Abstract
Patients with placenta accreta have abnormally adherent placentas and are at risk for massive hemorrhage at delivery. We report 2 cases of cesarean hysterectomy in patients with placenta accreta. These patients were cared for by a multidisciplinary team consisting of a maternal fetal medicine specialist, gynecologic oncologist, anesthesiologist, neonatologist, interventional radiologist, and urologist. Favorable maternal and fetal outcomes resulted from the use of this team.
Keywords: Cesarean delivery, hemorrhage, hysterectomy, placenta accreta
Placenta accreta (an abnormally adherent placenta) may be associated with significant maternal hemorrhage at delivery owing to incomplete placental separation. Normally, the placenta adheres to the decidua basalis layer, allowing for a smooth separation of the placenta from the uterine wall after delivery. In patients with abnormal placentation, the placenta has invaded past the decidua basalis layer. There are varying degrees of abnormal placentation. Placenta accreta vera occurs when the placenta adheres to the myometrium. Placental invasion into the myometrium is termed placenta increta. Placenta percreta involves invasion of the placenta through the myometrium to the uterine serosa and may include invasion into other pelvic organs. When placenta accreta is diagnosed before delivery, a multidisciplinary approach may improve patient outcome. We present 2 cases of cesarean hysterectomy in patients with placenta accreta and the multidisciplinary management of these patients.
CASE 1
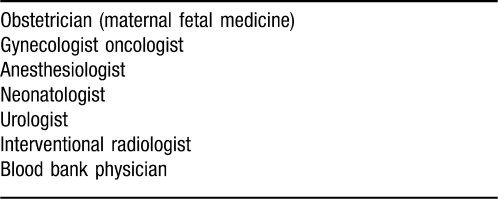
A 32-year-old woman, gravida 3, para 2, with a history of 2 prior cesarean deliveries, presented with hematuria at 32 weeks' gestation. Ultrasound imaging showed placenta previa and possible abnormal placentation. Magnetic resonance imaging (MRI) revealed possible placental invasion through the uterus and into the bladder. The patient was admitted for administration of steroids and consultation with the specialists that would be involved in her care (Table 1).
Table 1.
Multidisciplinary Team for Management of a Placenta Accreta
Physical examination revealed a narrow maxilla, retrognathia, a small mouth opening, and a high arched palate, and a Mallampati III classification was assessed. The decision was made to proceed with intravenous sedation for ureteral stent and iliac artery catheter placement and general anesthesia for cesarean hysterectomy.
The patient received aspiration prophylaxis and antibiotics before the procedures, and a labor and delivery nurse monitored fetal heart tones continuously until just before delivery. Initially, the patient was brought to the endovascular operative suite for the placement of ureteral stents and iliac artery balloon catheters under intravenous sedation with fentanyl and midazolam and standard noninvasive monitoring. The consulting urologist performed a cystoscopy and ruled out placental invasion of the bladder. Bilateral ureteral catheters were placed to aid in identification of the ureters during hysterectomy, followed by the placement of bilateral femoral sheaths and bilateral internal iliac artery balloon catheters under fluoroscopic guidance. The catheters were placed in the anterior division of the internal iliac arteries by 2 interventional radiologists, with 1 working on each side of the patient simultaneously to minimize radiation exposure to the mother and fetus. Thereafter, the patient remained supine with legs unflexed.
