Abstract
Wee1 inactivates the Cdc2–cyclin B complex during interphase by phosphorylating Cdc2 on Tyr-15. The activity of Wee1 is highly regulated during the cell cycle. In frog egg extracts, it has been established previously that Xenopus Wee1 (Xwee1) is present in a hypophosphorylated, active form during interphase and undergoes down-regulation by extensive phosphorylation at M-phase. We report that Xwee1 is also regulated by association with 14-3-3 proteins. Binding of 14-3-3 to Xwee1 occurs during interphase, but not M-phase, and requires phosphorylation of Xwee1 on Ser-549. A mutant of Xwee1 (S549A) that cannot bind 14-3-3 is substantially less active than wild-type Xwee1 in its ability to phosphorylate Cdc2. This mutation also affects the intranuclear distribution of Xwee1. In cell-free kinase assays, Xchk1 phosphorylates Xwee1 on Ser-549. The results of experiments in which Xwee1, Xchk1, or both were immunodepleted from Xenopus egg extracts suggested that these two enzymes are involved in a common pathway in the DNA replication checkpoint response. Replacement of endogenous Xwee1 with recombinant Xwee1-S549A in egg extracts attenuated the cell cycle delay induced by addition of excess recombinant Xchk1. Taken together, these results suggest that Xchk1 and 14-3-3 proteins act together as positive regulators of Xwee1.
INTRODUCTION
A pivotal regulatory step for the G2/M transition in eukaryotes is activation of the Cdc2–cyclin B complex, also known as maturation or M-phase promoting factor (Coleman and Dunphy, 1994; Morgan, 1997). The proper regulation of Cdc2 in vertebrates requires an activating phosphorylation on Thr-161 and inhibitory phosphorylations on Thr-14 and Tyr-15 (Morgan, 1997). The inhibitory phosphorylations are carried out by Wee1 and Myt1, which act on Tyr-15 or both Thr-14 and Tyr-15, respectively (Featherstone and Russell, 1991; Parker and Piwnica-Worms, 1992; McGowan and Russell, 1995; Mueller et al., 1995a,b; Watanabe et al., 1995). The Cdc2-activating dephosphorylation of these residues is catalyzed by the dual-specificity phosphatase Cdc25 (Dunphy and Kumagai, 1991; Gautier et al., 1991). These inhibitory phosphorylations maintain Cdc2–cyclin B in its inactive state if there is incompletely replicated DNA or damaged DNA in the cell (Morgan, 1997; Ohi and Gould, 1999; O'Connell et al., 2000). These surveillance processes, commonly referred to as checkpoints, are essential for the protection of genomic integrity during cell division (Elledge, 1996).
One of the important checkpoint targets most proximal to Cdc2 appears to be Cdc25. The catalytic activity of Cdc25 is dynamically regulated during the cell cycle: it is high during the mitotic (M) phase and low during interphase (Izumi et al., 1992; Kumagai and Dunphy, 1992). Another level of regulation involves the binding of Cdc25 to 14-3-3 proteins, which sequester Cdc25 in the cytoplasm before mitosis (Dalal et al., 1999; Kumagai and Dunphy, 1999; Lopez-Girona et al., 1999; Yang et al., 1999; Zeng and Piwnica-Worms, 1999). For the binding of 14-3-3 proteins, Cdc25 must be phosphorylated by kinases such as Chk1 and/or Cds1/Chk2 on Ser-216, Ser-287, or multiple serine residues in the case of human, Xenopus, or fission yeast Cdc25, respectively (Peng et al., 1997; Sanchez et al., 1997; Kumagai et al., 1998a; Matsuoka et al., 1998; Zeng et al., 1998; Guo and Dunphy, 2000).
Likewise, recent studies have suggested that regulation of Wee1 and/or its relatives, which compete against Cdc25, are also objectives of the damaged DNA and/or unreplicated DNA checkpoints in various systems (Rowley et al., 1992; O'Connell et al., 1997; Michael and Newport, 1998; Raleigh and O'Connell, 2000). Nonetheless, the mechanism(s) by which Wee1 participates in these control circuits is unclear. Fission yeast Chk1 can phosphorylate Wee1 in vitro, but the site(s) of phosphorylation and its physiological consequence(s) have not been elucidated (O'Connell et al., 1997). Similarly, both the fission yeast relative of Wee1 (Mik1) and a Xenopus homologue of Wee1 (Xwee1) are stabilized during DNA checkpoint responses, but the molecular mechanisms underlying these phenomena have not been established (Michael and Newport, 1998; Baber-Furnari et al., 2000). At this time, the most well characterized role of a Wee1 homologue in a checkpoint-related mechanism involves the stabilization of budding yeast Swe1 in response to morphogenetic defects (Lew, 2000).
Our laboratory has been using Xenopus egg extracts to study Cdc2 regulatory enzymes and their interaction with factors that are involved in sensing unreplicated or damaged DNA. A Xenopus homologue of Chk1 (Xchk1) is required to respond to stalled replication forks induced by replication inhibitors such as aphidicolin (Kumagai et al., 1998a). Moreover, Xchk1 responds to UV light-damaged DNA (Kumagai et al., 1998a). Xchk1 phosphorylates Cdc25 on Ser-287, thereby recruiting 14-3-3 proteins and inhibiting nuclear accumulation of Cdc25 (Kumagai and Dunphy, 1999). However, this process is most probably not the sole mechanism underlying the cell cycle delay in the DNA replication checkpoint response in this system.
In this report, we have assessed the role of Xwee1 in the DNA replication checkpoint in egg extracts. Our strategy has been to ask whether Xchk1 and 14-3-3 proteins, which are both known mediators of this pathway in egg extracts, control the action of Xwee1. Previous studies have indicated that the carboxyl half of mouse Wee1 binds to 14-3-3ζ and that a Wee1/14-3-3ζ complex coimmunoprecipitates with Cdc2 (Honda et al., 1997). Likewise, coexpression of human Wee1 with 14-3-3β in baculovirus-infected insect cells results in an increased yield of active Wee1, but the mechanism was not rigorously established (Wang et al., 2000). We find that 14-3-3 proteins bind to Xwee1 in a regulated manner in Xenopus egg extracts. This binding increases the kinase activity of Xwee1 and also affects its intranuclear distribution. Phosphorylation of Xwee1 on Ser-549 by Xchk1 is sufficient for the binding of 14-3-3 to Xwee1. In egg extracts, this phosphorylation of Xwee1 appears to be required for regulation of the cell cycle by Xchk1. Overall, these studies suggest that Xchk1 is a positive regulator of Xwee1.
