Abstract
In vivo cryotechnique (IVCT), which immediately cryofixes target organs in situ, was used to clarify the morphological features of beating heart tissue of living mice. IVCT was performed for diastolic heart tissue under the condition of monitoring with electrocardiogram (ECG). Other mouse hearts were prepared with conventional perfusion-fixation (PF-DH) or immersion-fixation followed by dehydration (IM-DH), and quick-freezing of resected heart tissues (FQF). Immunolocalizations of albumin, immunoglobulin G1 (IgG1), intravenously injected bovine serum albumin (BSA), and connexin 43 were examined after different intervals of BSA injection. In the case of IVCT, the exact stop time of beating mouse hearts was recorded by ECG, and open blood vessels with flowing erythrocytes were observed with less artificial tissue shrinkage than with conventional preparation methods. Both albumin and BSA were well preserved in intercalated discs and t-tubules of cardiomyocytes in addition to blood vessels and interstitial matrices. IgG1 was immunolocalized in interstitial matrices of heart tissues in addition to their blood vessels. At 4 hr after BSA injection, it was immunolocalized in the intercalated discs of cardiomyocytes and lost later at 8 hr. IVCT should prove to be more useful for the morphofunctional examination of dynamically changing heart tissue than conventional preparation methods.
Keywords: in vivo cryotechnique, serum protein, microenvironment, mouse heart tissue
I. Introduction
It is well known that the serum components of living animals are one of the important factors comprising the microenvironment of all animal organ tissues [2]. The dynamically changing microenvironment undoubtedly reflects various physiological and pathological states of cells and tissues. However, with conventional tissue preparation methods, such as perfusion- or immersion-fixation with chemical fixatives, it is difficult to morphofunctionally and immunohistochemically examine the soluble components of living animal tissues in situ because of the loss of blood circulation induced by tissue resection, artificial perfusion-fixation pressure, or tissue shrinkage with organic solvent dehydration [3, 5, 10, 18]. In recent years, in vivo cryotechnique (IVCT), a new preparation method for living animal organs, has drawn increasing attention because of its technical merits that overcome these problems [11, 13, 14].
For the past few decades, IVCT has been used to immediately cryofix any target organ of living animals in situ without tissue resection for immersion-fixation or perfusion-fixation. As such it has the ability to capture transient molecular movements such as rapid phosphorylation of signaling molecules in living mouse retina and also to preserve the intranuclear soluble components of various cells and tissues in situ [9, 20]. In addition, it has already been reported that immunolocalizations of soluble molecules or dynamic structural changes in vivo could be morphofunctionally revealed on paraffin-embedded sections prepared by IVCT [6, 10]. Therefore, IVCT is the rational choice to examine the dynamically changing microenvironment of living animal heart tissues.
In the present study, we have morphologically and immunohistochemically examined living mouse heart tissues, monitored by electrocardiogram, with IVCT, and mainly focused on the immunolocalization of various serum proteins that contribute to the homeostasis of the microenvironment around the cardiomyocytes. In addition, we have described the time-dependent double immunolocalizations of intrinsic and extrinsic serum proteins at different time-intervals from bovine serum albumin (BSA) injection.
II. Materials and Methods
Animals and experimental design
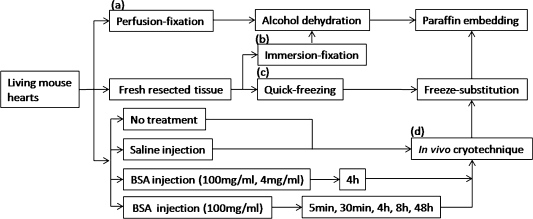
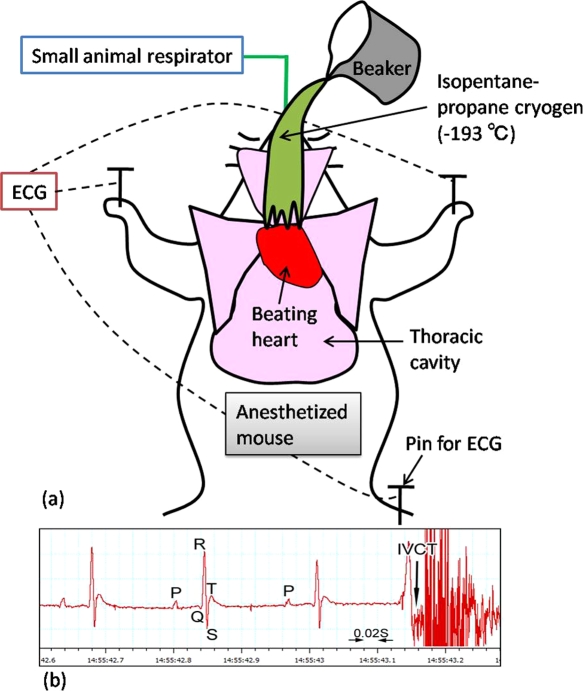
The present animal experiment was approved by the University of Yamanashi Animal Care and Use Committee. Male C57BL/6J mice, aged 6–8 weeks, were purchased from Japan SLC (Shizuoka, Japan) and prepared by IVCT method (Fig. 1d), as described below. Other conventional preparation methods were also used (Fig. 1a–c), including immersion-fixation (IM-DH) or perfusion-fixation followed by dehydration (PF-DH) and quick-freezing of resected tissues (FQF). Mice prepared with IVCT were divided into several experimental groups and a control group (3 mice in each group). In the experimental groups, 50 µl of 100 mg/ml BSA with low endotoxin (Sigma, St. Louis, MO, USA) in sterile saline was injected via tail veins into the anesthetized mice, as reported before [1, 12]. After BSA injection, IVCT was performed at different time-intervals of 5 min, 30 min, 4 hr, 8 hr, and 48 hr (Fig. 1d). For another experiment, 50 µl of 4 mg/ml or 100 mg/ml BSA was similarly injected into mice and IVCT was also performed at 4 hr time-intervals before cryofixation [1, 12]. In the control group, mice were prepared without BSA injection or only with an injection of 50 µl of physiological saline. All these mice were automatically monitored using an electrocardiogram (ECG) apparatus (Fig. 2) when IVCT was performed.
Fig. 1.
A flow chart of different experimental procedures, including perfusion-fixation (a), immersion-fixation (b), quick-freezing (c), and in vivo cryotechnique (d) for living mouse hearts. The letters (a)–(d) correspond to the procedures described in Materials and Methods.