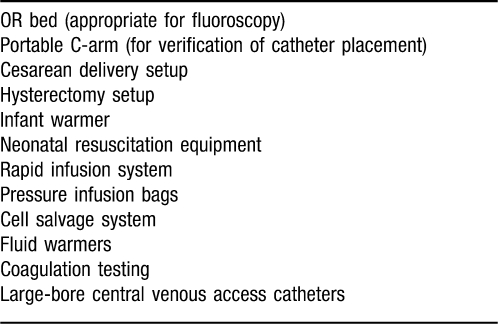
The patient was then carefully moved to a stretcher and transferred to a larger operating room equipped with a cell salvage system, a rapid infuser, neonatal resuscitation equipment, pressurized infusion bags and other necessary equipment (Table 2). Eight units of packed red blood cells and 4 units of fresh frozen plasma were cross-checked and available for immediate use. An interventional radiologist remained in the operating room until the hysterectomy was complete. A radial arterial line, two 14-gauge peripheral intravenous lines, and a triple-lumen internal jugular catheter were inserted. Once the patient was prepared and draped and the surgical team was in place, general anesthesia was induced. A rapid sequence intubation and a video laryngoscope easily achieved tracheal intubation. Cesarean delivery was performed without difficulty, and the neonate was handed to the neonatologist for further care. Upon removal of the uterus, copious bleeding began, and the balloon catheters were inflated by the interventional radiologist. The bleeding decreased, and the surgical team's visibility improved. After complete removal of the cervix and placenta, hemostasis was attained. The placenta strongly adhered to the bladder but had not invaded into the musculature, so a bladder resection was not required. Estimated blood loss was 3 L, which was replaced with 8 units of packed red blood cells, 4 units of fresh frozen plasma, and 3 liters of saline, administered using a rapid infusion device. At the conclusion of the procedure, the patient was extubated and monitored in the postanesthesia care unit (PACU) overnight. The balloon occlusion catheters were removed at the end of the procedure in the operating room, the bilateral femoral artery sheaths were removed in the PACU, and pressure was held for hemostasis. The patient was discharged 5 days after surgery.
Table 2.
Operating Room (OR) Equipment for Cesarean Delivery With or Without Hysterectomy
CASE 2
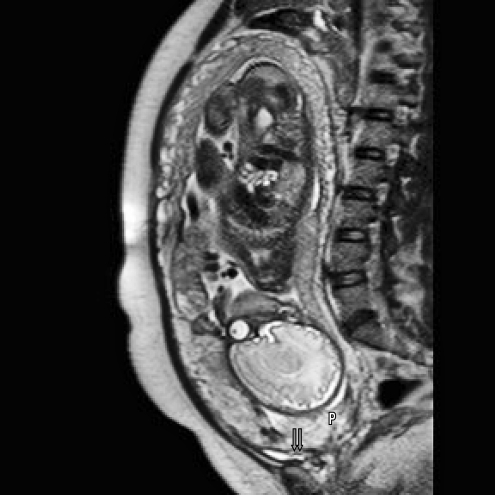
A 36-year-old woman, gravida 5, para 2 (with a history of 2 vaginal deliveries and 2 cervical dilation and curettages for elective pregnancy terminations) was admitted at 34 weeks' gestation. The patient had previously been admitted to an outside institution because of vaginal bleeding at 20 weeks' gestation, which required transfusion of blood products. At that time, the diagnosis was complete placenta previa and possible abnormal placentation by ultrasonography. An MRI (Figure 1) revealed invasion of the cervix and lower uterine segment by placental tissue. Images of the bladder demonstrated discontinuity of the posterior bladder wall, suggesting the possibility of placenta percreta with invasion of the lower posterior bladder. The obstetricians planned a cesarean hysterectomy at 36 weeks' gestation. Again, specialists in anesthesiology, urology, interventional radiology, gynecologic oncology, and neonatology were consulted. The blood bank was made aware of the possible need for massive transfusion for this patient, and prospective approval for the use of recombinant factor VII was obtained. After an anesthetic preoperative evaluation case review, anesthesiologists chose to use a neuraxial anesthetic. Contact information for all the physicians involved was kept on the patient's chart, in case of emergency.
Figure 1.
Magnetic resonance image showing the fetus, placenta previa (P), and 2 areas of possible vascular invasion of the posterior bladder wall (arrows).
