anti-P70S6K, anti-GAPDH, anti–caspase-8, anti–caspase-3, anti-caspase-9, anti-PARP, and anti-tubulin (Cell Signaling Technology Inc., Beverly, MA, USA).
Detection of apoptosis by Annexin V/PI staining
Detection of early apoptotic cells was performed with the annexin V-PI detection kit (Immunotech/Beckman Coulter). Briefly, 106 MM cells were exposed for 24-48 hours to rapamycin (10 nM), perifosine (5 uM), or combination, washed and then incubated in the dark at room temperature with annexin V-FITC and PI for 15 minutes. Annexin V+PI- apoptotic cells were enumerated using the Epics flow cytometer. Cells that were annexin V-FITC1 positive (with translocation of phosphatidylserine from the inner to the outer leaflet of the plasma membrane) and PI negative (with intact cellular membrane) were considered as early apoptotic cells, while positivity for both annexin V-FITC1 and PI was associated with late apoptosis or necrosis.
Immunocytochemical detection of LC3
MM.1S cells were cultured in the presence of media, 10 nM rapamycin, 5 uM perifosine, or combination for 3 hours at 37°C, and cytospins were prepared. Cells were fixed in 4% paraformaldehyde. The anti-LC3 polyclonal antibody (MBL I.C.) was diluted with PBS at 1:100 and incubated with cells overnight at 4°C. FITC-conjugated anti-rabbit IgG at 1:100 dilutions was added for 1 hour at 4°C, then DAPI containing mounting medium and cover slips added promptly. Samples were observed by fluorescence microscopy and digitally photographed.
Electron microscopy
MM.1S cells were cultured in the presence of media or 10 nM rapamycin, 5 uM perifosine, or combination for 3 and 16 hours at 37°C. Cells were collected and fixed with 2.0% paraformaldehyde/2.5% EM grade glutaraldehyde in 0.1 M sodium cacodylate buffer (pH 7.4) at 37°C. After fixation, samples were placed in 2% osmium tetroxide in 0.1 M sodium cacodylate buffer (pH 7.4), dehydrated in a graded series of ethyl alcohol, and embedded in resin. Ultrathin sections were cut and placed on formvar-coated slot copper grids. Sections were then counterstained with uranyl acetate and lead citrate, and viewed with a TecnaiTM G2 Spirit Bio TWIN electron microscope. Digital images were acquired with an AMT 2k CCD camera (direct magnification 1400X and 6800X).
In silico study
In silico study was performed using the iC-PHYS™ Oncology Technology (Cellworks Group Inc (CWG), India) (30, 31). The iC-PHYS™ Oncology platform consists of a dynamic representation of the signaling pathways underlying tumor physiology at the bio-molecular level. All the key relevant proteins and associated gene and mRNA transcripts with regard to tumor related signaling are comprehensively included in the system with their relationship quantitatively represented. The modeling of time dependent changes in the fluxes of the constituent pathways has been carried out utilizing modified Ordinary Differential Equations (ODE) and Mass Action Kinetics. The state of the system was set to simulate late tumor stage. The drug concentrations used in the model is assumed to be post ADME (Absorption, Distribution, Metabolism, Excretion). The bottom layer is the computational backplane which enables the system to be dynamic and computes all the mathematics in the middle layer. The Oncology platform is ported to iC-PHYS™ and is simulated so that all the molecules attain the control steady state values (~by 1 × 105 seconds), following which the triggers are introduced in to the system. This leads to a phase of disease progression and the model stabilizes at steady disease levels by 2 × 105 seconds.
In initial conditions, the model simulated the kinetic interactions of the PI3K/Akt/mTor interactome based on proteomic data characterizing the pathophysiology of late stage cancer disease. Rapamycin: 10 nM; Ki: 1e-2, perifosine: 5 uM; Ki: 3.79e-1 uM, and their combination were tested on the system to observe the consequent effects on mTOR, p-Akt, and caspases levels.
MM xenograft murine model
The in vivo anti-MM activity of both single agent nab-rapamycin, perifosine, and the combination of nab-rapamycin and perifosine treatment was evaluated in CB-17 severe combined immunodeficient (SCID) mice obtained from Charles River Laboratories (Wilmington, MA). Housed and monitored in the Animal Research Facility at the Dana-Farber Cancer Institute (DFCI), mice were subjected to animal studies according to the protocols approved by the Animal Ethics Committee. Forty male 5-6 week old mice were irradiated (2 Gy [200 rad]) using cesium 137 (137Cs) -irradiator source); 24 hours after irradiation 2.5 × 106 MM.1S cells suspended in 100 μL of RPMI medium were inoculated subcutaneously. When tumors were measurable, mice were randomly assigned into cohorts (10 mice per cohort) receiving nab-rapamycin (10 mg/kg by tail vein injections on days 1, 3, 5 weekly for 4 weeks), perifosine (125 mg/kg orally by gavage on day 5 weekly for 4 weeks), or both (nab-rapamycin on day 1, 3, 5 with perifosine added at day 5 weekly, for 4 weeks). Control mice (n=10) were administered vehicles: PBS orally and 0.9% sodium chloride by tail vein on the same schedule as the combination. Animals were monitored for body weight and tumor volume by caliper measurements every alternate day. Tumor volume was estimated using the following formula: ½ × (length) × (width) 2. Animals were euthanized in accordance with institutional guidelines by CO2 inhalation in the event of tumor size > 2cm or major compromise in their quality of life, due to tumor ulceration. Survival was evaluated from the first day of treatment until death. Tumor growth was evaluated using caliper measurements from the first day of treatment until day of first sacrifice, which was day 33 for controls, day 47 for nab-rapamycin-treated, day 47 for perifosine-treated and day 89 for combination-treated cohorts.
Immunohistochemical staining
Immunohistochemical staining was performed using the standard avidin-biotin complex-peroxidase method on formalin-fixed, paraffin embedded tissue sections of tumor excised from xenografts following one week treatment with either nab-rapamycin (10mg/kg by tail vein thrice weekly), perifosine (125 mg/kg orally by gavage once a week), both, or control vehicles. Tumor sections were stained with anti–phospho (p)-Akt (Ser473), or cleaved caspase-3 antibody (Cell Signaling Technology Inc.), or LC3 antibody APG8a N-term (Abgent, Inc., San Diego, CA, USA), or were subjected to TUNEL staining.
Statistical analysis
All in vitro experiments were performed in triplicate and repeated at least 3 times; a representative experiment was selected for figures. Statistical significances of differences were determined using Student t test, with minimal level of significance P < 0.05. All statistical analysis of the in vivo data was determined using GraphPad prism software (GraphPad Software, Inc. San Diego, CA, USA). Synergism was determined by using the Chou-Talalay method (32).
