Abstract
The information carried by transforming growth factor β (TGF-β) signaling molecules induces profound responses in target cells. To restrict this information to appropriate cells, TGF-β signaling pathways are tightly regulated by dynamic interactions with transcriptional activators and repressors. Numerous cross-species experiments have shown that TGF-β family members and their signal transduction machinery (receptors and Smad signal transducers) are functionally conserved between vertebrates and invertebrates. TG-interacting factor (TGIF) is a homeodomain-containing transcriptional corepressor of TGF-β-dependent gene expression in mammals that is associated with holoprosencephaly in humans. Here we report a biochemical analysis of TGIF from zebra fish and Drosophila. Our study reveals an unprecedented role reversal between vertebrate and invertebrate TGIF proteins. Zebra fish TGIF, like its mammalian relative, interacts with general corepressors and represses TGF-β-responsive gene expression. We identified a tandem duplication of TGIF genes in Drosophila. In contrast to vertebrate TGIFs, both Drosophila TGIFs strongly activate transcription. We also demonstrate that Drosophila TGIF proteins physically interact with both Mad and dSmad2, suggesting a role in Dpp and activin signaling. Thus, dTGIF may be the first transcription factor in the Drosophila activin pathway. Overall, our study suggests that assumptions about the functional equivalence of conserved proteins must be validated experimentally.
Homeodomain proteins were first discovered in Drosophila as regulators of segment identity. Homeobox genes have been identified in diverse organisms and encode transcriptional factors, which regulate multiple developmental processes (16, 36, 37). The homeodomain is a structurally conserved 60-amino-acid protein module that consists of three alpha helices (16, 17). Outside the homeodomain itself, homeodomain proteins are considerably divergent and play roles in transcriptional repression or activation. Homeodomains are both DNA binding and protein interaction domains. The third alpha helix plays the major role in DNA binding, and these proteins often bind to relatively simple DNA sequence motifs (5). The first two helices of many homeodomain proteins are important for protein-protein interactions, which can confer altered DNA-binding specificity (21, 25, 31). For example, interaction of Ubx with Exd or of Pbx1 with HoxB1 results in the recognition of an expanded composite binding site on DNA, with both proteins making specific DNA contacts (46, 47). Members of the TALE (three-amino-acid loop extension) superfamily of homeodomain proteins are characterized by the presence of a 3-amino-acid insertion between helices 1 and 2 of the homeodomain (5, 9). This insertion is unlikely to affect DNA binding, but it can play a role in interactions with other proteins (46, 47). TALE superfamily homeodomain proteins are present in many species and have been shown to activate and repress gene expression (1, 3, 4, 21, 23, 41, 44).
TG-interacting factor (TGIF), which is a TALE homeodomain protein, was first identified by its ability to bind to a specific retinoid response element (RXRE) from the cellular retinol binding protein II (CRBPII) gene (5). TGIF was shown to compete with retinoid receptors for binding to the CRBPII RXRE, resulting in reduced transcriptional activity. The consensus sequence to which TGIF binds was determined in vitro, and TGIF has recently been shown to bind to the dopamine 1A receptor (D1AR) promoter via a consensus TGIF site (5, 63). Interestingly, the Meis2 homeodomain protein also binds this site, and it has been suggested that Meis2 and TGIF compete with each other to activate or repress D1AR expression. TGIF is an active transcriptional repressor that interacts with multiple transcriptional corepressors, including mSin3, histone deacetylases, and CtBP (39, 53, 59-61). Thus, repression of gene expression by TGIF involves both competition with activators and the recruitment of general corepressor proteins. Recently, a second human TGIF-like protein (TGIF2) has been identified (20, 38). TGIF2 is a transcriptional repressor that interacts with histone deacetylase, but not with CtBP. However, TGIF2 appears to function similarly to TGIF in its ability to repress gene expression when bound directly to DNA or when recruited by other proteins (38).
Following the binding of TGF-β family ligands to their cell surface receptor complex, the receptors activate intracellular mediators, the Smad proteins (19, 33, 34, 65). Receptor-activated Smads (R-Smads) are phosphorylated directly by the type I receptors and then form a complex with the co-Smad (Smad4) and accumulate in the nucleus, where the activated Smad complex is recruited to specific target genes. In mammals, Smad1, Smad5, and Smad8 transduce signals of the BMP subfamily, while Smad2 and Smad3 transduce signals from the TGF-β/activin subfamily. Smads can bind directly to DNA or can be recruited by interactions with other transcription factors, giving them an important role in determining which genes are regulated in response to TGF-β family signals (12, 24, 35, 64). Once in the nucleus, a Smad complex activates gene expression, in part, by interactions with general coactivators such as p300/CBP (15, 22, 48). TGF-β/activin-activated Smads can also contact specific transcriptional corepressors, including TGIF, c-Ski, and SnoN (29, 54-56, 60). This results in the recruitment of a complex of general corepressors to the Smad target gene. It appears that the balance between coactivators and corepressors, with which Smads interact, can determine how efficiently they activate gene expression. Additionally, there is evidence for regulation of corepressor levels by TGF-β and by other signaling inputs, suggesting that competition between coactivators and corepressors may fine tune the cell's response to TGF-β/activin signals (28, 54).
Recent evidence points to an important role for TGIF in human brain development. Heterozygous mutation or deletion of the TGIF gene is associated with holoprosencephaly, a severe genetic defect affecting craniofacial development (18, 40, 45). However, it is not clear whether this is due to effects on TGF-β-dependent or TGF-β-independent pathways. Given the conservation of TGF-β family signaling pathways and the possible importance of TGIF in human brain development, we were interested to know how well conserved TGIF proteins are in nonmammalian species. Here we show that vertebrate TGIFs are functionally conserved and can repress TGF-β-activated gene expression. We also report that Drosophila melanogaster has two proteins (dTGIFs) with homeodomains that are structurally and functionally similar to human TGIF. However, in contrast to vertebrate TGIFs, dTGIFs are transcriptional activators that can physically interact with dMad and dSmad2, suggesting an important role in Drosophila TGF-β family signaling pathways, including the recently discovered dActivin pathway.
MATERIALS AND METHODS
Plasmids and DNA sequencing.
TGIF-related proteins were identified by searching with TGIF protein sequence against translated EST databases (tblastn) using the BLAST server (http://www.ncbi.nlm.nih.gov/BLAST/). EST clones for zebra fish and Drosophila TGIFs were obtained from the American Type Culture Collection and were fully sequenced on an ABI 377 Prism sequencer. The putative Xenopus TGIF sequence was derived by piecing together multiple sequences from the EST database. mTex1 was isolated by reverse transcription PCR (RT-PCR) from RNA from mouse testes prepared with TRIzol reagent. The predicted intron/exon structure for dTGIFa and dTGIFb was determined by comparing the EST sequences with the Drosophila genomic sequence. The 3TP-lux, and SBE-luc reporters have been described before (11, 64), and the (Gal)5TATA-luc contains five Gal4 binding sites upstream of a minimal E1b TATA element. The D1AR-luc was generated by PCR from D1AR-CAT (63), which was a kind gift of M. M. Mouradian. Mammalian expression constructs were created within modified versions of the pCMV5 vector, containing either a Flag or T7 epitope tag. dTGIFa, dTGIFb, dSmad2, and dMad coding sequences were transferred into pCMV5 by PCR and dTGIFa, and dTGIFb deletion constructs were generated by PCR. Human Smad3 was expressed from pCMV5 with no epitope tag. Gal4 DNA binding domain (GBD) fusions were created within pM (Clontech). Myc-Sin3 and T7-CtBP expression constructs are as previously described (39, 51, 59). Cyan fluorescent protein (CFP) and yellow fluorescent protein (YFP) fusions were created within a modified pCS2 vector, containing an amino-terminal enhanced CFP (eCFP) or eYFP tag (BD Biosciences). YFP-NLS, was created by inserting a double-stranded nuclear localization signal (NLS) oligonucleotide into the pCS2-YFP vector.
Cell culture and transfections.
HepG2 cells were grown in Dulbecco's modified Eagle's medium (DMEM), and mink lung epithelial L17 cells were grown in MEM with nonessential amino acids supplemented with 10% fetal bovine serum. L17 cells were transfected in six-well plates by using DEAE-dextran as previously described (60). HepG2 cells were transfected with Exgen 500 (MBI Fermentas) according to the manufacturer's instructions. COS-1 cells were maintained in DMEM with 10% fetal bovine serum and transfected in 60-mm-diameter dishes with Lipofectamine (Gibco BRL) according to the manufacturer's instructions.
Reporter assays.
L17 or HepG2 cells were cotransfected with the appropriate firefly luciferase reporter and pCMV-RL, and luciferase activity was assayed after 40 to 48 h. For assays involving TGF-β addition, a TGF-β type I receptor expression vector (10) was cotransfected, and 100 pM TGF-β (R & D Systems) was added 18 h prior to assaying. Firefly luciferase was assayed with a luciferase assay kit (Promega), and Renilla luciferase activity was assayed with 0.09 μM coelentrazine (Biosynth) in a mixture of 25 mM Tris (pH 7.5) and 100 mM NaCl. Luciferase activities were assayed with a Berthold LB 953 luminometer.
