Abstract
This study was designed to clarify the mechanism of the mammalian target of rapamycin (mTOR)-hypoxia inducible factor-1 (HIF-1) pathway using the cultured cell strain derived from human ovarian clear cell adenocarcinoma (CCA). Everolimus (a derivative of rapamycin)-treated cells and non-treated cells did not show any difference in mTOR expression. But, phosphorylated-mTOR (p-mTOR) expression significantly decreased in the treated cells, and mTOR-related factors such as phosphorylated-4E-BP1 (p-4E-BP1), HIF-1α, and vascular endothelial growth factor (VEGF) in the downstream region of mTOR revealed a marked decrease in expression. The analysis of influences of the drug on the HIF-1α degradation system showed an increase in von-Hippel Lindau (VHL) expression in the treated cells. Increase of cleaved caspase-3, one of key factors involved in apoptosis, was also shown in the treated cells. In the next step, using nude mice implanted with RMG-1 cells, a decrease in tumor size was demonstrated in 4 of the 7 mice which were orally administered with everolimus. As a result, it was suggested that everolimus administration would be helpful as an anti-tumor therapy for CCA not only via down-regulation of p-mTOR but also degradation of HIF-1α by VHL and induction of apoptosis by cleaved caspase-3.
Keywords: ovary, clear cell carcinoma, mTOR, anti-cancer therapy, rapamycin
I. Introduction
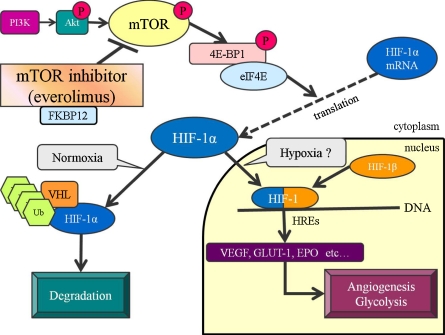
The mammalian target of rapamycin (mTOR) is a serine-threonine kinase, identified as the target of rapamycin which is one of macrolide antibiotics, and is a regulatory factor in cell division, growth, and survival (Fig. 1) [4, 6, 27]. mTOR is indispensable for the utilization of nutrients to maintain the survival capacity of the body [27]. Rapamycin as an mTOR inhibitor suppresses immune responses by inhibiting lymphocyte proliferation, and has been reported to suppress rejection on kidney or heart transplantation in rats [26]. Rapamycin forms a complex with FK506 binding protein (FKBP12), inhibiting kinase activity associated with lymphocyte proliferation [5, 7, 8]. Studies in recent years have shown the inhibitory effects of rapamycin mediated by vascular endothelial growth factor (VEGF) on angiogenesis (Fig. 1) [11, 13, 16, 18]. Rapamycin at a dose used for immunosuppression has been reported to exhibit marked inhibitory effects on tumor proliferation in metastasis and implantation models [13]. As its action mechanism, inhibition of both the proliferation and tube formation of vascular endothelial cells has been suggested [13]. Hudson et al. reported that mTOR acts as a positive regulatory factor in the PI3K-Akt-mTOR pathway, and that mTOR inhibition leads to inhibition of HIF-1α mRNA translation [14].
Fig. 1.
mTOR regulation pathway involved in hypoxia. Everolimus combines with FKBP12, and obstructs the function of mTOR. p-mTOR (phosphorylated-mTOR) is inhibited by everolimus. Inhibition of mTOR increases the binding of 4E-BP1 to elF-4E, consequently leading to inactivation of HIF-1α mRNA translation. HIF-1 composed of α unit and β unit binds to hypoxia response elements (HREs), resulting in the upregulation of hypoxia-related factors such as VEGF (vascular endothelial growth factor), GLUT-1 (glucose transporter-1), and EPO (erythropoietin).
Based on such evidence, as a first step, we evaluated the mechanism of the mTOR-HIF-1 pathway in vitro, and then the effectiveness of rapamycin in vivo, which is expected to be a promising new anti-tumor drug for CCA.
