Abstract
Porphyrias are a group of metabolic disorders resulting from enzymatic defects in the heme biosynthetic pathway. Erythropoietic protoporphyria is thought to be the second most common porphyria seen in clinical practice. It is, however, commonly under-recognized and can lead to both cutaneous manifestations as well as derangement in hepatic function in a minority of patients. This review summarizes the current understanding of this disorder. Different treatment options are discussed with the goal of preventing liver damage. The roles of liver and bone marrow transplantation are also addressed.
Keywords: Erythropoietic protoporphyria, metabolic disorders
Porphyrias are a group of metabolic disorders caused by defects in the heme biosynthetic pathway. Heme is the critical prosthetic group for numerous hemoproteins such as hemoglobin, myoglobin, catalase, and microsomal cytochromes. The term “porphyria” is derived from the Greek word “porphyra,” which means “purple.” The biochemical hallmark of porphyrias is overproduction and, ultimately, overexcretion of compounds called porphyrins, which have a deep red or purple color. The first clinical reports of porphyria appeared in the late nineteenth century and described a patient with severe cutaneous photosensitivity and brown pigmentation of the bones, which is characteristic of congenital erythropoietic porphyria. Soon thereafter, a case of acute porphyria was described in a person addicted to drugs who had urine the color of port wine and later died after taking the hypnotic drug sulfonomethane.
Porphyrias can present clinically either with neurovisceral symptoms and signs such as abdominal pain, constipation, and weakness or with cutaneous symptoms and signs. In hereditary coproporphyria and variegate porphyria, patients may present with both types of symptoms; in the other forms of porphyria, patients manifest only one type of clinical presentation. When considering therapy for porphyrias, it is useful to classify the disorders into two major categories: acute/inducible porphyrias or chronic cutaneous porphyrias (Table 1). Regardless of the specific type of acute porphyria or associated enzymatic defect, all of the acute porphyrias produce similar neurovisceral manifestations and should be managed in a similar manner. Management of cutaneous porphyria, though more specific to the particular type of porphyria, also involves the application of several general principles.
Table 1.
Classification and Major Features of Human Porphyrias
| Clinical features | |||||
|---|---|---|---|---|---|
| Disease | Primary enzymatic defect | Autosomal inheritance | Neurovisceral symptoms | Photosensitivity dermatosis | |
| Acute/inducible pofphyiias | |||||
| ALA-D deficiency porphyria | ALA dehydratase | Recessive | + | -- | |
| Acute intermittent porphyria | PBG deaminase | Dominant | + | -- | |
| Hereditary coproporphyria | Coproporphyrinogen oxidase | Dominant | + | + | |
| Variegate porphyria | Protoporphyrinogen oxidase | Dominant | + | + | |
| Chronic cutaneous porphyrias | |||||
| Congenital erythropoietic | Uroporphyrinogen III cosynthase | Recessive | -- | ++ | |
| Hepatoerythropoietic porphyria | Uroporphyrinogen decarboxylase | Recessive | +/- | + | |
| Porphyria cutanea tarda | Uroporphyrinogen decarboxylase | Dominant (acquired variant exists) | -- | + | |
| Protoporphyria | Ferrochelatase | Dominant† | --* | + | |
ALA=5-aminolevulinate; ALA-D=ALA dehydratase; PBG=porphobilinogen. ALA is the first intermediate in the heme biosynthetic pathway, and PBG is the second intermediate in the heme biosynthetic pathway.
A neurovisceral syndrome reminiscent of those observed in acute porphyrias has been described in several patients with protoporphyria and hepatic failure around the time of orthotopic liver transplantation;
autosomal recessive inheritance has been described.
