Abstract
α2-Agonists are a novel class of drugs with mechanisms of action that differ from other commonly used anesthetic drugs. They have neuroprotective, cardioprotective, and sedative effects. These unique characteristics make them potentially useful during neuroanesthesia and intensive care. We review the effects of dexmedetomidine on cerebral blood flow and cerebral metabolism, along with recent advances in using α2-agonists in neuroanesthesia and neurointensive care.
Keywords: Alpha-2 agonist, anesthesia, dexmedetomidine, neuroanesthesia, neuroprotection
INTRODUCTION
α2-Adrenergic agonists were introduced to clinical anesthesia for their sympatholytic, sedative, anesthetic-sparing, and hemodynamic-stabilizing properties.1 We review recent advances in the clinical use of α2-agonists in neuroanesthesia and neurointensive care, with an emphasis on dexmedetomidine. This discussion complements reviews that focus on other uses of α2-agonists, especially during anesthesia and in the critical care units.2-4 The sedative effects of α2-agonists are mediated via pathways that also make them promising neuroprotective agents. Much of the knowledge accumulated on the subject of neuroprotection by α2-agonists comes from the ophthalmology literature, where investigators have been searching for agents to decrease retinopathy in glaucoma patients. For example, brimonidine, an α2-agonist, has emerged as an antiglaucoma medication not only because it reduces intraocular pressure similarly to other antiglaucoma medications, but because it also reduces progression to glaucoma retinopathy.5-7
PUTATIVE MECHANISMS UNDERLYING NEUROPROTECTION OF α2-ADRENORECEPTOR AGONISTS
Effects on Catecholamine Release
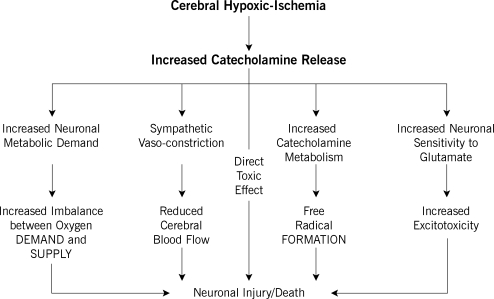
Engelhard and colleagues8 studied the effects of dexmedetomidine on cerebral and peripheral catecholamines during ischemia in anesthetized rats. Dexmedetomidine given intraperitoneally 30 minutes before ischemia did not inhibit the intraischemic increase in cerebral extracellular catecholamine or glutamate concentrations but did ameliorate the rise in peripheral catecholamine concentrations. The neuroprotective effect of dexmedetomidine during cerebral ischemia, as evident by increased expression of antiapoptotic proteins B cell lymphoma-2 (Bcl-2) and Mdm-2, is therefore primarily related to decreased peripheral catecholamine levels; dexmedetomidine did not inhibit the intraischemic increase of cerebral extracellular catecholamine or glutamate concentrations.9 Neuroprotection observed with reduced sympathetic activity may be related to several mechanisms: (1) high catecholamine concentrations increase the sensitivity of pyramidal neurons to excitatory neurotransmitters such as glutamate,10 which results in elevated intracellular Ca2+ concentrations with consecutive activation of intracellular catabolic enzymes (excitotoxicity); (2) an increase in catecholamine release might have a direct toxic effect on neuronal tissues11; (3) increased catecholamine metabolism could increase free radical formation9; and (4) increased sympathetic activity may reduce perfusion in the ischemic penumbra. Therefore, suppression of catecholamine concentrations may be neuroprotective by balancing the ratio between cerebral oxygen demand and oxygen supply, reducing excitotoxicity, ameliorating toxic effects, or improving perfusion in the ischemic penumbra8 (Figure 1).
Figure 1.
Effects of catecholamines during cerebral hypoxic ischemia. (Reproduced with permission of Oxford University Press. Ma D, et al. Br Med Bull. 2005;71:77-92.9)
Effects on Glutamate-Mediated Excitotoxicity and Intravascular Ca2+ Concentrations
The excitatory amino acid neurotransmitter glutamate triggers neuronal death by overexcitation of its receptors when released in excessive concentrations. Oxidative degradation to pyruvate and lactate is probably the main mechanism by which the brain disposes of excess glutamate.12 This process is halted during anoxia but is re-established after reoxygenation.13 Dexmedetomidine stimulates astrocytic α2-adrenergic receptors, which raises astrocytic [Ca2+] concentrations, in turn stimulating glutaminase activity and the ability of astrocytes to dispose of glutamine by oxidative metabolism.14 The α2-adrenergic agonist dexmedetomidine stimulates glutamine oxidation in plasma, an effect sufficiently large that it may affect glutamine content in the brain, thus representing a novel mechanism of action for a neuroprotectant drug. Dexmedetomidine may cause a substantial change in the balance between formation and disposal of glutamine, thus reducing the availability of glutamine as a precursor of neurotoxic glutamate.14
By activating α2-adrenergic receptors, brimonidine may reduce ischemic retinal injury as the accumulation of extracellular glutamate and aspartate is prevented.15 Brimonidine-mediated activation of α2-adrenoceptors appears to limit glutamate accumulation by maintaining the glutamate-buffering activity of Muller cells, which are retinal glial cells that are critical for maintaining low levels of extracellular glutamate via the electrogenic Na+-dependent glutamate/aspartate transporter, GLAST.15 It has been proposed that GLAST function reverses during ischemia so that intracellular glutamate is pumped into the extracellular space.16 Activation of α2-adrenoreceptors may therefore prevent glutamate and aspartate accumulation by maintaining activity of the glutamate/aspartate transporters.
Effects on Apoptotic Neuronal Death
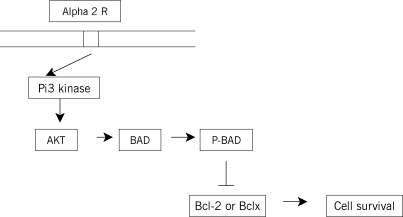
α2-Adrenergic receptor stimulation may inhibit proapoptotic mitochondrial signaling.17 α2-Adrenergic receptor stimulation can also activate the antiapoptotic phosphatidyl inositol-3-(Pi3) and protein kinase/AKT (protein kinase B) pathways (Figure 2). These are major pathways in the promotion of cell survival that block apoptosis by phosphorylation-dependent inhibition of proapoptotic signaling molecules, including Bcl-2–associated death promoter and Bcl-2–associated protein (BAX) and by increasing prosurvival molecules like Bcl-2. Engelhard and colleagues18 compared the effect of dexmedetomidine and S(+) ketamine on the expression of apoptosis-regulating proteins after 30 minutes of incomplete cerebral ischemia and reperfusion in rats. Thirty-two male rats were divided into 4 groups. In the dexmedetomidine group, 100 µg/kg of dexmedetomidine was administered intraperitoneally 30 minutes before ischemia; another group was given S(+) ketamine 1 mg/kg. The other groups were the control and sham groups. Dexmedetomidine increased the concentration of the antiapoptotic protein Bcl-2. The other important finding was that Mdm-2 was increased in animals treated with dexmedetomidine but not in animals treated with S(+) ketamine. Dexmedetomidine, by increasing Bcl-2 concentration, helps to decrease the mitochondrial membrane permeability during the time of ischemia and consequently the release of proapoptotic protease activity such as cytochrome C or the apoptosis-inducing factor from the mitochondria into the cytosol.18,19
Figure 2.
Possible mechanism by which brimonidine increases ganglion cell survival. Activation of α2-receptors by brimonidine activates Pi3 kinase, which subsequently activates AKT (protein kinase B). This phosphorylates Bcl-2–associated death promoter, which prevents it from interacting with the Bcl-2–associated protein and thus prevents apoptosis. (Reprinted with permission of Elsevier. Wheeler L, et al. Surv Ophthalmol. 2003;48(suppl 1):S47-S51.19) Pi3, phosphatidyl inositol-3; Bcl-2, B cell lymphoma-2; BAD, Bcl-2–associated death promoter; P-BAD, phosphorylated BAD; Bclx, Bcl-2–associated × protein.
