INTRODUCTION
Atrial fibrillation (AF) is the most common arrhythmia seen in adult practice and accounts for about one-third of hospitalizations for cardiac rhythm disturbances.1 The prevalence increases with age and can be found in about 0.1% of adults younger than 55 but is found in greater than 9% of adults older than 80. The prevalence in North America is 1%, affecting about 2.3 million adults, and it is expected that this will increase to 6 million by 2050.2,3 Given the increasing prevalence of AF, there has been intense effort to elucidate the mechanisms of this disease process. With the advent of newer technologies and the renewed focus on AF, the understanding of the pathophysiology of the disease process has evolved, and this has changed the approach to therapy. In particular, the insight into the mechanisms of AF has allowed for significant improvements in non-pharmacological therapy.
Although there are several classification systems for AF, the evolution in our understanding of AF has led the electrophysiology community at large to adopt the classification system developed by the American College of Cardiology/American Heart Association/European Society of Cardiology (ACC/AHA/ESC) Committee 2006 Guidelines for the Management of Patients with AF.1 Paroxysmal AF has been defined as recurrent AF that terminates spontaneously within 7 days. Persistent AF is that which is sustained for greater than 7 days or is terminated in less than 7 days due to pharmacologic or electrical cardioversion. Patients who have persistent AF can be further classified as having “longstanding persistent AF,” which is AF that is continuous for greater than 1 year. Patients who have AF for whom cardioversion has been attempted and failed or never attempted are classified as having permanent AF. Except for permanent AF, these descriptions are not mutually exclusive, in that patients may have episodes which fall into one or another category. In this case, it is recommended to categorize the patient based on their dominant pattern of AF.1,2,4
This article reviews the current understanding of the pathophysiology of the disease, as well as the non-pharmacological therapy utilized to treat AF, with particular emphasis on radiofrequency ablation (RFA).
RATIONALE FOR RADIOFREQUENCY ABLATION OF AF
The Atrial Fibrillation Follow-up Investigation of Rhythm Management (AFFIRM) Trial was a large randomized study which evaluated two treatment strategies for AF: rate control and rhythm control. Of 4,060 patients with AF and at least one other risk factor for stroke (e.g., congestive heart failure, diabetes, age…) followed for a mean of 3.5 years, there was no significant difference in the primary endpoint of overall mortality with either treatment strategy.5 The lack of superiority of the rhythm control strategy has been seen in multiple other studies as well4 and has raised questions as to the clinical value of pursuing restoration of normal sinus rhythm (NSR) in patients with AF. Of interest, however, is a post hoc investigation of AFFIRM, in which an “on treatment” as opposed to “intention to treat” analysis was performed. In this analysis, the presence of normal sinus rhythm was associated with a lower mortality, and the use of antiarrhythmic medications was associated with a higher risk of death. It was suggested that the lack of a mortality benefit seen in the original analysis may have been due to the benefits of restoration of normal sinus rhythm being negated by the adverse effects of the antiarrhythmic medications.6 Furthermore, the efficacy of antiarrhythmic medications in recent trials has been modest at best; only 23%–60% of patients on antiarrythmics were in sinus rhythm at the end of follow-up.7,8 Thus, a safer and more effective method for restoration and maintenance of normal sinus rhythm may provide a mortality benefit. While trials evaluating mortality are of value, AF can have a significant impact on morbidity, and there may be a significant impact in terms of quality of life and exercise tolerance with the restoration of normal sinus rhythm.7
