Abstract
The roles of PDZ domain-containing proteins such as Dlg and Scrib have been well described for Drosophila; however, their requirement for mammalian development is poorly understood. Here we show that Dlg, Scrib, MAGI1, MAGI3, and MPDZ are expressed in the mouse ocular lens. We demonstrate that the increase in proliferation and defects in cellular adhesion and differentiation observed in epithelia of lenses that express E6, a viral oncoprotein that can bind to several PDZ proteins, including the human homologs of Dlg and Scrib, is dependent on E6's ability to bind these proteins via their PDZ domains. Analyses of lenses from mice carrying an insertional mutation in Dlg (dlggt) show increased proliferation and proliferation in spatially inappropriate regions of the lens, a phenotype similar to that of lenses expressing E6. The results from this study indicate that multiple PDZ domain-containing proteins, including Dlg and Scrib, may be required for maintaining the normal pattern of growth and differentiation in the lens. Furthermore, the phenotypic similarities among the Drosophila dlg mutant, the lenses of dlggt mice, and the lenses of E6 transgenic mice suggest that Dlg may have a conserved function in regulating epithelial cell growth and differentiation across species.
Determining the molecular mechanisms that regulate cell growth and differentiation during the critical phase of organogenesis in vivo has been a central theme in developmental biology for many years. Inherent in this process is a fundamental switch of a cell from a state capable of proliferation to one that is irreversibly withdrawn from the cell cycle and undergoing terminal differentiation. Disruption of cell cycle control often has adverse consequences, such as defects in development, tumorigenesis, and cell death. The retinoblastoma susceptibility protein, pRb, is a critical regulator of cell proliferation during development (26, 31). Evidence from studies in invertebrates suggests that perhaps other proteins with tumor suppressor properties, such as the PDZ (PSD-95/Dlg/ZO-1) domain-containing proteins, which include Discs Large (Dlg) and Scribble (Scrib), are also important in regulating mammalian cell growth and differentiation (3, 42). To address the possible role of PDZ domain-containing proteins in regulating growth and differentiation of epithelial tissues in vertebrates, we have examined the consequences of the functional disruption of some of these PDZ proteins on cell growth and differentiation in the mouse ocular lens.
The mouse ocular lens is an ideal system in which to identify the cellular factors that are required for maintaining proper cell cycle control. In the postnatal mouse, the lens can be divided into two major compartments, the anterior epithelium and the fiber cell compartment. The anterior epithelium is a monolayer of cuboidal epithelial cells that covers the anterior surface. Within the epithelium reside specific groups of cells at spatially restricted positions that exhibit different proliferative characteristics. In the central region of the epithelium, cells are mitotically quiescent, while more peripherally located cells in the germinative zone are actively proliferating. Moving toward the posterior, cells in the transition zone are postmitotic and undergoing differentiation. These give rise to the postmitotic, terminally differentiated cells in the fiber cell compartment, which constitutes the bulk of the lens. In addition to changes in proliferative potential, the process of fiber cell differentiation involves extensive changes in cell shape; elimination of membrane-bound organelles, including the nucleus; and expression of differentiation-specific genes, including β and γ crystallins, MIP26, and genes for beaded filament proteins filesin and phakinin (reviewed in reference 33).
Previous studies have shown that the tumor suppressor protein pRb and pRb family members p107 and p130 are essential for cell cycle regulation in the lens. Inactivation of pRb at the time of cell cycle withdrawal and fiber cell differentiation leads to a failure in cell cycle withdrawal, failure of morphological differentiation, and ultimately the induction of apoptosis (26, 31). Additionally, inactivation of pRb family members throughout the epithelium leads to increased cell proliferation in that compartment and inhibition of differentiation (30).
While functional pRb family members are essential for cell cycle regulation in the lens, whether other proteins with potential tumor suppressor activity are also involved in regulating cell growth and differentiation in epithelial tissues such as the lens in vertebrates has yet to be determined. In Drosophila, the PDZ domain-containing proteins Dlg and Scrib have been shown to be tumor suppressors (4, 42). Analysis of null mutations of either of these genes indicates that they are necessary for maintaining normal cell growth control, cell polarity, and cell-cell adhesion in some epithelial tissues, such as the imaginal discs (3, 4, 42). Within the epithelial cells of Drosophila, Dlg and Scrib are found associated with septate junctions (4, 41). They act as scaffolding proteins, recruiting a number of transmembrane and signaling molecules to specific sites on the plasma membrane, through which they appear to be involved in stabilizing cell-cell junctions as well as intracellular signaling (2). The human Dlg protein is also known to interact with the gene product of the tumor suppressor gene adenomatous polyposis coli (APC), which is thought to affect cell cycle progression (14, 24).
Despite extensive knowledge of the cellular roles of Dlg and Scrib in Drosophila and Caenorhabditis elegans (3, 5, 20), very little is known about their roles in epithelial cell growth and differentiation in vivo in vertebrates. To date, our knowledge comes from the initial analysis of a mouse strain carrying an insertional mutation in Dlg, which showed that this gene is necessary for proper craniofacial development (6).
To determine if one or more of the mouse homologs of Drosophila Dlg, Scrib, and related PDZ domain proteins might play a role in regulating epithelial cell growth and differentiation in mice, we made use of a factor that acts as a potent and pan-dominant repressor of many PDZ domain proteins. This factor, the high-risk human anogenital papillomavirus type 16 (HPV-16) E6 oncoprotein, is a multifunctional protein implicated in human cervical cancer. While first determined to bind to and inactivate the cellular tumor suppressor p53 (40), it has since been found to bind many other cellular factors, including a number of PDZ domain proteins, such as the human homologs of Scrib (27), the multi-PDZ-domain-containing protein Mupp1 (19), members of the membrane-associated guanylate kinase proteins, Dlg (16, 18), Magi-1 (10), Magi-2, and Magi-3 (37). E6 interacts with these PDZ domain proteins through a PDZ binding motif located at its C terminus. In tissue culture, the ability of E6 to bind PDZ domain proteins is recognized to contribute to its transforming properties (16). In our prior studies in which we characterized the effects of HPV-16 E6 expression in the epithelium and the transition zone of the lens in K14HPV16E6 (K14E6WT) transgenic mice, we noted hyperplasia that was characterized by a multilayered and disorganized epithelium. In this hyperplastic epithelium there was an abundance of intercellular vacuoles, suggestive of defects in cell-cell adhesion (30). This phenotype is reminiscent of the phenotype of Drosophila embryos carrying null mutations in Dlg, Scrib, or Lgl (lethal giant larvae) (3) and suggested to us that E6's interference with the function of one or more PDZ proteins is the molecular basis for the lens phenotype in K14E6WT transgenic mice.
To determine if the loss of PDZ protein function is the molecular basis of the lens phenotype in K14E6WT transgenic mice, we first documented that multiple PDZ domain genes are expressed in the mouse lens. Next we generated and characterized transgenic mice expressing mutant forms of E6 that either retained (E6I128T) or lost (E6Δ146-151) the ability to bind PDZ proteins. Finally, we characterized the lenses in mice that carry a gene trap insertion in Dlg. The results of this study indicate that PDZ domain proteins function in controlling normal cell cycle control, cell structure, and cell adhesion in the mouse and further raise the possibility that PDZ factors may have tumor suppressor activity in mammalian species.
MATERIALS AND METHODS
RT-PCR of PDZ domain-containing genes.
Total RNA was isolated from lenses of nontransgenic neonatal mice by use of Trizol (Invitrogen). DNA was removed from the RNA preparation by use of a Message Clean kit (GenHunter Corp.). Five micrograms of RNA was used to generate cDNA with Ready To Go beads (Amersham) and oligo(dT) primer. DNA fragments of PDZ domain-containing proteins were amplified by PCR using primers specific for each transcript. Primer sequences for PDZ domain sequences were as follows: Dlg1 5′ (5′ GAGCATTGCATCTGTTGG 3′) and 3′ (5′ AGTGCAGCTGCTGCTTGTT 3′) (21); Scrib 5′ (5′ TGTCAGTGTCATCCAGTTCG 3′) and 3′ (5′ CCTCGTCATCTCCTTTGTAG 3′); Llglh 5′ (5′ CATCGCTTCCTGTGTCTTCA 3′) and 3′ (5′ AGGTTCCGCAGTTCTTCTCA 3′); MAGI-1a 5′ (5′ GGAAAGCCCTTTTTGTTTCC 3′) and 3′ (5′ TCCAAAACTTCACGCCTCTT 3′); MAGI-1b 5′ (5′ TTGGAAAGAAGGGAGAAGCA 3′) and 3′ (5′ ATGATTTCGTTTTGCGACCT 3′); MAGI-1c 5′ (5′ TGTTCCTTATTTGGGGCAAG 3′) and 3′ (5′ CTGAGCTAAGGCTGGGTTTG 3′); MAGI-3 5′ (5′ CCACAGGAGGCCTATGATGT 3′) and 3′ (5′ AGGCTGTGCAAGGTGCTTAT 3′); MUPP1 (MPDZ) 5′ (5′ GCGGACCTCAGCTCACTTAC 3′) and 3′ (5′ GCAGGGTCAGAAGCAAAGAC 3′). Reverse transcription (RT)-PCR products were then cloned into the pGEM-T vector and sequenced to verify their identities.
