Abstract
Three glycoproteins (ZP1, ZP2, and ZP3) are synthesized in growing mouse oocytes and secreted to form an extracellular zona pellucida that mediates sperm binding and fertilization. Each has a signal peptide to direct it into a secretory pathway, a “zona” domain implicated in matrix polymerization and a transmembrane domain from which the ectodomain must be released. Using confocal microscopy and enhanced green fluorescent protein (EGFP), the intracellular trafficking of ZP3 was observed in growing mouse oocytes. Replacement of the zona domain with EGFP did not prevent secretion of ZP3, suggesting the presence of trafficking signals and a cleavage site in the carboxyl terminus. Analysis of linker-scanning mutations of a ZP3-EGFP fusion protein in transient assays and in transgenic mice identified an eight-amino-acid hydrophobic region required for secretion and incorporation into the zona pellucida. The hydrophobic patch is conserved among mouse zona proteins and lies between a potential proprotein convertase (furin) cleavage site and the transmembrane domain. The cleavage site that releases the ectodomain from the transmembrane domain was defined by mass spectrometry of native zonae pellucidae and lies N-terminal to a proprotein convertase site that is distinct from the hydrophobic patch.
The mammalian zona pellucida is an extracellular matrix that surrounds growing oocytes, ovulated eggs and early embryos. It mediates the relative taxon-specific binding of sperm at the time of fertilization, provides a potent postfertilization block to polyspermy, and is essential for passage of the embryo through the oviduct prior to implantation in the uterus (40). The zona pellucida matrix is first observed as electron-dense amorphous clumps adjacent to the plasma membrane that become contiguous as oocytes enter into a growth phase prior to ovulation (9). Over a 2-week period, oocyte diameters increase from 15 to 80 μm, obliging the existing zona matrix to accommodate an ∼30-fold growth in the surface area while simultaneously increasing its width to 7 μm. These dramatic changes in the supramolecular structure must require considerable plasticity in the constituents of the zona pellucida surrounding the female germ cell.
The mouse zona is composed of three major glycoproteins designated ZP1, ZP2, and ZP3 (25). Although distinct from one another, the three proteins share motifs, including a signal peptide to direct them into a secretory pathway, an ∼260-amino-acid “zona” domain with eight conserved cysteine residues (6) implicated in matrix polymerization (14), and a transmembrane domain near their carboxyl termini. Each zona protein is encoded by a single-copy gene in the mouse genome, and mouse lines have been established in which either Zp1, Zp2, or Zp3 has been inactivated by targeted mutagenesis. Mice lacking ZP1 form a fairly robust zona matrix composed of ZP2 and ZP3 (27), mice lacking ZP2 form a thin matrix composed of ZP1 and ZP3 that does not persist past the early stages of oocyte growth (29), and no visible zona is observed in mice lacking ZP3 (20, 26). These data suggest that “building blocks” of either ZP1/ZP3 or ZP2/ZP3 can participate in the zona matrix and that ZP3 is critical to the formation of each of these unit subassemblies. Although mouse ZP3 has been a candidate for mediating sperm-egg recognition via O glycans, more recent genetic data in which replacement of ZP2 and/or ZP3 by human does not affect taxon-specific fertilization has led to an alternative model in which sperm binding is determined by the supramolecular structure of the zona pellucida matrix and mediated by the cleavage status of ZP2 (28).
These suggested roles, coupled with its relatively small size, have focused attention on ZP3 and the intracellular processing required for its assembly into the extracellular zona pellucida. Although initially the zona pellucida matrix is closely apposed to the plasma membrane of the growing oocyte, a distinct perivitelline space appears between the plasma membrane and the inner aspect of the zona pellucida matrix late in oogenesis. Thus, it appears that the N-terminal ectodomain (minus the signal peptide) of each zona protein must be released from the transmembrane domain located near the carboxyl termini. The immunolocalization of individual and epitope-tagged zona proteins at the periphery of growing oocytes in the absence of a zona pellucida suggests that this cleavage occurs at the surface of the plasma membrane (24, 26, 42).
A potential proprotein convertase (furin) cleavage site upstream of the transmembrane domain is well conserved among mammalian zona proteins and has been implicated in the release of the zona ectodomain (19, 41). Mutation of the site is reported to affect secretion of ZP3 in heterologous somatic cells to greater (39) and lesser (16) extents. However, mutation of the site (RNRR → ANAA) does not prevent the secretion or incorporation of ZP3 reporter proteins into the zona pellucida after nuclear injection of growing oocytes in vitro (24, 42) or in transgenic mouse lines (42). To further investigate the formation of the zona pellucida, linker-scanning mutagenesis has been used to identify regions required for the secretion of ZP3, and its carboxyl terminus has been defined by mass spectrometry (MS) of native zonae pellucidae.
MATERIALS AND METHODS
ZP3-EGFP expression vectors.
pZP31-31aa-EGFP-ZP3323-424aa was derived from pEGFP-C2 (BD Biosciences Clontech, Palo Alto, Calif.), to which the N-terminal signal peptide of mouse ZP3 (i.e., amino acids 1 to 31 [superscript “1-31aa” in the vector designation]) was fused in frame with enhanced green fluorescent protein (EGFP) downstream of a cytomegalovirus (CMV) promoter (42). The PstI/KpnI fragment (from bp 999 to 1317) from pZP3.5 (31) was cloned into the identical restriction enzyme sites in the multicloning site of pEGFP-C2 to complete the expression vector.
pMutA, pMutB, pMutC, and pMutD ZP3-EGFP were constructed by using pSZP3-EGFP (42) as a parental plasmid in which four, 24-bp segments of ZP3 cDNA (bp 1089 to 1184) were sequentially replaced with sequence encoding the FLAG epitope (DYKDDDDK) (42) by using the following primers: PA1 (5′-GACTACAAGGACGACGATGACAAGACTGTAGGGCCCCTGATATTCCTT-3′), PA2 (5′-CTTGTCATCGTCGTCCTTGTAGTCCCTGCGGTTTCGAGAAACTAGCTT-3′), PB1 (5′-GACTACAAGGACGACGATGACAAGGGAAAGGCCAACGACCAGACTGTG-3), PB2 (5′-CTTGTCATCGTCGTCCTTGTAGTCGACATCAGCTTCATCGGTCACGTG-3), PC1 (5′-GACTACAAGGACGACGATGACAAGGAAGGCTGGACTGCTTCTGCTCAA-3′), PC2 (5′-CTTGTCATCGTCGTCCTTGTAGTCAAGGAATATCAGGGGCCCTACAGT-3′), PD1 (5′-GACTACAAGGACGACGATGACAAGACCTCTGTGGCTCTTGGGTTAGGC-3′), PD2 (5′-CTTGTCATCGTCGTCCTTGTAGTCCACAGTCTGGTCGTTGGCCTTTCC-3′), UP (5′-TACATCACCTGCCATCTCCAA-3′), and DN (5′-TAATACGACCTCACTATAGGG-3′). An initial PCR used pZP3.5 as a template, along with the following primer pairs: PA1-DN, PA2-UP, PB1-DN, PB2-UP, PC1-DN, PC2-UP, PD1-DN, and PD2-UP. The PCR products were then gel purified and designated A1, A2, B1, B2, C1, C2, D1, and D2, respectively. Combinations of the above PCR productions—A1-A2, B1-B2, and C1-C2—were reamplified without additional primers or templets. The SapI-EcoRV fragments of the resultant PCR products (bp 1031 to 1317) containing the mutated ZP3 sequences were substituted into the corresponding region of pEGFP-MoZP3 (42) and designated pMutA, pMutB, pMutC, and pMutD, respectively. Taq polymerase was used for amplification, and all PCR reactions were performed for 30 cycles of 95°C for 30 s, 55°C for 30 s, and 72°C for 30 s with a Perkin-Elmer GeneAmp System (Perkin-Elmer, Norwalk, Conn.). The sequence of PCR fragments was confirmed by dideoxy sequencing (35).