II. Materials and Methods
In vitro
Cell culture
RMG-1, a cultured cell strain derived from human ovarian CCA, was cultured in Ham’s F-12 medium (Gibco Co., Grand Island, NY) supplemented with 10% FBS (Gibco Co., Grand Island, NY) and 1% penicillin-streptomycin (Gibco Co., Grand Island, NY) at 37°C under 5% CO2.
Drug preparation
As a derivative of rapamycin, everolimus (Certican: 0.25 mg tablet) was purchased from Novartis Pharma (Basel, Switzerland). For the in vitro study, everolimus was prepared in dimethylsulfoxide before addition to the cell cultures. For the in vivo study, everolimus was diluted at the appropriate concentration in 0.5% carboxymethyl cellulose just before administration by gavage to the mice.
Immunoblotting
Immunoblotting was used to analyze changes after everolimus treatment in the expression of mTOR and its related factors in the downward pathway (Fig. 1). RMG-1 cells were treated with everolimus at 80 nM or 100 nM for 6 hr. Cell lysates were subjected to SDS-PAGE; a prestained protein marker (Broad Range, Bio-Rad Laboratories, Hercules, CA, USA) was run on the outside lane to verify the molecular weight of mTOR, phosphorylated-mTOR (p-mTOR), phosphorylated-4E-BP1 (p-4E-BP1), HIF-1α, VEGF, von Hippel-Lindau (VHL) and cleaved caspase-3. The samples were then transferred to polyvinylidene fluoride membranes, which were blocked with 2% normal goat serum in 5% skim milk for 1 hr at room temperature (RT). After incubation overnight at RT with the antibodies as follows: mTOR (clone 7C10, diluted at 1:1000, Cell Signaling Technology, Beverly, MA, USA), p-mTOR (clone 49F9, diluted at 1:1000, Cell Signaling Technology), p-4EBP1 (clone 236B4, diluted at 1:1000, Cell Signaling Technology), HIF-1α (clone H-206, diluted at 1:200, Santa Cruz Biotech, Santa Cruz, CA, USA), VEGF (clone 5C3F8, diluted at 1:1000, Novus Biologicals, Littleton, CO, USA), VHL (clone G-7, diluted at 1:100, Santa Cruz Biotech), cleaved caspase-3 (clone Asp175, diluted at 1:1000, Cell Signaling Technology), the membranes were then incubated with a biotin labeling anti-mouse/-rabbit IgG (Nichirei Biosciences, Tokyo, Japan) and with avidin-horseradish peroxidase complex (Vectastain Elite ABC kit, Vector Laboratories Inc, Burlingame, CA, USA) or rabbit-labeled polymer (Envision kit, Dako, Copenhagen, Denmark).
Immunohistochemistry
Treated cells with everolimus at 100 nM and non-treated cells were smeared on glass slides and fixed in 100% ethanol. Endogenous peroxidase was then blocked with methanol containing 0.3% hydrogen peroxidase for 30 min at RT. These preparations were incubated for 1 hr at RT with the anti-Ki-67-antibody. The cells grown on the chamber slides were treated with everolimus at 80 nM, 100 nM for 12 hr and fixed by 4% paraformaldehyde. Then, after blocking endogenous peroxidase similarly as smear preparation, these preparations were incubated with the primary antibodies such as mTOR (diluted at 1:100), p-mTOR (diluted at 1:100), HIF-1α (diluted at 1:60), VHL (diluted at 1:50). The secondary detection systems were similarly used as immunoblotting.
The expressions were semi-quantitatively evaluated according to the following scoring scheme: –, no staining; 1+, <10% cells positive; 2+, 10%–50% cells positive; 3+, >50% cells positive.
Cell count
The cells treated with everolimus at 100 nM for 6 hr were collected and washed with a new medium. The extent of lyses was measured by a cell-counting chamber stained with trypan blue.
In vivo
To evaluate the anti-tumor effects, 7 nude mice aged 6 weeks were inoculated subcutaneously in the right flank with 1×107 RMG-1 cells in 200 µL of PBS. When the tumors reached approximately 150 mm3 in volume, the nude mice were orally administered with everolimus (2.5 mg/kg) once a week for 33 days. As a control, 3 mice were inoculated with buffered saline.