Enzymatic Defects and Genetics
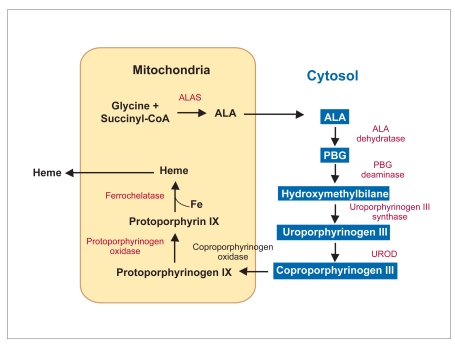
Erythropoietic protoporphyria (EPP) was first described by Magnus and associates in 1961.1 Bonkovsky and colleagues demonstrated that heme synthase or ferrochelatase (FECH), an inner mitochondrial enzyme, was deficient in this disorder (Figure 1).2 Since then, the gene for FECH has been localized to 18q21,3,4 and approximately 120 disease-producing mutations have been described to date. The gene has 11 exons and a minimum size of 45 kb. From early studies of kindreds with EPP, most investigators concluded that the disease displayed autosomal dominant inheritance with low penetrance and an increased propensity to develop chronic liver disease,5 though autosomal recessive forms of EPP have also been described.6 The activity of ferrochelatase in clinically affected patients is approximately 15–25% of normal activity. The parents of many patients are asymptomatic and phenotypically normal, though approximately one half demonstrate decreases in ferrochelatase activity. More recent familial studies have identified a single nucleotide polymorphism (IVS3-48C), which leads to a lower level of expression of the normal FECH gene allele.7 Patients develop clinical disease with less than 25% of normal FECH activity when a mutated allele is inherited along with the low-level-expressing, wild-type allele, which usually harbors the IVS3-48C polymorphism. One recent paper stressed the importance of large deletions in the FECH gene exons as an important cause of “mutation-negative” EPP.8
Figure 1.
The heme biosynthetic pathway.
ALA=5-aminolevulinate; ALAS=delta-aminolevulinate synthase; PBG=porphobilinogen; UROD = uroporphyrinogen decarboxylase.
FECH carries out the ultimate step in heme biosynthesis, namely the insertion of ferrous iron into protoporphyrin for the formation of heme. The only difference between heme and protoporphyrin is the insertion of an iron atom into the tetrapyrrole ring in heme. This insertion increases the stability of the structure and results in the loss of its fluorescent properties. In EPP, the major site of protoporphyrin overproduction is localized to the bone marrow, where erythrocytes are developed.9–12 Protoporphyrin, unlike other porphyrins, is water-insoluble and therefore removed from the body only through hepatic excretion into bile or feces. Protoporphyrin is not excreted in urine, though it does undergo some enterohepatic circulation.
Epidemiology and Clinical Presentation
Of all the porphyrias found in the United States, EPP is thought to be second in prevalence only to porphyria cutanea tarda. The exact prevalence of EPP has not been reliably estimated due to its frequently mild nature and normal urinary porphyrins, which increase the possibility of missing bona fide cases. There is no gender predominance in EPP, which is found across all ethnic and racial groups.
EPP, a disorder with highly variable clinical expression, has been previously well characterized as a chronic cutaneous porphyria.13–16 Most affected patients present in early childhood with sun sensitivity and, at times, an urticarial-type reaction. Infants and children classically develop intense burning pain from sun-exposed skin following brief exposure in the spring and summer. Several hours later, erythema, edema, and itching become prominent. Vesicles are uncommon and develop only with prolonged sun exposure. With chronic and repeated exposure, involved skin may become leathery and hyperkeratotic. This reaction is particularly prominent in a malar “butterfly” pattern on the face and over the knuckles of the hands. Patients usually have a chronic and stable disease course, with no identified aggravating factors.
Photoactivation of lipid-soluble protoporphyrin, which is deposited in the dermal blood vessels, leads to the characteristic skin burning from sun exposure. Chronic facial scarring, hirsutism, and fluorescent teeth, which are common in congenital erythropoietic porphyria or hepatoerythropoietic porphyria, are not usually found in EPP.16 Photoactivation of circulating water-soluble uroporphyrins, on the other hand, leads to chronic blistering, as seen in congenital erythropoietic porphyria and porphyria cutanea tarda.
The most serious complication of EPP is development of pigmentary cirrhosis of the liver.17,18 Fortunately, liver injury is evident in only a minority of patients with EPP (-10%), with approximately 3–5% of the patients eventually developing end-stage liver disease. The pattern of liver injury, when seen in this disorder, typically develops over a period of time and ranges from mild liver disease with elevations in liver enzymes to progressive liver disease with extensive bridging fibrosis or cirrhosis and other concomitant complications. By itself, EPP rarely presents as true acute liver injury, though there may be sudden acute worsening of underlying chronic liver disease.19 Liver disease is thought to develop due to the precipitation of protoporphyrin in hepatocytes and biliary radicles.20,21 No risk factors have been identified that are able to predict the development of liver disease in EPP thus far.