The justification for non-pharmacological therapy (i.e., catheter ablation) for the treatment of AF includes improvement in quality of life, decreased stroke risk, decreased heart failure risk, and improved survival. Ablation may prevent the hemodynamic abnormalities associated with AF and may prevent anatomical and electrical remodeling of the left atrium that is due to continuous AF.2 To date, however, the only documented benefit of restoration of normal sinus rhythm with RFA is symptom improvement.2,4 Current trials show that RFA is more effective in preventing recurrent AF compared to antiarrhythmic drugs.4,9–11 Studies also suggest that there are benefits of obtaining normal sinus rhythm via RFA compared to rate control. However, we await the results of large prospective multicenter randomized clinical trials to determine whether sinus rhythm achieved by ablation lowers morbidity and mortality compared with rate control alone or with treatment with antiarrhythmic therapy.4,12
MECHANISMS OF AF
The understanding of the pathophysiology of AF has evolved considerably over the past two decades. In general, sustained tachyarrhythmia requires a trigger for initiation, as well as an anatomical substrate that allows for perpetuation.2 Until the mid to late 1980s, AF was thought to be primarily a substrate-based disease. The most widely accepted theory was the Multiple Wavelet Hypothesis. Based on the initial work of Moe,13,14 and subsequently supported by animal studies by Allessie,4,15 AF was believed to be a random propagation of multiple electrical wavelets across the atria. It was thought that the abnormal atrial substrate allows for perpetuation of AF independent of the initial trigger. In this hypothesis, the atrial substrate that allows for the development of AF includes: 1) sufficiently large area of tissue (i.e., critical mass), 2) abnormal refractory period of the tissue and conduction velocity of the impulse, and 3) nonuniform distribution of the refractory periods and conduction velocities. Slow conduction, shortened refractory periods, and increased atrial mass favor the maintenance of AF by increasing fractionation of wavefronts propagating throughout the atria and thus resulting in a higher number of self-perpetuating “daughter wavelets.”1,4,13 Based on this model, the primary non-pharmacological therapy during that era, the Maze procedure, was developed (see below).
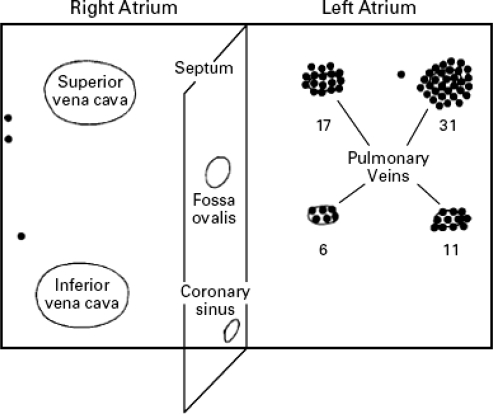
The Multiple Wavelet Hypothesis remained the accepted theory until the seminal work of Haissaguerre and his colleagues from Bordeaux, France.16,17 They were able to demonstrate the initiation of AF from focal triggers and that ablation of these triggers may lead to freedom from AF without the need for antiarrhythmic therapy. Furthermore, the source of these triggers was catalogued, and the majority of foci appeared to cluster in the pulmonary veins (PVs) (Figure 1).17 This prompted intense investigation into the PVs, and much has been learned about the anatomy and electrophysiological properties of these structures. The connection between the left atrium and pulmonary veins is via muscular sleeves, 1 cm to 3 cm in length, which extend from the left atrium and taper distally into the PVs in isolated strands (Figure 2).1,4 Although the mechanism has not been clearly elucidated, it is thought that the heterogeneous distribution of these muscle fibers lends itself to rapidly firing foci, which, when propagated into the left atrium proper, degenerate into AF. Muscular sleeves connecting to the left atrium in locations other than the PVs have also been identified, including the superior vena cava (SVC), inferior vena cava (IVC), Ligament of Marshall, left posterior free wall, crista terminalis, and coronary sinus (CS).1,4,16–18 The work by Haissaguerre and colleagues led to a paradigm shift in the perspective of AF. Instead of viewing AF as a substrate-based disease, the focus turned to targeting triggers that may be responsible for initiation. This is the theoretical basis for the radiofrequency ablation procedures that have been developed (see below).
Figure 1.
Diagram showing foci triggering atrial fibrillation. Note the clustering of these foci in the pulmonary veins.