RESULTS
Rapamycin induces p-Akt in MM cells, while perifosine inhibits p-Akt
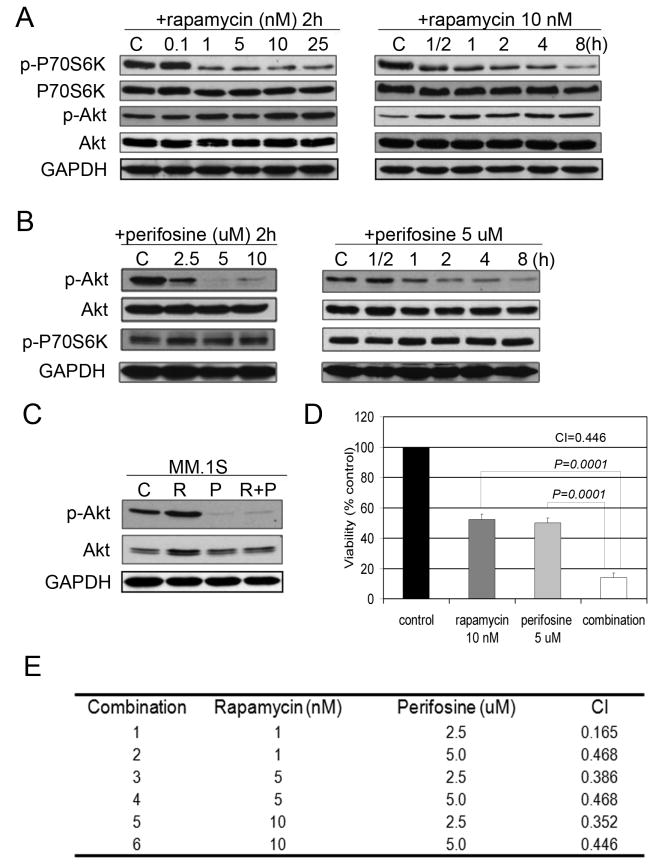
To confirm the effects of rapamycin signaling on MM cells, MM.1S cells were exposed to increasing concentrations of rapamycin for 2 hours. Rapamycin treatment resulted in a dose-dependent decrease of p-P70S6K. This was accompanied by an increase in phosphorylation of Akt at Ser473, starting at doses as low as 1 nM. Inhibition of p-P70S6K and activation of p-Akt were observed as early as 30 min after exposure of MM cells to rapamycin indicating that suppression of p-P70S6K and activation of Akt are early, concurrent, and lasting effects induced by rapamycin in MM.1S cells (Figure 1A).
Fig. 1. Rapamycin induces p-Akt in MM cells, while perifosine inhibits Akt.
(A) MM.1S cells were incubated with culture media or rapamycin (0.1-25 nM) for 2 hours, or rapamycin (10 nM) for the indicated periods. Whole-cell lysates were subjected to western blotting using anti-p-P70S6K, anti-P70S6K, anti-p-Akt, or anti-Akt antibodies.
(B) MM.1S cells were incubated for 2 hours with culture media, or perifosine (2.5-10 uM), or perifosine (5 uM) for the indicated time points. Whole-cell lysates were subjected to western blotting using anti-p-P70S6K, anti-P70S6K, anti-p-Akt, or anti-Akt antibodies.
(C) MM.1S cells were cultured for 6 hours in control media (C), rapamycin 10 nM (R), perifosine 5 uM (P), or rapamycin and perifosine (R+P). Whole-cell lysates were subjected to Western blotting using anti-p-Akt and anti-Akt antibodies.
(D) MM.1S cells were cultured for 48 hours in control media, rapamycin, perifosine, or their combination as indicated. Cytotoxicity was assessed by MTT assay.
(E) MM.1S cells were cultured at varying concentrations of rapamycin (1, 5, 10 nM) with perifosine (2.5, 5 uM) and cytotoxicity evaluated by MTT assay at 48 hours. Combination index (CIs) was calculated based upon the isobologram generated using the Chou-Talalay method.
We next examined the effects of perifosine on mTOR/Akt signaling in MM cells. MM.1S cells were cultured for 2 hours in the presence of increasing doses of perifosine (2.5–5 uM). Because perifosine was able to completely inhibit Akt phosphorylation at 5 uM, we next performed a time course (0.5 to 8 hours) to examine the effects of perifosine (5 uM) on Akt and P70S6K phosphorylation. Our data (Figure 1B) demonstrates that perifosine inhibited Akt, without exhibiting evident effects on P70S6K phosphorylation in a dose- and time-dependent manner.
We next incubated MM.1S cells with rapamycin (10 nM), perifosine (5 uM), or the combination for the specified times to study effects on cell signaling and cytotoxicity. As shown in Figure 1C, rapamycin treatment resulted in increased p-Akt, which was overcome by the combination as early as 6 hours, associated with enhanced cytotoxicity at 48 hours (Figure 1D).
To determine whether rapamycin effects were cell line specific we tested other MM cell lines. Our data demonstrates activation of Akt in OPM1, OPM2, and U266 MM cells in the presence of rapamycin (10 nM) at 6 hours. Similar to MM1.S cells, the combination of rapamycin and perifosine abrogated Akt phosphorylation in OPM1, OPM2, and U266 cells and resulted in enhanced cytotoxicity with the combination treatment in all 3 MM cell lines (supplementary data). Furthermore, 48 hour co-culture of MM.1S cells with rapamycin (10 nM) and the selective Akt kinase inhibitor Akti-½ (1.25 uM) potentiated rapamycin-induced cytotoxicity (supplementary data), confirming the enhanced cytocidal effect with dual mTOR and p-Akt inhibition.
Using Chou-Talalay method, we examined possible additive or synergistic anti-proliferative effects of rapamycin and perifosine following 48 hours treatment in MM.1S cells. Doses below IC50 for rapamycin (1-10 nM) and perifosine (2.5-5 uM) were used for combination studies. Combination index (CI) ranged from 0.468 to 0.165, suggesting synergistic growth inhibitory activity (Figure 1E).
Rapamycin and perifosine overcome the growth and survival advantage conferred by IL-6, IGF-1 and BMSCs in MM.1S cells
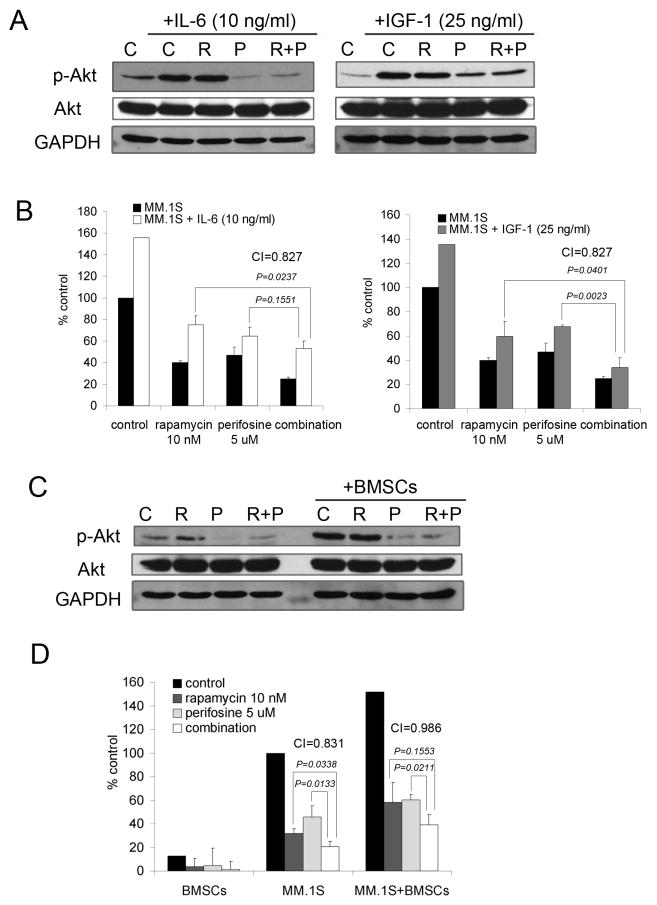
Because of the critical role played by BMSCs and cytokines such as IL-6 and IGF-1 on the growth and survival of MM cells and their impact on the PI3K/Akt pathway in the context of drug resistance, we examined the effects of rapamycin and perifosine combination in the presence of cytokines and stroma. As shown in Figure 2A, IL-6 triggered Akt phosphorylation, which was inhibited when rapamycin and perifosine were combined. The suppression of p-Akt by rapamycin and perifosine after IGF-1 stimulation was not as robust, suggesting that once activated IGF-1 signaling strongly upregulates Akt activity and there might be other signaling circuits contributing to p-Akt phosphorylation. However, when combined, rapamycin and perifosine increased the cytotoxicity in IL-6- and IGF-1-stimulated MM.1S cells (Figure 2B). Similarly, the combination was studied in the context of BMSCs. Adherence of MM.1S cells to BMSCs triggered upregulation of p-Akt; the combination blocked this effect, resulting in p-Akt downregulation (Figure 2C). Furthermore, the proliferative advantage conferred by BMSCs was overcome by the combination, as demonstrated by [3H]-thymidine uptake and confirmed by CI=0.986.
