Abstract
Inflammation plays a pivotal role in all phases of atherosclerosis. High-sensitivity C-reactive protein (hsCRP), the best characterized biomarker of inflammation, is an independent predictor of future cardiovascular (CV) events and can add further insight to risk stratification. Assessment of hsCRP levels in clinical practice is feasible and inexpensive. Justification for the Use of Statins in Primary Prevention: An Intervention Trial Evaluating Rosuvastatin (JUPITER) was a landmark primary prevention trial that enrolled 17,802 apparently healthy men and women with low-density lipoprotein cholesterol levels of less than 130 mg/dL and hsCRP levels of 2 mg/L or higher and randomly assigned them to rosuvastatin, 20 mg daily, or placebo. The trial demonstrated that treatment with statin was associated with significant lowering of hsCRP (37%), with 44% reduction in incident CV and 20% reduction in all-cause mortality. These compelling data from the JUPITER trial should encourage changes in our approach toward primary prevention of CV disease and lipid-lowering therapy, as these data shift the focus toward a link between inflammation, statin therapy, and prevention of atherosclerotic CV diseases.
Keywords: Cardiovascular disease, coronary artery disease, C-reactive protein, JUPITER, risk factor
INTRODUCTION
Recent data have highlighted the role of inflammation in the genesis of cardiovascular (CV) diseases and atherothrombosis.1 Inflammation plays an important role in all phases of atherosclerosis, from initiation of the fatty streak, plaque growth, and rupture, to final culmination in acute coronary syndromes.1,2
Circulating levels of several inflammatory markers—including cell adhesion molecules, cytokines, chemokines, and acute-phase reactants—have been assessed in predicting CV diseases. High-sensitivity C-reactive protein (hsCRP) is an easily measured, widely investigated, and established marker of systemic inflammation that has been implicated in the pathophysiology of CV diseases, including acute coronary syndromes and stroke.1,3 An increase in hsCRP has been associated with markers of disease activity, including atherosclerotic plaque macrophage content and frequency of thin fibrous caps.4 Levels of hsCRP are elevated in subjects with metabolic syndrome,5 and many measures of obesity, including body mass index, waist circumference, and waist-to-hip ratio, are significantly correlated with higher levels of hsCRP.3 Importantly, in asymptomatic populations, high hsCRP levels have been associated with higher risk of acute coronary syndromes and symptomatic peripheral arterial disease.6
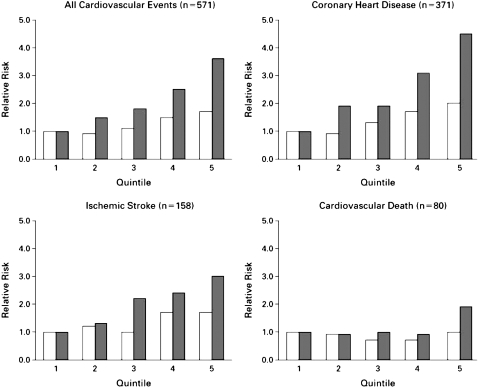
Several major prospective studies7–10 have established that elevated hsCRP levels contribute to increased CV risk (Figure 1); these include the Physicians' Health Study, Women's Health Study, Atherosclerosis Risk in Communities, and Air Force/Texas Coronary Atherosclerosis Prevention Study (AFCAPS/TexCAPS). Recently, the findings of JUPITER,12 a landmark primary prevention trial, were published. The major objective of JUPITER was to investigate whether treatment with rosuvastatin, 20 mg daily, compared to placebo, would decrease the rate of first major CV events in healthy subjects with normal low-density lipoprotein cholesterol (LDL-C) levels but elevated hsCRP levels.12 This review will discuss the role of hsCRP as a CV risk factor and the impact of JUPITER and its implications for the current preventive care guidelines.
Figure 1.
Age-adjusted relative risk of future cardiovascular events according to baseline C-reactive protein levels (solid bars) and low-density lipoprotein (LDL) cholesterol levels (open bars).