MATERIALS AND METHODS
In Vitro Translation of Xwee1 Proteins
The entire open reading frame of Xwee1 (Mueller et al., 1995a) was inserted into the NdeI and BamHI sites of pET-9a to prepare pET-Xwee1. To produce pET-Xwee1-S549A, two primers (S549A-T, 5′-CACTCGATCGCTAGCCTTCAC; and S549A-B, 5′-GCAGGTGAAGGCTAGCGATCG) were used for mutagenesis of serine-549 to alanine with Quickchange kit (Stratagene, La Jolla, CA). These plasmids were used as templates for in vitro protein synthesis with the TNT reticulocyte-lysate–coupled transcription and translation system (Promega, Madison, WI). For radiolabeling of Xwee1 with 35S, Trans35S-Label (ICN Biomedicals, Irvine, CA) was added to the reaction. For kinase assays, the Xwee1 proteins were synthesized on a large scale (100-μl reaction).
Expression of Recombinant Proteins in Insect Cells
For hexahistidine and glutathione S-transferase (GST) double tagging, GST was amplified by polymerase chain reaction (PCR) with pGEX as the template and was inserted into the EheI and NcoI sites of pFastBacHTa (Life Technologies, Gaithersburg, MD) to produce pFastBac-His6-GST (Kumagai and Dunphy, 2000). The following two primers were used to amplify Xwee1 with Pfu DNA polymerase: XW-Bam, 5′-CGGGATCCGCCATGAGGACGGCCATGTCATGC; and XW-HD2, 5′-CATGGGAAGCTTTTAATACCCTCCGCAGGTGAAGC. To prepare pFastBac-His6-GST-Xwee1, the 1.7-kb PCR product was cut with BamHI and HindIII and ligated into pFastBac-His6-GST that had been cut with BamHI and HindIII. With this template, two primers (XW-NA-5, 5′-GACATCAAGCCAAGCGCCATATTTATCTGCCG; and XW-NA-3, 5′-CGGCAGATAAATATGGCGCTTGGCTTGATGTC) were used for substitution of asparagine-342 to alanine, to yield pFastBac-His6-GST-Xwee1-N342A. The PCR product from a reaction with XW-Bam and XW-HD2 as primers and pET-Xwee1-S549A as the template was used to prepare pFastBac-His6-GST-Xwee1-S549A as described above. Baculoviruses were generated with the Bac-to-Bac system (Life Technologies) and used to infect Sf9 insect cells at a density of 2 × 106 cells/ml. Recombinant proteins were purified by sequential chromatography on nickel agarose and glutathione agarose. Binding to nickel agarose and elution with imidazole were carried out as described before (Kumagai and Dunphy, 1997). Next, imidazole-eluted proteins were bound to glutathione agarose (Amersham Pharmacia Biotech, Piscataway, NJ) at 4°C for 1 h, washed twice with buffer B (10 mM HEPES-KOH, pH 7.5, 0.5 M NaCl, 5 mM EGTA, and 0.1% NP-40) and twice with HEPES-buffered saline (HBS; 10 mM HEPES-KOH, pH 7.5, 150 mM NaCl). Proteins were eluted with 50 mM glutathione (pH 8.0) in HBS and kept frozen in aliquots at −80°C. All buffers contain 1 mM phenylmethylsulfonyl fluoride. Protein concentrations were determined by SDS-PAGE and Coomassie blue staining with bovine serum albumin as the standard. The net yield of His6-GST-Xwee1-S549A was usually 10-fold lower than that of the corresponding wild-type protein. His6-Xchk1 was prepared as described previously (Kumagai et al., 1998a).
Expression of Recombinant Proteins in Bacteria
Escherichia coli BL21-Codon Plus (DE3)-RIL (Stratagene) was transformed with pET-Xwee1, and the culture was treated with 0.4 mM isopropyl-1-thio-d-galactopyranoside. After 3 h, the expressed His6-Xwee1 proteins were solubilized, purified with nickel agarose beads, and electroeluted from SDS gel as described (Mueller et al., 1995a). DNA fragments encoding wild-type and S549A mutant Xwee1 were produced by PCR with the primers XW-Bam and XW-XH12 (5′-GGGCCCCTCGAGTTAATACCCTCCGCAGGTGAA) and then subcloned into pGEX-4T-3 (Amersham Pharmacia Biotech). The expressed proteins were directly purified with glutathione agarose from the soluble fraction of bacteria and used as substrates in kinase assays. Fragments containing the COOH-terminal 81 amino acids of wild-type and S549A Xwee1 were amplified with the primers XW-N4 (5′-CGCGGATCCGCCAAGAATTCTGTGCTGAGACG) and XW-XH12, cut with BamHI and XhoI, and inserted into the pGEX-4T-3 to produce pGEX-Xwee1(475-555) or pGEX-Xwee1(475-555)-S549A, respectively. E. coli BL21(DE3)pLysS was transformed with these plasmids. The expressed GST-Xwee1(475-555)-WT and GST-Xwee1(475-555)-S549A proteins were purified with glutathione agarose as described above.
Preparation of Egg Extracts
Xenopus egg extracts were prepared as described (Murray, 1991). Typically, demembranated Xenopus sperm nuclei were added to a concentration of 103/μl extract. Cell cycle progression was initiated by adding Ca2+ to 0.4 mM. For cell cycle arrest, 100 μg/ml aphidicolin or 10 μg/ml His6-Xchk1 was added to the extracts as indicated (Kumagai et al., 1998a). Cytostatic factor-arrested egg extracts were used as M-phase extracts.
Immunoprecipitation and Immunoblotting
Bacterially expressed His6-Xwee1 was coupled to CNBr-activated Sepharose 4B (Amersham Pharmacia Biotech) and used for affinity purification of anti-Xwee1 antibody as described (Mueller et al., 1995a). Anti-Xchk1 and anti-14-3-3ε antibodies were purified as described (Kumagai et al., 1998a,b). To detect insect cell 14-3-3 proteins, we used an anti-14-3-3β antibody (K-19) that cross-reacts broadly with 14-3-3 family members (Santa Cruz Biotechnology, Santa Cruz, CA). Egg extracts containing 0.1% NP-40 were incubated with antibody-coated Affiprep Protein-A beads (Bio-Rad, Richmond, CA) at 4°C for 1 h. After centrifugation at 4000 × g for 1 min, the beads were washed twice with immunoprecipitation wash buffer (10 mM HEPES-KOH, pH 7.5, 150 mM NaCl, 20 mM β-glycerolphosphate, and 0.1% NP-40), and twice with either HBS for immunoblotting or kinase wash buffer (20 mM Tris-HCl, pH 7.5, 10 mM MgCl2, 20 mM β-glycerolphosphate, and 0.1 mM Na3VO4) for kinase assays. For immunoblotting, proteins were transferred to a polyvinylidene difluoride membrane (Millipore, Bedford, MA) and detected with the enhanced chemiluminescence system (Amersham Pharmacia Biotech).