Transgenic mice.
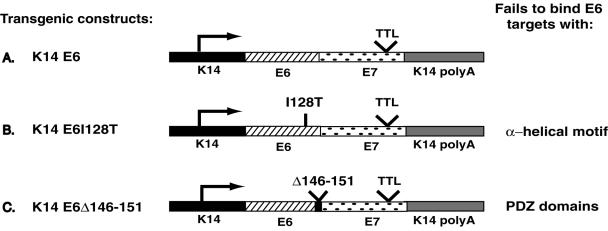
Transgenic mouse lines K1416E6WT (lines 5737 and 5743) (34) and K14E6I128T (lines 6061 and 6072) (28) were generated and characterized previously. The K14E6Δ146-151 transgene construct was generated by cloning the E6Δ146-151 mutant (obtained from D. Galloway, Fred Hutchinson Cancer Center) into the K14 cassette containing the human K14 promoter and E6/E7 translation termination linker (TTL) and K14 poly(A) sequences (see Fig. 2). Transgenic mice were generated by microinjecting DNA fragments into the male pronuclei of one-cell fertilized FVB/n embryos, as previously described (11, 12) by the University of Wisconsin Biotechnology Center's Transgenic Animal Facility. Mice were genotyped by PCR analysis on tail DNA as described previously (31). Animals were staged by designating the day of birth as neonate (neo) and subsequent days as P1, P2, etc. The dlggt mice were genotyped by Southern blot analysis of total genomic DNA digested with EcoRI probed with a 400-bp SalI fragment from pCR2.1DlgRP. Embryos were staged by designating the day of the plug as day E0.5.
FIG. 2.
Diagram of transgene DNAs used to generate transgenic mice. All E6/E7TTL variants were cloned into a human keratin 14 (K14) expression cassette that contains K14 promoter and poly(A) sequences. (A) K14E6WT transgene (35). The TTL was introduced into the E7 open reading frame to disrupt translation of E7. (B) K14E6I128T transgene. The E6WT sequences shown in panel A were replaced with an E6 mutant that contains an isoleucine-to-threonine substitution at amino acid 128. (C) K14E6Δ146-151 transgene. The E6WT sequences shown in panel A were replaced with a mutant E6 from which the final 18 nucleotides of E6, which correspond to amino acids 146 to 151, were deleted.
Histological analysis.
Eyes from nontransgenic and transgenic animals were fixed in 10% buffered formalin overnight at 4°C, transferred into phosphate-buffered saline (PBS), dehydrated in increasing concentrations of ethanol, and embedded in paraffin. Sections (5 μm) were cut, stained with hematoxylin and eosin, and viewed by light microscopy or used for in situ hybridization and immunohistochemical analyses.
In situ hybridization.
In situ hybridization reactions were performed as described previously (32). Briefly, eyes from nontransgenic and transgenic animals from neonate through P21 were fixed, embedded, and sectioned as described above. Probes for E6/E7 RNA were made by using a pABE7 plasmid in which the sequence for HPV16E7 was subcloned into pGEMI. pABE7 was linearized with EcoRI or HindIII to generate sense and antisense [α-35S]UTP-labeled riboprobes, respectively. A probe for p57KIP2 RNA was derived from the pBS-mp57 plasmid (provided by P. Zhang, Baylor College of Medicine). pBS-mp57 was digested with either NotI or KpnI to generate sense and antisense [α-35S]UTP-labeled riboprobes, respectively (Boehringer Mannheim or Ambion, Inc.). Probes for Dlg and Scrib were derived from RT-PCR products cloned into the pGEMT vector. pGEMT-Dlg was digested with ApaI or NdeI to generate sense and antisense [α-35S]UTP-labeled riboprobes, respectively. pGEMT-Scrib was digested with NdeI or NcoI to generate sense and antisense [α-35S]UTP-labeled riboprobes, respectively. Hybridized sections were exposed to Kodak NTB-2 emulsion in the dark for 7 to 21 days before developing. After developing, the sections were counterstained with 0.2% toluidine blue, mounted, and viewed under bright- and dark-field illumination.
In situ detection of proliferation.
For DNA synthesis studies, mice were injected with a solution of 100 ng of bromodeoxyuridine (BrdU)/6.7 ng of fluorodeoxyuridine per g of body weight 1 h prior to sacrifice. Eyes were removed, fixed, embedded, and sectioned as described above. Cells that had incorporated BrdU were identified immunohistochemically by using a primary antibody to BrdU from Oncogene Sciences. BrdU-positive cells were visualized by using diaminobenzine (DAB). Sections then were counterstained with hematoxylin, mounted, and viewed by bright-field microscopy.
Immunohistochemistry.
For detection of Dlg and Scrib, eyes from postnatal animals were fixed, embedded, and sectioned as described above. Tissue sections from paraffin-embedded eyes were deparaffinized in xylenes, rehydrated through graded ethanols, and treated with trypsin (Sigma) for 30 min at room temperature. Sections were blocked in 5% horse serum-PBS for 1 h at room temperature. Excess blocking solution was removed and sections were incubated with either a 1:500 dilution of anti-Dlg (anti-SAP97 antibody provided by J. Hell, University of Iowa) or a 1:100 dilution of anti-hScrib (Santa Cruz Biotechnology, Inc.). Sections were then washed in PBS and incubated with a 1:1,000 dilution of Texas Red-conjugated goat anti-rabbit antibody (for Dlg) (Molecular Probes) or a 1:50 dilution of Texas Red-conjugated donkey anti-goat antibody (for Scrib) (Jackson ImmunoReasearch) in a darkened chamber. Sections incubated with no primary antibody or with preimmune serum were used as negative controls. Sections were then washed in the dark with PBS and mounted in 50% glycerol-PBS-0.4% propylgallate. Sections were viewed by using Bio-Rad 1024 on a Nikon Diaphot 200 confocal microscope. For detection of β-crystallins, eyes from postnatal animals were fixed, embedded, and sectioned as described above. Sections were blocked in blocking solution (10% goat serum, 1% bovine serum albumin [BSA], 16% fetal bovine serum, 0.05% Tween 20). Sections then were incubated at 4°C overnight with a 1:300 dilution of rabbit anti-β-crystallin antibody (obtained from S. Zigler, National Eye Institute) in 1% BSA-PBS. Sections were washed with PBS and incubated with a 1:200 dilution of fluorescein isothiocyanate (FITC)-conjugated anti-rabbit immunoglobulin G in 1% BSA-PBS for 2 h at room temperature. Finally, sections were washed in PBS and mounted in 50% glycerol-PBS-0.4% propylgallate. Lens sections were viewed by UV microscopy with a FITC filter.
E6 inhibition of p53 induction following irradiation.
To monitor the ability of wild-type and mutant E6 transgenes to inhibit p53 induction following DNA damage in vivo, 9-day-old nontransgenic, K14E6WT, and K14E6Δ146-151 mice were irradiated with a 137Cs source at a dose rate of 3.1 Gy/min. A single dose of 4 Gy was delivered to the whole bodies of mice individually. The mice were sacrificed 24 h after irradiation. Groups of age-matched unirradiated mice were used as controls. Skin samples obtained from the dorsal area were fixed in 10% buffered formalin and embedded in paraffin, and histological sections of 5 μm in thickness were subjected to p53-specific immunohistochemistry as previously described (34). Briefly, histological sections were deparaffinized in xylenes, rehydrated in graded alcohol and PBS, and quenched of endogenous peroxidase by treatment of skin sections with 3% hydrogen peroxide for 15 min. The sections were heated in boiling 0.01 M citrate buffer, pH 6.0, in a microwave oven for 20 min to unmask antigens. Tissue sections were blocked with 5% nonfat dry milk-PBS and 5% normal goat serum for 30 min. After blocking, rabbit anti-mouse p53 antibody (CM5; Novocastra Laboratories), diluted 1:500, was added and incubated for 3 h at room temperature. After incubation with secondary antibody (30 min) and then with Vectastain ABC reagents (30 min), the slides were exposed to DAB substrate. p53-positive epithelial cells were quantified by microscopy with ×400 magnification.
Western blot analysis.
