Abstract
Proteins vital to presynaptic function are synthesized in the neuronal perikarya and delivered into synapses via two modes of axonal transport. While membrane-anchoring proteins are conveyed in fast axonal transport via motor-driven vesicles, cytosolic proteins travel in slow axonal transport; via mechanisms that are poorly understood. We found that in cultured axons, populations of cytosolic proteins tagged to photoactivable-GFP (PA-GFP) move with a slow motor-dependent anterograde bias; distinct from vesicular-trafficking or diffusion of untagged PA-GFP. The overall bias is likely generated by an intricate particle-kinetics involving transient assembly and short-range vectorial spurts. In-vivo biochemical studies reveal that cytosolic proteins are organized into higher-order structures within axon-enriched fractions that are largely segregated from vesicles. Data-driven biophysical modeling best predicts a scenario where soluble molecules dynamically assemble into mobile supra-molecular structures. We propose a model where cytosolic proteins are transported by dynamically assembling into multi-protein complexes that are directly/indirectly conveyed by motors.
Keywords: Cytosolic synaptic proteins, synapsin, CamKII, slow axonal transport, transport packets, cargo complexes, diffusion
INTRODUCTION
The vast majority of the proteins in a neuron are synthesized in cell-bodies and conveyed into axons and presynapses via axonal transport. Distal presynaptic boutons are critically dependent on axonal transport throughout life, and functional organization of the adult neuronal circuitry relies upon continued protein synthesis and transport (Kleim et al., 2003). Previous pulse-chase radiolabeling studies have shown that newly-synthesized proteins are conveyed via two distinct modes of transport. While proteins with membrane-spanning/anchoring domains are packaged into vesicles and conveyed via fast axonal transport at overall rates of 50-400 mm/day (0.5-4μm/s), cytosolic proteins lacking such domains are transported much more slowly in a transport-group historically called Slow Component-b (SCb), at rates of 1-10 mm/day (0.01-0.1 μm/s), reviewed in (Brown, 2003; Roy et al., 2005).
Of the 200+ cytosolic proteins that are conveyed in slow axonal transport, many are enriched at presynaptic terminals as shown by detailed radiolabeling studies (Garner and Mahler, 1987). Similar studies have also characterized the transport of two well-known presynaptic proteins - synapsin and CamKIIa - showing that after perikaryal synthesis, the vast majority of these synaptic proteins move with slow overall rates, whereas a smaller fraction (≈ 15%) is conveyed rapidly in fast axonal transport (Baitinger and Willard, 1987; Garner and Lasek, 1982; Lasek et al., 1984; Lund and McQuarrie, 2001, 2002; Paggi and Petrucci, 1992; Petrucci et al., 1991).
By their very nature, pulse-chase radiolabeling experiments offer a descriptive and indirect view of axonal transport and provide little insight into the mechanisms that generate this motion. Thus though the overall transport of these synaptic proteins was described decades ago, critical questions remain unanswered. How do inherently soluble proteins move in this slow, sustained manner? What is the underlying molecular basis of this movement? What (if any) is the role of diffusion in this process? What is the nature of the minor pool that is conveyed in fast axonal transport? To date, there is no evidence-based model that can provide the molecular details to adequately explain the slow sustained transit of soluble proteins that was seen in the classic radiolabeling studies decades ago.
To address these questions we developed an assay for cytosolic cargo transport in cultured neurons using photoactivable vectors, where we could visualize bulk cargo movement and particle-dynamics with high resolution. Coupled with in-vivo biochemical assays and data-driven biophysical modeling, we show that cytosolic synaptic proteins employ atypical transport strategies. These studies provide evidence for a detailed model that can explain the mechanistic logic behind the axonal transport of these cytosolic cargoes in neurons, providing insights into a long-standing scientific question.
RESULTS
Polarized bulk transport of cytosolic proteins in axons
To investigate bulk axonal transport of cytosolic protein populations we transfected cultured hippocampal neurons with synapsin (synapsin-Ia) and CamKIIa tagged to PA-GFP, selectively photoactivated discrete protein pools within the primary axon emerging from the soma (away from presynaptic boutons), and tracked the mobility of photoactivated cytosolic protein populations at various time compressions (fig. 1 and 2). We focused our studies on two cytosolic proteins enriched at synapses – synapsin and CamKIIa - as radiolabeling studies have established the overall transport of these proteins, showing that they are largely conveyed by slow axonal transport (Baitinger and Willard, 1987; Lund and McQuarrie, 2001, 2002; Petrucci et al., 1991). The GFP fusions of these synaptic proteins have been characterized in previous studies (Gitler et al., 2004; Sturgill et al., 2009) - also see Supplementary figure 1. Note that punctate particles are clearly visible both in axons expressing the fluorescent proteins as well as adjacent naive axons (Supplementary fig. 1B) suggesting that the fusion proteins generally mimic the behaviors of their in-situ counterparts. Figure 1A, B shows typical results from photoactivation experiments (also see Supplementary videos 1 and 2).
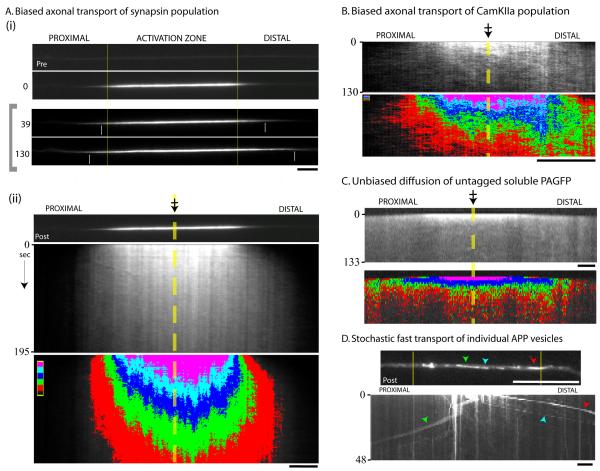
Figure 1. Axonal transport dynamics of cytosolic proteins.
(A) Neurons were co-transfected with PAGFP:synapsin (and soluble mRFP to identify transfected axons); a discrete region within the primary axon was photoactivated, and mobility of the photoactivated protein pool was tracked in living axons by time-lapse imaging. (i) Top: Representative example showing a transfected axon pre- and post- activation. The vertical yellow bars denote the boundaries of the photoactivated zone throughout the frames. Bottom: Selected frames from the time-lapse showing the anterograde migration of the photoactivated plume of fluorescence (time in seconds, left). The small white vertical bars within the images mark the approximate position of the ‘wave-fronts’. Note that the frames within the grey bracket (left) were linearly scaled to maximize the bit-depth within each image and highlight the leading edges of the fluorescent wave-fronts. (ii) Kymograph from the time-lapse above (dashed yellow line and crossed-arrow depicts center of the photoactivated-zone). In the lower panel, the grayscale values of the kymograph above were assigned five pseudo-colored intensity-bins (red: lowest intensity bin, pink: highest intensity bin above background), allowing clear visualization of the anterograde bias of the fluorescent plume. (B) A similar biased transit was seen for the cytosolic protein CamKIIa as shown in these representative images. (C) The movement of untagged PA-GFP was rapid, bidirectional and unbiased. (D) Using a similar photoactivation paradigm, PAGFP:APP vesicles stochastically departed the fluorescent pool over time. Green, blue and red arrowheads mark the position of individual photoactivated vesicles in the post-activation image (above) and the kymograph (below). Scale bars: lower right, elapsed time in seconds: left of kymograph.
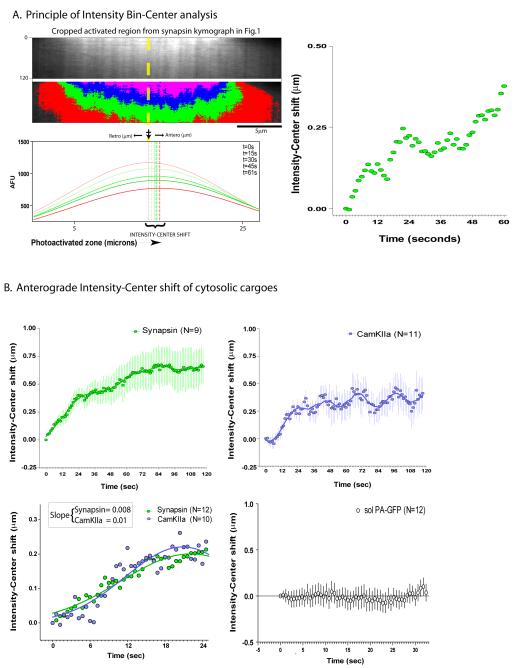
Figure 2. Quantitative strategy to analyze the bulk movement of photoactivated protein pools.