Kinase Assays
Recombinant or immunopurified Xwee1 was incubated in 20 μl of kinase buffer (20 mM Tris-HCl, pH 7.5, 10 mM MgCl2, and 1 mM dithiothreitol) containing 5 μCi of [γ-32P]ATP and a complex of Xenopus Cdc2 (the N133A mutant) and human Δcyclin B1 as the substrate. This substrate was prepared by published procedures (Kumagai and Dunphy, 1997). Xchk1 was assayed as described (Kumagai et al., 1998a). Kinase reactions were carried out for 30 min and terminated with SDS gel sample buffer.
Phosphopeptide Mapping
Nickel agarose beads (10 μl) containing either His6-GST-Xwee1 or its S549A mutant were incubated for 60 min in 100 μl of interphase egg extracts containing 100 μg/ml cycloheximide and 0.5 mCi of [32P]orthophosphate. Beads were washed twice with buffer B and twice with HBS. Labeled proteins were eluted with 150 mM imidazole in HBS and subjected to tryptic phosphopeptide mapping as described (Boyle et al., 1991). For this procedure, pH 1.9 electrophoresis buffer and phospho-chromatography buffer were used.
Immunodepletion of Xwee1 and Xchk1
M-phase extracts (100 μl) were incubated for 40 min at 4°C with 7.5 μl of Affiprep Protein-A beads coated with 20 μg of anti-Xwee1 antibodies. After removal of the beads by centrifugation at 4000 × g for 20 s, the extracts were incubated with another batch of antibody-coated beads in the same manner to prepare double-depleted extracts. Xchk1 was depleted as previously described (Kumagai et al., 1998a). For simultaneous depletion of both Xwee1 and Xchk1, beads were coated with both anti-Xwee1 and anti-Xchk1 antibodies. Purified immunoglobulin (IgG) (Zymed Laboratories, San Francisco, CA) was used to produce control, mock-depleted extracts. Two microliters of each egg extract was removed for confirmation of successful depletion of proteins by immunoblotting.
Binding of His6-14-3-3ε to GST-Xwee1 Peptides
Glutathione agarose (5 μl) containing GST-Xwee1 peptides was rotated in 50 μl of kinase buffer supplied with 1 mM ATP and 1 μg of either wild-type or kinase-dead His6-Xchk1 at 23°C. After 30 min, 800 ng of His6–14-3-3ε (Kumagai et al., 1998b) and 0.5% NP-40 were added. The mixture was then rotated at 4°C for an additional 1 h. The beads were washed four times with buffer B and four times with HBS. Proteins were eluted with 50 mM glutathione (pH 8.0) and characterized by immunoblotting.
Transfection of GFP-Xwee1 into XTC Cells
The coding sequence for kinase-dead Xwee1 from pFastBac-His6-GST-Xwee1-N342A was isolated by cutting with BamHI and HindIII and subcloned into pEGFP-C1 (Clontech Laboratories, Palo Alto, CA) that had been digested with BglII and HindIII to yield pGFP-Xwee1-N342A. Serine-549 was changed into alanine with primers S549A-T and S549A-B (see above) to produce pGFP-Xwee1-N342A-S549A. Vectors for expression of the Myc peptide (control) or Myc-tagged Xenopus 14-3-3ε were described previously (Kumagai and Dunphy, 1999). XTC cells were transfected using the FuGENE 6 reagent (Boehringer-Mannheim, Indianapolis, IN) according to the manufacturer's protocol. After 18 h at 27°C, the cells were stained with 4′6-diamidino-2-phenylindole (DAPI, 10 μg/ml) (Sigma, St. Louis, MO) and fixed with 3% p-formaldehyde in phosphate-buffered saline.
Activation of Xwee1 In Vitro with 14-3-3 Proteins
His6-GST-Xwee1 isolated from Sf9 insect cells and immobilized on glutathione agarose was washed once with 0.4% Empigen-BB in buffer B to remove the insect 14-3-3 proteins (Thorson et al., 1998) and washed again three times with HBS. The beads were incubated for 2 h at 4°C in 50 μl of kinase buffer (lacking ATP but containing 0.5% NP-40) in the presence or absence of 800 ng of His6–14-3-3ε. The beads were washed twice with buffer B and twice with HBS. Proteins were eluted with 50 mM glutathione and assayed for kinase activity.
Endogenous Xwee1 from interphase egg extracts was immunoprecipitated with anti-Xwee1 antibodies, washed with 0.4% Empigen-BB, incubated in the presence or absence of His6-14-3-3ε and assayed for kinase activity. In the case of Xwee1 from M-phase extracts, immunoprecipitated Xwee1 was washed again with phosphatase buffer (40 mM Tris-HCl, pH 7.5, 40 mM NaCl, 0.1 mM EDTA, 0.5 mM MgCl2, 0.5 mM MnCl2, 0.5 mM CaCl2, 0.1% Triton X-100, and 1 mg/ml bovine serum albumin). Next, Xwee1 was treated for 1 h at 23°C in the presence or absence of 1 unit of protein phosphatase 2A (PP2A) (Upstate Biotechnology, Lake Placid, NY) in 100 μl of phosphatase buffer containing 1 mM dithiothreitol. To inhibit the PP2A at the end of the reaction, okadaic acid was added to 5 μM. The beads were washed three times with immunoprecipitation wash buffer and twice with kinase wash buffer. Then, the beads were incubated for 30 min at 23°C in kinase buffer containing 1 mM ATP in the presence or absence of His6-Xchk1. Next, His6-14-3-3ε (800 ng) was added and the incubation was continued for an additional 2 h at 4°C. After washing as described above, the beads were assayed for kinase activity. The samples were separated by SDS-PAGE, and transferred to a polyvinylidene difluoride membrane for either quantitation of radioactivity with a PhosphorImager (Molecular Dynamics, Sunnyvale, CA) or for immunoblotting.
RESULTS
Xwee1 Binds to 14-3-3 Proteins in a Cell Cycle-regulated Manner
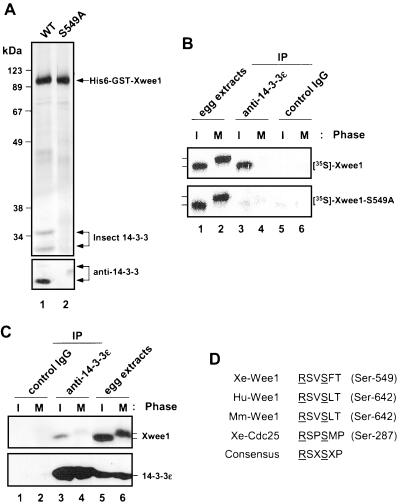
To identify Xwee1-associated proteins, we prepared a recombinant version of Xwee1 containing both the His6 and GST tags at its NH2-terminal end (hereafter referred to as His6-GST-Xwee1). As shown in Figure 1A (top, lane 1), by using sequential chromatography on nickel and glutathione agarose, we were able to obtain highly purified His6-GST-Xwee1 from baculovirus-infected insect cells. However, the preparation did contain two additional prominent bands (28–35 kDa) that could not be removed by washing in 1 M NaCl or 1% NP-40. From their sizes and the precedent that mouse Wee1 binds to 14-3-3 proteins (Honda et al., 1997), we suspected that these bands corresponded to 14-3-3 proteins. To test this possibility directly, the proteins were subjected to immunoblotting with an anti-14-3-3β antibody that cross-reacts with most 14-3-3 family members (see MATERIALS AND METHODS). Both proteins were recognized by this anti-14-3-3 antibody, though the smaller one typically reacted much more strongly (Figure 1A, bottom).