Transient expression of normal and mutant ZP3-EGFP.
Transient-transfection assays were performed in 10T[1/2] cells (42) with 1 μg of either normal, MutA, MutB, MutC, or MutD ZP3-EGFP. Cells were either incubated in Dulbecco modified Eagle medium (lacking fetal bovine serum) for 48 h with one refeeding prior to harvest or, 1 day after transfection, the cells were changed to medium containing 0.2 μg of brefeldin A (BFA)/ml and cultured for an additional 12 h. The cells were then washed with phosphate-buffered saline (PBS) and changed to Dulbecco modified Eagle medium with 10% fetal bovine serum medium (lacking BFA). After 30 min in culture, the cells were fixed with 2% paraformaldehyde for 40 min at room temperature, permeabilized, and then stained with rabbit anti-bovine protein disulfide isomerase (PDI) or rabbit anti-α-mannosidase. Cy5-labeled donkey anti-rabbit antibodies (Jackson Immunoresearch Laboratories, West Grove, Pa.) was used for imaging on a 510 LSM confocal microscope (Carl Zeiss, Thornwood, N.Y.) as previously described (42).
Western blot analyses.
Cell supernatants from transient transfections were concentrated by Microcon 10 (Millipore, Bedford, Mass.), and cell pellets were washed twice with PBS and lysed with 50 mM Tris-HCl (pH 7.5)-1% NP-40-10 mM EDTA, containing 1 mM AEBSF [4-(2-aminoethyl)-benzenesulfonyl fluoride], aprotinin (5 μg/ml), leupepstin (1 μg/ml), beneimidine (0.1 mM), and pepstatin (1 μg/ml). Protein samples (pellets or supernatants from 104 cells) were treated or not treated with endoglycosidase H (Endo-H), separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), and assayed by Western blot (7). For Endo-H treatment, samples were denatured at 100°C for 10 min and then incubated with 100 U of enzyme/sample at 37°C for 1 h according to the manufacturer's instructions (New England Biolabs, Beverly, Mass.).
Expression of normal and mutant ZP3-EGFP in microinjected oocytes.
Oocytes, isolated from 11- to 13-day-old mouse ovaries (23), were incubated in M199 medium supplemented with 0.28 mM sodium pyruvate, 25 mM HEPES (pH 7.4), and 2 mg of bovine serum albumin/ml at 37°C prior to microinjection. About 10 pl of solution, containing 50 ng of plasmid DNA/ml, was injected into the nucleus of each oocyte. The injected oocytes were cultured (37°C, 5% CO2) for 24 h in the same supplemented M199 medium. The oocytes were stained with 1-μg/ml lipophilic dye PM-R18 (octadecyl rhodamine B Cl−; Molecular Probes, Eugene, Oreg.) for 20 min and then separated into two groups. One group was fixed with 2% paraformaldehyde for 1 h at room temperature. The other group was transferred into 20 mM Tris-HCl (pH 7.4) containing 1% NP-40 and 0.5 M NaCl and freeze-thawed 10 times on ethanol-dry ice to isolate zona ghosts (36). The fixed oocytes and treated zona ghosts were washed three times with PBS and put on a slide chambered with Gene-Frame (20-μm cavity volume; Advanced Biotechnologies, Leatherhead, United Kingdom). Images were obtained by confocal microscopy using an Argon laser light (488 nm) to visualize EGFP at 515 to 530 nm and an HeNe 543 laser light to detect PM-R18 at 570 nm.
MutA and MutB ZP3-EGFP transgenic mice.
NheI-EcoRV fragments isolated from MutA or MutB ZP3-EGFP were inserted into SpeI-EcoRV sites of a plasmid containing 6 kbp of the mouse Zp3 promoter previously shown to direct oocyte-specific expression in transgenic mice (30). A bovine growth hormone polyadenylation signal was cloned into EcoRV-NotI sites downstream of the ZP3-EGFP sequences, and the 8.1-kbp MutA or MutB Zp3-ZP3-EGFP fragments were purified by agarose electrophoresis after digestion with Meganuclease I-SceI and NotI. After pronuclear injection, founders were identified, and transmission of the transgene in their progeny was monitored by PCR specific to EGFP and Southern analyses (42). Three to five founders were established for each mutant construct. All experiments with mice were conducted under protocols approved by the National Institute of Diabetes and Digestive and Kidney Diseases Animal Care and Use Committee.
MS.
Zonae pellucidae from 500 mice were isolated from an ovarian homogenate by density gradient ultracentrifugation (3), heat solubilized (65°C, 1 h), and lyophilized. Zona proteins (20 μg) were resuspended (250 mM Tris-HCl [pH 8.0]) and reduced (5 mM dithiothreitol) and alkylated (500 mM iodoacetamide) under denaturing conditions (8 M urea). The sample was then diluted and buffer exchanged (50 mM NH4HCO3 [pH 7.8]; Amicon Centriprep), digested with PNGase F, and lyophilized. After resuspension (50 mM NH4HCO3 [pH 6.1]), the sample was digested with Exo-O [sialidase A, β-(1,4)-galactosidase, and glucosaminidase] and Endo-O glycosidases under conditions prescribed by the manufacturer (Prozyme deglycosylation kit GE51). Trypsin, AspN, and trypsin-AspN double digests of deglycosylated samples were analyzed on a Micromass QTOF Ultima Global (Micromass, Manchester, United Kingdom) as described previously (5).
RESULTS
Signal peptide and carboxyl-terminal region direct ZP3 secretion.
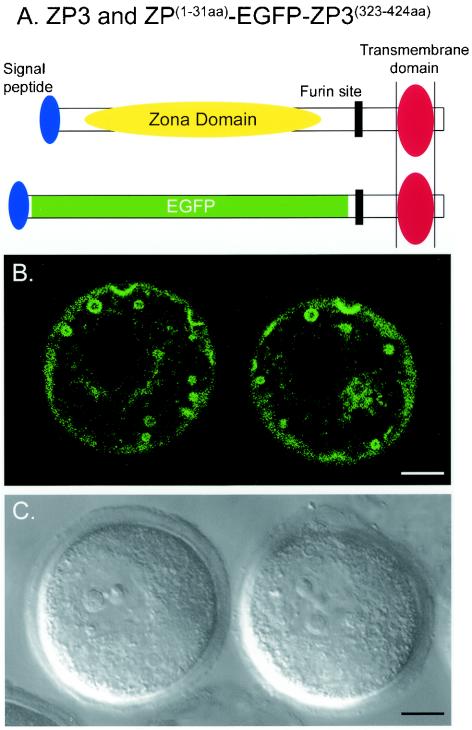
To determine whether the signal peptide and the carboxyl terminus of mouse ZP3 were sufficient to direct secretion, the zona domain was replaced with enhanced green fluorescent protein (EGFP) in a fusion protein, ZP31-31aa-EGFP-ZP3342-424aa. The protein contained the ZP3 signal peptide replete with a cleavage site (amino acids 1 to 31) fused in-frame with EGFP, followed by the 82-amino-acid carboxyl terminus of ZP3 that included the potential proprotein convertase (furin) site, as well as the predicted transmembrane domain (Fig. 1A). Plasmid DNA encoding the fusion protein downstream of a CMV promoter was microinjected into the nucleus of growing oocytes. After 24 to 30 h of culture, the EGFP fusion protein was imaged by confocal microscopy (Fig. 1B and C). In addition to a distribution consistent with localization in the endoplasmic reticulum and Golgi network (perinuclear and peripheral), there were a series of strikingly large circular structures that ranged in size from 0.5 to 3.0 μm in diameter. Each appeared with an empty lumen, suggesting that the ZP3-EGFP remained tethered to one or more lipid bilayers at the periphery of the structure. Thus, the carboxyl terminus appeared to be sufficient to direct intracellular trafficking of ZP3 into subcellular organelles, but the absence of a zona domain precluded incorporation into the extracellular zona pellucida matrix.