III. Results
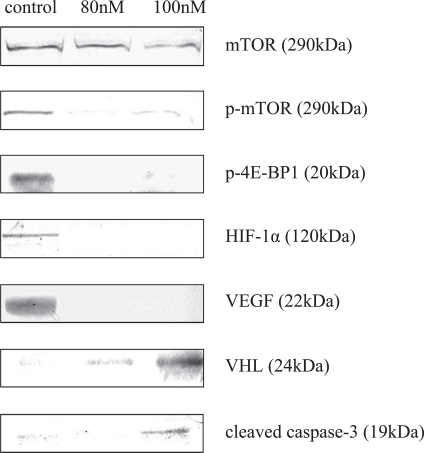
Although mTOR expression did not differ between the cells treated by everolimus and non-treated cells, p-mTOR expression was significantly decreased in the treated cells compared with the non-treated cells (Figs. 2, 3). Analysis of the expression of mTOR-related factors in the downstream region revealed a marked decrease in the expressions of p-4E-BP1, HIF-1α, and VEGF in the treated cells (Figs. 2, 3). However, there was no significant difference between 80 nM and 100 nM. On the other hand, analysis of the influences of the drug on the HIF-1α degradation system showed an increase of expression of VHL as a ubiquitin ligase, which is targeting HIF-1α, in the treated cells (especially at 100 nM) compared with the non-treated cells. Increase of cleaved caspase-3 expression was shown in the treated cells (especially at 100 nM).
Fig. 2.
Down- and upregulation of mTOR-involved proteins by immunoblotting. Expressions of p-mTOR, p-4E-BP1, HIF-1α, and VEGF were almost completely diminished due to treatment with everolimus. In contrast, the expressions of VHL and cleaved caspase-3 were strengthened in the treated cells.
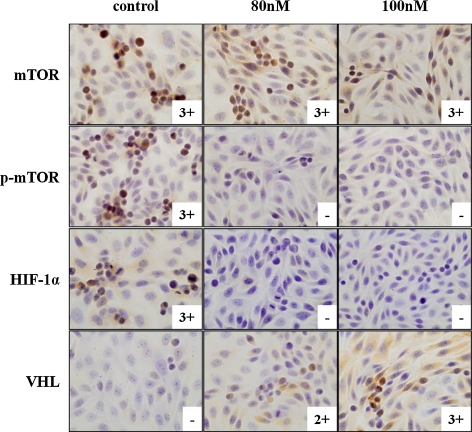
Fig. 3.
Down- and upregulation of mTOR-involved proteins by immunohistochemistry. m-TOR did not show a change of expression at all, but p-mTOR and HIF-1α expressions completely attenuated after the treatment. In contrast, VHL was clearly expressed at 100 nM (–, no staining; 1+, <10% cells positive; 2+, 10%–50% cells positive; 3+, >50% cells positive).
Ki-67 labeling index was estimated as 11% in the treated cells but 17% in the non-treated cells. In the analysis of cell survival, the cell count was 6.6×106 in the treated cells, but 8.7×106 in the non-treated cells, showing that 25% of the cells died with the treatment.
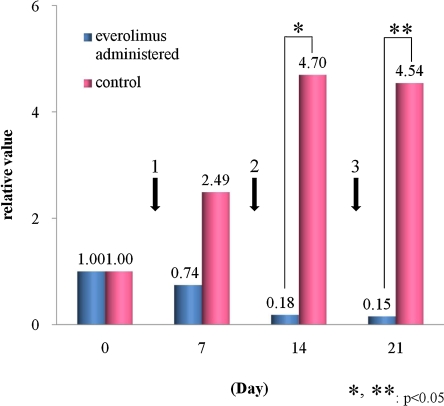
In the nude mice implanted with RMG-1 cells, the treated group showed a decrease in tumor size in 4 of the 7 mice. The tumor disappeared in 2 of the 4 mice (Figs. 4, 5), and decreased in size by 60–70% in the remaining two. Of the 3 mice not showing a significant decrease in the tumor size, 2 mice exhibited a decrease after the initial administration but no decrease after the second administration. The tumors subsequently increased in size and eventually became uncontrollable. In the remaining one, no significant change in tumor size was observed from the first administration.