During cerebral ischemia, the tumor suppressor gene, p53, is induced. p53 protein induces apoptosis by altering BAX and Bcl-2 expression. Mdm-2 (antiapoptotic protein) inhibits p53 by concealing the activation domain of the p53 gene. Mdm-2 concentration is increased by dexmedetomidine; therefore, modulation of p53 activity by Mdm-2 may be a cofactor in dexmedetomidine-induced neuroprotection.18
NEUROPROTECTIVE EFFECTS ON THE GROWING BRAIN
α2-Agonists clonidine and dexmedetomidine exert neuroprotective effects in animal models of perinatal excitotoxic injury and hypoxic-ischemic injury. For example, Laudenbach and colleagues20 report that intraperitoneal injection of clonidine and dexmedetomidine before an intracerebral stereotatic injection of the N-methyl d-aspartate receptor agonist ibotenate provided potent neuroprotection by stimulating the α2-adrenoreceptors in perinatal excitotoxic brain injury. The combination of xenon and dexmedetomidine was also shown to have neuroprotective effects in neonatal mice in vitro and synergistically in vivo. In vitro, a primary co-culture of neural and glial cells derived from neonatal mice was deprived of oxygen and glucose, and the resulting neuronal injury was assessed by the release of lactate dehydrogenase (LDH). In vivo, postnatal 7-day-old rats underwent right common carotid artery ligation followed by 90 minutes of hypoxia.
The combination of xenon and dexmedetomidine results in reducing LDH release in vitro and improving the long-term neurologic function with reduction of the infarction area in vivo. Dexmedetomidine and xenon may interact synergistically to reduce intracellular Ca2+ concentrations and upregulate the antiapoptotic protein Bcl-2 during hypoxic ischemia.21 Sanders and colleagues studied in vitro and in vivo the neuroprotective effects of dexmedetomidine against 0.75% isoflurane-induced neuroapoptosis in postnatal 7-day-old rats. Isoflurane exposure induced long-term memory impairment, which was prevented by administration of dexmedetomidine. In vitro, dexmedetomidine also inhibited isoflurane-induced capsase-3 expression in organotypic hippocampal slice cultures, suggesting that dexmedetomidine may be an important adjunct to prevent isoflurane-induced neurotoxicity.22 However, it remains unclear whether volatile anesthetics are harmful in human infants, much less whether dexmedetomidine is protective.
DEXMEDETOMIDINE AND CEREBRAL BLOOD FLOW
Early studies in animals raised concern over the potential of α2-agonists to reduce cerebral blood flow (CBF) out of proportion to their effect on cerebral metabolic rate of oxygen (CMRO2). For example, Karlsson and colleagues23 measured canine global CBF and CMRO2 during 1 minimal alveolar concentration (MAC) halothane anesthesia. At a dose of 10 µg/kg, dexmedetomidine caused significant reduction in CBF without influencing CMRO2. Decreasing the halothane concentration to 0.1% did not change CBF reduction but instead increased CMRO2 by 19%.
In an elegant study, Asano and colleagues24 compared the effects of locally applied dexmedetomidine versus systemically administered dexmedetomidine on pial arterioles with and without local application of the specific α2-antagonist atipamezole. Six groups of male rats were anesthetized with isoflurane. Local application of dexmedetomidine caused dose-dependent constriction, which was significant starting at 10−8 M for small and medium-sized arterioles and 10−7 M for large arterioles. Intravenous (IV) dexmedetomidine at 1 µg/kg caused modest constriction, which resolved after 8 minutes. However, the 10 µg/kg dose constricted the arterioles of all sizes. The constriction started to resolve after 10 minutes. The major finding in this study was that atipamezole was able to completely abolish the vasoconstricted effect of locally applied dexmedetomidine. However, it partially abolished the vasoconstriction effect of systemically administered dexmedetomidine. The vasoconstrictor response to systemically administered dexmedetomidine might be due to its direct vasoconstrictive actions via α2-receptors.
McPherson and colleagues25 studied the effect of intraventricular dexmedetomidine on CBF during normoxia and hypoxic hypoxia in dogs anesthetized with 1.4% isoflurane. Dexmedetomidine decreased normoxic flow in the cerebral hemispheres from 76 ± 6 to 44 ± 4 mL · min−1 · 100 g−1, with similar decreases in other regions. Regional blood flow during hypoxia, in all regions, was halved by dexmedetomidine (P < .05). However, the absolute change in cerebrovascular tone resulting from hypoxia is unaltered by dexmedetomidine, suggesting that dexmedetomidine does not alter the underlying mechanism for cerebrovascular response to hypoxia.
CMRO2 was maintained at control values during normoxia despite the reduction in CBF. Dexmedetomidine-induced vasoconstriction is sufficient to limit cerebral oxygen delivery during hypoxia and result in reduced CMRO2. This finding led the authors to hypothesize that the mechanism of dexmedetomidine-induced CBF reduction is not metabolically mediated. The other interesting finding of the study was that α2-receptors were mainly accumulated in periventricular brain structures as evidenced by the fact that the concentration of H3-clonidine in the cerebral cortex was approximately 1% of that in the ipsilateral caudate nucleus. This finding led the authors to speculate that if the mechanism of blood flow reduction was mediated by direct vascular effect it would have a greater effect on blood flow in the regions with the highest concentration of α2-receptors. However, this study demonstrated similar blood flow effect among the different brain regions. The authors therefore surmised that the reduction in CBF by dexmedetomidine was due mainly to its stimulatory effect on the locus ceruleus.
Iida and colleagues26 compared the constrictive effects of topically applied dexmedetomidine, clonidine, phenylephrine, or epinephrine in 3 different concentrations of spinal and cerebral pial vessels in pentobarbital-anesthetized dogs. In cerebral arterioles, dexmedetomidine and clonidine induced greater constriction than phenylephrine and epinephrine. However, in spinal vessels vasoconstriction was smaller than that induced by phenylephrine and epinephrine. Ogawa and colleagues27 studied the effect of low-dose dexmedetomidine (loading 3 µg · kg−1 · h−1 for 10 minutes, maintenance 0.2 µg · kg−1 · h−1 for 60 minutes) and high-dose dexmedetomidine (loading 6 µg · kg−1 · h−1 for 10 minutes, maintenance 0.4 µg · kg−1 · h−1 for 60 minutes) on dynamic cerebral autoregulation, assessed by transfer function using the thigh cuff method in 14 healthy male volunteers. The authors demonstrated that dexmedetomidine weakened dynamic cerebral autoregulation and delayed restoration in CBF velocity during temporary decreases in arterial pressure. The authors caution that dexmedetomidine may lead to further sustained reductions in CBF when arterial pressure decreases temporarily with postural changes or release of the tourniquet. Dexmedetomidine was shown to decrease cerebrovascular CO2 reactivity more than propofol sedation in patients with septic shock. This finding is important because maintaining CBF is crucial for avoiding septic encephalopathy.28
Another study by Iida and colleagues29 evaluated pial vessel diameters, cerebral oxygen extraction, and systemic hemodynamics in pentobarbital-anesthetized, mechanically ventilated dogs before and after cardiac arrest (5 minutes) and resuscitation in the presence or absence of dexmedetomidine. The use of dexmedetomidine during cardiopulmonary resuscitation (CPR) did not alter changes in cerebral arteriolar diameters or cerebral oxygen extraction that occurred after cardiac arrest at resuscitation. The major finding of this study was that dexmedetomidine reduced the dose of phenylephrine used to maintain blood pressure (BP) during CPR; it also reduced the number of ventricular ectopic beats observed after CPR. The authors suggested that dexmedetomidine-induced stabilization of the systemic circulation and maintenance of optimal cerebral perfusion pressure after CPR may result in improved outcomes after CPR.
In a landmark study, Drummond and colleagues30 investigated the relationship between middle cerebral artery velocity (CBFv) and cerebral metabolic rate equivalent (CMRe) in 6 volunteers under dexmedetomidine sedation. Dexmedetomidine was found to cause a dose-related reduction in both CBF and cerebral metabolic rate (CMR) in healthy subjects. Dexmedetomidine maintained CMR-CBF coupling with no decrease in the CBFv to CMRe ratio. However, the authors cautioned that their study results do not assure that dexmedetomidine will not cause adverse effects on the CBF to CMR ratio in patients with neurologic injuries. Bekker and colleagues31 demonstrated the safety of dexmedetomidine sedation during awake carotid endarterectomy, with no increase in the incidence of intracarotid shunting. The contradiction in the findings from different studies, especially in animal studies, can be attributed to the following factors:
Higher doses of dexmedetomidine were used in animal studies compared with human studies.
Dexmedetomidine causes greater reduction in CBF in isoflurane- and halothane-anesthetized dogs compared to pentobarbital-anesthetized dogs,32 which could explain the contradictory results in dog model studies.