Figure 1.
Xwee1 binds to 14-3-3 proteins in a regulated manner. (A) His6-GST-Xwee1-WT (lane 1) and His6-GST-Xwee1-S549A (lane 2) were purified from Sf9 insect cells as described in MATERIALS AND METHODS and subjected to silver staining (top) or immunoblotting with a broadly cross-reactive anti-14-3-3β antibody (bottom). (B) Binding of 14-3-3 to Xwee1 requires Ser-549 and is cell cycle-dependent. 35S-Labeled Xwee1 (top) and Xwee1-S549A (bottom) were incubated for 60 min in interphase (I) (lanes 1, 3, and 5) or M-phase (M) (lanes 2, 4, and 6) Xenopus egg extracts. The extracts were analyzed directly by SDS-PAGE (lanes 1 and 2) or immunoprecipitated with either anti-14-3-3ε antibodies (lanes 3 and 4) or control antibodies (lanes 5 and 6). (C) Binding of endogenous Xwee1 in egg extracts to 14-3-3 is also cell cycle-regulated. Immunoprecipitates from 100 μl of interphase (lanes 1 and 3) or M-phase (lanes 2 and 4) extracts prepared with either control (lanes 1 and 2) or anti-14-3-3ε antibodies (lanes 3 and 4) were immunoblotted with anti-Xwee1 antibodies (top) or anti-14-3-3ε antibodies (bottom). Egg extracts (2 μl) in interphase (lane 5) or M-phase (lane 6) were immunoblotted for comparison. (D) The COOH-terminal sequences of Wee1 proteins from Xenopus (Xe), human (Hu), and mouse (Mm) were aligned and compared with the 14-3-3 binding site in Xenopus Cdc25 (Mueller et al., 1995a; Honda et al., 1997; Kumagai et al., 1998b; Wang et al., 2000). The critical arginine (R) and serine (S) residues from the 14-3-3 binding consensus site are underlined (Yaffe et al., 1997).
Previously, we identified the ε and ζ forms of Xenopus 14-3-3 as Cdc25 binding proteins in Xenopus egg extracts. To assess whether Xwee1 is associated with 14-3-3 proteins in this system, we incubated the His6-GST-Xwee1 (immobilized on nickel beads) in Xenopus egg extracts for 1 h. Next, we recovered the beads, eluted the bound proteins with imidazole, and performed immunoblotting with either anti-14-3-3ε or anti-14-3-3ζ antibodies (neither of which cross-reacts with insect cell 14-3-3). By this analysis, we found that both Xenopus 14-3-3ε and 14-3-3ζ are associated with His6-GST-Xwee1 in egg extracts (our unpublished results).
The activity of Xwee1 is highly regulated during the cell cycle. In particular, it exists in an active, hypophosphorylated form during interphase but becomes hyperphosphorylated and loses activity at M-phase (Mueller et al., 1995a; Figure 1B, lanes 1 and 2). To ask whether the interaction between Xwee1 and 14-3-3 is similarly cell cycle-dependent, we added 35S-labeled Xwee1 to either interphase or M-phase egg extracts and subsequently performed immunoprecipitation with anti-14-3-3ε antibodies. Significantly, the binding of Xwee1 to 14-3-3 could be detected readily during interphase, but was strongly reduced at M-phase (Figure 1B, top, lanes 3–6). Furthermore, we showed by immunoprecipitation with anti-14-3-3ε antibodies that the binding of endogenous Xwee1 to 14-3-3 in egg extracts is regulated in the same manner (Figure 1C).
In Xwee1, there are three serines (Ser-62, Ser-275, and Ser-549) that reside in regions that contain the RXXS sequence from the consensus motif for binding to 14-3-3 proteins, though none is a perfect match to this motif (Muslin et al., 1996; Yaffe et al., 1997; Thorson et al., 1998). We mutated each of these serines to alanine individually and then assessed the binding of the respective 35S-labeled mutant Wee1 proteins to 14-3-3. As shown in Figure 1B (bottom), the Xwee1-S549A mutant was deficient for binding to 14-3-3, whereas the other two mutants did not show a significant loss of 14-3-3 binding (our unpublished results). Consistent with this observation, the mutant His6-GST-Xwee1-S549A protein purified from Sf9 insect cells was devoid of insect 14-3-3 proteins (Figure 1A, lane 2). This 14-3-3 binding site is well conserved in COOH-terminal end of human and mouse Wee1 (Figure 1D), but not Wee1 from Schizosaccharomyces pombe.
Chk1 Can Phosphorylate Xwee1 and Mediate the Binding of 14-3-3 Proteins
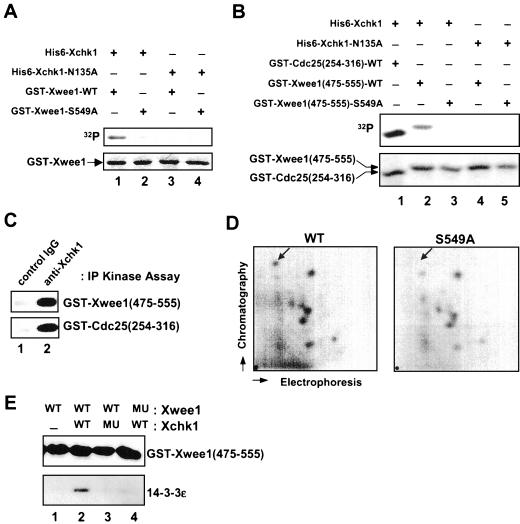
Chk1 and Cds1 have been identified in various organisms as kinases that phosphorylate Cdc25 on one or more serines, thereby mediating the binding of 14-3-3 proteins (Peng et al., 1997; Kumagai et al., 1998b; Matsuoka et al., 1998; Zeng et al., 1998; Blasina et al., 1999; Brown et al., 1999). First, we performed in vitro kinase assays to assess whether Xwee1 could serve as a substrate for Xchk1. For this purpose, we prepared bacterially expressed, full-length Xwee1 fused to GST. We used this substrate to minimize background phosphorylation of Xwee1 due to contaminating kinase activities and autophosphorylation of Xwee1 (bacterially expressed GST-Xwee1 is catalytically inactive). As shown in Figure 2A (lane 1), wild-type GST-Xwee1 was phosphorylated well by recombinant His6-Xchk1. In contrast, the S549A mutant of GST-Xwee1 was weakly phosphorylated by His6-Xchk1 (Figure 2A, lane 2), suggesting that Ser-549 is the major in vitro phosphorylation site. In control assays with kinase-inactive His6-Xchk1-N135A protein, there was no phosphorylation of GST-Xwee1 (Figure 2A, lanes 3 and 4).