(A) Top panels show cropped kymographs from the photoactivated zone in figure 1. Each curve below represents average-intensity bins along a line-scan within the photoactivated axonal segment for a given frame (time, upper right key, Gaussian fits shown). Dashed vertical lines represent the position of the peak-value for each intensity curve (intensity-center). Note that there is a left to right (anterograde) shift in the positions of the peaks over time (intensity-center shift). The graph on the right is a plot of the intensity-center shift over time. (B) Quantitative data from multiple synapsin and CamKIIa experiments (mean ± SEM) showing anterograde intensity-center shifts for both protein populations. In the bottom panel the first few seconds after photoactivation was imaged with higher time-compressions (Gaussian fits shown). Predicted average rates of the overall synapsin and CamKIIa population in these experiments (derived from linear-regression slopes) are comparable to known overall rates of synapsin and CamKIIa seen in previous radiolabeling studies (see text). No shifts are seen with untagged PA-GFP (bottom right, mean ± SEM). Each X-axes tick represents an image-acquisition time-point in all graphs. Scale bars: lower right, elapsed time in seconds: left of kymograph.
The photoactivated axonal protein pool of synapsin and CamKIIa dispersed as a plume of fluorescence with a distinct anterograde bias, as shown in the representative kymographs (fig. 1A, B). This directional bias of fluorescence is unlikely to be a result of some non-specific bulk axonal “flow” that moves all soluble proteins in its wake, as there was no bias in the axonal dispersion of untagged PA-GFP which showed bi-directional rapid diffusion as expected (fig. 1C; also see Supplementary video 3 and 5). Also, the intensity-center analyses are not likely influenced by photobleaching as similar trends of intensity-center shifts were observed under imaging conditions that greatly minimized photobleaching (Supplementary fig. 2A, B). The transport behavior of cytosolic proteins is also very different from the fast component amyloid precursor protein (APP), where discrete photoactivated vesicles rapidly escaped the activated zone over time (fig. 1D; also see Supplementary video 4), in line with conventional stochastic motor-driven transport (Kaether et al., 2000). Similar results were also obtained with PA-GFP:synaptophysin (not shown).
Biased axonal transport of cytosolic cargoes at expected overall rates
If the biased anterograde migration of the synapsin and CamKIIa population in our studies is the visual correlate of the slow axonal transport seen in pulse-chase radiolabeling studies, the overall vectorial bias of the fluorescence pool in our experiments should be similar to the overall rates of the radiolabeled population. To address this we quantified the bulk movement of the entire photoactivated pool using ‘intensity-center shift’ analysis (fig. 2A). Briefly, the intensity-center in a given frame is a quantitative center of the distribution of binned fluorescence intensities along a line-scan within the photoactivated zone. A bulk vectorial movement of fluorescent molecules within the axon would lead to a corresponding shift in the intensity-center as well. Anterograde shifts were consistently seen in the photoactivated pools of both synapsin and CamKIIa, though there were differences in the overall kinetics as shown in figure 2B. Specifically, for CamKIIa, there was a slight lag in the initiation of intensity-center shift as well as a periodic variation that was more pronounced than that for synapsin. Note that there is an expected flattening of the wave after the initial rise as the fluorescent molecules leave the analyzed area (photoactivated zone) over time.
Despite these differences in the overall nature of the intensity-center shifts between synapsin and CamKIIa, there were similarities in their initial intensity-shifts suggesting possible commonalities between mechanisms transporting these cytosolic proteins – a notion supported by radiolabeling studies as well (Garner and Lasek, 1982). To investigate this in more detail we focused on the initial intensity-shift, imaging the activated zone with higher time-compression. Indeed there was some similarity in the overall transport behaviors of both proteins (fig. 2B, lower panel). Linear-regression slopes were 0.008 and 0.01 respectively, equivalent to predicted average rates of 0.008-0.01 μm/sec for the entire population. These data are comparable to known slow rates of synapsin and CamKIIa from in-vivo radiolabeling experiments- ≈0.01-0.03 μm/sec (Baitinger and Willard, 1987; Lund and McQuarrie, 2001; Petrucci et al., 1991), providing confidence in the validity of this assay in evaluating axonal transport of these cytosolic cargoes. Note that similar analysis of untagged soluble PA-GFP diffusion does not show any bias in its mobility (fig. 2B, lower right panel).
The anterograde bias of cytosolic cargoes is microtubule- and motor- dependent
The anterogradely biased transit of synapsin and CamKIIa, distinct from the bidirectional, unbiased movement of untagged soluble PAGFP in axons suggest that the movement is not a simple diffusive process. We further tested this notion by analyzing synapsin and CamKIIa transport in the presence of micro-molar amounts of the alkylating agent N-ethyl maleimaide (NEM) - a known inhibitor of kinesins, dyneins and myosins (Pfister et al., 1989). Most recently, NEM was used to inhibit molecular motors to dissect their role in mobilizing vesicles at synapses (Shakiryanova et al., 2005), and when perfused in squid axons, 1μM NEM inhibits bidirectional vesicular transport within minutes (Pfister et al., 1989). This transport inhibition is due to the action of alkylating sulfhydryl groups on motors that profoundly alters its interactions with ATP and microtubules, stalling vesicle-motor complexes (Pfister et al., 1989). Without knowledge of the specific anterograde and retrograde motor(s) moving the assortment of cytosolic proteins, NEM provides a useful tool to examine the role of molecular motors in generating the intensity-center shift of synapsin and CamKIIa in our imaging experiments.
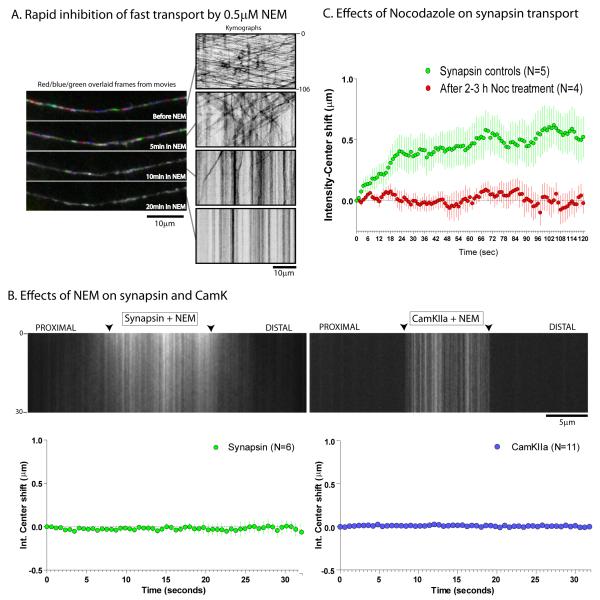
Upon adding 0.5μM NEM, there was a gradual perturbation of axonal transport of APP vesicles with a complete cessation of vesicular transport at 20 minutes (fig. 3A). Accordingly, for our experiments we visualized synapsin and CamKIIa transport after 10 minutes of NEM treatment. We found that such NEM treatment resulted in the accumulation of stationary puncta within axons with a dramatic inhibition of the anterogradely biased motion (fig. 3B). NEM-treatment did not lead to any discernable changes in the diffusion kinetics of untagged PAGFP (Supplementary fig. 2C, 2D). Addition of Nocodazole, a microtubule-depolymerizing agent also resulted in an inhibition of the anterograde bias (fig. 3C and Supplementary fig. 3A) as did the cellular metabolic poison (oxidative phosphorylation un-coupler) 2,4 Dinitrophenol (2,4 DNP) (Supplementary fig. 3B). Finally, co-expression of headless Kinesins-1A/1B/1C (also called Kif-5A/B/C), known to act in a dominant negative fashion to inhibit Kinesin-1 mediated transport (Kozielski et al., 1997; Uchida et al., 2009) also inhibited the anterograde bias of synapsin (Supplementary fig. 3C), further suggesting that the bias is dependent on the activity of motors.
Figure 3. Biased transport of cytosolic cargoes is distinct from untagged PAGFP and is motor-dependent.