Proteins from nontransgenic neonatal brain tissue and lenses from P10 nontransgenic, 512, 5737, 6061, and 6072 animals were isolated in 1× RIPA buffer containing protease inhibitors. One hundred micrograms of each protein sample was run on a 7.5% acrylamide gel. Proteins were then transferred to nitrocellulose. The blot was blocked in 5% nonfat dry milk in 1× PBS-Tween 20 and then incubated with goat anti-human Scrib antibody (Santa Cruz Biotechnology, Inc.) for 2 h at room temperature, incubated with horseradish peroxidase-conjugated mouse anti-goat secondary antibody (Amersham), and visualized by ECL (Amersham). To determine if the amount of each sample loaded was equivalent, the blot was stripped and incubated with mouse anti-glyceraldehyde-3-phosphate dehydrogenase (GAPDH) antibody (Chemicon International) and treated similarly as described above, with the exception of the use of a horseradish peroxidase-conjugated sheep anti-mouse secondary antibody (Amersham).
RESULTS
Expression of PDZ protein genes in the lens.
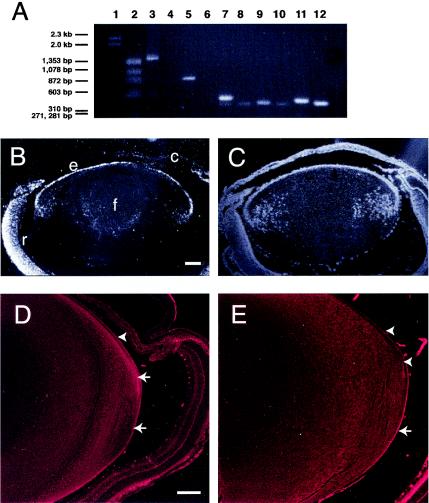
The phenotypes of K14E6WT transgenic mice (30) led us to hypothesize that the disruption of function of PDZ domain proteins targeted by E6, including Dlg and Scrib, contributes to the observed hyperplasia. If PDZ proteins play a role in growth and differentiation in the lens, they must be expressed in the lens. To determine if one or more PDZ domain-containing genes are expressed in the lens, RT-PCRs were carried out on total lens RNA from neonatal mice using gene-specific primers. As shown in Fig. 1, Dlg1, Scrib, Llglh (the mouse homolog of Drosophila Llgl), MAGI-1 (splice variants a, b, and c), MAGI-3, and MUPP1 (MPDZ) RT-PCR products were all detected in lens RNA, indicating that these genes are expressed in the mouse lens. To document the patterns of expression of Dlg and Scrib, in situ hybridization was carried out on eye sections from nontransgenic mice, using 35S-labeled riboprobes specific for Dlg and Scrib. Transcripts for Dlg1 (Fig. 1B) and Scrib (Fig. 1C) were found in both epithelium and fiber cell compartments. To determine if Dlg and Scrib proteins were similarly present, immunohistochemical analysis using antibodies specific for Dlg1 and Scrib was carried out on eye sections from nontransgenic mice. Both Dlg1 (Fig. 1D) and Scrib (Fig. 1E) proteins were found in both epithelium and fiber cells. The expression of multiple PDZ proteins in the lens provides the rationale to test the hypothesis that PDZ domain proteins play a role in regulating cell proliferation and maintaining normal cell structure in the cells of the lens epithelium.
FIG. 1.
Expression of genes for PDZ proteins in mouse lens tissue. (A) RT-PCR analysis. RNAs isolated from lenses of neonatal nontransgenic mice were subjected to RT-PCR analyses using primers specific for the indicated genes, and the products were resolved by electrophoresis on 1% agarose gels. Primer pairs were specific for mouse Dlg1 (lanes 3 and 4), Scrib (lanes 5 and 6), Llglh (lane 7), Magi-1a (lane 8), Magi-1b (lane 9), Magi-1c (lane 10), Magi-3 (lane 11), and MUPP1 (Mpdz) (lane 12). RT-PCR was carried out with Dlg (lane 2), Scrib (lane 4), and all others (not shown) on RNase-treated RNA as negative controls. Molecular mass markers λHindIII and φX174 HaeIII are shown in lanes 1 and 2, respectively. (B and C). In situ hybridization for Dlg and Scrib. Paraffin-embedded sections (5 μm) of eyes from control nontransgenic neonatal mice were hybridized to antisense [α-35S]UTP-labeled ribroprobes derived from pGEMT-Dlg (B) and pGEMT-Scrib (C). No signal was observed in sections hybridized with an [α-35S]UTP sense-strand riboprobe (data not shown). (D and E). Immunohistochemistry for Dlg and Scrib. Immunohistochemistry was carried out on paraffin-embedded sections (5 μm) of lenses from P10 nontransgenic mice. Sections were probed with an anti-SAP97 (Dlg) (D) or anti-hScrib (E) antibody. Antibody binding was detected by using fluorescent secondary antibodies and was viewed by confocal microscopy. No staining was observed in sections incubated with no primary antibody or preimmune serum (data not shown). Arrowheads, staining for Dlg or Scrib in the epithelium; arrows, staining for Dlg or Scrib in the fiber cells. e, lens epithelium; f, lens fiber cells; r, retina; c, cornea. Bars, 200 (B and C) and 100 μm (D and E).
Generation of transgenic mice.
To determine if functional disruption of PDZ proteins contributes to the hyperplastic epithelial phenotype seen in the K14E6WT transgenic mice, we characterized the phenotypes of transgenic mice expressing mutants of E6 that either retain (E6I128T) or lose (E6Δ146-151) the ability to interact with PDZ proteins. The generation of K14E6WT and K14E6I128T transgenic mice has been described previously (28, 35). The E6I128T mutant gene, containing an isoleucine-to-threonine substitution at amino acid position 128, leads to production of an E6 protein that fails to efficiently bind cellular proteins that contain an α-helical motif, such as E6AP, E6BP, E6TP, and paxillin, but is predicted to retain the ability to bind PDZ domain proteins (22). The K14E6Δ146-151 transgene generated for this study contains a mutant E6 gene encoding a protein from which the C-terminal six amino acids of the wild-type E6 protein are deleted. These last six amino acids include the PDZ interaction motif that mediates E6's interaction with its PDZ domain protein binding partners. The E6Δ146-151 mutant is unable to bind PDZ domain-containing proteins. However, it retains the ability to bind and degrade p53, and therefore to bind α-helical motif binding partners, and to activate telomerase (15). Figure 2 summarizes the structures of the three transgenes used in this study. The previously generated K14E6WT (lines 5737 and 5743) (35) and K14E6I128T (lines 6061 and 6072) (28) transgenic mouse lines all exhibited cataracts. For this study, six independent lines of mice carrying the K14E6Δ146-151 transgene were generated. None of the mice in these K14E6Δ146-151 lines exhibited cataracts in either the heterozygous or homozygous transgene states.
Characterization of transgene expression.
As previously reported, expression of the transgene under the K14 promoter directs expression to the basal layer of stratified squamous epithelia, such as those of the skin, tongue, and stomach (39). This K14 promoter has also been shown, in the cases of transgenes directing expression of the HPV-16 E6 and/or E7 genes, to direct expression to the anterior epithelium and the newly differentiating cells of the transition zone of the lens (30). For characterization of the pattern and levels of transgene expression, eye sections from 10-day-old heterozygous mice from two K14E6WT lines, two K14E6I128T lines, and six K14E6Δ146-151 lines were subjected to in situ hybridization using a cRNA probe specific for the transgene sequences. Within the anterior epithelium specifically, expression levels were similar in K14E6WT line 5743 (Fig. 3A), K14E6I128T line 6061 (Fig. 3B), and K14E6Δ146-151 line 512 (Fig. 3C). The expression domains of the K14E6WT and K14E6I128T transgenes within the transition zone were broader than that for the K14E6Δ146-151 transgene. This expanded pattern of expression corresponds to the expansion of the epithelium observed specifically in the lenses of K14E6WT and K14E6I128T mice (see Fig. 4, 5, and 6). Given their similar levels of transgene expression, these three lines of mice transgenic for wild-type or mutant E6 genes were chosen for further comparison, along with K14E6WT line 5737 (Fig. 3D), the reference line of K14E6WT mice used in our prior analyses (30). The latter line of mice, however, expressed the wild-type E6 transgene in the lens at a higher level.
FIG. 3.
Analysis of transgene mRNA expression in the lens by in situ hybridization. Paraffin-embedded sections (5 μm) of eyes from day P10 K14E6WT line 5743 (A) K14E6I128T line 6061 (B) K14E6Δ146-151 line 512 (C), and K14E6WT line 5737 (D) mice were hybridized to antisense [α-35S]UTP-labeled riboprobe derived from pABE7, dipped in emulsion, exposed for 7 days, processed, and viewed by dark-field microscopy. Representative sections are shown. e, lens epithelium; f, lens fiber cells; tz, transition zone. Bar, 100 μm. In all panels, the anterior part of the lens is oriented to the top.
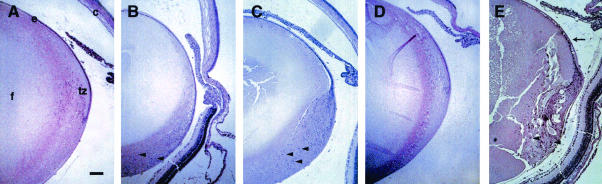
FIG. 4.
