Abstract
Introduction:
Sinus node dysfunction (SND) following orthotopic heart transplantation may lead to bradycardia, atrioventricular block, sick sinus syndrome, syncope, and death, with 6%-23% of patients requiring pacemakers.
Methods:
Permanent pacemakers were placed in 5% of orthotopic heart transplants conducted at our institution from January 2002 to October 2008.
Results:
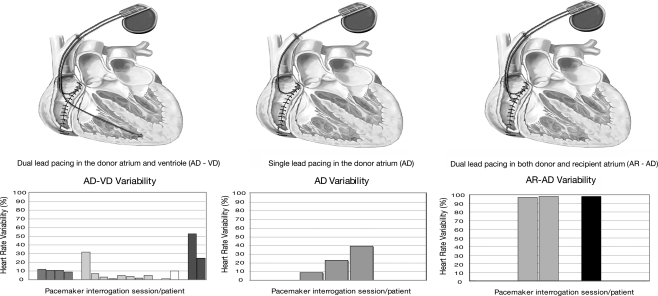
Three different implant techniques were used over this time: (1) dual-chamber pacing in the donor atrium and ventricle (AD-VD) (62.5%); (2) single lead in the donor atrium (AD) (12.5%); and (3) dual leads placed in both donor and recipient atrium (AR-AD) (25%). Using the percentage of paced histograms recorded in the device, heart rate variability for the types of lead placements were 14% for AD-VD, 35% for AD, and 97% for AR-AD.
Discussion:
The transplanted heart is characterized physiologically by autonomic denervation and chronotropic incompetence. Restoration of chronotropic competence by atrial pacing increases exercise duration and peak VO2. Rate responsiveness can be achieved in this patient population with the placement of one lead in the remnant right atrium and one lead in the transplanted donor right atrium.
Keywords: Chronotropic incompetence, dual-atrial pacing, orthotopic heart transplantation, permanent pacemaker, sinus node dysfunction
INTRODUCTION
When a patient undergoes cardiac transplantation and subsequently is considered for permanent pacemaker implantation, unique concerns arise that the implanting cardiologist must reconcile. Although current pacing guidelines address some of the different clinical situations in which a permanent pacemaker should be considered post transplantation, the recommendations regarding the timing of implantation, the location of lead placements, and the type of pacemaker system to be used in the transplanted heart remain unclear. In absence of prospective case-control clinical trials on which to base clear guidelines, the following review serves to contribute to the ongoing development of such guidelines by (1) describing a single transplant center's experience with permanent pacemaker implantation following orthotopic heart transplantation, (2) reviewing the current understanding of the electrophysiology of the denervated transplanted heart and the pathogenesis of both early and late bradycardias, and (3) suggesting an alternative mode of pacing in post–heart transplant patients.
METHODS
The present study was approved by the institutional review board of the Ochsner Clinic Foundation (IRB No. 2010.075.A) and uses data collected retrospectively by reviewing our institution's database of transplanted patients. The cohort included 153 patients who received orthotopic heart transplants between January 2002 and October 2008. Records of pacemaker implantation and interrogation were obtained from the medical record and used to calculate heart rate variability quotients for each patient based on the rate settings at the time of each interrogation. Heart rate variability was defined as the percentage of heartbeats outside the lower rate setting at the time of each pacemaker interrogation.
RESULTS
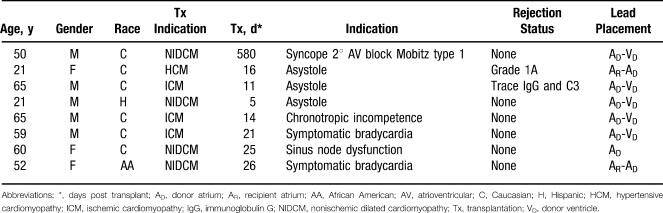
Permanent pacemakers were placed in 8 of the 153 orthotopic heart transplants conducted during the study period for symptomatic bradycardia (25%), asystole (38%), atrioventricular (AV) block (13%), sinus node dysfunction (13%), and chronotropic insufficiency (13%). Three lead placement techniques were used over time: (1) dual-chamber pacing in the donor atrium and ventricle (AD-VD) (62.5%), (2) single lead in the donor atrium (AD) (12.5%), and (3) dual leads placed in both donor and recipient atrium (AR-AD) (25%) (Figure). Patient demographics at the time of implant are displayed in the Table; all permanent pacemaker patients identified in this study underwent a standard Lower-Shumway (biatrial) orthotopic heart transplantation procedure. A variety of pacemaker settings and lead placement techniques were used, with a frequency of dual-chamber rate adaptive (DDDR) pacing, 78%; atrial-inhibited, rate-modulated (AAIR) pacing, 16%; and ventricular demand inhibited (VVI) pacing, 6%. Among the three lead placement groups—AD-VD (n = 5), AD (n = 1), and AR-AD (n = 2)—heart rate variability for the types of lead placements was 14%, 35%, and 97%, respectively (Figure). At the time of publication, 2 patients are deceased, 2 patients are no longer followed at our institution, and permanent pacing is still required in the 4 remaining patients.