Fig. 2.
A schematic drawing shows how to perform “in vivo cryotechnique” (IVCT) for living mouse heart with monitoring electrocardiogram (ECG) (a). Beating heart of an anesthetized mouse on a small animal respirator is exposed after opening the thoracic cavity, which is cryofixed by pouring isopentane-propane cryogen (–193°C) precooled in liquid nitrogen. (b) An example of ECG shows typical P, Q, R, S, and T points, and exact time of stopping with IVCT is indicated by an arrow.
Tissue preparation
Perfusion-fixation followed by alcohol dehydration (PF-DH)
PF-DH was performed as described previously (Fig. 1a) [10]. Briefly, three mice, starved for 24 hr, were anesthetized with pentobarbital sodium (100 mg/kg body weight, Nacalai Tesque, Kyoto, Japan) and transcardially perfused with 2% paraformaldehyde (PFA) in 0.1 M phosphate buffer, pH 7.4. Their hearts were resected and immersed in the same fixative overnight, and routinely embedded in paraffin wax.
Immersion-fixation followed by alcohol dehydration (IM-DH)
Three starved mice were also anesthetized as described above (Fig. 1b). Their hearts were resected and immersed in buffered 2% PFA overnight, which were then routinely embedded in paraffin wax.
Quick-freezing of fresh resected tissues (FQF) followed by freeze-substitution
Heart specimens were prepared as described previously (Fig. 1c) [9]. Briefly, heart tissues were removed from three starved mice under anesthesia and cut into small pieces with razor blades. Tissue pieces were quickly frozen by plunging into isopentane-propane cryogen (–193°C) immediately after tissue resection. After freeze-substitution with acetone containing 2% PFA as reported previously [20], they were embedded in paraffin wax.
In vivo cryotechnique (IVCT) followed by freeze-substitution
Mice were anesthetized with pentobarbital sodium. A plastic tubule of 22-gauge was used for intratracheal intubation, to allow them to automatically breathe with a respirator (Shinano Respirator, Japan; SN-480-7). Their thoracic cavity was opened, and IVCT was routinely performed by pouring isopentane-propane cryogen over the beating hearts while monitoring their ECG (Fig. 2). Then the frozen hearts in vivo were removed with a dental electric drill in liquid nitrogen, as previously described [10]. All cryofixed heart specimens were routinely freeze-substituted in acetone containing 2% PFA, and then embedded in paraffin wax.
Immunohistochemistry of intrinsic or extrinsic serum proteins
Paraffin sections of about 3 µm thick were mounted on glass slides (Matsunami Adhesive Slide, Matsunami Glass, Osaka, Japan) [12]. Some of them were routinely deparaffinized and stained with hematoxylin-eosin (HE). The other deparaffinized sections were rehydrated in phosphate-buffered saline (PBS) and immunostained for serum proteins, as previously described with some modifications [21]. Briefly, the serial sections for immunoperoxidase-DAB staining were first treated with 0.3% hydrogen peroxide in PBS for inhibition of enzyme activity of endogenous peroxidase, and additionally incubated in 5% fish gelatin (Sigma, St. Louis, MO, USA). They were then incubated in primary antibodies at 4°C overnight and their corresponding secondary antibodies at room temperature for 1 hr, as reported previously [10]. The primary antibodies were goat or rabbit anti-mouse albumin (Bethyl Laboratories, Montgomery, TX, USA), goat anti-mouse immunoglobulin G1 (IgG1), sheep anti-BSA, and rabbit anti-connexin 43 antibodies (Sigma, St. Louis, MO, USA). The secondary antibodies were biotinylated anti-goat IgG, biotinylated anti-rabbit IgG (Vector Laboratories, Burlingame, CA, USA), and biotinylated donkey anti-sheep IgG antibodies (Jackson ImmunoResearch Labs, West Grove, PA, USA). Furthermore, they were incubated with horseradish peroxidase (HRP)-conjugated avidin-biotin complex (ABC) for 1 hr and visualized with metal-enhanced 3,3'-diaminobenzidine (DAB) (Pierce, Rockford, IL, USA) for 5 min (ABC-DAB method). Finally, they were incubated in 0.04% osmium tetroxide solution for 2 min to increase the contrast, as previously described [6].
For immunofluorescence staining of the albumin, IgG1, and connexin 43, the secondary antibodies were Alexa Fluor 488-conjugated donkey anti-goat IgG and Alexa Fluor 594-conjugated donkey anti-rabbit IgG antibodies, which were incubated together with TOPRO-3 (Invitrogen, Carlsbad, CA, USA), as reported previously [10]. For the immunofluorescence staining of BSA, the tissue sections were visualized using Alexa Fluor 546-conjugated streptavidin (Invitrogen, Carlsbad, CA, USA) after incubation with primary sheep anti-BSA antibody (Sigma) and biotinylated donkey anti-sheep IgG antibody (Jackson ImmunoResearch Labs), as reported previously [1].
All the immunostained sections were embedded in glycerol or Vectashield (Vector Laboratories), and their light micrographs were taken using either a light microscope (BX-61; Olympus, Tokyo, Japan) or a confocal laser scanning microscope (FV1000; Olympus, Tokyo, Japan).
Semi-quantitative analyses of immunoreaction intensity
For the semi-quantitative analyses of IgG1 immunoreactivity as reported previously [23], twenty micrographs of heart tissue immunostained for IgG1 were randomly selected from the 4 hr BSA injection group and the normal group or four different preparation method groups. The immunoreactivity in their blood vessels, interstitial matrices, and cardiomyocytes was classified into five categories, which were negative (–), unclear (±), immunopositive (+), moderately immunopositive (2+), and strongly immunopositive (3+), by three investigators under a light microscope. Such semi-quantitative data of IgG1 immunoreactivity were statistically analyzed by the non-parametric Kruskal-Wallis H-test using SPSS 11.5 software for Windows (SPSS Inc., Chicago, IL, USA) [23]. A score of P<0.05 was considered to constitute a significant difference between the control and different experimental groups.