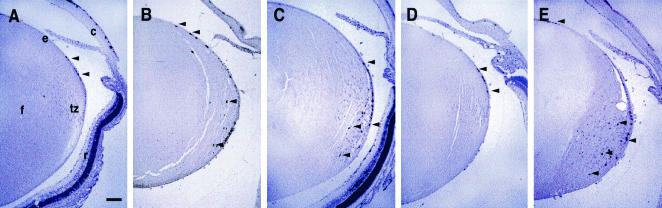
Histological analysis of lenses from K14E6 transgenic mice. Representative hematoxylin-and-eosin-stained eye sections from P15 nontransgenic (A), P15 K14E6WT line 5743 (B), P15 K14E6I128T line 6061 (C), P23 K14E6Δ146-151 line 512 (D), and P15 K14E6WT line 5737 (E) mice are shown. c, cornea; e, lens epithelium; f, lens fiber cells; tz, transition zone; arrowheads, nuclei in inappropriate regions of the fiber cell compartment; arrow, disorganized epithelium. In all panels, the anterior part of the lens is oriented to the top. Bar, 100 μm.
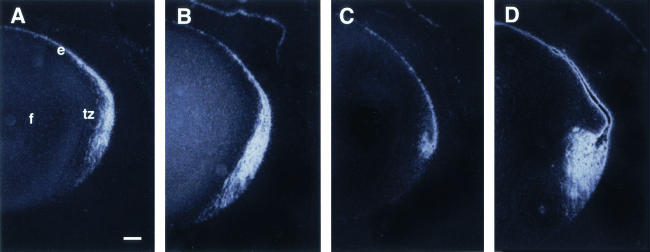
FIG. 5.
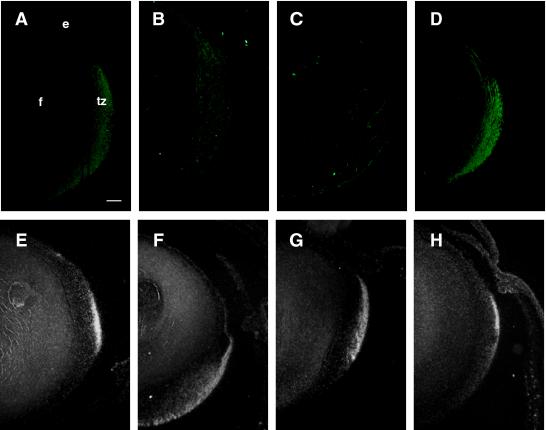
In situ detection of proliferation in lenses from K14E6 transgenic mice using BrdU incorporation assays. P10 mice were injected with BrdU 1 h prior to sacrifice. BrdU incorporation into newly synthesized DNA was detected in paraffin sections (5 μm) of eyes by immunohistochemistry using DAB color reagent and hematoxylin counterstaining. Representative immunostained eye sections from nontransgenic (A), K14E6WT line 5743 (B), K14E6I128T line 6061 (C), K14E6Δ146-151 line 512 (D), and K14E6WT line 5737 (E) mice are shown. c, cornea; e, lens epithelium; f, lens fiber cells; r, retina; tz, transition zone; arrows (dark nuclei), BrdU-positive nuclei. In all panels, the anterior part of the lens is oriented to the top. Bar, 100 μm.
FIG. 6.
In situ analyses of expression of lens differentiation markers. (A to D) Immunohistochemical detection of β-crystallin protein expression in K14E6 transgenic mouse lenses. Paraffin sections (5 μm) of eyes from P10 nontransgenic (A), P10 K14E6WT line 5743 (B), P15 K14E6I128T line 6061 (C), and P10 K14E6Δ146-151 line 512 (D) mice were incubated with anti-β-crystallin antibody followed by FITC-conjugated secondary antibody. Representative sections are shown. (E to H) In situ hybridization analysis of p57KIP2 mRNA expression in K14E6 transgenic mouse lenses. Paraffin sections (5 μm) from P10 nontransgenic (E), P10 K14E6WT line 5737 (F), P10 K14E6I128T line 6061(G), and P10 K14E6Δ146-151 line 512 (H) mice were hybridized to [α-35S]UTP-labeled antisense riboprobes derived from pBS-mp57, dipped in emulsion, exposed for 7 days, processed, and viewed by dark-field microscopy. Representative sections are shown. e, lens epithelium; f, lens fiber cells; tz, transition zone. In all panels, the anterior part of the lens is oriented to the top. Bar, 100 μm.
Microscopic analysis of transgenic lenses.
To determine if the defects noted in the lenses of transgenic mice expressing the K14E6WT transgene were retained in the lenses of K14E6I128T and K14E6Δ146-151 mice, we conducted microscopic analysis of hematoxylin-and-eosin-stained sections of paraffin-embedded eyes from postnatal mice of various ages. Figure 4A shows the appearance of the lens from a control nontransgenic mouse at postnatal day 10 (P10). A regularly organized monolayer of cuboidal cells in the epithelium was evident along the anterior surface of the lens, in addition to a well-organized transition zone at the equatorial plane, where cells begin their differentiation process to form the highly elongated differentiated fiber cells. In line 5737, the line of K14E6WT transgenic mice with higher expression levels, expression of the E6WT transgene in the epithelium resulted in multilayering and disorganization of the epithelium, with a loss of normal epithelial cell structure, movement posteriorly and disorganization of the transition zone, and an increased number of nucleated cells that failed to differentiate into fiber cells (Fig. 4E) (30). Lenses from an E6WT line with a lower expression level (line 5743) and from K14E6I128T line 6061, in which transgene expression levels were similar, also displayed these same defects, although the phenotypes were not as pronounced (Fig. 4B and C). The same phenotype was seen in a second line of K14E6I128T mice, line 6072, which expresses its transgene at commensurate levels based on in situ hybridization (data not shown). Collectively, the defects that arose in the lenses of K14E6WT and K14E6I128T mice as a consequence of transgene expression resulted in the expansion of the population of cells with epithelial characteristics into the fiber cell compartment. In contrast, the lenses from K14E6Δ146-151 line 512 mice had no unique phenotype; they were indistinguishable from the lenses of control nontransgenic mice (Fig. 4D). The other five K14E6Δ146-151 lines, albeit expressing their transgenes at levels lower than that of line 512, also failed to show any unique lens phenotypes (data not shown). Together, these analyses of matched lines of transgenic mice indicate that the ability of E6 to alter cell growth in the mouse lens specifically correlates with its capacity to bind to PDZ domain proteins. From these data, we conclude that one or more PDZ proteins function in maintaining normal lens structure.
Effect of the loss of PDZ protein function on cell proliferation.
In the normal lens, cell proliferation, which can be directly scored by the ability of a cell to incorporate the thymidine analog BrdU, is restricted to the germinative zone of the epithelium (Fig. 5A). In contrast, cells in the central epithelium and transition zone do not actively proliferate. Previously, we showed that E6 expression in the epithelium and transition zone led to increased numbers of BrdU-positive cells throughout the epithelium and transition zone (30). To determine the effect of the E6 mutants on cell proliferation, we likewise monitored BrdU incorporation in vivo. Increased numbers of BrdU-positive cells were noted throughout the epithelium and transition zone in lenses of K14E6I128T line 6061 mice (Fig. 5C), as was the case in lenses of K14E6WT line 5737 mice (Fig. 5E) and K14E6WT line 5743 mice (Fig. 5B). When these data were quantified, lenses of K14E6I128T line 6061 mice showed an increased frequency of BrdU-positive cells (8.0 ± 3.2%) compared to nontransgenic mice (4.3 ± 1.3%). In contrast, in the lenses of K14E6Δ146-151 line 512 mice, BrdU-positive cells were restricted to the anterior epithelium of the lens; none were found in the transition zone (Fig. 5D). The frequency of BrdU-positive cells in the lenses of K14E6Δ146-151 line 512 mice was indistinguishable from that in nontransgenic mice (4.0 ± 1.2% compared to 4.3 ± 1.3%). These data indicate that the hyperproliferation observed in the lens epithelium of K14E6WT mice is primarily due to E6's interaction with PDZ domain protein partners, not with α-helix partners. These observations further indicate that PDZ proteins are important for maintaining normal cell cycle regulation in the lens epithelium.
Effect of the loss of PDZ protein function on expression of differentiation-specific markers.