Immunoprecipitation and Western blotting.
COS-1 cells were lysed by sonication in MSHD (100 mM NaCl, 20 mM HEPES [pH 7.8], 10% glycerol, 1% NP-40) or phosphate-buffered saline (PBS) with 1% NP-40, supplemented with protease inhibitors (protease inhibitor cocktail; Roche). Following removal of cell debris by centrifugation, lysates were precleared with protein A Sepharose, and complexes were precipitated on Flag agarose (Sigma) or T7 agarose (Novagen). After washing, proteins were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to Immobilon-P (Millipore). Blots were incubated with the appropriate antisera (anti-Flag M2, Sigma; anti-T7, Novagen; anti-Smad2/3, Upstate Biotechnology; or anti-Myc, Sigma) and either horseradish peroxidase-conjugated goat anti-mouse or goat anti-rabbit (Pierce). Proteins were visualized by the ECL enhanced chemiluminescence system (Amersham). For direct Western blotting, a portion of the cleared lysate was subjected to SDS-PAGE and Western blotting as described above.
Fluorescence microscopy.
COS-1 cells were split onto four-well chamber slides (Nunc) and transfected with eCFP- or eYFP-tagged fusion proteins by using Fugene 6 (Roche). After 22 to 26 h, cells were imaged with a Zeiss Axiovert 135T inverted fluorescence microscope on a heated stage with CFP and YFP filter sets (Omega Opticals). Images were visualized and captured with a Zeiss 32×/0.40 objective and a Hamamatsu Orca II cooled charge-coupled device (CCD) camera controlled by Isee software (Inovision). Images were converted to 8-bit .tif files by using Isee and manipulated in Photoshop 6.0.
RESULTS
TGIF is conserved in vertebrates.
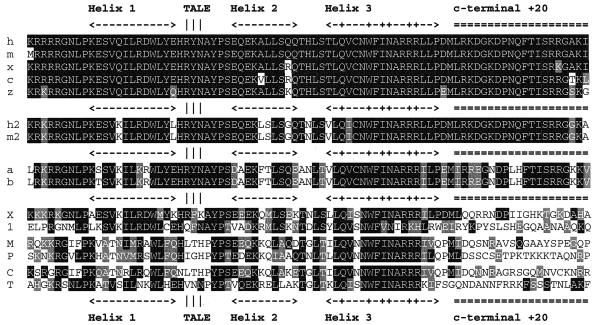
The TGIF protein is conserved between humans and mice, and a related protein (AKR) is present in chickens (5, 6, 50). We were interested to know whether TGIF was also conserved in other species. Screening of the EST databases revealed the presence of multiple overlapping sequences from both Xenopus and zebra fish, which were highly related to human TGIF (see Fig. 2). In particular, the homeodomain and a short region carboxyl terminal to it (HD +20) are highly conserved across all five species (Fig. 1 and 2). In addition, this region (HD +20) is present in the related TGIF2 protein from humans (20, 38). Interestingly, the +20 region is not conserved in other human TALE superfamily proteins. The two human proteins with homeodomains most similar to TGIF/TGIF2 are Meis2 and Prep1. As shown in Fig. 1 (M and P in the lowest homology group), neither of these shows any significant similarity to TGIF in the +20 region. To identify other TGIF-related proteins, we decided to search the sequence databases with the homeodomain plus the 20-amino-acid carboxyl-terminal extension (HD +20). With the availability of the full genome sequences of several nonvertebrate species, we were able to search for the presence of TGIF-like proteins in Saccharomyces cerevisiae, Caenorhabditis elegans, and D. melanogaster. We considered proteins to be TGIF related if the homeodomain was more than 70% identical and if there was greater than 50% identity in the 20 amino acids carboxyl terminal to it. Analysis of the S. cerevisiae and C. elegans genome databases revealed no TGIF-like proteins, although other more distantly related TALE superfamily homeodomain proteins were present. The homeodomains of the most similar protein from each species are shown in the lowest homology block in Fig. 1 (C, C. elegans CEH-25; and T, S. cerevisiae Tos8p). Interestingly, in the Drosophila genome database, two predicted proteins, which we term “dTGIFa” and “dTGIFb” (a and b in the third homology group, Fig. 1) were present, which had a high degree of similarity to the TGIF HD +20. Little similarity outside this region was observed, and these proteins are discussed in more detail below. In contrast, the vertebrate TGIF homologs share considerable sequence similarity outside the HD +20 region (Fig. 2). Sequences amino terminal to the homeodomain, including the CtBP-interaction motif (PLDLS) (14, 39, 57), are similar, and there is a major block of homology encompassing the carboxyl-terminal 44 amino acids. However, within the central region of TGIF from these species, there is little homology apart from a short stretch from amino acid 159 to amino acid 179, suggesting that the region between the homeodomain and carboxyl terminus may be a nonconserved linker region. In addition to the HD +20 region, the carboxyl-terminal homology block is also conserved in human and mouse TGIF2. This region is likely the major site of interaction with the corepressor mSin3 (59). Thus, it appears that TGIF is conserved in vertebrates, but direct homologs are not easily identified in nonvertebrate species.
FIG. 2.
Alignment of vertebrate TGIF sequences. The deduced amino acid sequences of TGIF from human, mouse, chicken, Xenopus, and zebra fish (h, m, c, x, and z, respectively) are shown in the upper group. Human and mouse (h2 and m2) TGIF2 sequences are shown in the middle block, and the TGIF-related proteins human TGIFLX (X) and mouse Tex1 (indicated by 1) are shown in the lower group. The homeodomain is indicated by single dashes, and the +20 region is indicated by double dashes. The CtBP interaction motif is shown by double dots, and the conserved Sin3 interaction domain at the carboxyl termini is indicated by dashes. Within this region, asterisks indicate the position of mitogen-activated protein kinase sites, which have been shown to be phosphorylated in TGIF and TGIF2. A small region of similarity between TGIF1s only is shown by open circles. Amino acids that are identical or similar in at least five sequences are shaded black and gray, respectively.
FIG. 1.
The TGIF HD +20 region. The homeodomain and 20 amino acids carboxyl terminal to it are shown from TGIF (upper group: human, mouse, chicken, Xenopus and zebra fish, shown as h, m, c, x, and z, respectively), TGIF2 (second group: human and mouse, shown as h2 and m2, respectively), Drosophila TGIFa and -b (third group), and a selection of less closely related proteins (lower group). The proteins in this group are designated as follows: X, human TGIFLX; 1, mouse Tex1; M, human Meis2; P, human Prep1; C, C. elegans CEH-25; T, S. cerevisiae Tos8p. These represent two recently identified TGIF-related proteins—human TGIF-like on the X (X) and mouse Tex1 (indicated by 1)—the homeodomain from the two next-most-similar human TALE proteins (Meis2 and Prep1), and the most-similar homeodomain from S. cerevisiae and C. elegans. The positions of each of the three alpha helices are shown, together with the +20 region and the TALE. A “+” within helix 3 indicates the DNA contact residues. Amino acids that are identical or similar to human TGIF are shaded black or gray, respectively.
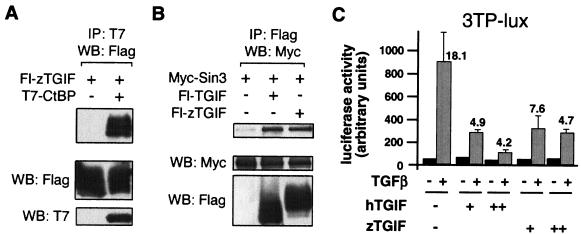
To determine whether the TGIF homologs shown in Fig. 1 are genuine functional homologs, we obtained EST clones for zebra fish TGIF, the most divergent of these five proteins. These clones were verified by DNA sequencing, and a Flag-tagged mammalian expression construct encoding amino acids 2 to 273 of zTGIF was created. COS-1 cells were cotransfected with the Flag-zTGIF expression vector together with T7-tagged CtBP, and proteins were precipitated on T7 agarose. As shown in Fig. 3A, Flag-zTGIF clearly coprecipitated with T7-CtBP, but was not present in a control immunoprecipitate. mSin3 has been shown to interact directly with the carboxyl-terminal region of human TGIF (59). To determine whether the zebra fish protein also interacts with mSin3, COS-1 cells were cotransfected with Flag-zTGIF and Myc-tagged mSin3. Proteins were precipitated on Flag-agarose and Western blotted for Myc-mSin3. Myc-mSin3 coprecipitated with both human and zebra fish TGIF (Fig. 3B), suggesting that the conserved carboxyl terminus of zTGIF is a site for interaction with Sin3. Human TGIF is a transcriptional corepressor, which interacts with TGF-β/activin-activated Smads and regulates the maximal transcriptional response to TGF-β (60). To test whether zTGIF performs the same function, mink lung epithelial (L17) cells were transfected with the TGF-β-responsive 3TP-lux reporter (11). As shown in Fig. 3C, coexpression of increasing amounts of either human or zebra fish TGIF resulted in repression of TGF-β-induced luciferase activity from the 3TP-lux reporter. Little repression by either human or zebra fish TGIF was observed in the absence of TGF-β. These results suggest that TGIF-like transcriptional repressors are present in many vertebrate species and that they are likely to function as transcriptional corepressors of TGF-β/activin-activated gene expression.