There are species differences in α2-receptors. In dogs, the concentration of receptors is higher than in humans.33
THERAPEUTIC ROLE IN NEUROANESTHESIA
Neuromonitoring and Depth of Anesthesia
Dexmedetomidine reduces MAC up to 90%.34,35 Similar to the effect of many anesthetics, the α2-agonist clonidine significantly increased the latency and reduced the amplitude of the cortical auditory evoked potential.36 MAC-sparing effects may be helpful to the clinician, especially in situations in which increased anesthetic concentration is undesirable or not tolerated, such as during multimodal neurophysiologic monitoring. Disappearance of motor-evoked potentials (MEPs) was attributed to the effect of dexmedetomidine in 2 pediatric cases.37 However, in this report larger than usual doses of dexmedetomidine were administered, which could explain the disappearance of evoked potentials.
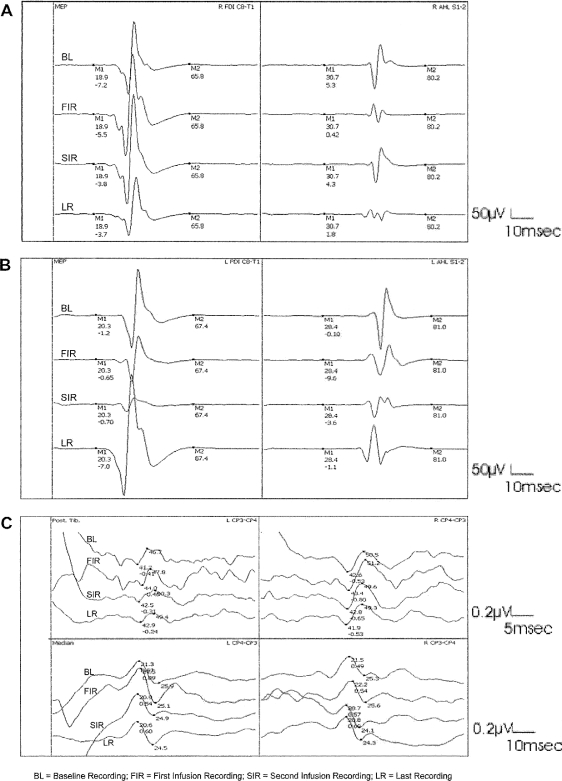
Tobias and colleagues38 showed that dexmedetomidine has no effect on sensory-evoked potentials and MEPs when it is used as a component of total IV anesthesia during posterior spinal fusion. In another report, dexmedetomidine infusion also did not affect somatosensory-evoked potentials (SSEPs) in 2 patients undergoing cervico-occipital fusion.39 Our own work showed that the use of dexmedetomidine as an anesthetic adjunct at target plasma concentrations up to 0.6 ng/mL does not change SSEPs or MEPs to a clinically significant degree during complex spine surgery40 (Figure 3). A unique feature of dexmedetomidine is that at plasma concentrations up to 1.60 ng/mL, it decreases the median frequency of the electroencephalogram but does not affect spike activity in patients with temporal lobe epilepsy anesthetized with 2.5% sevoflurane.41 This feature has been confirmed during awake craniotomy for seizure resection. Dexmedetomidine infusion at a dose of 0.2 to 0.5 µg · kg−1 · h−1 allows successful sedation for craniotomy and electrocorticography.42 It appears that dexmedetomidine at regular doses does not have harmful effects on evoked potential recordings; however, higher doses or boluses could abolish the evoked potential.
Figure 3.
Transcranial motor-evoked potentials (TceMEPs) and somatosensory-evoked potentials (SSEPs) at different study recording periods. (A) Right first dorsal interossei (R FD1 C8-T1) and abductor hallucis longus (R AHL S1-2) TceMEP. (B) Left first dorsal interossei (L FD1 C8-T1) and abductor hallucis longus (L AHL S1-2) TceMEP. (C) Left (L) and right posterior tibial nerve (Post Tib) and median nerve (Median) SSEP. (Reprinted with permission of Lippincott Williams & Wilkins. Bala E, et al. Anesthesiology. 2008;109(3):417-425.40)
Awake Craniotomy
Awake craniotomy is one of the most challenging procedures in neuroanesthesia practice. It requires that the patient be hemodynamically stable, comfortable, and sedated but awake enough to perform neuromotor and neurocognitive tests. Opioid-based sedation regimens for awake craniotomy are associated with respiratory depression and hypercapnia, which can result in brain swelling and increased intracranial pressure. Dexmedetomidine provides sedation, analgesia, and anxiolysis with minimal effect on respiratory function during awake craniotomy. Dexmedetomidine was used successfully as a primary anesthetic for brain mapping of the cortical speech area in children undergoing awake craniotomy.43 However, it was inadequate as a sole agent and required further supplementation with other anesthetics to achieve sedation for stereotactic radiosurgical ablation of an arteriovenous malformation (AVM) in a 6-year-old boy.44
In adult patients undergoing awake craniotomy, dexmedetomidine has been shown to improve patient safety and comfort with minimal effects on respiratory function, while facilitating sophisticated neurologic testing procedures during awake craniotomy.45-47 Dexmedetomidine inhibits hypercapnic cerebral vasodilation, a finding that could be beneficial during awake craniotomy procedures or neurointensive care.48 Thus, it could be used as an adjunct to other sedative drugs.
Implantation of Deep Brain Stimulation
Deep brain stimulation (DBS) is a procedure that presents many anesthetic challenges. The goal of this therapy is to enhance the quality of life for patients with Parkinson disease and other chronic neurologic disorders such as dystonia, depression, chronic pain syndromes, and minimally conscious states.49 Various subcortical structures within the basal ganglia, including the subthalamic nucleus, the globus pallidus, and the thalamus, are the targets of electrical stimulation.50 Anesthesia for DBS placement poses a challenge because of the complex neurocircuitry in the central nervous system of the areas to be stimulated, their sensitivity to anesthetic agents, and the co-morbidities suffered by patients with movement disorders.
The cessation of anti-Parkinsonian medications before the surgery (off-period) can be very unpleasant, particularly for patients with severe off-period pain, dystonia, or depression. Moreover, the avoidance of other narcotics and benzodiazepines to allow proper neurologic assessment in patients with chronic pain syndromes who undergo DBS therapy can be a very challenging task, especially because DBS is considered the last therapeutic measure where other measures failed.
The DBS procedure requires fixation of the patient's head to the stereotactic apparatus for accurate electrode placement. This can lead to considerable patient discomfort. Dexmedetomidine's properties make it well suited for sedation during DBS procedures. At doses up to 0.3 to 0.6 µg · kg−1 · h−1 dexmedetomidine does not interfere with microelectrode recordings, has little effect on the patient's motor symptoms, maintains respiratory status, and yet creates an environment in which the patient feels comfortable and relaxed.51,52 Dexmedetomidine also helps to maintain BP within the required target. The BP during DBS should be kept below 140 mmHg systolic to avoid the development of intracerebral hemorrhage during the procedure.
α2-Agonists are used to treat sympathetically maintained pain (pain dependent on efferent sympathetic activity) such as complex regional pain syndrome (CRPS).53 Our clinical experience has been that dexmedetomidine is very helpful in managing CRPS patients undergoing DBS by sparing narcotics during the procedure to avoid interference with neurologic testing.
Adjuncts to General Anesthesia During Craniotomy
One of the aims of anesthetic management during craniotomy is to avoid abrupt increases in arterial BP, which may cause bleeding or edema in the operating field.54 Low arterial pressures, on the other hand, predispose a patient to cerebral ischemia, because autoregulation of CBF is often impaired near tumors or surgically traumatized areas.55
In a recent study, dexmedetomidine increased perioperative hemodynamic stability in patients undergoing intracranial tumor surgery. It significantly attenuated the hypertensive hemodynamic responses to both intubation and emergence from anesthesia. In addition, it increased intraoperative cardiovascular stability. Most of the effects were concentration dependent, and a higher dose was more effective. Furthermore, patients receiving dexmedetomidine had their endotracheal tubes removed earlier than those in the placebo group, suggesting that the former group of patients had better respiratory function.54
Insertion of a skull-pin head holder during craniotomy results in substantial acute hypertension and possibly increased CBF. These hemodynamic responses may lead to brain edema, increased intracranial pressure (ICP), or intracranial hemorrhage. A single bolus dose of dexmedetomidine before induction of anesthesia attenuated the hemodynamic and neuroendocranial responses to skull-pin insertion in patients undergoing craniotomy.56 Dexmedetomidine also significantly attenuated isoflurane- and sevoflurane-induced dilatation of cerebral arterioles.57 The cerebral vasoconstrictive effect of dexmedetomidine might be beneficial to patients requiring higher concentrations of inhalation anesthetics because the attendant augmentation of CBF and brain volume might be avoided during craniotomy operations.