During the transition of an epithelial cell to a fiber cell, it undergoes withdrawal from the cell cycle and upregulation of differentiation-specific markers. The results of the BrdU analysis indicated that cells in the lenses of K14E6WT and K14E6I128T mice failed to exit the cell cycle appropriately, whereas cells in the lenses of K14E6Δ146-151mice did exit the cell cycle appropriately. In K14E6WT mice, the abnormalities in cell cycle regulation were also accompanied by changes in the expression patterns of differentiation markers (30). To determine the effect of the E6I128T and E6Δ146-151 mutations on differentiation, we assessed the expression patterns of two lens fiber cell differentiation markers, p57KIP2 and β-crystallin, by in situ hybridization and immunohistochemistry, respectively. p57KIP2 is a cyclin-dependent kinase inhibitor which has been demonstrated to be critical for control of lens cell differentiation (45). In nontransgenic lenses, upregulation of p57KIP2 is found in the transition zone and is concomitant with the withdrawal of cells from the cell cycle and the onset of differentiation (23). β-Crystallins are normally first expressed just as cells leave the epithelium and enter the fiber cell compartment and are continually expressed throughout this compartment (25).
As noted previously, expression of p57KIP2 in lenses of K14E6WT line 5737 mice showed a posteriorly expanded pattern of expression, consistent with the movement posteriorly of the transition zone and failure of these cells to undergo normal differentiation (Fig. 6F). The pattern of p57KIP2 expression in lenses of K14E6I128T line 6061(Fig. 6G) was similar to that for K14E6WT line 5743 lenses (data not shown), showing an expansion in the pattern of p57KIP2 expression that is less severe than that seen in K14E6WT line 5737 (Fig. 6F). In contrast, the expression pattern of p57KIP2 in lenses of K14E6Δ146-151 line 512 mice (Fig. 6H) was indistinguishable from that in lenses of nontransgenic control mice (Fig. 6E), consistent with the absence of a unique phenotype at the cellular level in the lenses of these mice. As previously noted, β-crystallin expression in lenses of K14E6WT line 5737 mice was reduced compared to that in nontransgenic mice (30). Expression of β-crystallin in the lenses of K14E6WT line 5743 (Fig. 6B) and K14E6I128T line 6061 mice (Fig. 6C) was also reduced compared to that in nontransgenic mice (Fig. 6A). In contrast, expression of β-crystallin in lenses of K14E6Δ146-151 line 512 mice (Fig. 6D) was indistinguishable from that in nontransgenic mice (Fig. 6A). These data collectively indicate that the dysregulation of normal lens cell differentiation correlates with the ability of E6 to interact with PDZ domain partners, not α-helix partners. These data suggest that, as with the maintenance of cell cycle control, PDZ domain proteins are important for maintaining normal patterns of differentiation at the biochemical level in the lens.
Expression of functional E6 protein in lenses of K14E6Δ146-151line 512 mice.
The data described so far collectively demonstrate an absence of unique phenotypes for mice expressing the E6Δ146-151 transgene at levels comparable to that in mice expressing wild-type E6 (line 5743) or the E6I128T mutant (line 6061). Those transgene expression studies were performed at the RNA level. We wanted to determine whether the K14E6Δ146-151 line 512 mice stably expressed E6 protein. It has been shown previously that the wild-type E6 protein can eliminate DNA damage responses in vivo and that this capacity is primarily due to E6's inactivation of p53 (28, 29). TheE6Δ146-151 protein is predicted to retain the ability to bind to and induce the degradation of p53 protein in vivo. Therefore, we determined whether in K14E6Δ146-151 line 512 mice, as is found in K14E6Δ146-151 mouse lines, E6 can inhibit the induction of p53 protein following irradiation with 5 Gy of 137Cs. In contrast to what was observed for nontransgenic mice, p53 protein was not induced in either K14E6Δ146-151 or K14E6WT mice (Table 1). These data demonstrate that in line 512 mice, a mutant E6 protein retaining the ability to inactivate p53 is stably expressed. This finding provides a compelling basis on which to interpret the significance of the absence of other phenotypes.
TABLE 1.
Quantification of p53 protein induction in response to ionizing irradiation
| Mouse strain | Irradiationa | % p53-positive cellsb |
|---|---|---|
| Nontransgenic | − | 0 |
| + | 59 | |
| K14E6WT | − | 0 |
| + | 3.5c | |
| K14E6Δ146-151 | − | 0 |
| + | 2.6c |
Nine-day-old animals were subjected to 4 Gy of 137Cs ionizing radiation. Tissues were harvested at 24 h postirradiation. Tissues from unirradiated controls were likewise harvested at 10 days of age.
p53-positive cells were identified by immunohistochemistry as described in Materials and Methods. Over 250 cells were scored per sample. Shown are results from representative mice; similar results were seen with at least three animals per genotype.
The few p53-positive cells in these two samples were markedly lower in their intensity of staining compared to that seen in irradiated nontransgenic mice.
Effect of E6 expression on PDZ target proteins.
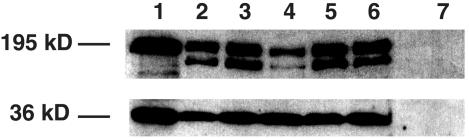
It has been shown in studies in vitro that E6 not only binds to the PDZ proteins Dlg and Scrib, but also promotes their degradation through ubiquitin-mediated proteolysis (9, 27). To determine if E6 expression affects the levels of Dlg or Scrib protein and whether this might pertain to the observed phenotypes, we measured the levels of these proteins in lens extracts prepared from postnatal day 10 mice from nontransgenic as well as K14E6WT, K14E6I128T, and K14E6Δ146-151 transgenic mouse lines. Western blot analysis of Dlg expression showed that the levels of Dlg protein are not reduced by wild-type or mutant E6 proteins (data not shown). Western blot analysis of Scrib protein showed that in the presence of wild-type E6 protein, the level of Scrib protein in the lens was reduced (Fig. 7, lane 4). However, the levels of Scrib protein in the lenses of K14E6I128T and K14E6Δ146-151 mice were unaffected (Fig. 7, lanes 3, 5, and 6). These data are consistent with a prior study that demonstrated E6 to bind and degrade Scrib via an E6AP-mediated pathway. E6I128T protein is grossly defective in binding E6AP (22). These data show that reduced levels of at least one PDZ protein targeted by E6, but not all PDZ proteins targeted by E6, accompanies the disruption in cell cycle and differentiation control in the lens.
FIG. 7.
Determination of Scrib protein levels in lenses of K14E6 transgenic mice by Western blot analysis. Protein lysates (100 μg) from the brains of nontransgenic mice (lane 1) and the lenses of P10 nontransgenic (lane 2), K14E6Δ146-151 line 512 (lane 3), K14E6WT line 5737 (lane 4), K14E6I128T line 6061 (lane 5), and K14E6I128T line 6072 (lane 6) mice were probed with goat anti-human Scrib antibody, followed by horseradish peroxidase-conjugated secondary antibody and ECL detection. Lens lysates from nontransgenic mice in which the primary antibodies were omitted from the reaction were used as negative controls (lane 7). The blot was then stripped and reprobed with an anti-GAPDH antibody. The molecular masses, 195 and 36 kDa, are sizes of Scrib and GAPDH proteins, respectively.
Effect of a dlg mutant on lens cell growth and differentiation.
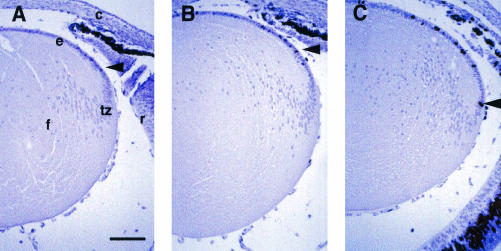
Since the epithelial phenotype of the lenses from K14E6WT mice bore a strong resemblance to the phenotype of Drosophila mutants defective in expression of Dlg and Scrib and since the E6 mutant defective for interaction with PDZ domain-containing proteins was absent from the lens phenotype seen for K14E6WT mice, it would be informative to assess the phenotypes of mice deficient in one or more of the PDZ domain-containing proteins. In addition to having documented Dlg expression in the mouse lens by RT-PCR (Fig. 1A), we have found that the Dlg message is expressed in the epithelium and transition zone and that Dlg and Scrib proteins are present in the lens based upon in situ hybridization and immunohistochemical analyses, respectively (Fig. 1B to E). Recently, Caruana and Bernstein generated a mutant mouse line that carries a β-geo cassette insertion in the Dlg locus (dlggt) (6). This insertion resulted in the loss of the SH3, 4.1, and guanylate kinase domains of Dlg; however, the region that coded for the three PDZ domains remained intact. The dlggt mutant mice died perinatally, with craniofacial defects that included cleft palates. Caruana and Bernstein reported LacZ expression in the lens. No overt lens phenotype was noted; however, a detailed examination of the lens was not performed. To determine if there was a defect in the lenses of dlggt animals, we performed histological and BrdU incorporation analyses. BrdU incorporation analysis demonstrated the presence of proliferating cells in the transition zone of dlggt/dlggt mice (Fig. 8C), in contrast to the normal restriction of BrdU-positive cells to the germinative zone within the anterior epithelium in the lenses of wild-type mice (Fig. 8A). Consistent with this expansion in the germinative zone, lenses of day E18.5 dlggt/dlggt mice had an increased number of total cells in the epithelium compared to the lenses of wild-type mice (191 versus 172; P = 4.0 × 10−7) as well as an increased number of BrdU-positive cells in the epithelium compared to wild-type lenses (34.3 versus 29.3; P = 0.001). These results indicate that intact Dlg is necessary for proper control of cell proliferation in the lens epithelium.
