Abstract
Fukutin is a gene responsible for Fukuyama-type congenital muscular dystrophy (FCMD), accompanying ocular and brain malformations represented by cobblestone lissencephaly. Fukutin is related to basement membrane formation via the glycosylation of α-dystoglycan (α-DG), and astrocytes play a crucial role in the pathogenesis of the brain lesion. On the other hand, its precise function in neurons is unknown. In this experiment, the roles of fukutin in mature and immature neurons were examined using brains from control subjects and FCMD patients and cultured neuronal cell lines. In quantitative PCR, the expression level of fukutin looked different depending on the region of the brain examined. A similar tendency in DG expression appears to indicate a relation between fukutin and α-DG in mature neurons. An increase of DG mRNA and core α-DG in the FCMD cerebrum also supports the relation. In immunohistochemistry, dot-like positive reactions for VIA4-1, one of the antibodies detecting the glycosylated α-DG, in Purkinje cells suggest that fukutin is related to at least a post-synaptic function via the glycosylation of α-DG. As for immature neurons, VIA4-1 was predominantly positive in cells before and during migration with expression of fukutin, which suggest a participation of fukutin in neuronal migration via the glycosylation of α-DG. Moreover, fukutin may prevent neuronal differentiation, because its expression was significantly lower in the adult cerebrum and in differentiated cultured cells. A knockdown of fukutin was considered to induce differentiation in cultured cells. Fukutin seems to be necessary to keep migrating neurons immature during migration, and also to support migration via α-DG.
Keywords: fukutin, α-dystroglycan, synapse, migration, differentiation
I. Introduction
Fukuyama-type congenital muscular dystrophy (FCMD) is an autosomal recessive disease, which manifests as muscular dystrophy with central nervous system (CNS) and ocular malformations [2, 20]. The most common CNS lesion is cobblestone lissencephaly of the cerebrum and cerebellum. A gene responsible for FCMD is fukutin [9]. Fukutin is related to the glycosylation of α-dystroglycan (DG), which is involved in basement membrane formation. A product from single DG mRNA is cleaved into α-DG and β-DG [13, 16]. α-DG is a heavily glycosylated extracellular protein and one of the components of the dystrophin-glycoprotein complex (DGC). The sugar chains of α-DG are receptors for extracellular matrix proteins such as laminin, and glycosylated α-DG is observed in the sarcolemma of the skeletal muscle [13, 16]. Reduced glycosylation of α-DG causes muscular dystrophy [5].
The basement membrane covers the glia limitans, a structure observed in the CNS surface, where the glycosylated α-DG is observed [34]. In CNS lesions of FCMD fetuses, glioneuronal tissues protrude into the leptomeninges through disruptions of the glia limitans [18, 32, 34], in which the glycosylation of α-DG is reduced [34]. Therefore, hypoglycosylation of α-DG is considered to be a main cause of the disruption of the glia limitans, resulting in the cobblestone lissencephaly. The glia limitans is formed by astrocytic endfeet, and in FCMD patients, the basement membrane and cell membrane of astrocytes is abnormal, electron microscopically [7, 31]. Astrocytes appear to be greatly involved in the pathogenesis of the CNS lesion [34, 37]. On the other hand, its function in neurons is unknown. Although neurons do not form the basement membrane, both fukutin and α-DG are expressed in mature and immature neurons [24–26, 33, 37]. It has been supposed that fukutin participates in neuronal migration and synaptic function through the glycosylation of α-DG by immunohistochemical studies. However, quantitative analysis has yet to be done, and to the best of our knowledge there are no biochemical analyses that directly prove the relationship between fukutin and the glycosylation of α-DG in neurons. Neurons are the leading component in the CNS. To clarify the roles of fukutin in neurons is indispensable for understanding the whole pathomechanism of CNS lesions of FCMD and for supporting a future gene therapy.
In this study, we investigated whether expressions of fukutin and α-DG differ in different regions of the brain, whether fukutin works via α-DG in neurons, and whether these expressions show developmental changes in neurons, using brain tissues from neurologically normal control subjects and FCMD individuals, as well as neuron cell lines. Quantitative PCR analysis (qPCR) and immunohistochemistry were performed. Moreover, RNA interference (RNAi) was performed in a human neuroblastoma cell line to evaluate influence on cellular differentiation of fukutin.
II. Materials and Methods
Brain tissues
For qPCR, brain tissues from a FCMD patient (aged 27 years) and 8 neurologically normal control subjects (5 fetuses aged 22 to 39 weeks of gestation [gw] and 3 adults aged 56 to 77 years) were obtained at autopsy within 16 hr after death. Tissue samples were immediately frozen and stored at −80°C. Immunohistochemistry was performed on brain tissues from 6 postnatal FCMD patients (aged 6 to 27 years) and 7 control subjects (aged 23 gw to 31 years) within 37 hr after death.
Each autopsy was performed after family members granted informed consent in accordance with the Ethical Guidelines of Tokyo Women’s Medical University and the Helsinki Declaration.
Cell lines
Hybrid cell line consisting of murine spinal motor neuron and neuroblastoma (NSC34) was purchased from CELLutions Biosystems Inc (Ontario, Canada). Cells were grown in high-glucose Dulbecco’s MEM (Invitrogen, Carlsbad, CA, USA), supplemented with 10% fetal bovine serum (Thermo Fisher Scientific, Waltham, MA, USA), 1% penicillin-streptomycin (Invitrogen) and 2 mM glutamine (Invitrogen). Cells were differentiated by treatment with 1:1 DMEM and Ham’s F12 (Invitrogen), supplemented with 1% FBS, 1% penicillin-streptomycin, 1% glutamine and 1% MEM and non-essential amino acids solution (Invitrogen) for 48 hr.
Human neuroblastoma cell line (IMR-32) was purchased from Health Science Research Resources Bank (Osaka, Japan). Cells were grown in MEM (Invitrogen), supplemented with 10% fetal bovine serum and 1% penicillin-streptomycin.
Cells were maintained at 37°C in a humidified incubator with a 5% CO2 atmosphere.