III. Results
Morphological analyses of heart specimens
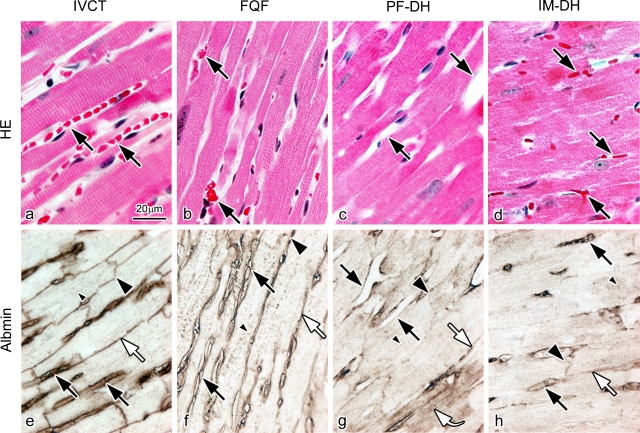
Serial sections prepared by IVCT, FQF, PF-DH, and IM-DH were first examined with HE staining (Fig. 3a–d) and immunostaining for serum albumin on apical parts of left ventricles. As shown in Figure 3a, with IVCT, the blood vessels are clearly open, and contain flowing erythrocytes. However, with FQF (Fig. 3b), erythrocytes are aggregated in the blood vessels of the resected heart tissue owing to loss of blood pressure. With PF-DH, no erythrocytes are seen in the widely opened blood vessels (Fig. 3c), although, with IM-DH, erythrocytes are seen in the collapsed blood vessels (Fig. 3d).
Fig. 3.
Light micrographs of hematoxylin-eosin staining (HE; a–d) and immunostaining for serum albumin (Albmin; e–h) on serial sections prepared by in vivo cryotechnique (IVCT; a, e), quick-freezing of resected fresh tissues (FQF; b, f), and perfusion-fixation (PF-DH; c, g) or immersion-fixation (IM-DH; d, h) followed by alcohol dehydration. (a), (e) In the specimens prepared by IVCT, blood vessels are open, containing flowing erythrocytes (a; black arrows). Immunoreactivity of albumin is clearly detected in blood vessels (e; black arrows), interstitium (white arrow), intercalated discs (large black arrowhead), and t-tubules (small black arrowhead). (b), (f) In the specimens prepared by FQF, the morphology with aggregated erythrocytes in collapsed blood vessels (b; black arrows) and albumin immunolocalization (f) are similar to those in the specimens prepared by IVCT. (c), (g) In the specimens prepared by PF-DH, no erythrocytes are seen in widely opened blood vessels (c; black arrows), and albumin is diffused into cardiomyocytes in some areas (g; curve white arrow). A curved arrow indicates immunoreactivity in the cytoplasm of cardiomyocytes. (d), (h) In contrast, congested erythrocytes are seen in collapsed blood vessels in the specimens prepared by IM-DH (d; black arrows). (g), (h) In the specimens prepared by PF-DH (g) or IM-DH (h), immunoreactivities of albumin are decreased in the interstitium (white arrows), intercalated discs (large black arrowheads), and t-tubules (small black arrowheads). Bar=20 µm.
Then, we also examined the immunolocalization of albumin (Fig. 3e–h) in heart tissues on the serial sections, corresponding to the tissue areas with HE staining (Fig. 3a–d). In specimens prepared with IVCT, immunoreactivity of albumin was detected not only in the blood vessels, but also on the lateral side among the cardiomyocytes representing the interstitial matrices. It was also detected in the intercalated discs and t-tubules of cardiomyocytes (Fig. 3e). The immunolocalization of albumin observed with FQF was similar to that with IVCT (Fig. 3f), showing that it was detected in the intercalated discs and t-tubules of cardiomyocytes in addition to the blood vessels and interstitial matrices. In contrast, in specimens prepared with PF-DH (Fig. 3g), the immunoreactivity of albumin became obscure in all parts. Some albumin was washed out from the blood vessels or the interstitium and some got into the cytoplasm of some cardiomyocytes, showing diffusion artifacts because of perfusion pressure (Fig. 3g, curved arrow). In specimens prepared with IM-DH (Fig. 3h), less albumin was seen in the intercalated discs and t-tubules of cardiomyocytes. Semi-quantitative analyses of serum albumin immunoreactivity among the four different preparation methods were carried out for the immunostained heart tissue of normal mice (Table 1). All these findings indicate that the morphology of heart tissue in various areas and the immunolocalization of serum proteins in vivo were significantly affected by conventional chemical fixation followed by alcohol dehydration during the preparation procedures of hearts. These findings also show that IVCT was the most useful for the morphological study of heart tissues in situ and immunohistochemical analyses of soluble serum proteins in living mouse hearts.
Table 1.
Semi-quantitative comparison among immunoreactivities of serum albumin in mouse heart tissues with different preparation methods
| IVCT | FQF | PF-DH | IM-DH | |
|---|---|---|---|---|
| Blood vessel | 2+ | + | ± | + |
| Interstitium | 2+ | + | – ~ + | ± |
| Intercalated disc | 2+ | + | – ~ + | – ~ + |
| T-tubule | + | + | – ~ + | – ~ + |
| Cytoplasm of cardiomyocyte | – | – | – ~ + | ± |
Negative: (–), Unclear: (±), Immunopositive: (+), Moderately immunopositive: (2+).
Immunolocalization of serum proteins in living mouse heart tissue
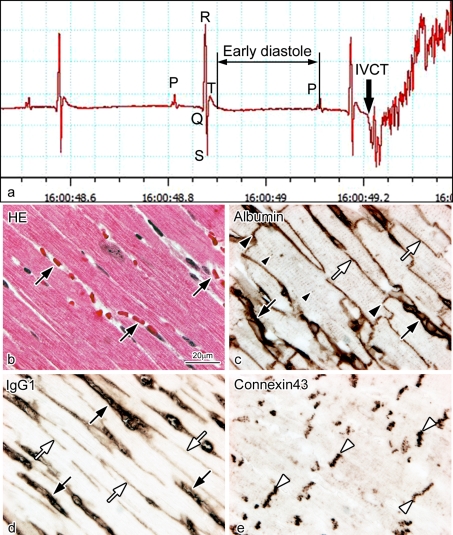
To determine the immunolocalization of different serum proteins in living mouse heart tissue monitored with electrocardiogram (ECG), serial paraffin sections prepared with IVCT were stained with HE and immunostained for albumin, IgG1, and connexin 43 (Fig. 4). With IVCT, the exact stopping time of a beating heart was accurately recorded by ECG (Fig. 4a), reflecting the living heart morphology at the time of freezing. In the present study, albumin passed across the vascular endothelium into the interstitium (Fig. 4c), and IgG1 also passed across the vascular endothelium and immunolocalized in the interstitium in addition to the blood vessels (Fig. 4d). Furthermore, serum albumin also got into the intercalated discs and t-tubules of cardiomyocytes (Fig. 4c). In addition, connexin 43 was clearly immunolocalized at gap junctions distributed in the intercalated discs (Fig. 4e).