(Reproduced with permission from Ridker PM, Rifai N, Rose L, Buring JE, Cook NR. N Engl J Med 2002; 347:1557–1565.11)
INFLAMMATION AND THE EVOLVING ROLE OF hsCRP IN ATHEROSCLEROSIS
CRP is the prototypic marker of systemic inflammation and a member of a highly conserved family of proteins called the pentraxins. CRP is produced in the atherosclerotic lesion (especially by smooth muscle cells and macrophages),13–15 and the mRNA and protein for CRP are expressed in higher concentrations in arterial plaque tissue.15 Increased levels of hsCRP have been associated with increased expression of intercellular adhesion molecule 1 (ICAM-1), vascular cell adhesion molecule 1 (VCAM-1), E-selectin, and monocyte chemoattractant protein 1 (MCP-1)16 and with poor endothelial cell function.17 Studies17,18 have shown that CRP impairs endothelial vasoreactivity and decreases endothelial nitric oxide synthase activity. Endothelial dysfunction plays a critical role in CV diseases and has been linked to various risk factors for CV diseases such as obesity, hypertriglyceridemia, low levels of high-density lipoprotein cholesterol (HDL-C), impaired glucose tolerance, and hypertension.19,20 Similarly, CRP has been shown to increase the activity of plasminogen activator inhibitor-1 (PAI-1), a marker of impaired fibrinolysis and atherothrombosis, which are increased in patients with coronary artery disease (CAD).21 These properties have been postulated to make CRP a proatherogenic, prothrombotic molecule.
hsCRP AND CV RISK ASSESSMENT
In the United States alone, nearly 1.5 million patients experience an acute myocardial infarction (MI) or major cerebrovascular accident or stroke annually.22 Cardiovascular diseases have been the leading cause of morbidity and mortality for the past 80 years and are associated with substantial health care expenditures; for example, CAD is projected to cost an estimated $151,600 million in direct and indirect costs in 2007.23 Physicians in practice commonly use the Framingham risk score equation to estimate the 10-year absolute risk of major CV disease events (MI, death due to CV disease, major CAD events)24 and to classify patients as having low risk (10-year absolute risk <10%), intermediate risk (10-year absolute risk of 10%–20%), or high risk (10-year absolute risk >20%) for disease. Because a small proportion of asymptomatic adult subjects are classified as being at high risk for disease with these criteria, large gaps remain in the application of prevention guidelines and, thus, in the opportunities to reduce the morbidity and mortality of CV diseases.
More than 20 prospective studies with distinct cohorts have assessed the impact of elevated levels of CRP on future CV events and found that elevated hsCRP values were associated with an increased risk of incident CV events after adjusting for 4 major traditional risk factors, including Framingham risk factor scores and/or diabetes mellitus and obesity.25 Danesh and colleagues26 published a meta-analysis of prospective population-based studies that compared persons in the lower tertile of hsCRP (hsCRP <1 mg/L) with those in the upper tertile (values >3 mg/L) and demonstrated a 45% increase in CV events in subjects in the upper tertile. In general, most studies show a dose-response relationship between the level of hsCRP and risk of incident CAD; the magnitude of the association of hsCRP with incident CV diseases is comparable to that for LDL-C concentration, systolic blood pressure, or cigarette smoking. Numerous studies have examined the predictive ability of hsCRP to further the information already gained with the traditionally established risk factors. In multivariate models, hsCRP was found to be significantly predictive of incident CAD events after adjustment for age, total cholesterol, HDL-C, smoking, body mass index, diabetes mellitus, history of hypertension, exercise level, and family history of CAD.11,27 Furthermore, Ridker and colleagues,28 using hsCRP along with traditional risk factors (age, cholesterol level, blood pressure, smoking history, diabetes mellitus, and parental history of premature MI before age 60 years), showed that the Reynolds Risk Score improved risk classification, with better prediction of the CV events than with standard risk equations. In a number of secondary prevention trials, hsCRP has proved to be an important predictor of recurrent CV disease, of risk of restenosis after percutaneous coronary intervention, and of death, both in short- and long-term follow-ups.29–31
The Cholesterol and Recurrent Events trial30 demonstrated early on that statin therapy lowered hsCRP levels. Statins have reduced hsCRP levels by 20% to 30% in various trials and have demonstrated a drug class effect in lowering hsCRP concentrations. The more potent statins usually produce a greater reduction in LDL-C and hsCRP levels.32 Post hoc analyses from the randomized controlled trial AFCAPS/TexCAPS,10 evaluating lovastatin versus placebo, showed that subjects with low LDL-C and high hsCRP levels benefited from the statin therapy and those with low LDL-C and low hsCRP levels did not, which supports the potential utility of hsCRP for targeting patients for primary preventive interventions.