Dexmedetomidine reduces rocuronium requirements during sevoflurane anesthesia, according to a study by Memis and colleagues.58 The authors hypothesized that dexmedetomidine might alter rocuronium pharmacokinetics. This effect may be helpful in decreasing muscle relaxant requirements during surgery, thereby potentially further reducing the risk of residual muscle weakness during recovery from anesthesia.
α2-Agonists induce hypothermia in volunteers by stimulating inhibitory α2-adrenoceptors on central neurons. This effect could be helpful to clinicians attempting to induce or maintain hypothermia for neuroprotection, treatment of stroke, or ICP treatment.36
Interventional Neuroradiology Procedures
Dexmedetomidine has been used as an anesthetic adjunct during interventional neuroradiology procedures, during which maintaining proper cerebral perfusion pressure and hemodynamic stability represent capstones of anesthetic management. We used dexmedetomidine successfully for managing cocaine and opioid withdrawal in patients undergoing coiling for cerebral aneurysms or angioplasty for cerebral vasospasm.59 Stenting procedures for intracerebral atherosclerotic disease (ICAD) are gaining popularity. Cerebral ischemia caused by ICAD is associated with increased circulating and extracellular catecholamine levels,8 which are associated with severe hypertension and hemodynamic instability. Dexmedetomidine for ICAD stenting procedures may attenuate catecholamine-related hemodynamic instability while potentially mitigating the toxic effect of catecholamines.8,60 Endovascular therapy for patients with cerebral AVM bears the risk of postocclusion hypertension, which may result in cerebral hemorrhage. The authors combine dexmedetomidine, nicardipine, and inhalation anesthetics to control BP optimally during the procedure and continue dexmedetomidine infusion postoperatively to reduce the requirements for antihypertension medications.
α2-Agonists decrease the secretion of vasopressin and antagonize its action on renal tubules.61 α2-Adrenoceptors are also thought to inhibit the release of renin62 and increase the release of atrial natriuretic factor in the rat.63 Billings and colleagues64 investigated the renal protective effect of α2-agonists in radio-contrast nephropathy (RCN) in mice. The authors concluded that the α2-agonists clonidine and dexmedetomidine protect mice against RCN by preserving outer medullary renal blood flow.
POSTANESTHESIA CARE UNIT AND NEUROLOGIC INTENSIVE CARE UNIT
Animal Studies Support Applications for α2-Agonists in Neurologic Intensive Care Unit and Intensive Care Unit Settings
Zornow and colleagues65 studied the effect of IV dexmedetomidine on ICP in 24 New Zealand white rabbits under halothane anesthesia. Dexmedetomidine (20, 80, or 320 µg/kg IV) or saline solution was infused during a 10-minute period. The ICP transiently decreased by 31% in the 20 µg/kg group (from a mean value of 9.4 ± 1.3 to 6.5 ± 1.0 mmHg, P < .05). In the 320 µg/kg group, ICP remained unchanged despite a significant increase in arterial BP (32 mmHg). The authors induced intracranial hypertension (ICH) by a cryogenic lesion. Dexmedetomidine was found to have no effect on ICP in this model of ICH. In the same study, sagittal sinus blood flow was measured by the hydrogen clearance technique after the administration of dexmedetomidine (320 µg/kg IV). Dexmedetomidine was associated with a 14% decrease in sagittal sinus blood flow that was not statistically significant. The authors concluded that dexmedetomidine has minimal effects on ICP in halothane-anesthetized rabbits.
Hofer and colleagues66 investigated the therapeutic potential of prophylactic administration of either clonidine or dexmedetomidine in a prospective randomized study. They used a murine model of cecal ligation and puncture-induced sepsis. Animals receiving preemptive injections were treated with either clonidine (5 µg/kg) or dexmedetomidine (40 µg/kg) 1 hour and 12 hours before the operation, as well as 1, 6, and 12 hours afterward. Another group of animals received only clonidine (5 µg/kg) 1 hour, 6 hours, and 12 hours after the procedure. The control group received solvent injections at the respective periods.
Preemptive administration of clonidine or dexmedetomidine induced a central sympatholysis and significantly reduced mortality (clonidine: P = .015, dexmedetomidine: P = .029), while postoperative administration of clonidine failed to significantly prolong survival. The prophylactic administration of α2-agonists was accompanied by a reduction in the proinflammatory mediators interleukin (IL)-1B, IL-6, and tumor necrosis factor-α as well as a decrease in NF-RB binding activity. Thus, an overwhelming response to sepsis was inhibited, and BP was controlled. The authors advocated the use of clonidine or dexmedetomidine as an adjunct sedative in an intensive care unit (ICU) setting to reduce the occurrence of sepsis.
Kumagai and colleagues67 investigated the effects of IV dexmedetomidine on lung permeability induced by ICH in halothane-anesthetized and mechanically ventilated rats. Researchers divided the rats into 4 groups. In 2 groups, a subdural balloon catheter was inflated for 60 seconds to produce ICH. The dexmedetomidine group (n = 8) received IV dexmedetomidine 80 µg/kg followed by 6 µg · kg−1 · min−1 for 10 minutes, and the control group (n = 8) received IV saline. The surgery was performed without ICH with dexmedetomidine (sham-control, n = 5). In all groups, pulmonary permeability was measured using a modification of Evans blue dye extravasation technique. The authors concluded that prophylactic IV dexmedetomidine decreased lung permeability and attenuated hemodynamic changes induced by ICH in rats. Another interesting finding was that PaO2 was higher in the dexmedetomidine group than in the control group after ICH (P < .01). Human studies are needed to prove the beneficial effects of dexmedetomidine sedation on oxygenation and lung permeability in patients with ICH managed in the neurologic intensive care unit (NICU).
Clinical Studies for Using Dexmedetomidine in the ICU
Dexmedetomidine represents a suitable sedative for the postoperative period and intensive care environments. The major goals of sedation for patients in the postanesthesia care unit (PACU) and NICU are to provide anxiolysis and analgesia and to facilitate therapeutic and diagnostic procedures. It is also crucial to avoid additional cardiorespiratory work and metabolic alterations caused by increased levels of catecholamines and other stress hormones. The ideal sedative should also avoid respiratory depression and hypotension.2 Dexmedetomidine and other α2-agonists induce sedation by initiating processes similar to those found during natural sleep.68 Dexmedetomidine induced a pattern of c-Fos (an immediate early gene product) expression that was qualitatively similar to that occurring during sleep. The maintenance of natural sleep during sedation might speed recovery time in the ICU and counteract the effects of sleep deprivation, a common problem for critically ill patients and patients recovering from surgery.69 Dexmedetomidine increases activity in the pulvinar nucleus of the thalamus. As this region mediates the ability of arousal stimuli to produce attention,70 a sedative state with good patient cooperation might result.
Early work on sedation of critical care patients with dexmedetomidine demonstrated that 18 of 66 patients experienced significant hypotension or bradycardia; most of those events occurred when the patients received a full loading dose of dexmedetomidine.71 More recent work has shown that halving the loading dose preserves dexmedetomidine's sedative action while eliminating adverse cardiovascular events.72 Dexmedetomidine pharmacokinetics (clearance, steady state, volume of distribution, area under the curve) did not differ among young, middle-aged, and elderly adults or between men and women. A given dexmedetomidine infusion is expected to produce the same dexmedetomidine concentration irrespective of age or gender.2 Although this might be beneficial for clinical use in that pharmacokinetic variation is reduced, pharmacodynamically mediated differences in patient sensitivity to dexmedetomidine may still occur.
Alpha agonists increase the release of growth hormone and inhibit adipose tissue lipolysis.73 Clonidine increases the secretion of thyroid-stimulating hormone.36 These effects might prove beneficial in the ICU setting by counteracting catabolic processes. Administration of dexmedetomidine for up to 7 days did not suggest any adrenal shock or impairment of the hypothalamic-pituitary axis in dogs.74 However, prolonged use of high dosages of dexmedetomidine may result in accumulation and inhibit cortisol synthesis.75 α2-Agonists, through their central sympatholytic action, can induce hypothermia and inhibit the shivering response to hypothermia.76 Shivering frequently limits the effectiveness of hypothermia measures, and α2-agonists may be useful in the management of hypothermia for neuroprotection or ICP control.