FIG. 1.
Intracellular trafficking of ZP3-EGFP reporter protein lacking a zona domain. (A) Expression plasmid ZP31-31aa-EGFP-ZP3342-424aa, in which EGFP replaced the zona domain while the N-terminal signal peptide (amino acids 1 to 31) and the carboxyl terminus (amino acids 342 to 424) of ZP3 were preserved, including the potential proprotein convertase (furin) site. (B) The ZP3-EGFP reporter construct was placed downstream of a CMV promoter within a circular plasmid and injected into the nucleus of growing oocytes. After 20 h of incubation, lacy EGFP signals were observed as a lacy pattern in the perinuclear region, in the periphery, and in large (up to 3 μm in diameter) circular structures. (C) Light microscopic image of (B) by using differential interference optics to visualize the nucleus and extracellular zona pellucida matrix. Bars, 10 μm.
Linker-scanning mutagenesis of the carboxyl terminus of mouse ZP3.
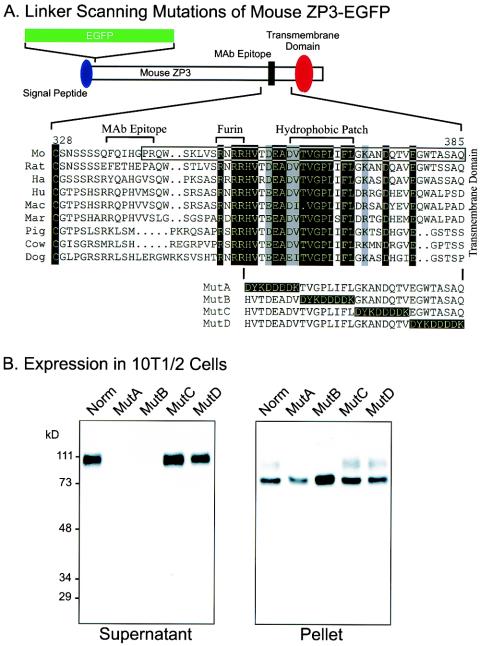
To investigate carboxyl-terminal sequences involved in secretion of ZP3, we used linker-scanning mutagenesis of the region bounded by the potential proprotein convertase cleavage site and the transmembrane domain (i.e., amino acids 353 to 386). Utilizing the ZP3-EGFP construct from an earlier study (42), four regions, each eight amino acids long, C-terminal to the proprotein convertase site were individually mutated to the FLAG epitope (DYKDDDDK) and designated MutA, MutB, MutC, and MutD ZP3-EGFP (Fig. 2A).
FIG. 2.
Linker-scanning mutations of the carboxyl terminus of ZP3. (A) A second ZP3-EGFP expression vector (top) was used to construct four linker-scanning mutations (MutA, MutB, MutC, and MutD) with a FLAG epitope (DYKDDDDK) to sequentially replace 8-amino-acid domains between the potential proprotein convertase (Furin) cleavage site and the transmembrane domain. Nine amino acid sequences of ZP3 (mouse [Mo], rat, hamster [Ha], human [Hu], macaque [Mac], marmoset [Mar], pig, cow, and dog) were aligned from the terminal, conserved cysteine residue of the zona domain to the predicted transmembrane domain. Residues that are identical in all sequences are indicated as white on a black background; those that were conservative substitutions in all sequences are indicated as black on a gray background. The sequence included in the mouse ZP31-31-EGFP-ZP3342-424aa construct (Fig. 1A) was enclosed in a box. The monoclonal antibody binding site on mouse ZP3 (amino acids 336 to 342), the potential proprotein convertase (furin) recognition site (amino acids 350 to 354), and the hydrophobic patch (amino acids 361 to 369) are indicated above the alignment. (B) Western blot of ZP3-EGFP mutants expressed in heterologous somatic cells. At 3 days after transfection with plasmid vectors encoding either normal or MutA, MutB, MutC, or MutD ZP3-EGFP, 10T[1/2] cell supernatants (left) and pellets (right) were harvested. Proteins in each were separated by SDS-PAGE and transferred to nitrocellulose membranes. Blots were probed with a monoclonal antibody specific to mouse ZP3. Molecular mass markers are indicated on the left in kilodaltons.
cDNAs encoding the mutant ZP3-EGFP proteins were cloned into an expression vector driven by a CMV promoter and transiently expressed in mouse embryonic fibroblast 10T[1/2] cells. Culture supernatants and cell pellets of transfected cells were collected and analyzed by SDS-PAGE and immunoblot with a monoclonal antibody specific to mouse ZP3 (Fig. 2B). As previously reported (42), ZP3-EGFP was synthesized, posttranslationally modified, and secreted into the culture medium. After SDS-PAGE, recombinant reporter protein was detected by immunoblot as a broad band with molecular mass of ca. 110 kDa reflecting the combined molecular mass of EGFP (27 kDa) and mouse ZP3 (83 kDa). Similar results were obtained from cells transfected with either MutC or MutD ZP3-EGFP. Thus, the amino acid sequence included in MutC and MutD (GKANDQTVEGWTASAQ) that lies immediately upstream of the transmembrane domain was not required for the synthesis and intracellular processing of mouse ZP3 in 10T[1/2] cells. However, ZP3-EGFP was not detected in the culture supernatants of cells transfected with MutA or MutB ZP3-EGFP expression vectors. These data suggest that the region immediately downstream of the proprotein convertase site (HVTDEADVTVGPLIFL) affected either the synthesis, the intracellular processing, or the secretion of ZP3-EGFP from somatic cells. This region is well conserved among ZP3 proteins (Fig. 2A), although the MutA region (HVTDEAD) is poorly recapitulated in mouse ZP1 and ZP2 compared to the MutB region (VTVGPLIFL) (Fig. 7).
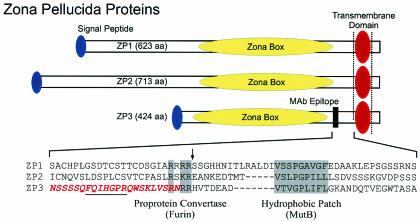
FIG. 7.
Zona pellucida proteins. Mouse ZP1 (623 amino acids), ZP2 (713 amino acids), and ZP3 (424 amino acids) each has a signal peptide (blue oval) to direct it into a secretory pathway, an ∼260-amino-acid zona domain that contains eight conserved cysteine residues (yellow oval) and a transmembrane domain (red oval) near the carboxyl termini followed by a short cytoplasmic tail. The amino acid sequences of the three mouse zona proteins are aligned between a ZP3-specific monoclonal antibody binding site (darkened box, amino acids 336 to 342) and the ZP3 transmembrane domain (amino acids 387 to 410). Each zona protein has a conserved cleavage site (arrow) for proprotein convertase/furin (R-X-R/K-R) and a hydrophobic patch that are 36 to 53 and 25 to 40 amino acids, respectively, N terminal to the transmembrane domain. The carboxyl terminus of ZP3 defined by MS is shown in red letters. The binding site of the monoclonal antibody that recognizes ZP3 in the extracellular zona pellucida is underlined.