(Reprinted with permission from Haissaguerre M, Jais P, Shah DC, et al. N Engl J Med. 1998;339:659–666.17)
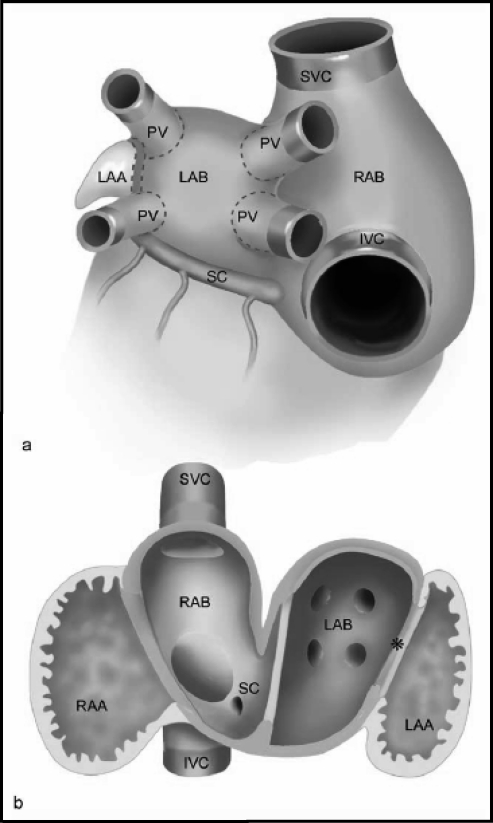
Figure 2.
(a) Schematic depicting the outer sides of the atrial chambers with pulmonary veins (PV), superior vena cava (SVC), and inferior venal cava (IVC). The right and left atrial bodies (RAB and LAB) are covered by smooth-walled inner myocardium that stretches into the extracardiac PVs and partially into the systemic veins. (b) Schematic depicting the tissue seen from inside the left and right atria and how the smooth-walled myocardial tissue extends into the systemic veins. Left-sided sinus venosus tissue (*) and SC = coronary sinus.
(Reprinted with permission from Douglas YL, Jongbloed MR, Gittenberger-de Groot AD, et al. Am J Cardiol. 2006;97:662–670.18)
However, for some patients, targeting of triggers will not be sufficient, and the abnormal underlying substrate may be more important. Also, other factors may be at play. For instance, cardiac autonomic innervation, in particular parasympathetic tone, has an impact in some patients with AF. Acetylcholine shortens refractory periods and increases conduction time, both of which can lead to AF. Furthermore, regional differences in the distribution of vagal innervation lead to a nonuniform effect on refractory periods and conduction times, thus setting up the milieu for micro-reentry, spiral waves and daughter wavelets. In animal studies that support this, conversion from PV tachycardia to AF with acetylcholine has been observed in canine hearts.19
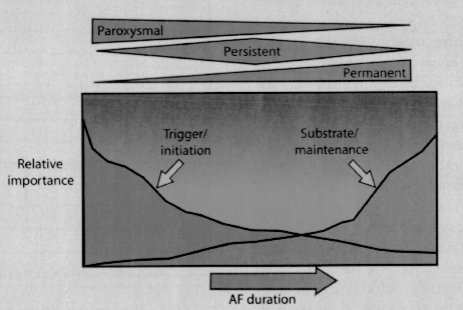
Although we have gained significant insight into the mechanisms of AF, our understanding of the disease process remains a work in progress. What has become clear is that AF is likely a heterogeneous disorder, with different mechanisms at play within different patient populations, and likely even in each individual patient. To date, although we remain unable to “fingerprint” an individual patient as to the cause of their AF, what has emerged is a perspective of the mechanisms involved in the general patient population. Specifically, it is believed that triggers are most likely to be the dominant mechanism for those with paroxysmal AF and relatively normal hearts. As one progresses to persistent and ultimately permanent atrial fibrillation, with subsequent atrial remodeling, abnormal atrial substrate becomes the dominant factor and thus the target for therapies (Figure 3).2,7
Figure 3.
Spectrum of importance of mechanisms of atrial fibrillation as they relate to atrial fibrillation classification and how these change over time.