Dexmedetomidine and other α2-agonists have been used successfully to manage the sympathetic hyperactivity resulting from drug withdrawal syndromes in substance abuse.77 In patients anesthetized with ketamine, premedication with an α2-agonist may prevent postanesthetic delirium.78
The α2-agonists have been found to have the potential to be prophylactic agents in treating sepsis. Kim and Hahn79 demonstrated that clonidine premedication was able to significantly reduce the proinflammatory cytokines—IL-1B and IL-6—in patients undergoing hysterectomy. Another study used clonidine as an adjunct sedative in critically ill patients to reduce the occurrence of pneumonia.80
Pandharipande and colleagues81 randomized 103 adults in medical and surgical ICUs into either dexmedetomidine or lorazepam groups for periods greater than 4 hours. This prospective, randomized, and double-blinded study showed that the dexmedetomidine group had more days alive and days without delirium. Ruokonen and colleagues82 suggested that in long-term ICU sedation for 48 hours or more, dexmedetomidine was comparable to propofol and midazolam in maintaining sedation targets of the Richmond Agitation Sedation Scale (RASS) score 0 to 3 but not suitable for deep sedation (RASS 4 or less). Dexmedetomidine had no effect on length of ICU stay. The authors suggested further studies to examine the effects of dexmedetomidine on duration of mechanical ventilation.
A double-blind randomized trial was conducted in 68 centers in 5 countries between 2005 and 2007 among 375 medical and surgical ICU patients with expected mechanical ventilation of more than 24 hours. Sedation level and delirium were assessed using RASS and the Confusion Assessment Method in the ICU. Dexmedetomidine (0.2-1.4 µg/kg per hour; n = 244) or midazolam 0.02-0.1 µg/kg per hour; n = 122) was titrated to achieve light sedation (RASS scores between −2 and +1) from enrollment until extubation or 30 days. No difference was found between dexmedetomidine and midazolam in the time-targeted sedation level in mechanically ventilated ICU patients. Dexmedetomidine-treated patients spent less time on the ventilator, experienced less delirium, and developed less tachycardia and hypertension.83 The most notable adverse effect of dexmedetomidine was bradycardia.83
Use of Dexmedetomidine for Neurointensive Care
Talke and colleagues,84 in a placebo-controlled, double-blind design using a single dose of dexmedetomidine (plasma concentration 600 pg/mL) versus placebo (0.9% NaCl), studied the effect of dexmedetomidine on lumbar cerebrospinal fluid (CSF) pressure in patients after transsphenoidal pituitary tumor surgery. Sixteen transsphenoidal pituitary tumor surgery patients were randomized to receive placebo (n = 9) or dexmedetomidine (n = 7) for 60 minutes in the PACU. Dexmedetomidine had no effect on lumbar CSF pressure.
In a retrospective study by Aryan and colleagues,85 the authors reviewed the data of 39 patients who received dexmedetomidine sedation in neurosurgical intensive care. The results of the study showed increases in cerebral perfusion pressure with decreased ICP.
In a case report about a 38-year-old man with severe traumatic brain injury (TBI) associated with paroxysmal autonomic instability with dystonia (PAID), dexmedetomidine was the only agent to aid in abrogating PAID after standard medical therapy failed to control the symptoms.86
In the pediatric intensive care unit (PICU), dexmedetomidine is used for long-term sedation of children during mechanical ventilation after TBI. This practice has not been without complications. For example, 1 case report showed dexmedetomidine-induced hypertension in a 32-month-old boy with TBI following a motor vehicle accident.87 The authors increased the infusion from 2 µg · kg−1 · h−1 to 4 µg · kg−1 · h−1 to control the patient's increasing agitation. The following morning, hypertension developed with systolic BP peaking up to 152 mmHg and was maintained between 130 and 140 mmHg with a diastolic BP of 60 to 70 mmHg (93rd percentile for the upper limits of BP in a child this age is 105/59 mmHg). No increase in ICP despite the increase in BP might be attributed to dexmedetomidine's cerebral vasoconstrictor effect. BP returned to normal after the dose was reduced to 2 µg · kg−1 · h−1. The patient made an uneventful recovery. In another case report, dexmedetomidine induced bradycardia when it was given during TBI treatment using therapeutic hypothermia in 2 children aged 12 and 4 years.88 The authors speculated that the concomitant use of remifentanil infusion and hypothermia with dexmedetomidine could accentuate the negative chronotropic effect of dexmedetomidine. The authors in these case reports advocated using dexmedetomidine sedation in PICU patients with TBI because of its neuroprotective effects, its lack of effect on ICP, and its inhibitory effect on shivering with induced hypothermia for ICP treatment.87,88 Long-term propofol infusion (more than 48 hours) in the PICU poses a potential risk for the development of propofol-infusion syndrome.89 The use of dexmedetomidine sedation can serve as a good alternative to propofol for long-term sedation in the PICU. Nevertheless, large studies are still needed on the use of dexmedetomidine sedation in PICU patients with neurologic injuries.
CONCLUSION
Dexmedetomidine is a new α2-agonist sedative agent that has neuroprotective and myocardial protective effects. It is considered the near-ideal sedative agent in the ICU, for it maintains the patient's cooperation with minimal effect on respiratory function. Dexmedetomidine is unique among other sedative agents in that it maintains the normal sleep pattern, which is essential for the patient's well-being. However, despite its beneficial effects, the drug's safety as a sole sedative agent in patients with neurologic diseases or patients with sepsis in the ICU is still controversial. Dexmedetomidine decreases CBF without proportional reduction in CMRO2. Large randomized studies are still needed to assess its safety in patients with neurologic diseases or patients with severe sepsis.
Acknowledgments
This article meets the Accreditation Council for Graduate Medical Education competencies for Patient Care and Medical Knowledge.
Footnotes
Financial disclosure: None of the authors has a financial or proprietary interest in the subject matter of this article.
REFERENCES
- 1.Maze M., Tranquilli W. Alpha-2 adrenoceptor agonists: defining the role in clinical anesthesia. Anesthesiology. 1991;74((3)):581–605. [PubMed] [Google Scholar]
- 2.Maze M., Scarfini C., Cavaliere F. New agents for sedation in the intensive care unit. Crit Care Clin. 2001;17((4)):881–897. doi: 10.1016/s0749-0704(05)70185-8. [DOI] [PubMed] [Google Scholar]
- 3.Kamibayashi T., Maze M. Clinical uses of alpha2-adrenergic agonists. Anesthesiology. 2000;93((5)):1345–1349. doi: 10.1097/00000542-200011000-00030. [DOI] [PubMed] [Google Scholar]
- 4.Khan Z. P., Ferguson C. N., Jones R. M. Alpha-2 and imidazoline receptor agonists: their pharmacology and therapeutic role. Anaesthesia. 1999;54((2)):146–165. doi: 10.1046/j.1365-2044.1999.00659.x. [DOI] [PubMed] [Google Scholar]