The cell pellets of these transiently transfected cells also were analyzed by SDS-PAGE and immunoblot with a monoclonal antibody specific to mouse ZP3. Each of the five NP-40 extracted pellets had a readily visible band, indicating that all five ZP3-EGFP constructs expressed proteins in 10T[1/2] cells, even though MutA and MutB ZP3-EGFP proteins were not detected in the supernatant (Fig. 2B). The major band of each pellet had a molecular mass of 75 kDa that was shifted to 70 kDa after treatment with Endo-H (data not shown) as previously demonstrated with normal ZP3-EGFP (42). These data indicated the removal of multiple N-linked high-mannose oligosaccharides and were consistent with N glycosylation of normal and all four mutant ZP3-EGFP in the endoplasmic reticulum. The minor band (∼100 kDa) present in the pellets obtained from cells transfected with normal, MutC, and MutD ZP3-EGFP could reflect further posttranslational modifications. Similar results were obtained when analyzing supernatants and pellets with antibodies to EGFP, and neither supernatants nor pellets of untransfected 10T[1/2] cells reacted with the monoclonal antibody specific to ZP3 (data not shown).
Examination of the intracellular trafficking of ZP3-EGFP.
To further define the intracellular progression of normal and mutated protein in 10T[1/2] cells, ZP3-EGFP was colocalized with antibodies specific to subcellular organelles. These studies were performed with BFA to initially block translocation of proteins from the endoplasmic reticulum to the Golgi apparatus. When BFA was present in the culture medium, ZP3-EGFP protein was retained in the endoplasmic reticulum. Within 30 min of BFA removal from the culture medium, normal ZP3-EGFP was detected in the Golgi apparatus in a perinuclear location (data not shown). Cells transfected with normal, MutA, MutB, MutC, or MutD ZP3-EGFP expression plasmids were treated and released from BFA prior to fixation and staining either with antibodies to PDI, a marker of the endoplasmic reticulum or with antibodies to α-ManII (α-mannosidase II), a marker of the Golgi network.
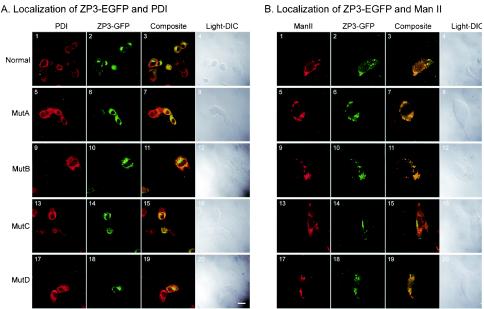
The PDI signal is normally distributed equally throughout the endoplasmic reticulum and excluded from the nucleus. In cells transfected with normal and mutant ZP3-EGFP, the localization of ZP3-EGFP and PDI was determined by confocal microscopy (Fig. 3A). For the most part, ZP3-EGFP (green) was concentrated and close to the nuclear membrane in a typical Golgi structure staining pattern, whereas PDI (red) was more ubiquitously present within the cell. The overlap (yellow) of the two signals most likely reflected the presence of newly synthesized ZP3-EGFP that had yet to leave the endoplasmic reticulum and was not consistently observed in cells transfected with a particular mutation. The subcellular localization of ZP3-EGFP in the Golgi was confirmed with antibodies to α-Man II (Fig. 3B) that resulted in coincident signals (yellow) of the antibody (red) and EGFP (green). All four ZP3-EGFP mutants showed, to various degrees, overlapping staining pattern, indicating that MutA, MutB, MutC, and MutD ZP3-EGFP successfully translocated from the endoplasmic reticulum to the Golgi network in 10T[1/2] cells.
FIG. 3.
Confocal microscopy of ZP3-EGFP mutants expressed in heterologous somatic cells. Cells transfected with plasmid expression vectors were incubated with BFA overnight, allowed to recover for 30 min, and fixed for imaging. Cells expressing normal (A1 to 4 and B1 to 4), MutA (A5 to 8 and B5 to 8), MutB (A9 to 12 and B9 to 12), MutC (A13 to 16 and B13 to 16), or MutD (A17 to 20 and B17 to 20) ZP3-EGFP were incubated with antibodies to PDI (A) or α-ManII (B) and imaged to detect antibody binding with a Cy5-conjugated secondary antibody (A1, 5, 9, 13, and 17 and B1, 5, 9, 13, and 17), ZP3-EGFP (A2, 6, 10, 14, and 18 and B2, 6, 10, 14, and 18) or both (A3, 7, 11, 15, and 19 and B3, 7, 11, 15, and 19). Differential interference optic images were obtained for each transfected cell line (A4, 8, 12, 16, and 20 and B4, 8, 12, 16, and 20). ManII, α-ManII, DIC, differential interference optics. Scale bar, 2 μm.
Expression and processing of ZP3-EGFP proteins in growing oocytes.
Intracellular processing of ZP3-EGFP proteins was further studied in growing mouse oocytes at a developmental time when endogenous zona proteins are synthesized, secreted, and incorporated into the zona matrix. Groups of 10 to 15 oocytes were nuclear injected with plasmids encoding normal, MutA, MutB, MutC or MutD ZP3-EGFP and cultured for 40 h. Oocytes were stained briefly with PM-R18 to detect the plasma membrane and imaged by confocal microscopy (Fig. 4A). The presence of ZP3-EGFP in >80% of injected oocytes indicated that each of the five plasmid constructs was transcribed and translated into protein. The localization of the EGFP signal in a lacy, nonnuclear pattern is consistent with expression and correct folding of the ZP3-EGFP proteins in the endoplasmic reticulum. Depending on the focal plane, large (0.5 to 3.0 μm in diameter) circular structures with ZP3-EGFP at their periphery were detected in oocytes injected with normal and mutant ZP3-EGFP constructs (Fig. 4A).
FIG. 4.
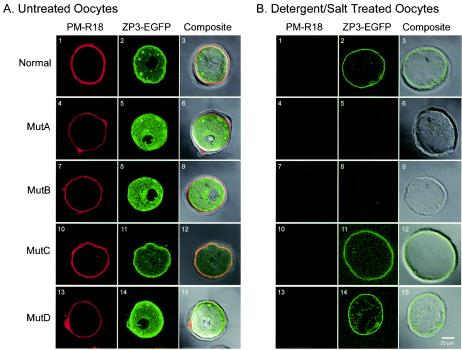
Confocal microscopy of mutant ZP3-EGFP expressed in growing oocytes. Plasmid vectors expressing normal, MutA, MutB, MutC, or MutD ZP3-EGFP were injected into the nucleus of growing oocytes and cultured for 40 h. Oocytes were incubated with a lipid membrane stain (PM-R18) before (A1, 4, 7, 10, and 13) or after (B1, 4, 7, 10, and 13) freeze-thawing in the presence of 0.5 M NaCl and 1% NP-40. PM-18 (A1, 4, 7, 10, and 13 and B1, 4, 7, 10, and 13) and ZP3-EGFP (A2, 5, 8, 11, and 14 and B2, 5, 8, 11, and 14) were viewed by individually and as a composite (A3, 6, 9, 12, and 16 and B3, 6, 9, 12, and 15) after superimposition on a light microscopic image. Scale bar, 20 μm.
Because the zona pellucida matrix is closely apposed to the plasma membrane during oocyte growth, it was unclear whether the peripheral localization in the composite images reflected persistence of ZP3-EGFP in the plasma membrane or incorporation into the zona pellucida matrix (Fig. 4A3, 12, and 15). Therefore, a second group of injected oocytes was treated with nonionic detergent and high salt (1% NP-40, 0.5 M NaCl), a previously described procedure to remove membranes and cytoplasmic contents (36, 42). The absence of staining with PM-R18 ensured that the zona “ghosts” were not contaminated with membrane fragments (Fig. 4B1, 4, 7, 10, and 13). The persistence of the EGFP signal at the periphery (Fig. 4B2, 11, and 14) was consistent with incorporation of the normal, MutC and MutD ZP3-EGFP into the inner aspect of the zona pellucida (Fig. 4B3, 12, and 15).