Serum levels of hsCRP may be used best as guidelines for patients classified as intermediate risk per the traditional risk stratification; assessment of the levels of hsCRP allows the patient to be reclassified as being at low or high risk for disease. This reclassification may provide a greater means to augment risk assessment in the identification of persons who should be considered for lipid-lowering, antiplatelet, or other cardioprotective drug therapies.
Strokes and peripheral arterial disease have also been associated with higher levels of hsCRP, independent of traditional risk factors.25,33,34 Thus, hsCRP may play an important role in risk stratification of patients with established CV disease.35 The metabolic syndrome is a constellation of risk factors that predispose to increased CV risk and has been associated with higher levels of hsCRP.36 Elevated hsCRP levels have also been implicated in the development of type 2 diabetes mellitus, a powerful risk factor for CV disease.37
JUPITER AND IMPLICATION FOR THE PRIMARY PREVENTION OF CV DISEASE
JUPITER,12 a landmark primary prevention trial whose findings were recently published in the New England Journal of Medicine, was a large multicenter randomized trial designed to assess the effectiveness of statin therapy in reducing CV events for healthy individuals with low LDL-C concentrations but increased hsCRP levels, a population that currently is not recommended for statin therapy. The primary outcome was the occurrence of a first major CV event, defined as nonfatal MI, nonfatal cerebrovascular accident, hospitalization for unstable angina, an arterial revascularization procedure, or confirmed death from CV causes. Secondary end points included the components of the primary end point considered individually—arterial revascularization or hospitalization for unstable angina, MI, cerebrovascular accident, or death from CV causes or from any cause. Men, 50 years of age or older, and women, 60 years of age or older, were eligible for the trial if they did not have a history of CV disease and if, at the initial screening visit, they had an LDL-C level of 3.4 mmol/L (<130 mg/dL) and a hsCRP level of >2 mg/L. Eligible subjects were randomly assigned in a 1∶1 ratio to receive either rosuvastatin, 20 mg daily, or matching placebo.12 The trial was terminated early by the independent data monitoring board after approximately 2 years of the proposed 4 years of follow-up because of a significant reduction in the primary end point in the rosuvastatin group.