- 5.Wheeler L. A., Gil D. W., WoldeMussie E. Role of alpha-2 adrenergic receptors in neuroprotection and glaucoma. Surv Ophthalmol. 2001;45((suppl 3)):S291–S294. doi: 10.1016/s0039-6257(01)00206-5. [DOI] [PubMed] [Google Scholar]
- 6.Danylkova N. O., Alcala S. R., Pomeranz H. D., McLoon L. K. Neuroprotective effects of brimonidine treatment in a rodent model of ischemic optic neuropathy. Exp Eye Res. 2007;84((2)):293–301. doi: 10.1016/j.exer.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 7.Merin S., Obolensky A., Farber M. D., Chowers I. A pilot study of topical treatment with an alpha2-agonist in patients with retinal dystrophies. J Ocul Pharmacol Ther. 2008;24((1)):80–86. doi: 10.1089/jop.2007.0022. [DOI] [PubMed] [Google Scholar]
- 8.Engelhard K., Werner C., Kaspar S., et al. Effect of the alpha2-agonist dexmedetomidine on cerebral neurotransmitter concentrations during cerebral ischemia in rats. Anesthesiology. 2002;96((2)):450–457. doi: 10.1097/00000542-200202000-00034. [DOI] [PubMed] [Google Scholar]
- 9.Ma D., Rajakumaraswamy N., Maze M. alpha2-Adrenoceptor agonists: shedding light on neuroprotection? Br Med Bull. 2005;71:77–92. doi: 10.1093/bmb/ldh036. [DOI] [PubMed] [Google Scholar]
- 10.Madison D. V., Nicoll R. A. Actions of noradrenaline recorded intracellularly in rat hippocampal CA1 pyramidal neurones, in vitro. J Physiol. 1986;372:221–244. doi: 10.1113/jphysiol.1986.sp016006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stein S. C., Cracco R. Q. Cortical injury without ischemia produced by topical monoamines. Stroke. 1982;13((1)):74–83. doi: 10.1161/01.str.13.1.74. [DOI] [PubMed] [Google Scholar]
- 12.Hertz L., Dringen R., Schousboe A., Robinson S. R. Astrocytes: glutamate producers for neurons. J Neurosci Res. 1999;57((4)):417–428. [PubMed] [Google Scholar]
- 13.Pascual J. M., Carceller F., Roda J. M., Cerdán S. Glutamate, glutamine, and GABA as substrates for the neuronal and glial compartments after focal cerebral ischemia in rats. Stroke. 1998;29((5)):1048–1056. doi: 10.1161/01.str.29.5.1048. [DOI] [PubMed] [Google Scholar]
- 14.Huang R., Chen Y., Yu A. C., Hertz L. Dexmedetomidine-induced stimulation of glutamine oxidation in astrocytes: a possible mechanism for its neuroprotective activity. J Cereb Blood Flow Metab. 2000;20((6)):895–898. doi: 10.1097/00004647-200006000-00001. [DOI] [PubMed] [Google Scholar]
- 15.Donello J. E., Padillo E. U., Webster M. L., Wheeler L. A., Gil D. W. alpha(2)-Adrenoceptor agonists inhibit vitreal glutamate and aspartate accumulation and preserve retinal function after transient ischemia. J Pharmacol Exp Ther. 2001;296((1)):216–223. [PubMed] [Google Scholar]
- 16.Rossi D. J., Oshima T., Attwell D. Glutamate release in severe brain ischaemia is mainly by reversed uptake. Nature. 2000;403((6767)):316–321. doi: 10.1038/35002090. [DOI] [PubMed] [Google Scholar]
- 17.Wheeler L. A., Tatton N. A., Elstner M., et al. Alpha-2 adrenergic receptor activation by brimonidine reduces neuronal apoptosis through AKT (protein kinase B) dependent new synthesis of BCL-2. Invest Ophthalmol Vis Sci. 2001;42:S411. [Google Scholar]
- 18.Engelhard K., Werner C., Eberspacher E., et al. The effect of the alpha 2-agonist dexmedetomidine and the N-methyl-D-aspartate antagonist S(+)-ketamine on the expression of apoptosis-regulating proteins after incomplete cerebral ischemia and reperfusion in rats. Anesth Analg. 2003;96((2)):524–531. doi: 10.1097/00000539-200302000-00041. [DOI] [PubMed] [Google Scholar]
- 19.Wheeler L., WoldeMussie E., Lai R. Role of alpha-2 agonists in neuroprotection. Surv Ophthalmol. 2003;48((suppl 1)):S47–S51. doi: 10.1016/s0039-6257(03)00004-3. [DOI] [PubMed] [Google Scholar]
- 20.Laudenbach V., Mantz J., Lagercrantz H., Desmonts J. M., Evrard P., Gressens P. Effects of alpha(2)-adrenoceptor agonists on perinatal excitotoxic brain injury: comparison of clonidine and dexmedetomidine. Anesthesiology. 2002;96((1)):134–141. doi: 10.1097/00000542-200201000-00026. [DOI] [PubMed] [Google Scholar]
- 21.Ma D., Hossain M., Rajakumaraswamy N., et al. Dexmedetomidine produces its neuroprotective effect via the alpha 2A-adrenoceptor subtype. Eur J Pharmacol. 2004;502((1-2)):87–97. doi: 10.1016/j.ejphar.2004.08.044. [DOI] [PubMed] [Google Scholar]
- 22.Sanders R. D., Xu J., Shu Y., et al. Dexmedetomidine attenuates isoflurane-induced neurocognitive impairment in neonatal rats. Anesthesiology. 2009;110((5)):1077–1085. doi: 10.1097/ALN.0b013e31819daedd. [DOI] [PubMed] [Google Scholar]
- 23.Karlsson B. R., Forsman M., Roald O. K., Heier M. S., Steen P. A. Effect of dexmedetomidine, a selective and potent alpha 2-agonist, on cerebral blood flow and oxygen consumption during halothane anesthesia in dogs. Anesth Analg. 1990;71((2)):125–129. doi: 10.1213/00000539-199008000-00003. [DOI] [PubMed] [Google Scholar]
- 24.Asano Y., Koehler R. C., Kawaguchi T., McPherson R. W. Pial arteriolar constriction to alpha 2-adrenergic agonist dexmedetomidine in the rat. Am J Physiol. 1997;272((6, pt 2)):H2547–H2556. doi: 10.1152/ajpheart.1997.272.6.H2547. [DOI] [PubMed] [Google Scholar]
- 25.McPherson R. W., Koehler R. C., Kirsch J. R., Traystman R. J. Intraventricular dexmedetomidine decreases cerebral blood flow during normoxia and hypoxia in dogs. Anesth Analg. 1997;84((1)):139–147. doi: 10.1097/00000539-199701000-00026. [DOI] [PubMed] [Google Scholar]
- 26.Iida H., Ohata H., Iida M., Watanabe Y., Dohi S. Direct effects of alpha1- and alpha2-adrenergic agonists on spinal and cerebral pial vessels in dogs. Anesthesiology. 1999;91((2)):479–485. doi: 10.1097/00000542-199908000-00023. [DOI] [PubMed] [Google Scholar]
- 27.Ogawa Y., Iwasaki K., Aoki K., Kojima W., Kato J., Ogawa S. Dexmedetomidine weakens dynamic cerebral autoregulation as assessed by transfer function analysis and the thigh cuff method. Anesthesiology. 2008;109((4)):642–650. doi: 10.1097/ALN.0b013e3181862a33. [DOI] [PubMed] [Google Scholar]
- 28.Kadoi Y., Saito S., Kawauchi C., Hinohara H., Kunimoto F. Comparative effects of propofol vs dexmedetomidine on cerebrovascular carbon dioxide reactivity in patients with septic shock. Br J Anaesth. 2008;100((2)):224–229. doi: 10.1093/bja/aem343. [DOI] [PubMed] [Google Scholar]
- 29.Iida H., Iida M., Ohata H., Michino T., Dohi S. Effects of dexmedetomidine on cerebral circulation and systemic hemodynamics after cardiopulmonary resuscitation in dogs. J Anesth. 2006;20((3)):202–207. doi: 10.1007/s00540-006-0402-0. [DOI] [PubMed] [Google Scholar]
- 30.Drummond J. C., Dao A. V., Roth D. M., et al. Effect of dexmedetomidine on cerebral blood flow velocity, cerebral metabolic rate, and carbon dioxide response in normal humans. Anesthesiology. 2008;108((2)):225–232. doi: 10.1097/01.anes.0000299576.00302.4c. [DOI] [PubMed] [Google Scholar]
- 31.Bekker A., Gold M., Ahmed R., et al. Dexmedetomidine does not increase the incidence of intracarotid shunting in patients undergoing awake carotid endarterectomy. Anesth Analg. 2006;103((4)):955–958. doi: 10.1213/01.ane.0000237288.46912.39. [DOI] [PubMed] [Google Scholar]
- 32.Fale A., Kirsch J. R., McPherson R. W. Alpha 2-adrenergic agonist effects on normocapnic and hypercapnic cerebral blood flow in the dog are anesthetic dependent. Anesth Analg. 1994;79((5)):892–898. doi: 10.1213/00000539-199411000-00013. [DOI] [PubMed] [Google Scholar]