(Reprinted with permission from Crandall MA, Bradley DJ, Packer DL, Asirvatham SJ. Mayo Clin Proc. 2009;84:643–662.2)
NON-PHARMACOLOGICAL THERAPY FOR AF
As noted above, the primary non-pharmacological therapy through the 1980s was the surgical Maze procedure, developed by Dr. James Cox.20,21 Predicated on the Multiple Wavelet Hypothesis, the goal of this procedure is to alter the atrial substrate so as to prevent the perpetuation of AF. Incisions are made in specific atrial locations to create anatomical barriers to conduction. The incisions are made in a pattern that allows for conduction of the electrical impulse to the ventricles via a controlled path or “maze.” By decreasing the mass of atria depolarized, the number of wavelets that can be created is minimized, and thus AF cannot perpetuate.20,21 The Maze procedure is effective at restoration of normal sinus rhythm, but its clinical use has been limited by significant morbidity associated with sternotomy. In attempts to avoid the invasiveness associated with surgery, electrophysiologists attempted to reproduce the Maze procedure endocardially with radiofrequency ablation. Consisting of multiple lines created in the left and right atria, this approach has a lower success rate and is associated with significantly more complications.1,4,22,23
Based on the work of the Bordeaux group in the 1990s,16,17 the endocardial ablative approach evolved from compartmentalizing the atrium in a manner similar to the Maze procedure to targeting the triggers, in particular those arising from the PVs. Initially, RFA was performed by mapping and then ablating the sites which triggered AF. However, this led to extremely long procedure times as well as high recurrence rates. It was noted that if one eliminated the triggers arising from one PV, during a repeat procedure another trigger was often found in another PV. For this reason, the procedure evolved to empiric isolation of all four PVs. In order to achieve isolation, RFA was performed at the ostium of the PV until no further electrical activity was noted inside the vein. Ablation at the opening of the PV, however, carries a risk for PV stenosis. Furthermore, it has been recognized that sites responsible for initiation or perpetuation of AF are often located within the PV antrum (left atrial tissue just proximal to the PVs) as opposed to within the PVs themselves.2,4,8,22–24 For these reasons, most centers now perform RFA within the antrum of the left atrium to achieve electrical isolation of the PVs.4,8,23
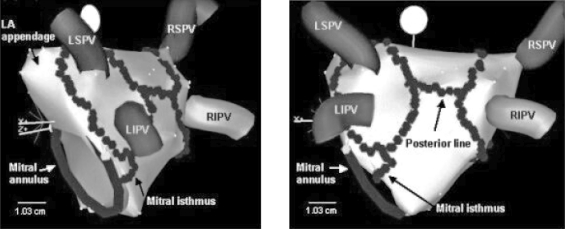
PV isolation as described above is most effective in those patients with paroxysmal AF. Because paroxysmal AF is more likely to be trigger-mediated, RFA targeting triggers is likely to be effective. However, as patients progress to persistent and longstanding persistent AF, abnormal atrial substrate likely plays a more significant role.2,22 Multiple strategies have been utilized to target the substrate in patients with more advanced disease. One such method involves the creation of linear ablation lesions in the LA that “anchor” PV isolation lines to other ablation sites or to the mitral valve. The most common linear ablation lines are those connecting the superior/posterior aspects of the left and right upper PV isolation lesions (LA “roof” line), and the tissue between the mitral valve (MV) and the left inferior PV (the mitral isthmus) (Figure 4). As noted in the mechanisms section of this article, the parasympathetic nervous system may play a role in initiation and maintenance of AF in some patients. For this reason, another method of substrate modification is to target the ganglionated plexi of the parasympathetic system, which tend to cluster around the PV antra.2,4,8,24 Yet another method of targeting the atrial substrate is to perform RFA at sites at which complex fractionated atrial electrograms (CFAEs) are noted during mapping. These abnormal electrograms potentially represent areas of abnormal conduction that may be important for the perpetuation of AF.2,4,8,24
Figure 4.
Electroanatomic maps of the left atrium demonstrating the left atrial radiofrequency ablation lines (series of connected dots) created during pulmonary vein isolation and two additional linear lines. The posterior line connects the two circular lesions that surround the ipsalateral pulmonary veins, and one line connects the mitral valve annulus to the circular lesion that surrounds the left pulmonary veins (mitral isthmus line). LSPV = left superior pulmonary vein; LIPV = left inferior pulmonary vein; RSPV = right superior pulmonary vein; and RIPV = right inferior pulmonary vein.