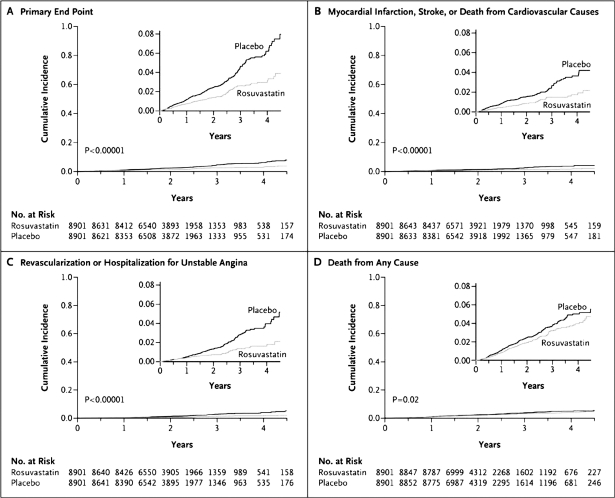
The median concentration of LDC-C in the JUPITER population was 2.79 mmol/L (108 mg/dL), which was lower than that of the previous trials. According to the design of the trial, the median hsCRP concentration was 4.3 mg/L. At the 12-month visit, the rosuvastatin group, compared to the placebo group, had a 50% lower median LDL-C concentration (mean difference, 1.2 mmol/L [47 mg/dL]), a 37% lower median hsCRP level, and a 17% lower median triglyceride level (P < .001 for all 3 comparisons). These effects persisted throughout the study period. At the time of study termination (median follow-up, 1.9 years; maximal follow-up, 5 years), 142 first major CV events had occurred in the rosuvastatin group, as compared with 251 in the placebo group. These results indicate an absolute reduction of events, from 1.8% in the placebo group to 0.9% in the rosuvastatin group. The primary end point of combined CV events was reduced by 44% (Figure 2), with a significant 20% reduction in total mortality. Rosuvastatin was also associated with significant reductions in the rates of the individual components of the primary trial end point: 54% for MI, 47% for revascularization, and 48% for cerebrovascular accident.12 In the JUPITER trial, relative hazard reductions in the rosuvastatin group were similar for women (46%) and men (42%) and were observed across various subgroups of participants evaluated, including subgroups tailored to age, race or ethnic group, status with regard to traditional risk factors, and presence or absence of the metabolic syndrome. Importantly, for subjects with elevated hsCRP levels but no other major risk factor than increased age, the benefit of rosuvastatin was similar to that for higher-risk subjects.12
Figure 2.
Cumulative incidence of cardiovascular events according to study group. Panel A shows the cumulative incidence of the primary end point (nonfatal myocardial infarction, nonfatal stroke, arterial revascularization, hospitalization for unstable angina, or confirmed death from cardiovascular causes). The hazard ratio for rosuvastatin, as compared with placebo, was 0.56 (95% confidence interval [CI], 0.46–0.69; P < .00001). Panel B shows the cumulative incidence of nonfatal myocardial infarction, nonfatal stroke, or death from cardiovascular causes, for which the hazard ratio in the rosuvastatin group was 0.53 (95% CI, 0.40–0.69; P < .00001). Panel C shows the cumulative incidence of arterial revascularization or hospitalization for unstable angina, for which the hazard ratio in the rosuvastatin group was 0.53 (95% CI, 0.40–0.70; P < .00001). Panel D shows the cumulative incidence of death from any cause, for which the hazard ratio in the rosuvastatin group was 0.80 (95% CI, 0.67–0.97; P = .02).
(Reproduced with permission from Ridker PM, Danielson E, Fonseca FA, et al. N Engl J Med 2008; 359: 2195–2207.12)
POTENTIAL IMPLICATION OF THE JUPITER TRIAL ON PUBLIC HEALTH AND PREVENTIVE GUIDELINES
JUPITER was a landmark primary prevention trial that could potentially impact how physicians practice preventive CV care; it is bound to have far-reaching implications for public health and future preventive care guidelines. Although LDL-C is a major risk factor for CV disease and future CV events, and lowering its levels reduces adverse CV events, atherosclerosis is now being increasingly regarded as an inflammatory process, with hsCRP playing a pivotal role in risk stratification.