A common complication of EPP is the development of pigment gallstones, which have a high content of protoporphyrin. In bile, protoporphyrin may crystallize, providing the nidus for development of gallstones. When evident in children, gallstones should prompt evaluation for EPP.22 Mild anemia with or without hemolysis is occasionally noted. In EPP, abdominal pain and neuropathy-like symptoms are seen only rarely and in the setting of advanced liver disease.23–26 Of note, motor neuropathy can develop both pre- and postoperatively at the time of liver transplantation.
Diagnosis
The diagnosis of EPP requires the demonstration of increased levels of protoporphyrin, without increased levels of coproporphyrin, in the stool, RBCs, or both.27 In EPP, elevated levels of erythrocyte protoporphyrin, plasma protoporphyrin, and stool protoporphyrin are evident. Urinary porphyrin levels are usually normal because, as already mentioned, protoporphyrin is not excreted into the urine. Analysis of the plasma porphyrin emission spectrum reveals a characteristic peak at approximately 634–636 nm, following excitation with light in the Soret band (-400 nm). Erythrocyte protoporphyrin elevations are also evident in severe iron deficiency and lead poisoning. In EPP, the protoporphyrin is primarily free protoporphyrin, in contrast to iron and lead toxicity, in which predominantly Zn-protoporphyrin is found.28 In EPP patients, rising plasma protoporphyrin levels, along with severe elevations in RBC protoporphyrin levels, may portend impending liver failure.29,30 Patients with EPP who develop significant liver disease have been shown to have significant increases in urinary coproporphyrin levels. An inversion of the physiological urinary coproporphyrin isomer III/I ratio has also been observed in patients with protoporphyria who develop cholestatic cirrhosis.31 Liver biopsy is performed to assess the severity of suspected liver disease, particularly in patients with very high RBC (>5,000 µg/dL) or plasma protoporphyrin (>1,000 µg/dL) concentrations. Polarized microscopy of the biopsy may demonstrate birefringent crystal deposits with characteristic Maltese cross patterns in EPP. Plugging of biliary canaliculi with protoporphyrin may also be evident in patients with advanced liver disease.32
Management
A multitude of treatment options are available for EPP,27 ranging from efforts to reduce protoporphyrin overproduction in the bone marrow to augmenting its excretion into bile (Table 2). Other agents are used for their cytoprotective properties in conjunction with measures to reduce the circulating pool of protoporphyrin and, on occasion, liver transplantation to correct end-organ damage. Cutaneous protection with opaque sunscreens and barrier clothing cannot be overemphasized. Standard sunscreens are not useful; the only topical sunscreens that are effective at blocking wavelengths greater than 400 nm, with a high sun protection factor (>30), are light-opaque, contain zinc oxide or titanium dioxide, and may be cosmetically unacceptable to some patients. Oral beta-carotene (Solatene, Roche; 60—180 mg orally per day) reduces photosensitivity in approximately 80% of patients over a 1—3 month period after initiation of therapy. Beta-carotene causes yellow-orange discoloration of the skin, and topical beta-carotene cream is ineffective. Beta-carotene is thought to scavenge the free radicals generated by the excitation of protoporphyrin and is dosed to maintain a therapeutic serum level of 600–800 pg/dL.33 Beta-carotene's efficacy has been questioned in the recent literature, and many patients have found their resulting yellow skin difficult to accept. Oral cysteine has also been used and is thought to have a similar mechanism of action.34 Bile-acid binding agents such as cholestyramine35,36 have been used to decrease the enterohepatic circulation of protoporphyrin. Patients have attempted to use long-term activated charcoal as a safe and cheap alternative in the hopes of decreasing intestinal reabsorption of protoporphyrin.37 Intravenous vitamin E was reported in a case report as being effective for reversing liver disease.38 Ursodeoxycholic acid has been used in patients with early liver disease,31 not only because of its ability to enhance the biliary excretion of protoporphyrin, but also because of its cytoprotective properties.39–41 However, a recent study in an autosomal recessive mouse model of EPP demonstrated no benefit with the use of ursodeoxycholic acid and heme arginate.42 Patients are advised against caloric restriction and should undergo iron replacement only if they are found to be iron-deficient.43–47
Table 2.