Fig. 2. Rapamycin and perifosine combination overcomes the growth and survival advantage in MM.1S cells conferred by IL-6 and IGF or BMSCs.
(A) MM.1S cells were cultured for 6 hours in control media (C), rapamycin 10 nM (R), perifosine 5 uM (P), or rapamycin and perifosine combination (R+P). Cells were then stimulated with IL-6 (10 ng/ml) or IGF-1 (25 ng/ml) for 10 min. Whole-cell lysates were subjected to Western blotting using anti-p-Akt and anti-Akt antibodies.
(B) MM.1S cells were cultured for 48 hours in control media, or rapamycin 10 nM, perifosine 5 uM, or their combination in the presence or absence of IL-6 (10 ng/ml) or IGF-1 (25 ng/ml). Cell proliferation was assessed by [3H]-thymidine uptake; data represent means (+/-SD) of triplicate cultures. Combination indices (CIs) were calculated based upon the isobologram generated using the Chou-Talalay method.
(C) MM.1S cells were cultured in control media (C), rapamycin 10 nM (R), perifosine 5 uM (P), rapamycin and perifosine combination (R+P) in the presence or absence of BMSCs. MM.1S cells were harvested, lysed, and subjected to Western blotting using anti-p-Akt and anti-Akt antibodies.
(D) MM.1S cells were cultured for 48 hours in control media, rapamycin 10 nM, perifosine 5 uM, or their combination in the presence or absence of BMSCs. Cell growth was assessed by [3H]-thymidine uptake; data represent means (+/-SD) of triplicate cultures. Combination indices (CIs) were calculated based upon the isobologram generated using the Chou-Talalay method.
Rapamycin-induced autophagy resulted in apoptosis when combined with perifosine
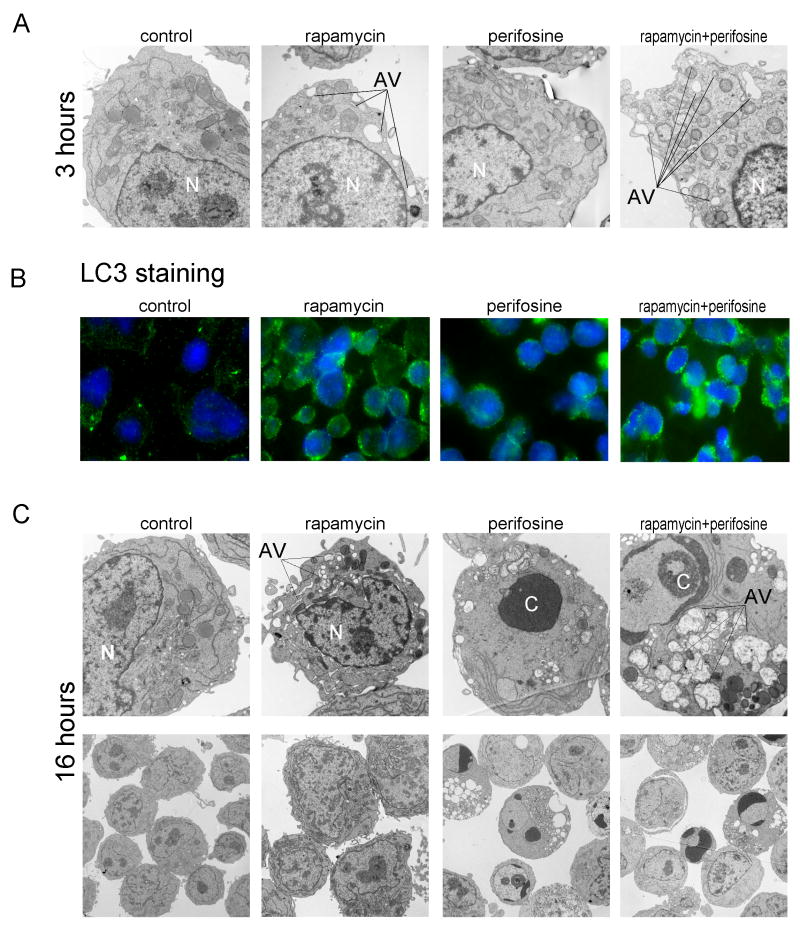
Since an increasing number of studies indicate that inhibition of mTOR results in induction of autophagy, we examined whether rapamycin treatment triggers autophagy in MM.1S cells. Because our data demonstrates rapamycin-induced downregulation of p-P70S6K as early as 30 min suggesting rapid mTOR inhibition, we first determined whether rapamycin treatment triggered early autophagy. Second, because of p-Akt’s ability to disinhibit mTOR (33), we hypothesized that inhibition of rapamycin-induced p-Akt activity by the combination of rapamycin and perifosine might facilitate initiation of autophagy. MM.1S cells were exposed to rapamycin (10 nM), perifosine (5 uM), the combination, or media alone for 3 hours, and ultrastructural morphology of the cells were analyzed by electron microscopy. As seen in Figure 3A, rapamycin-treated cells exhibited morphological changes characteristic of autophagy with presence of single-and double-membrane limiting vesicles sequestering the cytosolic material, which were not evident in perifosine-treated cells. These were more abundant when rapamycin and perifosine were combined. These microscopic observations suggested that rapamycin results in autophagy in MM.1S cells at early time points, and that rapamycin-induced autophagy was enhanced when rapamycin and perifosine were combined.
Fig. 3. Rapamycin-mediated autophagy resulted in apoptosis when combined with perifosine.
(A) Electronmicroscopy (direct mag: 4800x) of representative MM.1S cells cultured for 3 hours in the presence or absence of rapamycin 10 nM, perifosine 5 uM or their combination (N = nucleus and AV = autophagic vacuole). Note the abundance of membranous vacuoles in rapamycin and perifosine combination treated cells.
(B) Immunofluorescence analysis for LC3 localization in MM.1S cells grown in the absence or presence of single drugs or their combination for 3 hours. The control and perifosine-treated cells exhibited less intense diffuse distribution of LC3-associated green fluorescence compared to rapamycin- and combination-treated cells. Although rapamycin- and combination-treated cells displayed increased LC3-immunostaining, the punctate pattern was more evident when cells where treated with rapamycin in combination with perifosine.
(C) Representative transmission electron micrographs (direct magnification: 4800x upper panel and 1400x lower panel) depicting ultrastructures of MM.1S cells cultured in media or treated with rapamycin 10 nM, perifosine 5 uM, or their combination for 16 hours. While rapamycin-treated cells expressed multiple membranous vesicles (autophagy) with no evidence of nuclear “N” piknosis, the perifosine-treated cells manifested morphologic features of apoptosis (chromatin condensation “C”). Cells treated with the combination of rapamycin and perifosine developed features of both autophagy and apoptosis.