RNA extraction and qPCR
On adult control subjects, tissue samples from the gray matter of the frontal lobe, putamen, thalamus, hippocampal head, parahippocampal gyrus, substantia nigra, red nucleus, and inferior olivary nucleus were used. The inferior olivary nucleus was obtained only from one case. On fetal control subjects, tissue samples from the cerebrum and cerebellum were used. On the FCMD patient, a tissue sample from the cerebrum was used. RNA was extracted from brain tissues with RNeasy Lipid Tissue Mini kit (Qiagen, Valencia, CA, USA). Reverse-transcription was performed using Prime Script RT-PCR kit (Takara, Tokyo, Japan).
qPCR was performed using the SYBR green method on a Thermal Cycler Dice TP800 real time system (Takara) according to the manufacturer’s instruction. Reactions were set up with cDNA corresponding to 50 ng total RNA, primers (50 pM final concentration), and SYBR Premix ExTaq II (Takara). The sequences of gene specific primers used were as follows: fukutin forward 5'-CAT TCT GGC TGA GCA GTG GAA C-3' /reverse 5'-GAA GTC CTG CAT CCT GAA ATG CTA A-3', DG forward 5'-GCG CTC ATT TCG AGT GAC CA-3' /reverse 5'-TGA GTC CCA GTG CAG CCA AG-3'. The amplification profiles consisted of 95°C for 10 sec, followed by 40 cycles at 95°C for 5 sec and 60°C for 30 sec. At the end of each run, a melting point analysis was performed to validate the specificity of the PCR products. The quality of the PCR products was also confirmed by agarose-gel electrophoresis. All samples were analyzed in triplicate. Four different housekeeping genes, β-actin, GAPDH, HPRT1, and β2-microglobulin, were used to qualify the expression levels of fukutin and DG mRNA. Since all the housekeeping genes showed a similar result, β-actin was used for the experiment of brain tissues. Primers of housekeeping genes were purchased from Takara. Relative quantification was performed using the ΔΔ Ct method. For comparison of data between fetuses and adults, tissue sample from white matter of the neurologically normal adult brain was used for standardization.
Antibodies
Primary antibodies used were anti-fukutin antibody (rabbit, polyclonal [33]), anti-α-DG antibody against the laminin binding site of glycosylated epitope of α-DG (IIH6C4, mouse, monoclonal, Upstate Biotechnology, Lake Placid, NY, USA), anti-α-DG antibody against glycosylated epitope of α-DG (VIA4-1, mouse, monoclonal, Upstate Biotechnology), and anti-α-DG antibody against core peptide of α-DG (α-DG-p, sheep, polyclonal, generously provided by Dr. S. Kröger [6]).
Immunohistochemistry
All brain tissues were fixed in 10% formalin, embedded in paraffin, and cut into 4-µm-thick sections. The frontal or parietal lobe, thalamus, medulla oblongata, and cerebellum were examined. Details of antibody dilution, antigen retrieval, and detection methods are shown in Table 1. Briefly, after deparaffinization, antigen retrieval was performed. Sections were immersed in 0.3% hydrogen peroxide for 10 min to quench endogenous peroxidase activity. Nonspecific binding was blocked with 5% skim milk for 10 min. The sections were then incubated overnight at 4°C with primary antibody. The immunoproducts were visualized with Liquid DAB+Substrate Chromogen System (DAKO, Glostrup, Denmark). The specimens were counterstained with hematoxylin.
Table 1.
Primary antibodies for immunohistochemistry
| antigen | dilution | antigen retrieval | detection |
|---|---|---|---|
| fukutin | 1:50 | 0.1% trypsin, 37°C 30 min. | CSA or polymer HRP |
| IIH6C4 | 1:1000 | citrate pH 6.0, MW 20 min. | polymer HRP |
| VIA4-1 | 1:50 | citrate pH 6.0, MW 20 min. | polymer HRP |
| α-DG-p | 1:1000 | EDTA pH 9.0, MW 40 min. | ABC |
CSA: catalyzed signal amplification (CSA system, Dako).
polymer HRP: polymer horseradish peroxidase (EnVision, Dako).
ABC: avidin-biotin-peroxidase complex (Vectastatin ABC system, Vector laboratories).
RNAi
RNAi was performed as described previously [36]. Stealth siRNA duplex for fukutin mRNA was designed and synthesized by Invitrogen. The target sense for fukutin was 5'-UUUGGAAGGGAACAAAUUUCCUGUC-3' (F697). Scrambled negative control StealthTM RNA (SNC, Invitrogen) was used for negative control. Omission of siRNA was always performed as a negative control in each experiment. Neuroblastoma cells were plated one day before transfection at a density of about 200,000 cells in each 35-mm dish. Antibiotics were omitted from the medium. Then siRNA (40 nM final concentration) was transfected into the cells using lipofectamine MAX (Invitrogen) and Opti-MEM (Invitrogen) according to the manufacturer’s instructions. The culture medium was changed to the standard formulation one day after transfection. Four days after transfection, cells were harvested and suppression of each gene was confirmed by RT-PCR and/or real time PCR. Moreover the length of neuronal processes was measured and the mean length was calculated (control: n=128 neuronal processes, and fukutin-knockdown cells: n=183 neuronal processes). The measurement was performed on neuronal processes whose branching from the cell body was clearly determined, and a length between the cell surface to the terminal of neuronal process was measured.
Statistical analysis
The significance of differences between groups was evaluated by unpaired t-test, Mann-Whitney test, and non-repeated ANOVA. Values of p<0.05 were considered significant.
III. Results
Regional differences in fukutin and DG expressions in the adult control brain
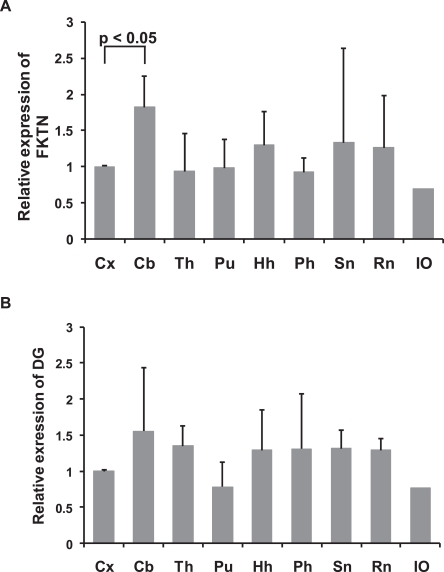
On qPCR, the relative expression level of fukutin mRNA was significantly higher in the cerebellum than in the cerebral cortex. Its expression was relatively equivalent in the cerebral cortex, thalamus, putamen, hippocampal head, parahippocampal gyrus, substantia nigra, and red nucleus (Fig. 1A). The expression level of DG mRNA tended to be similar to that of fukutin mRNA (Fig. 1B). Its expression in the cerebellum tended to be increased, but the difference was not significant.
Fig. 1.