(A) To determine the effects of NEM on generic motor-driven transport, neurons were transfected with APP:YFP and transport of APP vesicles was determined before and after adding 0.5μM NEM. Each panel on left is created by overlapping three consecutive time-lapse frames that were pseudo-colored red/blue/green respectively; before (top frame) and at incremental time-points after adding NEM. Corresponding kymographs from the movies are shown on the right. Note that the robust bidirectional vesicular transport was gradually inhibited with NEM-treatment. (B) Neurons transfected with PAGFP:synapsin or PAGFP:CamKIIa were incubated with 0.5mM NEM for 10 minutes and photoactivation experiments were performed as described in text. As shown in the representative kymographs (top), NEM treatment greatly inhibited the overall mobility of the photoactivated pool, leading to stalled fluorescent particles within axons and essentially eliminated the anterograde bias, as shown in the graphs below (mean ± SEM). Scale bars: lower right, elapsed time in seconds: right/left of kymograph. (C) A similar inhibition of the anterograde bias of synapsin was seen upon treatment of neurons with the microtubule-depolymerizing drug Nocodazole (mean ± SEM, p<0.0001, paired t-test).
Intricate particle-kinetics underlie the slow transport of cytosolic proteins
The above experiments show that populations of synapsin and CamKIIa move along axons with a motor-dependent anterograde bias. This movement seems different from fast transport, where individual vesicles are stochastically transported by molecular motors. What is the underlying molecular basis for this unconventional motion of cytosolic protein populations? We reasoned that the overall biased transit of these proteins is ultimately mediated by the movement of particles that are transport-competent. First, when neurons are stained for endogenous cytosolic synaptic proteins, particulate structures are seen within naive axons (Supplementary fig. 1A) and also see (Fletcher et al., 1991; Roy et al., 2007; Withers et al., 1997), suggesting that these are the native structural form of cytosolic proteins within axons. Second, particulate structures are also clearly present within photoactivated zones in our experiments (note vertical lines in kymographs, fig. 1A and elsewhere). Third, when the anterograde bias of the photo-activated pool was inhibited with NEM or Nocodazole, stalled particles are seen in axons (fig. 3B and Supplementary fig. 3A). Furthermore, upon carefully examining kymographs, we could occasionally pick out diagonal tracks suggestive of vectorial particle-motion (see kymographs in fig. 1, for example).
However our experiments designed to visualize bulk axonal transport of soluble proteins by maximally photo-activating synapsin and CamKIIa protein pools were not ideal for detecting the movement of individual particles. Accordingly we photoactivated smaller protein pools, reasoning that stochastic incorporation of fluorescent molecules on synapsin and CamKIIa particles would allow us to see their movement; adopting methods from the “speckle microscopy” field (Dorn et al., 2008). Indeed such methods revealed numerous mobile particles within the photoactivated zone as shown in the kymograph examples in Supplementary figure 4. These movements were surprisingly intricate, consisting of rapid assembly/disassembly and vectorial spurts of the speckles. Manual analyses of a subset of vectorial tracks from the kymographs show that the average velocities of synapsin and CamKIIa speckles are comparable to known rates of kinesins and dyneins (1.981 ± 0.14 μm/s and 1.931 ± 0.13 μm/s – mean ± SEM for anterogradely moving synapsin and CamKIIa respectively, N≈60).
Bimodal axonal transport of synapsin
Previous radiolabeling studies have shown that small fractions (≈15%) of somatically-synthesized cytosolic synaptic proteins are conveyed in fast axonal transport (Baitinger and Willard, 1987; Paggi and Petrucci, 1992; Petrucci et al., 1991). These data have always been puzzling, and the nature of this smaller, rapidly transported pool is poorly understood. To more closely simulate the radiolabeling paradigm, we photoactivated perikaryal PAGFP:synapsin and immediately thereafter imaged the emanating axon to track the migration of the photoactivated protein population from the soma into the axon (fig. 4A; also see Supplementary video 6). We reasoned that such experiments would photoactivate large protein pools and allow us to visualize both the slowly transported wave as well as potential persistent particles as they emerged into an axon devoid of background fluorescence.
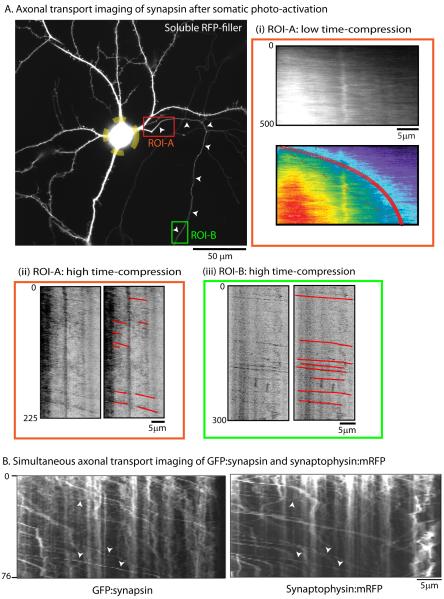
Figure 4. A subset of rapid and persistent synapsin particles in axons.
(A) Neurons were co-transfected with PA-GFP:synapsin and soluble mRFP (to identify morphology, image on left) and the cell-body was selectively photoactivated (dashed yellow circle marks the boundaries of photoactivation-zone; small arrowheads mark the emanating axon). Thereafter, the photoactivated protein pool was imaged as it entered the axon, using varying time-compressions in proximal (ROI-A, red box) and distal (ROI-B, green box) regions. Imaging of the proximal axon in ROI-A at low time-compressions (1 frame/5sec) revealed a slow, anterogradely moving plume of fluorescence matching the known kinetics of slow transport (kymographs in A-I, slope= 0.02, equivalent to average velocity of 0.02μm/sec). However, when the same ROI was imaged at a higher time compression (1 frame/0.75 sec), we also saw rapid, persistent particles emerging from the front of the slowly migrating fluorescence plume (kymographs in A-ii). This was further confirmed by subsequent imaging of a distal portion of the same axon (ROI-B) at the higher frame-rate, where many rapid and persistent particles were clearly seen, moving with an exclusively anterograde bias (kymographs in A-iii). Axonal transport of the fast-component protein APP using a similar paradigm is shown in Supplementary figure 5. (B) Simultaneous imaging of steady-state GFP:synapsin and synaptophysin:mRFP in thin distal axons revealed mostly persistent synapsin particles that co-localized with synaptophysin (arrowheads). Scale bars: lower right, elapsed time in seconds: left of kymograph.
Focusing on the proximal axonal region (fig. 4A, ROI-A) we saw a gradual migration of synapsin into the axon over time at rates closely resembling slow axonal transport [fig. 4A(i)-representative of 4 such experiments]; likely representing the slow transport of synapsin from the perikarya into axons. Next, after the slow moving front had entered the axon, we imaged the advancing synapsin wave-front within ROI-A at higher time-compressions [fig. 5A(ii)]. Surprisingly, we saw numerous particles emerging from the leading edge of the wave and move rapidly and persistently along the axon [fig. 5A(ii)]. Such persistent, anterogradely moving particles were also readily visible when we imaged a more distal region of the same axon [fig. 5A(iii), kymograph from ROI-B] (note that the persistently mobile particles in the distal axon originated in the cell-body as only the soma was photoactivated in these experiments). Imaging of the fast axonal transport protein APP using a similar experimental strategy gave entirely different results, where a deluge of photoactivated APP vesicles rapidly entered the axon a few seconds after perikarya photoactivation as expected (Supplementary fig. 5; representative of 4 such experiments, also see Supplementary video 7), and no slowly migrating wave was observed, as seen with synapsin. To determine if the persistent synapsin particles seen in the above experiments were associated with vesicular cargoes moving in fast axonal transport, we co-transfected neurons with GFP:synapsin-I (labeling all synapsin at steady-state) and synaptophysin:mRFP (transmembrane protein moving in fast transport) and simultaneously imaged both cargoes in thin distal axons using dual-cam imaging. We found that a large fraction of persistent synapsin particles were co-localized with synaptophysin as shown in figure 4B. Collectively the data show that while a large population of synapsin moves as a slowly-migrating wave (likely with intricate particle kinetics), a sub-population of somatically-derived synapsin associates with vesicles conveying other fast synaptic proteins and moves persistently. These data help reconcile the seemingly conflicting observations by live imaging that synapsin particles clearly move rapidly as “transport-packets” (Ahmari et al., 2000) and the fact that radiolabeling studies have established that the majority of synapsin is conveyed in slow axonal transport (Baitinger and Willard, 1987; Petrucci et al., 1991).