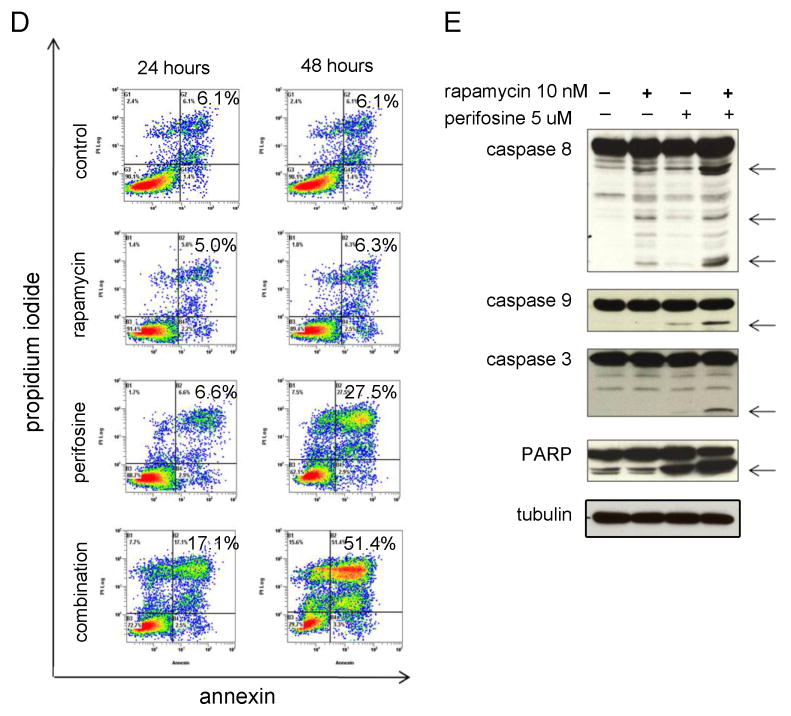
(D) FACS analysis for Annexin/PI. MM.1S cells were cultured in the presence or absence of rapamycin and perifosine, or their combination for 24 and 48 hours. Annexin/PI staining was then performed to assess apoptosis/necrosis. Annexin labeling (lower right quadrants) represents the population undergoing apoptosis. Annexin and PI double labeling (upper right quadrants) represent cells that have already died by apoptosis. Live cells are represented in the lower left quadrants. Increasing percentages of Annexin-positive and PI-positive cells with increasing exposure to rapamycin perifosine combination treatment is shown in the upper right quadrants.
(E) MM.1S cells were cultured for 24 hours in control media, rapamycin 10 nM, perifosine 5 uM, or rapamycin and perifosine combination. The cells were harvested, lysed, and subjected to Western blotting using anti-caspase 8, anti-caspase 3, anti-caspase 9, anti-PARP antibodies, and anti-tubulin.
To confirm rapamycin-induced autophagy and gain insights into the extent of increased autophagy triggered by the combination, we examined the effect of these drugs on localization of LC3, which serves as a marker of autophagy. We tested the effect of 3-hour treatment with rapamycin, perifosine, or both on localization of LC3 in MM.1S cells by immunofluorescence microscopy (Figure 3B). Untreated control cells exhibited diffuse distribution of LC3-associated green fluorescence, while rapamycin-treated MM.1S cells displayed a punctate pattern of LC3 immunostaining with increased fluorescence indicating co-localization with autophagosomes. Perifosine-treated cells expressed less intense and mostly perinuclear staining, while the combination demonstrated more focal LC3 green fluorescence predominantly in conglomerates, which suggests maturation of autophagic vacuoles.
Although autophagy is a response to various anticancer therapies, the extent to which autophagy contributes to cell death, known as type 2 or autophagic cell death, remains unclear. Shown in Figure 3C are morphological changes in MM.1S cells induced after 16 hours of treatment with rapamycin, perifosine, or the combination. Whereas untreated cells had normal nuclear and cytoplasmic morphology, rapamycin–treated cells developed typical features of autophagy with centrally condensed nuclear chromatin and numerous membranous vesicles. Higher magnification revealed double or multiple membrane boundaries surrounding cytoplasmic material and alternating with electron dense vesicles. Conversely, perifosine–treated cells manifested morphological characteristics of apoptosis, with nuclear condensation (pyknosis) and fragmentation (karyorhexis), cell shrinkage, plasma membrane blebbing, and vacuolization. Rapamycin and perifosine co-treatment resulted in morphological features of both autophagy and apoptosis, with evidence of double-membrane autophagolysosomes containing cytoplasmic fragments and disintegrated organelles typical of autophagy as well as condensation and margination of chromatin characteristic of apoptosis.
Given that rapamycin-perifosine co-treatment induced both apoptosis and autophagy features in MM.1S cells, we investigated the effect of this combination on apoptosis. As shown in Figure 3D-E, although rapamycin-induced caspase 8 cleavage, it did not result in significant apoptosis of MM cells at 24 or 48 hours. However perifosine resulted in apoptosis and necrosis of 30% of MM cells at 48 hours. The combination resulted in enhanced caspase-dependent apoptosis, manifested by increased caspase 3, 8, 9 and PARP cleavage (Figure 3E).
Since the combination of rapamycin and perifosine was able to activate both autophagy and apoptosis in MM cells, we next investigated whether these cell death-associated phenomena were interconnected and defined their role in rapamycin and perifosine combination-induced programmed MM cell death. We evaluated whether blocking of either apoptosis or autophagy would compromise rapamycin and perifosine combination induced cytotoxicity by assessing viability of MM.1S cells in the presence or absence of z-VAD-fmk or 3-MA pretreatment (supplementary data). Neither blockade of autophagy nor inhibition of apoptosis rescued MM cells from death induced by the combination, suggesting that cell death resulted once either mechanism was initiated.
In silico rapamycin and perifosine combination study confirms the Akt/mTOR kinase downregulation, and activated caspases
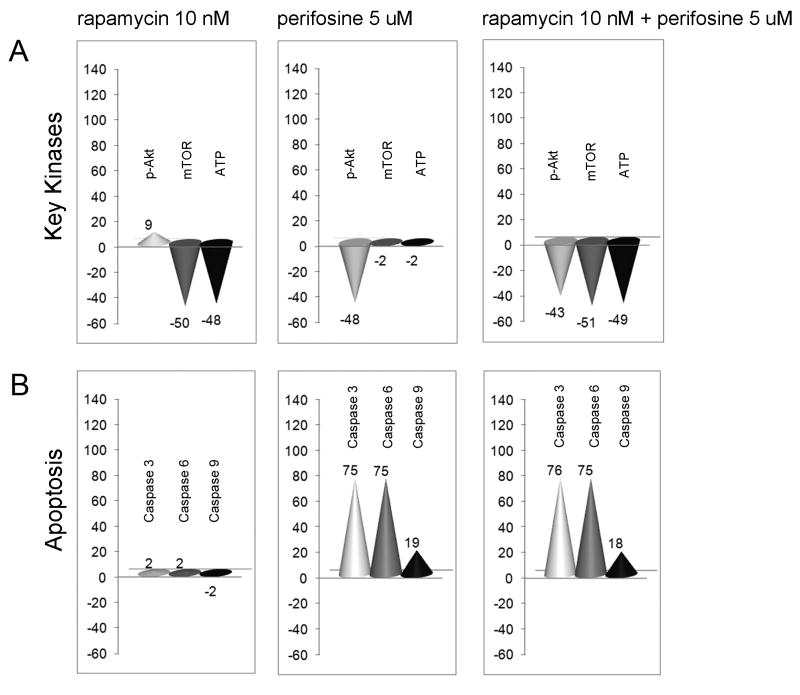
For a more comprehensive understanding of the cellular mechanisms underlying the synergism of this combination we proceeded with in silico tumor cell modeling. The objective was to analyze the predictive effects of the mTOR inhibitor rapamycin and the AKT inhibitor perifosine on the key kinases up-regulated in cancer and on other major end points for cancer phenotypes of proliferation, survival, and tumor microenvironment. The in silico study was performed on the iC-PHYS™ Oncology platform. Various clinically important markers were observed and their levels quantitatively compared under conditions of untreated control, rapamycin alone (10 nM), perifosine alone (5 uM), or the combination.