Fig. 4.
Anti-tumor effects in the model mice depending on dosage. The contractive tendency of the tumor was seen in the everolimus-administered group compared with the control group (1, 2, 3: 1st, 2nd, 3rd administration of everolimus). The values express the average of each group using the relative ratio to the non-administered state.
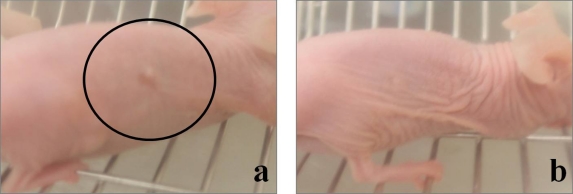
Fig. 5.
Anti-tumor effects in the model mouse shown by gross appearance. The tumor recognized by the naked eye before administration of everolimus (a) was completely diminished after the administration (b).
IV. Discussion
In this study, the marked expression of HIF-1-related factors was initially observed in the CCA strain. Among them, expression of mTOR was unchangeable, but p-mTOR, p-4E-BP1, HIF-1α, and VEGF expressions were dose-dependently inhibited. Everolimus is known to inhibit the phosphorylation of mTOR, resulting in inhibition of HIF-1α translation via the downstream effectors. These results suggest that mTOR is universally present and may be maintained in a stable state without phosphorylation.
The non-treated cells showed slight expression of VHL, which suggests that VHL is not activated in this CCA strain. Loss of heterozygosity at the VHL gene locus was reported to be frequently detected in ovarian carcinomas [22]. Therefore, it is speculated that inactivation of VHL may occur in ovarian carcinomas and be involved in excessive HIF-1α expression. In renal carcinomas, inactivation of VHL by mutation, deficiency or methylation has been reported [12, 23, 25]. However, the detailed mechanism of the overexpression of VHL induced by everolimus remains to clarified. This phenomenon may play a role in acceleration of degradation of HIF-1α through another unknown pathway which is directly related with overexpression of VHL. In future study, it is necessary to explore the mechanism of everolimus-induced overexpression of VHL.
The analysis of changes in the expression of apoptosis-related factor, represented by cleaved caspase-3, showed no expression in the non-treated cells. Kleinberg et al. reported cleaved caspase-3 expression in less than 10% of ovarian carcinomas [17]. They also evaluated the possible correlation between cleaved caspase-3 and the outcome, and reported the involvement of high-level cleaved caspase-3 expression in outcome improvement [17]. Our study showed that mTOR inhibition leads to cleaved caspase-3 overexpression and a decrease in the viable cells. It is considered that the effect of mTOR inhibition is also closely related to cell death by apoptosis. Albert et al. reported an increase in cleaved caspase-3 expression through the combination of radiotherapy and mTOR inhibitor administration in breast cancer [1], but its detailed mechanism has yet to be clarified.
Analysis of the cell growth ability revealed a decrease in Ki-67 labeling index from 17% in the non-treated cells to 11% in the treated cells. According to Itamochi et al., the growth ability of CCA is significantly lower compared to other types of ovarian carcinomas: Ki-67 labeling index, CCA 18.4%; serous adenocarcinoma 38.8% [15]. Interestingly, a low survival rate in CCA is considered to be related to the Ki-67 labeling index <18.4% [15]. Wang et al. also observed a decrease in the Ki-67 labeling index after mTOR inhibitor administration using a hepatocellular carcinoma mice model [28]. An increase in p27kip1 protein after mTOR inhibition has been reported to decrease the activities of cyclin-dependent kinase 4 (CDK4)/cyclin D and CDK2/cyclin E. It is speculated that a decrease in the Ki-67 labeling index is caused by termination of the cell cycle by increasing p27kip1 protein due to mTOR inhibition [9].