FIG. 3.
Zebra fish TGIF is a Smad transcriptional corepressor. (A) COS-1 cells were cotransfected with Flag-zTGIF and T7-CtBP expression vectors as indicated, and proteins were isolated on T7 agarose (immunoprecipitation [IP]) and analyzed by Western blotting (WB) for the presence of Flag-zTGIF. A portion of the lysates was analyzed by direct Western blotting for the presence of Flag-zTGIF and T7-CtBP (shown below). (B) COS-1 cells were cotransfected with Myc-Sin3 and Flag-tagged human or zebra fish TGIF expression vectors. Proteins were collected on Flag agarose and Western blotted for Myc-Sin3. Expression controls are shown below. (C) L17 cells were transfected with the 3TP-lux reporter with or without increasing amounts (30 or 100 ng per well) of a human or zebra fish TGIF expression vector. Cells were treated with TGF-β and assayed for luciferase activity 18 h later. Activity is presented as mean ± standard deviation of triplicate transfections in arbitrary units. The fold induction by TGF-β is shown for each condition.
Other vertebrate TGIF-related proteins.
Recent reports have described the presence of TGIF-related proteins in both mice and humans, termed mTex1 and TGIFLX (7, 26). To test whether these two proteins fit our criteria for being TGIF-related, we first compared the HD +20 regions from these proteins. As shown in Fig. 1 (lowest homology block, X, human TGIFLX; 1, mouse Tex1), neither mTex1 nor TGIFLX had a high degree of conservation with TGIF in the 20 amino acids carboxyl terminal to the homeodomain. Additionally, conservation in the homeodomain itself was considerably lower than for the vertebrate TGIFs, TGIF2s, and dTGIFa and -b. However, the third alpha helices, which are the major DNA binding helices, including the residues that contact DNA (marked “+” in Fig. 1), are similar between TGIF, TGIFLX, and mTex1, suggesting that at least TGIFLX will bind to the same DNA element as TGIF. Interestingly, both TGIFLX and mTex1 also have some homology with the conserved carboxyl-terminal region of TGIF and TGIF2 (Fig. 2), which interacts with mSin3. From this analysis, it appears that TGIFLX and particularly mTex1 may be distant relatives of TGIF, more similar than Meis2 or Prep1, because they both contain a second region of homology to TGIF.
TGIF-related proteins in Drosophila.
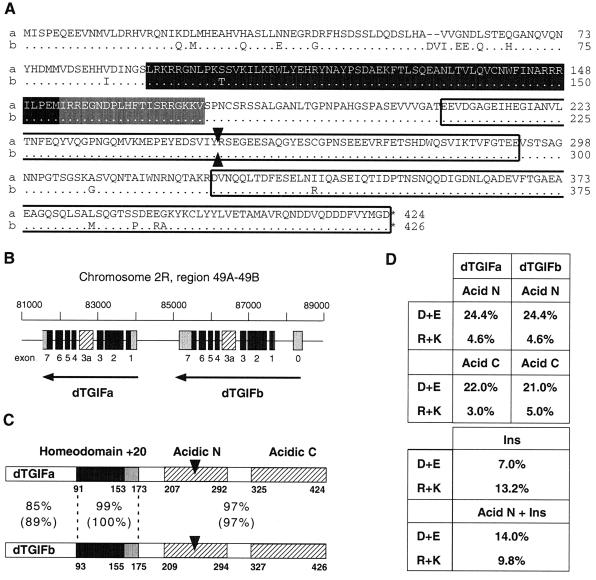
As shown in Fig. 1, the homeodomain, plus a 20-amino-acid region carboxyl terminal to it, is highly conserved across several vertebrate species. Interestingly, in the Drosophila genome database, two predicted proteins were present, which had a high degree of similarity to the TGIF HD +20. These two proteins (which we term dTGIFa and dTGIFb) appeared to be expressed from a pair of adjacent genes (Fig. 4B) and were originally annotated as CG8819 (dTGIFb) and CG8821 (dTGIFa). We obtained ESTs for both proteins and sequenced the entire open reading frames (ORFs), revealing two highly related ORFs of 424 and 426 codons. These two ORFs have recently been renamed Achintya and Vismay (dTGIFb and dTGIFa, respectively) and they have been shown to be important in spermatogenesis (2, 58).
FIG. 4.
TGIF-related proteins from Drosophila. (A) dTGIFa and dTGIFb protein products are almost identical at the primary amino acid level. The dTGIFa sequence is shown in full, with amino acids that are different in dTGIFb shown below (dots indicate identity). The homeodomain is shaded black, the +20 region is gray, and two regions with a high proportion of acidic residues are boxed. The arrowheads indicate the position of the extra exon. (B) dTGIFa and dTGIFb are expressed from an adjacent pair of genes. Coding exons are in black, noncoding exons are in gray, and an alternatively spliced exon, not present in the clones analyzed here, is striped (see text for details). (C) dTGIFa and dTGIFb are shown schematically, with the percent identity and similarity between the HD +20 and the regions amino and carboxyl terminal to it. The two acid regions are shown, together with the position of insertion of the extra exon. (D) The proportions of acidic (D+E [aspartic acid and glutamic acid]) and basic (R+K [arginine and lysine]) residues in each of the acidic regions are shown. Below is the proportion of acidic or basic residues in the inserted extra exon alone and in the acidic N region with the extra exon.
dTGIFa and dTGIFb encode extremely highly related proteins (Fig. 4A): the homeodomains are essentially identical in sequence, and even the least similar domain (amino terminal to the homeodomains) is 85% identical between them. The HD +20 region is very similar to that of human TGIF and TGIF2 (see Fig. 1; HD, 73% identity; +20, 60% identity to hTGIF), and the DNA contact residues within the homeodomains are identical, suggesting that they will be able to bind to the same DNA sequence as human TGIF. Further analysis of the primary amino acid sequence of the dTGIFs revealed the presence of two large acidic regions within the carboxyl-terminal halves of the proteins (Fig. 4C and D). However, no homology to human TGIF outside the HD +20 region is present, suggesting that they may function very differently. The recent reports of these proteins as Achintya and Vismay also identify an extra exon (Fig. 4B), which is included by alternative splicing, specifically in testes. Inclusion of this exon results in the insertion of 129 extra codons within the first acidic region (Fig. 4C), disrupting the acidic nature of this domain.
dTGIFs are transcriptional activators.
The presence of the homeodomain in dTGIFs suggests a nuclear function for these proteins. To determine their subcellular localization, the full-length ORFs of both dTGIFa and dTGIFb were fused in frame to an amino-terminal eCFP tag. CFP-dTGIFa or CFP-dTGIFb was coexpressed in COS-1 cells with a YFP-NLS fusion that identifies the nucleus. As shown in Fig. 5A, both proteins were nuclear at steady state, consistent with a role in transcription.
FIG. 5.
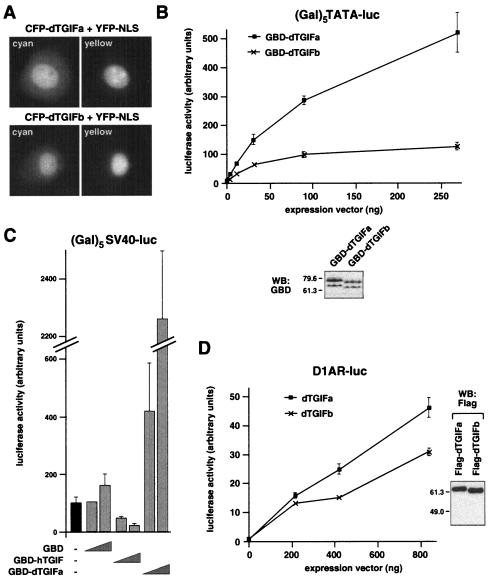
dTGIFa and dTGIFb are transcriptional activators. (A) dTGIFs are nuclear. Fusion proteins consisting of amino-terminal fusions of eCFP to either dTGIFa or dTGIFb were coexpressed in COS-1 cells with an eYFP-NLS protein to mark the nucleus. Individual cyan and yellow images are shown. (B) The full-length coding sequence of either dTGIFa or dTGIFb was fused to the GBD. Increasing amounts of GBD-dTGIF fusions were coexpressed in L17 cells transfected with a luciferase reporter in which the luciferase gene is activated by a minimal TATA element and multiple Gal4 binding sites. Relative expression of the maximum levels of transfected GBD-dTGIF fusions was assayed by Western blotting (WB) with an antibody against the GBD as shown below. (C) GBD, GBD-dTGIFa, or GBD-TGIF expression vectors (10 or 100 ng per well) were cotransfected into HepG2 cells with a (Gal)5-SV40 luciferase reporter, and luciferase activity was assayed as described for panel B. (D) L17 cells were cotransfected with increasing amounts of Flag-dTGIF expression vectors and a D1AR-luc reporter, in which the D1AR promoter drives luciferase expression. Relative expression levels of the Flag-dTGIFa and dTGIFb proteins are shown by Western blotting. Luciferase activity (mean ± standard deviation of triplicate transfections) is shown in arbitrary units.