Fig. 4.
Light micrographs of serial sections of mouse heart tissues prepared by IVCT. They were stained with hematoxylin-eosin (HE; b) or immunostained for albumin (c), immunoglobulin G1 (IgG1; d), and connexin 43 (e). By electrocardiogram (a), the exact stopping time of a diastolic heart is accurately recorded, reflecting its living morphology (b). Serum albumin and IgG1 are immunolocalized in blood vessels (c, d; black arrows) and interstitium (c, d; white arrows). The albumin is also immunolocalized in intercalated discs (c; black arrowheads) and dotted t-tubules (c; small black arrowheads). The connexin 43 (e; white arrowheads) is immunolocalized at gap junctions distributed in the intercalated discs. Bar=20 µm.
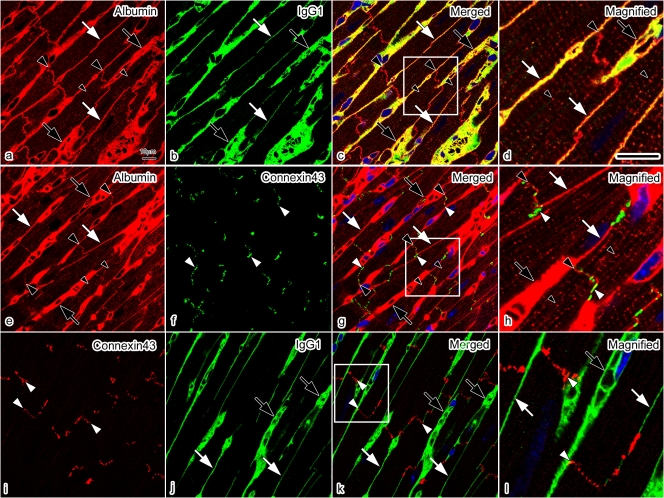
To clarify the relative immunolocalizations of albumin and IgG1 in the intercalated discs, we performed double-immunofluorescence staining for albumin and IgG1, albumin and connexin 43, and connexin 43 and IgG1 (Fig. 5). Double-immunofluorescence staining for albumin and IgG1 revealed that albumin was immunolocalized in the intercalated discs and t-tubules (Fig. 5a–d), and that both albumin and IgG1 were also immunolocalized in both the blood vessels and interstitium. Double-immunofluorescence staining for albumin and connexin 43 (Fig. 5e–h) revealed that the albumin was detected close to connexin 43, without overlapping with it, which represents the intercalated disc at the longitudinal terminal of the cardiomyocytes [19]. Double-immunofluorescence staining for connexin 43 and IgG1 revealed that IgG1 was not immunolocalized in the intercalated disc (Fig. 5i–l). They were more clearly shown at higher magnification (Fig. 5d, h, l).
Fig. 5.
Confocal laser scanning micrographs of double-immunofluorescence staining for albumin (red) and IgG1 (green) (a–d); albumin (red) and connexin 43 (green) (e–h); and connexin 43 (red) and IgG1 (green) (i–l) in living mouse heart tissues with IVCT. (a)–(d) Both albumin and IgG1 are immunolocalized in blood vessels (black arrows) and interstitium (white arrows). The albumin is also immunolocalized in intercalated discs (c; large black arrowheads) and t-tubules (c; small black arrowheads) without overlapping IgG1. At higher magnification (d), albumin is clearly immunolocalized in t-tubules (d; small black arrowheads), showing dot patterns. (e)–(h) By double-immunofluorescence staining for albumin and connexin 43, the albumin is shown to be closely immunolocalized in intercalated discs (e, g, h; large black arrowheads) with connexin 43 (white arrowheads) without overlapping each other. (i)–(l) By double-immunofluorescence staining for connexin 43 and IgG1, the IgG1 is shown to be immunolocalized in blood vessels (j; black arrows) and interstitium (j; white arrows), but not in the intercalated discs where connexin 43 is immunolocalized (k, l; white arrowheads). Bars=10 µm.
Time-dependent immunolocalizations of albumin and IgG1 after BSA injection
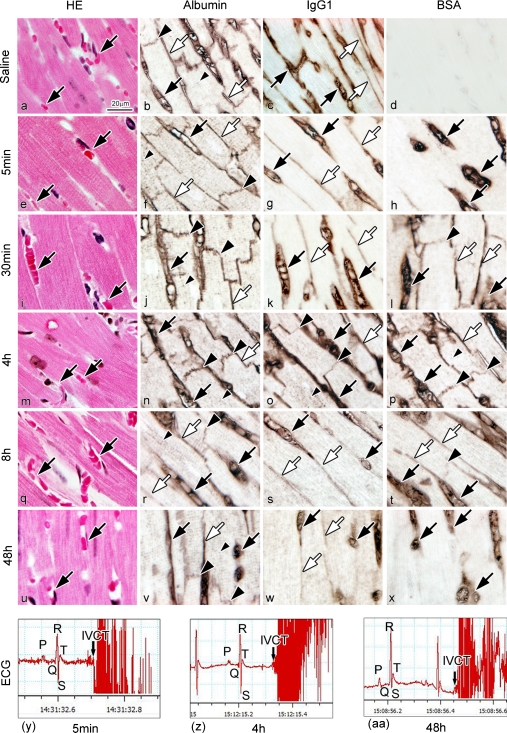
As described above, we found that endogenous serum albumin normally entered the intercalated discs and t-tubules of cardiomyocytes. To confirm immunolocalization of the mouse serum albumin, exogenous BSA with almost the same molecular weight, was injected into mouse tail veins at a concentration of 100 mg/ml, as reported previously [1, 12]. First, the immunolocalizations of endogenous mouse albumin or exogenous BSA were compared following various time-intervals after BSA injection, in addition to immunolocalization of serum IgG1. A series of paraffin sections were immunohistochemically examined for BSA, albumin, and IgG1 after saline injection and at 5 min, 30 min and 4 hr, 8 hr, and 48 hr after BSA injection (Fig. 6). With the saline injection, BSA immunoreactivity was not detected in living mouse heart tissue (Fig. 6d). At 5 min after BSA injection, BSA was immunolocalized only in the blood vessels (Fig. 6h). At 30 min, it was immunolocalized in both the blood vessels and interstitium, and also slightly in intercalated discs and t-tubules of cardiomyocytes (Fig. 6l), in a pattern similar to that of albumin (Fig. 6j). At 48 hr, it disappeared from the intercalated discs, t-tubules, and also the interstitium, and was only immunolocalized in the blood vessels (Fig. 6x). As both the endogenous and exogenous serum albumins were immunolocalized in the intercalated discs and t-tubules of the cardiomyocytes, it is undoubtedly confirmed that the mouse serum albumin in vivo could enter both the intercalated discs and t-tubules, and was one of the important components comprising the extracellular microenvironment around cardiomyocytes. IgG1 was also slightly immunolocalized in the intercalated discs at 4 hr after the BSA injection (Fig. 6o), and recovered to its normal immunolocalization of mouse heart tissue at 8 hr (Fig. 6s). Semi-quantitative comparison among relative immunoreactivities of albumin, BSA, and IgG1 in different areas of the heart tissue was performed for normal mice and the mice with different BSA injections (Table 2). Semi-quantitative analyses of IgG1 immunoreactivity in different areas of the heart tissue were also performed in normal mice and the 4 hr BSA injection mice (Table 3). The recorded ECGs were first compared between the experimental and control groups to check whether there were any changes of electrical impulses after the BSA injection. As shown in Figure 6y–aa, there were no significant differences between BSA injection samples.