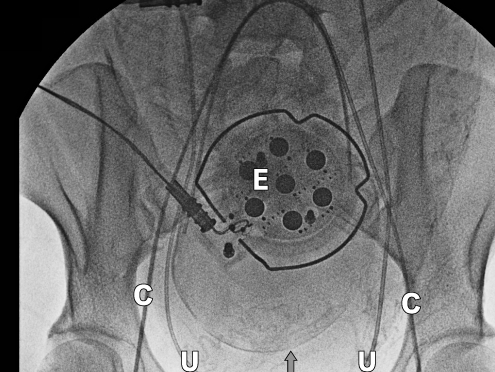
On the day of the procedure, two 16-gauge intravenous lines were placed. Fetal heart tones were monitored, and aspiration prophylaxis and antibiotics were administered. An epidural was placed in the preoperative area, and an epidural test dose was administered. The patient was transferred to the endovascular operative suite, and epidural anesthesia was initiated with 2% lidocaine with epinephrine and bicarbonate to achieve a T4 sensory level. The patient also received small doses of intravenous midazolam for anxiety-related sedation. Bilateral ureteral catheters and bilateral internal iliac artery catheters were placed and visualized by radiography (Figure 2), as in the previous case. Again, care was taken to keep the patient in the supine position with unflexed legs while she was being moved to a larger operating room that contained neonatal resuscitation equipment and a cell salvage system.
Figure 2.
Radiograph showing bilateral internal iliac artery catheters (C), bilateral ureteral catheters (U), and the external fetal monitor (E).
In the larger operating room, a portable C-arm system obtained an image, and the interventional radiologist verified that the iliac artery catheters were still correctly situated. Again, the interventional radiologist remained with the patient until completion of the hysterectomy. Crossed and checked blood products (8 units of packed red blood cells and 4 units of fresh frozen plasma) and a rapid infusion device were immediately available. A radial arterial line and a double-lumen internal jugular central line were placed before incision. Epidural lidocaine was administered as needed to maintain a T4 sensory level, and 100 µg of fentanyl was also given via the epidural catheter. A vigorous infant was delivered via cesarean. The lower uterine segment appeared markedly distended. Increased vascularity was noted between the uterine wall and the bladder wall, but the placenta did not invade through the myometrium. The bladder and uterus were gently dissected, and the areas of increased vascularity were cauterized or suture ligated. Estimated blood loss for the procedure was 700 mL, and neither transfusions nor inflation of the iliac artery balloon catheters was required. The iliac artery catheters and femoral sheaths were removed in the PACU. The patient had an uneventful recovery and was discharged on postoperative day 3.
DISCUSSION
The incidence of placenta accreta has increased steadily during the past several decades—most likely secondary to the rising rate of cesarean deliveries—and currently occurs at a rate of 1∶500 deliveries.1 Placenta previa, especially with a history of cesarean delivery, is a major risk factor for placenta accreta.2 The rate of cesarean delivery in the United States has risen by 53% from 1996 to 2007. As of 2007, one-third of all deliveries in the United States were by cesarean, with Louisiana having the third highest cesarean delivery rate in the country.3 The incidence of abnormal placentation in patients with a placenta previa increases from 3% with no history of cesarean delivery to greater than 60% in patients with more than 2 prior cesarean deliveries.4 As the incidence of cesarean delivery rises, so will cases of abnormal placentation.4 However, it is important to remember that abnormal placentation can occur even in patients without a prior history of cesarean delivery, as illustrated by the second case. Multiparity, advanced maternal age, previous dilation and curettage, hypertensive disorders, and tobacco use are also risk factors for accreta in patients with a placenta previa.5
With a prenatal diagnosis of placenta accreta confirmed or suspected, the implementation of a multidisciplinary team approach is appropriate. Prenatal diagnosis of placenta accreta is associated with a decrease in maternal morbidity.6 When risk factors are present, early diagnosis is most often performed with ultrasonography.7 When ultrasound findings are not definitive, MRI is often used to provide more information.8,9 When placenta accreta is suspected, the following specialties become involved: maternal fetal medicine, gynecologic oncology, anesthesiology, and neonatology. After initial case review, specialists in interventional radiology, urology, and the blood bank may provide additional consultation as necessary.