Oocytes injected with MutA or MutB ZP3-EGFP displayed increased fluorescence near the nucleus, suggesting a higher concentration of ZP3-EGFP in the Golgi apparatus compared to oocytes injected with normal ZP3-EGFP (Fig. 4A5 and 8). The absence of MutA and MutB ZP3-EGFP at the periphery of injected oocytes (Fig. 4A5 and 8) further suggested disruption of post-Golgi intracellular trafficking. The inability of MutA or MutB ZP3-EGFP to be secreted and incorporated into the zona pellucida was confirmed by the absence of an EGFP signal after removal of the plasma membrane (Fig. 4B4 to 9). Thus, although synthesized and apparently transported to the Golgi apparatus, MutA and MutB ZP3-EGFP were not detected at the plasma membrane and were not incorporated into the zona pellucida in these transient assays.
ZP3-EGFP incorporation into the zona pellucida of transgenic mice.
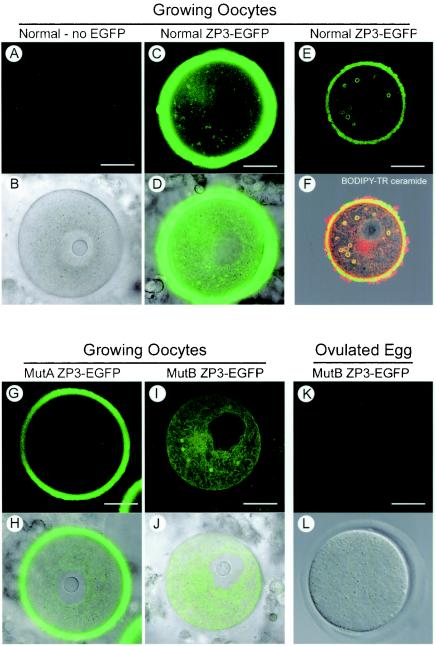
To confirm in vivo the results obtained in transient assays in somatic 10T[1/2] cells and growing oocytes, transgenic mouse lines were established that express normal, MutA or MutB ZP3-EGFP by using a 6.5-kb oocyte-specific promoter (30). Three to five transgenic lines were founded for each construct, and the expression and localization of the MutA and MutB ZP3-EGFP proteins were examined by confocal microscopy of growing oocytes and ovulated eggs. As previously reported (42), ZP3-EGFP encoded by the normal transgene was present in the zona pellucida (Fig. 5C and D). The MutA ZP3-EGFP protein was also processed and incorporated into the zona matrix of growing oocytes and ovulated eggs of transgenic mice (Fig. 5G and H). The signal was less robust than that observed with normal ZP3-EGFP, which could reflect positional effects of the integration site on expression of the transgene or impaired intracellular trafficking of MutA. As noted above, MutA was not detected in the supernatantof somatic 10T[1/2] cells (Fig. 2B) nor incorporated into the zona pellucida in transient assays (Fig. 4). It may be that the acidic residues (DDDD) in the linker-scanning mutation (DYKDDDDK) were sufficiently similar to those of the endogenous ZP3 sequence (DEAD) such that MutA ZP3-EGFP underwent processing and secretion in vivo that was not observed in short-term in vitro culture.
FIG. 5.
Confocal microscopic imaging of ZP3-EGFP mutants expressed in transgenic mice. Mouse lines with transgenes expressing either normal, MutA, or MutB ZP3-EGFP were established and compared to normal, nontransgenic mice. Almost fully grown oocytes or eggs were isolated and imaged by confocal microscopy to detect EGFP alone (A, C, G, I, and K) or superimposed on images obtained by differential interference contrast optics (B, D, H, J, and L). No background signal was detected in the cytoplasm or zona pellucida of normal mice (A and B), and the strongest signal was observed in fully grown oocytes from normal ZP3-EGFP transgenic mice (C and D). In smaller growing oocytes, normal ZP3-EGFP also was observed in large circular structures (E) that costained with BODIPY-TR ceramide (F). (G and H) A diminished, although significant signal, was observed in MutA ZP3-EGFP mice. (I and J) No EGFP was observed in the zona pellucida of MutB ZP3-EGFP mice, but reporter protein was present in the cytoplasm and incorporated in the circular structures. (K and L) EGFP was not observed in the cytoplasm or in the zona pellucida of ovulated eggs isolated from MutB ZP3-EGFP transgenic mice. Scale bar, 20 μm.
In contrast, but concordant with the transient assays, MutB ZP3-EGFP was not incorporated into the extracellular zona pellucida in five independently derived transgenic mouse lines (Fig. 5I and J). Although MutB ZP3-EGFP was synthesized in growing oocytes isolated from 10-day-old transgenic mice, this cytoplasmic localization did not persist in ovulated eggs (Fig. 5K and L). The absence of ZP3-EGFP in the cytoplasm of ovulated eggs was consistent with the observation that zona proteins are not synthesized after ovulation and suggested that MutB ZP3-EGFP protein made earlier in oogenesis was degraded. The large circular structures observed in transient assays were also present in growing oocytes isolated from transgenic mice expressing normal ZP3-EGFP (Fig. 5E). The peripheral localization of the EGFP signal in the circular structures and costaining with BODIPY Texas red ceramide suggested that ZP3 remained associated with a delimiting membrane(s), reflecting either its C-terminal transmembrane domain or interactions with independent membrane-associated protein(s). Similar circular structures were observed in growing oocytes obtained from MutB ZP3-EGFP transgenic mice, but there was only minimal localization of the reporter protein at the plasma membrane (as observed in transient assays) and no evidence of incorporation into the extracellular zona pellucida (Fig. 5I). Thus, we conclude that the region TVGPLIFL (amino acids 363 to 370) plays a role in intracellular trafficking and incorporation of mouse ZP3 into the zona pellucida matrix.
Cleavage of ZP3 occurs N-terminal to a conserved dibasic site.
The absence of MutB ZP3-EGFP incorporation into the zona pellucida of transgenic mice suggested that the mutation resulted either in improper trafficking to the cell surface or failed release of the ectodomain from the transmembrane domain. To ascertain whether the cleavage site of the ZP3 ectodomain lay within the MutB sequence, the carboxyl terminus of mature mouse ZP3 was determined. Initially, native mouse zonae pellucidae were isolated, digested with trypsin, and analyzed by liquid chromatography-quadrupole time of flight (LC-QTOF) MS. Although no tryptic peptides were identified beyond Arg350, the carboxyl-terminal peptide LVSR (amino acids 347 to 350) was uninformative because it was bounded by tryptic digestion sites.
