Abstract
The elderly population comprises the fastest growing segment of the world's population. As patients age, the incidence and prevalence of certain pain syndromes increase. Pain may be underreported as some elderly patients incorrectly believe that pain is a normal process of aging. A comprehensive pain assessment includes a thorough medical history and physical examination, review of systems and pertinent laboratory results, imaging studies, and diagnostic tests. Pain physicians should have a broad range of understanding of the pharmacologic and physiological changes that occur in the geriatric population. The present review on pain management in the elderly focuses on relevant information for the pain clinician. Included are appropriate pain assessment, physical examination, pathophysiologic changes in the elderly, pharmacokinetic and pharmacodynamic changes, and present pain management modalities. Elderly patients present with increased fat mass, decreased muscle mass, and decreased body water, all of which have important ramifications on drug distribution. Hepatic phase I reactions involving oxidation, hydrolysis, and reduction appear to be more altered by age than phase II conjugation such as acetylation, glucuronidation, sulfation, and glycine conjugation. There is a predictable age-related decline in cytochrome P-450 function and, combined with the polypharmacy that much of the elderly population experiences, this may lead to a toxic reaction of medications. One of the newer opiates, oxymorphone, has recently been studied as it is metabolized in a non-cytochrome P-450 pathway and therefore bypasses many of the drug-drug interactions common to the elderly. A multidisciplinary approach is recommended to investigate all possible options for optimal management, including pharmacotherapy, interventional procedures, physical rehabilitation, and psychological support.
Keywords: Geriatric, pain management, pharmacotherapy, polypharmacy pharmacodynamic
THE IMPORTANCE OF UNDERSTANDING PAIN IN THE ELDERLY
Currently, elderly patients comprise the fastest growing segment of the world's population. The number of people worldwide 65 years and older was estimated at 506 million as of 2008 and by 2040 will increase to 1.3 billion. The United States Census Bureau asserts that there were 38.9 million people 65 and older in 2008, making up 12.8% of the total population. Of this population segment, 5.7 million are 85 years old and older, and this number is growing.
Chronic geriatric pain may be defined as “an unpleasant sensory and emotional experience associated with actual or potential tissue damage, or described in terms of such damage, for persons who are either aged (65 to 79 years old) or very aged (80 and over) and who have had pain for greater than 3 months.”1 The consequences of this pain include impaired activities of daily living (ADLs) and ambulation, depression, and strain on the health care economy.2 Pain may also be related to complications associated with deconditioning, gait abnormalities, accidents, polypharmacy, and cognitive decline.
The prevalence of persistent pain increases with age3; increases in joint pain and neuralgias are particularly common.4 A majority of elderly persons have significant pain problems and are undertreated. Between 25% and 40% of older cancer patients studied had daily pain. Among these patients, 21% who were between 65 and 74 years of age received no pain medication; of patients who were 75 to 84 years old, 26% received no pain medication; and for those above the age of 84, 30% were left untreated.5 Moreover, detection and management of chronic pain remain inadequate.6 In one study, 66% of geriatric nursing home residents had chronic pain, but in almost half of the cases (34%) it was not detected by the treating physician.7
PAIN ASSESSMENT AND THE PHYSICAL EXAMINATION
The treatment of pain begins with the assessment of what instigated the pain, how it can be terminated, and what management modalities are most effective for a particular patient. However, assessment is rarely that simple. Clinical manifestations of persistent pain are often complex and multifactorial in the older population. Even the perception of pain may differ from that perceived by those of less advanced years. Issues of physical accessibility to treatment, cost of drugs, the presence of coexisting illness, the use of concomitant medication, and the ability to understand the complaints of the patient who has cognitive impairment are only some of the factors that contribute to the complexity of the situation. Furthermore, the elderly patient's condition is often complicated by depression, psychosocial concerns, denial, poor health, and poor memory. Without a thorough assessment, pain that is causing severe impairment may not be revealed for an array of personal, cultural, or psychological reasons.
Pain may be underreported because some elderly patients incorrectly believe that pain is a normal process of aging. In other cases, such as with cancer pain, it is underreported because of fear of disease progression. Further, the caregivers and relatives are often the most reliable source of information.8 To address the need to adequately identify and diagnose pain, an increasing number of articles are being written on pain assessment in patients with dementia as well as research focusing on the measurement of pain.9–11
The complexity of pain assessment in geriatric patients often requires a multidisciplinary approach to diagnosis and to management. The pain physician should work together with a psychologist or psychiatrist as depression is oftentimes present in the patient with chronic pain. A physical therapist should be part of the team as well, to help with functionality. Laboratory and imaging studies may be ordered to help pinpoint a diagnosis if a detailed history and physical examination is not enough.