In our previous study with subcutaneous injection of everolimus to the tumor in mice, a tendency toward a decrease in tumor volume was observed [20]. The tumor was histologically degradated in close association with decreases in the expressions of HIF-1-related factors. In the present study, subcutaneous injection of everolimus was changed to its oral administration, and the tumor volume in mice also tended to decrease. In terms of clinical application, administration of mTOR inhibitor is expected to have great anti-tumor effects on CCA in combination with other chemotherapeutic drugs such as cisplatin [3, 24].
Specific-molecule targeting therapy for various tumors is being conducted as a promising strategy [19, 29]. It is considered that tumor therapy should be specifically targeted for each patient according to propensity of the tumor. Instead of mTOR, overexpression of p-mTOR is a hallmark for the optimal administration of m-TOR inhibitor [2, 10, 21]. The detailed analysis of the novel function of mTOR inhibitors, which is involved in the activation of apoptosis and HIF-1α degradation system, should be further studied for more effective administration of mTOR inhibitor.
V. References
- 1.Albert J. M., Kim K. W., Cao C., Lu B. Targeting the Akt/mammalian target of rapamycin pathway for radiosensitization of breast cancer. Mol. Cancer Ther. 2006;5:1183–1189. doi: 10.1158/1535-7163.MCT-05-0400. [DOI] [PubMed] [Google Scholar]
- 2.Baselga J., Semiglazov V., van Dam P., Manikhas A., Bellet M., Mayordomo J., Campone M., Kubista E., Greil R., Bianchi G., Steinseifer J., Molloy B., Tokaji E., Gardner H., Phillips P., Stumm M., Lane H. A., Dixon J. M., Jonat W., Rugo H. S. Phase II randomized study of neoadjuvant everolimus plus letrozole compared with placebo plus letrozole in patients with estrogen receptor-positive breast cancer. J. Clin. Oncol. 2009;27:2630–2637. doi: 10.1200/JCO.2008.18.8391. [DOI] [PubMed] [Google Scholar]
- 3.Beuvink I., Boulay A., Fumagalli S., Zilbermann F., Ruetz S., O’Reilly T., Natt F., Hall J., Lane H. A., Thomas G. The mTOR inhibitor RAD001 sensitizes tumor cells to DNA-damaged induced apoptosis through inhibition of p21 translation. Cell. 2005;120:747–759. doi: 10.1016/j.cell.2004.12.040. [DOI] [PubMed] [Google Scholar]
- 4.Bjornsti M. A., Houghton P. J. The TOR pathway: a target for cancer therapy. Nat. Rev. Cancer. 2004;4:335–348. doi: 10.1038/nrc1362. [DOI] [PubMed] [Google Scholar]
- 5.Chen J., Zheng X. F., Brown E. J., Schreiber S. L. Identification of an 11-kDa FKBP12-rapamycin-binding domain within the 289-kDa FKBP12-rapamycin-associated protein and characterization of a critical serine residue. Proc. Natl. Acad. Sci. U S A. 1995;92:4947–4951. doi: 10.1073/pnas.92.11.4947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen Y. J. Targeted mTOR in human gynecologic cancers. Cancer Mol. 2007;3:101–106. [Google Scholar]
- 7.Chiu M. I., Katz H., Berlin V. RAPT1, a mammalian homolog of yeast Tor, interacts with the FKBP12/rapamycin complex. Proc. Natl. Acad. Sci. U S A. 1994;91:12574–12578. doi: 10.1073/pnas.91.26.12574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Choi J., Chen J., Schreiber S. L., Clardy J. Structure of the FKBP12-rapamycin complex interacting with the binding domain of human FRAP. Science. 1996;273:239–242. doi: 10.1126/science.273.5272.239. [DOI] [PubMed] [Google Scholar]