Targets for Intervention
|
Chronic therapy with intravenous hematin and erythrocyte transfusions is thought to suppress heme production48–51 by decreasing protoporphyrin levels. Exchange transfusions and plasmapheresis have been used to remove the protoporphyrin in transit, as a bridge to liver transplantation or other more definitive treatments. Heme therapy, along with plasmapheresis, has been noted to stabilize patients with advanced liver disease.52–54 Nonbiologic liver assist devices have been used recently in an attempt to decrease the morbidity associated with motor neuropathy, both in the pre- and postoperative period.55
Since the first liver transplantation for EPP-related liver disease in 1980, EPP patients with progressive liver disease have been transplanted with acceptable outcomes. Liver transplant recipients from US centers have been shown to have 1-, 5-, and 10-year survival rates of 85%, 69%, and 47%, respectively.56 These survival rates are similar to those for liver transplants performed for other indications. However, recurrent EPP was found in 65% (11/17) of patients who survived more than 2 months post-transplant. The high incidence of recurrent disease post-transplant is not surprising, as the transplant itself does not correct the primary cause and major source of protoporphyrin overproduction, which has been shown to be in the bone marrow.57 Of all the porphyrias, EPP has the best established indication for liver transplantation as a therapeutic option.58 The previous medical literature, as well as more recent reports, have demonstrated a benefit in EPP patients who receive a bone marrow transplant.59,60 Successful bone marrow transplantation with or without liver transplantation, depending upon the severity of the liver disease, is considered the definitive treatment for EPP.
Patients with EPP are at increased risk of developing chronic liver disease and should be vaccinated for hepatitis A and B (and possibly hepatitis E in the near future) in case they are found not to be immune. They should be counseled to avoid hepatotoxins such as alcohol because of the risk of accelerating their liver disease.61 EPP patients with no evident liver disease should be monitored with liver enzymes and porphyrin levels, both serum and RBC protoporphyrin, at least every 6 months, if not more frequently, to detect early signs of liver injury.62 Patients with known chronic liver disease should also be screened regularly for hepatocellular carcinoma.
Effective management of EPP involves the judicious use of all available treatment options to prevent disease progression and possibly achieve cure. Treatment is directed at minimizing complications from sun damage, monitoring for development of liver disease, and stabilizing cholestatic liver disease once it develops, in the hopes of a more definitive treatment such as bone marrow transplantation with or without liver transplantation. The initial experience with bone marrow transplantation is promising, though the opportune moment to intervene remains unclear.
References
- 1.Magnus IA, Jarrett A, Prankerd TA, Rimington C. Erythropoietic protoporphyria. A new porphyria syndrome with solar urticaria due to protoporphyrinaemia. Lancet. 1961;2:448–451. doi: 10.1016/s0140-6736(61)92427-8. [DOI] [PubMed] [Google Scholar]