Figure 2.
Phosphorylation of Ser-549 on Xwee1 by Xchk1 mediates the binding of 14-3-3 proteins. (A) His6-Xchk1 phosphorylates full-length Xwee1 on Ser-549. Bacterially expressed GST-Xwee1-WT (lanes 1 and 3) and GST-Xwee1-S549A (lanes 2 and 4) were incubated with wild-type His6-Xchk1 (lanes 1 and 2) or kinase-inactive His6-Xchk1-N135A (lanes 3 and 4). The kinase reactions were subjected to SDS-PAGE and analyzed by autoradiography (top) and Coomassie blue staining (bottom). (B) His6-Xchk1 phosphorylates a C-terminal peptide derived from Xwee1. Bacterially expressed GST-Cdc25(254-316)-WT (lane 1), GST-Xwee1(475-555)-WT (lanes 2 and 4), and GST-Xwee1(475-555)-S549A (lanes 3 and 5) were incubated with His6-Xchk1 (lanes 1–3) or His6-Xchk1-N135A (lanes 4 and 5). The kinase reactions were processed as in A. (C) Interphase egg extracts were subjected to immunoprecipitation with control antibodies (lane 1) or anti-Xchk1 antibodies (lane 2). The resulting immunoprecipitates were assayed for kinase activity toward GST-Xwee1(475-555)-WT (top) or GST-Cdc25(254-316)-WT (bottom). The kinase reactions were then processed for SDS-PAGE and autoradiography. (D) Ser-549 is a physiological site of phosphorylation in interphase Xenopus egg extracts. His6-GST-Xwee1 (left) and His6-GST-Xwee1-S549A (right) were labeled with 32P and subjected to two-dimensional tryptic phosphopeptide mapping as described in MATERIALS AND METHODS. The position of the radioactive spot that is missing from the map of the S549A mutant is marked with an arrow. The mobility of this phosphopeptide was confirmed by mixing the tryptic digests of the wild-type and S549A proteins. The origin is indicated by a dot in the lower left-hand corner. (E) GST-Xwee1(475-555)-WT (lanes 1–3) and GST-Xwee1(475-555)-S549A (lane 4), both of which were immobilized on glutathione agarose, were treated with no kinase (lane 1), wild-type His6-Xchk1 (lanes 2 and 4), or His6-Xchk1-N135A (lane 3) in the presence of ATP. Next, His6–14-3-3ε was added to all four incubations. After extensive washing, bound proteins were eluted with 50 mM glutathione, and the eluates were analyzed by immunoblotting with anti-Xwee1 (top) or anti-14-3-3ε (bottom) antibodies.
Because only a small fraction of full-length GST-Xwee1 is soluble in bacteria, we investigated whether a GST fusion protein containing the COOH-terminal 81 amino acids of Xwee1, GST-Xwee1(475-555)-WT, would be a more convenient substrate. For comparison, we used the GST-Cdc25(254-316)-WT protein, which contains the 14-3-3 binding site of Xenopus Cdc25 and is an excellent substrate for Xchk1. We observed that soluble GST-Xwee1(475-555)-WT was expressed at high levels in bacteria and was phosphorylated by His6-Xchk1 nearly as well as GST-Cdc25(254-316)-WT (Figure 2B, lanes 1 and 2). The S549A mutant of GST-Xwee1(475–555) was not phosphorylated under these conditions (Figure 2B, lane 3). Furthermore, both GST fusion proteins derived from Xwee1 and Cdc25, respectively, were phosphorylated by anti-Xchk1 immunoprecipitates from interphase Xenopus egg extracts (Figure 2C), indicating that endogenous and recombinant Xchk1 have similar properties.
To evaluate whether Ser-549 is a site of phosphorylation on Xwee1 in Xenopus egg extracts, we performed tryptic phosphopeptide mapping studies. For this experiment, we incubated the His6-GST-Xwee1-WT or His6-GST-Xwee1-S549A protein (immobilized on nickel agarose) in interphase egg extracts containing [32P]orthophosphate. Both proteins were reisolated, digested with trypsin, and the resulting peptides were separated by two-dimensional electrophoresis and thin-layer chromatography (Figure 2D). We found that a single 32P-labeled tryptic phosphopeptide was missing from the map of the S549A mutant (see arrow), indicating that Ser-549 is a site of phosphorylation in egg extracts.
Another issue to be addressed is whether phosphorylation of Ser-549 in Xwee1 is sufficient for 14-3-3 binding. For this experiment, we incubated wild-type or S549A GST-Xwee1(475-555), both of which were immobilized on glutathione agarose, with wild-type His6-Xchk1 or the kinase-inactive His6-Xchk1-N135A protein. Then, we added recombinant His6–14-3-3ε protein to the reactions. After subjecting the incubations to SDS-PAGE and immunoblotting with anti-14-3-3ε antibodies, we found that both Ser-549 and active Xchk1 are required for the binding of 14-3-3 to GST-Xwee1(475–555) (Figure 2E).
Binding of 14-3-3 Affects the Intranuclear Distribution of Xwee1
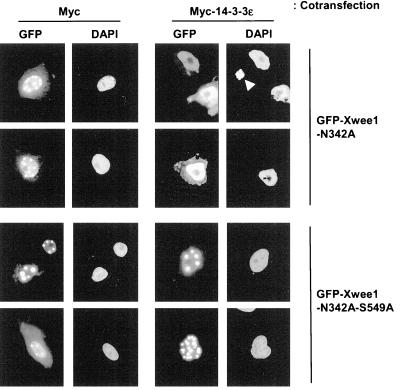
Spatial regulation has been established as an important feature of cell cycle regulation. For example, a number of studies have demonstrated that 14-3-3 proteins help to sequester Cdc25 in the cytoplasm during interphase (Kumagai and Dunphy, 1999; Lopez-Girona et al., 1999; Yang et al., 1999; Zeng and Piwnica-Worms, 1999). To investigate the possibility that 14-3-3 proteins affect the intracellular localization of Wee1, we prepared various green fluorescent protein (GFP)-tagged versions of Xwee1 that could be expressed in Xenopus tissue culture (XTC) cells. For these experiments, we used a kinase-dead version of Xwee1 (the N342A mutant) to avoid deleterious effects in the transfected cells due to overexpression of active Wee1, a cell cycle inhibitory kinase. As shown in Figure 3, when the GFP-Xwee1-N342A protein was expressed, most of the signal (>80%) was detected in nuclei, which were stained with DAPI. This was to be expected on the basis of reports that Wee1 is a nuclear protein (McGowan and Russell, 1995; Aligue et al., 1997; Murakami and Vande Woude, 1998). Interestingly, however, a portion of the nuclear GFP-Xwee1-N342A protein was present in punctate structures within the nucleus. Previously, our laboratory observed that the supply of 14-3-3 is limiting in XTC cells transfected with GFP-tagged Cdc25 (Kumagai and Dunphy, 1999). For this reason, we also expressed GFP-Xwee1-N342A in cells cotransfected with a vector encoding either Myc-14-3-3ε or the Myc tag only. Coexpression with Myc-14-3-3ε, but not the Myc tag alone, resulted in an even dispersion of Xwee1 throughout the nuclear interior. In contrast, the corresponding mutant of GFP-tagged Xwee1 that cannot bind 14-3-3 proteins (GFP-Xwee1-N432A-S549A) was present in a distinctly punctate distribution in both the presence and absence of Myc-14-3-3ε. These data suggest that binding of 14-3-3 proteins helps to keep Xwee1 evenly distributed throughout the nuclear interior.