FIG. 8.
In situ detection of proliferation in lenses of dlggt mice using BrdU incorporation assays. Pregnant dams at E19.5 in gestation were injected with BrdU 1 h prior to sacrifice. Embryos were isolated and heads were embedded in paraffin. BrdU incorporation into newly synthesized DNA in lenses was detected in paraffin sections (5 μm) by immunohistochemistry using DAB color reagent and hematoxylin counterstaining. Representative immunostained eye sections from dlg+/+ (wild-type) (A), dlggt/+ (B), and dlggt/gt (C) embryos are shown. c, cornea; e, lens epithelium; f, lens fiber cells; r, retina; tz, transition zone; arrows (dark nuclei), BrdU-positive nuclei. In all panels, the anterior part of the lens is oriented to the top. Bar, 100 μm.
DISCUSSION
The mechanisms by which normal epithelial cell growth and differentiation are maintained in vivo in mammalian cells have long been thought to involve the activity of tumor suppressor proteins. In Drosophila, PDZ domain proteins, including Dlg and Scrib, which display tumor suppressor properties, determine proper cell growth and polarity in embryonic epithelial sheets of the imaginal discs. In this study, we demonstrated that multiple PDZ protein genes, including but not limited to Dlg and Scrib, are expressed in the mouse ocular lens. We provided evidence that the lens cell growth and differentiation are dependent on these PDZ domain proteins, in particular Dlg. Furthermore, we provided evidence to suggest that one mechanism through which the E6 oncoprotein from HPV-16 alters cell growth and differentiation is through interference with the function of PDZ proteins. This finding has potential implications for the mechanism by which E6 contributes to HPV-associated cervical cancer. Finally, these studies highlighted the conservation of function of PDZ domain proteins in controlling epithelial cell growth and differentiation from Drosophila to the mouse.
Multiple PDZ proteins are expressed in the lens.
To date, there is little information regarding the expression and function of PDZ proteins in mammalian epithelial tissues. In addition to their role in maintaining normal cell growth, PDZ proteins such as Dlg and Scrib are thought to localize to cell-cell junctional complexes and to be responsible, at least in part, for maintaining epithelial cell integrity and polarity. The lens is known to contain adherens junctions in the epithelium, newly differentiating fiber cells, and central epithelium (44). We demonstrated that Dlg and Scrib, as well as MAGI-1, MAGI-3, and MPDZ, are expressed at the RNA level in the lenses of neonatal mice (Fig. 1). The expression domains of Dlg and Scrib RNA message (Fig. 1B and C) and protein (Fig. 1D and E) overlap with the domain of transgene expression (Fig. 3). The presence of these proteins in the lens, the defects in lens epithelium noted in the dlggt/dlggt mice, and the reduced levels of Scrib protein in the lenses of K14E6WT mice (Fig. 7) together suggest that Dlg and Scrib play a similar role in epithelial cells in mammalian hosts as that seen in invertebrates; additional studies will be required to determine if these proteins carry out their function by similar mechanisms, as has been implicated by their analysis with Drosophila and C. elegans (3, 5, 20).
PDZ protein function is essential for normal lens cell growth and differentiation.
To address the possible requirement for PDZ proteins in controlling lens growth and differentiation, we used the human papillomavirus protein E6 and mutants thereof as probes. Our choice was based on the fact that multiple PDZ domain-containing proteins appear to be expressed in the lens (Fig. 1), and to date, there is no evidence to indicate that any one PDZ protein contributes to maintaining normal cell growth and structure in mammalian tissues. Expression in the mouse lens of the E6WT protein, which among its biochemical properties has the ability to bind multiple PDZ domain proteins, resulted in a wide range of defects, including an increased rate of proliferation and induction of proliferation in spatially inappropriate regions of the lens (Fig. 5), inhibition of differentiation at the morphological and molecular level (Fig. 4 and 6), and defects in cell structure and adhesion (Fig. 4) (29; data not shown). Collectively, these defects resulted in an expansion of the population of lens cells exhibiting epithelial rather than terminally differentiated characteristics and an expansion in the population of cells expressing the transgenes (Fig. 3). These defects were absent from the lenses of mice expressing the E6Δ146-151 mutant, which does not have the ability to bind to PDZ proteins. In contrast, mice expressing the K14E6I128T transgene, in which the expressed E6 protein retains the ability to bind PDZ protein partners but loses the ability to bind α-helical protein partners, displayed the same defects as did mice expressing the E6WT transgene. These data strongly support the hypothesis that the function of PDZ proteins is required for regulating normal growth and differentiation of epithelial tissues in vivo in the mouse.
The results of this study implicate a second family of proteins with putative tumor suppressor function in maintaining the normal growth and differentiation of mammalian epithelial cells. While it is known that the loss of pRb function directly results in deregulated E2F activity (8, 13), it is not entirely clear how PDZ domain-containing tumor suppressors affect the cell cycle. It is thought that proteins such as Dlg and Scrib do not act directly on the cell cycle but rather that they act as molecular scaffolds that recruit components necessary for signal transduction of transmembrane complexes (3, 7). In the case of Dlg, the effect may also be a result of disruption of APC or PTEN (phosphatase and tensin homolog deleted on chromosome 10) (1, 14). Although it may seem that effects of the disruption of pRb and PDZ proteins occur through different pathways, the observation that both proteins affect the expression of the differentiation markers p57KIP and β-crystallin (Fig. 6) (30) suggests that these two pathways converge on the same pathway or proceed in parallel.
Disruption of Scrib may contribute to defects in the lens.
In this study, we found that Scrib protein levels are reduced in lenses expressing E6WT but not in those expressing either the E6Δ146-151 or E6I128T mutant. The simplest explanation for this observation is that E6 leads to the degradation of Scrib. It is known that E6 is capable of degrading PDZ proteins such as Scrib via an E6AP-dependent mechanism (27). This observation supports two important contentions. First, functional E6 protein is expressed in the lenses of K14E6WT mice. Second, Scrib is targeted by E6 in the lens and therefore could be a PDZ protein whose function contributes to the regulation of normal cell growth, cell structure, and differentiation of lens epithelial cells. Alternatively, were Scrib protein absent from the epithelium or at a substantially lower level in the epithelium than in the fiber cells, it would be conceivable that the expansion of the epithelial compartment at the expense of the fiber cell compartment could result in the apparent reduction in Scrib levels that we observed by Western blot analysis. Since Scrib protein is found in the epithelium, we favor the former interpretation, although we cannot at this time rule out the latter. Interestingly, the E6 protein also has been argued, at least under certain conditions, to target Dlg for degradation through a ubiquitin-mediated pathway (9, 17). However, Dlg protein levels are not reduced in the lenses of K14E6WT mice (data not shown). Nevertheless, Dlg appears to be required for proper regulation of lens cell growth and differentiation (Fig. 8). It is important to note that mice expressing the E6I128T transgene, which should be defective for E6AP-mediated targeting of E6-associated proteins for degradation, retained the same phenotype as that for mice expressing similar levels of the wild-type E6 protein. These data suggest that the degradation of neither Dlg nor Scrib is necessary for the phenotype induced by E6. Instead, the results argue that binding of E6 through PDZ domain interactions is sufficient. This fact then raises the alternative possibility that the phenotypes of mice expressing wild-type E6 arise because E6's binding to Dlg and Scrib leads to their mislocalization within the cell and/or interferes with the ability of these proteins to associate properly with the junctional complexes. Preliminary immunohistochemical analysis of PDZ protein localization in the lenses of control and K14E6 transgenic mice provides some support for this possibility. In the lenses of nontransgenic and K14E6Δ146-151 mice, Scrib was restricted mainly to the apical and basal membranes of epithelial cells in the transition zone. In comparison, in the lenses of K14E6WT and K14E6I128T mice, Scrib appeared more widely distributed across apical, lateral, and basal surfaces of the epithelial cells (not shown). Additionally, in the posterior region of the lenses of nontransgenic or K14E6Δ146-151 mice, Scrib appeared to be basally restricted. In comparison, there was an expansion of Scrib onto the lateral membranes in the lenses of K14E6WT and K14E6I128T mice (not shown). Mislocalization of other PDZ proteins such as ZO-1 was also observed (C. Rivera, M. M. Nguyen, and A. E. Griep, unpublished observations).
Dlg is required for cell cycle control in the lens epithelium.