- 2.Bonkovsky HL, Bloomer JR, Ebert PS, Mahoney MJ. Heme synthetase deficiency in human protoporphyria. Demonstration of the defect in liver and cultured skin fibroblasts. J Clin Invest. 1975;56:1139–1148. doi: 10.1172/JCI108189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Taketani S, Inazawa J, Nakahashi Y, Abe T, Tokunaga R. Structure of the human ferrochelatase gene. Exon/intron gene organization and location of the gene to chromosome 18. EurJ Biochem. 1992;205:217–222. doi: 10.1111/j.1432-1033.1992.tb16771.x. [DOI] [PubMed] [Google Scholar]
- 4.Whitcombe DM, Carter NP, Albertson DG, Smith SJ, Rhodes DA, Cox TM. Assignment of the human ferrochelatase gene (FECH) and a locus for protoporphyria to chromosome 18q22. Genomics. 1991;11:1152–1154. doi: 10.1016/0888-7543(91)90044-f. [DOI] [PubMed] [Google Scholar]
- 5.Sarkany RP, Alexander GJ, Cox TM. Recessive inheritance of erythropoietic protoporphyria with liver failure. Lancet. 1994;343:1394–1396. doi: 10.1016/s0140-6736(94)92525-9. [DOI] [PubMed] [Google Scholar]
- 6.Gouya L, Martin-Schmitt C, Robreau AM, Austerlitz F, Da Silva V, et al. Contribution of a common single-nucleotide polymorphism to the genetic predisposition for erythropoietic protoporphyria. Am J Hum Genet. 2006;78:2–14. doi: 10.1086/498620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gouya L, Puy H, Robreau AM, Bourgeois M, Lamoril J, et al. The penetrance of dominant erythropoietic protoporphyria is modulated by expression of wildtype FECH. Nat Genet. 2002;30:27–28. doi: 10.1038/ng809. [DOI] [PubMed] [Google Scholar]
- 8.Whatley SD, Mason NG, Holme SA, Anstey AV, Elder GH, Badminton MN. Gene dosage analysis identifies large deletions of the FECH gene in 10% of families with erythropoietic protoporphyria. J Invest Dermatol. 2007;127:2790–2794. doi: 10.1038/sj.jid.5700924. [DOI] [PubMed] [Google Scholar]
- 9.Straka JG, Hill HD, Krikava JM, Kools AM, Bloomer JR. Immunochemical studies of ferrochelatase protein: characterization of the normal and mutant protein in bovine and human protoporphyria. Am J Hum Genet. 1991;48:72–78. [PMC free article] [PubMed] [Google Scholar]
- 10.Rufenacht UB, Gouya L, Schneider-Yin X, Puy H, Schäfer BW, et al. Systematic analysis of molecular defects in the ferrochelatase gene from patients with erythropoietic protoporphyria. Am J Hum Genet. 1998;62:1341–1352. doi: 10.1086/301870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bloomer JR, Hill HD, Kools AM, Straka JG. Heme synthesis in protoporphyria. Curr Probl Dermatol. 1991;20:135–147. [PubMed] [Google Scholar]
- 12.Samuel D, Boboc B, Bernuau J, Bismuth H, Benhamou JP. Liver transplantation for protoporphyria. Evidence for the predominant role of the erythropoietic tissue in protoporphyrin overproduction. Gastroenterology. 1988;95:816–819. [PubMed] [Google Scholar]
- 13.Schmidt H, Snitker G, Thomsen K, Lintrup J. Erythropoietic protoporphyria. A clinical study based on 29 cases in 14 families. Arch Dermatol. 1974;110:58–64. doi: 10.1001/archderm.110.1.58. [DOI] [PubMed] [Google Scholar]
- 14.DeLeo VA, Poh-Fitzpatrick M, Mathews-Roth M, Harber LC. Erythropoietic protoporphyria. 10 years experience. Am J Med. 1976;60:8–22. doi: 10.1016/0002-9343(76)90528-3. [DOI] [PubMed] [Google Scholar]
- 15.Baart de la Faille H, Bijlmer-Iest JC, van Hattum J, Koningsberger J, Rade-makers LH, van Weelden H. Erythropoietic protoporphyria: clinical aspects with emphasis on the skin. Curr Probl Dermatol. 1991;20:123–134. doi: 10.1159/000420016. [DOI] [PubMed] [Google Scholar]
- 16.Holme SA, Anstey AV, Finlay AY, Elder GH, Badminton MN. Erythropoietic protoporphyria in the U.K.: clinical features and effect on quality of life. Br J Dermatol. 2006;155:574–581. doi: 10.1111/j.1365-2133.2006.07472.x. [DOI] [PubMed] [Google Scholar]