Anesthesiology
The type of anesthesia provided is based on both maternal and surgical factors. General anesthesia was chosen in the first case because of the patient's airway examination and because the placental invasion through the uterus was thought to be more extensive. The patient's airway examination indicated that intubation would probably be difficult; general anesthesia allowed her airway to be secured in a controlled setting. Neuraxial anesthesia was also avoided because of an increased likelihood of massive hemorrhage and subsequent coagulopathy, based on suspected bladder involvement. To limit the fetal exposure to volatile anesthetics, intravenous sedation was administered for the urology and interventional radiology procedures.
In the second case, the patient's airway examination was more favorable, and the MRI findings indicated less extensive placental invasion than in the first case. The anesthesiologist decided to use neuraxial anesthesia. When balloon catheters are in place, it is extremely important to avoid any hip flexion, as this could dislodge the catheters.6 Therefore, the epidural was placed before the internal iliac artery balloon catheters were inserted, and the urology and interventional radiology procedures were performed after the epidural.
One of the challenges of managing these patients was the involvement of 6 different specialty groups working in 2 different operating rooms. Preoperatively, in both cases, the anesthesiologist played a crucial role in coordinating the different subspecialties. This role involved preparing for possible emergency situations while the patient was in the antepartum unit, choreographing the operating room schedule, and facilitating communication among specialists. The important role of the anesthesiologist in cases of expected hemorrhage has been previously described.10
Maternal Fetal Medicine and Gynecologic Oncology
Patients with placenta accreta usually require hysterectomy. When placenta accreta is diagnosed antenatally, generally the placenta is left attached while hysterectomy is performed.11 Conservative management may be an option in select situations and may allow subsequent pregnancy.12,13 Conservative management was not considered for either of the cases discussed above, partially because fertility preservation was not a concern.
A gynecologic oncologist was part of the operative team in both cases. The potential for massive hemorrhage or urinary tract injury with cesarean hysterectomy usually calls for the presence of 2 obstetricians in the operating room, and the surgical expertise of a gynecologic oncologist may help avoid these complications.
Urology
Urologic consultation before the scheduled procedure has been associated with a decreased incidence of urologic complications.14 Also, preoperative ureteral stent placement has been shown to decrease the incidence of ureteral injury during cesarean hysterectomy for abnormal placentation.15 In cases of placental involvement of the bladder, the urologist can perform bladder reconstruction.
Interventional Radiology
When massive hemorrhage is a concern, the interventional radiologist is consulted to determine whether the patient is an appropriate candidate for the use of internal iliac artery balloon catheters, as they are not without risk.16,17 The internal iliac artery is divided into anterior and posterior divisions. The uterine artery, which is a branch of the anterior division, provides most of the blood supply to the uterus. Ideally, the balloon catheter should be placed in the anterior division to avoid possible ischemic effects of decreased blood flow to the posterior division.
Controversy exists regarding the efficacy of internal iliac artery balloon catheters for cesarean hysterectomy. Tan et al18 have shown a reduction in intraoperative blood loss, whereas others19 have shown no difference in blood loss or transfusion requirements. The use of this technique must be decided on a case-by-case basis. In our experience, the use of internal iliac balloon catheters appeared beneficial in the first case, as there was a marked decrease in blood loss and improved surgical visualization after inflation.
Although cesarean delivery has been described within the interventional radiology suite, this is not possible at our institution because the rooms are not large enough to accommodate all of the equipment necessary for this procedure.20 At our institution, an endovascular operating suite is available within our main surgical area. The permanent radiology equipment in this endovascular suite provides the interventional radiologists with superior imaging compared with portable equipment and results in reduced exposure of the patient and fetus to radiation. Having 2 interventional radiologists working simultaneously on each side of the patient to place bilateral catheters also reduces exposure. After placement of the balloon catheters, we then transfer the patient to an adjacent, larger operating room that easily holds all of the surgical, anesthetic, and neonatal equipment. A portable C-arm is available if there is any question about catheter position before delivery.