Figure.
Depiction of lead placement techniques in orthotopic heart transplant recipients and graphic representation of calculated heart rate variability.
Table.
Patient Characteristics at Permanent Pacemaker Implant
DISCUSSION
Since 1960, the traditional biatrial technique reported by Lower and Shumway1 was the predominant surgical method used in orthotopic heart transplantation. The loss of atrial anatomy resulting from this technique is responsible for the development of posttransplant complications such as mitral and tricuspid regurgitation, atrial septal aneurysm, atrial thrombus formation, bradycardia, and tachyarrhythmia.2,3 As a result, alternative techniques and modifications, such as the domino procedure and the bicaval technique, have been used to address the problem of large atrial cavities with abnormal geometry created by the Lower and Shumway technique.
Regardless of the specific technique, in all cases the donor heart is denervated from the autonomic nervous system. Depending on which technique is used, however, two sinus nodes may be present: an innervated recipient sinus node and a denervated donor sinus node. The donor sinus node in the biatrial technique displays properties of variable atrial conduction and electrical isolation because of the atrial suture line. The activity of both sinus nodes is frequently visible on electrocardiograms as two distinct P waves that differ in morphology and rate. The recipient P wave can be dissociated from the QRS complex, while the donor sinus node, lacking parasympathetic input, typically demonstrates a rate between 80 and 100 beats per minute.
Electrophysiology
According to multiple studies,4,5 the electrophysiology of the transplanted heart, assessed in the resting state, demonstrates atrial and ventricular refractory periods and AV nodal conduction properties following orthotopic cardiac transplantation that are similar to those observed in normal controls. Neurochemically, the response of the transplanted heart to β-adrenergic agonists and antagonists is qualitatively normal.6 However, the donor heart demonstrates supersensitivity to catecholamines. Studies showing a greater increase in donor heart rate compared with the recipient sinus node suggest a presynaptic origin for this supersensitivity.7 This denervated supersensitivity also occurs in response to the neurotransmitter acetylcholine and the endogenous metabolite adenosine.8
Chronotropic Incompetence
It is imperative that physicians understand the effects of denervation in determining the medical management of posttransplant bradycardias and conduction abnormalities, as well as in explaining why chronotropic incompetence is the rule following orthotopic heart transplant. The rate response to exercise, in the posttransplant heart, is characterized by a delayed onset, a reduced rate of rise, and a lower maximal rate at peak exercise. Following cessation of exercise, heart rate typically increases further before decelerating slowly.9 In a 31-patient series, Scott and colleagues10 reviewed chronotropic incompetence associated with the transplanted heart. In most patients, responsiveness continually improved, peaking between the third and sixth postoperative weeks. This majority, however, peaked at only 40% of the expected chronotropic response. A small minority (5 of 31) progressively normalized by the sixth postoperative month. Additional studies looking at oxygen consumption (VO2) have found that although transplant patients generally demonstrate up to a 43% increase in peak oxygen consumption compared with pretransplant levels, their maximal exercise capacity is subnormal.11 When compared with normal controls, a multivariate analysis revealed chronotropic response was the factor most strongly associated with peak VO2.12
Posttransplant Bradycardia
Following orthotopic transplantation, most hearts are functionally depressed and require support with positive chronotropic agents or temporary pacing for the first 24 hours. Bradycardias that occur or persist beyond this point are seen in 14% to 44% of patients.9 The myriad of studies characterizing these bradycardias find that those secondary to sinus node dysfunction predominate early in the posttransplant period, with AV block being relatively uncommon. Illustrating that most patients with sinus node dysfunction will regain function with time, a Pittsburgh series13 showed that 55 of 72 patients with bradycardia had improvement in heart rate by the third week following transplantation, with 50 of the 55 showing improvement in less than 7 days. Multiple potential etiologies—including the ischemic time of the donor heart, surgical trauma, donor age, total bypass time, and preoperative use of amiodarone—may explain bradycardia in cardiac transplantation. However, studies have not shown consistently which, if any, of these factors are predictors of sinus node dysfunction or the need for permanent pacing. In contrast, late bradyarrhythmias generally present with AV block14 and with a slightly increased association with severe rejection.15
Permanent Pacemaker Implantation