Fig. 6.
Light micrographs of HE-stained heart sections with IVCT after saline injection (a) or at various times after the BSA injection (e, i, m, q, u). Serial sections are immunostained for albumin (b, f, j, n, r, v), IgG1 (c, g, k, o, s, w), and BSA (d, h, l, p, t, x). After the saline injection (a–d), the BSA immunostaining is not detected (d). At 5 min after the BSA injection (e–h), BSA is immunolocalized only in blood vessels (h; black arrows). At 30 min (i–l), BSA is immunolocalized in blood vessels (l; black arrows), interstitium (l; white arrows), and intercalated discs (l; large black arrowhead), in the same pattern as the albumin immunolocalization (j). At 48 hr (u–x), BSA is immunolocalized in blood vessels (x; black arrows), but not in intercalated discs and interstitium. At 4 hr (m–p), IgG1 is immunolocalized in intercalated discs (o; large black arrowheads), blood vessels (o; black arrows), and interstitium (o; white arrow). At 8 hr (q–t), IgG1 is only immunolocalized in blood vessels (s; black arrows) and interstitium (s; white arrows). (y)–(aa) Arrows in ECG show the exact times of freezing hearts with IVCT at 5 min (y), 4 hr (z), and 48 hr (aa) after the BSA injection, showing early diastolic phases. Bar=20 µm.
Table 2.
Semi-quantitative comparison among immunoreactivities of albumin (Alb), BSA, and IgG1 in living mouse hearts with IVCT under normal and saline injection or at various time-intervals after BSA (100 mg/ml) injection
| Normal or saline injection | BSA injection | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5 min | 30 min | 4 hr | 8 hr | 48 hr | |||||||||||||||||||
| Alb | BSA | IgG1 | Alb | BSA | IgG1 | Alb | BSA | IgG1 | Alb | BSA | IgG1 | Alb | BSA | IgG1 | Alb | BSA | IgG1 | ||||||
| Blood vessel | 3+ | – | 2+ | 3+ | 3+ | 2+ | 3+ | 3+ | 2+ | 3+ | 3+ | 2+ | 3+ | 3+ | 2+ | 3+ | 2+ | 2+ | |||||
| Interstitium | 2+ | – | + | 2+ | – | + | 2+ | 2+ | + | 2+ | 2+ | + | 2+ | 2+ | + | 2+ | – | + | |||||
| Intercalated disc | + | – | – | + | – | – | + | ± | – | + | ± | + ~ ± | + | ± | ∠ | + | ∠ | ∠ | |||||
| T-tubule | + | – | – | + | – | – | + | ± | – | + | ± | + ~ ± | + | ± | ∠ | + | ∠ | ∠ | |||||
Negative: (–), Unclear: (±), Immunopositive: (+), Moderately immunopositive: (2+), Strongly immunopositive: (3+).
Table 3.