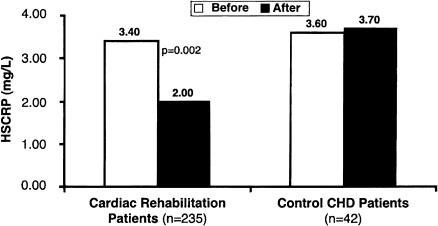
The JUPITER results extend primary prevention to those individuals who, by current risk models, are regarded as being at intermediate or low risk for disease and would not normally be prescribed a statin. The JUPITER trial stresses the fact that therapy with statins, such as rosuvastatin, is effective in lowering CV risk not only because of a lower LDL-C level achieved—despite a low starting level of LDL-C—but also because of its anti-inflammatory, pleitropic, and endothelial function-preserving properties. Studies17 have shown an inverse relationship between CRP levels and endothelial function. In addition, various risk factors for atherosclerosis such as obesity, low levels of HDL-C, hypertriglyceridemia, impaired fasting glucose, and raised blood pressure—all components of metabolic syndrome—are associated with impaired endothelium-dependent vasodilatation.38 In the JUPITER trial, about 40% of subjects had metabolic syndrome but had LDL-C levels below currently recommended thresholds for treatment. As we have stated above, hsCRP measurement would provide additional risk stratification of patients who are deemed to be at intermediate risk by the Framingham risk model, which uses several traditional risk factors to estimate CV disease risk in asymptomatic individuals. We can then further stratify these individuals into low- or high-risk groups on the basis of their high hsCRP levels. In a low-risk individual, hsCRP assessment would not add further insight to risk stratification or increase the need for further interventions. On the other hand, very high-risk individuals (10-year risk >20%) are probable candidates for aggressive preventive therapies, such as lipid-lowering and long-term aspirin therapy, regardless of their hsCRP values. The findings from JUPITER will initiate a debate to reconsider the role of statin therapy in the current Adult Treatment Panel III guidelines.39 The number of subjects needed for treatment in the JUPITER trial was 25 for the primary end point, with a reduction of 47% in CV events and about 20% in all-cause deaths.12 These values are of great significance for public health and in primary prevention, as they extend the benefit of statins to asymptomatic individuals with high hsCRP and low LDL-C levels and to populations that currently would not be eligible for statin therapy. Additionally, therapeutic lifestyle changes, particularly exercise training, also reduce levels of hsCRP by nearly 40%, independently of the statin effects (Figure 3).35,40,41
Figure 3.
Median changes in high-sensitivity C-reactive protein (hsCRP) in patients undergoing cardiac rehabilitation and in control subjects with coronary artery disease (CAD).
(Reproduced with permission from Milani RV, Lavie CJ, Mehra MR. J Am Coll Cardiol 2004; 43: 1056–1061.40)
On the other hand, in patients with low hsCRP and low LDL-C levels, the absolute risk of CV disease remains low; thus, these patients would not normally require any kind of aggressive statin therapy or change in physicians' practice behavior, and we would recommend only lifestyle and behavioral modifications for such individuals.
CONCLUSION
The JUPITER trial is a landmark trial that motivates change in our approach towards primary prevention of CV disease and lipid-lowering therapy, as it shifts the focus towards a link between inflammation, statin therapy, and prevention of atherosclerotic CV diseases. The implications for the public health are enormous, as a high proportion of the CV events that are otherwise expected to occur in adults worldwide could be prevented by treating populations with a statin drug, who otherwise would not be prescribed such therapy.