Evaluation of the patient's level of function is important as it affects the degree of independence, level of need for caregivers, as well as overall quality of life. Activities of daily living—eating, bathing, dressing—and instrumental ADLs—light housework, shopping, managing money, preparing meals—should be assessed. After a diagnosis is made, a consensus treatment plan should be outlined that includes modalities to decrease pain perception and increase patient function.12
The visual analogy scale (VAS), verbal descriptor scale, and numerical rating scale are frequently used to assess pain intensity. Available data support the use of these methods; however, the VAS should be used with caution as it is associated with a higher frequency of responses from the elderly that are incomplete or unable to be given a score.13,14 Moreover, elderly patients report difficulty in completing the VAS.13,15,16 It has, however, proven reliability in clinical and research settings, and offers the advantages of simplicity, ease of administration, and minimal intrusiveness.12
The McGill Pain Questionnaire has evidence for validity, reliability, and discriminative abilities that are not age-related. The McGill Pain Questionnaire can be used to assess the sensory, affective, evaluative, and miscellaneous components of pain.17
After assessing the intensity of pain, one should perform a thorough examination. An overview is discussed here:
Complete history and physical examination, with focus on most pressing pain issues
Review of location of pain, intensity, exacerbating and/or alleviating factors, and impact on mood and sleep
A screen for cognitive impairment such as the Folstein minimental examination
A screen for depression
A review of the patient's ADLs (bathing, dressing, toileting, transfers, feeding, and continence) and instrumental ADLs (use of phone, travel, shopping, food preparation, housework, laundry, taking medicine, handling finances)
Assessment of gait and balance
A screen for sensory depression to examine basic visual and auditory function
The pain physician should assess for evidence of chronic pain. The pain should be considered significant if it is persistent, recurrent, and affecting the patient's functional capacity and/or quality of life. Because pain may be manifested in multiple ways, a variety of terms should be used to screen for symptoms in older patients, such as burning, aching, soreness, tightness, discomfort, sharp, dull, and throbbing. One may also use vocalizations or changes in function as cues to underlying pain, especially in those patients with cognitive or language impairments. These cues may manifest as crying, groaning, changes in gait or posture, or withdrawn/agitated behavior. Furthermore, if cognitive or language impairments are present, the pain physician should seek reports from a caregiver or close relative. The underlying reason for this impairment should be optimally treated, and consultations for skilled procedures or knowledge should be sought when appropriate. A multidisciplinary approach is always recommended.
The examination continues with a comprehensive pain assessment including thorough medical history and physical examination, review of systems and pertinent laboratory results, imaging studies, and diagnostic tests. Noting the temporal relationships among events, medical interventions, and complaints helps elucidate the diagnosis and likely prognosis. The intensity, character, frequency, location, and duration of the pain should be probed. Ameliorating and exacerbating factors help show the nature of the pain as well. Afterward, the medication history should be reviewed, as well as over-the-counter herbal supplementation. A list of adverse effects should be noted. The physical examination should focus on neuromuscular systems with attention to impairments, weakness, hyperalgesia/hypoalgesia, hyperpathia, allodynia, numbness, and tingling. There may be trigger points, bony deformities, or local inflammation at certain sites that may suggest certain pathologies.
Physical function may be determined by assessing the ability of the patient to perform ADLs. Range of motion testing, gait, and balance testing are appropriate at this stage. The patient's psychosocial function may be determined by assessment of mood, social support groups, family relationships, and any appointed caregivers. Next, a quantitative assessment of the patient's pain may be ascertained with a VAS, numerical rating scale, or other pain scale. Finally, a pain log or diary may help keep track of how different treatment modalities are affecting the patient's pain intensity and function.
The follow-up interval should be determined by the severity of pain and dysfunction. This may be anywhere from 1 to 4 weeks depending on the patient's situation and compliance with medication. Regular visits help to reassess improvement or worsening of the condition, complications with medications, and patient compliance. Some patients who may be unable to drive to meet a physician may require house calls or the assistance of home health care for follow-up. Positive and negative effects of analgesics and therapeutic modalities should be noted, then the treatment plan modified.18
PATHOPHYSIOLOGIC CHANGES IN THE ELDERLY
A steady decline of homeostatic mechanisms and organ system function occurs during normal aging. The most important organ systems affected are described in the following sections.