To elucidate the mechanism by which PDZ proteins regulate lens development, it is necessary to first identify the specific members of the family that are required and the individual role of each protein or proteins in the process. To begin to address this issue, we evaluated the consequences of an insertional mutation in Dlg on the growth and structural properties in the lens. Our BrdU incorporation analysis of lenses from dlggt/dlggt mice (Fig. 8) showed that this gene is required for normal cell cycle regulation in a population of cells that retain proliferative potential (the anterior epithelium) as well as in cells within the transition zone that normally have entered a postmitotic state leading to differentiation. Although prior study of dlggt mutant mice demonstrated that Dlg is required for proper craniofacial development (6), the underlying mechanism leading to developmental defects was not identified. Thus, the results of our present study provide the first evidence that Dlg plays a role in maintaining normal cell cycle control in the mouse in vivo.
The similarity between the proliferation defects in the lenses of the dlggt mice (Fig. 8) and the K14E6WT mice (Fig. 5) suggests that interference with Dlg function contributes to the E6 phenotype. The phenotype of the lenses of dlggt/dlggt mice is, however, not as severe as that for K14E6WT lenses, in which defects in cell structure and adhesion were clearly evident. There are several possible explanations for the observed differences in phenotype. An obvious explanation is that the E6 phenotype is generated due to interference with the function of multiple PDZ proteins and, due to redundancy or compensation, interference with the function of only one member does not lead to a discernible phenotype. Second, the difference may be due to the disparity in the ages of the dlggt/dlggt mice and the K14E6WT mice. The BrdU analysis on the dlggt/dlggt mice was carried out on embryos because these mice die perinatally. In contrast, analysis of lens phenotype in nontransgenic and E6 mutant mice was carried out on postnatal animals because transgene expression is not observed until after birth and the first indication of altered cell growth is not observed until postnatal day 4 (P4) (data not shown). This explanation suggests that Dlg may play a more important role in controlling lens epithelial growth and differentiation in the postnatal animal than in the embryo. Finally, the difference in severity of phenotype between K14E6WT mice and dlggt homozygous mice may reflect the possibility that dlggt is a hypomorphic rather than null allele. The insertional mutation effectively removes the SH3 domain, the 4.1 domain, and the guanylate kinase domain of Dlg. However, the three PDZ domains remain intact in this mutant, and therefore the gene product from this allele may retain partial function. That the SH3 and GUK domains of Dlg may be involved in cell growth regulation is supported by mutational analysis of both Drosophila Dlg and its human homolog, in which the SH3 or GUK-like domain can also abolish inhibition of cell cycle progression (14, 42). Ultimately, a systematic analysis of the individual and combined phenotypes of null mutations in each of these genes and a quantitative comparison to the phenotype arising as a consequence of E6 expression will be required to answer this question. This likely will require the generation of conditional null alleles, at least for a subset of the genes encoding PDZ domain proteins.
E6 may contribute to carcinogenesis by targeting PDZ proteins.
E6 is one of two HPV genes expressed in cervical cancer. HPV-16 E6 is highly transforming in tissue culture and is tumorigenic in vivo. The most evident, acute effect of E6 in vivo is the induction of proliferation of undifferentiated epithelial cells within the lens (30) and epidermis (35). Here we demonstrate that the induction of cell proliferation in the mouse lens is dependent upon E6's binding to PDZ domain protein partners. The same requirement is true in the epidermis (29). Current studies are directed at learning whether this induction of epithelial hyperplasia contributes to E6's oncogenic potential. Were this to be the case, E6's targeting of PDZ proteins would represent a second mechanism through which E6 could contribute to cancer. It has been shown that E6, when coexpressed with E7 in lens fiber cells, supported tumor formation (11, 31), and in part, this capacity of E6 was thought to be due to E6's ability to inhibit E7-induced apoptosis through both p53-dependent and p53-independent pathways (32). This p53-dependent effect of E6 was distinct, however, from the effect of E6 expression on lens cell growth and differentiation when E6 was expressed either in the epithelium (this study) or in fiber cells (31, 32). In both cases, the effects of E6 that were described were solely p53 independent.
Cross-species conservation of function of PDZ proteins.
Studies in Drosophila have often led to important findings in vertebrates and for human diseases. However, there are currently few examples in which there is a conservation of tumor suppressor properties between Drosophila and mammals. One such example is the Drosophilia gene Warts and its human homolog LATS (36, 43). In the present study, we provide evidence for conservation of function between another Drosophila tumor suppressor gene, Dlg, and its mouse homolog. Multiple studies using Drosophila and mammalian cell culture systems have indicated that Dlg and Scrib appear to localize to similar cellular regions and have conserved physiological functions. This is highlighted in the case of Dlg, for which it was found that human Dlg is able to functionally compensate for a Drosophila dlg hypomorphic mutation (38). Taken together, these studies provide justification for determining the individual contributions of these as well as other PDZ proteins in epithelial cell cycle control and differentiation, the mechanism through which these proteins act, and ultimately, if protein factors possess tumor suppressor activity in vivo in the mouse.
Acknowledgments
We thank Denise Galloway for the E6Δ146-151 mutant, Sam Zigler for the anti-β-crystallin antibody, Johannes Hell for the anti-SAP97 antibody, Pumin Zhang for the pBS-mp57 plasmid, Joe Warren and Kathy Helmuth of the Transgenic Animal Facility for generating the K14E6Δ146-151 transgenic mice and rederiving some of the transgenic mouse strains, respectively, Amy Liem for assistance with the irradiator, and Denis Lee for assistance with p53 immunohistochemistry.
This work was supported by NIH grants EY09091, CA14520, CA09135, and CA22443 and ACS grant RPG-96-043-04-MGO.
REFERENCES
- 1.Adey, N. B., L. W. Huang, P. A. Ormonde, M. L. Baumgard, R. Pero, D. V. Byreddy, S. V. Tavtigian, and P. L. Bartel. 2000. Threonine phosphorylation of the MMAC1/PTEN PDZ binding domain both inhibits and stimulates PDZ binding. Cancer Res. 60:35-37. [PubMed] [Google Scholar]
- 2.Bilder, D. 2001. PDZ proteins and polarity: functions from the fly. Trends Genet. 17:511-519. [DOI] [PubMed] [Google Scholar]
- 3.Bilder, D., M. Li, and N. Perrimon. 2000. Cooperative regulation of cell polarity and growth by Drosophila tumor suppressors. Science 289:113-116. [DOI] [PubMed] [Google Scholar]
- 4.Bilder, D., and N. Perrimon. 2000. Localization of apical epithelial determinants by the basolateral PDZ protein Scribble. Nature 403:676-680. [DOI] [PubMed] [Google Scholar]
- 5.Bossinger, O., A. Klebes, C. Segbert, C. Theres, and E. Knust. 2001. Zonula adherens formation in Caenorhabditis elegans requires dlg-1, the homologue of the Drosophila gene discs large. Dev. Biol. 230:29-42. [DOI] [PubMed] [Google Scholar]
- 6.Caruana, G., and A. Bernstein. 2001. Craniofacial dysmorphogenesis including cleft palate in mice with an insertional mutation in the discs large gene. Mol. Cell. Biol. 21:1475-1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dimitratos, S. D., D. F. Woods, D. G. Stathakis, and P. J. Bryant. 1999. Signaling pathways are focused at specialized regions of the plasma membrane by scaffolding proteins of the MAGUK family. Bioessays 21:912-921. [DOI] [PubMed] [Google Scholar]
- 8.Dyson, N. 1998. The regulation of E2F by pRB-family proteins. Genes Dev. 12:2245-2262. [DOI] [PubMed] [Google Scholar]
- 9.Gardiol, D., C. Kuhne, B. Glaunsinger, S. S. Lee, R. Javier, and L. Banks. 1999. Oncogenic human papillomavirus E6 proteins target the discs large tumour suppressor for proteasome-mediated degradation. Oncogene 18:5487-5496. [DOI] [PubMed] [Google Scholar]