Semi-quantitative comparison of relative IgG1 immunoreactivity in different areas of living mouse heart tissues
| – | ± | + | 2+ | |
|---|---|---|---|---|
| Blood vessels | ||||
| Normal | 0 | 0 | 0 | 20 |
| BSA injection 4 hr | 0 | 0 | 0 | 20 |
| Interstitium | ||||
| Normal | 0 | 5 | 15 | 0 |
| BSA injection 4 hr | 0 | 2 | 18 | 0 |
| Intercalated disc* | ||||
| Normal | 20 | 0 | 0 | 0 |
| BSA injection 4 hr | 0 | 12 | 8 | 0 |
Negative: (–), Unclear: (±), Immunopositive: (+), Moderately immunopositive: (2+). *P<0.05
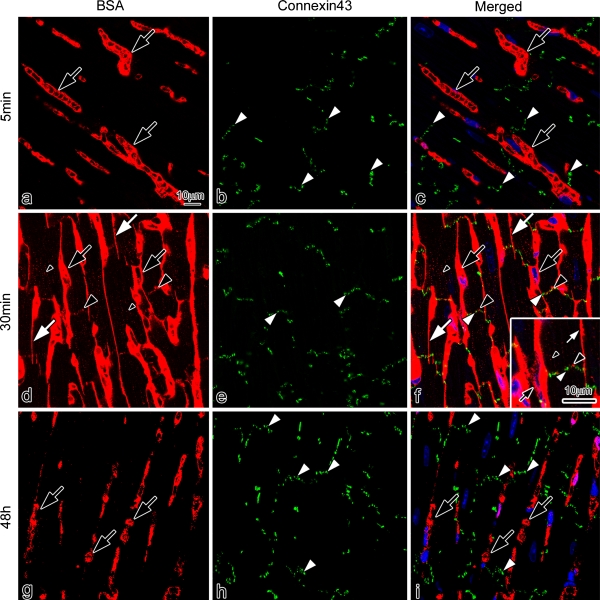
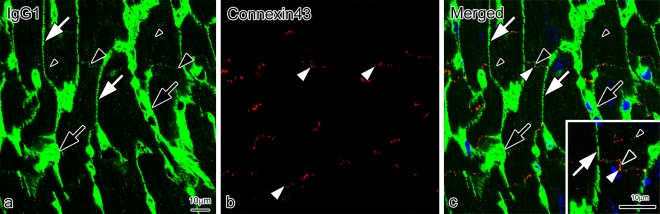
To more clearly characterize the immunolocalization of BSA, double-immunofluorescence staining for BSA and connexin 43 was performed in a single section (Fig. 7). The BSA was immunolocalized only in blood vessels at 5 min after BSA injection (Fig. 7a, c). At 30 min, it was immunolocalized in the blood vessels, interstitium, and both the intercalated discs and t-tubules of cardiomyocytes (Fig. 7d, f), in the same pattern as that of mouse serum albumin. At higher magnification (Fig. 7f: inset), the BSA was closely immunolocalized in the intercalated discs with connexin 43. At 48 hr, it was immunolocalized only in the blood vessels, and disappeared from the interstitium, intercalated discs, and t-tubules (Fig. 7g, i). To clarify the translocation of mouse serum IgG1 entering the intercalated discs at 4 hr after BSA injection, double-immunofluorescence staining for both IgG1 (Fig. 8a, c) and connexin 43 (Fig. 8b, c) was performed in a single section. The serum IgG1 was immunolocalized not only in the blood vessels and interstitium, but also slightly in the intercalated discs and t-tubules of cardiomyocytes (Fig. 8a, c). At higher magnification (Fig. 8c, inset), IgG1 and connexin 43 were closely immunolocalized in the intercalated discs.
Fig. 7.
Confocal laser scanning micrographs of double-immunofluorescence staining for BSA (a, d, g; red color) and connexin 43 (b, e, h; green color) on the sections of mouse heart tissues prepared by IVCT at 5 min (a–c), 30 min (d–f), and 48 hr (g–i) after the BSA injection. The BSA is immunolocalized only in blood vessels (a, c; black arrows) at 5 min. (d)–(f) At 30 min, it is immunolocalized in blood vessels (black arrows), interstitium (white arrows), intercalated discs (d, f; large black arrowheads), and t-tubules (small black arrowheads). At a higher magnification (f; inset), the BSA is closely immunolocalized in intercalated discs with connexin 43 (green color; white arrowhead). (g)–(i) At 48 hr, it is immunolocalized only in blood vessels (black arrows), and lost from the interstitium, intercalated discs, and t-tubules. Bars=10 µm.
Fig. 8.
Confocal laser scanning micrographs of double-immunofluorescence staining for IgG1 (a, c; green color) and connexin 43 (b, c; red color) on sections of mouse heart tissues prepared by IVCT at 4 hr after BSA injection. The IgG1 (green color) is immunolocalized in blood vessels (black arrows), interstitium (white arrows), slightly in intercalated discs (large black arrowheads), and t-tubules (small black arrowheads) at 4 hr after BSA injection. At the higher magnification (c; inset), IgG1 and connexin 43 (white arrowhead) are closely immunolocalized in the intercalated discs (large black arrowhead). Bars=10 µm.
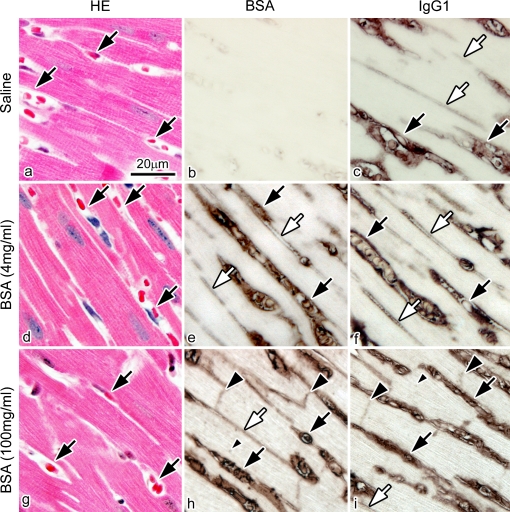
Then, to determine whether the immunolocalization of IgG1 at 4 hr after BSA injection was associated with high concentrations of injected BSA, the physiological saline and the different concentrations of BSA were injected into the tail veins. Serial paraffin sections were immunostained for BSA and IgG1 at 4 hr after the BSA injection, as shown in Figure 9. Compared with the group of BSA injection at a high concentration of 100 mg/ml, both BSA and IgG1 were immunolocalized only in blood vessels and interstitium with an injection of a low concentration of 4 mg/ml BSA, without IgG1 immunolocalization in either the intercalated discs or t-tubules of cardiomyocytes (Fig. 9e, f). These findings indicate that the concentration of injected BSA was a factor that affected the abnormal immunolocalization of native IgG1 in living mouse heart tissue.
Fig. 9.
Light micrographs of serial sections of mouse heart tissues prepared by IVCT at 4 hr after the saline injection or at two concentrations of 4 mg/ml or 100 mg/ml BSA injection. The serial sections are stained with hematoxylin-eosin (HE; a, d, g) or immunostained for BSA (b, e, h) and IgG1 (c, f, i). (b) There is no immunopositive staining for BSA at 4 hr after the saline injection. (e), (f) The BSA and IgG1 are immunolocalized only in blood vessels (black arrows) and interstitium (white arrows) at 4 hr at the concentration of 4 mg/ml BSA. (h), (i) However, they are immunolocalized in the intercalated discs (large black arrowheads) and t-tubules (small black arrowhead) in addition to blood vessels (black arrows) and interstitium (white arrow) at the concentration of 100 mg/ml. Bar=20 µm.