Human TGIF is a transcriptional repressor that effectively represses the activity of several promoters when a GBD fusion of TGIF is tethered to DNA via multiple Gal4 binding sites (61). We created GBD fusions to full-length dTGIFa and dTGIFb and tested the effect of targeting these fusions to a minimal TATA-containing promoter via five Gal4 binding sites [(Gal)5TATA-luc]. As shown in Fig. 5B, transfection of L17 cells with increasing amounts of the GBD-dTGIFa construct together with the (Gal)5TATA-luc reporter resulted in increasing transcriptional activation of this promoter. Similar results were obtained with a GBD-dTGIFb fusion construct, although the level of transcriptional activation from this construct appeared to reach a plateau at a lower level. The GBD-dTGIFb fusion appeared to be maximally expressed at a slightly lower level than GBD-dTGIFa, which may partly account for this difference (Fig. 5B). Since this reporter has a very low basal activity, we performed a similar assay using the (Gal)5SV40-luc reporter to allow us to see both activation and repression. Again, GBD-dTGIFa activated this reporter, whereas a GBD fusion to human TGIF clearly repressed it (Fig. 5C).
Since the homeodomain, and specifically the third alpha helix, which is the major DNA binding helix, is highly conserved between human and Drosophila TGIFs, we tested the ability of dTGIFa and dTGIFb to activate expression via a TGIF binding site (CTGTCAA) (5). The promoter region of the D1AR gene contains a single TGIF binding site, and overexpression of human TGIF or Meis2 has been shown to modulate activity of a reporter (D1AR-CAT) regulated by this promoter (63). Cotransfection of increasing amounts of either dTGIFa or dTGIFb with a D1AR-luciferase reporter resulted in a clear activation of luciferase expression (Fig. 5D). In this case, the difference in activity between dTGIFa and dTGIFb was considerably less than with the GBD fusions, and both proteins appeared to be expressed to similar levels (Fig. 5D). This may be because the activity in this experiment has not reached a plateau or because the GBD fusions may not be expressed at equivalent levels. Taken together, these data demonstrate that, unlike human TGIF, Drosophila TGIF proteins are potent transcriptional activators.
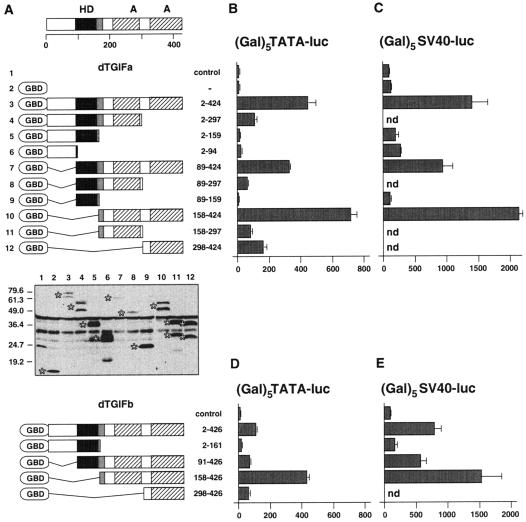
Drosophila TGIFs contain acidic activation domains.
To identify the region within dTGIFa that is responsible for the observed transcriptional activation, we created a series of GBD fusions to different portions of the dTGIFa protein (Fig. 6A). Each of these fusions was transfected into L17 cells with the (Gal)5TATA-luc reporter to determine their ability to activate expression from this reporter. As shown in Fig. 6B, deletion of the carboxyl-terminal 127 amino acids (to amino acid 297) greatly reduced transcriptional activation compared to the full-length fusion. Further truncation, to amino acid 159, almost completely abolished activation. In contrast, amino-terminal deletion to either amino acid 89 or 158 did not result in a loss of transcriptional activation. Thus, it appears that the region of dTGIFa carboxyl terminal to the homeodomain is required for activation of gene expression. This part of dTGIFa contains two acidic regions, both of which appear to be required for full transcriptional activation (compare construct 158-424 with constructs 158-297 and 298-424; Fig. 6B). The pattern of activation seen with these deletions appears to be largely independent of relative expression levels (Fig. 6A). To ensure that we had not uncovered the activity of a repression domain by deleting the activation domains of dTGIFa, we tested the activities of various GBD fusions on the (Gal)5SV40-luc reporter (Fig. 6C). A similar pattern of activation was seen, and none of the deletions tested appeared to repress transcription from this reporter. We also created and tested a smaller set of GBD fusions to dTGIFb, with similar results (Fig. 6D and E). Again it appeared that the transcriptional activation function resides in the region carboxyl terminal to the homeodomain and that both acidic regions are required. In the case of dTGIFb, deletion of the homeodomain and sequences amino terminal to it (construct 158-426; Fig. 6D) significantly enhanced activation relative to that seen with the full-length fusion, up to a level similar to that seen with dTGIFa. Together, these results demonstrate that dTGIFa and dTGIFb each contain a bipartite acid-rich transcriptional activation domain. This finding is consistent with recently reported genetic analyses of dTGIFa and dTGIFb (2, 58). These groups both showed that the testis-specific versions of dTGIFa and dTGIFb (with the additional exon in the first acidic domain) are required for expression of spermiogenesis and meiosis genes.
FIG. 6.
dTGIFs contain acidic activation domains. (A) The domains of dTGIFa are shown schematically above. The letter A represents the acidic region. A series of deletion mutants of dTGIFa fused to the GBD were created and assayed for expression levels by Western blotting with an antibody specific for the GBD. The specific bands are indicated by stars to the left of each lane. Lane numbers 1 to 12 refer to the numbers to the left of the schematic representation of the GBD-dTGIFa deletion series shown above. Amino acids contained in the fusion are shown to the right. The positions of molecular mass markers (in kilodaltons) are shown. A smaller series of GBD-dTGIFb deletions are shown schematically below. (B) The GBD-dTGIFa deletion series was cotransfected into L17 cells with the (Gal)5TATA-luc reporter. Luciferase activity (mean ± standard deviation of triplicate transfections) is shown, together with that from cells expressing the GBD alone or without any GBD fusion. (C) A subset of GBD-dTGIFa fusions were coexpressed with the (Gal)5-SV40 luciferase reporter to determine whether any of the nonactivating fusions could repress the activity of a promoter with a high basal activity. Luciferase activity was assayed, and the results are presented as in panel B. A series of GBD-dTGIFb deletions were cotransfected with the (Gal)5TATA-luc reporter (D) or the (Gal)5-SV40 luciferase reporter (E), and luciferase activity was assayed. The results are presented as described for panel B.
Human TGIF has been reported to repress transcription in part by competing with Meis2d for binding to a site within the D1AR promoter (63). To test whether dTGIFa could repress transcription by competition with another transcriptional activator, we coexpressed different combinations of Meis2d and dTGIFa in the presence of a transfected D1AR-luc reporter. Both proteins activated this reporter, and dTGIFa did not prevent Meis2 from activating transcription (data not shown). Thus, it appears unlikely that dTGIFs repress transcription by competing with other activators. However, we cannot rule out the possibility that the alternate splice forms of dTGIFa and -b are compromised for transcriptional activation and may be able to repress by competing with other activators.
Divergence of function between vertebrate and Drosophila TGIF-related proteins.