- 17.Chen FP, Risheg H, Liu Y, Bloomer J. Ferrochelatase gene mutations in erythropoietic protoporphyria: focus on liver disease. Cell Mol Biol (Noisy-le-grand) 2002;48:83–89. [PubMed] [Google Scholar]
- 18.Poh-Fitzpatrick MB. The erythropoietic porphyrias. Dermatol Clin. 1986;4:291–296. [PubMed] [Google Scholar]
- 19.Reisenauer AK, Soon SL, Lee KK, Hanifin JM. Erythropoietic protoporphyria presenting with liver failure in adulthood. Dermatology. 2005;210:72–73. doi: 10.1159/000081490. [DOI] [PubMed] [Google Scholar]
- 20.Lee RG, Avner DL, Berenson MM. Structure-function relationships of protoporphyrin-induced liver injury. Arch Pathol Lab Med. 1984;108:744–746. [PubMed] [Google Scholar]
- 21.Cox TM, Alexander GJ, Sarkany RP. Protoporphyria. Semin Liver Dis. 1998;18:85–93. doi: 10.1055/s-2007-1007144. [DOI] [PubMed] [Google Scholar]
- 22.Todd DJ. Gallstones in children. Am J Dis Child. 1991;145:971–972. doi: 10.1001/archpedi.1991.02160090021011. [DOI] [PubMed] [Google Scholar]
- 23.Rank JM, Carithers R, Bloomer J. Evidence for neurological dysfunction in end-stage protoporphyric liver disease. Hepatology. 1993;18:1404–1409. [PubMed] [Google Scholar]
- 24.Muley SA, Midani HA, Rank JM, Carithers R, Parry GJ. Neuropathy in erythropoietic protoporphyrias. Neurology. 1998;51:262–265. doi: 10.1212/wnl.51.1.262. [DOI] [PubMed] [Google Scholar]
- 25.Lock G, Holstege A, Mueller AR, Christe W, Doss MO, et al. Liver failure in erythropoietic protoporphyria associated with choledocholithiasis and severe posttransplantation polyneuropathy. Liver. 1996;16:211–217. doi: 10.1111/j.1600-0676.1996.tb00730.x. [DOI] [PubMed] [Google Scholar]
- 26.Nguyen L, Blust M, Bailin M, Melendez L, Raines DE. Photosensitivity and perioperative polyneuropathy complicating orthotopic liver transplantation in a patient with erythropoietic protoporphyria. Anesthesiology. 1999;91:1173–1175. doi: 10.1097/00000542-199910000-00038. [DOI] [PubMed] [Google Scholar]
- 27.Bonkovsky HL, Thapar M. Porphyria. In: Rakel RE, Bope ET, editors. Conn's Current Therapy. Philadelphia, PA: Elsevier Health; 2008. pp. 469–474. [Google Scholar]
- 28.Lamola AA, Piomelli S, Poh-Fitzpatrick MG, Yamane T, Harber LC. Erythropoietic protoporphyria and lead intoxication: the molecular basis for difference in cutaneous photosensitivity. II. Different binding of erythrocyte protoporphyrin to hemoglobin. J Clin Invest. 1975;56:1528–1535. doi: 10.1172/JCI108234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Poh-Fitzpatrick MB. Protoporphyrin metabolic balance in human protoporphyria. Gastroenterology. 1985;88:1239–1242. doi: 10.1016/s0016-5085(85)80085-8. [DOI] [PubMed] [Google Scholar]
- 30.Bloomer JR. The liver in protoporphyria. Hepatology. 1988;8:402–407. doi: 10.1002/hep.1840080235. [DOI] [PubMed] [Google Scholar]
- 31.Gross U, Frank M, Doss MO. Hepatic complications of erythropoietic protoporphyria. Photodermatol Photoimmunol Photomed. 1998;14:52–57. doi: 10.1111/j.1600-0781.1998.tb00011.x. [DOI] [PubMed] [Google Scholar]
- 32.MacDonald DM, Germain D, Perrot H. The histopathology and ultrastructure of liver disease in erythropoietic protoporphyria. Br J Dermatol. 1981;104:7–17. doi: 10.1111/j.1365-2133.1981.tb01705.x. [DOI] [PubMed] [Google Scholar]
- 33.Mathews-Roth MM, Pathak MA, Fitzpatrick TB, Harber LH, Kass EH. Beta carotene therapy for erythropoietic protoporphyria and other photosensitivity diseases. Arch Dermatol. 1977;113:1229–1232. [PubMed] [Google Scholar]
- 34.Mathews-Roth MM, Rosner B. Long-term treatment of erythropoietic protoporphyria with cysteine. Photodermatol Photoimmunol Photomed. 2002;18:307–309. doi: 10.1034/j.1600-0781.2002.02790.x. [DOI] [PubMed] [Google Scholar]