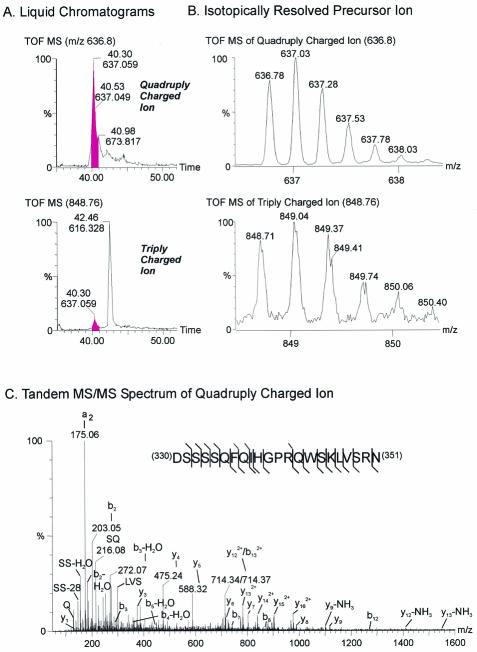
Therefore, native zonae pellucidae were digested with a mixture of Exo-O glycanases, Endo-O glycanase, and PNGase F to remove sugar residues. Asn330 is N glycosylated (5), and PNGase F treatment releases the N-linked glycan chain. The mechanism of hydrolysis involves cleavage of the amide bond between the N-linked sugar and the side chain of asparagine, converting it into aspartic acid. This results in a mass increase of 0.9840 Da (monoisotopic, 0.9847 Da average) and generates an additional cleavage site for AspN protease that enabled the isolation of the carboxyl-terminal peptide 330DSSSSQFQIHGPRQWSKLVSRN351 (Asn converted to Asp at position 330). The parent ion MH+ of 2544.25 Da with a mass deviation of −0.02 Da corresponding to this peptide was corroborated by the presence of both triple and quadruply charged ions at m/z 848.746 and 636.802 (Fig. 6A and B). Furthermore, the “b series” ions, including b2, b2-H2O, b3, b3-H2O, b4-H2O, b5-H2O, b7, b8, b12, and b132+, as well as y1, y3, y4, y5, y6, y7, y8, y9, y12-NH3, y122+, y13-NH3, y132+, y142+, y152+, and y162+ in the spectrum of the quadruply charged ion (as determined by tandem MS/MS) confirmed the sequence identity of this peptide (Fig. 6C). This ion was obtained from two separate samples either with or without reduction and alkylation of disulfide bonds. These results are consistent with the C terminus of mature, secreted ZP3 at Asn351, two amino acids N-terminal to the conserved proprotein convertase site and well upstream of the MutB site (Fig. 7). Thus, rather than a cleavage site to release the ectodomain, the importance of the hydrophobic patch more likely lies in facilitating the proper progression of ZP3 to the cell surface.
FIG. 6.
MS defines C terminus of ZP3. (A) Microscale electrospray QTOF-MS analysis of an AspN digest of deglycosylated zona pellucida proteins detected quadruply (upper) and triply (lower) charged ions of 330DSSSSQFQIHGPRQWSKLVSRN351 at 40.3 min by reversed-phase liquid chromatography. (B) The triply and quadruply charged ions of the carboxyl terminus peptide at m/z 848.71 and 636.78. (C) The identity of the quadruply charged ion of 330DSSSSQFQIHGPRQWSKLVSRN351 was confirmed by MS/MS.
DISCUSSION
The intracellular trafficking of ZP3, the smallest of three major sulfated glycoproteins in the extracellular zona pellucida, has been monitored by determining the expression of ZP3-EGFP fusion proteins in transient assays and transgenic mice. Taken together, these data indicate that, directed by its signal peptide, ZP3 enters into the oocyte's secretory pathway to transit the endoplasmic reticulum and the Golgi apparatus, where it is posttranslationally glycosylated. Despite the presence of a potential proprotein convertase (furin) cleavage site and the ubiquitous presence of the endoprotease within the trans-Golgi network (37), ZP3 remains associated with the its carboxyl-terminal transmembrane domain as it migrates to the cell surface (24, 26, 42). There, its ectodomain is released from a transmembrane domain by a yet-to-be-identified sheddase/endoprotease prior to integration into the extracellular zona pellucida matrix. It seems likely that the zona proteins contain signals that facilitate their interactions with one another and direct their trafficking through the growing oocytes.
Intracellular trafficking.
Shortly after birth, primordial follicles form within the ovary in which each oocyte is surrounded by a single layer of flattened granulosa cell and encased in a basal lamina. These primordial follicles represent the entire complement of germ cells available to the female during her reproductive life. Cyclically, throughout adult life, cohorts of follicles are induced to enter into a growth phase that culminates in meiotic maturation and ovulation of an egg into the oviduct. Expression of the oocyte-specific zona genes (Zp1, Zp2, and Zp3) is first detected perinatally, early in oogenesis, but the zona pellucida structure is not observed until recruitment into the follicular growth phase (25).
The primary structure (424 amino acids, 46,307 Da) of mouse ZP3 has been deduced from full-length cDNA and is well conserved among mammals (25). During its synthesis and prior to incorporation into the extracellular zona pellucida, ZP3 undergoes extensive modification. A cleavage site for a signal peptide predicted after amino acid 22 (38) and the suggestion that the resultant N-terminal glutamine is cyclized to pyroglutamate (31) have been confirmed recently by microscale MS (5). Also predicted is a 19-amino-acid transmembrane domain (17) immediately adjacent to a carboxyl-terminal, hydrophilic, 14-amino-acid cytoplasmic tail. During its intracellular trafficking, ZP3 is posttranslationally modified by the attachment of glycans such that the mature protein isolated from the extracellular zona pellucida has a molecular mass of ∼83 kDa (2, 36).
A notable observation in the current study is the presence of ZP3 in unusually large (0.5- to 3.0-μm) circular structures that stain with BODIPY-TR ceramide. The peripheral localization ZP3-EGFP in the large circular structures suggests continued attachment to a lipid membrane and the copresence of synaptobrevin (VAMP) (24) suggest that they arise in the post-Golgi compartment. However, if circular structures arise earlier in the secretory pathway, they might reflect the concentric arrays of the endoplasmic reticulum observed by electron microscopy in growing rat oocytes (15). Although a linear progression from the endoplasmic reticulum to the Golgi to the plasma membrane is envisioned for constitutively secreted proteins, a more integrated approach in which a “specialized” endoplasmic reticulum may play a role in the sorting and exit of proteins from the trans-Golgi has been hypothesized (19). If unique to growing oocytes, these structures may meet the need to deliver large packets of subassembled zona proteins for release into the extracellular milieu, as suggested by the presence of clumps of electron-dense amorphous material detected by electron microscopy prior to the formation of a continuous zona pellucida matrix (8, 12).
Two mutations, MutA and MutB ZP3-EGFP, which modify amino acid sequences immediately C terminal to the potential furin cleavage site, affect secretion of ZP3 in somatic cells and prevent incorporation of ZP3 into the zona pellucida of oocytes grown in short-term culture. The presence, albeit at decreased levels, of MutA ZP3-EGFP in the extracellular zona pellucida of transgenic mice suggests that with time at least some MutA ZP3-EGFP can traffic through the oocyte and participate in the extracelluar zona pellucida. In contrast, although MutB ZP3-EGFP present in the Golgi apparatus, it does not progress to the plasma membrane and is not incorporated into the zona pellucida.
The hydrophobic patch (VTVGPLIFL) modified in MutB ZP3-EGFP is well conserved among mammals, as are similarly positioned hydrophobic motifs in mouse ZP1 (VSSPGAVGF) and ZP2 (VSLPGPILL) (Fig. 7). However, the role of these short hydrophobic domains remains to be determined. They could serve as binding sites for chaperone proteins important for intracellular trafficking of individual zona proteins. Alternatively, they could serve as a nidus for the stoichiometric subassembly of the three zona proteins as homo- or heteromeric complexes which, if a prerequisite for trafficking to the cell surface, would account for the phenotype observed with MutB ZP3-EGFP. It remains unclear how the directing signal would be transmitted to the cytoplasmic surface of a transport vesicle. Each of the three mouse zona proteins have short (8 to 14 amino acids) carboxyl-terminal tails predicted to extend into the cytoplasm which could be involved in the binding of adaptor or organizing macromolecules critical for transport to the cell surface. These tails are notably hydrophilic due primarily to basic amino acids residues but do not have other obvious defining characteristics. Interestingly, ZP3 lacking its transmembrane domain and cytoplasmic tail (amino acids 380 to 424) is secreted from somatic cells but not incorporated into the zona pellucida matrix in transient assays (14).