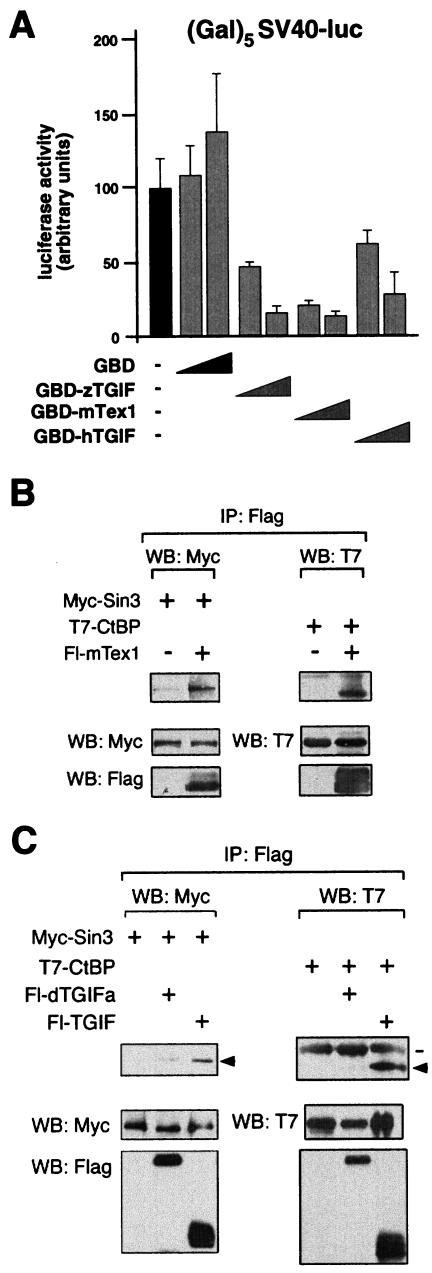
The fact that dTGIFs are potent activators, whereas human TGIF and TGIF2 and zebra fish TGIF are repressors, led us to test the transcriptional activity of a more distantly related mammalian TGIF-like protein. We isolated the coding sequence of mTex1 by RT-PCR from RNA isolated from mouse testes. Clones were verified by DNA sequencing and used to create both a Flag-tagged mTex1 expression construct and a GBD-mTex1 fusion. To determine whether mTex1 is an activator or repressor, HepG2 cells were cotransfected with the (Gal)5SV40-luc reporter and increasing amounts of expression vectors encoding GBD fusions to human or zebra fish TGIF or mTex1. As shown in Fig. 7A, all three fusions were able to repress transcription from this reporter, whereas the GBD alone resulted in a slight increase in activity. Next we tested the ability of mTex1 to interact with the corepressors mSin3A and CtBP. When Flag mTex1 was immunoprecipitated from transfected COS-1 cells, both mSin3A and CtBP were present in these immunocomplexes (Fig. 7B). In contrast, when we performed similar coimmunoprecipitation experiments with dTGIFa and either T7-CtBP or Myc-tagged mSin3, we were unable to detect a clear interaction with either protein, compared to the interactions seen with human TGIF (Fig. 7C). This result is consistent with the lack of transcriptional repression by dTGIFa. Together, these results suggest that vertebrate TGIF, TGIF2, and more distantly related TGIF-like proteins (such as mTex1) are transcriptional repressors, whereas the Drosophila TGIFs have the opposite function.
FIG. 7.
mTex1 is a transcriptional repressor. (A) HepG2 cells were cotransfected with the (Gal)5-SV40 luciferase reporter and two amounts (5 or 30 ng) of expression vectors encoding GBD alone or fusions of full-length human or zebra fish TGIF to the GBD or aGBD-mTex1 fusion. Luciferase activity (mean ± standard deviation of triplicate transfections) is shown, together with that from cells without any GBD fusion. (B) Flag-tagged mTex1 was coexpressed in COS-1 cells with expression vectors encoding either Myc-tagged mSin3 or T7-tagged CtBP, and proteins were isolated on Flag agarose. Coprecipitating proteins are indicated by arrows, and expression in the lysates (assayed by direct Western blotting [WB]) is shown below. (C) Flag-tagged dTGIFa, or human TGIF as a positive control, was coexpressed in COS-1 cells with expression vectors encoding either Myc-tagged mSin3 or T7-tagged CtBP, and proteins were isolated on Flag agarose. Coprecipitating proteins are indicated by arrows, and immunoglobulin heavy chain is indicated by a line. Protein expression in the lysates was assayed by direct Western blotting and is shown below.
dTGIFa interacts with dSmad2 and dMad.
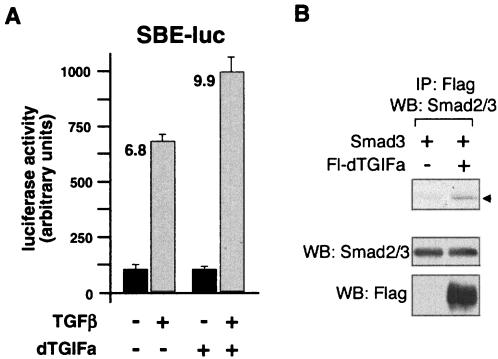
Human and zebra fish TGIF are able to specifically repress the activity of TGF-β/activin-responsive transcriptional reporters. In contrast, dTGIFs are potent transcriptional activators, suggesting that if they function in TGF-β family pathways, they may be activators of signaling. To test whether dTGIFa could have any effect on TGF-β/activin-activated gene expression, we tested its effect on the activity of the SBE-luc reporter in mink lung epithelial cells (L17). This reporter contains four copies of the Smad binding element (SBE) driving the luciferase reporter gene and is induced by TGF-β signaling (64). Cells were transfected with the SBE-luc reporter, with or without cotransfection of a dTGIFa expression vector, and luciferase activity was assayed after cells had been treated with TGF-β for 18 h or left untreated. As shown in Fig. 8A, coexpression of dTGIFa resulted in an increase in activation of this reporter in the presence of added TGF-β, suggesting that dTGIFa may be able to interact with mammalian TGF-β-activated Smads.
FIG. 8.
dTGIFa and TGF-β signaling. (A) L17 cells were transfected with either a control or a dTGIFa expression vector together with a reporter with eight copies of the SBE driving luciferase (SBE-luc). Cells were either treated with TGF-β for 18 h or left untreated and then assayed for luciferase activity, which is presented as mean ± standard deviation of triplicate transfections. Fold activation by TGF-β is shown. (B) COS-1 cells were cotransfected with an expression vector encoding Smad3 together with either a Flag-dTGIFa or control vector. Proteins were immunoprecipitated (IP) on Flag agarose and Western blotted (WB) for the presence of Smad3. Lysates were analyzed for protein expression by direct Western blotting (shown below). Coprecipitating Smad3 is indicated with an arrowhead.
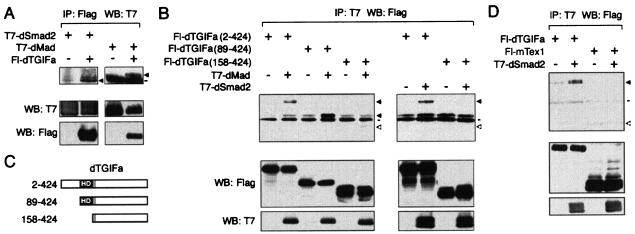
Since human TGIF interacts with Smad proteins to regulate TGF-β/activin signaling, the effect of dTGIFa overexpression on TGF-β/activin-responsive reporters in mink lung cells may be due to an interaction with Smad2 or Smad3. To test whether dTGIFa could interact with human Smad3, we cotransfected COS-1 cells with expression vectors encoding both proteins, and immunoprecipitated dTGIFa via the Flag epitope. As shown in Fig. 8B, Smad3 appeared to coprecipitate with Flag-dTGIFa, whereas very little Smad3 was present in control immunocomplexes. These results raised the possibility that dTGIFs may participate in TGF-β family signaling pathways in Drosophila. To test this possibility, we coexpressed dTGIFa with either dSmad2 or dMad in COS-1 cells. dMad is a founding member of the Smad family and is a critical mediator of Dpp signaling in Drosophila. Dpp is a member of the BMP subfamily (42, 49, 52). dSmad2 is homologous to the vertebrate Smad2 and Smad3 proteins (43). dSmad2 has recently been shown to transduce dActivin signals in Drosophila (8, 32, 43), just as Smad2 and Smad3 transduce signals of the TGF-β/activin subfamily in vertebrates. As shown in Fig. 9A, T7-tagged dSmad2 clearly coprecipitated with Flag-dTGIFa, suggesting that these two proteins are capable of interacting and that dTGIFs may regulate dActivin signaling. In addition, dTGIFa interacted with dMad (Fig. 9A), suggesting that dTGIFa may also play a role in regulating Dpp signaling. To further confirm these results, we performed similar coimmunoprecipitation experiments, in which we isolated T7-tagged dMad or dSmad2 on T7-agarose and Western blotted for the presence of various Flag-tagged dTGIFa constructs (Fig. 9C). As shown in Fig. 9B, full-length dTGIFa (amino acids 2 to 424) coprecipitated with both dMad and dSmad2, but was not present in a control precipitate. In contrast, deletion of the first 157 amino acids from dTGIFa, which removes the homeodomain, abolished the interaction with both dMad and dSmad2. Fl-dTGIFa (construct 89-424), which retains the homeodomain, but lacks the region amino terminal to it, was still able to interact with dMad (Fig. 9B). Thus it appears that dTGIFa interacts with Drosophila Smad proteins via the homeodomain. To test whether this interaction of dSmad2 and dMad with dTGIFa was simply a reflection of their ability to interact with any homeodomain protein, we tested interaction of mTex1 with dSmad2. As shown in Fig. 9D, mTex1 did not interact with dSmad2. Although this does not rule out the possibility of an interaction of mTex1 with mouse Smad proteins, it does suggest that not all TALE homeodomains interact with dSmad2. Together, these results suggest that dTGIF proteins may play a role in regulating the Dpp and dActivin signaling pathways in Drosophila.