- 35.McCullough AJ, Barron D, Mullen KD, Petrelli M, Park MC, et al. Fecal protoporphyrin excretion in erythropoietic protoporphyria: effect of cholestyramine and bile acid feeding. Gastroenterology. 1988;94:177–181. doi: 10.1016/0016-5085(88)90627-0. [DOI] [PubMed] [Google Scholar]
- 36.Bloomer JR. Pathogenesis and therapy of liver disease in protoporphyria. Yale J Biol Med. 1979;52:39–48. [PMC free article] [PubMed] [Google Scholar]
- 37.Gorchein A, Foster GR. Liver failure in protoporphyria: long-term treatment with oral charcoal. Hepatology. 1999;29:995–996. doi: 10.1002/hep.510290314. [DOI] [PubMed] [Google Scholar]
- 38.Komatsu H, Ishii K, Imamura K, Maruyama K, Yonei Y, et al. A case of erythropoietic protoporphyria with liver cirrhosis suggesting a therapeutic value of supplementation with alpha-tocopherol. Hepatol Res. 2000;18:298–309. doi: 10.1016/s1386-6346(00)00077-2. [DOI] [PubMed] [Google Scholar]
- 39.Pirlich M, Lochs H, Schmidt HH. Liver cirrhosis in erythropoietic protoporphyria: improvement of liver function with ursodeoxycholic acid. Am J Gastroenterol. 2001;96:3468–3469. doi: 10.1111/j.1572-0241.2001.05363.x. [DOI] [PubMed] [Google Scholar]
- 40.Rademakers LH, Cleton MI, Kooijman C, Baart de la Faille H, van Hattum J. Early involvement of hepatic parenchymal cells in erythrohepatic protoporphyria? An ultrastructural study of patients with and without overt liver disease and the effect of chenodeoxycholic acid treatment. Hepatology. 1990;11:449–457. doi: 10.1002/hep.1840110316. [DOI] [PubMed] [Google Scholar]
- 41.Van Hattum J, Baart de la Faille H, Van den Berg JW, Edixhoven-Bosdijk A, Wilson JH. Chenodeoxycholic acid therapy in erythrohepatic protoporphyria. J Hepatol. 1986;3:407–412. doi: 10.1016/s0168-8278(86)80496-2. [DOI] [PubMed] [Google Scholar]
- 42.Abitbol M, Puy H, Sabaté JM, Guénet JL, Deybach JC, Montagutelli X. Ursodesoxycholic acid and heme-arginate are unable to improve hematopoiesis and liver injury in an erythropoietic protoporphyria mouse model. Physiol Res. 2006;55(suppl 2):S93–S101. doi: 10.33549/physiolres.930000.55.S2.93. [DOI] [PubMed] [Google Scholar]
- 43.Holme SA, Worwood M, Anstey AV, Elder GH, Badminton MN. Erythropoiesis and iron metabolism in dominant erythropoietic protoporphyria. Blood. 2007;110:4108–4110. doi: 10.1182/blood-2007-04-088120. [DOI] [PubMed] [Google Scholar]
- 44.Holme SA, Thomas CL, Whatley SD, Bentley DP, Anstey AV, Badminton MN. Symptomatic response of erythropoietic protoporphyria to iron supplementation. J Am Acad Dermatol. 2007;56:1070–1072. doi: 10.1016/j.jaad.2006.11.030. [DOI] [PubMed] [Google Scholar]
- 45.Gordeuk VR, Brittenham GM, Hawkins CW, Mukhtar H, Bickers DR. Iron therapy for hepatic dysfunction in erythropoietic protoporphyria. Ann Intern Med. 1986;105:27–31. doi: 10.7326/0003-4819-105-1-27. [DOI] [PubMed] [Google Scholar]
- 46.Milligan A, Graham-Brown RA, Sarkany I, Baker H. Erythropoietic protoporphyria exacerbated by oral iron therapy. Br J Dermatol. 1988;119:63–66. doi: 10.1111/j.1365-2133.1988.tb07102.x. [DOI] [PubMed] [Google Scholar]
- 47.McClements BM, Bingham A, Callender ME, Trimble ER. Erythropoietic protoporphyria and iron therapy. Br J Dermatol. 1990;122:423–424. doi: 10.1111/j.1365-2133.1990.tb08293.x. [DOI] [PubMed] [Google Scholar]
- 48.van Wijk HJ, van Hattum J, Baart de la Faille H, van den Berg JW, Edixhoven-Bosdijk A, Wilson JH. Blood exchange and transfusion therapy for acute cholestasis in protoporphyria. Dig Dis Sci. 1988;33:1621–1625. doi: 10.1007/BF01535955. [DOI] [PubMed] [Google Scholar]