Central Nervous System
Many elderly patients may present with neurologic disease and dysfunction, including transient ischemic attacks, strokes, dementia, or movement disorders. The pain physician should be aware that these problems may affect accurate assessment of pain as well as the efficacy of treatment.19
Although the mechanisms are not totally clear, symptoms of CNS and peripheral nervous system dysfunction may occur as early as 50 years of age. Heredity, concomitant disease, and stress from daily activities may play a role.20 The neurons of elderly patients are not rejuvenated when these cells die and are instead replaced by proliferating glial cells.21 Furthermore, the number of dendritic synapses, cell receptors, and intracellular enzymes is decreased.22
Alzheimer disease constitutes approximately 60% of all cases of dementia, although one must also look for other causes such as idiopathic degenerative processes, vascular disorders, normal-pressure hydrocephalus, neoplastic diseases, CNS infections, metabolic disorders, and pseudodementia.23 Parkinson disease is another common pathology in the elderly.
Hepatic
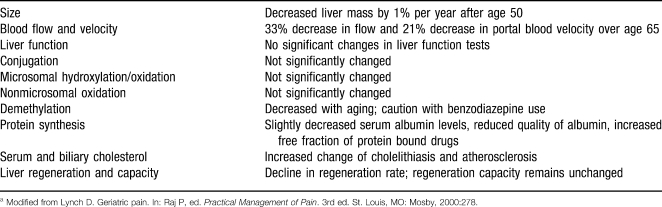
An aged liver may prolong the clearance of drugs from the body secondary to prehepatic, intrahepatic, or posthepatic causes (Table 1). Prehepatic dysfunction includes decreased first-pass and blood extraction, which may be secondary to lower gastrointestinal absorption or decreased portal and arterial blood flow. Intrahepatic dysfunction may be caused by hepatocellular pathology such as cirrhosis. Posthepatic dysfunction is usually due to either biliary tree or enterohepatic circulation blockage or pathology. Liver function tests are often normal despite these changes in the elderly liver.
Table 1.
Hepatic Changes in the Elderlya
Renal System
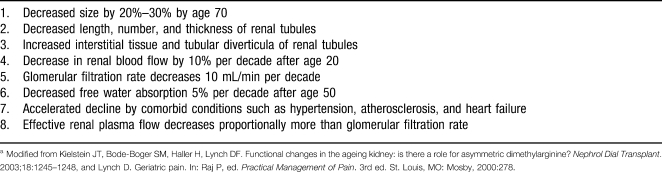
The decline in renal function begins after the age of 40 at a rate of approximately 1% per year, or a 1 mL/min per year decline in creatinine clearance.24 Although the structure and function of the kidney declines, clinically the function of the kidney seems to be maintained in healthy elderly patients.25 Typical changes in the aging kidney are noted in Table 2.
Table 2.
Renal Changes in the Elderlya
PAIN THRESHOLD
Multiple studies have been undertaken to determine the effect of aging on pain threshold. Gibson26 conducted a meta-analysis of over 50 studies that examined age differences in sensitivity to induced pain. The effect size was 0.074 (P < .0005), indicating that there is definite evidence of an increase in pain threshold with advancing age. There may be a difference in pain threshold depending on the type of pain, as well. Moreover, a study by Latienbacher et al27 compared pain perception in 40 men, half with a mean age of 27.1 years and the other with a mean age of 71.6 years. The results demonstrated that somatosensory thresholds for nonnoxious stimuli increase with age, whereas pressure pain thresholds decrease and heat pain thresholds show no age-related changes, which confirm previous studies as well.28
COMMON COMORBIDITIES IN THE ELDERLY
As patients age, the incidence and prevalence of certain pain syndromes increase (Table 3). The pain physician should be ready to deal with the following list of chronic geriatric pain syndromes commonly found in this patient population.
Table 3.
Chronic Geriatric Pain Syndromes
Pharmacokinetic Changes
Elderly patients present with increased fat mass, decreased muscle mass, and decreased body water, which have important ramifications on drug distribution.29,30 Blood volume may be decreased as well, secondary to diuretic use. Lipophilic medications such as fentanyl and lidocaine may have an increased duration of effect as more of these medications are absorbed by fat mass and will have an increased volume of distribution. Water-soluble drugs, however, are less efficiently distributed and result in higher plasma concentrations at equivalent doses, and therefore result in a higher frequency of side effects.
Decreases in serum albumin increase the amount of free drug availability. This is even more accentuated in patients with chronic disease and malnutrition, leading to higher levels of adverse effects when using highly protein-bound analgesics such as nonsteroidal anti-inflammatory drugs (NSAIDs) and antiepileptic drugs.