(Reprinted with permission from Padanilam BJ, Prystowsky EN. Medscape Cardiology 2005:9.23)
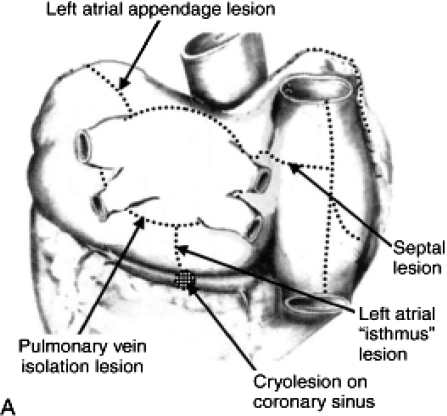
In parallel with the efforts undertaken by the electrophysiologists to treat AF, there has been an evolution in the surgical technique. For instance, the most recent iteration of the Maze procedure, the Maze III, includes incisions creating transmural lesions in the following manner: (i) isolation of the PVs; (ii) formation of lines connecting the lesions created around the PVs to each other, the LAA (with LAA resection) and the mitral valve (with cryoablation within the coronary sinus to complete the mitral isthmus line); and (iii) a cavo-tricuspid isthmus line in the right atrium (Figure 5). This technique can now be performed in a minimally invasive fashion, significantly decreasing the morbidity involved.1,24–26
Figure 5.
Lesions created in the Maze III procedure: (i) isolation of the PVs; (ii) formation of lines connecting the lesions created around the PVs to each other, the LAA (with LAA resection), and the mitral valve (with cryoablation within the coronary sinus to complete the mitral isthmus line); and (iii) a cavo-tricuspid isthmus line in the right atrium.
(Reprinted with permission from Cox JL. J Thorac Cardiovasc Surg. 2003;126:1693–1699 and Saunders Elsevier publishing.25,27)
During the past decade, RFA for AF has evolved rapidly, and it remains a work in progress. There is now no one standard approach to the procedure, and it is likely that no two major centers use the same technology, methodology, and endpoints. Despite this, general consensus has been achieved on certain aspects of the procedure, as summarized in Table 1.
Table 1.
Task Force Consensus on Ablation Techniques4
Indications for Non-pharmacological Therapy for AF
At this time, non-pharmacological therapy is reserved for patients who have symptomatic AF refractory or are intolerant to at least one Class I or III antiarrhythmic medication. The only absolute contraindication to catheter ablation is the presence of LA thrombus.1,4
In terms of patient selection, the patients with highest likelihood of success with this procedure are those without structural heart disease who have paroxysmal AF and are otherwise healthy and young. Their success rate is significantly higher than that in those with chronic AF and structural heart disease (e.g., severe LA enlargement). There is controversy as to the role of AF RFA in the elderly, as data indicate this patient population may have lower success rates and may be at increased risk of myocardial perforation and thromboembolic complications.2,4,24 Because RFA for patients with permanent AF is not likely to be successful, this procedure is not recommended for them. Patients with asymptomatic AF may request catheter ablation to eliminate AF as opposed to long-term anticoagulation with warfarin. However, no large prospective randomized clinical trials have shown that discontinuation of warfarin is safe after RFA of AF. Symptomatic and/or asymptomatic AF may recur in long-term follow-up after RFA, and thus it is not recommended that warfarin be discontinued in patients with moderate to high risk for stroke (CHADS II score ≥2). Thus, patients who wish to undergo RFA of AF only to eliminate the need for long-term anticoagulation are not considered appropriate for this procedure at this time.4
Conclusion
Atrial fibrillation, the most common arrhythmia seen in adult clinical practice, is becoming increasingly more prevalent.1–3 The mechanisms that initiate and maintain this arrhythmia are complex and still are not completely understood. At this point, non-pharmacologic therapy is indicated for symptomatic patients who are resistant or intolerant to antiarrhythmic medications.1 As we gain further understanding of the mechanisms driving this arrhythmia, we anticipate that the non-pharmacologic therapies will continue to evolve.
Footnotes
Disclosures: Dr. Saad has no potential conflict of interest to disclose. Drs. Morin and Khatib have received honoraria from Boston Scientific, Inc.; St. Jude Medical, Inc.; and Medtronic, Inc.