Figure 3.
Role of 14-3-3 binding in the nuclear distribution of Xwee1. A plasmid encoding GFP-Xwee1-N342A (top) or GFP-Xwee1-N342A-S549A (bottom) was transfected into XTC cells along with a plasmid encoding the Myc peptide alone (left) or Myc-14-3-3ε (right). The N342A and S549A mutations abolish kinase activity and 14-3-3 binding, respectively. The signal from GFP or DAPI was observed by fluorescence microscopy. The arrowhead in the top panel on the right indicates condensed chromosomes from a mitotic cell. Typically, we did not observe a GFP signal from mitotic cells.
Binding of 14-3-3 Stimulates the Kinase Activity of Xwee1 against Cdc2
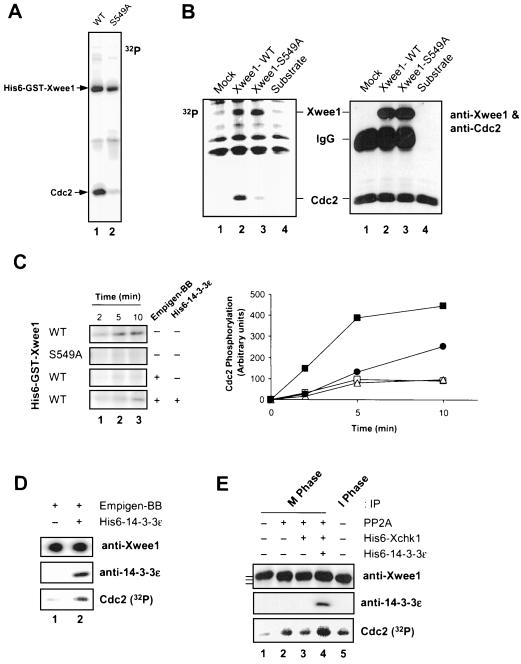
Next, we investigated whether binding of 14-3-3 would affect other functional properties of Xwee1. Toward this end, we compared the kinase activities of His6-GST-Xwee1-S549A and wild-type His6-GST-Xwee1, the latter of which is quantitatively associated with insect cell 14-3-3 proteins (Figure 1A). Significantly, as shown in Figure 4A, the S549A mutant was considerably less active (∼5-fold) than wild-type His6-GST-Xwee1 in its ability to phosphorylate the Cdc2 subunit of a recombinant Cdc2–cyclin B complex. By contrast, the autophosphorylation activities of His6-GST-Xwee1-WT and His6-GST-Xwee1-S549A were essentially identical, suggesting that 14-3-3 binding does not stimulate the intrinsic catalytic activity of Xwee1 but instead affects in some manner its ability to recognize Cdc2 properly (Figure 4A). We obtained similar results with recombinant Xwee1 proteins that were produced by in vitro translation in reticulocyte lysates, immunoprecipitated with anti-Xwee1 antibodies, and assayed for phosphorylation of Cdc2 (Figure 4B).
Figure 4.
Binding of 14-3-3 proteins to Xwee1 increases its kinase activity toward Cdc2. (A) Baculovirus-expressed wild-type (WT) (lane 1) and S549A (lane 2) His6-GST-Xwee1 were assayed for kinase activity toward a recombinant, kinase-inactive Cdc2–cyclin B complex (see MATERIALS AND METHODS). The kinase reactions were subjected to SDS-PAGE and autoradiography to detect incorporation of 32P into Cdc2. (B) Reticulocyte lysates containing no expressed Xwee1 (lane 1), Xwee1-WT (lane 2), or Xwee1-S549A (lane 3) were immunoprecipitated with anti-Xwee1 antibodies. The immunoprecipitates were incubated with a recombinant Cdc2–cyclin B complex (lanes 1–3) in the presence of [32P]ATP. Lane 4 depicts a control in which Cdc2–cyclin B was incubated without any anti-Xwee1 immunoprecipitate. The incubations were subjected to SDS-PAGE and analyzed by both autoradiography (left) and immunoblotting with a mixture of anti-Xwee1 and anti-Cdc2 antibodies (right). (C) Removal and rebinding of 14-3-3 reversibly affects the kinase activity of Xwee1. His6-GST-Xwee1-WT and His6-GST-Xwee1-S549A (as indicated) were incubated in the absence (−) or presence (+) of 0.4% Empigen-BB (a detergent that removes 14-3-3 proteins from Xwee1). After washing, the samples were incubated in the absence (−) or presence (+) of His6–14-3-3ε. Finally, the samples were mixed with a recombinant Cdc2–cyclin B complex. The kinase reactions were terminated after 2 min (lane 1), 5 min (lane 2), or 10 min (lane 3) and processed for SDS-PAGE and autoradiography to detect phosphorylation of Cdc2 (left). The right panel depicts quantitation of the data. Symbols: wild-type Xwee1 (▪); S549A mutant Xwee1 (□); Empigen-treated, wild-type Xwee1 that was incubated further in the absence (▵) or presence of His6–14-3-3ε (●). (D) Endogenous Xwee1 from interphase Xenopus egg extracts was immunoprecipitated with anti-Xwee1 antibodies, treated with Empigen-BB, washed, incubated in the absence (lane 1) or presence (lane 2) of His6–14-3-3ε, and assayed for Cdc2-specific kinase activity. The samples were subjected to SDS-PAGE and processed for immunoblotting with anti-Xwee1 antibodies (top), immunoblotting with anti-14-3-3ε antibodies (middle), or autoradiography to detect phosphorylation of Cdc2 (bottom). (E) Respective contributions of M-phase phosphorylation and Xchk1/14-3-3 to the regulation of Xwee1 during the cell cycle. Endogenous Xwee1 was immunoprecipitated with anti-Xwee1 antibodies from M-phase (lanes 1–4) or interphase (lane 5) egg extracts. The immunoprecipitates were treated in the presence (lanes 2–4) or absence (lanes 1 and 5) of PP2A. Subsequently, the samples were incubated with (lanes 3 and 4) or without (lanes 1, 2, and 5) His6-Xchk1. Next, His6–14-3-3ε (lane 4) was added to one incubation. Finally, a recombinant Cdc2–cyclin B complex and [32P]ATP were added. The kinase reaction samples were subjected to SDS-PAGE and processed for immunoblotting with anti-Xwee1 (top) or anti-14-3-3ε (middle) antibodies or for autoradiography to detect phosphorylation of Cdc2 (bottom). Kinase activity toward Cdc2 was quantitated and normalized to lane 1: lane 2 (3.6-fold), lane 3 (3.4-fold), lane 4 (5.5 fold), and lane 5 (3.5-fold). The bars to the left of top panel denote the various forms of Xwee1 with different electrophoretic mobilities.