Drug half-life, the ratio of the volume of distribution to clearance, is notably increased for several benzodiazepines and tricyclic antidepressants related to decreased kidney and liver clearance. Dose-related side effects from analgesics that undergo significant first-pass metabolism will be increased. These drugs, such as lidocaine and opioids, should be initiated slowly and at lower doses to avoid complications.31
Hepatic phase I reactions involving oxidation, hydrolysis, and reduction appear to be more altered by age than phase II conjugation such as acetylation, glucuronidation, sulfation, and glycine conjugation. There is a predictable age-related decline in cytochrome P-450 function and, combined with the polypharmacy that much of the elderly population experiences, this may lead to a toxic reaction of medications. Selective serotonin reuptake inhibitors and the newer serotonin-norepinephrine reuptake inhibitors both inhibit the cytochrome system and can lead to a buildup of other drugs. Narcotic accumulation when concurrently administered with other medications—specifically the aforementioned selective serotonin reuptake inhibitors and serotonin-norepinephrine reuptake inhibitors—is always a risk, especially in the elderly population with declining liver function. High doses of narcotics may also act as cytochrome enzyme inhibitors. Although a drug like acetaminophen is metabolized at an equal rate at older ages, a drug like diazepam is metabolized at a reduced rate in the elderly. Further, carbamazepine, lidocaine, and fentanyl are subject to reduced metabolism by the same enzyme systems in older patients even though they are metabolized by the same enzymes. Glucuronidation of morphine and glutathione conjugation of acetaminophen are examples of reduced and unaltered phase II reactions, respectively. The frequency of slow and rapid metabolizing genetic polymorphisms seems to be unaffected by age. Reduction in renal clearance, however, seems to have the largest pharmacodynamic effect on the elderly. Caution should be taken when using drugs that primarily undergo renal metabolism and clearance, such as gabapentin, to avoid side effects.32
Pharmacodynamic Changes
Generally speaking, geriatric patients usually have increased sensitivity to centrally acting drugs such as benzodiazepines and opioids. The adrenergic and cholinergic autonomic nervous systems, however, generally have decreased sensitivity to receptor-specific drugs such as beta blockers.33 These changes are strongly coupled with age-related decline in CNS function.
COMPLIANCE ISSUES
Many factors are associated with poor medical compliance. These include poor physician-patient communication, cost, race, drug and dosage form, and insurance coverage.34 Multiple studies have been conducted to determine the best ways to address this issue, but inconsistent methods and findings among studies prevent drawing a firm conclusion in favor of any particular intervention. Methods to increase compliance include improving communication of the purpose and side effect profile of medications, making simple dosing regimens, decreasing the total number of drugs needed, regularly scheduled follow-ups, group education, individualized medication cards, medication review by pharmacists, and multicompartment dose administration aids.35,36
PAIN MANAGEMENT MODALITIES IN THE ELDERLY
Treatment modalities for pain in the elderly may be categorized into the following areas. A multidisciplinary approach is recommended to investigate all possible options for optimal management: (1) pharmacotherapy (most commonly employed), (2) psychological support, (3) physical rehabilitation, and (4) interventional procedures.
Pharmacotherapy
Drug treatment is generally the first and most widely used treatment modality to control geriatric pain. It is relatively simple to implement and consists of NSAIDs, muscle relaxants, opioids, and other adjuvant therapy. Prescribing these medications is not without risks, however. The patient's cognitive, physiological, and functional status may be affected. The American Geriatric Society and the World Health Organization (WHO) have put together counsel to arrive at some form of consensus as to the best approach in this patient population.37
Summary of 2009 American Geriatric Society Recommendations
Nonopioids
-
Acetaminophen should be considered as initial and ongoing pharmacotherapy in the treatment of persistent pain, particularly musculoskeletal pain, owing to its demonstrated effectiveness and good safety profile (high quality of evidence, strong recommendation).
A. Absolute contraindications: liver failure (high quality of evidence, strong recommendation)
B. Relative contraindications and cautions: hepatic insufficiency, chronic alcohol abuse or dependence (moderate quality of evidence, strong recommendation)
C. Maximum daily recommended dosages of 4 g per 24 hours should not be exceeded and must include “hidden sources” such as from combination pills (moderate quality of evidence, strong recommendation)
-
Nonselective NSAIDs and cyclooxygenase 2 (COX-2) selective inhibitors may be considered rarely, and with extreme caution, in highly selected individuals (high quality of evidence, strong recommendation).
A. Patient selection: other (safer) therapies have failed, evidence of continuing therapeutic goals not met, ongoing assessment of risks and complications outweighed by therapeutic benefits (low quality of evidence, strong recommendation)
B. Absolute contraindications: current active peptic ulcer disease (low quality of evidence, strong recommendation); chronic kidney disease (moderate level of evidence, strong recommendation); heart failure (moderate level of evidence, weak recommendation)
C. Relative contraindications and cautions: hypertension, Helicobacter pylori, history of peptic ulcer disease, concomitant use of corticosteroids or selective serotonin reuptake inhibitors (moderate quality of evidence, strong recommendation)
Older persons taking nonselective NSAIDs should use a proton pump inhibitor or misoprostol for gastrointestinal protection (high quality of evidence, strong recommendation).
