Duodenal tumors are extremely rare and can become quite large. Endoscopic biopsies are typically nondiagnostic, and endoscopic removal may be difficult. These lesions may mimic tumors such as leiomyomas, gastrointestinal stromal tumors, carcinoid tumors, neurogenic tumors, or aberrant pancreas. For giant hamartomas, surgical extirpation is often necessary and malignant degeneration is rare. We present a case of gastric outlet obstruction secondary to a giant Brunner gland hamartoma, which created an intussusception.
Case Report
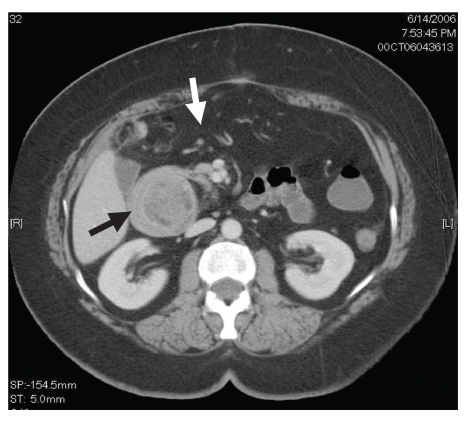
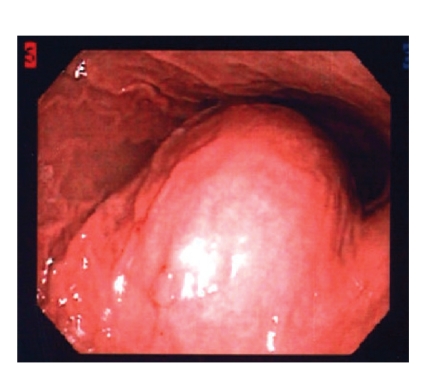
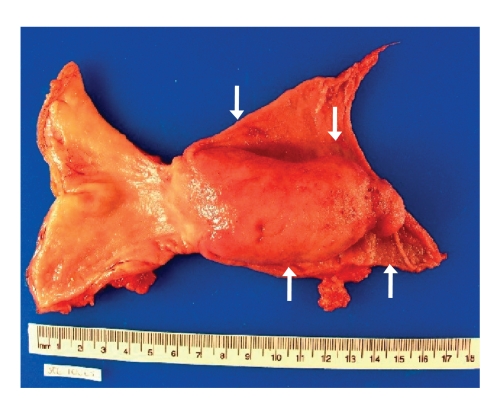
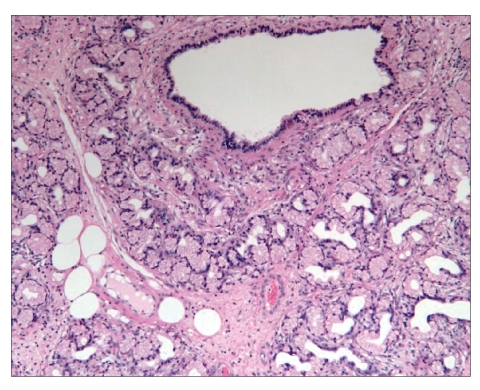
A 56-year-old white woman was admitted to our medical center with nausea, frequent bouts of vomiting, a 30-pound weight loss over 6 weeks, dehydration, anorexia, upper abdominal pain, and postprandial early satiety. The patient was vomiting partially digested materials typically within 30 minutes of a light liquid meal. She did not mention any recent upper endoscopy or imaging studies or any history of peptic disease. In addition, the patient did not have a history of smoking, alcohol use, or anti-inflammatory drug use. Six weeks earlier, she had undergone a right hemicolectomy for a tubulovillous adenoma of the cecum that could not be removed endoscopically. Since her discharge, she had been unable to eat. When she presented to our hospital, kidneys, ureters, and bladder imaging performed in the emergency room revealed gastrectasis. Subsequent abdominal computed tomography (CT) revealed a gastroduodenal intussusception with gastric outlet obstruction (Figure 1). No adenopathy, ascites, or other mass lesions were noted. Upper endoscopy showed an obstructed pyloric channel with a large submucosal prepyloric mass effect (Figure 2). The pylorus was traversed by a long tubular mass telescoping into the second portion of the duodenum with a friable leading edge juxtaposed with the ampulla. This mass could not be reduced into the stomach, and mucosal biopsies were nondiagnostic. During laparotomy, a gastroduodenal intussusception was noted with the leading edge of a firm mass in the distal second portion of the duodenum. The lesion could not be reduced, and a Billroth I reconstruction was performed. The exposed specimen measured 8.5 cm × 7 cm (Figure 3). The surface was lobulated and fibrous, with an ivory fleshy appearance when cut, resembling the pancreas or salivary gland. Histologically, the mass was a giant Brunner gland hamartoma (Figure 4). The postoperative course was uneventful, and the patient is eating well and thriving.