Neonatology
The neonatologist is consulted after the patient has been admitted to discuss postdelivery management of the neonate.11 Preterm delivery, the potential for maternal hemorrhage, and the systemic medications administered to the mother are all concerns. The neonatology team will also have to coordinate placement of their equipment in the operating room.
Blood Bank
Early communication with the blood bank team to plan for possible massive transfusion requirements is very important. Based on the patient's presentation and discussion with the blood bank physician, a decision is made regarding the type and quantity of blood products that should be readily available. It is important for the blood bank to be aware of the scheduled day and time of the procedure to prevent delays in obtaining the products required for the case. Factor VII has also been shown to be effective in cases of postpartum hemorrhage, and its use should be discussed with a blood bank physician during preparation for the case.21
CONCLUSION
Patients with placenta accreta are at risk for significant hemorrhage at delivery. The key to a successful outcome in these cases is a multidisciplinary approach, appropriate communication, and early planning. At our institution, we have assembled an obstetric hemorrhage team that consists of a maternal fetal medicine specialist, gynecologic oncologist, anesthesiologist, and neonatologist. After initial case review, an interventional radiologist, urologist, and a blood bank physician are consulted if deemed necessary. Favorable maternal and fetal outcomes have resulted from this team-based approach.
This article meets the Accreditation Council for Graduate Medical Education competencies for Patient Care and Medical Knowledge.
Footnotes
Financial disclosure: None of the authors has a financial or proprietary interest in the subject matter of this article.
REFERENCES
- 1.Wu S., Kocherginsky M., Hibbard J. U. Abnormal placentation: twenty year analysis. Am J Obstet Gynecol. 2005;192((5)):1458–1461. doi: 10.1016/j.ajog.2004.12.074. [DOI] [PubMed] [Google Scholar]
- 2.Hung T. H., Shau W. Y., Hsieh C. C., Chiu T. H., Hsu J. J., Hsieh T. T. Risk factors for placenta accreta. Obstet Gynecol. 1999;93((4)):545–550. doi: 10.1016/s0029-7844(98)00460-8. [DOI] [PubMed] [Google Scholar]
- 3.Menacker F., Hamilton B. E. Recent trends in cesarean delivery in the United States. NCHS Data Brief. 2010;35:1–8. [PubMed] [Google Scholar]
- 4.Silver R. M., Landon M. B., Rouse D. J., et al. Maternal morbidity associated with multiple repeat cesarean deliveries. Obstet Gynecol. 2006;107((6)):1226–1232. doi: 10.1097/01.AOG.0000219750.79480.84. [DOI] [PubMed] [Google Scholar]
- 5.Usta I. M., Hobeika E. M., Musa A. A., Gabriel G. E., Nassar A. H. Placenta previa-accreta: risk factors and complications. Am J Obstet Gynecol. 2005;193((3, pt 2)):1045–1049. doi: 10.1016/j.ajog.2005.06.037. [DOI] [PubMed] [Google Scholar]
- 6.Warshak C. R., Ramos G. A., Eskander R., et al. Effect of predelivery diagnosis in 99 consecutive cases of placenta accreta. Obstet Gynecol. 2010;115((1)):65–69. doi: 10.1097/AOG.0b013e3181c4f12a. [DOI] [PubMed] [Google Scholar]
- 7.Baysinger C. L. Imaging during pregnancy. Anesth Analg. 2010;110((3)):863–867. doi: 10.1213/ANE.0b013e3181ca767e. [DOI] [PubMed] [Google Scholar]