Relative expression of fukutin (A) and DG mRNA (B) in different regions of the adult control brain. A. The expression of fukutin mRNA is significantly higher in the cerebellum compared to that in the cerebrum. Its expressions in the other 6 portions are relatively equivalent. B. The pattern of expression tends to be similar to that of fukutin, but the difference does not reach significance. Whiskers indicate the standard deviation. FKTN, fukutin; DG, dystroglycan; Cx, cerebral cortex; Cb, cerebellum; Th, thalamus; Pu, putamen; Hh, hippocampal head; Ph, parahippocampal gyrus; SN, substantia nigra; RN, red nucleus; IO, inferior olivary nucleus.
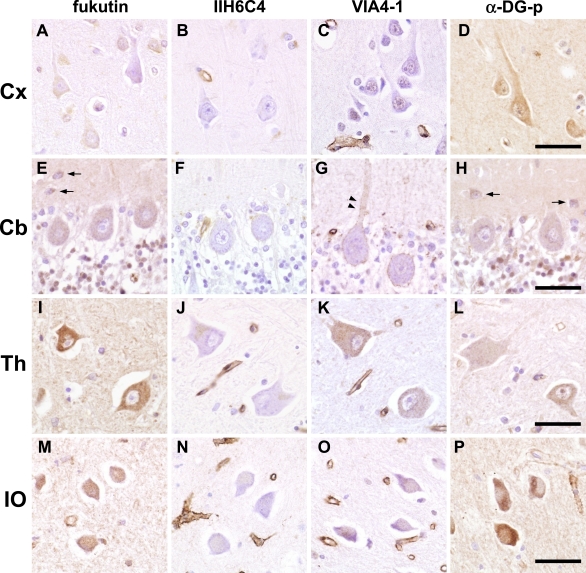
On immunohistochemistry of the adult brain, neurons were basically positive for fukutin, VIA4-1, and α-DG-p. The cytoplasm, nucleus, and neuropil were stained, but there were some differences in expression levels. In contrast, IIH6C4 was negative in neurons and neuropil in all regions of the brain (Fig. 2A–P).
Fig. 2.
Immunohistochemistry of fukutin and α-DG in the adult control brain. Fukutin, α-DG-p and VIA4-1 are basically positive in neurons and neuropil, while IIH6C4 is negative in neurons and neuropil. A–D. Cerebral cortex. Pyramidal cells are focally positive for fukutin, weakly positive in the nucleus for VIA4-1, and positive for α-DG-p. E–H. Cerebellum. Many cells in granular layer and small cells in the deep molecular layer (arrows) are positive and Purkinje cells are weakly positive for fukutin and α-DG-p. VIA4-1-positive dots fringed the cell soma and proximal dendrite of Purkinje cells (arrowheads). I–L. Thalamus. Thalamic neurons are positive for fukutin, VIA4-1 and α-DG-p. M–P. Inferior olivary nucleus. Neurons are positive for fukutin, weakly positive for VIA4-1, and strongly positive for α-DG-p. DG, dystroglycan; Cx, cerebral cortex; Cb, cerebellum; Th, thalamus; IO, inferior olivary nucleus. Bar=50 µm.
In the cerebral cortex (Fig. 2A–D), neurons were focally positive for fukutin and positive for α-DG-p, while VIA4-1 was weakly positive in the nuclei of neurons.
In the cerebellum (Fig. 2E–H), most of the cells in the granular layer were positive for fukutin and α-DG-p and weakly positive for VIA4-1. Purkinje cells were weakly positive for fukutin, VIA4-1, and α-DG-p. Interestingly, cell soma and proximal dendrite of Purkinje cells were fringed by VIA4-1-positive dots. Small cells, suggestive of basket cells, in the molecular layer were positive for fukutin and α-DG-p.
Thalamic neurons were positive for fukutin, VIA4-1, and α-DG-p (Fig. 2I–L). Neurons of the medulla oblongata were generally positive for fukutin, VIA4-1, and α-DG-p (Fig. 2M–P). Especially, neurons of the inferior olive were strongly positive for α-DG-p.
Alteration of α-DG expression in the postnatal FCMD cerebrum
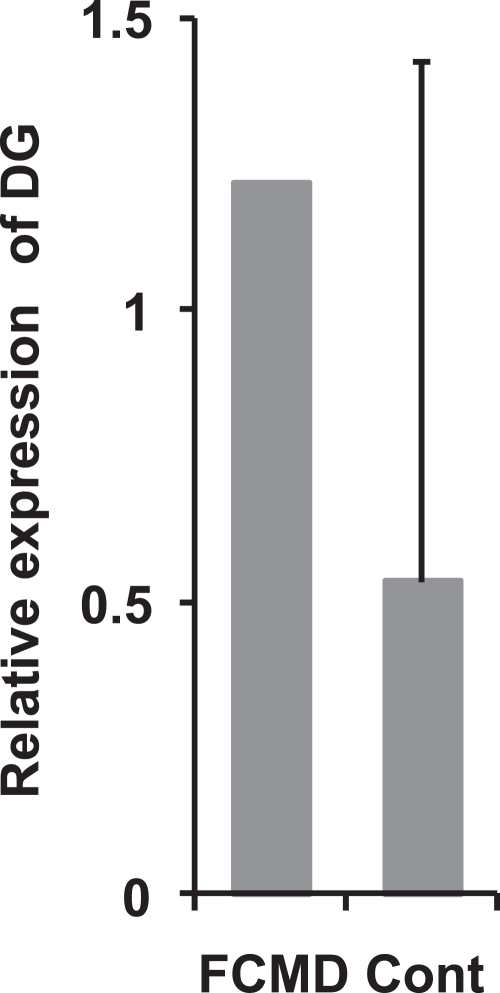
On qPCR, the relative expression level of DG mRNA in the cerebral cortex of FCMD tended to be increased compared to that in controls (Fig. 3).
Fig. 3.
Relative expression of DG mRNA in the cerebrum of postnatal FCMD and adult controls. DG mRNA expression level in FCMD tends to be higher than that in the control. Whisker indicates the standard deviation. FCMD, Fukuyama-type congenital muscular dystrophy; DG, dystroglycan; Cont, control.
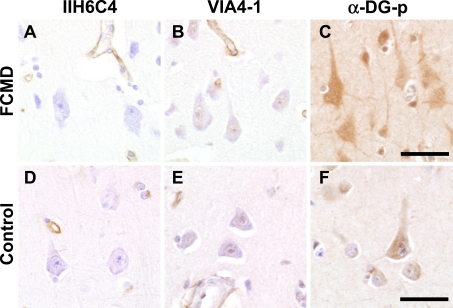
On immunohistochemistry of the FCMD cerebral cortex (Fig. 4A–C), IIH6C4 was negative, VIA4-1 was mostly negative except nucleus and α-DG-p was strongly positive in neurons and neuropil. Compared to that in age-matched controls (Fig. 4D–F), immunoreactivity to α-DG-p showed a clear difference, being stronger in the neuronal cytoplasm of FCMD patients than in that of adult control subjects (Fig. 4C, F).