To pursue these findings further, we attempted to strip 14-3-3 proteins from wild-type Xwee1 and then examine the consequences for its kinase activity. It has been reported that the zwitterionic detergent Empigen can be used to dislodge 14-3-3 proteins from the kinase Raf (Thorson et al., 1998). In pilot experiments, we found that 0.4% Empigen could remove 14-3-3 proteins from Xwee1 quantitatively (our unpublished results). As depicted in Figure 4C, the removal of 14-3-3 from wild-type His6-GST-Xwee1 by treatment with Empigen resulted in a drop of its kinase activity to the level of the S549A mutant. Importantly, readdition of recombinant His6–14-3-3ε to wild-type, Empigen-treated Xwee1 restored its kinase activity to ∼50% of the initial level. We believe that this partial restoration may be explained by the fact that Empigen is somewhat deleterious to Xwee1 (e.g., a higher concentration of this detergent, such as 1%, results in inactivation of Xwee1). It is important to note that incubation of Empigen-treated Xwee1 with His6-Xchk1 alone did not affect its activity (our unpublished results). Taken together, these observations indicate that phosphorylation of Ser-549 is not sufficient for activation of Xwee1. Instead, the binding of 14-3-3 to Xwee1 that is phosphorylated on Ser-549 seems most critical. We obtained similar results with endogenous Xwee1 that had been immunoprecipitated from interphase egg extracts. In particular, the kinase activity of interphase Xwee1 that had been treated with 0.4% Empigen was strongly stimulated by the addition of recombinant His6–14-3-3ε (Figure 4D).
In previous studies, our laboratory reported that Xwee1 is inactivated by extensive phosphorylation at M-phase (Mueller et al., 1995a). Therefore, we carried out experiments to assess the respective contributions of inhibitory M-phase phosphorylation and 14-3-3 binding to the regulation of Xwee1 in the Xenopus system. For this purpose, we immunoprecipitated interphase (hypophosphorylated) and M-phase (hyperphosphorylated) Xwee1 from Xenopus egg extracts. Consistent with previous results, interphase Wee1 was more active than M-phase Wee1, and treatment of M-phase Xwee1 with PP2A resulted in stimulation of Xwee1-associated kinase activity (Figure 4E). Next, we incubated the PP2A-treated Xwee1 either with His6-Xchk1 alone or with both His6-Xchk1 and His6–14-3-3ε. Subsequently, we assayed kinase activity toward Cdc2 (Figure 4E). We observed that treatment with His6-Xchk1 alone had a negligible effect on Xwee1 activity. However, treatment of Xwee1 with both His6-Xchk1 and His6–14-3-3ε resulted in a large increase (∼5-fold) in the activity of Xwee1 against Cdc2. Collectively, these findings indicate that Xwee1 is most active during interphase when it can bind to 14-3-3 proteins. At M-phase, the dissociation of 14-3-3 leads to a substantial decrease in the activity of Xwee1, whereas M-phase phosphorylation of Xwee1 results in a further reduction in its ability to phosphorylate Cdc2.
Binding of 14-3-3 Affects the Biological Properties of Xwee1 in Xenopus Egg Extracts
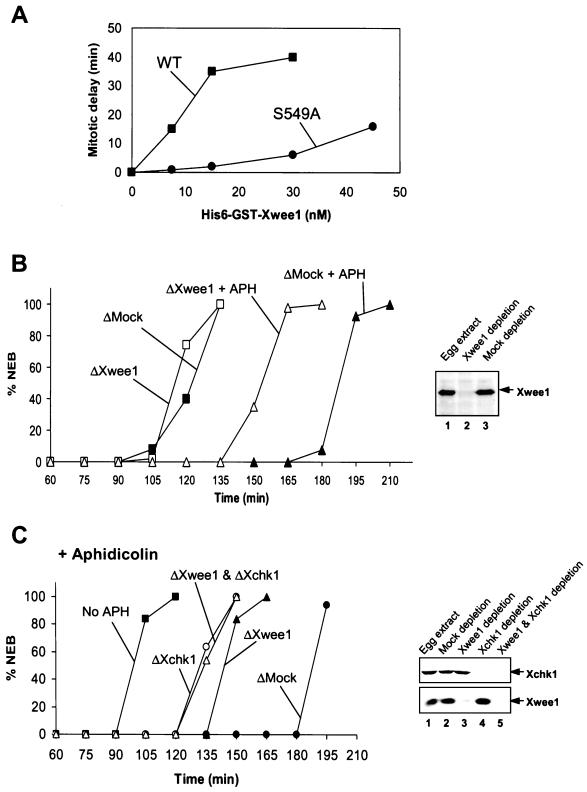
In view of the finding that binding of 14-3-3 proteins affects both the kinase activity and intranuclear localization of Xwee1, we examined whether the S549A mutant of Xwee1 would show altered biological properties in Xenopus egg extracts. As demonstrated previously, the addition of exogenous, wild-type Xwee1 protein results in a dose-dependent delay of mitotic initiation in egg extracts (Mueller et al., 1995a; Figure 5A). However, as shown in Figure 5A, we observed that the S549A mutant of Xwee1 is much less effective than wild-type Xwee1 at inducing a delay of mitotic entry. The difference between wild-type and S549A Xwee1 in this assay is similar to the disparity in their kinase activities. In control experiments with 35S-labeled wild-type and S549A Xwee1, we found that both proteins were stable throughout the time course of this experiment (our unpublished results), indicating that differential stability cannot explain these observations.
Figure 5.