- 8.Warshak C. R., Eskander R., Hull A., et al. Accuracy of ultrasonography and magnetic resonance imaging in the diagnosis of placenta accreta. Obstet Gynecol. 2006;108((3, pt 1)):573–581. doi: 10.1097/01.AOG.0000233155.62906.6d. [DOI] [PubMed] [Google Scholar]
- 9.Dwyer B. K., Belogolovkin V., Tran L., et al. Prenatal diagnosis of placenta accreta: sonography or magnetic resonance imaging? J Ultrasound Med. 2008;27((9)):1275–1281. doi: 10.7863/jum.2008.27.9.1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gallos G., Redai I., Smiley R. M. The role of the anesthesiologist in the management of obstetric hemorrhage. Semin Perinatol. 2009;33((2)):116–123. doi: 10.1053/j.semperi.2008.12.005. [DOI] [PubMed] [Google Scholar]
- 11.Oyelese K. O., Smulian J. C. Placenta previa, placenta accreta, and vasa previa. Obstet Gynecol. 2006;107((4)):927–941. doi: 10.1097/01.AOG.0000207559.15715.98. [DOI] [PubMed] [Google Scholar]
- 12.Timmermans S., van Hof A. C., Duvekot J. J. Conservative management of abnormally invasive placentation. Obstet Gynecol Surv. 2007;62((8)):529–539. doi: 10.1097/01.ogx.0000271133.27011.05. [DOI] [PubMed] [Google Scholar]
- 13.Sentilhes L., Ambroselli C., Kayem G., et al. Maternal outcome after conservative treatment of placenta accreta. Obstet Gynecol. 2010;115((3)):526–534. doi: 10.1097/AOG.0b013e3181d066d4. [DOI] [PubMed] [Google Scholar]
- 14.Ng M. K., Jack G. S., Bolton D. M., Lawrentschuk N. Placenta percreta with urinary tract involvement: the case for multidisciplinary approach. Urology. 2009;74((4)):778–782. doi: 10.1016/j.urology.2009.01.071. [DOI] [PubMed] [Google Scholar]
- 15.Eller A. G., Porter T. F., Soisson P., Silver R. M. Optimal management strategies for placenta accreta. BJOG. 2009;116((5)):648–654. doi: 10.1111/j.1471-0528.2008.02037.x. [DOI] [PubMed] [Google Scholar]
- 16.Greenberg J. I., Suliman A., Iranpour P., Angle N. Prophylactic balloon occlusion of the internal iliac arteries to treat abnormal placentation: a cautionary case. Am J Obstet Gynecol. 2007;197((5)):470.e1–470.e4. doi: 10.1016/j.ajog.2007.05.017. [DOI] [PubMed] [Google Scholar]
- 17.Harnett M. J. P., Carabuena J. M., Tsen L. C., Kodali B. S. Anesthesia for interventional radiology in parturients at risk of major hemorrhage at cesarean section delivery. Anesth Analg. 2006;103((5)):1329–1330. doi: 10.1213/01.ane.0000242324.31955.2b. [DOI] [PubMed] [Google Scholar]
- 18.Tan C. H., Tay K. H., Sheah K., et al. Perioperative endovascular internal iliac artery occlusion balloon placement in management of placenta accreta. AJR Am J Roentgenol. 2007;189((5)):1158–1163. doi: 10.2214/AJR.07.2417. [DOI] [PubMed] [Google Scholar]
- 19.Shrivastava V., Nageotte M., Major C., Haydon M., Wing D. Case-control comparison of cesarean hysterectomy with and without prophylactic placement of intravascular balloon catheters for placenta accreta. Am J Obstet Gynecol. 2007;197((4)):402.e1–402.e5. doi: 10.1016/j.ajog.2007.08.001. [DOI] [PubMed] [Google Scholar]
- 20.O'Rourke N., McElrath T., Baum R., et al. Cesarean delivery in the interventional radiology suite: a novel approach to obstetric hemostasis. Anesth Analg. 2007;104((5)):1193–1194. doi: 10.1213/01.ane.0000260264.89337.9e. [DOI] [PubMed] [Google Scholar]
- 21.Karalapillai D., Popham P. Recombinant factor VIIa in massive postpartum haemorrhage. Int J Obstet Anesth. 2007;16((1)):29–34. doi: 10.1016/j.ijoa.2006.09.001. [DOI] [PubMed] [Google Scholar]