Patients taking a COX-2 selective inhibitor with aspirin should use a proton pump inhibitor or misoprostol for gastrointestinal protection (high quality of evidence, strong recommendation).
Patients should not take more than one nonselective NSAID or COX-2 selective inhibitor for pain control (low quality of evidence, strong recommendation).
Patients taking aspirin for cardioprophylaxis should not use ibuprofen (moderate quality of evidence, weak recommendation).
Patients taking nonselective NSAIDs and COX-2 selective inhibitors should be routinely assessed for gastrointestinal and renal toxicity, hypertension, heart failure, and other drug-drug and drug-disease interactions (weak quality of evidence, strong recommendation).
Opioids
Patients with moderate to severe pain, pain-related functional impairment, or diminished quality of life because of pain should be considered for opioid therapy (low quality of evidence, strong recommendation).
Patients with frequent or continuous pain on a daily basis may be treated with around-the-clock time-contingent dosing aimed at achieving steady-state opioid therapy (low quality of evidence, weak recommendation).
Clinicians should anticipate, assess for, and identify potential opioid-associated adverse effects (moderate quality of evidence, strong recommendation).
Maximal safe doses of acetaminophen or NSAIDs should not be exceeded when using fixed-dose opioid combination agents as part of an analgesic regimen (moderate quality of evidence, strong recommendation).
When long-acting opioid preparations are prescribed, breakthrough pain should be anticipated, assessed, and prevented or treated using short-acting immediate-release opioid medications (moderate quality of evidence, strong recommendation).
Clinicians well versed in the use and risks of methadone should initiate it and titrate it cautiously (moderate quality of evidence, strong recommendation).
Patients taking opioid analgesics should be reassessed for ongoing attainment of therapeutic goals, adverse effects, and safe and responsible medication use (moderate quality of evidence, strong recommendation).
Adjuvant Analgesic Drugs
All patients with neuropathic pain are candidates for adjuvant analgesics (strong quality of evidence, strong recommendation).
Patients with fibromyalgia are candidates for a trial of approved adjuvant analgesics (moderate quality of evidence, strong recommendation).
Patients with other types of refractory persistent pain may be candidates for certain adjuvant analgesics (eg, back pain, headache, diffuse bone pain, temporomandibular disorder) (low quality of evidence, weak recommendation).
Tertiary tricyclic antidepressants (amitriptyline, imipramine, doxepin) should be avoided because of higher risk for adverse effects such as anticholinergic effects and cognitive impairment (moderate quality of evidence, strong recommendation).
Agents may be used alone, but often the effects are enhanced when used in combination with other pain analgesics and nondrug strategies (moderate quality of evidence, strong recommendation).
Therapy should begin with the lowest possible dose and increase slowly based on response and side effects, with the caveat that some agents have a delayed onset of action and therapeutic benefits are slow to develop. For example, gabapentin may require 2 to 3 weeks for onset of efficacy (moderate quality of evidence, strong recommendation).
An adequate therapeutic trial should be conducted before discontinuation of a seemingly ineffective treatment (weak quality of evidence, strong recommendation).
Other Drugs
Long-term systemic corticosteroids should be reserved for patients with pain-associated inflammatory disorders or metastatic bone pain. Osteoarthritis should not be considered an inflammatory disorder (moderate quality of evidence, strong recommendation).
Patients with localized neuropathic pain are candidates for topical lidocaine (moderate quality of evidence, strong recommendation).
Patients with localized nonneuropathic pain may be candidates for topical lidocaine (low quality of evidence, weak recommendation).
Patients with other localized nonneuropathic persistent pain may be candidates for topical NSAIDs (moderate quality of evidence, weak recommendation).
Other topical agents, including capsaicin or menthol, may be considered for regional pain syndromes (moderate quality of evidence, weak recommendation).
Many other agents for specific pain syndromes may require caution in older persons and merit further research (eg, glucosamine, chondroitin, cannabinoids, botulinum toxin, alpha-2 adrenergic agonists, calcitonin, vitamin D, bisphosphonates, ketamine) (low quality of evidence, weak recommendation).
Overview of the WHO Recommendations: Analgesic Ladder Significant overlap occurs between chronic geriatric pain and cancer pain. For this reason, following the WHO recommendations for pain management is appropriate. In order to maintain freedom from pain, WHO recommends (1) administration of drugs “by the clock” (eg, every 3–6 hours), (2) medication by mouth individualized for the patient, and lastly (3) following the “analgesic ladder” (which was modified from ref. 38 and follows):
For mild pain, the most appropriate first choice for relatively safe analgesia is acetaminophen.
For mild to moderate pain or pain uncontrolled with acetaminophen, the use of NSAIDs is appropriate.