The incidence and reasons for pacemaker implantation following cardiac transplantation vary among different studies, with reported rates of 6% to 23%.9 Most of these studies report sinus node dysfunction, slow junction rhythm, sinus arrest, or sinus bradycardia as the primary reasons, with about 10% resulting from abnormal AV conduction, primarily second- and third-degree heart block. Most of these studies involve pacemaker implantation prior to hospital discharge, usually between 7 and 21 days post transplant. Interestingly, when evaluated on follow-up, these same studies demonstrate that only around 45% of the patients were still bradycardic at 1 year.9
An important factor to account for in implanting a pacemaker in cardiac transplant recipients is the distorted right atrial anatomy. The posttransplant heart's position varies based on surgical techniques but usually is more inferior and medial in an anterior-posterior projection. When selecting leads for implantation, 2 considerations should be remembered: First, the risk of dislodgment is higher, making active fixation preferred16; second, bipolar leads provide superior sensing to limit far-field sensing of ventricular or recipient atrial activity.
Lead Placement
Standard implantation techniques have been used to place only ventricular leads (VD) in patients deemed at risk for AV block with or without concomitant signs of sinus node dysfunction. Some investigators advocate this method as the most cost-effective and appropriate therapy for 2 main reasons.17 First, pacing independence at 6 months is reportedly achieved in greater than 75% of patients initially identified as requiring permanent pacemakers.18 Second, deaths reported due to bradyarrhythmias following transplantation are usually associated with rejection and severe graft vasculopathy.18
Another single lead system, using a single lead in the donor atrium (AD), can be used in cases of sinus node dysfunction, as long as intrinsic conduction to the ventricle can be demonstrated (Figure). Loria and colleagues19 used this method with success in a case series of patients with persistent and symptomatic sinus node dysfunction in the early postoperative period. The theoretical benefit of this method comes from the lack of a cumbersome ventricular lead interfering with the frequent transvenous right ventricular biopsies required in posttransplant patients.
Currently, though, the most common system is a dual-chamber pacemaker, with leads in the donor atrium and donor ventricle (AD-VD). This pacing system may be adjusted to achieve a large variety of atrial and ventricular pacing settings in patients with and without AV conduction blocks. Zieroth and colleagues20 recently described a retrospective series that found, similar to our research, incidence of single- and dual-chamber permanent pacemakers of 31% and 69%, respectively.
Restoring Chronotropic Competence
As previously mentioned, chronotropic incompetence is prevalent and functionally limiting in the posttransplant population. Although most modern pacemakers are capable of rate-responsive pacing, this study illustrates that even with that feature active, dual-chamber and single-chamber lead systems placed in the donor heart do not maximally correct the chronotropic incompetence that comes from a denervated, transplanted heart (Figure).
Restoration of physiologic responsiveness has been studied, and various reports have documented the effectiveness of physiologic pacing in posttransplant patients with sinus node disease.21 This study confirms that physiologic pacing can restore rate responsiveness in patients with intact AV conduction by using the innervated recipient (remnant) atrium as an atrial sensor for pacing the donor atrium (AR-AD).22 This method was previously described in the medical literature using a dual-chamber device with an atrial lead positioned in the recipient atrium, while the lead connected to the ventricular channel was positioned in the donor atrium (Figure).22 To allow for the recipient atrium to serve as a biosensor, the dual-chamber system is programmed with ventricular pacing and the shortest possible AV delay. Alternatively, a bipolar single-chamber device with a Y-adapter connecting 2 unipolar leads implanted in the respective atria has been used to create physiologic pacing.23 The technical challenges of these systems are mapping and localizing donor and recipient atrial activity, which Bexton et al24 reported was possible in about 70% of patients. A comparative study looking at the impact of various pacing modalities on exercise performance in patients without rejection demonstrated that restoration of chronotropic competence by recipient-to-donor atrial pacing increased exercise duration and peak VO2.25
CONCLUSION
Despite the simplicity of a VVI system, the relative merits of physiologic pacing have seen DDDR or AAIR pacing become the standard for pacing in posttransplant patients.9 A unique mode of pacing for posttransplant patients, biatrial, uses the remnant as a physiologic sensor to achieve both AV synchrony and chronotropic competence. In patients with intact AV conduction, this method most closely approximates the chronotropic variability of the native heart, giving patients the maximum benefit from transplantation. Further studies are needed that assess the outcomes and long-term effects of the biatrial approach for pacemaker implantation following orthotopic heart transplantation.