- 33.Toda N. Alpha adrenergic receptor subtypes in human, monkey and dog cerebral arteries. J Pharmacol Exp Ther. 1983;226((3)):861–868. [PubMed] [Google Scholar]
- 34.Segal I. S., Vickery R. G., Walton J. K., Doze V. A., Maze M. Dexmedetomidine diminishes halothane anesthetic requirements in rats through a postsynaptic alpha 2 adrenergic receptor. Anesthesiology. 1988;69((6)):818–823. doi: 10.1097/00000542-198812000-00004. [DOI] [PubMed] [Google Scholar]
- 35.Aho M., Erkola O., Kallio A., Scheinin H., Korttila K. Dexmedetomidine infusion for maintenance of anesthesia in patients undergoing abdominal hysterectomy. Anesth Analg. 1992;75((6)):940–946. [PubMed] [Google Scholar]
- 36.Samuels E. R., Hou R. H., Langley R. W., Szabadi E., Bradshaw C. M. Modulation of the acoustic startle response by the level of arousal: comparison of clonidine and modafinil in healthy volunteers. Neuropsychopharmacology. 2007;32((11)):2405–2421. doi: 10.1038/sj.npp.1301363. [DOI] [PubMed] [Google Scholar]
- 37.Mahmoud M., Sadhasivam S., Sestokas A. K., Samuels P., McAuliffe J. Loss of transcranial electric motor evoked potentials during pediatric spine surgery with dexmedetomidine. Anesthesiology. 2007;106((2)):393–396. doi: 10.1097/00000542-200702000-00027. [DOI] [PubMed] [Google Scholar]
- 38.Tobias J. D., Goble T. J., Bates G., Anderson J. T., Hoernschemeyer D. G. Effects of dexmedetomidine on intraoperative motor and somatosensory evoked potential monitoring during spinal surgery in adolescents. Paediatr Anaesth. 2008;18((11)):1082–1088. doi: 10.1111/j.1460-9592.2008.02733.x. [DOI] [PubMed] [Google Scholar]
- 39.Bloom M., Beric A., Bekker A. Dexmedetomidine infusion and somatosensory evoked potentials. J Neurosurg Anesthesiol. 2001;13((4)):320–322. doi: 10.1097/00008506-200110000-00007. [DOI] [PubMed] [Google Scholar]
- 40.Bala E., Sessler D. I., Nair D. R., McLain R., Dalton J. E., Farag E. Motor and somatosensory evoked potentials are well maintained in patients given dexmedetomidine during spine surgery. Anesthesiology. 2008;109((3)):417–425. doi: 10.1097/ALN.0b013e318182a467. [DOI] [PubMed] [Google Scholar]
- 41.Oda Y., Toriyama S., Tanaka K., et al. The effect of dexmedetomidine on electrocorticography in patients with temporal lobe epilepsy under sevoflurane anesthesia. Anesth Analg. 2007;105((5)):1272–1277. doi: 10.1213/01.ane.0000281075.77316.98. [DOI] [PubMed] [Google Scholar]
- 42.Souter M. J., Rozet I., Ojemann J. G., et al. Dexmedetomidine sedation during awake craniotomy for seizure resection: effects on electrocorticography. J Neurosurg Anesthesiol. 2007;19((1)):38–44. doi: 10.1097/01.ana.0000211027.26550.24. [DOI] [PubMed] [Google Scholar]
- 43.Ard J., Doyle W., Bekker A. Awake craniotomy with dexmedetomidine in pediatric patients. J Neurosurg Anesthesiol. 2003;15((3)):263–266. doi: 10.1097/00008506-200307000-00015. [DOI] [PubMed] [Google Scholar]
- 44.Fahy C. J., Okumura M. Sedation for paediatric stereotactic radiosurgery: the dexmedetomidine experience. Anaesth Intensive Care. 2004;32((6)):809–811. doi: 10.1177/0310057X0403200613. [DOI] [PubMed] [Google Scholar]
- 45.Ard J. L., Jr, Bekker A. Y., Doyle W. K. Dexmedetomidine in awake craniotomy: a technical note. Surg Neurol. 2005;63((2)):114–116. doi: 10.1016/j.surneu.2004.02.029. [DOI] [PubMed] [Google Scholar]
- 46.Bekker A. Y., Kaufman B., Samir H., Doyle W. The use of dexmedetomidine infusion for awake craniotomy. Anesth Analg. 2001;92((5)):1251–1253. doi: 10.1097/00000539-200105000-00031. [DOI] [PubMed] [Google Scholar]
- 47.Mack P. F., Perrine K., Kobylarz E., Schwartz T. H., Lien C. A. Dexmedetomidine and neurocognitive testing in awake craniotomy. J Neurosurg Anesthesiol. 2004;16((1)):20–25. doi: 10.1097/00008506-200401000-00005. [DOI] [PubMed] [Google Scholar]
- 48.Takenaka M., Iida H., Iida M., Dohi S. Intrathecal dexmedetomidine attenuates hypercapnic but not hypoxic cerebral vasodilation in anesthetized rabbits. Anesthesiology. 2000;92((5)):1376–1384. doi: 10.1097/00000542-200005000-00028. [DOI] [PubMed] [Google Scholar]
- 49.Kenney C., Simpson R., Hunter C., et al. Short-term and long-term safety of deep brain stimulation in the treatment of movement disorders. J Neurosurg. 2007;106((4)):621–625. doi: 10.3171/jns.2007.106.4.621. [DOI] [PubMed] [Google Scholar]
- 50.Vesper J., Haak S., Ostertag C., Nikkhah G. Subthalamic nucleus deep brain stimulation in elderly patients—analysis of outcome and complications. BMC Neurol. 2007;7:7. doi: 10.1186/1471-2377-7-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Khatib R., Ebrahim Z., Rezai A., et al. Perioperative events during deep brain stimulation: the experience at Cleveland Clinic. J Neurosurg Anesthesiol. 2008;20((1)):36–40. doi: 10.1097/ANA.0b013e318157a15a. [DOI] [PubMed] [Google Scholar]
- 52.Rozet I., Muangman S., Vavilala M. S., et al. Clinical experience with dexmedetomidine for implantation of deep brain stimulators in Parkinson's disease. Anesth Analg. 2006;103((5)):1224–1228. doi: 10.1213/01.ane.0000239331.53085.94. [DOI] [PubMed] [Google Scholar]
- 53.Rauck R. L., Eisenach J. C., Jackson K., Young L. D., Southern J. Epidural clonidine treatment for refractory reflex sympathetic dystrophy. Anesthesiology. 1993;79((6)):1163–1169. [PubMed] [Google Scholar]
- 54.Tanskanen P. E., Kytta J. V., Randell T. T., Aantaa R. E. Dexmedetomidine as an anaesthetic adjuvant in patients undergoing intracranial tumour surgery: a double-blind, randomized and placebo-controlled study. Br J Anaesth. 2006;97((5)):658–665. doi: 10.1093/bja/ael220. [DOI] [PubMed] [Google Scholar]
- 55.Fieschi C., Agnoli A., Battistini N., Bozzao L., Prencipe M. Derangement of regional cerebral blood flow and of its regulatory mechanisms in acute cerebrovascular lesions. Neurology. 1968;18((12)):1166–1179. doi: 10.1212/wnl.18.12.1166. [DOI] [PubMed] [Google Scholar]
- 56.Uyar A. S., Yagmurdur H., Fidan Y., Topkaya C., Basar H. Dexmedetomidine attenuates the hemodynamic and neuroendocrinal responses to skull-pin head-holder application during craniotomy. J Neurosurg Anesthesiol. 2008;20((3)):174–179. doi: 10.1097/ANA.0b013e318177e5eb. [DOI] [PubMed] [Google Scholar]
- 57.Ohata H., Iida H., Dohi S., Watanabe Y. Intravenous dexmedetomidine inhibits cerebrovascular dilation induced by isoflurane and sevoflurane in dogs. Anesth Analg. 1999;89((2)):370–377. doi: 10.1097/00000539-199908000-00023. [DOI] [PubMed] [Google Scholar]
- 58.Memis D., Turan A., Karamanlioglu B., Şeker Ş, Pamukçu Z. Dexmedetomidine reduces rocuronium dose requirement in sevoflurane anaesthesia. Curr Anaesth Crit Care. 2008;19((3)):169–174. [Google Scholar]
- 59.Farag E., Chahlavi A., Argalious M., et al. Using dexmedetomidine to manage patients with cocaine and opioid withdrawal, who are undergoing cerebral angioplasty for cerebral vasospasm. Anesth Analg. 2006;103((6)):1618–1620. doi: 10.1213/01.ane.0000246399.10396.15. [DOI] [PubMed] [Google Scholar]
- 60.Farag E., Fiorella D., Robinson H., Anderson M., Ebrahim Z. Anesthetic management for wingspan stent insertion. Anesthesiology. 2008;109((4)):A485. [Google Scholar]