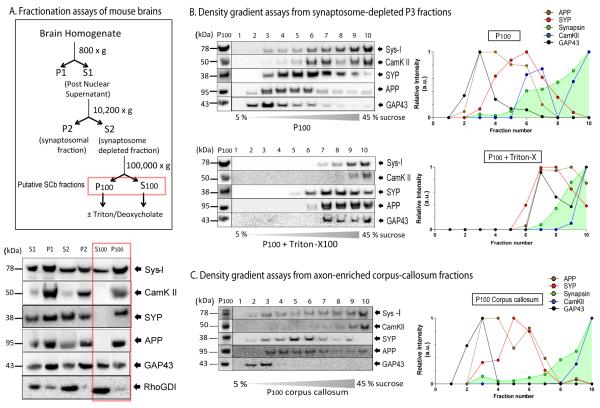
Figure 5. Biochemical analysis of synapsin and CamKII in-vivo from axon-enriched fractions.
(A) Mouse brains were homogenized and fractionated as outlined in the experimental strategy on the left, reasoning that axonal synapsin/CamKIIa cargoes would exist within P100 and S100 (derivates of S2, the synaptosome-depleted fractions, boxed in red). Fractions were blotted with antibodies to synapsin (Sys-I) and CamKII as well as the vesicular proteins synaptophysin (SYP), APP and GAP-43. The gel below shows that large proportions of these proteins were also present in the high-speed pellet (P100) indicating that they are not entirely soluble. Note that the presumptively diffusive signaling molecule RhoGDI remains largely in the supernate in such experiments. (B) Top: In density-gradient assays from the P100 fractions, large populations of synapsin and CamKII are found in the high-density fractions 7-10 (P100, top panel) distinct from vesicular proteins. Bottom: Treatment of P100 with Triton attenuated the associations of vesicular proteins with little effect on the higher-density fractions containing synapsin/CamKII. Densitometry analysis of the gels is shown on the right. (C) Similar supernate/pellet proportions and separation of cytosolic and vesicular proteins was observed in density-gradients from axon-enriched corpus-callosum fractions from mouse brains as well.
Evidence that cytosolic proteins exist as proteinaceous complexes in brains
The data above supports a model where the majority of cytosolic protein population organizes into particles that undergo slow transport via intricate kinetics. Particles are invariably seen upon immunostaining of cytosolic proteins in neurons, and (at least a fraction of them) likely represent the cargo structure moving in slow transport. What is the nature of these punctate structures and how are they being transported? One possibility is that they represent the association of synapsin and CamKIIa molecules with a proteinaceous particle or macromolecular complex that is transported by motors (Lasek et al., 1984). Another possibility is that these puncta represent the association of individual monomers with vesicles. Indeed association of both synapsin and CamK with synaptic vesicles is well-established (Takamori et al., 2006), and it is possible that they have transient vesicular associations in non-synaptic compartments as well.
To address the biochemical nature of synapsin and CamKII cargoes in mouse brains in-vivo we first used classical assays to isolate synaptosomal-rich and synaptosomal-depleted fractions (P2 and S2 respectively, see strategy in fig. 5A). We reasoned that the S2 fractions, largely free from the pre- and post-synaptic terminals (Dunkley et al., 2008; Whittaker, 1993) would likely contain the putative cargo elements transporting these proteins, and characterized the nature of synapsin and CamKIIa in these fractions. For these experiments, 4-weeks old mouse brains were homogenized under conditions that aim to preserve native interactions and separated into various soluble (S) and pellet (P) fractions (see methods for further details). If the entire synapsin and CamKII population was completely soluble in non-synaptic S2 fractions, one would expect that these proteins would exclusively migrate in the supernatant (S100) fractions. In line with that, we found that the small signaling molecule RhoGDI, previously used as a soluble marker in neurons (Kimura et al., 2005), is predominantly enriched in the S100 fractions (fig. 5A, bottom). Though the axonal transport of signaling molecules has not been rigorously evaluated by radiolabeling, it is generally believed that their intracellular motion is largely diffusive (Brangwynne et al., 2008; Lillemeier et al., 2001; Zeng et al., 2001). However, we found significant amounts of both synapsin and CamKII in the P100 pellet fractions (fig. 5A, below, see fractions within red box) indicating that fractions of these proteins in-vivo exist in a state that is not entirely soluble (Miller and Heidemann, 2008).
As vesicles are also present in the P100 fractions, we next asked if synapsin and CamKII are associated with vesicles in these fractions. To determine this we subjected the pellet fractions to sucrose-gradient floatation assays and probed them for synapsin and CamKII, as well as classic trans-membrane proteins APP and synaptophysin and a peripherally-associated membrane protein (GAP43) – all of which are conveyed in fast axonal transport. We found that while all vesicular proteins floated in lighter fractions (as expected), significant quantities of both synapsin and CamKII were present in the high-density fractions that were largely distinct (but partially overlapping) from the transmembrane proteins (fig. 5B, top). Also, while detergent-treatments disrupted the vesicular proteins, they had little effect on the higher-density fractions of the two cytosolic proteins (Fig. 5B, below and Supplementary fig. 6A). The separation of membranous and cytosolic proteins was also observed in density gradients from axon-enriched corpus callosum preparations from mouse brains (fig. 5C). In contrast, within the synaptosomal (P2) preparations, both synapsin and CamKII were largely (though not exclusively) associated with lighter fractions (Supplementary fig. 6B) and this association was disrupted by detergent treatment (Supplementary fig. 6C); suggesting that in synaptic domains, these proteins are largely associated with synaptic vesicles (as expected). The presence of synapsin and CamK in higher-density fractions within high-speed P100 pellets and their resistance to detergents further suggest that synapsin and CamK in these fractions are organized into proteinaceous complexes.
Data-driven biophysical simulation of cytosolic cargo transport
Next we generated a biophysical simulation model of synapsin transport driven by our imaging data to test specific scenarios that could not be readily addressed by experimentation. To simulate our photoactivation experiments we assumed an aqueous cylindrical environment (axon) containing hypothetical synapsin particles distributed within a central zone at time=0 (fig. 6A, green particles), mimicking the photo-activated pool of synapsin molecules in our imaging experiments immediately post-activation. At all times, each simulated synapsin particle was allowed to diffuse randomly within the axonal compartment with known diffusion coefficients of GFP:synapsin in axons (Tsuriel et al., 2006), and also collide with other intracellular components.
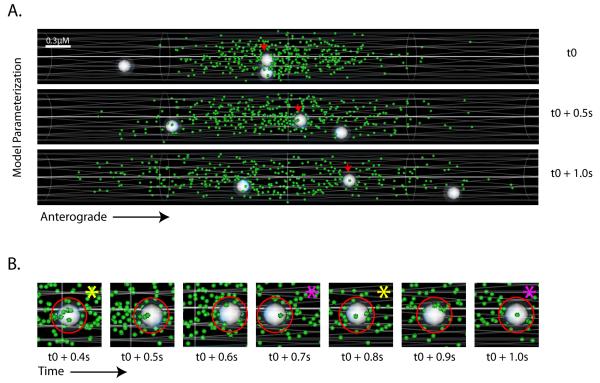
Figure 6. A biophysical simulation model of slow axonal transport.
(A) A simulation model was developed as described in the text, consisting of a hypothetical axon cylinder, synapsin particles (green) distributed in the central zone and vectorial ‘mobile-units’ (white spheres). Scale bar is shown in the upper-left and elapsed time to the right of the frames. Screen-shots from a simulated time-lapse show mobile-units traversing through a pool of synapsin particles; one anterogradely moving particle is marked with small red arrows (also see Supplementary video #7). (B) Dynamics of association/dissociation and clustering of synapsin particles on a mobile unit shown in representative zoomed screen-shots from a simulated time-lapse. The red circle represents the ‘dissociation radii’ around a mobile unit that restricts the movements of the synapsin particles that collide with the mobile-units (see text). Note the stochastic clustering and dispersion of synapsin particles upon the mobile unit in successive frames; marked by yellow and magenta asterisks respectively.
To further simulate our experimental data we allowed several hypothetical motor-driven “mobile-units” to traverse along the axon (white spheres in fig. 6A and B; also see Supplementary video 8). These mobile units were allowed to move persistently with a range of velocities similar to those detected by our SPT assay (≤3 μm/sec, only a few anterograde units shown in figure for simplicity), shooting through the cluster of synapsin particles in the model. Besides free diffusion, the synapsin particles within the axon were allowed to either a) randomly collide with vectorial mobile-units as they passed through, or b) specifically associate with mobile-units for user-defined times to simulate the clustering/association behavior of particles that we found in our imaging experiments (Supplementary fig. 4). Virtual kymographs and intensity-center shifts were generated from simulations (see methods for further details).