- 9.Costa L. F., Balcells M., Edelman E. R., Nadler L. M., Cardoso A. A. Proangiogenic stimulation of bone marrow endothelium engages mTOR and is inhibited by simultaneous blockade of mTOR and NF-kappaB. Blood. 2006;107:285–292. doi: 10.1182/blood-2005-06-2208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Doi T., Muro K., Boku N., Yamada Y., Nishina T., Takiuchi H., Komatsu Y., Hamamoto Y., Ohno N., Fujita Y., Robson M., Ohtsu A. Multicenter phase II study of everolimus in patients with previously treated metastatic gastric cancer. J. Clin. Oncol. 2010;28:1904–1910. doi: 10.1200/JCO.2009.26.2923. [DOI] [PubMed] [Google Scholar]
- 11.Fujita M., Yasuda M., Kitatani K., Miyazawa M., Hirabayashi K., Takekoshi S., Iida T., Hirasawa T., Murakami M., Mikami M., Ishiwata I., Shimizu M., Osamura R. Y. An up-to-date anti-cancer treatment strategy focusing on HIF-1alpha suppression: its application for refractory ovarian cancer. Acta Histochem. Cytochem. 2007;40:139–142. doi: 10.1267/ahc.07024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gnarra J. R., Tory K., Weng Y., Schmidt L., Wei M. H., Li H., Latif F., Liu S., Chen F., Duh F. M., Lubensky I., Duan D. R., Florence C., Pozzatti R., Walther M. M., Bander N. H., Grossman H. B., Brauch H., Pomer S., Brooks J. D., Isaacs W. B., Lerman M. I., Zbar B., Linehan W. M. Mutations of the VHL tumour suppressor gene in renal carcinoma. Nat. Genet. 1994;7:85–90. doi: 10.1038/ng0594-85. [DOI] [PubMed] [Google Scholar]
- 13.Guba M., von Breitenbuch P., Steinbauer M., Koehl G., Flegel S., Hornung M., Bruns C. J., Zuelke C., Farkas S., Anthuber M., Janch K. W., Geissler E. K. Rapamycin inhibits primary and metastatic tumor growth by antiangiogenesis: involvement of vascular endothelial growth factor. Nat. Med. 2002;8:128–135. doi: 10.1038/nm0202-128. [DOI] [PubMed] [Google Scholar]
- 14.Hudson C. C., Liu M., Chiang G. G., Otterness D. M., Loomis D. C., Kaper F., Giaccia A. J., Abraham R. T. Regulation of hypoxia-inducible factor 1alpha expression and function by the mammalian target of rapamycin. Mol. Cell Biol. 2002;22:7004–7014. doi: 10.1128/MCB.22.20.7004-7014.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Itamochi H., Kigawa J., Sugiyama T., Kikuchi Y., Suzuki M., Terakawa N. Low proliferation activity may be associated with chemoresistance in clear cell carcinoma of the ovary. Obstet. Gynecol. 2002;100:281–287. doi: 10.1016/s0029-7844(02)02040-9. [DOI] [PubMed] [Google Scholar]
- 16.Jiang H., Feng Y. Hypoxia-inducible factor 1alpha (HIF-1alpha) correlated with tumor growth and apoptosis in ovarian cancer. Int. J. Gynecol. Cancer. 2006;1:405–412. doi: 10.1111/j.1525-1438.2006.00310.x. [DOI] [PubMed] [Google Scholar]
- 17.Kleinberg L., Dong H. P., Holth A., Risberg B., Trope C. G., Nesland J. M., Flørenes V. A., Davidson B. Cleaved caspase-3 and nuclear factor-κB p65 are prognostic factors in metastatic serous ovarian carcinoma. Hum. Pathol. 2009;40:795–806. doi: 10.1016/j.humpath.2008.10.019. [DOI] [PubMed] [Google Scholar]
- 18.Mayerhofer M., Valent P., Sperr W. R., Griffin J. D., Sillaber C. BCR/ABL induces expression of vascular endothelial growth factor and its transcriptional activator, hypoxia inducible factor-1alpha, through a pathway involving phosphoinositide 3-kinase and the mammalian target of rapamycin. Blood. 2002;100:3767–3775. doi: 10.1182/blood-2002-01-0109. [DOI] [PubMed] [Google Scholar]