- 61.Smyth D. D., Umemura S., Pettinger W. A. Alpha 2-adrenoceptor antagonism of vasopressin-induced changes in sodium excretion. Am J Physiol. 1985;248((6, pt 2)):F767–F772. doi: 10.1152/ajprenal.1985.248.6.F767. [DOI] [PubMed] [Google Scholar]
- 62.Pettinger W. A. Renal alpha 2-adrenergic receptors and hypertension. Hypertension. 1987;9((1)):3–6. doi: 10.1161/01.hyp.9.1.3. [DOI] [PubMed] [Google Scholar]
- 63.Chen M., Lee J., Huang B. S., Grekin R. J., Malvin R. L. Clonidine and morphine increase atrial natriuretic peptide secretion in anesthetized rats. Proc Soc Exp Biol Med. 1989;191((3)):299–303. doi: 10.3181/00379727-191-42924. [DOI] [PubMed] [Google Scholar]
- 64.Billings F. T., 4th, Chen S. W., Kim M., et al. alpha2-Adrenergic agonists protect against radiocontrast-induced nephropathy in mice. Am J Physiol Renal Physiol. 2008;295((3)):F741–F748. doi: 10.1152/ajprenal.90244.2008. [DOI] [PubMed] [Google Scholar]
- 65.Zornow M. H., Scheller M. S., Sheehan P. B., Strnat M. A., Matsumoto M. Intracranial pressure effects of dexmedetomidine in rabbits. Anesth Analg. 1992;75((2)):232–237. doi: 10.1213/00000539-199208000-00014. [DOI] [PubMed] [Google Scholar]
- 66.Hofer S., Steppan J., Wagner T., et al. Central sympatholytics prolong survival in experimental sepsis. Crit Care. 2009;13((1)):R11. doi: 10.1186/cc7709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kumagai M., Horiguchi T., Nishikawa T., Masaki Y., Tobe Y. Intravenous dexmedetomidine decreases lung permeability induced by intracranial hypertension in rats. Anesth Analg. 2008;107((2)):643–647. doi: 10.1213/ane.0b013e3181770e6f. [DOI] [PubMed] [Google Scholar]
- 68.Nelson L. E., Lu J., Guo T., Saper C. B., Franks N. P., Maze M. The alpha2-adrenoceptor agonist dexmedetomidine converges on an endogenous sleep-promoting pathway to exert its sedative effects. Anesthesiology. 2003;98((2)):428–436. doi: 10.1097/00000542-200302000-00024. [DOI] [PubMed] [Google Scholar]
- 69.Maze M., Bonnet F. Receptor ligands-alpha 2-adrenergic receptor agonists. In: Evers A. S., Maze M., editors. Anesthetic Pharmacology: Physiologic Principles and Clinical Practice. Philadelphia, PA: Churchill Livingstone; 2004. pp. 473–489. [Google Scholar]
- 70.Coull J. T., Jones M. E., Egan T. D., Frith C. D., Maze M. Attentional effects of noradrenaline vary with arousal level: selective activation of thalamic pulvinar in humans. Neuroimage. 2004;22((1)):315–322. doi: 10.1016/j.neuroimage.2003.12.022. [DOI] [PubMed] [Google Scholar]
- 71.Venn R. M., Bradshaw C. J., Spencer R., et al. Preliminary UK experience of dexmedetomidine, a novel agent for postoperative sedation in the intensive care unit. Anaesthesia. 1999;54((12)):1136–1142. doi: 10.1046/j.1365-2044.1999.01114.x. [DOI] [PubMed] [Google Scholar]
- 72.Venn R. M., Karol M. D., Grounds R. M. Pharmacokinetics of dexmedetomidine infusions for sedation of postoperative patients requiring intensive care. Br J Anaesth. 2002;88((5)):669–675. doi: 10.1093/bja/88.5.669. [DOI] [PubMed] [Google Scholar]
- 73.Grossman A., Weerasuriya K., Al-Damluji S., Turner P., Besser G. M. Alpha 2-adrenoceptor agonists stimulate growth hormone secretion but have no acute effects on plasma cortisol under basal conditions. Horm Res. 1987;25((2)):65–71. doi: 10.1159/000180635. [DOI] [PubMed] [Google Scholar]
- 74.Maze M., Virtanen R., Daunt D., Banks S. J., Stover E. P., Feldman D. Effects of dexmedetomidine, a novel imidazole sedative-anesthetic agent, on adrenal steroidogenesis: in vivo and in vitro studies. Anesth Analg. 1991;73((2)):204–208. doi: 10.1213/00000539-199108000-00015. [DOI] [PubMed] [Google Scholar]
- 75.Wagner D. S., Brummett C. M. Dexmedetomidine: as safe as safe can be. Semin Anesth Perioperative Med Pain. 2006;25((2)):77–83. [Google Scholar]
- 76.Joris J., Banache M., Bonnet F., Sessler D. I., Lamy M. Clonidine and ketanserin both are effective treatment for postanesthetic shivering. Anesthesiology. 1993;79((3)):532–539. doi: 10.1097/00000542-199309000-00017. [DOI] [PubMed] [Google Scholar]
- 77.Multz A. S. Prolonged dexmedetomidine infusion as an adjunct in treating sedation-induced withdrawal. Anesth Analg. 2003;96((4)):1054–1055. doi: 10.1213/01.ANE.0000050773.70232.08. [DOI] [PubMed] [Google Scholar]
- 78.Levanen J., Makela M. L., Scheinin H. Dexmedetomidine premedication attenuates ketamine-induced cardiostimulatory effects and postanesthetic delirium. Anesthesiology. 1995;82((5)):1117–1125. doi: 10.1097/00000542-199505000-00005. [DOI] [PubMed] [Google Scholar]
- 79.Kim M. H., Hahn T. H. The effect of clonidine pretreatment on the perioperative proinflammatory cytokines, cortisol, and ACTH responses in patients undergoing total abdominal hysterectomy. Anesth Analg. 2000;90((6)):1441–1444. doi: 10.1097/00000539-200006000-00035. [DOI] [PubMed] [Google Scholar]
- 80.Spies C. D., Dubisz N., Neumann T., et al. Therapy of alcohol withdrawal syndrome in intensive care unit patients following trauma: results of a prospective, randomized trial. Crit Care Med. 1996;24((3)):414–422. doi: 10.1097/00003246-199603000-00009. [DOI] [PubMed] [Google Scholar]
- 81.Pandharipande P. P., Pun B. T., Herr D. L., et al. Effect of sedation with dexmedetomidine vs lorazepam on acute brain dysfunction in mechanically ventilated patients: the MENDS randomized controlled trial. JAMA. 2007;298((22)):2644–2653. doi: 10.1001/jama.298.22.2644. [DOI] [PubMed] [Google Scholar]
- 82.Ruokonen E., Parviainen I., Jakob S. M., et al. Dexmedetomidine versus propofol/midazolam for long-term sedation during mechanical ventilation. Intensive Care Med. 2009;35((2)):282–290. doi: 10.1007/s00134-008-1296-0. [DOI] [PubMed] [Google Scholar]
- 83.Riker R. R., Shehabi Y., Bokesch P. M., et al. Dexmedetomidine vs midazolam for sedation of critically ill patients: a randomized trial. JAMA. 2009;301((5)):489–499. doi: 10.1001/jama.2009.56. [DOI] [PubMed] [Google Scholar]
- 84.Talke P., Tong C., Lee H. W., Caldwell J., Eisenach J. C., Richardson C. A. Effect of dexmedetomidine on lumbar cerebrospinal fluid pressure in humans. Anesth Analg. 1997;85((2)):358–364. doi: 10.1097/00000539-199708000-00021. [DOI] [PubMed] [Google Scholar]
- 85.Aryan H. E., Box K. W., Ibrahim D., Desiraju U., Ames C. P. Safety and efficacy of dexmedetomidine in neurosurgical patients. Brain Inj. 2006;20((8)):791–798. doi: 10.1080/02699050600789447. [DOI] [PubMed] [Google Scholar]
- 86.Goddeau R. P., Jr, Silverman S. B., Sims J. R. Dexmedetomidine for the treatment of paroxysmal autonomic instability with dystonia. Neurocrit Care. 2007;7((2)):217–220. doi: 10.1007/s12028-007-0066-0. [DOI] [PubMed] [Google Scholar]
- 87.Erkonen G., Lamb F., Tobias J. D. High-dose dexmedetomidine-induced hypertension in a child with traumatic brain injury. Neurocrit Care. 2008;9((3)):366–369. doi: 10.1007/s12028-008-9102-y. [DOI] [PubMed] [Google Scholar]
- 88.Tobias J. D. Bradycardia during dexmedetomidine and therapeutic hypothermia. J Intensive Care Med. 2008;23((6)):403–408. doi: 10.1177/0885066608324389. [DOI] [PubMed] [Google Scholar]
- 89.Spitzfaden A. C., Jimenez D. F., Tobias J. D. Propofol for sedation and control of intracranial pressure in children. Pediatr Neurosurg. 1999;31((4)):194–200. doi: 10.1159/000028861. [DOI] [PubMed] [Google Scholar]