For pain refractory to NSAIDs, or pain rated as moderate initially, a weaker opioid (eg, codeine) is the appropriate first choice. Other weak opioids that may be used include hydrocodone, propoxyphene, and oxycodone in combination with acetaminophen.
For pain refractory to the previous plan, or pain rated as severe, a purse opioid agonist (eg, morphine) is selected. Other pure opioids to consider include hydromorphone, fentanyl, levorphanol, and oxycodone.
Adjuvant medication may be used to relieve fear and anxiety in the patient as well as for synergism with the previously named medications.
Adjuvants
Adjuvant drug therapy should be considered at all times to enhance the analgesic effects of other medications. It is often necessary to try different drugs to determine the best regimen for a particular patient. Some of the adjuvant drugs used to treat pain include but are not limited to the following:
Antidepressants
Anticonvulsants
Alpha-2 adrenergic agonists
Local anesthetics
Corticosteroids
Baclofen
N-methyl-d-aspartate receptor agonists
Muscle relaxants
Topical creams and gels
Neuroleptics
Antihistamines
Psychostimulants
Calcitonin
Newer Opiates and the Elderly
As new guidelines are released discussing the adverse reactions of NSAIDs and the elderly and there is a move toward opiate conversion, the search for new and safer opiates is inevitable. Most of the older opiates have a know efficacy and safety profile when used in an older population. One of the newer opiates, oxymorphone, has recently been studied as it is metabolized in a non-cytochrome P-450 pathway and therefore bypasses many of the drug-drug interactions common to the elderly. Moreover, the drug is still renally excreted, so it should be used with caution in elderly patients who already have a decreased glomerular filtration rate. The problem arises as it is not as familiar as many of the other opiates typically used; however, indications suggest that it is safe in the elderly and should be used in the same way as the other opiates, starting with a low dose and increasing it slowly.37
Psychological Support
Because pain is a complex sensory and emotional experience, psychological modalities should be employed in the pain management model. The psychological branch of pain also explains why some patients with minimal disease may have excruciating pain, whereas others with severe disease may have minimal complaints. Pain-coping strategies may include relaxation, prayer, and attention-diversion techniques. Depression and anxiety in the geriatric patient must be addressed with psychotherapy, meditation, and medication. Furthermore, the socioenvironmental variables of each patient should be adjusted to help the patient cope with pain. A solid support system including relatives and caregivers should be established.
Physical Rehabilitation
The rehabilitative aspect of pain management may help the patient live a more independent and functional life. Rehabilitation may involve adapting to loss of physical, psychological, or social skills. The assessment of ADLs can help assess the level of function and direct treatment. The objectives of rehabilitation include stabilizing the primary disorder, preventing secondary injuries, decreasing pain perception via a multidisciplinary approach, treating functional deficits, and promoting adaptations to current disabilities.39
Interventional Modalities
Interventional pain modalities may help to determine the underlying cause of pain and help to arrive at a precise diagnosis. It often alleviates the need for heavy medication use, thereby sparing the patient from unwanted side effects associated with larger doses of drugs. Nerve blocks are some of the most commonly used interventional procedures employed by pain physicians; these help not only with diagnosis but also prognosis, preemptive analgesia, and sometimes definitive therapy. Other interventions that may be used include chemical neurolysis, radiofrequency lesioning, cryoneurolysis, neuroaugmentation, and neuraxial drug delivery.
SUMMARY
Persistent pain is not an inevitable part of aging but is fairly common among the elderly. The treatment of pain may be complicated by multiple problems that are far less likely to occur in younger adults. Barriers to effective management include challenges to proper pain assessment, underreporting of pain by patients, atypical manifestations of pain in the elderly, and a need for increased appreciation of the pharmacokinetic and pharmacodynamic changes of aging. Physicians can provide appropriate analgesia in geriatric patients through proper assessment, a multidisciplinary approach, and appropriate use of treatment modalities.
Acknowledgments
The authors wish to thank Mark V. Boswell, MD, PhD, MBA, Professor and Medical Director, International Pain Center and Surgical Center for Pain Management, Texas Tech Health Sciences Center, Lubbock, Texas, for his editorial assistance and helpful comments in the preparation of this article.