- 19.Miyajima K., Takekoshi S., Itoh J., Kakimoto K., Miyakoshi T., Osamura R. Y. Inhibitory effects of anti-VEGF antibody on the growth and angiogenesis of estrogen-induced pituitary prolactinoma in Fischer 344 rats: animal model of VEGF-targeted therapy for human endocrine tumors. Acta Histochem. Cytochem. 2010;43:33–44. doi: 10.1267/ahc.09034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Miyazawa M., Yasuda M., Fujita M., Kajiwara H., Hirabayashi K., Takekoshi S., Hirasawa T., Murakami M., Ogane N., Kiguchi K., Ishiwata I., Mikami M., Osamura R. Y. Therapeutic strategy targeting at mTOR-HIF-1α-VEGF pathway for ovarian clear cell adenocarcinoma. Pathol. Int. 2009;59:19–27. doi: 10.1111/j.1440-1827.2008.02320.x. [DOI] [PubMed] [Google Scholar]
- 21.Motzer R. J., Escudier B., Oudard S., Hutson T. E., Porta C., Bracarda S., Grünwald V., Thompson J. A., Figlin R. A., Hollaender N., Urbanowitz G., Berg W. J., Kay A., Lebwohl D., Ravaud A., RECORD-1 Study Group. Efficacy of everolimus in advanced renal cell carcinoma: a double-blind, randomised, placebo-controlled phase III trial. Lancet. 2008;372:449–456. doi: 10.1016/S0140-6736(08)61039-9. [DOI] [PubMed] [Google Scholar]
- 22.Osada R., Horiuchi A., Kikuchi N., Yoshida J., Hayashi A., Ota M., Katsuyama Y., Melillo G., Konishi I. Expression of hypoxia-inducible factor 1alpha, hypoxia-inducible factor 2alpha, and von Hippel-Lindau protein in epithelial ovarian neoplasms and allelic loss of von Hippel-Lindau gene: nuclear expression of hypoxia-inducible factor 1alpha is an independent prognostic factor in ovarian carcinoma. Hum. Pathol. 2007;38:1310–1320. doi: 10.1016/j.humpath.2007.02.010. [DOI] [PubMed] [Google Scholar]
- 23.Pavlovich C. P., Schmidt L. S., Phillips J. L. The genetic basis of renal cell carcinoma. Urol. Clin. North Am. 2003;30:437–454. doi: 10.1016/s0094-0143(03)00023-5. [DOI] [PubMed] [Google Scholar]
- 24.Peng D. J., Wang J., Zhou J. Y., Wu G. S. Role of the Akt/mTOR survival pathway in cisplatin resistance in ovarian cancer cells. Biochem. Biophys. Res. Commun. 2010;394:600–605. doi: 10.1016/j.bbrc.2010.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rathmell W. K., Chen S. VHL inactivation in renal cell carcinoma: implications for diagnosis, prognosis and treatment. Expert Rev. Anticancer Ther. 2008;8:63–73. doi: 10.1586/14737140.8.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schuler W., Sedrani R., Cottens S., Häberlin B., Schulz M., Schuurman H. J., Zenke G., Zerwes H. G., Schreier M. H. SDZ RAD, a new rapamycin derivative: pharmacological properties in vitro and in vivo. Transplantation. 1997;64:36–42. doi: 10.1097/00007890-199707150-00008. [DOI] [PubMed] [Google Scholar]
- 27.Tokunaga C., Yoshino K., Yonezawa K. mTOR integrates amino acid- and energy-sensing pathways. Biochem. Biophys. Res. Commun. 2004;313:443–446. doi: 10.1016/j.bbrc.2003.07.019. [DOI] [PubMed] [Google Scholar]
- 28.Wang Z., Zhou J., Fan J., Qiu S. J., Yu Y., Huang X. W., Tang Z. Y. Effect of rapamycin alone and in combination with sorafenib in an orthotopic model of human hepatocellular carcinoma. Clin. Cancer Res. 2008;14:5124–5130. doi: 10.1158/1078-0432.CCR-07-4774. [DOI] [PubMed] [Google Scholar]
- 29.Yuan R., Kay A., Berg W. J., Lebwohl D. Targeting tumorigenesis: development and use of mTOR inhibitors in cancer therapy. J. Hematol. Oncol. 2009;2:45. doi: 10.1186/1756-8722-2-45. [DOI] [PMC free article] [PubMed] [Google Scholar]