FIG. 9.
dTGIFa interacts with dSmad2 and dMad. (A) COS-1 cells were cotransfected with expression vectors encoding T7-dSmad2 or T7-dMad, together with either a Flag-dTGIFa or control vector. Proteins were immunoprecipitated (IP) on Flag agarose and Western blotted (WB) for the presence of T7-tagged dSmad2 and dMad. Lysates were analyzed for protein expression by direct Western blotting (shown below). Coprecipitating proteins are indicated with arrows. The line indicates the position of the immunoglobulin heavy chain. (B) COS-1 cells were cotransfected with expression vectors encoding either Fl-dTGIFa (construct 2-424), Fl-dTGIFa (construct 89-424), or Fl-dTGIFa (construct 158-424), with either a control vector or one encoding T7-dSmad2 or T7-dMad, as indicated. Proteins were precipitated on T7-agarose and analyzed for the presence of Flag-dTGIFa proteins. Coprecipitating Fl-dTGIFa proteins are indicated by an arrow, and the expected position of Fl-dTGIFa (construct 158-424) is indicated by an open arrow. Immunoglobulin heavy chain is indicated by a bar. Protein expression in the lysates analyzed by direct Western blotting is shown below. (C) The dTGIFa expression constructs used in panel B are shown schematically. (D) COS-1 cells were cotransfected with Flag-tagged dTGIFa or mTex1, with or without T7-dSmad2, and immunocomplexes were isolated on T7-agarose. Coprecipitating dTGIFa is indicated by a solid arrowhead, and the expected position of Fl-mTex1 is indicated by an open arrowhead. Expression of proteins was analyzed by direct Western blotting (shown below).
DISCUSSION
Evolutionary conservation of TGIF.
Alignment of TGIF homologs from several vertebrate species suggests the presence of two highly conserved domains: the homeodomain and an approximately 40-amino-acid region at the extreme carboxyl-terminus, which we have previously identified as a Sin3-binding transcriptional repression domain (59). Conserved sequences surrounding the homeodomain include a 20-amino-acid region carboxyl terminal to it and the region amino terminal to the homeodomain. This region includes the PLDLS motif that binds CtBP and is required for full transcriptional repression by TGIF (39). Interestingly, the related protein, TGIF2 contains both of these conserved regions, but lacks the PLDLS and does not interact with CtBP (38). The region separating the two homology blocks is considerably less well conserved among vertebrate TGIFs and shares almost no significant homology between TGIF and TGIF2. Thus, it appears that vertebrate TGIF/TGIF2 proteins are comprised of two conserved regions separated by a more variable linker region.
TGIF-like homeodomain proteins in Drosophila.
The best-described function of human TGIF is as a repressor of TGF-β/activin-activated gene expression (60). In Drosophila, two genes appear to encode highly related TGIF-like homeodomain proteins. These two genes are very similar to each other in sequence and intron/exon structure and are located directly adjacent to each other, suggesting a tandem duplication. We have termed the protein products of these genes “dTGIFa” and “dTGIFb,” since it is likely that they can bind a TGIF consensus sequence (CTGTCAA) (5), for which mammalian TGIF was named. The dTGIFa and dTGIFb homeodomains are more similar to that of vertebrate TGIF than to any other homeodomain protein. Further reinforcing this similarity is the presence of a 20-amino-acid block of similarity directly carboxyl terminal to the homeodomain, which is not found in other TALE superfamily homeodomains. Many homeodomain proteins, particularly those of the TALE superfamily, bind to DNA as multiprotein complexes, often including two or three homeodomain proteins. The similarity between human and Drosophila TGIF may therefore extend to the type of proteins with which these factors interact when bound to DNA. For both vertebrate and Drosophila TGIFs, it is of interest to identify other factors they associate with when bound to DNA.
dTGIFs are activators.
Human TGIF and TGIF2 are active transcriptional repressors, which interact with multiple corepressor proteins. Interestingly, even the more distantly related mTex1 is also a transcriptional repressor, suggesting that all TGIF family members in vertebrates are likely to be repressors. In contrast, we have shown that dTGIFa and dTGIFb are potent transcriptional activators and that they activate gene expression whether bound directly to DNA via the homeodomain or when tethered to DNA by the heterologous GBD. dTGIFs appear to contain a relatively large transcriptional activation domain, which is rich in acidic residues. Acidic activation domains are common in transcriptional regulators (30), and it will be interesting to uncover the mechanism of action of the dTGIF activation domain. Despite their overall similarity, dTGIFb appears to be a slightly weaker transcriptional activator, and there are some differences between the carboxyl-terminal acidic regions of the two proteins. However, most of the sequence differences between dTGIFa and dTGIFb are amino terminal to the homeodomain. Interestingly, deletion of the amino-terminal region and the homeodomain results in better transcriptional activation in the GBD fusion assay by both proteins, but particularly by dTGIFb. It is therefore tempting to speculate that the amino termini may contain a regulatory region that modulates the activity of the activation domain. However, we cannot rule out the possibility that the lower activity of the full-length GBD fusion is due to an effect of the fusion itself.
dTGIFs and TGF-β signaling.
Comparison of the primary amino acid sequences of human and Drosophila TGIFs reveals no significant homology outside the HD +20 region. However, it appears that dTGIFa, like human TGIF interacts with Smad proteins, which are the critical mediators of TGF-β family signaling. Additionally, we show that the interaction of dTGIFa with dSmad2 and dMad is mediated at least in part by the homeodomain, suggesting that the homeodomain of human TGIF may also contribute to interactions with Smads. In support of this possibility, we have data to suggest that the interaction between human TGIF and Smad3 is at least partly mediated via the TGIF homeodomain (C.A.H. and D.W., unpublished observations). We have shown here that dTGIFa interacts with human Smad3 and more importantly with both dSmad2 and dMad and that this interaction requires the dTGIFa homeodomain. Human Smad1, a mediator of BMP signaling, has been shown to interact with Hoxc8, and this interaction appears to require the Hoxc8 homeodomain (62). Thus, it is possible that the interaction of homeodomain-containing proteins with Smad family proteins may be a common type of protein-protein interaction. However, we were unable to observe an interaction between mTex1 and dSmad2, suggesting that there is some further level of specificity in Smad-homeodomain interactions.
The interaction of dTGIFa with dSmad2 or dMad might be expected to function in one of three ways. First, dTGIFa might act as a transcriptional coactivator for dSmad2/dMad, whereby dSmad2 or dMad would be the factor that recruits dTGIFa to DNA, analogous to the recruitment of human TGIF by human Smads. Alternatively, dTGIFa could bind to its cognate DNA site and recruit dSmad2 or dMad to further activate gene expression in response to signaling. We have so far been unable to observe this second kind of complex with human TGIF- and TGFβ-activated Smads. Third, it is possible that both dTGIFa and dSmad2 bind to DNA together, creating a composite binding site, resulting in the activation of genes that would not be fully activated by either protein alone. This mode of action would be similar to the recruitment of vertebrate TGF-β-responsive Smads by FAST proteins to an activin response element (12, 13, 27). The effect of dTGIFa on the TGF-β-responsive SBE-luc reporter suggests that the first possibility—dTGIFa acting as a Smad coactivator—is perhaps more likely.
Our results provide strong biochemical support for the genetic analysis (and predictions derived from them about TGIF function) recently reported by Wang and Mann (58) and Ayyar et al. (2). Our demonstration that both dTGIFs are transcriptional activators agrees with their data showing that the dTGIFs appear to act as transcriptional activators. For example, Ayyar et al. (2) show that mutations eliminating both dTGIF genes markedly decrease the expression of Cyclin B and Twine, two genes required for entry into meiosis. Wang and Mann (58) show that mutations eliminating both dTGIF genes block spermatocyte development prior to spermatid differentiation. In addition, Wang and Mann (58) end a discussion of physical interactions between vertebrate TGIF and Smad 2, by proposing that the activity of an as-yet-unidentified TGF-β pathway is implied by their spermatogenesis data. Our demonstration that dTGIF physically interacts with dSmad2 (a signal transducer for the dActivin pathway) supports this proposal. However, genetic analysis of spermatogeneisis in dSmad2 mutants (66) is needed to provide clear evidence for the hypothesis that the dActivin pathway is involved in spermatogenesis.
In summary, we show that the proteins encoded by the Drosophila TGIF genes are potent transcriptional activators and that they may regulate both Dpp and dActivin signaling. Further, dTGIFa is the first dSmad2-interacting transcription factor and, as such, likely plays an important positive role in the dActivin pathway. Finally, our results raise the interesting possibility that TGIF proteins have opposite functions in TGF-β/activin subfamily signaling in Drosophila and vertebrates.