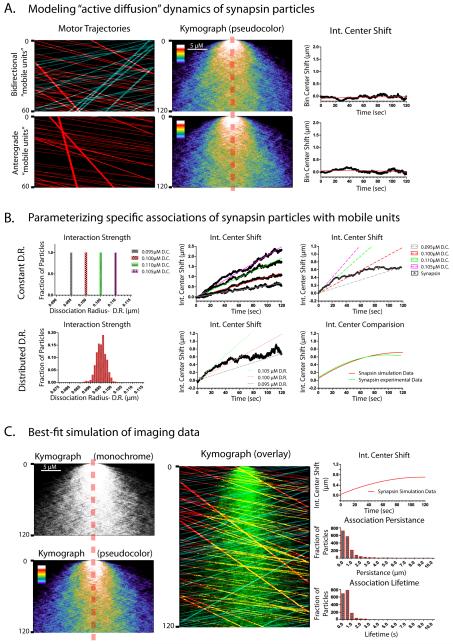
Three basic scenarios were simulated. 1) First we assumed that the synapsin molecules were only diffusing passively and randomly colliding with the bidirectional vectorial mobile-units (fig. 7A, kymographs- top panel). No significant intensity-center shift was noted under these conditions (fig. 7A, graph- top panel). 2) Even when retrogradely moving particles were completely eliminated from the simulation, there was no significant shift in the intensity-center (fig. 7A, bottom panel) indicating that non-specific movement within the axonal shaft, even when polarized, is insufficient to create a population shift in this model. Recent studies have shown that movement of motors/motor-driven cargoes can generate intracellular turbulence that can cause fluctuating motion of cytoskeletal polymers (Brangwynne et al., 2008; Brangwynne et al., 2007), and it is possible that such motion can also generate transport of cytosolic proteins. However these simulation data argue that the anterograde bias of synapsin seen in our experiments is unlikely to be generated simply by non-specific movement of other fast/persistent particles within the axonal shaft, but must involve additional mechanisms. Towards this, we next tested scenario # 3) asking whether the intensity-center shifts in our imaging experiments could be simulated by assuming that the hypothetical synapsin particles were associating with (and subsequently clustering on) vectorial mobile-units as they moved anterogradely.
Figure 7. Data-driven modeling of specific transport scenarios.
Various parameters were introduced into the simulation-model to test specific scenarios that could not be readily addressed by experimentation, and the data-output was analyzed using kymographs and intensity-center analysis as described in the text. In all cases, synapsin particles were allowed to undergo Brownian motion and biophysical collisions. (A) Kymographs on the left show anterograde (red tracks) and/or retrograde (blue tracks) mobile-units traversing across a simulated axon containing synapsin particles. Middle kymographs show the dispersion of synapsin particles and the graphs on right show the corresponding intensity-center shifts. Note that these simulation parameters are not sufficient to generate a shift in the intensity center. (B) In these simulations, specific interactions were allowed between the synapsin particles and the mobile-units by creating a ‘dissociation radii’ around the mobile-units as detailed in the methods. Alteration of ‘dissociation radii’ led to changes in hypothetical interaction strengths, allowing us to test the effects of a range of interaction strengths in the simulation. Assumption of such interactions was necessary and sufficient to generate an anterograde shift of the synapsin population. However, when we assumed invariable dissociation radius/interaction strengths in the simulation, the intensity-center shifts were continuously linear and did not match the actual imaging data as shown in the top panels (also see Supplementary fig. 7). Instead, the imaging data was best-fitted by assuming a range of interactions as shown in the bottom panels. (C) Detailed data from the simulation above that best-fitted the actual imaging data. Note the anterograde shift of the synapsin population, qualitatively seen in the kymographs (left). In the simulated multiple-parameter overlaid kymograph (middle) synapsin particles are shown in green and tracks of anterograde and retrograde mobile-units are depicted in red and blue respectively. The yellow tracks represent instances of colocalization of anterograde mobile unit tracks (red) and synapsin particles (green). Note numerous instances of transient associations and vectorial motion evident in this kymograph. Graphs on the right show the various other outcomes of the simulation.
Indeed we found that introduction of this singular parameter to the simulation (ability to transiently associate) was sufficient to generate a vectorial shift in the synapsin population (fig. 7B). Furthermore, we found that the magnitude of the intensity-center shift correlated with changes in the interaction strengths between the synapsin particles and the mobile-units (Supplementary fig. 7), suggesting that such interactions/associations were key determinants of intensity-center shifts. However we found that when individual synapsin particles were allowed to associate with the mobile-units for a constant (invariable) time-period, the intensity-center shifts obtained rose linearly over time (fig. 7B, upper panels and Supplementary fig. 7) and did not match the actual imaging data where the intensity-shifts plateau after an initial rise (see graphs in fig. 2). Instead we found that to match the simulated intensity-center shifts to the experimental data, it was necessary to assign a range of interaction strengths to the synapsin particles as shown in figure 7B, lower panels. Figure 7C shows further details of the simulation that most closely matched the actual imaging experiments.
Collectively, the simulation results indicate that 1) the anterogradely biased motion of photoactivated synapsin molecules in our experiments is unlikely to be a result of a non-specific axonal flow; 2) clustering/association of individual synapsin molecules into larger transport competent supra-molecular structures is necessary and sufficient to generate the biased vectorial motion of the synapsin population seen in our imaging experiments, and 3) the interaction strengths of synapsin molecules with the mobile-units is not invariant, but likely encompasses a range of interaction strengths.
DISCUSSION
The problem: How are cytosolic proteins delivered to their destinations?
Proteins are delivered into synapses by both fast and slow axonal transport (Garner and Mahler, 1987). However, while the basic principles underlying the fast axonal transport of vesicular proteins are well-understood, mechanisms underlying the slow transport of cytosolic proteins - that have much slower overall velocities - are unclear. While previous pulse-chase radiolabeling studies have generally characterized the movement of these cytosolic cargoes, they have not provided much mechanistic insight into how such inherently soluble, cytosolic proteins can be conveyed slowly and efficiently along axons. Thus though overall transport of synapsin and CamKIIa was described decades ago, to this date the underlying mechanisms that lead to this motion remain unclear. In this study we adopted an imaging strategy to visualize the bulk transport as well as single particle kinetics of the presynaptically enriched cytosolic cargoes synapsin and CamKIIa in living neurons, combining them with in-vivo biochemical assays and data-driven computational modeling.
Unconventional transport behavior of cytosolic proteins- qualitative and mechanistic differences from other transport modes
We found that pools of cytosolic cargoes are transported in a streaming fashion and with a motor-dependent anterograde bias, very different from the stochastic transport of individual vesicles. This overall biased motion is likely generated by an intricate particle kinetics including transient assembly, short rapid vectorial spurts (Supplementary fig. 4) – very different from fast vesicular transport that is typically persistent. The anterograde bias of the population, the dependence on molecular motors, the dissimilarity of cytosolic cargo motion to untagged PA-GFP, the short-range, rapid vectorial speckle-movement and the modeling data, all argue that this movement is not entirely a simple diffusive process. In brains, we found that these proteins are also present within high-speed pellet fractions, migrate distinctly from vesicular proteins in density gradients and are resistant to detergent treatments that solubilize membranes. Finally, our modeling data suggest that clustering/association of these cytosolic proteins is necessary and sufficient to generate the vectorial shift seen in our imaging experiments.
Collectively the imaging, biochemical and biophysical data support the following scenario. After synthesis, a small fraction of soluble proteins associate with vesicles, and are rapidly transported (at least for vesicle-associated proteins like synapsins). However the bulk of soluble proteins transiently assemble into supra-molecular assemblies like multi-protein complexes that are conveyed by associations with molecular motors. This association may be direct; or indirectly mediated via association with vesicles (note partial overlap of cytosolic cargoes with vesicles in fig. 5B, C). We are struck by the peculiarity of this slower motion, when compared to other modes of intracellular transport- axonal transport or otherwise. In the classic mode of vesicular transport for instance, individual vesicles bind to motors, and the motor-cargo unit is then transported stochastically for long distances. In the case of cytosolic proteins however, fluorescent molecules are transported in streaming plumes, qualitatively distinct from other transport modalities. As cytosolic proteins are ubiquitous, it is tempting to speculate that all cell-types employ such mechanisms to convey them to their destinations within the cell.