- 49.Bloomer JR, Pierach CA. Effect of hematin administration to patients with protoporphyria and liver disease. Hepatology. 1982;2:817–821. doi: 10.1002/hep.1840020613. [DOI] [PubMed] [Google Scholar]
- 50.Todd DJ, Callender ME, Mayne EE, Walsh M, Burrows D. Erythropoietic protoporphyria, transfusion therapy and liver disease. Br J Dermatol. 1992;127:534–537. doi: 10.1111/j.1365-2133.1992.tb14855.x. [DOI] [PubMed] [Google Scholar]
- 51.Lamon JM, Poh-Fitzpatrick MB, Lamola AA. Hepatic protoporphyrin production in human protoporphyria. Effects of intravenous hematin and analysis of erythrocyte protoporphyrin distribution. Gastroenterology. 1980;79:115–125. [PubMed] [Google Scholar]
- 52.Reichheld JH, Katz E, Banner BF, Szymanski IO, Saltzman JR, Bonkovsky HL. The value of intravenous heme-albumin and plasmapheresis in reducing postoperative complications of orthotopic liver transplantation for erythropoietic protoporphyria. Transplantation. 1999;67:922–928. doi: 10.1097/00007890-199903270-00023. [DOI] [PubMed] [Google Scholar]
- 53.Dellon ES, Szczepiorkowski ZM, Dzik WH, Graeme-Cook F, Ades A, et al. Treatment of recurrent allograft dysfunction with intravenous hematin after liver transplantation for erythropoietic protoporphyria. Transplantation. 2002;73:911–915. doi: 10.1097/00007890-200203270-00014. [DOI] [PubMed] [Google Scholar]
- 54.Do KD, Banner BF, Katz E, Szymanski IO, Bonkovsky HL. Benefits of chronic plasmapheresis and intravenous heme-albumin in erythropoietic protoporphyria after orthotopic liver transplantation. Transplantation. 2002;73:469–472. doi: 10.1097/00007890-200202150-00024. [DOI] [PubMed] [Google Scholar]
- 55.Eefsen M, Rasmussen A, Wulf HC, Brock A, Hansen BA. Erythropoietic protoporphyria and pretransplantation treatment with nonbiological liver assist devices. Liver Transpl. 2007;13:655–657. doi: 10.1002/lt.21049. [DOI] [PubMed] [Google Scholar]
- 56.McGuire BM, Bonkovsky HL, Carithers RL, Jr, Chung RT, Goldstein LI, et al. Liver transplantation for erythropoietic protoporphyria liver disease. Liver Transpl. 2005;11:1590–1596. doi: 10.1002/lt.20620. [DOI] [PubMed] [Google Scholar]
- 57.Poh-Fitzpatrick MB, Wang X, Anderson KE, Bloomer JR, Bolwell B, Lichtin AE. Erythropoietic protoporphyria: altered phenotype after bone marrow transplantation for myelogenous leukemia in a patient heteroallelic for ferrochelatase gene mutations. J Am Acad Dermatol. 2002;46:861–866. doi: 10.1067/mjd.2002.120460. [DOI] [PubMed] [Google Scholar]
- 58.Seth AK, Badminton MN, Mirza D, Russell S, Elias E. Liver transplantation for porphyria: who, when, and how? Liver Transpl. 2007;13:1219–1227. doi: 10.1002/lt.21261. [DOI] [PubMed] [Google Scholar]
- 59.Wahlin S, Aschan J, Bjornstedt M, Broome U, Harper P. Curative bone marrow transplantation in erythropoietic protoporphyria after reversal of severe cholestasis. J Hepatol. 2007;46:174–179. doi: 10.1016/j.jhep.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 60.Rand EB, Bunin N, Cochran W, Ruchelli E, Olthoff KM, Bloomer JR. Sequential liver and bone marrow transplantation for treatment of erythropoietic protoporphyria. Pediatrics. 2006;118:e1896–e1899. doi: 10.1542/peds.2006-0833. [DOI] [PubMed] [Google Scholar]
- 61.Bonkovsky HL, Schned AR. Fatal liver failure in protoporphyria. Synergism between ethanol excess and the genetic defect. Gastroenterology. 1986;90:191–201. [PubMed] [Google Scholar]
- 62.Mathews-Roth MM. The consequences of not diagnosing erythropoietic protoporphyria. Arch Dermatol. 1980;116:407. doi: 10.1001/archderm.116.4.407. [DOI] [PubMed] [Google Scholar]