IV. Discussion
The conventional chemical fixation of resected animal tissues is well known to easily cause some preparation artifacts of the dynamically changing microenvironment in cells and tissues, because of the loss of blood flow or the translocation of soluble serum proteins. As the dynamic microenvironment around the cardiomyocytes is closely associated with the transduction of electrical signals and mechanical contraction or relaxation coupling between them at intercalated discs [7], the histopathological characteristics of such a microenvironment is more significant for indicating the physiological and pathological states of the beating heart than was previously thought. ECG can record the cardiac cycle, which reflects the mechanical contraction and relaxation of living animal hearts. During each cardiac cycle of ECG, the early or the late diastole corresponds to the TP or the PR interval, respectively [17]. Therefore, the early diastolic morphology of living mouse heart could be accurately captured by combining the present IVCT with ECG monitoring, as shown in Figure 4. However, both the isovolumic contraction and ejection phases are too short to be captured by ECG. The present immunohistochemical findings with ECG monitoring suggest that the new IVCT could prevent the common artificial changes in cells and tissues due to ischemia and anoxia, and could contribute to morphofunctional and immunohistochemical examination of living mouse heart with fewer preparation artifacts during chemical fixation and alcohol dehydration, as shown in Figure 3.
The intercalated discs between individual cardiomyocytes are a main site of their interconnection, and possess considerable functional significance in terms of the mechanism of contraction or relaxation of the total myocardium. The specific membrane areas of the intercalated discs have already been established to exhibit a close association with a variety of intracellular and extracellular structures [4]. Although many studies have been reported to describe the characterization of the intercalated discs, little convincing data have been provided as to whether soluble components in vivo can freely pass through the intercalated discs of living animal hearts, and create the extracellular microenvironment around the functioning cardiomyocytes. The present study with IVCT is the first to immunohistochemically demonstrate the localization of endogenous serum proteins with different molecular sizes at the intercalated discs of the beating hearts in situ, and also to indicate more clearly the effect of BSA injection on serum components in the extracellular microenvironment.
It is well known that the interstitial fluid of living animal tissues consists of water solvent containing various chemical components, such as amino acids, proteins, carbohydrates, fatty acids, hormones, and ions, but large molecular-weight plasma proteins in vivo usually have difficulty passing through the commonly sealed walls of the blood capillaries, depending on dynamically different structures of various organ tissues. In the present study, it was shown that mouse serum albumin of about 69 kDa molecular weight was translocated across the vascular endothelium into interstitial matrices of living mouse heart tissue in addition to its localization within the blood vessel. It was also immunolocalized at the intercalated discs of cardiomyocytes and their t-tubules under normal blood circulation, as clearly shown in Figure 5. Then, we confirmed that the exogenous BSA also crossed the vascular endothelium into the interstitium of living mouse hearts and entered the intercalated discs and t-tubules of cardiomyocytes 30 min after BSA injection, as shown in Figure 6. The distribution in vivo of its own serum albumin in the interstitium, intercalated discs, and t-tubules of living mice heart tissues indicated that the mouse albumin might be one of the components that support the extracellular microenviroment around each cardiomyocyte of the functioning heart. The dynamically flowing serum proteins, probably as molecular carriers crossing the microvascular walls dynamically changed by beating hearts, could provide the living mouse heart tissues with not only various antibodies, protein-bound hormones, cytokines, and chemokines, but also calcium, various ions, and transferrin [2, 16].
In the present study, IgG1 of about 150 kDa molecular weight was also translocated across the vascular endothelium into the interstitium of living mouse heart tissue. Although one previous morphological study clarified the nitrated albumin transport across the continuous vascular endothelium [15], the present study with IVCT showed the immunolocalization of such intermediate-sized serum proteins, IgG1, in the living mouse heart interstitium [16]. This is possibly due to the cardiac microvascular endothelium of living mice possessing functionally much less permselectivity, which more efficiently transports various serum proteins with relatively large molecular weights. In addition, compared with serum albumin, the larger IgG1 could not enter either the intercalated discs or the t-tubules of cardiomyocytes under normal blood circulation conditions, as shown in Figure 5a–d. These immunohistochemical differences of serum proteins after BSA injection indicated that the molecular sizes might also be responsible for the intercalated disc and t-tubule selectivity in beating heart tissues. On the other hand, all these morphofunctional findings could also be explained partly by the difference of tissue preparation methods because the immunoreactivity of serum proteins within blood vessels was significantly modified, when prepared by IM-DH or PF-DH because of the loss of blood circulation, the washing of perfusion fixation, and chemical fixation with alcohol dehydration [8, 22].
In the present study, the time-dependent immunolocalization of exogenous BSA was revealed in the interstitial matrix and cardiomyocytes by IVCT. The abnormal immunolocalization of serum IgG1 at the intercalated discs and t-tubules was displayed 4 hr after BSA injection with a concentration of 100 mg/ml, although there was no abnormal immunolocalization of serum IgG1 when the concentration of injected BSA was decreased to 4 mg/ml. Although the mechanism of such abnormal IgG1 localization has yet to be clarified, the time-intervals after the BSA injection and its concentrations were both critical factors for the translocation of serum proteins in vivo through the blood capillaries of living mouse heart.
In conclusion, the present immunohistochemical study with ECG monitoring demonstrated that the distribution of soluble serum proteins was precisely revealed by the IVCT without the preparation artifacts usually caused by the conventional chemical fixation and dehydration. With IVCT, serum albumin in vivo was found to be one of the components that contributed to the extracellular microenvironment around cardiomyocytes, and the different immunolocalizations of serum proteins indicated molecular selectivity at the intercalated discs. When the cardiac microcirculation in vivo, including the regulatory mechanisms of functional coupling between cardiomyocytes and the propagation of electrical impulses at the intercalated discs, can be intensely studied because of its clinical significance in heart disease, IVCT will provide a useful approach for immunohistochemical and morphofunctional studies of cardiac microcirculation and microenvironment under various heart conditions in living animals.