REFERENCES
- 1.Ross R. Atherosclerosis: an inflammatory disease. N Engl J Med. 1999;340:115–126. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 2.Libby P., Ridker P. M., Maseri A. Inflammation and atherosclerosis. Circulation. 2002;105:1135–1143. doi: 10.1161/hc0902.104353. [DOI] [PubMed] [Google Scholar]
- 3.Hansson G. K. Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med. 2005;352:1685–1695. doi: 10.1056/NEJMra043430. [DOI] [PubMed] [Google Scholar]
- 4.Burke A. P., Tracy R. P., Kolodgie F., et al. Elevated C-reactive protein values and atherosclerosis in sudden coronary death: association with different pathologies. Circulation. 2002;105:2019–2023. doi: 10.1161/01.cir.0000015507.29953.38. [DOI] [PubMed] [Google Scholar]
- 5.Ridker P. M., Hennekens C. H., Buring J. E., Rifai N. C-reactive protein and other markers of inflammation in the prediction of cardiovascular disease in women. N Engl J Med. 2000;342:836–843. doi: 10.1056/NEJM200003233421202. [DOI] [PubMed] [Google Scholar]
- 6.Ridker P. M., Cushman M., Stampfer M. J., Tracy R. P., Hennekens C. H. Plasma concentration of C-reactive protein and risk of developing peripheral vascular disease. Circulation. 1998;97:425–428. doi: 10.1161/01.cir.97.5.425. [DOI] [PubMed] [Google Scholar]
- 7.Ridker P. M., Glynn R. J., Hennekens C. H. C-reactive protein adds to the predictive value of total and HDL cholesterol in determining risk of first myocardial infarction. Circulation. 1998;97:2007–2011. doi: 10.1161/01.cir.97.20.2007. [DOI] [PubMed] [Google Scholar]
- 8.Ridker P. M., Buring J. E., Cook N. R., Rifai N. C-reactive protein, the metabolic syndrome, and risk of incident cardiovascular events: an 8-year follow-up of 14719 initially healthy American women. Circulation. 2003;107:391–397. doi: 10.1161/01.cir.0000055014.62083.05. [DOI] [PubMed] [Google Scholar]
- 9.Ballantyne C. M., Hoogeveen R. C., Bang H., et al. Lipoprotein-associated phospholipase A2, high-sensitivity C-reactive protein, and risk for incident ischemic stroke in middle-aged men and women in the Atherosclerosis Risk in Communities (ARIC) study. Arch Intern Med. 2005;165:2479–2484. doi: 10.1001/archinte.165.21.2479. [DOI] [PubMed] [Google Scholar]
- 10.Downs J. R., Clearfield M., Weis S., et al. Primary prevention of acute coronary events with lovastatin in men and women with average cholesterol levels: results of AFCAPS/TexCAPS—Air Force/Texas Coronary Atherosclerosis Prevention Study. JAMA. 1998;279:1615–1622. doi: 10.1001/jama.279.20.1615. [DOI] [PubMed] [Google Scholar]
- 11.Ridker P. M., Rifai N., Rose L., Buring J. E., Cook N. R. Comparison of C-reactive protein and low-density lipoprotein cholesterol levels in the prediction of first cardiovascular events. N Engl J Med. 2002;347:1557–1565. doi: 10.1056/NEJMoa021993. [DOI] [PubMed] [Google Scholar]
- 12.Ridker P. M., Danielson E., Fonseca F. A., et al. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med. 2008;359:2195–2207. doi: 10.1056/NEJMoa0807646. [DOI] [PubMed] [Google Scholar]
- 13.Calabró P., Willerson J. T., Yeh E. T. Inflammatory cytokines stimulated C-reactive protein production by human coronary artery smooth muscle cells. Circulation. 2003;108:1930–1932. doi: 10.1161/01.CIR.0000096055.62724.C5. [DOI] [PubMed] [Google Scholar]
- 14.Yasojima K., Schwab C., McGeer E. G., McGeer P. L. Generation of C-reactive protein and complement components in atherosclerotic plaques. Am J Pathol. 2001;158:1039–1051. doi: 10.1016/S0002-9440(10)64051-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kobayashi S., Inoue N., Ohashi Y., et al. Interaction of oxidative stress and inflammatory response in coronary plaque instability: important role of C-reactive protein. Arterioscler Thromb Vasc Biol. 2003;23:1398–1404. doi: 10.1161/01.ATV.0000081637.36475.BC. [DOI] [PubMed] [Google Scholar]