Footnotes
Dr. Patel has been a consultant and speaker for Gilead Sciences, Actelion Pharmaceuticals, and United Therapeutics Corporation.
REFERENCES
- 1.Lower R. R., Shumway N. E. Studies on orthotopic transplantation of the canine heart. Surg Form. 1960;11:18–19. [PubMed] [Google Scholar]
- 2.Aziz T. M., Burgess M. I., El-Gamel A., et al. Orthotopic cardiac transplantation technique: a survey of current practice. Ann Thor Surg. 1999;68((4)):1242–1246. doi: 10.1016/s0003-4975(99)00796-1. [DOI] [PubMed] [Google Scholar]
- 3.Pavri B. B., O'Nunain S. S., Newell J. B., Ruskin J. N., William G. Prevalence and prognostic significance of atrial arrhythmias after orthotopic cardiac transplantation. J Am Coll Cardiol. 1995;25((7)):1673–1680. doi: 10.1016/0735-1097(95)00047-8. [DOI] [PubMed] [Google Scholar]
- 4.Cannom D. S., Graham A. F., Harrison D. C. Electrophysiological studies in the denervated transplanted human heart: response to trial pacing and atropine. Circ Res. 1973;32((2)):268–278. doi: 10.1161/01.res.32.2.268. [DOI] [PubMed] [Google Scholar]
- 5.Bexton R. S., Nathan A. W., Hellestrand K. J., et al. The electrophysiologic characteristics of the transplanted heart. Am Heart J. 1984;107((1)):1–7. doi: 10.1016/0002-8703(84)90124-8. [DOI] [PubMed] [Google Scholar]
- 6.Cannom D. S., Rider A. K., Stinson E. B., Harrison D. C. Electrophysiologic studies in the denervated transplanted human heart II: response to norepinephrine, isoproterenol and propranolol. Am J Cardiol. 1975;36((7)):859–866. doi: 10.1016/0002-9149(75)90074-0. [DOI] [PubMed] [Google Scholar]
- 7.Gilbert E. M., Eiswith C. C., Mealey P. C., Larrabee P., Herrick C. M., Bristow M. R. Beta-adrenergic supersensitivity of the transplanted human heart is presynaptic in origin. Circulation. 1989;79((2)):344–349. doi: 10.1161/01.cir.79.2.344. [DOI] [PubMed] [Google Scholar]
- 8.Ellenbogen K. A., Thames M. D., DiMarco J. P., Sheehan H., Lerman B. B. Electrophysiological effects of adenosine in the transplanted human heart: evidence of supersensitivity. Circulation. 1990;81((3)):821–828. doi: 10.1161/01.cir.81.3.821. [DOI] [PubMed] [Google Scholar]
- 9.Melton I. C., Gilligan D. M., Wood M. A., Ellenbogen K. A. Optimal cardiac pacing after heart transplantation. Pacing Clin Electrophysiol. 1999;22((10)):1510–1527. doi: 10.1111/j.1540-8159.1999.tb00356.x. [DOI] [PubMed] [Google Scholar]
- 10.Scott C. D., Dark J. H., McComb J. M. Evolution of the chronotropic response to exercise after cardiac transplantation. Am J Cardiol. 1995;76((17)):1292–1296. doi: 10.1016/s0002-9149(99)80358-0. [DOI] [PubMed] [Google Scholar]
- 11.Douard H., Parrens E., Billes M. A., Labbe L., Baudet E., Broustet J. P. Predictive factors of maximal aerobic capacity after cardiac transplantation. Eur Heart J. 1997;18((11)):1823–1828. doi: 10.1093/oxfordjournals.eurheartj.a015178. [DOI] [PubMed] [Google Scholar]
- 12.Givertz M. M., Hartley L. H., Colucci W. S. Long-term sequential changes in exercise capacity and chronotropic responsiveness after cardiac transplantation. Circulation. 1997;96((1)):232–237. doi: 10.1161/01.cir.96.1.232. [DOI] [PubMed] [Google Scholar]
- 13.Miyamoto Y., Curtiss E. I., Kormos R. L., Armitage J. M., Hardesty R. L., Griffith B. P. Bradyarrhythmia after heart transplantation: incidence, time course, and outcome. Circulation. 1990;82((5)):IV313–IV317. [PubMed] [Google Scholar]