The key marker values are presented as the percentage difference between control versus each drug alone or the combination (Figure 4). The in silico study confirmed that rapamycin-induced mTOR/ATP inhibition associates with upregulated p-Akt. As expected, perifosine alone reduced Akt activity, but did not have any effect on mTOR kinase level. Meanwhile, the combination decreased both Akt and mTOR kinases (Figure 4A). Rapamycin alone had no effect on caspases activation, while perifosine, as expected, increased the activity of caspase 3, 6, 9, and the combination ultimately resulted in cumulative signaling effects (Figure 4B).
Fig. 4. Rapamycin and perifosine combination effects on major cancer phenotypes by in silico analysis.
(A-B) Quantitative in silico analysis of late stage cancer tumor cell model under conditions of treatment in terms of key targeted kinases (A) and caspases (B) levels presented as percentage difference between disease versus rapamycin 10 nM, perifosine 5 uM, or rapamycin and perifosine combination.
Effects of nab-rapamycin and perifosine alone or in combination on MM tumor growth in vivo
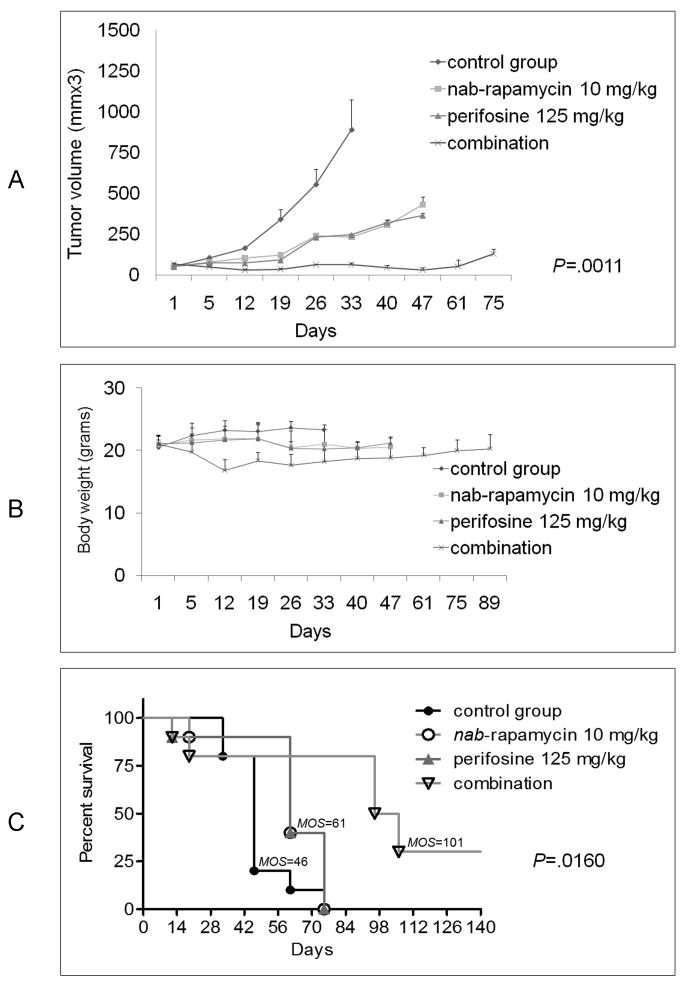
We finally sought to establish whether our in vitro observations would translate to anti-MM activity in vivo using our MM murine xenograft model. Due to the poor water solubility of rapamycin, we studied nab-rapamycin as a promising candidate for our in vivo MM studies. We first evaluated the toxicity and anti-MM activity of nab-rapamycin treatment for 4-weeks in our MM xenografts SCID mouse model. Both intravenous daily (30 mg/kg/day) and 3x weekly (20 mg/kg/day and 40 mg/kg/day) administration of nab-rapamycin resulted in significant inhibition of MM tumor growth and increased the survival of animals (data not shown). To investigate whether combined treatment with nab-rapamycin and perifosine would augment the anti-MM activity of each agent alone, MM tumor bearing SCID mice were treated for 4 weeks with nab-rapamycin (10 mg/kg/day; n = 10 mice) by tail vein injections on days 1, 3 and 5 for 4 weeks, perifosine (125 mg/kg; n = 10 mice) via oral gavage on day 5 for 4 weeks, or combination (n = 10 mice), nab-rapamycin on days 1, 3, 5 and perifosine given on day 5, for 4 weeks). Combined treatment with nab-rapamycin and perifosine significantly inhibited the growth of MM cell xenografts compared to administration of solvent alone (Figure 5A). Although each drug as a single agent inhibited tumor expansion (P< 0.05), combined nab-rapamycin and perifosine induced tumor growth arrest, assessed by tumor growth inhibition index (TGI) of 90% at the end of treatment.
Fig. 5. Combination of nab-rapamycin and perifosine significantly inhibits MM cell growth in vivo.
(A-C) SCID mice were inoculated subcutaneously with 3×106 MM.1S cells in 100 uL RPMI medium. 40 tumor-bearing mice were randomly assigned to 4 groups and treated for 4 weeks with control-vehicle, nab-rapamycin 10 mg/kg i.v. qwx3, perifosine 125 mg/kg gavage qwx1, or the combination (perifosine administered on day 5 of every week, while nab-rapamycin was given thrice-weekly on days 1,3 and 5 for a period of 4 weeks). At 28 days the treatment was discontinued and mice monitored for tumor dynamics and body weight.
(A) nab-rapamycin and perifosine combination therapy triggered more potent inhibition of tumor growth than nab-rapamycin or perifosine alone (P≤ .0011).
(B) Weight was used to assess toxicity of treatment.
(C) Combination therapy markedly prolonged survival (P≤ .0016) compared to control animals treated with vehicle or single agents.
Moreover, at 5 week follow-up after completion of nab-rapamycin or perifosine treatment, tumors started to regrow as early as 2 weeks. In contrast, all mice treated with the combination had smaller tumors, suggesting that therapeutic effects were maintained even after treatment was terminated.
Toxicity observed with the combination of nab-rapamycin and perifosine was evidenced by 20% weight loss at day 12 after initiation of treatment, which reversed after completion of treatment (Figure 5B).
The control and treated animals were maintained for their natural life span or sacrificed in the presence of a very large (≥ 1000 mm3 tumor volume) or ulcerated tumor. A significant survival advantage was observed when nab-rapamycin was combined with perifosine, as shown in Figure 5C. At day 61 after the beginning of treatment, only 10% of the animals survived in the control group versus 40% in each single-drug treated groups; in contrast, 80% of the animals were alive in the combination-treated mice. Moreover, 80% of mice in the combination–treated arm were still alive at day 75 following treatment initiation. There were no survivors in the control or monotherapy cohorts.