- 16.Pasceri V., Willerson J. T., Yeh E. T. Direct proinflammatory effect of C-reactive protein on human endothelial cells. Circulation. 2000;102:2165–2168. doi: 10.1161/01.cir.102.18.2165. [DOI] [PubMed] [Google Scholar]
- 17.Fichtlscherer S., Rosenberger G., Walter D. H., Breuer S., Dimmeler S., Zeiher A. M. Elevated C-reactive protein levels and impaired endothelial vasoreactivity in patients with coronary artery disease. Circulation. 2000;102:1000–1006. doi: 10.1161/01.cir.102.9.1000. [DOI] [PubMed] [Google Scholar]
- 18.Schwartz R., Osborne-Lawrence S., Hahner L., et al. C-reactive protein downregulates endothelial NO synthase and attenuates reendothelialization in vivo in mice. Circ Res. 2007;100:1452–1459. doi: 10.1161/01.RES.0000267745.03488.47. [DOI] [PubMed] [Google Scholar]
- 19.Brook R. D., Bard R. L., Rubenfire M., Ridker P. M., Rajagopalan S. Usefulness of visceral obesity (waist/hip ratio) in predicting vascular endothelial function in healthy overweight adults. Am J Cardiol. 2001;88:1264–1269. doi: 10.1016/s0002-9149(01)02088-4. [DOI] [PubMed] [Google Scholar]
- 20.Rodriguez C. J., Miyake Y., Grahame-Clarke C., et al. Relation of plasma glucose and endothelial function in a population-based multiethnic sample of subjects without diabetes mellitus. Am J Cardiol. 2005;96:1273–1277. doi: 10.1016/j.amjcard.2005.06.070. [DOI] [PubMed] [Google Scholar]
- 21.Devaraj S., Xu D. Y., Jialal I. C-reactive protein increases plasminogen activator inhibitor-1 expression and activity in human aortic endothelial cells: implications for the metabolic syndrome and atherothrombosis. Circulation. 2003;107:398–404. doi: 10.1161/01.cir.0000052617.91920.fd. [DOI] [PubMed] [Google Scholar]
- 22.Thom T., Haase N., Rosamond W., et al. Heart disease and stroke statistics—2006 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2006;113:e85–e151. doi: 10.1161/CIRCULATIONAHA.105.171600. [DOI] [PubMed] [Google Scholar]
- 23.Rosamond W., Flegal K., Friday G., et al. Heart disease and stroke statistics—2007 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2007;115:e69–e171. doi: 10.1161/CIRCULATIONAHA.106.179918. [DOI] [PubMed] [Google Scholar]
- 24.Wilson P. W., D'Agostino R. B., Levy D., Belanger A. M., Silbershatz H., Kannel W. B. Prediction of coronary heart disease using risk factor categories. Circulation. 1998;97:1837–1847. doi: 10.1161/01.cir.97.18.1837. [DOI] [PubMed] [Google Scholar]
- 25.Musunuru K., Kral B. G., Blumenthal R. S., et al. The use of high-sensitivity assays for C-reactive protein in clinical practice. Nat Clin Pract Cardiovasc Med. 2008;5:621–635. doi: 10.1038/ncpcardio1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Danesh J., Wheeler J. G., Hirschfield G. M., et al. C-reactive protein and other circulating markers of inflammation in the prediction of coronary heart disease. N Engl J Med. 2004;350:1387–1397. doi: 10.1056/NEJMoa032804. [DOI] [PubMed] [Google Scholar]
- 27.Ridker P. M. High-sensitivity C-reactive protein: potential adjunct for global risk assessment in the primary prevention of cardiovascular disease. Circulation. 2001;103:1813–1818. doi: 10.1161/01.cir.103.13.1813. [DOI] [PubMed] [Google Scholar]
- 28.Ridker P. M., Buring J. E., Rifai N., Cook N. R. Development and validation of improved algorithms for the assessment of global cardiovascular risk in women: the Reynolds Risk Score. JAMA. 2007;297:611–619. doi: 10.1001/jama.297.6.611. [DOI] [PubMed] [Google Scholar]