Release from transmembrane domain.
Fully grown oocytes form a perivitelline space between the zona pellucida and the plasma membrane of the oocyte. The primary structure of all zona pellucida proteins predicts a transmembrane domain near the carboxyl terminus from which the N-terminal ectodomain must be released to participate in the zona matrix (25). A monoclonal antibody that binds to ZP3 recognizes the extracellular zona pellucida matrix surrounding growing oocytes (11). Thus, we have reasoned that cleavage of the ZP3 ectodomain must occur between the antibody-binding site (amino acids 336 to 342) (22) and the transmembrane domain (amino acids 387 to 410). Although a potential furin cleavage site (amino acids 350 to 353) is ideally positioned between these two limits, the integrity of the site is not required for secretion of ZP3 in tissue culture or its incorporation into the extracellular zona pellucida of transgenic mice (16, 24, 42).
Analysis of the linker-scanning mutations (MutA, MutB, MutC, and MutD) of mouse ZP3 does not suggest that they provide potential cleavage sites for release of the ectodomain. Either the mutation does not preclude incorporation into the zona pellucida in transient assays (MutC and MutD) and transgenic mice (MutA) or it prevents progression of ZP3 to the cell surface (MutB), where cleavage is thought to occur. The further observation that digestion of mouse zonae pellucidae with commercially available furin does not alter the mobility of ZP3 on Western blots (data not shown) suggests that the cleavage site is in close proximity to the furin site. Because of the paucity of biological material, we utilized MS to determine the carboxyl terminus of ZP3.
After deglycosylation with PNGase F, Asn330 of mouse ZP3 is converted to aspartic acid, allowing its cleavage by AspN endoprotease. The carboxyl-terminal triple and quadruply charged peptide, 330DSSSSQFQIHGPRQWSKLVSRN351, was detected by LC-QTOF MS, and its identity was confirmed by MS/MS. Thus, the carboxyl terminus of native mouse ZP3, Asn351, lies two amino acids N-terminal to the furin site (amino acids 350 to 353), immediately upstream of two basic residues. The furin cleavage site (RNR/KR↓) is imperfectly conserved among mammalian zona proteins (e.g., it is not present in cat or human ZPB), but all mammalian zona proteins contain a dibasic motif in the same region. This conservation is striking; a similar cleavage site is observed in mouse ZP2 and ZP3 (5), and the dibasic motif may serve as the primary cleavage site for a yet-to-be-identified cell surface endoprotease(s). Alternatively, C termini may result from cleavage by a proprotein converase, followed by carboxylpeptidase trimming of two amino acids, as has been suggested for quail and Xenopus homologues of ZP3 (18, 32). The observation that secretion of human ZP3 with a mutant furin site that affects the dibasic motif (RNRR → ANAA) from somatic 293T cells (16) and the incorporation of mouse ZP3 with an identical (42) or a different mutation (RNRR → RNGE) (24) into the zona pellucida suggests that alternative cleavage site(s) are available.
The additional mechanisms by which the ZP3 ectodomain might be shed from its transmembrane domain at the cell surface is unclear. The role of sheddases in releasing ectodomains from membrane-spanning domains has been documented in a variety of proteins (1, 4, 13). The signals that regulate the cleavage site in the juxtamembrane region need not be sequence specific, vary among substrates, and have more than one sheddase that can release the ectodomain even in the same cell type (33, 34). The sorting of proteins to enter into a regulated secretory pathway occurs in the trans-Golgi (10, 21), and we suggest that zona proteins progress to the plasma membrane via a sheddase regulated pathway, either separately or in conjunction with other zona proteins. Exposure to the extracellular environment may trigger cleavage of the ectodomains of zona proteins by a membrane-anchored endoprotease(s) prior to incorporation into the extracellular zona pellucida.
Acknowledgments
We appreciate the critical reading of the manuscript by Julie Donaldson and the useful discussions with members of our laboratory.
REFERENCES
- 1.Arribas, J., and A. Borroto. 2002. Protein ectodomain shedding. Chem. Rev. 102:4627-4638. [DOI] [PubMed] [Google Scholar]
- 2.Bleil, J. D., and P. M. Wassarman. 1980. Structure and function of the zona pellucida: identification and characterization of the proteins of the mouse oocyte's zona pellucida. Dev. Biol. 76:185-202. [DOI] [PubMed] [Google Scholar]
- 3.Bleil, J. D., and P. M. Wassarman. 1988. Galactose at the nonreducing terminus of O-linked oligosaccharides of mouse egg zona pellucida glycoprotein ZP3 is essential for the glycoprotein's sperm receptor activity. Proc. Natl. Acad. Sci. USA 85:6778-6782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blobel, C. P. 2000. Remarkable roles of proteolysis on and beyond the cell surface. Curr. Opin. Cell Biol. 12:606-612. [DOI] [PubMed] [Google Scholar]
- 5.Boja, E., T. Hoodbhoy, H. Fales, and J. Dean. 2003. Structural characterization of native mouse zona pellucida proteins using mass spectrometry. J. Biol. Chem. 278:34189-34202. [DOI] [PubMed] [Google Scholar]
- 6.Bork, P., and C. Sander. 1992. A large domain common to sperm receptors (Zp2 and Zp3) and TGF-β type III receptor. FEBS Lett. 300:237-240. [DOI] [PubMed] [Google Scholar]
- 7.Burnette, W. N. 1981. “Western blotting”: electrophoretic transfer of proteins from sodium dodecyl sulfate-polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal. Biochem. 112:195-203. [DOI] [PubMed] [Google Scholar]
- 8.Chiquoine, A. D. 1960. The development of the zona pellucida of the mammalian ovum. Am. J. Anat. 106:149-169. [DOI] [PubMed] [Google Scholar]
- 9.Dietl, J. 1989. Ultrastructural aspects of the developing mammalian zona pellucida, p. 49-60. In J. Dietl (ed.), The mammalian egg coat. Springer-Verlag, Berlin, Germany.