Figure 1.
Computed tomography scan showing the gastroduodenal mass (black arrow) with obstruction and gastrectasis (white arrow).
Figure 2.
Esophagogastroduodenoscopy image of gastric outlet with submucosal mass effect.
Figure 3.
The surgical specimen after Billroth I resection, revealing the giant Brunner hamartoma of the duodenum (arrows).
Figure 4.
Hematoxylin and eosin stain (150 ×) of the Brunner gland hamartoma.
Discussion
Brunner gland hamartomas are rare duodenal tumors, occurring in middle age, with no gender prevalence. They may present with gastrointestinal hemorrhage or obstructive symptoms, or as incidental findings detected by endoscopy, CT, magnetic resonance imaging (MRI), or barium imaging.1 The majority of these lesions begin in the posterior duodenal bulb and are pedunculated. The mean diameter of reported lesions falls within the range of 2 cm, with the largest reported lesion being a 12-cm hamartoma that caused intussusception in a 15-year-old patient.2 Since the first description, in 1835, of a fatal Brunner gland hamartoma causing duodenal intussusception, less than 200 cases have been described in the worldwide literature.3 Benign duodenal neoplasms are exceedingly rare, with Brunner gland tumors comprising less than 10% of these lesions. Histologically, Brunner gland hamartomas are thought to be nonneoplastic and lacking in any true dysplasia. They contain an admixture of normal tissues, including Brunner glands, ducts, adipose, pancreatic acini, and lymphoid materials. Focal sclerotic areas within a Brunner hamartoma may mimic adenocarcinoma.4,5 Submucosal Brunner glands extend from the duodenal bulb to the jejunum, and they produce mucus that protects small-bowel mucosa from the harmful effects of gastric acid. Brunner glands also secrete the antacid proteins pepsinogen and urogastrone. Brunner gland hamartomas are usually covered by normal mucosa, making pinch biopsy obtained during endoscopy typically nondiagnostic. There have also been reports of these hamartomas presenting as painless jaundice when associated with ampullary growth, chronic pancreatitis caused by obstruction, and chronic diarrhea associated with the chronic pancreatitis and steatorrhea.6
Brunner gland hyperplasia may be associated with uremia, chronic pancreatitis, and Helicobacter pylori infection.7,8 There have been reports of recurrence following endoscopic or surgical resection, and malignant transformation is exceedingly rare.9,10 Increased expression of p53 antigens has been demonstrated in these hamartomatous lesions by the use of immunohistochemistry.11
Duodenal obstruction or gastric outlet obstruction, with or without intussusception, is extremely rare with these tumors. No more than 25 cases with these features have been reported in the literature.8 Lesions less than 5 cm in size may be amenable to endoscopic or laparoscopic excision.12 CT or MRI does not distinguish a Brunner gland hamartoma from other duodenal tumors but may define its relationship to other structures such as the pyloric channel, gastric antrum, ampulla of Vater, or pancreatic head. The differential diagnosis of smooth-walled duodenal filling defects includes leiomyomas, gastrointestinal stromal tumors, lipomas, neurogenic tumors, carcinoid tumors, hamartomas, aberrant pancreas, prolapsed pyloric mucosa, or Peutz-Jeghers polyps.5
In summary, Brunner gland hyperplasia is a common finding on upper endoscopy. However, hamartomas are rare and, when accompanied by bleeding or obstruction, commonly require endoscopic or surgical resection. Brunner hamartomas can become giant-sized, resulting in gastroduodenal intussusception and gastric outlet obstruction. These tumors are polypoid, typically bulbar, often pedunculated, and can be quite vascular. Endoscopic surveillance is not required due to extremely infrequent malignant degeneration and no evidence of recurrence following endoscopic or surgical excision.