- 29.Lindahl B., Toss H., Siegbahn A., Venge P., Wallentin L. for The FRISC Study Group. Markers of myocardial damage and inflammation in relation to long-term mortality in unstable coronary artery disease. N Engl J Med. 2000;343:1139–1147. doi: 10.1056/NEJM200010193431602. [DOI] [PubMed] [Google Scholar]
- 30.Ridker P. M., Rifai N., Pfeffer M. A., et al. Inflammation, pravastatin, and the risk of coronary events after myocardial infarction in patients with average cholesterol levels—Cholesterol and Recurrent Events (CARE) Investigators. Circulation. 1998;98:839–844. doi: 10.1161/01.cir.98.9.839. [DOI] [PubMed] [Google Scholar]
- 31.Zebrack J. S., Muhlestein J. B., Horne B. D., Anderson J. L. Intermountain Heart Collaboration Study Group. C-reactive protein and angiographic coronary artery disease: independent and additive predictors of risk in subjects with angina. J Am Coll Cardiol. 2002;39:632–637. doi: 10.1016/s0735-1097(01)01804-6. [DOI] [PubMed] [Google Scholar]
- 32.Ridker P. M., Cannon C. P., Morrow D., et al. C-Reactive protein levels and outcomes after statin therapy. N Engl J Med. 2005;352:20–28. doi: 10.1056/NEJMoa042378. [DOI] [PubMed] [Google Scholar]
- 33.Rost N. S., Wolf P. A., Kase C. S., et al. Plasma concentration of C-reactive protein and risk of ischemic stroke and transient ischemic attack: the Framingham study. Stroke. 2001;32:2575–2579. doi: 10.1161/hs1101.098151. [DOI] [PubMed] [Google Scholar]
- 34.Rossi E., Biasucci L. M., Citterio F., et al. Risk of myocardial infarction and angina in patients with severe peripheral vascular disease: predictive role of C-reactive protein. Circulation. 2002;105:800–803. doi: 10.1161/hc0702.104126. [DOI] [PubMed] [Google Scholar]
- 35.Lavie C. J., Milani R. V., Verma A., O'Keefe J. H. C-reactive protein and cardiovascular diseases—is it ready for primetime? Am J Med Sci. In press. [DOI] [PubMed]
- 36.Milani R. V., Lavie C. J. Prevalence and profile of metabolic syndrome in patients following acute coronary events and effects of therapeutic lifestyle change with cardiac rehabilitation. Am J Cardiol. 2003;92:50–54. doi: 10.1016/s0002-9149(03)00464-8. [DOI] [PubMed] [Google Scholar]
- 37.Pradhan A. D., Manson J. E., Rifai N., Buring J. E., Ridker P. M. C-reactive protein, interleukin 6, and risk of developing type 2 diabetes mellitus. JAMA. 2001;286:327–334. doi: 10.1001/jama.286.3.327. [DOI] [PubMed] [Google Scholar]
- 38.Devaraj S., Singh U., Jialal I. Human C-reactive protein and the metabolic syndrome [review] Curr Opin Lipidol. 2009;20:182–189. doi: 10.1097/MOL.0b013e32832ac03e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.O'Keefe J. H., Carter M. D., Lavie C. J., Bell D. S. The gravity of JUPITER (Justification for the Use of Statins in Primary Prevention: An Intervention Trial Evaluating Rosuvastatin) Postgrad Med. 2009;121:113–118. doi: 10.3810/pgm.2009.05.2010. [DOI] [PubMed] [Google Scholar]
- 40.Milani R. V., Lavie C. J., Mehra M. R. Reduction in C-reactive protein through cardiac rehabilitation and exercise training. J Am Coll Cardiol. 2004;43:1056–1061. doi: 10.1016/j.jacc.2003.10.041. [DOI] [PubMed] [Google Scholar]
- 41.Lavie C. J., Thomas R. J., Squires R. W., Allison T. G., Milani R. V. Exercise training and cardiac rehabilitation in primary and secondary prevention of coronary heart disease. Mayo Clin Proc. 2009;84:373–383. doi: 10.1016/S0025-6196(11)60548-X. [DOI] [PMC free article] [PubMed] [Google Scholar]