Fig. 4.
Immunohistochemistry of α-DG in postnatal FCMD patients (A–C) and age-matched controls (D–F). In FCMD, pyramidal neurons are negative for IIH6C4 and positive reaction for VIA4-1 appears to be reduced. Immunoreaction for α-DG-p in neurons and neuropil is stronger in FCMD than in the control (C, F). FCMD, Fukuyama-type congenital muscular dystrophy; DG, dystroglycan. Bar=50 µm.
Developmental changes in fukutin and α-DG in the control cerebrum and cerebellum
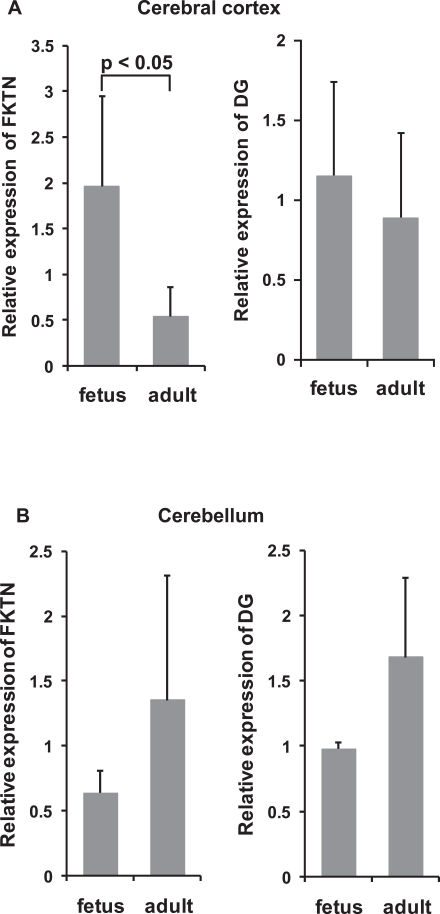
On qPCR, the relative expression of fukutin mRNA was significantly higher in the fetal cerebral cortex than in the adult cerebral cortex (Fig. 5A). In contrast, the adult cerebellar cortex tended to show greater fukutin expression than the fetal cerebellar cortex (Fig. 5B). The expression of DG mRNA tended to be similar to that of fukutin (Fig. 5A, B).
Fig. 5.
Relative expression of fukutin and DG mRNA in control fetuses and adults. A. Cerebral cortex. The expression of fukutin mRNA is significantly higher in the fetal cerebrum than in the adult cerebrum. The expression of DG mRNA tends to be similar to that of fukutin. B. Cerebellum. In contrast to the cerebrum, the adult cerebellum tends to show higher expressions of fukutin and DG mRNA than the fetal cerebellum. Whiskers indicate the standard deviation. FKTN, fukutin; DG, dystroglycan.
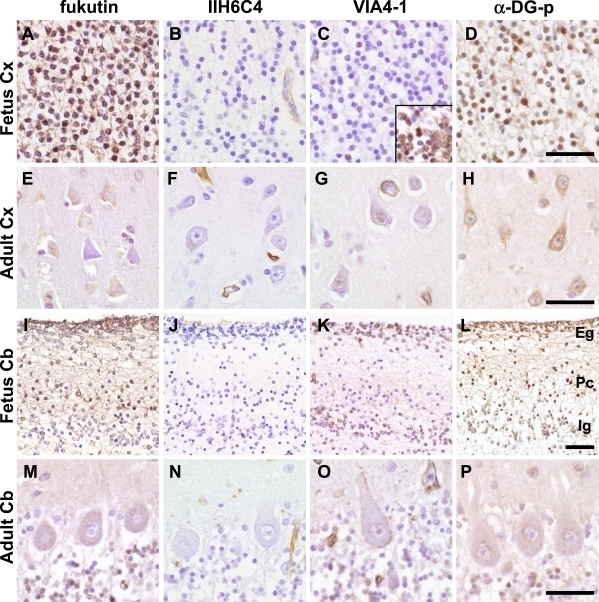
On immunohistochemistry of the fetal cerebrum (Fig. 6A–D), fukutin and α-DG-p were positive in the cytoplasm and nucleus of many cortical neurons, cells in the white matter, probably migrating neurons, and periventricular cells including cells in the ventricular and periventricular zone. IIH6C4 was negative, but VIA4-1 was positive in some cortical neurons and the majority of cells in the white matter and periventricular cells, and the positive reactions of later two areas were at a similar level. Compared to those in the adult cerebrum (Fig. 6E–H), fukutin and α-DG-p expressions were stronger in the fetal cerebrum. In the fetal cerebellar cortex (Fig. 6I–L), fukutin was positive in the external granular and Purkinje cell layers and weakly positive in some neurons of the internal granular layer. IIH6C4 was negative. VIA4-1 was positive in the external and internal granular layers and in some neurons of the Purkinje cell layer, while α-DG-p was positive in all layers. Periventricular cells and cells in the white matter were positive for fukutin, VIA4-1 and α-DG-p. Compared to those in the adult cerebellum (Fig. 6M–P), positive reactions for fukutin and α-DG-p were stronger in Purkinje cells. Cells in the internal granular layer appeared to maintain their expression after maturation.
Fig. 6.
Immunohistochemistry of fukutin and α-DG in control fetuses and adults. Neurons are negative for IIH6C4 in both fetuses and adults. A–D. Fetal cerebral cortex. Many cortical neurons are positive for fukutin (A) and α-DG-p (D). Some cortical neurons and many periventricular cells including cells in the ventricular and periventricular zone (inset) are positive for VIA4-1 (C). E–H. Adult cerebral cortex. Compared to those in the fetal cortex, the expressions of fukutin and α-DG are apparently decreased. I–L. Fetal cerebellar cortex. Fukutin is positive in the external granular and Purkinje cell layers and weakly positive in some neurons of the internal granular layer. VIA4-1 is positive in the external and internal granular layers and in some neurons of the Purkinje cell layer. α-DG-p is positive in all layers. M–P. Adult cerebellum. Compared to the fetal cerebellum, the expressions of fukutin and α-DG are apparently decreased in Purkinje cells. Immunoreaction in the granular layer appears to be preserved. DG, dystroglycan; Cx, cerebral cortex; Cb, cerebellum; Eg, external granular layer; Pc, Purkinje cell layer; Ig, Internal granular layer. Bar=50 µm.