REFERENCES
- 1.Fuster V., Rydén L. E., Cannom D. S., et al. ACC/AHA/ESC 2006 Guidelines for the Management of Patients with Atrial Fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the European Society of Cardiology Committee for Practice Guidelines (Writing Committee to Revise the 2001 Guidelines for the Management of Patients With Atrial Fibrillation): developed in collaboration with the European Heart Rhythm Association and the Heart Rhythm Society. J Am Coll Cardiol. 2006;48(4):e149–e246. doi: 10.1016/j.jacc.2006.07.009. [DOI] [PubMed] [Google Scholar]
- 2.Crandall M. A., Bradley D. J., Packer D. L., Asirvatham S. J. Contemporary management of atrial fibrillation: update on anticoagulation and invasive management strategies. Mayo Clin Proc. 2009;84(7):643–662. doi: 10.1016/S0025-6196(11)60754-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Go A. S., Hylek E. M., Phillips K. A., et al. Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study. JAMA. 2001;285(18):2370–2375. doi: 10.1001/jama.285.18.2370. [DOI] [PubMed] [Google Scholar]
- 4.Calkins H., Brugada J., Packer D. L., et al. European Heart Rhythm Association (EHRA); European Cardiac Arrhythmia Society (ECAS); American College of Cardiology (ACC); American Heart Association (AHA); Society of Thoracic Surgeons (STS) HRS/EHRA/ECAS expert Consensus Statement on catheter and surgical ablation of atrial fibrillation: recommendations for personnel, policy, procedures and follow-up. A report of the Heart Rhythm Society (HRS) Task Force on catheter and surgical ablation of atrial fibrillation. Heart Rhythm. 2007;4(6):816–861. doi: 10.1016/j.hrthm.2007.04.005. Erratum in: Heart Rhythm. 2009;6(1):148. [DOI] [PubMed] [Google Scholar]
- 5.Wyse D. G., Waldo A. L., DiMarco J. P., et al. A comparison of rate control and rhythm control in patients with atrial fibrillation. N Engl J Med. 2002;347(23):1825–1833. doi: 10.1056/NEJMoa021328. [DOI] [PubMed] [Google Scholar]
- 6.Corley S. D., Epstein A. E., DiMarco J. P., et al. Relationships between sinus rhythm, treatment, and survival in the Atrial Fibrillation Follow-Up Investigation of Rhythm Management (AFFIRM) Study. Circulation. 2004;109(12):1509–1513. doi: 10.1161/01.CIR.0000121736.16643.11. [DOI] [PubMed] [Google Scholar]
- 7.Van Gelder I. C., Hemels M. E. The progressive nature of atrial fibrillation: a rationale for early restoration and maintenance of sinus rhythm. Europace. 2006;8(11):943–949. doi: 10.1093/europace/eul107. [DOI] [PubMed] [Google Scholar]
- 8.Aliot E., Ruskin J. N. Controversies in ablation of atrial fibrillation. Eur Heart J Suppl. 2008;10(Suppl H):H32–H54. [Google Scholar]
- 9.Jais P., Cauchemez B., Macle L., et al. Atrial fibrillation ablation versus antiarrhythmic drugs: A multicenter randomized trial. Heart Rhythm Society 2006 Scientific Sessions. 2006. Boston, MA.