Acknowledgments
We thank Guillermo Marques for the dSmad2 cDNA and for valuable discussions and M. M. Mouradian for the D1AR-CAT reporter.
This work was supported by an NIH grant to D.W. (HD39926). S.J.N.'s laboratory is also supported by NIH grant CA95875.
REFERENCES
- 1.Asahara, H., S. Dutta, H.-Y. Kao, R. M. Evans, and M. Montminy. 1999. Pbx-Hox heterodimers recruit coactivator-corepressor complexes in an isoform-specific manner. Mol. Cell. Biol. 19:8219-8225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ayyar, S., J. Jiang, A. Collu, H. White-Cooper, and R. A. White. 2003. Drosophila TGIF is essential for developmentally regulated transcription in spermatogenesis. Development 30:2841-2852. [DOI] [PubMed] [Google Scholar]
- 3.Berthelsen, J., V. Zappavigna, E. Ferretti, F. Mavilio, and F. Blasi. 1998. The novel homeoprotein Prep1 modulates Pbx-Hox protein cooperativity. EMBO J. 17:1434-1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berthelsen, J., V. Zappavigna, F. Mavilio, and F. Blasi. 1998. Prep1, a novel functional partner of Pbx proteins. EMBO J. 17:1423-1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bertolino, E., B. Reimund, D. Wildt-Perinic, and R. Clerc. 1995. A novel homeobox protein which recognizes a TGT core and functionally interferes with a retinoid-responsive motif. J. Biol. Chem. 270:31178-31188. [DOI] [PubMed] [Google Scholar]
- 6.Bertolino, E., S. Wildt, G. Richards, and R. G. Clerc. 1996. Expression of a novel murine homeobox gene in the developing cerebellar external granular layer during its proliferation. Dev. Dyn. 205:410-420. [DOI] [PubMed] [Google Scholar]
- 7.Blanco-Arias, P., C. A. Sargent, and N. A. Affara. 2002. The human-specific Yp11.2/Xq21.3 homology block encodes a potentially functional testis-specific TGIF-like retroposon. Mamm. Genome 13:463-468. [DOI] [PubMed] [Google Scholar]
- 8.Brummel, T., S. Abdollah, T. E. Haerry, M. J. Shimell, J. Merriam, L. Raftery, J. L. Wrana, and M. B. O'Connor. 1999. The Drosophila activin receptor baboon signals through dSmad2 and controls cell proliferation but not patterning during larval development. Genes Dev. 13:98-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Burglin, T. R. 1997. Analysis of TALE superclass homeobox genes (MEIS, PBC, KNOX, Iroquois, TGIF) reveals a novel domain conserved between plants and animals. Nucleic Acids Res. 25:4173-4180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cárcamo, J., F. M. B. Weis, F. Ventura, R. Wieser, J. L. Wrana, L. Attisano, and J. Massagué. 1994. Type I receptors specify growth-inhibitory and transcriptional responses to transforming growth factor β and activin. Mol. Cell. Biol. 14:3810-3821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cárcamo, J., A. Zentella, and J. Massagué. 1995. Disruption of transforming growth factor β signaling by a mutation that prevents transphosphorylation within the receptor complex. Mol. Cell. Biol. 15:1573-1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen, X., M. J. Rubock, and M. Whitman. 1996. A transcriptional partner of MAD proteins in TGF-β signalling. Nature 383:691-696. [DOI] [PubMed] [Google Scholar]
- 13.Chen, X., E. Weisberg, V. Fridmacher, M. Watanabe, G. Naco, and M. Whitman. 1997. Smad4 and FAST-1 in the assembly of activin-responsive factor. Nature 389:85-89. [DOI] [PubMed] [Google Scholar]
- 14.Chinnadurai, G. 2002. CtBP, an unconventional transcriptional corepressor in development and oncogenesis. Mol. Cell 9:213-224. [DOI] [PubMed] [Google Scholar]
- 15.Feng, X.-H., Y. Zhang, R.-Y. Wu, and R. Derynck. 1998. The tumor suppressor Smad4/DPC4 and transcriptional adaptor CBP/p300 are coactivators for Smad3 in TGF-β-induced transcriptional activation. Genes Dev. 12:2153-2163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gehring, W. J., M. Affolter, and T. Burglin. 1994. Homeodomain proteins. Annu. Rev. Biochem. 63:487-526. [DOI] [PubMed] [Google Scholar]
- 17.Gehring, W. J., Y. Q. Qian, M. Billeter, K. Furukubo-Tokunaga, A. F. Schier, D. Resendez-Perez, M. Affolter, G. Otting, and K. Wuthrich. 1994. Homeodomain-DNA recognition. Cell 78:211-223. [DOI] [PubMed] [Google Scholar]
- 18.Gripp, K. W., D. Wotton, M. C. Edwards, E. Roessler, L. Ades, P. Meinecke, A. Richieri-Costa, E. H. Zackai, J. Massague, M. Muenke, and S. J. Elledge. 2000. Mutations in TGIF cause holoprosencephaly and link NODAL signalling to human neural axis determination. Nat. Genet. 25:205-208. [DOI] [PubMed] [Google Scholar]
- 19.Heldin, C.-H., K. Miyazono, and P. ten Dijke. 1997. TGF-β signalling from cell membrane to nucleus through SMAD proteins. Nature 390:465-471. [DOI] [PubMed] [Google Scholar]
- 20.Imoto, I., A. Pimkhaokham, T. Watanabe, F. Saito-Ohara, E. Soeda, and J. Inazawa. 2000. Amplification and overexpression of TGIF2, a novel homeobox gene of the TALE superclass, in ovarian cancer cell lines. Biochem. Biophys. Res. Commun. 276:264-270. [DOI] [PubMed] [Google Scholar]
- 21.Jacobs, Y., C. A. Schnabel, and M. L. Cleary. 1999. Trimeric association of Hox and TALE homeodomain proteins mediates Hoxb2 hindbrain enhancer activity. Mol. Cell. Biol. 19:5134-5142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Janknecht, R., N. J. Wells, and T. Hunter. 1998. TGF-β-stimulated cooperation of Smad proteins with the coactivators CBP/p300. Genes Dev. 12:2114-2119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kamps, M. P., C. Murre, X. H. Sun, and D. Baltimore. 1990. A new homeobox gene contributes the DNA binding domain of the t(1;19) translocation protein in pre-B ALL. Cell 60:547-555. [DOI] [PubMed] [Google Scholar]
- 24.Kim, J., K. Johnson, H. J. Chen, S. Carroll, and A. Laughon. 1997. Drosophila Mad binds to DNA and directly mediates activation of vestigial by Decapentaplegic. Nature 388:304-308. [DOI] [PubMed] [Google Scholar]
- 25.Knoepfler, P. S., Q. Lu, and M. P. Kamps. 1996. Pbx-1 Hox heterodimers bind DNA on inseparable half-sites that permit intrinsic DNA binding specificity of the Hox partner at nucleotides 3′ to a TAAT motif. Nucleic Acids Res. 24:2288-2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lai, Y. L., H. Li, H. S. Chiang, and H. M. Hsieh-Li. 2002. Expression of a novel TGIF subclass homeobox gene, Tex1, in the spermatids of mouse testis during spermatogenesis. Mech. Dev. 113:185-187. [DOI] [PubMed] [Google Scholar]
- 27.Liu, F., C. Pouponnot, and J. Massagué. 1997. Dual role of the Smad4/DPC4 tumor suppressor in TGFβ-inducible transcriptional responses. Genes Dev. 11:3157-3167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lo, R. S., D. Wotton, and J. Massague. 2001. Epidermal growth factor signaling via Ras controls the Smad transcriptional co-repressor TGIF. EMBO J. 20:128-136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Luo, K., S. L. Stroschein, W. Wang, D. Chen, E. Martens, S. Zhou, and Q. Zhou. 1999. The Ski oncoprotein interacts with the Smad proteins to repress TGFbeta signaling. Genes Dev. 13:2196-2206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ma, J., and M. Ptashne. 1987. A new class of yeast transcriptional activators. Cell 51:113-119. [DOI] [PubMed] [Google Scholar]
- 31.Mann, R. S., and S. K. Chan. 1996. Extra specificity from extradenticle: the partnership between HOX and PBX/EXD homeodomain proteins. Trends Genet. 12:258-262. (Erratum, 12:328.) [DOI] [PubMed] [Google Scholar]