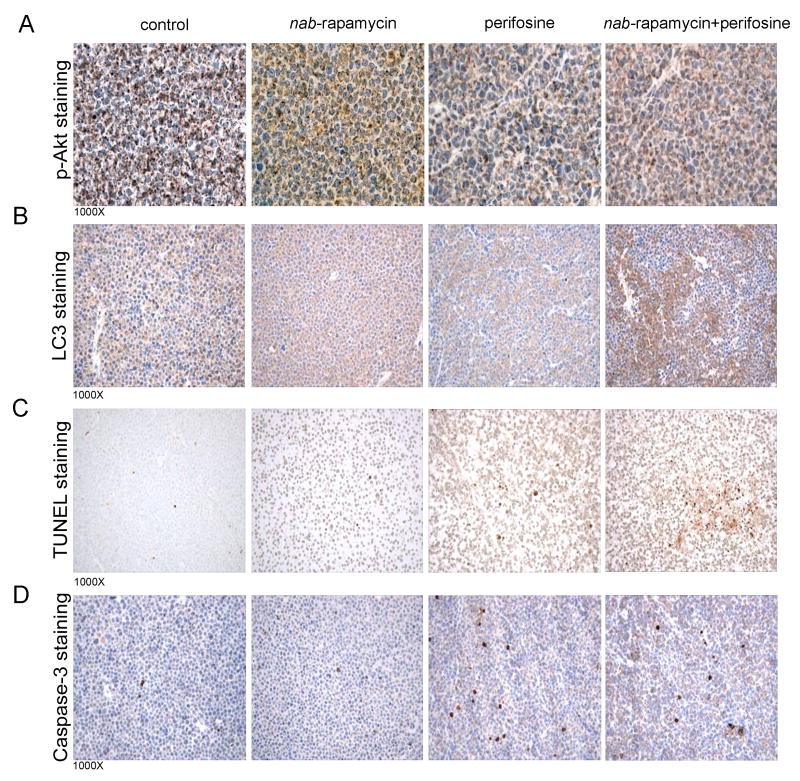
Given the therapeutic efficacy of nab-rapamycin and perifosine combination in our in vivo MM model, we next examined the associated histological events. Four mice were subjected to a similar in vivo study; mice were sacrificed and tumors collected after 1 week-treatment. As seen in Figure 6A, nab-rapamycin induced p-Akt in tumor tissue, which was inhibited when nab-rapamycin was combined to perifosine. LC3 immunohistochemical staining identified distinct patterns: LC-3 diffuse cytoplasmic expression (basal) in vehicle- and nab-rapamycin-treated tumors versus patchy-distribution staining in perifosine-treated tumor (Figure 6B). Interestingly, the combination-treated tumor showed increased LC3 staining in both diffuse and patchy patterns, along with more cleaved Caspase 3 and TUNEL-positive cells (Figure 6C-D). These findings therefore support our in vitro data showing amplification of both autophagy and apoptosis.
Fig. 6. Ex vivo immunohistochemistry staining for p-Akt, LC3, TUNEL, and cleaved caspase 3 in MM xenografts.
(A) p-Akt was detected by immunohistochemistry using paraffin-embedded sections of tumor excised from MM mouse xenografts. Increased staining for pAKT was observed in the nab-rapamycin and this was inhibited in perifosine-combination treated cases relative to untreated (control) or perifosine-treated mice.
(B) LC3 protein was detected by immunohistochemistry using paraffin-embedded sections of tumor excised from MM mouse xenografts. A more pronounced staining for LC3 was observed in the nab-rapamycin and perifosine-combination treated case relative to untreated (control), nab-rapamycin-, or perifosine-treated mice.
(C-D) Representative photomicrographs of TUNEL and cleaved Caspase 3 immunohistochemical staining of both tumors excised from MM xenograft mice treated with control vehicle-, nab-rapamycin-, perifosine-, or both nab-rapamycin- and perifosine. Greater Caspase-3 and TUNEL staining was observed in tumor from combination-treated relative to control and single agent-treated mice.
DISCUSSION
There is growing interest in targeting the PI3K/Akt/mTOR signaling cascade because of its critical role in the development of drug resistance. Indeed, the discovery that rapamycin specifically blocks mTOR suggested its potential in cancer therapy. However, the cytoreduction and G1 arrest triggered by rapamycin in vitro did not translate into significant single agent clinical anti-tumor activity, highlighting the need for studying combination and alternative strategies.
Several studies performed on various cancer types (lung cancer, glioma, colorectal carcinoma) including MM have characterized the molecular mechanisms of reduced sensitivity to rapamycin. Specifically, rapamycin induced activation of Akt follows the disinhibition of insulin-like growth factor receptor/insulin receptor substrate-1 (IGF-I/IRS-1) signaling subsequent to downregulation of p-P70S6K (20, 25). Additionally, a recent study in rhabdomyosarcoma cell lines and xenografts suggested that mTOR/S6K1 inhibition-mediated feedback activation of Akt can also occur via an IRS-1-independent mechanism (18) due to the ability of rapamycin insensitive mTORC2 (complexed with Rictor) to directly phosphorylate and activate Akt at serine 473, thus providing a level of additional positive feedback to the pathway.
Because mTOR may function both upstream and downstream of Akt, an agent directly targeting Akt, rather than targeting its precursors such as IGF-1/IRS-1 and PI3K, would more likely overcome reduced sensitivity to rapamycin. After confirming that suppression of mTORC1 signaling by rapamycin in MM cells was associated with upregulation of Akt phosphorylation; and that inhibition of p-p70S6K and activation of Akt occurred as concurrent, early and lasting effects; we used the Akt inhibitor perifosine for the direct inactivation of rapamycin-induced Akt. Consistent with previous data, perifosine resulted in inhibition of constitutive phosphorylation of Akt. Importantly, because the lowest dose (5 uM) at which perifosine exhibited strong p-Akt inhibition had minor effect on P70S6K phosphorylation status, we demonstrate that combining rapamycin with perifosine results in inhibition of rapamycin-induced Akt without influencing rapamycin-mediated mTORC1 signaling, thereby enhancing rapamycin-mediated cytotoxicity.
Since rapamycin does not cause apoptosis in MM at low concentrations, and a growing body of evidence indicates that rapamycin-induced antitumor effect is likely mediated through autophagy, we studied autophagy in MM cells to elucidate the mechanism of rapamycin induced anti-MM activity. Essential for maintaining cell autonomous survival in normal growing conditions, autophagy is necessarily self-limited; various intra- and extra-cellular stimuli enhance autophagic cell death (type II programmed cell death) if the stress is sustained. Through inhibition of mTOR, which suppresses autophagy, rapamycin activates the autophagic process. The observation that inhibition of autophagy by small interfering RNA (siRNA) directed against the autophagy-related gene beclin 1 abrogates rapamycin induced cytotoxicity, and that silencing of mTOR with siRNA increases the inhibitory effect of rapamycin by stimulating autophagy (34), suggest that rapamycin-induced autophagy is primarily an anti-tumor, rather than a cell protective effect. However, whether mTOR inhibitors promote autophagy and autophagic cell death in MM was previously unknown. Moreover, recent data have suggested that pro-autophagic rapamycin activity may prevent apoptosis (35). Here we have shown that autophagy occurs in MM cells shortly after rapamycin treatment, correlating with the inhibition of mTOR as an early- and low-dose response to rapamycin. Because the extent of autophagy increased in a dose- and time-dependent manner with no notable apoptosis, as assessed by Annexin/PI analysis, we suggest that rapamycin’s cytotoxic effect on MM cells is mainly mediated via autophagy rather than apoptosis. Since activated Akt has been shown to inhibit mTOR and suppress autophagy, we augmented rapamycin-induced autophagy by perifosine inactivation of Akt.