- 14.Cataldo R., Olsen S., Freedman R. A. Atrioventricular block occurring late after heart transplantation: presentation of three cases and literature review. Pacing Clin Electrophysiol. 1996;19((3)):325–330. doi: 10.1111/j.1540-8159.1996.tb03334.x. Comment in: Pacing Clin Electrophysiol. 1996;19(8):1272. [DOI] [PubMed] [Google Scholar]
- 15.Montero J. A., Anguita M., Concha M., et al. Pacing requirements after orthotopic heart transplantation: incidence and related factors. J Heart Lung Transplant. 1992;11((4, pt 1)):799–802. Comment in: J Heart Lung Transplant. 1993;12(3):536. [PubMed] [Google Scholar]
- 16.Woodard D. A., Conti J. B., Mills R. M., Jr, Williams R. A., Curtis A. B. Permanent atrial pacing in cardiac transplant patients. Pacing Clin Electrophysiol. 1997;20((10, pt 1)):2398–2404. doi: 10.1111/j.1540-8159.1997.tb06077.x. [DOI] [PubMed] [Google Scholar]
- 17.Scott C. D., Omar L., McComb J. M., Dark J. H., Bexton R. S. Long-term pacing in heart transplant recipients is usually unnecessary. Pacing Clin Electrophysiol. 1991;14((11, pt 2)):1792–1796. doi: 10.1111/j.1540-8159.1991.tb02768.x. [DOI] [PubMed] [Google Scholar]
- 18.Scott C. D., Dark J. H., McComb J. M. Sinus node function after cardiac transplantation. J Am Coll Cardiol. 1994;24((5)):1334–1341. doi: 10.1016/0735-1097(94)90117-1. [DOI] [PubMed] [Google Scholar]
- 19.Loria K., Salinger M., McDonough T., Frohlich T., Arentzen C. Activitrax AAIR pacing for sinus node dysfunction after orthotopic heart transplantation: an initial report. J Heart Transplant. 1988;7((5)):380–384. [PubMed] [Google Scholar]
- 20.Zieroth S., Ross H., Rao V., et al. Permanent pacing after cardiac transplantation in the era of extended donors. J Heart Lung Transplant. 2006;25((9)):1142–1147. doi: 10.1016/j.healun.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 21.Markewitz A., Kemkes B. M., Reble B., et al. Particularities of dual chamber pacemaker therapy in patients after orthotopic heart transplantation. Pacing Clin Electrophysiol. 1987;10((2)):326–332. doi: 10.1111/j.1540-8159.1987.tb05972.x. [DOI] [PubMed] [Google Scholar]
- 22.Markewitz A., Osterholzer G., Weinhold C., Kemkes B. M., Feruglio G. A. Recipient P wave synchronized pacing of the donor atrium in a heart-transplanted patient: a case study. Pacing Clin Electrophysiol. 1988;11((10)):1402–1404. doi: 10.1111/j.1540-8159.1988.tb04987.x. [DOI] [PubMed] [Google Scholar]
- 23.Kacet S., Molin F., Lacroix D., et al. Bipolar atrial triggered pacing to restore normal chronotropic responsiveness in an orthotopic cardiac transplant patient. Pacing Clin Electrophysiol. 1991;14((10)):1444–1447. doi: 10.1111/j.1540-8159.1991.tb04062.x. [DOI] [PubMed] [Google Scholar]
- 24.Bexton R. S., Nathan A. W., Hellestrand K. J., et al. Sinoatrial function after cardiac transplantation. J Am Coll Cardiol. 1984;3((3)):712–723. doi: 10.1016/s0735-1097(84)80247-8. [DOI] [PubMed] [Google Scholar]
- 25.Beniaminovitz A., Coromilas J., Oz M., Galantowicz M., Donchez L., Mancini D. Electrical connection of native and transplanted sinus nodes via atrial to atrial pacing improves exercise performance after cardiac transplantation. Am J Cardiol. 1998;81((11)):1373–1377. doi: 10.1016/s0002-9149(98)00173-8. [DOI] [PubMed] [Google Scholar]