- 32.Marquez, R. M., M. A. Singer, N. T. Takaesu, W. R. Waldrip, Y. Kraytsberg, and S. J. Newfeld. 2001. Transgenic analysis of the Smad family of TGF-beta signal transducers in Drosophila melanogaster suggests new roles and new interactions between family members. Genetics 157:1639-1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Massagué, J. 1998. TGFβ signal transduction. Annu. Rev. Biochem. 67:753-791. [DOI] [PubMed] [Google Scholar]
- 34.Massagué, J., A. Hata, and F. Liu. 1997. TGFβ signaling through the Smad pathway. Trends Cell Biol. 7:187-192. [DOI] [PubMed] [Google Scholar]
- 35.Massagué, J., and D. Wotton. 2000. Transcriptional control by the TGF-beta/Smad signaling system. EMBO J. 19:1745-1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McGinnis, W., R. L. Garber, J. Wirz, A. Kuroiwa, and W. J. Gehring. 1984. A homologous protein-coding sequence in Drosophila homeotic genes and its conservation in other metazoans. Cell 37:403-408. [DOI] [PubMed] [Google Scholar]
- 37.McGinnis, W., M. S. Levine, E. Hafen, A. Kuroiwa, and W. J. Gehring. 1984. A conserved DNA sequence in homoeotic genes of the Drosophila Antennapedia and bithorax complexes. Nature 308:428-433. [DOI] [PubMed] [Google Scholar]
- 38.Melhuish, T. A., C. M. Gallo, and D. Wotton. 2001. TGIF2 interacts with histone deacetylase 1 and represses transcription. J. Biol. Chem. 276:32109-32114. [DOI] [PubMed] [Google Scholar]
- 39.Melhuish, T. A., and D. Wotton. 2000. The interaction of C-terminal binding protein with the Smad corepressor TG-interacting factor is disrupted by a holoprosencephaly mutation in TGIF. J. Biol. Chem. 275:39762-39766. [DOI] [PubMed] [Google Scholar]
- 40.Ming, J. E., and M. Muenke. 1998. Holoprosencephaly: from Homer to Hedgehog. Clin. Genet. 53:155-163. [DOI] [PubMed] [Google Scholar]
- 41.Moskow, J. J., F. Bullrich, K. Huebner, I. O. Daar, and A. M. Buchberg. 1995. Meis1, a PBX1-related homeobox gene involved in myeloid leukemia in BXH-2 mice. Mol. Cell. Biol. 15:5434-5443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Newfeld, S. J., E. H. Chartoff, J. M. Graff, D. M. Melton, and W. M. Gelbart. 1996. Mothers against dpp encodes a conserved cytoplasmic protein required in DPP/TGFβ responsive cells. Development 122:2099-2108. [DOI] [PubMed] [Google Scholar]
- 43.Newfeld, S. J., R. G. Wisotzkey, and S. Kumar. 1999. Molecular evolution of a developmental pathway: phylogenetic analyses of transforming growth factor-beta family ligands, receptors and Smad signal transducers. Genetics 152:783-795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nourse, J., J. D. Mellentin, N. Galili, J. Wilkinson, E. Stanbridge, S. D. Smith, and M. L. Cleary. 1990. Chromosomal translocation t(1;19) results in synthesis of a homeobox fusion mRNA that codes for a potential chimeric transcription factor. Cell 60:535-545. [DOI] [PubMed] [Google Scholar]
- 45.Overhauser, J., H. F. Mitchell, E. H. Zackai, D. B. Tick, K. Rojas, and M. Muenke. 1995. Physical mapping of the holoprosencephaly critical region in 18p11.3. Am. J. Hum. Genet. 57:1080-1085. [PMC free article] [PubMed] [Google Scholar]
- 46.Passner, J. M., H. D. Ryoo, L. Shen, R. S. Mann, and A. K. Aggarwal. 1999. Structure of a DNA-bound Ultrabithorax-Extradenticle homeodomain complex. Nature 397:714-719. [DOI] [PubMed] [Google Scholar]
- 47.Piper, D. E., A. H. Batchelor, C.-P. Chang, M. L. Cleary, and C. Wolberger. 1999. Structure of a HoxB1-Pbx1 heterodimer bound to DNA: role of the hexapeptide and a fourth homeodomain helix in complex formation. Cell 96:587-597. [DOI] [PubMed] [Google Scholar]
- 48.Pouponnot, C., L. Jayaraman, and J. Massagué. 1998. Physical and functional interactions of Smads and p300/CBP. J. Biol. Chem. 273:22865-22868. [DOI] [PubMed] [Google Scholar]
- 49.Raftery, L. A., V. Twombly, K. Wharton, and W. M. Gelbart. 1995. Genetic screens to identify elements of the decapentaplegic signaling pathway in Drosophila. Genetics 139:241-254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ryan, A. K., M. L. Tejada, D. L. May, M. Dubaova, and R. G. Deeley. 1995. Isolation and characterization of the chicken homeodomain protein AKR. Nucleic Acids Res. 23:3252-3259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schaeper, U., J. M. Boyd, S. Verma, E. Uhlmann, T. Subramanian, and G. Chinnadurai. 1995. Molecular cloning and characterization of a cellular phosphoprotein that interacts with a conserved C-terminal domain of adenovirus E1A involved in negative modulation of oncogenic transformation. Proc. Natl. Acad. Sci. USA 92:10467-10471. (Erratum, 95:14584, 1998.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sekelsky, J. J., S. J. Newfeld, L. A. Raftery, E. H. Chartoff, and W. M. Gelbart. 1995. Genetic characterization and cloning of Mothers against dpp, a gene required for decapentaplegic function in Drosophila melanogaster. Genetics 139:1347-1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sharma, M., and Z. Sun. 2001. 5′TG3′ interacting factor interacts with Sin3A and represses AR-mediated transcription. Mol. Endocrinol. 15:1918-1928. [DOI] [PubMed] [Google Scholar]
- 54.Stroschein, S. L., W. Wang, S. Zhou, Q. Zhou, and K. Luo. 1999. Negative feedback regulation of TGF-beta signaling by the SnoN oncoprotein. Science 286:771-774. [DOI] [PubMed] [Google Scholar]
- 55.Sun, Y., X. Liu, E. N. Eaton, W. S. Lane, H. F. Lodish, and R. A. Weinberg. 1999. Interaction of the Ski oncoprotein with Smad3 regulates TGF-beta signaling. Mol. Cell 4:499-509. [DOI] [PubMed] [Google Scholar]
- 56.Sun, Y., X. Liu, E. Ng-Eaton, H. F. Lodish, and R. A. Weinberg. 1999. SnoN and ski protooncoproteins are rapidly degraded in response to transforming growth factor beta signaling. Proc. Natl. Acad. Sci. USA 96:12442-12447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Turner, J., and M. Crossley. 2001. The CtBP family: enigmatic and enzymatic transcriptional co-repressors. BioEssays 23:683-690. [DOI] [PubMed] [Google Scholar]
- 58.Wang, Z., and R. S. Mann. 2003. Requirement for two nearly identical TGIF-related homeobox genes in Drosophila spermatogenesis. Development 30:2853-2865. [DOI] [PubMed] [Google Scholar]
- 59.Wotton, D., P. S. Knoepfler, C. D. Laherty, R. N. Eisenman, and J. Massague. 2001. The Smad transcriptional corepressor TGIF recruits mSin3. Cell Growth Differ. 12:457-463. [PubMed] [Google Scholar]
- 60.Wotton, D., R. S. Lo, S. Lee, and J. Massague. 1999. A Smad transcriptional corepressor. Cell 97:29-39. [DOI] [PubMed] [Google Scholar]
- 61.Wotton, D., R. S. Lo, L. A. Swaby, and J. Massague. 1999. Multiple modes of repression by the smad transcriptional corepressor TGIF. J. Biol. Chem. 274:37105-37110. [DOI] [PubMed] [Google Scholar]
- 62.Yang, X., X. Ji, X. Shi, and X. Cao. 2000. Smad1 domains interacting with Hoxc-8 induce osteoblast differentiation. J. Biol. Chem. 275:1065-1072. [DOI] [PubMed] [Google Scholar]
- 63.Yang, Y., C. K. Hwang, U. M. D'Souza, S. H. Lee, E. Junn, and M. M. Mouradian. 2000. Tale homeodomain proteins Meis2 and TGIF differentially regulate transcription. J. Biol. Chem. 275:20734-20741. [DOI] [PubMed] [Google Scholar]
- 64.Zawel, L., J. L. Dai, P. Buckhaults, S. Zhou, K. W. Kinzler, B. Vogelstein, and S. E. Kern. 1998. Human Smad3 and Smad4 are sequence-specific transcription activators. Mol. Cell 1:611-617. [DOI] [PubMed] [Google Scholar]
- 65.Zhang, Y., and R. Derynck. 1999. Regulation of Smad signaling by protein associations and signaling crosstalk. Trends Cell Biol. 9:274-279. [DOI] [PubMed] [Google Scholar]
- 66.Zheng, C., J. Wang, T. E. Haerry, A. Y.-H. Wu, J. Martin, M. B. O'Connor, C.-H. J. Lee, and T. Lee. 2003. TGFb signaling activates steroid hormone receptor expression during neuronal remodeling in the Drosophila brain. Cell 112:303-315. [DOI] [PubMed] [Google Scholar]