- 10.Glaunsinger, B. A., S. S. Lee, M. Thomas, L. Banks, and R. Javier. 2000. Interactions of the PDZ-protein MAGI-1 with adenovirus E4-ORF1 and high-risk papillomavirus E6 oncoproteins. Oncogene 19:5270-5280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Griep, A., R. Herber, S. Jeon, J. Lohse, R. Dubielzig, and P. Lambert. 1993. Tumorigenicity by human papillomavirus type 16 E6 and E7 in transgenic mice correlates with alterations in epithelial cell growth and differentiation. J. Virol. 67:1373-1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hogan, B., R. Beddington, F. Costantini, and E. Lacy. 1994. Manipulating the mouse embryo: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 13.Hyde, R. K., and A. E. Griep. 2002. Unique roles for E2F1 in the mouse lens in the absence of functional pRB proteins. Investig. Ophthalmol. Vis. Sci. 43:1509-1516. [PubMed] [Google Scholar]
- 14.Ishidate, T., A. Matsumine, K. Toyoshima, and T. Akiyama. 2000. The APC-hDLG complex negatively regulates cell cycle progression from the G0/G1 to S phase. Oncogene 19:365-372. [DOI] [PubMed] [Google Scholar]
- 15.Kiyono, T., S. A. Foster, J. I. Koop, J. K. McDougall, D. A. Galloway, and A. J. Klingelhutz. 1998. Both Rb/P16(Ink4a) inactivation and telomerase activity are required to immortalize human epithelial cells. Nature 396:84-88. [DOI] [PubMed] [Google Scholar]
- 16.Kiyono, T., A. Hiraiwa, M. Fujita, Y. Hayashi, T. Akiyama, and M. Ishibashi. 1997. Binding of high-risk human papillomavirus E6 oncoproteins to the human homologue of the Drosophila discs large tumor suppressor protein. Proc. Natl. Acad. Sci. USA 94:11612-11616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kuhne, C., D. Gardiol, C. Guarnaccia, H. Amenitsch, and L. Banks. 2000. Differential regulation of human papillomavirus E6 by protein kinase A: conditional degradation of human discs large protein by oncogenic E6. Oncogene 19:5884-5891. [DOI] [PubMed] [Google Scholar]
- 18.Lee, S. S., R. S. Weiss, and R. T. Javier. 1997. Binding of human virus oncoprotein to hDlg/SAP97, a mammalian homolog of the Drosophila discs large tumor suppressor protein. Proc. Natl. Acad. Sci. USA 94:6670-6675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee, S. S., B. Glaunsinger, F. Mantovani, L. Banks, and R. T. Javier. 2000. Multi-PDZ domain protein MUPP1 is a cellular target for both adenovirus E4-ORF1 and high-risk papillomavirus type 18 E6 oncoproteins. J. Virol. 74:9680-9693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Legouis, R., A. Gansmuller, S. Sookhareea, J. M. Bosher, D. L. Baillie, and M. Labouesse. 2000. LET-413 is a basolateral protein required for the assembly of adherens junctions in Caenorhabditis elegans. Nat. Cell Biol. 2:415-422. [DOI] [PubMed] [Google Scholar]
- 21.Lin, L. H., K. E. Sahr, and A. H. Chishti. 1997. Identification of the mouse homologue of human discs large and rat Sap97 genes. Biochim. Biophys. Acta 1362:1-5. [DOI] [PubMed] [Google Scholar]
- 22.Liu, Y., J. J. Chen, Q. S. Gao, S. Dalal, Y. H. Hong, C. P. Mansur, V. Band, and E. J. Androphy. 1999. Multiple functions of human papillomavirus type 16 E6 contribute to the immortalization of mammary epithelial cells. J. Virol. 73:7297-7307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lovicu, F. J., and J. W. McAvoy. 1999. Spatial and temporal expression of p57(KIP2) during murine lens development. Mech. Dev. 86:165-169. [DOI] [PubMed] [Google Scholar]
- 24.Matsumine, A., A. Ogai, T. Senda, N. Okumura, K. Satoh, G. H. Baeg, T. Kawahara, S. Kobayashi, M. Okada, K. Toyoshima, and T. Akiyama. 1996. Binding of Apc to the human homolog of the Drosophila discs large tumor suppressor protein. Science 272:1020-1023. [DOI] [PubMed] [Google Scholar]
- 25.McAvoy, J. 1978. Cell division, cell elongation and distribution of α, β, and γ-crystallins in the rat lens. J. Embryol. Exp. Morphol. 44:149-165. [PubMed] [Google Scholar]
- 26.Morgenbesser, S. D., B. O. Williams, T. Jacks, and R. A. DePinho. 1994. p53-dependent apoptosis produced by Rb-deficiency in the developing mouse lens. Nature 371:72-74. [DOI] [PubMed] [Google Scholar]
- 27.Nakagawa, S., and J. M. Huibregtse. 2000. Human scribble (Vartul) is targeted for ubiquitin-mediated degradation by the high-risk papillomavirus E6 proteins and the E6AP ubiquitin-protein ligase. Mol. Cell. Biol. 20:8244-8253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nguyen, M., S. Song, A. Liem, E. Androphy, Y. Liu, and P. F. Lambert. 2002. A mutant of human papillomavirus type 16 E6 deficient in binding alpha-helix partners displays reduced oncogenic potential in vivo. J. Virol. 76:13039-13048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nguyen, M. L., M. M. Nguyen, D. Lee, A. E. Griep, and P. F. Lambert. 2003. The PDZ ligand domain of the human papillomavirus type 16 E6 protein is required for E6's induction of epithelial hyperplasia in vivo. J. Virol. 77:6957-6964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nguyen, M. M., S. J. Potter, and A. E. Griep. 2002. Deregulated cell cycle control in lens epithelial cells by expression of inhibitors of tumor suppressor function. Mech. Dev. 112:101-113. [DOI] [PubMed] [Google Scholar]
- 31.Pan, H. C., and A. E. Griep. 1994. Altered cell cycle regulation in the lens of HPV-16 E6 or E7 transgenic mice—implications for tumor suppressor gene function in development. Genes Dev. 8:1285-1299. [DOI] [PubMed] [Google Scholar]
- 32.Pan, H. C., and A. E. Griep. 1995. Temporally distinct patterns of p53-dependent and p53-independent apoptosis during mouse lens development. Genes Dev. 9:2157-2169. [DOI] [PubMed] [Google Scholar]
- 33.Piatigorsky, J. 1981. Lens differentiation in vertebrates. A review of cellular and molecular features. Differentiation 19:134-153. [DOI] [PubMed] [Google Scholar]
- 34.Song, S. Y., and P. F. Lambert. 1999. Different responses of epidermal and hair follicular cells to radiation correlate with distinct patterns of p53 and p21 induction. Am. J. Pathol. 155:1121-1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Song, S. Y., H. C. Pitot, and P. F. Lambert. 1999. The human papillomavirus type 16 E6 gene alone is sufficient to induce carcinomas in transgenic animals. J. Virol. 73:5887-5893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tao, W. F., S. Zhang, G. S. Turenchalk, R. A. Stewart, M. A. R. St. John, W. L. Chen, and T. Xu. 1999. Human homologue of the Drosophila melanogaster lats tumour suppressor modulates CDC2 activity. Nat. Genet. 21:177-181. [DOI] [PubMed] [Google Scholar]
- 37.Thomas, M., R. Laura, K. Hepner, E. Guccione, C. Sawyers, L. Lasky, and L. Banks. 2002. Oncogenic human papillomavirus E6 proteins target the MAGI-2 and MAGI-3 proteins for degradation. Oncogene 21:5088-5096. [DOI] [PubMed] [Google Scholar]
- 38.Thomas, U., B. Phannavong, B. Muller, C. C. Garner, and E. D. Gundelfinger. 1997. Functional expression of rat synapse-associated proteins Sap97 and Sap102 in Drosophila Dlg-1 mutants—effects on tumor suppression and synaptic Bouton structure. Mech. Dev. 62:161-174. [DOI] [PubMed] [Google Scholar]
- 39.Vassar, R., M. Rosenberg, S. Ross, A. Tyner, and E. Fuchs. 1989. Tissue-specific and differentiation-specific expression of a human K14 keratin gene in transgenic mice. Proc. Natl. Acad. Sci. USA 86:1563-1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Werness, B. A., A. J. Levine, and P. M. Howley. 1990. Association of human papillomavirus types 16 and 18 E6 proteins with p53. Science 248:76-79. [DOI] [PubMed] [Google Scholar]
- 41.Woods, D. F., and P. J. Bryant. 1991. The discs-large tumor suppressor gene of Drosophila encodes a guanylate kinase homolog localized at septate junctions. Cell 66:451-464. [DOI] [PubMed] [Google Scholar]
- 42.Woods, D. F., C. Hough, D. Peel, G. Callaini, and P. J. Bryant. 1996. Dlg protein is required for junction structure, cell polarity, and proliferation control in Drosophila epithelia. J. Cell Biol. 134:1469-1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yabuta, N., T. Fujii, N. G. Copeland, D. J. Gilbert, N. A. Jenkins, H. Nishiguchi, Y. Endo, S. Toji, H. Tanaka, Y. Nishimune, and H. Nojima. 2000. Structure, expression, and chromosome mapping of LATS2, a mammalian homologue of the Drosophila tumor suppressor gene lats/warts. Genomics 63:263-270. [DOI] [PubMed] [Google Scholar]
- 44.Zampighi, G. A., S. Eskandari, and M. Kreman. 2000. Epithelial organization of the mammalian lens. Exp. Eye Res. 71:415-435. [DOI] [PubMed] [Google Scholar]
- 45.Zhang, P., N. J. Liegeois, C. Wong, M. Finegold, H. Hou, J. C. Thompson, A. Silverman, J. W. Harper, R. A. DePinho, and S. J. Elledge. 1997. Altered cell differentiation and proliferation in mice lacking p57KIP2 indicates a role in Beckwith-Wiedemann syndrome. Nature 387:151-158. [DOI] [PubMed] [Google Scholar]