- 10.Donaldson, J. G., and J. Lippincott-Schwartz. 2000. Sorting and signaling at the Golgi complex. Cell 101:693-696. [DOI] [PubMed] [Google Scholar]
- 11.East, I. J., B. J. Gulyas, and J. Dean. 1985. Monoclonal antibodies to the murine zona pellucida protein with sperm receptor activity: effects on fertilization and early development. Dev. Biol. 109:268-273. [DOI] [PubMed] [Google Scholar]
- 12.Hadek, R. 1965. The structure of the mammalian egg. Int. Rev. Cytol. 18:29-68. [DOI] [PubMed] [Google Scholar]
- 13.Hospital, V., V. Chesneau, A. Balogh, C. Joulie, N. G. Seidah, P. Cohen, and A. Prat. 2000. N-arginine dibasic convertase (nardilysin) isoforms are soluble dibasic-specific metalloendopeptidases that localize in the cytoplasm and at the cell surface. Biochem. J. 349:587-597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jovine, L., H. Qi, Z. Williams, E. Litscher, and P. M. Wassarman. 2002. The ZP domain is a conserved module for polymerization of extracellular proteins. Nat. Cell Biol. 4:457-461. [DOI] [PubMed] [Google Scholar]
- 15.Kang, Y.-H. 1974. Development of the zona pellucida in the rat oocyte. Am. J. Anat. 139:535-566. [DOI] [PubMed] [Google Scholar]
- 16.Kiefer, S. M., and P. Saling. 2002. Proteolytic processing of human zona pellucida proteins. Biol. Reprod. 66:407-414. [DOI] [PubMed] [Google Scholar]
- 17.Klein, P., M. Kanehisa, and C. Delisi. 1985. The detection and classification of membrane-spanning proteins. Biochim. Biophys. Acta 815:468-476. [DOI] [PubMed] [Google Scholar]
- 18.Kubo, H., M. Matsushita, M. Kotani, H. Kawasaki, T. C. Saido, S. Kawashima, C. Katagiri, and A. Suzuki. 1999. Molecular basis for oviductin-mediated processing from gp43 to gp41, the predominant glycoproteins of Xenopus egg envelopes. Dev. Genet. 25:123-129. [DOI] [PubMed] [Google Scholar]
- 19.Litscher, E. S., H. Qi, and P. M. Wassarman. 1999. Mouse zona pellucida glycoproteins mZP2 and mZP3 undergo carboxy-terminal proteolytic processing in growing oocytes. Biochemistry 38:12280-12287. [DOI] [PubMed] [Google Scholar]
- 20.Liu, C., E. S. Litscher, S. Mortillo, Y. Sakai, R. A. Kinloch, C. L. Stewart, and P. M. Wassarman. 1996. Targeted disruption of the mZP3 gene results in production of eggs lacking a zona pellucida and infertility in female mice. Proc. Natl. Acad. Sci. USA 93:5431-5436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marsh, B. J., and K. E. Howell. 2002. The mammalian Golgi-complex debates. Nat. Rev. Mol. Cell. Biol. 3:789-795. [DOI] [PubMed] [Google Scholar]
- 22.Millar, S. E., S. M. Chamow, A. W. Baur, C. Oliver, F. Robey, and J. Dean. 1989. Vaccination with a synthetic zona pellucida peptide produces long-term contraception in female mice. Science 246:935-938. [DOI] [PubMed] [Google Scholar]
- 23.Millar, S. E., E. Lader, L.-F. Liang, and J. Dean. 1991. Oocyte-specific factors bind a conserved upstream sequence required for mouse zona pellucida promoter activity. Mol. Cell. Biol. 12:6197-6204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Qi, H., Z. Williams, and P. M. Wassarman. 2002. Secretion and assembly of zona pellucida glycoproteins by growing mouse oocytes microinjected with epitope-tagged cDNAs for mZP2 and mZP3. Mol. Biol. Cell 13:530-541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rankin, T., and J. Dean. 2000. The zona pellucida: using molecular genetics to study the mammalian egg coat. Rev. Reprod. 5:114-121. [DOI] [PubMed] [Google Scholar]
- 26.Rankin, T., M. Familari, E. Lee, A. M. Ginsberg, N. Dwyer, J. Blanchette-Mackie, J. Drago, H. Westphal, and J. Dean. 1996. Mice homozygous for an insertional mutation in the Zp3 gene lack a zona pellucida and are infertile. Development 122:2903-2910. [DOI] [PubMed] [Google Scholar]
- 27.Rankin, T., P. Talbot, E. Lee, and J. Dean. 1999. Abnormal zonae pellucidae in mice lacking ZP1 result in early embryonic loss. Development 126:3847-3855. [DOI] [PubMed] [Google Scholar]
- 28.Rankin, T. L., J. S. Coleman, O. Epifano, T. Hoodbhoy, S. G. Turner, P. E. Castle, E. Lee, R. Gore-Langton, and J. Dean. 2003. Fertility and taxon-specific sperm binding persist after replacement of mouse “sperm receptors” with human homologues. Dev. Cell 5:33-43. [DOI] [PubMed] [Google Scholar]
- 29.Rankin, T. L., M. O'Brien, E. Lee, K. E. J. J. Wigglesworth, and J. Dean. 2001. Defective zonae pellucidae in Zp2 null mice disrupt folliculogenesis, fertility and development. Development 128:1119-1126. [DOI] [PubMed] [Google Scholar]
- 30.Rankin, T. L., Z.-B. Tong, P. E. Castle, E. Lee, R. Gore-Langton, L. M. Nelson, and J. Dean. 1998. Human ZP3 restores fertility in Zp3 null mice without affecting order-specific sperm binding. Development 125:2415-2424. [DOI] [PubMed] [Google Scholar]
- 31.Ringuette, M. J., M. E. Chamberlin, A. W. Baur, D. A. Sobieski, and J. Dean. 1988. Molecular analysis of cDNA coding for ZP3, a sperm binding protein of the mouse zona pellucida. Dev. Biol. 127:287-295. [DOI] [PubMed] [Google Scholar]
- 32.Sasanami, T., J. Pan, Y. Doi, M. Hisada, T. Kohsaka, and M. Toriyama. 2002. Secretion of egg envelope protein ZPC after C-terminal proteolytic processing in quail granulosa cells. Eur. J. Biochem. 269:2223-2231. [DOI] [PubMed] [Google Scholar]
- 33.Schlondorff, J., L. Lum, and C. P. Blobel. 2001. Biochemical and pharmacological criteria define two shedding activities for TRANCE/OPGL that are distinct from the tumor necrosis factor alpha convertase. J. Biol. Chem. 276:14665-14674. [DOI] [PubMed] [Google Scholar]
- 34.Schwager, S. L., A. J. Chubb, Z. L. Woodman, L. Yan, R. Mentele, M. R. Ehlers, and E. D. Sturrock. 2001. Cleavage of disulfide-bridged stalk domains during shedding of angiotensin-converting enzyme occurs at multiple juxtamembrane sites. Biochemistry 40:15624-15630. [DOI] [PubMed] [Google Scholar]
- 35.Seto, D. 1990. An improved method for sequencing double stranded plasmid DNA from minipreps using DMSO and modified template preparation. Nucleic Acids Res. 18:5905-5906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shimizu, S., M. Tsuji, and J. Dean. 1983. In vitro biosynthesis of three sulfated glycoproteins of murine zonae pellucidae by oocytes grown in follicle culture. J. Biol. Chem. 258:5858-5863. [PubMed] [Google Scholar]
- 37.Thomas, G. 2002. Furin at the cutting edge: from protein traffic to embryogenesis and disease. Nat. Rev. Mol. Cell. Biol. 3:753-766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Von Heijne, G. 1986. A new method for predicting signal sequence cleavage sites. Nucleic Acids Res. 14:4683-4690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Williams, Z., and P. M. Wassarman. 2001. Secretion of mouse ZP3, the sperm receptor, requires cleavage of its polypeptide at a consensus furin cleavage-site. Biochemistry 40:929-937. [DOI] [PubMed] [Google Scholar]
- 40.Yanagimachi, R. 1994. Mammalian fertilization, p. 189-317. In E. Knobil and J. Neil (ed.), The physiology of reproduction. Raven Press, New York, N.Y.
- 41.Yurewicz, E. C., A. G. Sacco, and M. G. Subramanian. 1987. Structural characterization of the Mr = 55, 000 antigen (ZP3) of porcine oocyte zona pellucida. Purification and characterization of α- and β-glycoproteins following digestion of lactosaminoglycan with endo-β-galactosidase. J. Biol. Chem. 262:564-571. [PubMed] [Google Scholar]
- 42.Zhao, M., L. Gold, A. M. Ginsberg, L.-F. Liang, and J. Dean. 2002. Conserved furin cleavage site not essential for secretion and integration of ZP3 into the extracellular egg coat of transgenic mice. Mol. Cell. Biol. 22:3111-3120. [DOI] [PMC free article] [PubMed] [Google Scholar]