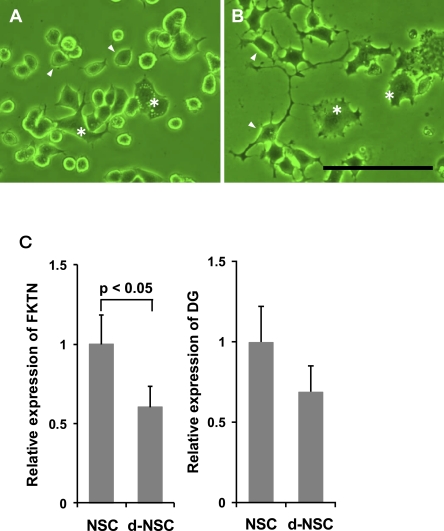
Differentiation-related alteration in the murine neuronal cell line, NSC34
NSC34 contains two populations of cells: small cells derived from murine spinal motor neurons, and larger multinucleated cells derived from murine neuroblastoma cells (Fig. 7A). After differentiation (d-NSC34), small cells extended neuronal processes prominently (Fig. 7B). The expression of fukutin mRNA was significantly lower in d-NSC34 than in NSC34 (Fig. 7C). The expression of DG mRNA was apparently decreased in d-NSC34, but the difference did not reach significance (Fig. 7D).
Fig. 7.
Differentiation-related alteration in murine neuronal cells, NSC34. A. NSC34. NSC34 contains small cells (arrowhead) derived from murine spinal motor neurons, and larger multinucleated cells derived from mouse neuroblastoma cells (asterisk). B. d-NSC34. Small cells extend neuronal processes (arrowhead). Asterisk: neuroblastoma cells. Bar=50 µm. C. Relative expression of fukutin and DG mRNA. The expression of fukutin mRNA is significantly lower in d-NSC than in NSC34. The expression of DG tends to be similar to that of fukutin. Whiskers indicate the standard deviation. FKTN, fukutin; DG, dystroglycan.
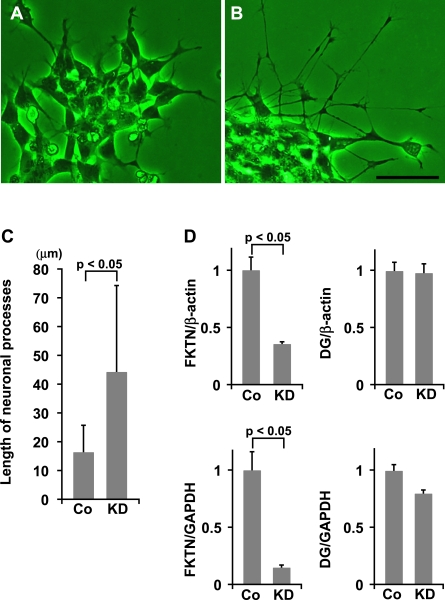
RNAi in human neuroblastoma cells, IMR-32
After knockdown of fukutin, cells showed marked outgrowth of neuronal processes compared to control cells (Fig. 8A, B). The length of neuronal processes of fukutin-knockdown cells (44.2±30.3 µm) was significantly longer than that of control cells (16.4±9.6 µm) (Fig. 8C). The expression of fukutin mRNA was significantly decreased in fukutin-knockdown cells compared to that of control cells (Fig. 8D). There was no decrease of fukutin mRNA in cells treated with SNC. There was no significant difference in DG mRNA expression between fukutin-knockdown cells and control cells (Fig. 8D).
Fig. 8.
RNAi in neuroblastoma cells, IMR-32. Compared to the control (A), cells show marked outgrowth of neuronal processes after knockdown of fukutin (B). Bar: 50=µm. C. Length of neuronal processes. Length of neuronal processes is significantly longer in fukutin-knockdown cells than in control cells. D. Relative expression of fukutin and DG mRNA. The expression of fukutin mRNA is significantly decreased in fukutin-knockdown cells compared to that in control cells. The expression of DG mRNA does not show a significant difference. mRNA level of fukutin and DG normalized by GAPDH tends to be similar to those normalized by β-actin. Whiskers indicate the standard deviation. FKTN, fukutin; DG, dystroglycan; Cont, control; KD, knockdown.
IV. Discussion
Fukutin is known to be expressed in neurons, but it remains to be clarified whether the expression and function of fukutin in each kind of neuron are the same or not. The present qPCR study demonstrated that fukutin was expressed in 9 different regions of the neurologically normal adult brain. Moreover, the amounts of fukutin mRNA differed somewhat from region to region. On immunohistochemistry of adult control brains, the cytoplasm and nucleus of most of the neurons was basically positive for fukutin, indicating that both excitatory and inhibitory neurons express fukutin. Some variations in positive reactions support the result of qPCR: the expression of fukutin may be somewhat different depending on the kind of neuron involved.
The relative expression of fukutin mRNA was significantly higher in the cerebellum compared to that in other regions of the brain. This difference may be derived from differences in synapse numbers and the presence of cerebellum-specific cells such as Purkinje cells and granule cells. Actually, the positive reaction in the internal granular layer remains after development [24, 33], as seen in this study.
On immunohistochemistry, the co-expression of fukutin and α-DG has been reported in various neurons in mice [19]. In the human brain, the relation between fukutin and the glycosylation of α-DG has been suggested only in hippocampal neurons [25]. In this study, the expression of fukutin and glycosylated α-DG was observed in the neurons of other regions, immunohistochemically. Moreover, on qPCR, the variations in the expression levels were similar between fukutin and DG. Although tissue samples for qPCR contained neurons and other components of the CNS, this finding supports the view that fukutin and α-DG are closely related in neurons as well, and that their expression showed regional differences.
In the cerebral cortex of FCMD patient, qPCR showed that the expression of DG mRNA tended to be increased compared to that in the control, and that the immunoreactivity against α-DG-p was stronger in neurons of FCMD patients than in those of control subjects. These findings may reflect a negative feedback of DG production against hypoglycosylation of α-DG, disrupted intracellular transport of unglycosylated DG, and/or impaired DG-degradation. Alteration of neuronal DG expression associated with abnormal fukutin reduction in FCMD patients also supports a functional linkage between fukutin and α-DG in human neurons. RNAi of IMR-32 could not reveal a significant change in DG mRNA. This is probably because the experimental period was short.