REFERENCES
- 1.Lynch D. Geriatric pain. In: Raj P. P., editor. Practical Management of Pain. 3rd ed. St. Louis, MO: Mosby; 2000. pp. 270–271. [Google Scholar]
- 2.Manchikanti L., Boswell M. V., Singh V., et al. Comprehensive review of epidemiology, scope, and impact of spinal pain. Pain Phys. 2009;12((4)):E35–70. [PubMed] [Google Scholar]
- 3.Crook J., Rideout E., Browne G. The prevalence of pain complaints in a general population. Pain. 1984;18((3)):299–314. doi: 10.1016/0304-3959(84)90824-8. [DOI] [PubMed] [Google Scholar]
- 4.Badley E. M., Tennant A. Changing profile of joint disorders with age: findings from a postal survey of the population of Calderdale, West Yorkshire, United Kingdom. Ann Rheum Dis. 1992;51((3)):366–371. doi: 10.1136/ard.51.3.366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cleeland C. S. Undertreatment of cancer pain in elderly patients. JAMA. 1998;279((23)):1914–1915. doi: 10.1001/jama.279.23.1914. [DOI] [PubMed] [Google Scholar]
- 6.Woo J., Ho S. C., Lau J., Leung P. C. Musculoskeletal complaints and associated consequences in elderly Chinese aged 70 years and over. J Rheumatol. 1994;21((10)):1927–1931. [PubMed] [Google Scholar]
- 7.Sengstaken E. A., King S. A. The problems of pain and its detection among geriatric nursing home residents. J Am Geriatr Soc. 1993;41((5)):541–544. doi: 10.1111/j.1532-5415.1993.tb01892.x. [DOI] [PubMed] [Google Scholar]
- 8.Ferrell B. A. Pain management in elderly people. J Am Geriatr Soc. 1991;39((1)):64–73. doi: 10.1111/j.1532-5415.1991.tb05908.x. [DOI] [PubMed] [Google Scholar]
- 9.Ferrell B. A., Ferrell B. R., Rivera L. M. Pain in cognitively impaired nursing home patients. J Pain Symptom Manage. 1995;10((8)):591–598. doi: 10.1016/0885-3924(95)00121-2. [DOI] [PubMed] [Google Scholar]
- 10.Kovach C., Weissman D., Griffie J., Matson S., Muchka S. Assessment and treatment of discomfort for people with late-stage dementia. J Pain Symptom Manage. 1999;18((6)):412–419. doi: 10.1016/s0885-3924(99)00094-9. [DOI] [PubMed] [Google Scholar]
- 11.Warden V., Hurley A. C., Volicer L. Development and psychometric evaluation of the Pain Assessment In Advanced Dementia (PAINAD) scale. J Am Med Dir Assoc. 2003;4((1)):9–15. doi: 10.1097/01.JAM.0000043422.31640.F7. [DOI] [PubMed] [Google Scholar]
- 12.Lynch D. Geriatric pain. In: Raj P., editor. Practical Management of Pain. 3rd ed. St. Louis, MO: Mosby; 2000. pp. 271–275. [Google Scholar]
- 13.Gagliese L. Assessment of pain in the elderly. In: Turk D. C., Melzack R., editors. Handbook of Pain Assessment. New York, NY: Guilford Press; 2002. pp. 119–133. [Google Scholar]
- 14.Gagliese L., Melzack R. Age differences in the quality of chronic pain: a preliminary study. Pain Res Manage. 1997;2:157–162. [Google Scholar]
- 15.Herr K. A., Mobily P. R. Comparison of selected pain assessment tools for use with the elderly. Appl Nurs Res. 1993;6((1)):39–46. doi: 10.1016/s0897-1897(05)80041-2. [DOI] [PubMed] [Google Scholar]
- 16.Benesh L. R., Szigeti E., Ferraro F. R., Gullicks J. N. Tools for assessing chronic pain in rural elderly women. Home Healthc Nurse. 1997;15((3)):207–211. doi: 10.1097/00004045-199703000-00009. [DOI] [PubMed] [Google Scholar]
- 17.Melzack R. The McGill Pain Questionnaire: appraisal and current status. In: Turk D. C., Melzack R., editors. Handbook of Pain Assessment. New York, NY: Guilford Press; 1975. pp. 35–52. [Google Scholar]
- 18.American Geriatrics Society Panel on Chronic Pain in Older Persons. The management of chronic pain in older persons. J Am Geriatr Soc. 1998;46((5)):635–651. doi: 10.1111/j.1532-5415.1998.tb01084.x. [DOI] [PubMed] [Google Scholar]
- 19.Broe G. A. The neuroepidemiology of old age. In: Tallis R., editor. The Clinical Neurology of Old Age. Chichester, UK: Wiley; 1989. pp. 50–80. [Google Scholar]