Data from several studies point out that autophagy and apoptosis may be interconnected in some settings, and even simultaneously regulated by the same trigger resulting in different cellular outcomes. Akt/mTOR is one of the few converging molecular links in both autophagy and apoptosis signaling. Our data suggests that rapamycin-induced autophagy in MM cells results in apoptosis when combined with perifosine. However, neither alternative, nor concomitant inhibition of apoptosis and autophagy rescued MM cell when rapamycin and perifosine were combined, suggesting a more complex signaling interaction underlying the synergistic effects of this promising anticancer drug combination.
To this end, we used the in silico predictive modeling system based on mathematical analysis of cellular networks provided by a systems biology approach. Multiscale in silico study of the predicted biology of rapamycin and perifosine combined effects on the tumor cell confirmed and complemented our in vitro experimental findings.
Although mTOR inhibitors such as rapamycin analogs CCI-779, RAD001 and AP23573 have shown preclinical promise, their roles as single agents in phase 2 and 3 studies have resulted in only modest responses. Pre-clinical studies of nab-rapamycin (ABI-009) in breast and colon cancer in in vivo models demonstrated anti-tumor activity, suggesting potential clinical utility. Moreover nab-rapamycin was well tolerated (no observed hypercholesterolemia and hypertriglyceridemia) overcoming the limitations posed by the poor water solubility of rapamycin (36). Specifically the binding of water-insoluble rapamycin to nanoparticle albumin permits albumin-mediated transcytosis of rapamycin by microvessel endothelial cells and the SPARC-albumin interaction may further increase accumulation of albumin-bound drug in the tumor. While the role of SPARC in MM is not fully understood, there is evidence that SPARC is upregulated in extramedullary tumor growth of MM (37, 38). Additionally, nab-rapamycin recently demonstrated promising data in phase I clinical trials in patients with advanced non-hematologic malignances prompting us to test nab-rapamycin in our studies.
We examined the effects of nab-rapamycin with the Akt inhibitor perifosine in vivo in our MM murine xenograft models, hypothesizing that anti-MM therapeutic effects would be enhanced both by dual inhibition of the Akt/mTOR pathway and also due to lower doses and better tolerability of nab-rapamycin. Our in vivo results demonstrated that combination treatment led to statistically significant MM tumor growth inhibition and increased survival in mice. Collectively our data suggest that mutual suppression of the PI3K/Akt/mTOR pathway by rapamycin and perifosine co-treatment induces both autophagy and apoptosis resulting in synergistic cytotoxicity in MM, providing the rationale for combination clinical trials in patients with MM.
Supplementary Material
Acknowledgments
We are grateful to all members of Leebow Institute of Myeloma Therapeutics and Jerome Lipper Multiple Myeloma Disease Center for collaboration and stimulating discussions throughout the preparation of this work. We thank Cellworks Group Inc. staff, in particular Ashish Sharma and Anay Talawdekar for the in silico study efforts.
Grant Support: ASCO CDA, LLS CDA and MMRF (NR)
References
- 1.Hideshima T, Bergsagel PL, Kuehl WM, Anderson KC. Advances in biology of multiple myeloma: clinical applications. Blood. 2004 Aug 1;104(3):607–18. doi: 10.1182/blood-2004-01-0037. [DOI] [PubMed] [Google Scholar]
- 2.Hideshima T, Mitsiades C, Tonon G, Richardson PG, Anderson KC. Understanding multiple myeloma pathogenesis in the bone marrow to identify new therapeutic targets. Nat Rev Cancer. 2007 Aug;7(8):585–98. doi: 10.1038/nrc2189. [DOI] [PubMed] [Google Scholar]
- 3.Hideshima T, Chauhan D, Richardson P, Anderson KC. Identification and validation of novel therapeutic targets for multiple myeloma. J Clin Oncol. 2005 Sep 10;23(26):6345–50. doi: 10.1200/JCO.2005.05.024. [DOI] [PubMed] [Google Scholar]
- 4.Hideshima T, Raje N. Akt as a therapeutic target in mutiple myeloma. Haematologica. 2007;92(suppl 2) abstract n S83. [Google Scholar]
- 5.Anderson KC. Future Perspectives in the Management of Myeloma. Haematologica. 2007;92(suppl 2) abstract n S134. [Google Scholar]
- 6.Hsu J, Shi Y, Krajewski S, et al. The AKT kinase is activated in multiple myeloma tumor cells. Blood. 2001 Nov 1;98(9):2853–5. doi: 10.1182/blood.v98.9.2853. [DOI] [PubMed] [Google Scholar]
- 7.Shi Y, Hsu JH, Hu L, Gera J, Lichtenstein A. Signal pathways involved in activation of p70S6K and phosphorylation of 4E-BP1 following exposure of multiple myeloma tumor cells to interleukin-6. J Biol Chem. 2002 May 3;277(18):15712–20. doi: 10.1074/jbc.M200043200. [DOI] [PubMed] [Google Scholar]
- 8.Shi Y, Gera J, Hu L, et al. Enhanced sensitivity of multiple myeloma cells containing PTEN mutations to CCI-779. Cancer Res. 2002 Sep 1;62(17):5027–34. [PubMed] [Google Scholar]
- 9.Hu L, Shi Y, Hsu JH, Gera J, Van Ness B, Lichtenstein A. Downstream effectors of oncogenic ras in multiple myeloma cells. Blood. 2003 Apr 15;101(8):3126–35. doi: 10.1182/blood-2002-08-2640. [DOI] [PubMed] [Google Scholar]
- 10.Raje N, Kumar S, Hideshima T, et al. Combination of the mTOR inhibitor rapamycin and CC-5013 has synergistic activity in multiple myeloma. Blood. 2004 Dec 15;104(13):4188–93. doi: 10.1182/blood-2004-06-2281. [DOI] [PubMed] [Google Scholar]
- 11.Stromberg T, Dimberg A, Hammarberg A, et al. Rapamycin sensitizes multiple myeloma cells to apoptosis induced by dexamethasone. Blood. 2004 Apr 15;103(8):3138–47. doi: 10.1182/blood-2003-05-1543. [DOI] [PubMed] [Google Scholar]
- 12.Yan H, Frost P, Shi Y, et al. Mechanism by which mammalian target of rapamycin inhibitors sensitize multiple myeloma cells to dexamethasone-induced apoptosis. Cancer Res. 2006 Feb 15;66(4):2305–13. doi: 10.1158/0008-5472.CAN-05-2447. [DOI] [PubMed] [Google Scholar]
- 13.Frost P, Moatamed F, Hoang B, et al. In vivo antitumor effects of the mTOR inhibitor CCI-779 against human multiple myeloma cells in a xenograft model. Blood. 2004 Dec 15;104(13):4181–7. doi: 10.1182/blood-2004-03-1153. [DOI] [PubMed] [Google Scholar]