References
- 1.Nakanishi T, Takeuchi T, Hara K, Sugimoto A. A great Brunner's gland adenoma of the duodenal bulb. Dig Dis Sci. 1984;29:81–85. doi: 10.1007/BF01296867. [DOI] [PubMed] [Google Scholar]
- 2.Chuang JH, Chen WJ. Duodenojejunal intussusception secondary to hamarto-matous polyp of Brunner's glands. J Pediatr GastroenterolNutr. 1991;13:96–100. doi: 10.1097/00005176-199107000-00018. [DOI] [PubMed] [Google Scholar]
- 3.Cruveilhier J. Paris, France: JB Balliere; Anatomie Pathologique du Corpshumain; p. 1835. [Google Scholar]
- 4.De Castella H. Brunner's gland adenoma. An unusual cause of intestinal bleeding. Br J Surg. 1966;53:153–156. doi: 10.1002/bjs.1800530216. [DOI] [PubMed] [Google Scholar]
- 5.Walden DT, Marcon NE. Endoscopic injection and polypectomy for bleeding Brunner's gland hamartoma: case report and expanded literature review. Gastrointest Endosc. 1998;47:403–407. doi: 10.1016/s0016-5107(98)70228-7. [DOI] [PubMed] [Google Scholar]
- 6.Mayoral W, Salcedo JA, Montgomery E, Al-Kawas FH. Biliary obstruction and pancreatitis caused by a Brunner's gland hyperplasia of the ampulla of Vater: a case report and review of the literature. Endoscopy. 2000;32:998–1001. doi: 10.1055/s-2000-9623. [DOI] [PubMed] [Google Scholar]
- 7.Levine JA, Burgart LJ, Batts KP, Wang KK. Brunner's gland hamartomas: clinical presentation and pathological features of 27 cases. Am J Gastroenterol. 1995;90:290–294. [PubMed] [Google Scholar]
- 8.Krishnamurthy P, Junaid O, Moezzi J, Ali SA, Gopalswamy N. Gastric outlet obstruction caused by Brunner's gland hyperplasia: case report and review of literature. Gastrointest Endosc. 2006;64:464–467. doi: 10.1016/j.gie.2006.02.026. [DOI] [PubMed] [Google Scholar]
- 9.Zanetti G, Casadei G. Brunner's gland hamartoma with incipient ductal malignancy. Tumori. 1981;67:75–78. doi: 10.1177/030089168106700114. [DOI] [PubMed] [Google Scholar]
- 10.Itsuno M, Makiyama K, Omagari K, Tanaka T, Hara K, et al. Carcinoma of duodenal bulb arising from the Brunner's gland. Gastroenterol Jpn. 1993;28:118–125. doi: 10.1007/BF02775012. [DOI] [PubMed] [Google Scholar]
- 11.Faller G, Kirchner T. Low-grade intraepithelial neoplasia of Brunner's gland. Histopathology. 2005;47:118–119. doi: 10.1111/j.1365-2559.2005.02066.x. [DOI] [PubMed] [Google Scholar]
- 12.Kehl O, Buhler H, Stamm B, Amman RW. Endoscopic removal of a large, obstructing and bleeding Brunner's gland adenoma. Endoscopy. 1985;17:231–232. doi: 10.1055/s-2007-1018512. [DOI] [PubMed] [Google Scholar]