Interestingly, cell soma and proximal dendrite of Purkinje cells were fringed by VIA4-1-positive dots. This dot-like positivity of VIA4-1 could correspond to both inhibitory and excitatory synapses, because similar dot-like immunodeposits in neurons including Purkinje cells have been reported on immunostaining of GABAA and glutamate receptors in the brain and cultured cells of rodents [4, 8], and inhibitory and excitatory synapses are distributed respectively in cell soma and proximal dendrite of Purkinje cells [21]. α-DG co-localizes with GABAA receptors and post-synaptic densities of excitatory synapses in the mouse brain [11, 38]. Brain-selective DG-knockout mice show severely blunted hippocampal long-term potentiation [17]. Taken together, fukutin may be involved in at least post-synaptic function of mature human neurons through the glycosylation of α-DG. In this study, the dot-like positivity of VIA4-1 was observed only in Purkinje cells. This reaction may be unique to Purkinje cell, because Purkinje cell has a higher molecular weight of α-DG than other kinds of neurons in mice [27]. However, diffuse immunoreactions of the cytoplasm might mask dot-like reactions in other neurons, because of the differences of α-DG amount and/or cell size.
In this study, we used three kinds of anti-α-DG antibodies, recognizing the core peptide of α-DG (α-DG-p), the glycosylated epitope of α-DG (VIA4-1), and the laminin binding site (IIH6C4). α-DG-p and VIA4-1 were positive in the cytoplasm and nucleus of neurons and neuropil. In contrast, IIH6C4 was negative. The target molecule of VIA4-1 is still unidentified [12], but VIA4-1 probably recognizes glycosylated epitopes other than the laminin binding site, because the molecule reacting with VIA4-1 does not bind to laminin [1, 15]. Although an amount of α-DG detected by IIH6C4 might be very small in neurons, the α-DG expressed in neurons might have its own glycosylated epitopes.
The function of fukutin and α-DG in immature neurons is unclear. However, in addition to the over-migration of immature neurons due to abnormal glia limitans, migration arrest is suggested from several neuropathological findings, like heterotopic neurons of the white matter of the FCMD brain [37]. This phenomenon may be derived from a neuronal abnormality. In the human fetal cerebrum, VIA4-1 was positive in some cortical neurons and the majority of migrating neurons and periventricular cells including cells in the ventricular and subventricular zone, while fukutin and α-DG-p were positive in all areas. Fukutin may be involved in the neuronal migration, relating to the glycosylation of α-DG. The histology of the cerebral cortex appeared normal in NEX-Cre/DG-null mice showing neuron-specific deletion of DG, and it was suggested that neuronal DG is not critical for neuronal migration [27]. However, the defect of DG in more primitive cells may induce abnormal neuronal migration [17, 27]. Immature neurons migrate along the so-called radial glial processes [29], interacting with various extracellular proteins including agrin [22, 23, 30]. Some of the extracellular matrix proteins might react with a glycosylated epitope of α-DG other than the laminin-binding site, because the sugar chains of α-DG are the receptors of several extracellular matrix proteins [13, 16, 23]. In the cerebellum, immature cells in the lateral surface of the fourth ventricle migrate in a tangential manner to form the external granular layer, cells of which then migrate inwardly. Positive reaction of fukutin and VIA4-1 in external granular layer cells that are in the way of migration, also support the view that fukutin participates in cerebellar neuronal migration via the glycosylation of α-DG.
The present study demonstrated that the expression of fukutin mRNA was decreased with maturation in the human cerebrum. DG mRNA showed a tendency similar to fukutin mRNA. This is compatible with the findings of several previous studies [24, 33]. In the murine cell line, NSC34, the expression of fukutin mRNA was decreased with cell differentiation. In the human neuroblastoma cell line, IMR-32, showed a significant neuronal process-outgrowth by knockdown of fukutin. Because fukutin appears to prevent cellular differentiation in astrocytoma cell line (data not shown), a similar role could be considered in IMR-32. Functional analyses were not performed in this experiment, but the elongation of neuronal processes seems to indicate cellular differentiation. DG-knockout chick embryos show an increase in the thickness of the neuroepithelial cell layer and loss of their radial morphology [28]. Fukutin might be involved in cellular differentiation via glycosylation of α-DG. Immature neurons are in an immature state during migration, and begin to develop to a mature form once they reach the proper position after migration. Fukutin appears to be important not only simply for migration, but also for preventing differentiation during migration. However, further investigation is needed to clarify this point.
In contrast to those in the cerebrum, the expressions of fukutin and DG mRNA were increased with maturation in the cerebellum. The reason for this contradictory finding is obscure. There is a possibility of sampling error because the fetal cerebellum is very small and fragile, or a difference in components comprising the cerebellum.
Taken together, fukutin and α-DG are closely related in mature and immature human neurons in vivo, such as skeletal muscles and astrocytes. Fukutin may participate not only in basement membrane formation but in synaptic function and neuronal migration via the glycosylation of α-DG. Moreover, it may have other functions presumably relating to cellular differentiation.
In fukutin-transfected cultured cells, the transgene products are restricted to the Golgi apparatus which is the site of α-DG glycosylation [9, 14]. If the function of fukutin is only related to the glycosylation of α-DG, fukutin expression should be restricted to the Golgi apparatus, but our immunohistochemistry showed positive reactions of fukutin in the entire neuronal cytoplasm, nucleus, and neuropil. Possible nuclear and cytoplasmic localizations besides the Golgi apparatus have been observed in carcinoma [36] and astrocytoma cell lines (data not shown). Furthermore, β-DG localizes at the nucleus and cytoplasm besides the Golgi apparatus in cultured cells [3]. Because α- and β-DG generally co-localize, α-DG could also localize in the nucleus and cytoplasm of neurons. Because a binding of fukutin and α-DG is suggested [35], it is likely that fukutin has as of yet unknown distinctive functions that co-operate with α-DG. Since there is an alternative splicing in fukutin [10], nuclear and cytoplasmic staining might be derived from isoforms. However, these isoforms are originated from a single mRNA and our present results seems to elucidate at least a part of the characteristics and functions of fukutin.
V. Acknowledgments
The authors wish to thank Dr. Stephan Kröger, Physiologisches Institut, Ludwig-Maximilians-Universität, for kindly providing the anti-α-dystroglycan antibody, and also thank Dr. Tatsuo Sawada and Dr. Yoichiro Kato for valuable advice and encouragement. The authors are also grateful to Mr. Mizuho Karita, Mr. Hideaki Takeiri, Mr. Fumiaki Muramatsu, Mrs. Noriko Sakayori and Mr. Shuichi Iwasaki for their excellent technical assistance.