- 14.Mitsiades N, McMullan C, Poulaki V, et al. The mTOR inhibitor RAD001 (everolimus) is active against multiple myeloma cells in vitro and in vivo [Google Scholar]
- 15.Easton JB, Houghton PJ. mTOR and cancer therapy. Oncogene. 2006 Oct 16;25(48):6436–46. doi: 10.1038/sj.onc.1209886. [DOI] [PubMed] [Google Scholar]
- 16.Faivre S, Kroemer G, Raymond E. Current development of mTOR inhibitors as anticancer agents. Nat Rev Drug Discov. 2006 Aug;5(8):671–88. doi: 10.1038/nrd2062. [DOI] [PubMed] [Google Scholar]
- 17.Guertin DA, Sabatini DM. Defining the role of mTOR in cancer. Cancer Cell. 2007 Jul;12(1):9–22. doi: 10.1016/j.ccr.2007.05.008. [DOI] [PubMed] [Google Scholar]
- 18.Wan X, Harkavy B, Shen N, Grohar P, Helman LJ. Rapamycin induces feedback activation of Akt signaling through an IGF-1R-dependent mechanism. Oncogene. 2007 Mar 22;26(13):1932–40. doi: 10.1038/sj.onc.1209990. [DOI] [PubMed] [Google Scholar]
- 19.Sun SY, Rosenberg LM, Wang X, et al. Activation of Akt and eIF4E survival pathways by rapamycin-mediated mammalian target of rapamycin inhibition. Cancer Res. 2005 Aug 15;65(16):7052–8. doi: 10.1158/0008-5472.CAN-05-0917. [DOI] [PubMed] [Google Scholar]
- 20.O’Reilly KE, Rojo F, She QB, et al. mTOR inhibition induces upstream receptor tyrosine kinase signaling and activates Akt. Cancer Res. 2006 Feb 1;66(3):1500–8. doi: 10.1158/0008-5472.CAN-05-2925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Witzig TE, Geyer SM, Ghobrial I, et al. Phase II trial of single-agent temsirolimus (CCI-779) for relapsed mantle cell lymphoma. J Clin Oncol. 2005 Aug 10;23(23):5347–56. doi: 10.1200/JCO.2005.13.466. [DOI] [PubMed] [Google Scholar]
- 22.Tabernero J, et al. A phase study with tumor molecular pharmacodynamic (MPD) evaluation of dose and schedule of the oral mTOR inhibitor everolimus (RAD001) in patients (pts) with advanced solid tumors. Proc Am Soc Clin Oncol 2005. 2005 [Google Scholar]
- 23.Trieu V, De T, Cordia J, et al. Preclinical pharmacokinetics, tissue distribution and antitumor activity of the intravenously administered mTOR inhibitior, nanoparticle albumin-bound (nab) rapamycin. Submitted to Cancer Chemotherapy and Pharmacology. 2008 [Google Scholar]
- 24.Hawkins MJ, Soon-Shiong P, Desai N. Protein nanoparticles as drug carriers in clinical medicine. Adv Drug Deliv Rev. 2008 May 22;60(8):876–85. doi: 10.1016/j.addr.2007.08.044. [DOI] [PubMed] [Google Scholar]
- 25.Shi Y, Yan H, Frost P, Gera J, Lichtenstein A. Mammalian target of rapamycin inhibitors activate the AKT kinase in multiple myeloma cells by up-regulating the insulin-like growth factor receptor/insulin receptor substrate-1/phosphatidylinositol 3-kinase cascade. Mol Cancer Ther. 2005 Oct;4(10):1533–40. doi: 10.1158/1535-7163.MCT-05-0068. [DOI] [PubMed] [Google Scholar]
- 26.Hideshima T, Catley L, Yasui H, et al. Perifosine, an oral bioactive novel alkylphospholipid, inhibits Akt and induces in vitro and in vivo cytotoxicity in human multiple myeloma cells. Blood. 2006 May 15;107(10):4053–62. doi: 10.1182/blood-2005-08-3434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.LoPiccolo J, Granville CA, Gills JJ, Dennis PA. Targeting Akt in cancer therapy. Anticancer Drugs. 2007 Sep;18(8):861–74. doi: 10.1097/CAD.0b013e3280cc2c6f. [DOI] [PubMed] [Google Scholar]
- 28.Aksenov SV, Church B, Dhiman A, et al. An integrated approach for inference and mechanistic modeling for advancing drug development. FEBS Lett. 2005 Mar 21;579(8):1878–83. doi: 10.1016/j.febslet.2005.02.012. [DOI] [PubMed] [Google Scholar]
- 29.Fitzgerald JB, Schoeberl B, Nielsen UB, Sorger PK. Systems biology and combination therapy in the quest for clinical efficacy. Nat Chem Biol. 2006 Sep;2(9):458–66. doi: 10.1038/nchembio817. [DOI] [PubMed] [Google Scholar]
- 30.Jagatha B, Mythri RB, Vali S, Bharath MM. Curcumin treatment alleviates the effects of glutathione depletion in vitro and in vivo: therapeutic implications for Parkinson’s disease explained via in silico studies. Free Radic Biol Med. 2008 Mar 1;44(5):907–17. doi: 10.1016/j.freeradbiomed.2007.11.011. [DOI] [PubMed] [Google Scholar]
- 31.Vali S, Mythri RB, Jagatha B, et al. Integrating glutathione metabolism and mitochondrial dysfunction with implications for Parkinson’s disease: a dynamic model. Neuroscience. 2007 Nov 23;149(4):917–30. doi: 10.1016/j.neuroscience.2007.08.028. [DOI] [PubMed] [Google Scholar]
- 32.Chou TC, Talalay P. Quantitative analysis of dose-effect relationships: the combined effects of multiple drugs or enzyme inhibitors. Adv Enzyme Regul. 1984;22:27–55. doi: 10.1016/0065-2571(84)90007-4. [DOI] [PubMed] [Google Scholar]
- 33.Sabatini DM. mTOR and cancer: insights into a complex relationship. Nat Rev Cancer. 2006 Sep;6(9):729–34. doi: 10.1038/nrc1974. [DOI] [PubMed] [Google Scholar]
- 34.Iwamaru A, Kondo Y, Iwado E, et al. Silencing mammalian target of rapamycin signaling by small interfering RNA enhances rapamycin-induced autophagy in malignant glioma cells. Oncogene. 2007 Mar 22;26(13):1840–51. doi: 10.1038/sj.onc.1209992. [DOI] [PubMed] [Google Scholar]
- 35.Ravikumar B, Berger Z, Vacher C, O’Kane CJ, Rubinsztein DC. Rapamycin pretreatment protects against apoptosis. Hum Mol Genet. 2006 Apr 1;15(7):1209–16. doi: 10.1093/hmg/ddl036. [DOI] [PubMed] [Google Scholar]
- 36.Trieu V. Preclinical evidence for the effectiveness of mTOR inhibitor, nanoparticle albumin-bound (nab®) rapamycin as an anticancer agent 20th EORTC-NCI-AACR Symposium (Molecular Targets and Cancer Therapeutics) 2008 [Google Scholar]
- 37.De Vos J, Thykjaer T, Tarte K, et al. Comparison of gene expression profiling between malignant and normal plasma cells with oligonucleotide arrays. Oncogene. 2002 Oct 3;21(44):6848–57. doi: 10.1038/sj.onc.1205868. [DOI] [PubMed] [Google Scholar]
- 38.Hedvat CV, Comenzo RL, Teruya-Feldstein J, et al. Insights into extramedullary tumour cell growth revealed by expression profiling of human plasmacytomas and multiple myeloma. Br J Haematol. 2003 Sep;122(5):728–44. doi: 10.1046/j.1365-2141.2003.04481.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.