- 20.Samorajski T. How the human brain responds to aging. J Am Geriatr Soc. 1976;24((1)):4–11. doi: 10.1111/j.1532-5415.1976.tb03246.x. [DOI] [PubMed] [Google Scholar]
- 21.Long D. M. Aging in the nervous system. Neurosurgery. 1985;17((2)):348–354. doi: 10.1227/00006123-198508000-00022. [DOI] [PubMed] [Google Scholar]
- 22.Coleman P. D., Flood D. G. Neuron numbers and dendritic extent in normal aging and Alzheimer's disease. Neurobiol Aging. 1987;8((6)):521–545. doi: 10.1016/0197-4580(87)90127-8. [DOI] [PubMed] [Google Scholar]
- 23.Geldmacher D. S., Whitehouse P. J. Evaluation of dementia. N Engl J Med. 1996;335((5)):330–336. doi: 10.1056/NEJM199608013350507. [DOI] [PubMed] [Google Scholar]
- 24.Stiff J. L. Evaluations of the geriatric patient. In: Rogers M. C., Covino B. G., Tinker J. H., editors. Principles and Practice of Anesthesiology. St. Louis, MO: Mosby-Year Book; 1993. pp. 480–492. [Google Scholar]
- 25.Beck L. H. Changes in renal function with aging. Clin Geriatr Med. 1998;14:199–209. [PubMed] [Google Scholar]
- 26.Gibson S. J. In: Proceedings of the 10th World Congress on Pain, Progress in Pain Research and Management. Vol. 24. Dostrovsky J. O., Carr D. B., Kaltenzburg M., editors. Seattle, WA: IASP Press; 2003. pp. 767–790. [Google Scholar]
- 27.Latienbacher S., Kunz M., Strate P., Nielsen J., Arendt-Nielsen L. Age effects on pain thresholds, temporal summation and spatial summation of heat and pressure pain. Pain. 2005;115((3)):410–418. doi: 10.1016/j.pain.2005.03.025. [DOI] [PubMed] [Google Scholar]
- 28.Lin Y. H., Hsieh S. C., Chao C. C., Chang Y. C., Hsieh S. T. Influence of aging on thermal and vibratory thresholds of quantitative sensory testing. J Peripher Nerv Syst. 2005;10((3)):269–281. doi: 10.1111/j.1085-9489.2005.10305.x. [DOI] [PubMed] [Google Scholar]
- 29.Turnheim K. When drug therapy gets old: pharmacokinetics and pharmacodynamics in the elderly. Exp Gerontol. 2003;38((8)):843–853. doi: 10.1016/s0531-5565(03)00133-5. [DOI] [PubMed] [Google Scholar]
- 30.Vuyk J. Pharmacodynamics in the elderly. Best Prac Res Clin Anaesthesiol. 2003;17((2)):207–218. doi: 10.1016/s1521-6896(03)00008-9. [DOI] [PubMed] [Google Scholar]
- 31.Bresler R., Bahl J. J. Principles of drug therapy for the elderly patient. Mayo Clin Proc. 2003;78((12)):1564–1577. doi: 10.4065/78.12.1564. [DOI] [PubMed] [Google Scholar]
- 32.Herrlinger C., Klotz V. Drug metabolism and drug interactions in the elderly. Best Pract Res Clin Gastroenterol. 2001;15((6)):897–918. doi: 10.1053/bega.2001.0249. [DOI] [PubMed] [Google Scholar]
- 33.Muravchick S. The effects of aging on anesthetic pharmacology. Acta Anaesthesiol Belg. 1998;49((2)):79–84. [PubMed] [Google Scholar]
- 34.Balkrishnan R. Predictors of medication adherence in the elderly. Clin Ther. 1998;29((4)):764–771. doi: 10.1016/s0149-2918(98)80139-2. [DOI] [PubMed] [Google Scholar]
- 35.George J., Elliott R. A., Stewart D. C. A systematic review of interventions to improve medication taking in elderly patients prescribed multiple medications. Drugs Aging. 2008;25((4)):307–324. doi: 10.2165/00002512-200825040-00004. [DOI] [PubMed] [Google Scholar]
- 36.American Geriatrics Society Panel on Pharmacological Management of Persistent Pain in Older Persons. Pharmacological management of persistent pain in older persons. J Am Geriatr Soc. 2009;57((8)):1331–1346. doi: 10.1111/j.1532-5415.2009.02376.x. [DOI] [PubMed] [Google Scholar]
- 37.Chamberloin K., Cottle M., Neville R., Tan J. Oral oxymorphone for pain management. Ann Pharmacother. 2004;1((7)):1144–1152. doi: 10.1345/aph.1H451. [DOI] [PubMed] [Google Scholar]
- 38.Davies E., Higginson I. J., editors. Better Palliative Care for Older People. Copenhagen, Denmark: World Health Organization; 2004. [Google Scholar]
- 39.Brummel-Smith K. Rehabilitation. In: Cassel C. K., Cohen H. J., Larson E. B., editors. Geriatric Medicine. 3rd ed. New York, NY: Springer-Verlag; 1997. pp. 211–226. [Google Scholar]