VI. References
- 1.Ervasti J. M., Campbell K. P. A role for the dystrophin-glycoprotein complex as a transmembrane linker between laminin and actin. J. Cell Biol. 1993;122:809–823. doi: 10.1083/jcb.122.4.809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fukuyama Y., Osawa M., Suzuki H. Congenital progressive muscular dystrophy of the Fukuyama type. Clinical, genetic and pathological considerations. Brain Dev. 1981;3:1–29. doi: 10.1016/s0387-7604(81)80002-2. [DOI] [PubMed] [Google Scholar]
- 3.González-Ramírez R., Morales-Lázaro S. L., Tapia-Ramírez V., Mornet D., Cineros B. Nuclear and nuclear envelope localization of dystrophin Dp71 and dystrophin-associated proteins (DAPs) in the C2C12 muscle cells: DAPs nuclear localization is modulated during myogenesis. J. Cell. Biochem. 2008;105:735–745. doi: 10.1002/jcb.21870. [DOI] [PubMed] [Google Scholar]
- 4.Graf E. R., Zhang X., Jin S., Linhoff M. W., Craig A. M. Neurexins induce differentiation of GABA and glutamate postsynaptic specializations via neuroligins. Cell. 2004;119:1013–1026. doi: 10.1016/j.cell.2004.11.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hayashi Y. K., Ogawa M., Tagawa K., Noguchi S., Ishihara T., Nonaka I., Arahata K. Selective deficiency of α-dystroglycan in Fukuyama-type congenital muscular dystrophy. Neurology. 2001;57:115–121. doi: 10.1212/wnl.57.1.115. [DOI] [PubMed] [Google Scholar]
- 6.Herrmann R., Straub V., Blank M., Kutzick C., Franke N., Jacob E. N., Lenard H. G., Kröger S., Voit T. Dissociation of the dystroglycan complex in caveolin-3-deficient limb girdle muscular dystrophy. Hum. Mol. Genet. 2000;9:2335–2340. doi: 10.1093/oxfordjournals.hmg.a018926. [DOI] [PubMed] [Google Scholar]
- 7.Ishii H., Hayashi Y. K., Nonaka I., Arahata K. Electron microscopic examination of basal lamina in Fukuyama congenital muscular dystrophy. Neuromuscul. Disord. 1997;7:191–197. doi: 10.1016/s0960-8966(97)00462-8. [DOI] [PubMed] [Google Scholar]
- 8.Knuesel I., Mastrocola M., Zuellig R. A., Bornhauser B., Schaub M. C., Fritschy J. M. Altered synaptic clustering of GABAA receptors in mice lacking dystrophin (mdx mice) Eur. J. Neurosci. 1999;11:4457–4462. doi: 10.1046/j.1460-9568.1999.00887.x. [DOI] [PubMed] [Google Scholar]
- 9.Kobayashi K., Nakahori Y., Miyake M., Matsumura K., Kondo-Iida E., Nomura Y., Segawa M., Yoshioka M., Saito K., Osawa M., Hamano K., Sakakihara Y., Nonaka I., Nakagome Y., Kanazawa I., Nakamura Y., Tokunaga K., Toda T. An ancient retrotransposal insertion causes Fukuyama-type congenital muscular dystrophy. Nature. 1998;394:388–392. doi: 10.1038/28653. [DOI] [PubMed] [Google Scholar]
- 10.Kobayashi K., Sasaki J., Kondo-Iida E., Hukuda Y., Kinoshita M., Sunada Y., Nakamura Y., Toda T. Structural organization, complete genomic sequences and mutational analyses of the Fukuyama-type congenital muscular dystrophy gene, fukutin. FEBS. Lett. 2001;489:192–196. doi: 10.1016/s0014-5793(01)02088-9. [DOI] [PubMed] [Google Scholar]
- 11.Lévi S., Grade R. M., Henry M. D., Campbell K. P., Sanes J. R., Craig A. M. Dystroglycan is selectively associate with inhibitory GABAergic synapses but is dispensable for their differentiation. J. Neurosci. 2002;22:4274–4285. doi: 10.1523/JNEUROSCI.22-11-04274.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Longman C., Brockington M., Torelli S., Jimenez-Mallebrera C., Kennedy C., Khalil N., Feng L., Saran R. K., Voit T., Merlini L., Sewry C. A., Brown S. C., Muntoni F. Mutations in the human Large gene cause MDC1D, a novel form of congenital muscular dystrophy with severe mental retardation and abnormal glycosylation of α-dystroglycan. Hum. Mol. Genet. 2003;12:2853–2861. doi: 10.1093/hmg/ddg307. [DOI] [PubMed] [Google Scholar]
- 13.Martin P. T. The dystroglycanopathies: The new disorders of O-linked glycosylation. Semin. Pediat. Neurol. 2005;152:152–158. doi: 10.1016/j.spen.2005.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Matsumoto H., Noguchi S., Sugie K., Ogawa M., Murayama K., Hayashi Y. K., Nishino I. Subcellular localization of fukutin and fukutin-related protein in muscle cells. J. Biochem. 2004;135:709–712. doi: 10.1093/jb/mvh086. [DOI] [PubMed] [Google Scholar]
- 15.Matsumura K., Chiba A., Yamada H., Hukuta-Ohi H., Fujita S., Endo T., Kobata A., Anderson L. V. B., Kanazawa I., Campbell K. P., Shimizu T. A role of dystroglycan in schwannoma cell adhesion to laminin. J. Biol. Chem. 1997;272:13904–13910. doi: 10.1074/jbc.272.21.13904. [DOI] [PubMed] [Google Scholar]
- 16.Michele D. E., Campbell K. P. Dystrophin-glycoprotein complex: Post-translational processing and dystroglycan function. J. Biol. Chem. 2003;278:15457–15460. doi: 10.1074/jbc.R200031200. [DOI] [PubMed] [Google Scholar]
- 17.Moore S., Saito F., Chen J., Michele D. E., Henry M. D., Messing A., Chorn R. D., Ross-Barta S. E., Westra S., Williamson R. A., Hoshi T., Campbell K. P. Deletion of brain dystroglycan recapitulates aspects of congenital muscular dystrophy. Nature. 2002;418:422–425. doi: 10.1038/nature00838. [DOI] [PubMed] [Google Scholar]
- 18.Nakano I., Funahashi M., Takada K., Toda T. Are breaches in the glia limitans the primary cause of the micropolygyria in Fukuyama-type congenital muscular dystrophy (FCMD)?—Pathological study of the cerebral cortex of a FCMD fetus. Acta Neuropathol. 1996;91:313–321. doi: 10.1007/s004010050431. [DOI] [PubMed] [Google Scholar]