The role of diffusion in the axonal transport of cytosolic cargoes
Unlike membranous proteins that are anchored to vesicles, cytosolic proteins invariably have diffusible pools, raising the question if diffusion can play a role in cytosolic cargo transport (Miller and Heidemann, 2008). In that regard, recent studies have revealed a novel intracellular motion where random intracellular agitation due to the activity of motors can create cytoplasmic stirring that can then move proteins in its wake (Brangwynne et al., 2008, 2009). In the context of cytosolic cargo transport, it seems plausible that soluble, freely diffusible molecules of cytosolic proteins can be simply transported secondary to a polarized flow generated by other moving organelles; as opposed to specific interactions of higher-order structures (like cargo-complexes) with motors.
Several observations argue against this notion. First, though individual cargoes can exhibit a biased transport in axons, when overall axonal transport is examined (as in the squid and xenopus system), a plethora of organelles are seen to move bi-directionally, and there is little evidence of a bias in overall movement (Grafstein and Forman, 1980). Thus there is no reason to believe that there is a biased flow within axons in the steady-state situation. Even if we assume that there was some polarized axonal flow that could carry soluble proteins in its wake; one would expect that a purely soluble protein with no significant molecular interactions within neurons would be conveyed by such mechanisms. However as shown in fig. 1C, soluble untagged PAGFP has no bias in axons. Secondly, average velocities of mobile speckles within the photoactivated pool (≈1 μm/sec) encompass the expected range of motor-driven cargoes (Supplementary fig. 4), which would be difficult to reconcile if the motion was solely generated by passive flows. Furthermore the wide diversity of axonal transport rates seen in our studies indicates an overall motion that would also be inconsistent with active diffusion, which would likely generate homogenous transport velocities. Finally, in our biophysical modeling, we specifically simulated situations that would be analogous to passive flows within the axon, allowing hypothetical ‘mobile-units’ to move within the simulation and physically collide with the cytosolic particles, but as shown in figure 7A, such passive movements were not sufficient to generate any biased flow of the population. Instead, shifts in the simulated population were only possible when we assumed specific interactions between the cytosolic molecules and the mobile units. In fact we could alter the magnitudes of the shifts in the population, simply by altering the association/dissociation rates between the synapsin particles and the mobile-units in the model (Supplementary fig. 7), suggesting that such interactions were necessary and sufficient to create the biased population dynamics in our studies.
Thus, though there is good evidence that diffusion can generate some intracellular motion such as fluctuation of cytoskeletal polymers (Brangwynne et al., 2007), and it is clear that cytosolic proteins can diffuse, we favor the view that though passive diffusion is a component of cytosolic cargo transport - simply due to the biophysical properties of these proteins - such mechanisms do not actively contribute to the vectorial slow transport seen within axons.
A working model for intracellular transport of soluble cargos
Based on the data, we propose a model for the axonal transport of cytosolic synaptic cargoes where soluble proteins dynamically organize into multi-protein complexes that are conveyed by motors. Our biophysical simulations provides substantial strength to the overall model by showing that clustering/associations of freely diffusible synapsin molecules is necessary and sufficient to generate the anterograde flux seen in our imaging experiments. The existence of protein complexes is supported by the following data. 1) Imaging experiments directly reveal assembly and vectorial transport of punctate fluorescent speckles containing these proteins (Supplementary fig. 4). 2) Such particles are also seen natively by immunostaining (Supplementary fig. 1). 3) Upon motor-inhibition, clusters of stalled synapsin and CamKIIa particles can be clearly seen in axons (fig. 3B), suggesting that their mobility was interrupted by these manipulations. 4) Finally, biochemical experiments show that subsets of these cytosolic proteins are present in high-speed pellets from synaptosome-depleted (P100) as well as axon-enriched corpus callosum brain fractions that are detergent-resistant, indicating the existence of higher-order macromolecular structures within axons in-vivo (fig. 5). We posit that the vast majority of these complexes transiently engage with motors (directly or indirectly) within axons, leading to a slow overall movement of the synapsin/CamKIIa population.
Minor fractions of synapsin are co-transported with vesicles in fast axonal transport
Early nerve ligation/crushing studies showed that synapsin was associated with vesicles accumulating proximally in ligated/crushed sites (Booj et al., 1986). More detailed pulse-chase radiolabeling studies showed that while small population of newly-synthesized synapsin (≈15%) departed the soma immediately afterwards, the vast majority (≈85%) was released from the cell-body only after several days, and this pool moved much more slowly, at rates consistent with slow axonal transport (Baitinger and Willard, 1987; Petrucci et al., 1991). More recent studies have shown that in cultured neurons, synapsin is associated with mobile synaptic vesicular precursors (“transport packets”) likely conveyed in fast axonal transport (Ahmari et al., 2000). Synapsin is also an established component of synaptic vesicles (Takamori et al., 2006).
Our data (fig. 4 A) directly show that photoactivated somatic synapsin is transported into proximal axons both as punctate particles that are highly persistent, as well as a slow wave that departs the soma with a transport behavior consistent with slow axonal transport. The persistent punctate synapsin particles co-localize with synaptophysin, a vesicular protein conveyed in fast axonal transport (fig. 4B). Collectively, the data indicate that a small fraction of newly-synthesized synapsin is associated with vesicles and conveyed in fast axonal transport, while the remainder is conveyed in slow axonal transport with intricate particle kinetics. Though the biological basis for this bimodal transport behavior is unclear, it may have some evolutionary significance, where cytosolic proteins in higher organisms may have acquired novel roles at synapses, requiring them to localize to boutons and necessitating rapid transport in the fast component as well.
In contrast to vesicular transport, mechanisms of slow axonal transport have been harder to unravel, largely due to difficulties in visualizing the phenomena (Brown, 2003). This is particularly true for transport of cytosolic cargoes where the inherent solubility of these proteins makes optical imaging challenging. In previous studies, we transfected fluorescent-tagged soluble proteins in cultured hippocampal neurons and imaged thin distal axons, visualizing discernable individual particles within a diffuse background of fluorescent molecules (Roy et al., 2007, 2008). Based on observations that cytosolic particles moved rapidly but more infrequently than FC proteins, we speculated that population dynamics of cytosolic cargoes were akin to neurofilament transport, where compelling evidence indicates that the intermittent fast movements of individual neurofilaments leads to the overall slow rates seen in the radio-labeling studies (Roy et al., 2000; Wang et al., 2000; Yan and Brown, 2005). However; limited to the analysis of observable particles in thin distal axons, our previous methods did not allow us to visualize and analyze the population as a whole, or consider potential roles of non-particulate/diffusible protein pools on overall transport, an issue that we overcame using our current imaging paradigm. Moreover these studies did not take into account the minor pools of cytosolic proteins moving in fast transport. In hindsight, it seems likely that the vast majority of the transient, short-range movements that we see with our photoactivation paradigm were not apparent using steady-state labeling where they were perhaps hidden within the background fluorescence. The presence of fast-moving particles also complicates the interpretation of those studies. Finally, some studies have reported the biased movement of soluble, cytoskeletal proteins in extruded squid axons by exogenously introducing (stabbing) these proteins within the axon shaft (Galbraith et al., 1999; Terada et al., 2000). The physiologic relevance of this experimental paradigm is unclear, and these studies do not provide much mechanistic insight beyond what is known using pulse-chase radiolabeling.
In summary, our experiments using live-imaging, in-vivo biochemical assays and biophysical modeling suggest a working model that can explain the mechanistic logic behind the slow axonal transport of cytosolic proteins. Though the model can explain how clusters of cytosolic proteins can be transported efficiently, further insights into the rules of cytosolic protein transport will have to await identification of specific transport machineries and the detailed characterization of the complexes themselves.
EXPERIMENTAL PROCEDURES
Hippocampal cell cultures, transfections and plasmids
Hippocampal cultures were obtained from brains of postnatal (P0-P2) CD-1 mice following standard protocols. Briefly, dissociated cells were plated at a density of 50,000 cells/cm2 in poly-D-lysine-coated glass-bottom culture dishes (MatTek, Ashland, MA) and maintained in Neurobasal/B27 media (Invitrogen, Carlsbad, CA) supplemented with 0.5 mM glutamine. Neurons were transiently transfected with the respective PA-GFP construct and a soluble mRFP (to identify transfected neurons) using Lipofectamine-2000 (Invitrogen) at 7–9 d in vitro (DIV) according to the manufacturer’s instructions. All axonal transport studies were performed 17-24 hours after transfection. For CamKIIa, some neurons were allowed to express the proteins for up-to 48 hours due to low levels of photoactivable protein pools (empiric observations). All animal studies were performed in accordance with University of California guidelines. The GFP:synapsin-Ia and the APP constructs were sub-cloned into the PAGFP vector using standard cloning techniques. All constructs used in this study were confirmed by sequencing.