V. References
- 1.Bai Y., Ohno N., Terada N., Saitoh S., Nakazawa T., Nakamura N., Katoh R., Ohno S. Immunolocalization of serum proteins in xenografted mouse model of human tumor cells by various cryotechniques. Histol. Histopathol. 2009;24:717–728. doi: 10.14670/HH-24.717. [DOI] [PubMed] [Google Scholar]
- 2.Fitzpatrick F. A., Liggett W. F., Wynalda M. A. Albumin-eicosanoid interactions. A model system to determine their attributes and inhibition. J. Biol. Chem. 1984;259:2722–2727. [PubMed] [Google Scholar]
- 3.Hippe-Sanwald S. Impact of freeze substitution on biological electron microscopy. Microsc. Res. Tech. 1993;24:400–422. doi: 10.1002/jemt.1070240506. [DOI] [PubMed] [Google Scholar]
- 4.Hoyt R. H., Cohen M. L., Saffitz J. E. Distribution and three-dimensional structure of intercellular junctions in canine myocardium. Circ. Res. 1989;64:563–574. doi: 10.1161/01.res.64.3.563. [DOI] [PubMed] [Google Scholar]
- 5.Kellenberger E. The potential of cryofixation and freeze substitution: observations and theoretical considerations. J. Micro. 1991;161:183–203. doi: 10.1111/j.1365-2818.1991.tb03083.x. [DOI] [PubMed] [Google Scholar]
- 6.Li Z., Ohno N., Terada N., Ohno S. Immunolocalization of serum proteins in living mouse glomeruli under various hemodynamic conditions by ‘in vivo cryotechnique’. Histochem. Cell Biol. 2006;126:399–406. doi: 10.1007/s00418-006-0175-4. [DOI] [PubMed] [Google Scholar]
- 7.Noorman M., van der Heyden M. A., Cox M. G., Hauer R. N., de Bakker J. M., van Rijen H. V. Cardiac cell-cell junctions in health and disease: Electrical versus mechanical coupling. J. Mol. Cell. Cardiol. 2009;47:23–31. doi: 10.1016/j.yjmcc.2009.03.016. [DOI] [PubMed] [Google Scholar]
- 8.Ohno N., Terada N., Ohno S. Advanced application of the in vivo cryotechnique to immunohistochemistry for animal organs. Acta Histochem. Cytochem. 2004;37:357–364. [Google Scholar]
- 9.Ohno N., Terada N., Murata S., Katoh R., Ohno S. Application of cryotechniques with freeze-substitution for the immunohistochemical demonstration of intranuclear pCREB and chromosome territory. J. Histochem. Cytochem. 2005;53:55–62. doi: 10.1177/002215540505300107. [DOI] [PubMed] [Google Scholar]
- 10.Ohno N., Terada N., Ohno S. Histochemical analyses of living mouse liver under different hemodynamic conditions by ‘in vivo cryotechnique’. Histochem. Cell Biol. 2006;126:389–398. doi: 10.1007/s00418-006-0173-6. [DOI] [PubMed] [Google Scholar]
- 11.Ohno N., Terada N., Saitoh S., Zhou H., Fujii Y., Ohno S. Recent development of in vivo cryotechnique to cryobiopsy for living animals. Histol. Histopathol. 2007;22:1281–1290. doi: 10.14670/HH-22.1281. [DOI] [PubMed] [Google Scholar]
- 12.Ohno N., Terada N., Bai Y., Saitoh S., Nakazawa T., Nakamura N., Naito I., Fujii Y., Katoh R., Ohno S. Application of cryobiopsy to morphological and immunohistochemical analyses of xenografted human lung cancer tissues and functional blood vessels. Cancer. 2008;113:1068–1079. doi: 10.1002/cncr.23701. [DOI] [PubMed] [Google Scholar]
- 13.Ohno S., Terada N., Fujii Y., Ueda H., Takayama I. Dynamic structure of glomerular capillary loop as revealed by an in vivo cryotechnique. Virchows Arch. 1996;427:519–527. doi: 10.1007/BF00199513. [DOI] [PubMed] [Google Scholar]
- 14.Ohno S., Terada N., Ohno N., Saitoh S., Saitoh Y., Fujii Y. Significance of ‘in vivo cryotechnique’ for morphofunctional analyses of living animal organs. J. Electron Microsc. 2010;59:395–408. doi: 10.1093/jmicro/dfq058. [DOI] [PubMed] [Google Scholar]
- 15.Predescu D., Predescu S., Malik A. B. Transport of nitrated albumin across continuous vascular endothelium. Proc. Natl. Acad. Sci. U S A. 2002;99:13932–13937. doi: 10.1073/pnas.212253499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rippe B., Rosengren B. I., Carlsson O., Venturoli D. Transendothelial transport: the vesicle controversy. J. Vasc. Res. 2002;39:375–390. doi: 10.1159/000064521. [DOI] [PubMed] [Google Scholar]
- 17.Sengupta P. P., Korinek J., Belohlavek M., Narula J., Vannan M. A., Jahangir A., Khandheria B. K. Left ventricular structure and function basic science for cardiac imaging. J. Am. Coll. Cardiol. 2006;48:1988–2001. doi: 10.1016/j.jacc.2006.08.030. [DOI] [PubMed] [Google Scholar]
- 18.Shiurba R. Freeze-substitution: origins and applications. Int. Rev. Cytol. 2001;206:45–96. doi: 10.1016/s0074-7696(01)06019-3. [DOI] [PubMed] [Google Scholar]
- 19.Smith J. H., Green C. R., Peters N. S., Rothery S., Severs N. J. Altered Patterns of gap junction distribution in ischemic heart disease: an immunohistochemical study of human myocardium using laser scanning confocal microscopy. Am. J. Pathol. 1991;139:801–821. [PMC free article] [PubMed] [Google Scholar]
- 20.Terada N., Ohno N., Ohguro H., Li Z., Ohno S. Immunohistochemical detection of phosphorylated rhodopsin in light-exposed retina of living mouse with in vivo cryotechnique. J. Histochem. Cytochem. 2006;54:479–486. doi: 10.1369/jhc.5A6844.2006. [DOI] [PubMed] [Google Scholar]
- 21.Terada N., Ohno N., Saitoh S., Ohno S. Immunohistochemical detection of hypoxia in mouse liver tissues treated with pimonidazole using “in vivo cryotechnique”. Histochem. Cell Biol. 2007;128:253–261. doi: 10.1007/s00418-007-0324-4. [DOI] [PubMed] [Google Scholar]
- 22.Tong J., Huang L., Huang J., Wang H., Chen D., Zeng L., Zhou J., Luo X. Improved method of ink-gelatin perfusion for visualising rat retinal microvessels. Acta Histochem. Cytochem. 2008;41:127–133. doi: 10.1267/ahc.08015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhou H., Ohno N., Terada N., Saitoh S., Naito I., Ohno S. Permselectivity of blood follicle barriers in mouse ovaries of the mifepristone-induced polycystic ovary model revealed by in vivo cryotechnique. Reproduction. 2008;136:599–610. doi: 10.1530/REP-08-0022. [DOI] [PubMed] [Google Scholar]