- 19.Ohtsuka-Tsurumi E., Saito Y., Yamamoto T., Voit T., Kobayashi M., Osawa M. Co-localization of fukutin and α-dystroglycan in the mouse central nervous system. Dev. Brain Res. 2004;152:121–127. doi: 10.1016/j.devbrainres.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 20.Osawa M., Sumida S., Suzuki N., Arai Y., Ikenaka H., Murasugi H., Shishikura K., Suzuki H., Saito K., Fukuyama Y. In “Congenital Muscular Dystrophies”, ed. by Y. Fukuyama, M. Osawa and K. Saito. Elsevier; Amsterdam: 1997. Fukuyama type congenital progressive muscular dystrophy; pp. 31–68. [Google Scholar]
- 21.Parent A. In “Carpenter’s Human Neuroanatomy”, 9th ed., ed. by P. Coryell. Williams & Wilkins; Baltimore: 1996. Cerebellum; pp. 583–629. [Google Scholar]
- 22.Porcionatto M. A. The extracellular matrix provides directional cues for neuronal migration during cerebellar development. Braz. J. Med. Biol. Res. 2006;39:313–320. doi: 10.1590/s0100-879x2006000300001. [DOI] [PubMed] [Google Scholar]
- 23.Qu Q., Smith F. I. Alpha-dystroglycan interactions affect cerebellar granule neuron migration. J. Neurosci. Res. 2004;76:771–782. doi: 10.1002/jnr.20129. [DOI] [PubMed] [Google Scholar]
- 24.Saito Y., Mizuguchi M., Oka A., Takashima S. Fukutin protein is expressed in neurons of the normal developing human brain but is reduced in Fukuyama-type congenital muscular dystrophy brain. Ann. Neurol. 2000;47:756–764. [PubMed] [Google Scholar]
- 25.Saito Y., Yamamoto T., Mizuguchi M., Kobayashi M., Saito K., Ohno K., Osawa M. Altered glycosylation of α-dystroglycan in neurons of Fukuyama congenital muscular dystrophy brains. Brain Res. 2006;1075:223–228. doi: 10.1016/j.brainres.2005.12.108. [DOI] [PubMed] [Google Scholar]
- 26.Sasaki J., Ishikawa K., Kobayashi K., Kondo-Iida E., Fukuyama M., Mizusawa H., Takashima S., Sakakihara Y., Nakamura Y., Toda T. Neuronal expression of the fukutin gene. Hum. Mol. Genet. 2000;9:3083–3090. doi: 10.1093/hmg/9.20.3083. [DOI] [PubMed] [Google Scholar]
- 27.Satz J. S., Ostendorf A. P., Hou S., Turner A., Kusano H., Lee J. C., Turk R., Nguyen H., Ross-Barta S. E., Westra S., Hoshi T., Moore S. A. Distinct functions of glial and neuronal dystroglycan in the developing and adult mouse brain. J. Neurosci. 2010;30:14560–14572. doi: 10.1523/JNEUROSCI.3247-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shröder J. E., Tegeler M. R., Groβhans U., Porten E., Blank M., Lee J., Esapa C., Blake D. J., Kroger S. Dystroglycan regulates structure, proliferation and differentiation of neuroepithelial cells in the developing vertebrate CNS. Dev. Biol. 2007;307:62–78. doi: 10.1016/j.ydbio.2007.04.020. [DOI] [PubMed] [Google Scholar]
- 29.Sidman R. L., Rakik P. Neuronal migration, with special reference to developing human brain: A review. Brain Res. 1973;62:1–35. doi: 10.1016/0006-8993(73)90617-3. [DOI] [PubMed] [Google Scholar]
- 30.Sobeih M. M., Corfas G. Extracellular factors that regulate neuronal migration in the central nervous system. Int. J. Devel. Neurosci. 2002;20:349–357. doi: 10.1016/s0736-5748(02)00040-0. [DOI] [PubMed] [Google Scholar]
- 31.Yamamoto T., Shibata N., Kanazawa M., Kobayashi M., Komori T., Kondo E., Saito K., Osawa M. Early ultrastructural changes in the central nervous system in Fukuyama congenital muscular dystrophy. Ultrastruct. Pathol. 1997;21:355–360. doi: 10.3109/01913129709021933. [DOI] [PubMed] [Google Scholar]
- 32.Yamamoto T., Toyoda C., Kobayashi M., Kondo E., Saito K., Osawa M. Pial-glial barrier abnormalities in fetuses with Fukuyama congenital muscular dystrophy. Brain Dev. 1997;19:35–42. doi: 10.1016/s0387-7604(96)00056-3. [DOI] [PubMed] [Google Scholar]
- 33.Yamamoto T., Kato Y., Karita M., Takeiri H., Muramatsu F., Kobayashi M., Saito K., Osawa M. Fukutin expression in glial cells and neurons: implication in the brain lesions of Fukuyama congenital muscular dystrophy. Acta Neuropathol. 2002;104:217–224. doi: 10.1007/s00401-002-0542-8. [DOI] [PubMed] [Google Scholar]
- 34.Yamamoto T., Kato Y., Kawaguchi M., Shibata N., Kobayashi M. Expression and localization of fukutin, POMGnT1, and POMT1 in the central nervous system: consideration for functions of fukutin. Med. Electron Microsc. 2004;37:200–207. doi: 10.1007/s00795-004-0260-5. [DOI] [PubMed] [Google Scholar]
- 35.Yamamoto T., Kawaguchi M., Sakayori N., Muramatsu F., Morikawa S., Kato Y., Shibata N., Kobayashi M. Intracellular binding of fukutin and α-dystroglycan: Relation to glycosylation of α-dystroglycan. Neurosci. Res. 2006;56:391–399. doi: 10.1016/j.neures.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 36.Yamamoto T., Kato Y., Shibata N., Sawada T., Osawa M., Kobayashi M. A role of fukutin, a gene responsible for Fukuyama type congenital muscular dystrophy, in cancer cells: a possible role to suppress cell proliferation. Int. J. Exp. Pathol. 2008;89:332–341. doi: 10.1111/j.1365-2613.2008.00599.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yamamoto T., Shibata N., Saito Y., Osawa M., Kobayashi M. Functions of fukutin, a gene responsible for Fukuyama type congenital muscular dystrophy, in neuromuscular system and other somatic organs. Cent. Nerv. Syst. Agents Med. Chem. 2010;10:169–179. doi: 10.2174/187152410791196369. [DOI] [PubMed] [Google Scholar]
- 38.Zaccaria M. L., Tommaso F. D., Brancaccio A., Paggi P., Petrucci T. Dystroglycan distribution in adult mouse brain: S light and electron microscopy study. Neuroscience. 2001;104:311–324. doi: 10.1016/s0306-4522(01)00092-6. [DOI] [PubMed] [Google Scholar]