Microscopy, live-imaging and photoactivation set-up
All time-lapse images were acquired using an Olympus IX81 inverted motorized epifluorescence microscope equipped with a Z-controller (IX81, Olympus), a motorized X-Y stage controller (Prior Scientific) and a fast electronic shutter (Smartshutter). Images were acquired using ultra-stable light source (Exfo exacte) and CCD cameras (Coolsnap HQ2, Photometrics); photoactivation was performed using a 100W mercury lamp (Olympus). For live imaging, neurons were transferred to a ‘live-cell imaging media’- low-fluorescence Hibernate E (Brainbits), 2% B27, 2 mM Glutamax, 0.4% D-glucose, and 37.5 mM NaCl (Roy et al., 2008) and maintained at 37°C using an air-curtain incubator (Nevtek) mounted on the microscope. All images were acquired using Metamorph software (Molecular Devices) and processed using either Metamorph or Matlab (MathWorks). For simultaneous photoactivation and visualization, we used the IX2-RFAW; dual-input illuminator (Olympus) attached to the microscope. The photoactivation input contained a violet excitation filter (D405/40, Chroma), a pinhole to focus the incident activation beam and an electronic shutter (Olympus) in the light-path. The visualization input contained a GFP excitation filter (HQ480/40, Chroma) and a neutral-density filter (reducing the incident-light intensity by 94-97%). The GFP filter cube within the microscope housing consisted of 1) no excitation filter (placed on the respective light-paths as described above), 2) a dichroic (T495pxr, Chroma) that blocked transmission of (and reflected) low-wavelength violet light and 3) an emission filter (HQ535/50, Chroma). Using these settings we were able to photoactivate our region of interest while visualizing it. Consistent imaging parameters were used throughout all experiments.
Quantitative image analyses: Intensity-Center analysis
The intensity-center analysis function represents the arithmetic peak of the average values of fluorescence intensities along the photoactivated zone and was determined as follows. After background subtraction, the photoactivated region of interest (ROI) was cropped. An average-intensity line-scan was then performed within this ROI to generate kymographs using Metamorph. Subsequent analysis was performed in MatLab. Line-scans representing each time-point within the kymograph were subjected to the following equation to assay the intensity center of the pulse-labeled protein population at each time-point: ([X1,X2,X3,…Xn]*[I1,I2,I3,…,In])/sum([I1,I2,I3,…,In]). Here [X1,X2,X3…Xn] defines an array of X coordinates of pixels along the axon from 1 to Xn, were Xn represents the length of the photoactivated zone. [I1,I2,I3,…,In] defines an array representing the intensity at each X coordinate. These intensity-center values were then normalized by subtracting the X-coordinate of the intensity center at t=0 from the intensity center at each subsequent time-point. The data were then graphical represented as intensity-center shift v/s time.
Biochemical assays
For fractionation assays, brains from 4 weeks old CD-1 mice were homogenized in buffer containing 20 mM HEPES, 40mM KCl, 5mM EGTA, 5mM EDTA, and protease inhibitors, pH 7.2. The resulting homogenate was centrifuged at 1000g for 20min to obtain a nuclear pellet (P1) and a post-nuclear supernatant (S1). The supernatant S1 was centrifuged at 10,200g for 20 min to obtain the crude synaptosomal fraction (P2) and synaptosome depleted fraction (S2). Finally, the S2 supernatant was then centrifuged at high-speeds (100,000g for 1 h at 4°C) to obtain supernatant S100 and pellet P100. For floatation assays, P100 fractions were adjusted to 45 % sucrose, bottom loaded on a 5-45 % sucrose gradient and centrifuged at 160,000g for 16 h at 4°C in a SW55-Ti swinging bucket rotor in an Optima L-100 ultracentrifuge (Beckmancoulter). Ten 0.5 ml fractions were collected from top of the gradient column and equal volumes were used for SDS-PAGE and western blot analysis. For some experiments, 1% Triton-X 100 or 1% deoxycholate was added and floatation assay were performed as mentioned above. The following antibodies were used for Western blotting: anti-Synapsin-I at 1:5000 (Synaptic Systems), anti-APP at 1:2000 (Epitomics), anti-CamKII at 1:2000 (Thermo Scientific), and anti-synaptophysin at 1:1000 (Millipore, USA), anti-GAP43 (sc-17790, Santa Cruz). Blots were developed using Pierce ECL Western blotting Kit, visualized using Versa Doc Imaging system (Bio-Rad) and quantified by densitometry.
Computational modeling of slow axonal transport
Details regarding generating the simulation and analyzing the data are provided in the Supplemental experimental procedures.
Statistical Methods
Routine statistics were used to analyze data and are indicated in the respective figure legends.
Supplementary Material
Acknowledgements
This work was supported by start-up funds from UCSD and a grant from the March of Dimes foundation (Basil O’Connor award) to SR. The authors thank Dr. Ge Yang of Carnegie Mellon University for his suggestion of and initial assistance with the intensity center analysis. We are grateful to Drs. George Patterson and Lippincott Schwartz (NIH) for the PAGFP construct. We also thank Drs. George Augustine (Duke University), Bernardo Sabatini (Harvard), Christoph Kaether (Leibniz Institute, Germany) and Leon Laganado (MRC, Cambridge, UK) and for providing the GFP:synapsin-I, PAGFP:CamKIIa, APP:YFP and synaptophysin:mRFP respectively.
ABBREVIATIONS LIST
- (GFP)
Green fluorescent protein
- (mRFP)
monomeric red fluorescent protein
- (sys-I)
Synapsin-I
- (CamK)
calcium/calmodulin-dependent kinase
- (NEM)
N-ethyl maleimide
- (SPT)
single particle tracking
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Ahmari SE, Buchanan J, Smith SJ. Assembly of presynaptic active zones from cytoplasmic transport packets. Nat.Neurosci. 2000;3:445–451. doi: 10.1038/74814. [DOI] [PubMed] [Google Scholar]
- Baitinger C, Willard M. Axonal transport of synapsin I-like proteins in rabbit retinal ganglion cells. J.Neurosci. 1987;7:3723–3735. doi: 10.1523/JNEUROSCI.07-11-03723.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booj S, Larsson PA, Dahllof AG, Dahlstrom A. Axonal transport of synapsin I- and cholinergic synaptic vesicle-like material; further immunohistochemical evidence for transport of axonal cholinergic transmitter vesicles in motor neurons. Acta Physiol Scand. 1986;128:155–165. doi: 10.1111/j.1748-1716.1986.tb07962.x. [DOI] [PubMed] [Google Scholar]
- Brangwynne CP, Koenderink GH, MacKintosh FC, Weitz DA. Cytoplasmic diffusion: molecular motors mix it up. J Cell Biol. 2008;183:583–587. doi: 10.1083/jcb.200806149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brangwynne CP, Koenderink GH, MacKintosh FC, Weitz DA. Intracellular transport by active diffusion. Trends Cell Biol. 2009;19:423–427. doi: 10.1016/j.tcb.2009.04.004. [DOI] [PubMed] [Google Scholar]
- Brangwynne CP, MacKintosh FC, Weitz DA. Force fluctuations and polymerization dynamics of intracellular microtubules. Proc Natl Acad Sci U S A. 2007;104:16128–16133. doi: 10.1073/pnas.0703094104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown A. Axonal transport of membranous and nonmembranous cargoes: a unified perspective. J.Cell Biol. 2003;160:817–821. doi: 10.1083/jcb.200212017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorn JF, Danuser G, Yang G. Computational processing and analysis of dynamic fluorescence image data. Methods Cell Biol. 2008;85:497–538. doi: 10.1016/S0091-679X(08)85022-4. [DOI] [PubMed] [Google Scholar]
- Dunkley PR, Jarvie PE, Robinson PJ. A rapid Percoll gradient procedure for preparation of synaptosomes. Nat Protoc. 2008;3:1718–1728. doi: 10.1038/nprot.2008.171. [DOI] [PubMed] [Google Scholar]