- 10.Pappone C., Augello G., Sala S., et al. A randomized trial of circumferential pulmonary vein ablation versus antiarrhythmic drug therapy in paroxysmal atrial fibrillation: the APAF Study. J Am Coll Cardiol. 2006;48(11):2340–2347. doi: 10.1016/j.jacc.2006.08.037. [DOI] [PubMed] [Google Scholar]
- 11.Wazni O. M., Marrouche N. F., Martin D. O., et al. Radiofrequency ablation vs antiarrhythmic drugs as first-line treatment of symptomatic atrial fibrillation: a randomized trial. JAMA. 2005;293(21):2634–2640. doi: 10.1001/jama.293.21.2634. [DOI] [PubMed] [Google Scholar]
- 12.Packer D. Catheter ablation versus antiarrhythmic drug therapy for atrial fibrillation—pilot trial (CABANA) Government Clinical Trials Study NCT00578617. http://clinicaltrials.gov. Accessed 3 August 2009. [DOI] [PMC free article] [PubMed]
- 13.Moe G. K., Abildskov J. A. Atrial fibrillation as a self-sustaining arrhythmia independent of focal discharge. Am Heart J. 1959;58(1):59–70. doi: 10.1016/0002-8703(59)90274-1. [DOI] [PubMed] [Google Scholar]
- 14.Moe G. K. On the multiple wavelet hypothesis of atrial fibrillation. Arch Int Pharmacodyn Ther. 1962;140:183–188. [Google Scholar]
- 15.Allessie M. A., Lammers W. J., Bonke F. I., et al. Experimental evaluation of Moe’s multiple wavelet hypothesis of atrial fibrillation. In: Zipes D. P., Jalife J., editors. Cardiac Electrophysiology and Arrhythmias. Orlando, FL: Grune & Stratton; 1985. [Google Scholar]
- 16.Jaïs P., Haïssaguerre M., Shah D. C., et al. A focal source of atrial fibrillation treated by discrete radiofrequency ablation. Circulation. 1997;95(3):572–576. doi: 10.1161/01.cir.95.3.572. [DOI] [PubMed] [Google Scholar]
- 17.Haïssaguerre M., Jaïs P., Shah D. C., et al. Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins. N Engl J Med. 1998;339(10):659–666. doi: 10.1056/NEJM199809033391003. [DOI] [PubMed] [Google Scholar]
- 18.Douglas Y. L., Jongbloed M. R., Gittenberger-de Groot A. C., et al. Histology of vascular myocardial wall of left atrial body after pulmonary venous incorporation. Am J Cardiol. 2006;97(5):662–670. doi: 10.1016/j.amjcard.2005.11.019. [DOI] [PubMed] [Google Scholar]
- 19.Horikawa-Tanami T., Hirao K., Furukawa T., Isobe M. Mechanism of the conversion of a pulmonary vein tachycardia to atrial fibrillation in normal canine hearts: role of autonomic nerve stimulation. J Cardiovasc Electrophysiol. 2007;18(5):534–541. doi: 10.1111/j.1540-8167.2007.00772.x. [DOI] [PubMed] [Google Scholar]
- 20.Cox J. L., Canavan T. E., Schuessler R. B., et al. The surgical treatment of atrial fibrillation. II. Intraoperative electrophysiologic mapping and description of the electrophysiologic basis of atrial flutter and atrial fibrillation. J Thorac Cardiovasc Surg. 1991;101(3):406–426. [PubMed] [Google Scholar]
- 21.Cox J. L., Sundt TM 3rd. The surgical management of atrial fibrillation. Annu Rev Med. 1997;48:511–523. doi: 10.1146/annurev.med.48.1.511. [DOI] [PubMed] [Google Scholar]
- 22.Gauri A. J., Knight B. P. Catheter ablation for atrial fibrillation. Indian Pacing Electrophysiol J. 2003;3(4):210–223. [PMC free article] [PubMed] [Google Scholar]
- 23.Padanilam B. J., Prystowsky E. N. Atrial fibrillation: techniques and results. Medscape Cardiology. 2005;9(2) http://cmemedscape.com/viewarticle/513372. Accessed 22 May 2009. [Google Scholar]
- 24.Chugh A., Morady F. Atrial fibrillation: catheter ablation. J Interv Card Electrophysiol. 2006;16(1):15–26. doi: 10.1007/s10840-006-9018-4. [DOI] [PubMed] [Google Scholar]
- 25.Cox J. L. Atrial fibrillation II: rationale for surgical treatment. J Thorac Cardiovasc Surg. 2003;126(6):1693–1699. doi: 10.1016/j.jtcvs.2003.06.003. [DOI] [PubMed] [Google Scholar]
- 26.Wright M., Haïssaguerre M., Knecht S., et al. State of the art: catheter ablation of atrial fibrillation. J Cardiovasc Electrophysiol. 2008;19(6):583–592. doi: 10.1111/j.1540-8167.2008.01187.x. [DOI] [PubMed] [Google Scholar]
- 27.Oral H. Atrial fibrillation: mechanisms, features, and management. In: Zipes D. P., Jalife J., editors. Cardiac Electrophysiology: From Cell to Bedside. Philadelphia: Saunders Elsevier; 2009. pp. 577–588. [Google Scholar]