- Fletcher TL, Cameron P, De CP, Banker G. The distribution of synapsin I and synaptophysin in hippocampal neurons developing in culture. J.Neurosci. 1991;11:1617–1626. doi: 10.1523/JNEUROSCI.11-06-01617.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galbraith JA, Reese TS, Schlief ML, Gallant PE. Slow transport of unpolymerized tubulin and polymerized neurofilament in the squid giant axon. Proc.Natl.Acad.Sci.U.S.A. 1999;96:11589–11594. doi: 10.1073/pnas.96.20.11589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garner JA, Lasek RJ. Cohesive axonal transport of the slow component b complex of polypeptides. J.Neurosci. 1982;2:1824–1835. doi: 10.1523/JNEUROSCI.02-12-01824.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garner JA, Mahler HR. Biogenesis of presynaptic terminal proteins. J.Neurochem. 1987;49:905–915. doi: 10.1111/j.1471-4159.1987.tb00979.x. [DOI] [PubMed] [Google Scholar]
- Gitler D, Xu Y, Kao HT, Lin D, Lim S, Feng J, Greengard P, Augustine GJ. Molecular determinants of synapsin targeting to presynaptic terminals. J.Neurosci. 2004;24:3711–3720. doi: 10.1523/JNEUROSCI.5225-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grafstein B, Forman DS. Intracellular transport in neurons. Physiol Rev. 1980;60:1167–1283. doi: 10.1152/physrev.1980.60.4.1167. [DOI] [PubMed] [Google Scholar]
- Kaether C, Skehel P, Dotti CG. Axonal membrane proteins are transported in distinct carriers: a two-color video microscopy study in cultured hippocampal neurons. Mol.Biol.Cell. 2000;11:1213–1224. doi: 10.1091/mbc.11.4.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura T, Watanabe H, Iwamatsu A, Kaibuchi K. Tubulin and CRMP-2 complex is transported via Kinesin-1. J Neurochem. 2005;93:1371–1382. doi: 10.1111/j.1471-4159.2005.03063.x. [DOI] [PubMed] [Google Scholar]
- Kleim JA, Bruneau R, Calder K, Pocock D, VandenBerg PM, MacDonald E, Monfils MH, Sutherland RJ, Nader K. Functional organization of adult motor cortex is dependent upon continued protein synthesis. Neuron. 2003;40:167–176. doi: 10.1016/s0896-6273(03)00592-0. [DOI] [PubMed] [Google Scholar]
- Kozielski F, Sack S, Marx A, Thormahlen M, Schonbrunn E, Biou V, Thompson A, Mandelkow EM, Mandelkow E. The crystal structure of dimeric kinesin and implications for microtubule-dependent motility. Cell. 1997;91:985–994. doi: 10.1016/s0092-8674(00)80489-4. [DOI] [PubMed] [Google Scholar]
- Lasek RJ, Garner JA, Brady ST. Axonal transport of the cytoplasmic matrix. J.Cell Biol. 1984;99:212s–221s. doi: 10.1083/jcb.99.1.212s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lillemeier BF, Koster M, Kerr IM. STAT1 from the cell membrane to the DNA. EMBO J. 2001;20:2508–2517. doi: 10.1093/emboj/20.10.2508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund LM, McQuarrie IG. Calcium/calmodulin-dependent protein kinase IIalpha in optic axons moves with slow axonal transport and undergoes posttranslational modification. Biochem.Biophys.Res.Commun. 2001;289:1157–1161. doi: 10.1006/bbrc.2001.6111. [DOI] [PubMed] [Google Scholar]
- Lund LM, McQuarrie IG. Calcium/calmodulin-dependent protein kinase IIbeta isoform is expressed in motor neurons during axon outgrowth and is part of slow axonal transport. J.Neurosci.Res. 2002;67:720–728. doi: 10.1002/jnr.10162. [DOI] [PubMed] [Google Scholar]
- Miller KE, Heidemann SR. What is slow axonal transport? Exp Cell Res. 2008;314:1981–1990. doi: 10.1016/j.yexcr.2008.03.004. [DOI] [PubMed] [Google Scholar]
- Paggi P, Petrucci TC. Neuronal compartments and axonal transport of synapsin I. Mol.Neurobiol. 1992;6:239–251. doi: 10.1007/BF02780556. [DOI] [PubMed] [Google Scholar]
- Petrucci TC, Macioce P, Paggi P. Axonal transport kinetics and posttranslational modification of synapsin I in mouse retinal ganglion cells. J.Neurosci. 1991;11:2938–2946. doi: 10.1523/JNEUROSCI.11-09-02938.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfister KK, Wagner MC, Bloom GS, Brady ST. Modification of the microtubule-binding and ATPase activities of kinesin by N-ethylmaleimide (NEM) suggests a role for sulfhydryls in fast axonal transport. Biochemistry. 1989;28:9006–9012. doi: 10.1021/bi00449a008. [DOI] [PubMed] [Google Scholar]
- Roy S, Coffee P, Smith G, Liem RK, Brady ST, Black MM. Neurofilaments are transported rapidly but intermittently in axons: implications for slow axonal transport. J.Neurosci. 2000;20:6849–6861. doi: 10.1523/JNEUROSCI.20-18-06849.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy S, Winton MJ, Black MM, Trojanowski JQ, Lee VM. Rapid and intermittent cotransport of slow component-b proteins. J.Neurosci. 2007;27:3131–3138. doi: 10.1523/JNEUROSCI.4999-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy S, Winton MJ, Black MM, Trojanowski JQ, Lee VM. Cytoskeletal requirements in axonal transport of slow component-b. J Neurosci. 2008;28:5248–5256. doi: 10.1523/JNEUROSCI.0309-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy S, Zhang B, Lee VM, Trojanowski JQ. Axonal transport defects: a common theme in neurodegenerative diseases. Acta Neuropathol.(Berl) 2005;109:5–13. doi: 10.1007/s00401-004-0952-x. [DOI] [PubMed] [Google Scholar]
- Shakiryanova D, Tully A, Hewes RS, Deitcher DL, Levitan ES. Activity-dependent liberation of synaptic neuropeptide vesicles. Nat.Neurosci. 2005;8:173–178. doi: 10.1038/nn1377. [DOI] [PubMed] [Google Scholar]
- Sturgill JF, Steiner P, Czervionke BL, Sabatini BL. Distinct domains within PSD-95 mediate synaptic incorporation, stabilization, and activity-dependent trafficking. J Neurosci. 2009;29:12845–12854. doi: 10.1523/JNEUROSCI.1841-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takamori S, Holt M, Stenius K, Lemke EA, Gronborg M, Riedel D, Urlaub H, Schenck S, Brugger B, Ringler P, et al. Molecular anatomy of a trafficking organelle. Cell. 2006;127:831–846. doi: 10.1016/j.cell.2006.10.030. [DOI] [PubMed] [Google Scholar]
- Terada S, Kinjo M, Hirokawa N. Oligomeric tubulin in large transporting complex is transported via kinesin in squid giant axons. Cell. 2000;103:141–155. doi: 10.1016/s0092-8674(00)00094-5. [DOI] [PubMed] [Google Scholar]
- Tsuriel S, Geva R, Zamorano P, Dresbach T, Boeckers T, Gundelfinger ED, Garner CC, Ziv NE. Local sharing as a predominant determinant of synaptic matrix molecular dynamics. PLoS Biol. 2006;4:e271. doi: 10.1371/journal.pbio.0040271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchida A, Alami NH, Brown A. Tight functional coupling of kinesin-1A and dynein motors in the bidirectional transport of neurofilaments. Mol Biol Cell. 2009;20:4997–5006. doi: 10.1091/mbc.E09-04-0304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Ho CL, Sun D, Liem RK, Brown A. Rapid movement of axonal neurofilaments interrupted by prolonged pauses. Nat.Cell Biol. 2000;2:137–141. doi: 10.1038/35004008. [DOI] [PubMed] [Google Scholar]
- Whittaker VP. Thirty years of synaptosome research. J Neurocytol. 1993;22:735–742. doi: 10.1007/BF01181319. [DOI] [PubMed] [Google Scholar]
- Withers GS, George JM, Banker GA, Clayton DF. Delayed localization of synelfin (synuclein, NACP) to presynaptic terminals in cultured rat hippocampal neurons. Brain Res.Dev.Brain Res. 1997;99:87–94. doi: 10.1016/s0165-3806(96)00210-6. [DOI] [PubMed] [Google Scholar]
- Yan Y, Brown A. Neurofilament polymer transport in axons. J.Neurosci. 2005;25:7014–7021. doi: 10.1523/JNEUROSCI.2001-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng X, Goetz JA, Suber LM, Scott WJ, Jr., Schreiner CM, Robbins DJ. A freely diffusible form of Sonic hedgehog mediates long-range signalling. Nature. 2001;411:716–720. doi: 10.1038/35079648. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.