Effects of 14-3-3 binding on the action of Xwee1. (A) Exogenously added His6-GST-Xwee1-S549A is much less efficient than His6-GST-Xwee1-WT in delaying the cell cycle. Wild-type or S549A mutant His6-GST-Xwee1 was added to interphase egg extracts at the indicated concentrations. Note that the endogenous concentration of Xwee1 is ∼15 nM. Mitotic entry was monitored by observing nuclear envelope breakdown (NEB) by microscopy. The timing of half-maximal NEB is plotted in the figure. (B) Immunodepletion of Xwee1 compromises the replication checkpoint. Egg extracts were subjected to an immunodepletion procedure with control antibodies (ΔMock) (closed symbols) or anti-Xwee1 antibodies (ΔXwee1) (open symbols). The extracts were treated with (triangles) or without (squares) 100 μg/ml aphidicolin (APH). The extracts also contained demembranated sperm nuclei (1000/μl). NEB was monitored at 15-min intervals. The content of Xwee1 in untreated (lane 1), Xwee1-depleted (lane 2), and mock-depleted (lane 3) egg extract is depicted in the anti-Xwee1 immunoblot in the right panel. (C) Effects of immunodepletion of Xwee1, Xchk1, or both on the DNA replication checkpoint. Egg extracts were immunodepleted with control antibodies (●), anti-Xwee1 antibodies (▴), anti-Xchk1 antibodies (○), or both anti-Xwee1 and anti-Xchk1 antibodies (▵). Aphidicolin (100 μg/ml) was added to these extracts, and the timing of NEB was monitored by microscopy. For comparison, the timing of mitosis in untreated extracts lacking aphidicolin was examined (▪). The immunoblots on the right-hand side indicate the content of Xchk1 (top) and Xwee1 (bottom) in the various extracts.
The next issue we considered is whether Xwee1 is a target of Xchk1, a major mediator of the DNA replication checkpoint in Xenopus egg extracts. First, we assessed the contribution of Xwee1 to the aphidicolin-induced replication checkpoint under the standard conditions used in our laboratory. For this purpose, we immunodepleted Xwee1 from aphidicolin-treated or control egg extracts and then monitored the timing of mitosis. As shown in Figure 5B, Xwee1-depleted extracts containing aphidicolin entered mitosis much earlier than mock-depleted extracts treated with aphidicolin. However, there was still a significant delay of mitosis in aphidicolin-treated, Xwee1-depleted extracts compared with extracts lacking aphidicolin (either mock-depleted or Xwee1-depleted). Our findings differ somewhat from those of Michael and Newport (1998), who found that removal of Xwee1 completely abolished the aphidicolin-induced checkpoint in egg extracts. However, since these studies were performed with cycloheximide-containing extracts supplemented with recombinant cyclin B, whereas our experiments were carried out with cycling egg extracts lacking cycloheximide, the two results may not be directly comparable.
Then, we investigated the potential relationship between Xwee1 and Xchk1 in egg extracts. For this analysis, we immunodepleted Xwee1, Xchk1, or both from aphidicolin-treated extracts. Consistent with the observations on Xwee1 described above (Figure 5B) and previously published results with Xchk1 (Kumagai et al., 1998a), immunodepletion of either Xwee1 or Xchk1 substantially compromised the replication checkpoint, though the removal of Xchk1 led to a more severe defect (Figure 5C). Significantly, extracts lacking Xchk1 or both Xchk1 and Xwee1 entered mitosis at essentially the same time. Because the effects of removing Xchk1 and Xwee1 are not additive, this result implies that Xchk1 and Xwee1 are involved in the same pathway.
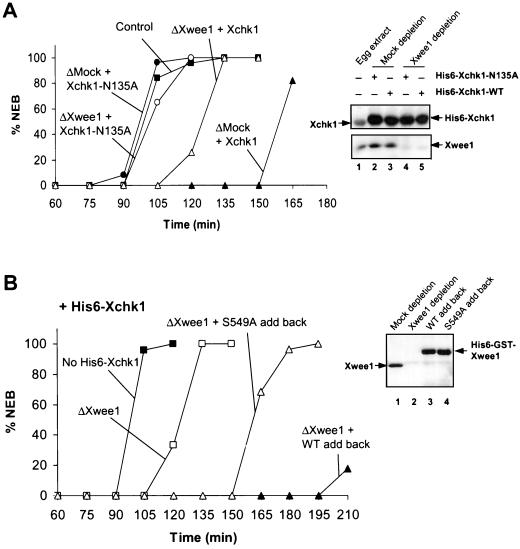
As another means to explore the relationship between Xchk1 and Xwee1, we took advantage of the previous observation that addition of exogenous His6-Xchk1 to egg extracts results in a strong inhibition of mitotic entry (Kumagai et al., 1998a). As depicted in Figure 6A, the addition of recombinant His6-Xchk1 to control, mock-depleted extracts resulted in a pronounced delay of mitosis. However, this delay was substantially reduced when Xwee1 was immunodepleted from the extracts, suggesting that Xwee1 is required for recombinant His6-Xchk1 to elicit its maximal effect in delaying the cell cycle. As might have been expected, removal of Xwee1 did not fully abolish the delay, most probably because Xchk1 possesses at least one additional target (e.g., Cdc25). In control experiments, addition of kinase-inactive His6-Xchk1-N135A did not affect cell cycle timing in mock-depleted or Xwee1-depleted extracts.
Figure 6.
Xwee1 is an effector of Xchk1 in Xenopus egg extracts. (A) Xwee1 is involved in the cell cycle delay induced by excess His6-Xchk1. Wild-type His6-Xchk1 (triangles) and His6-Xchk1-N135A (circles) were added to mock-depleted (▴ and ●) or Xwee1-depleted extracts (▵ and ○). The timing of NEB was scored by microscopy. NEB was also monitored in control, untreated extracts (▪) for comparison. Recombinant His6-Xchk1 proteins were added to a fivefold excess over the concentration of endogenous Xchk1. The immunoblots on the right depict the content of endogenous Xchk1 and recombinant His6-Xchk1 proteins (top) and endogenous Xwee1 (bottom) in the various extracts. (B) The S549A mutant of Xwee1 is deficient in mediating the cell cycle delay induced by excess recombinant His6-Xchk1. Egg extracts were immunodepleted of endogenous Xwee1 with anti-Xwee1 antibodies (as indicated) and then supplemented with excess recombinant His6-Xchk1 (to a fivefold excess over endogenous Xchk1). Next, no additional protein (□), 15 nM His6-GST-Xwee1-WT (▴), or 15 nM His6-GST-Xwee1-S549A (▵) was added to the extracts. NEB was monitored by microscopy. Untreated extracts lacking His6-Xchk1 were also examined (▪). The immunoblots on the right indicate the content of endogenous Xwee1 and recombinant His6-GST-Xwee1 proteins in indicated extracts.
We also compared the abilities of His6-GST-Xwee1-WT and His6-GST-Xwee1-S549A to function in egg extracts containing excess recombinant His6-Xchk1 (Figure 6B). To perform this experiment, we first immunodepleted endogenous Xwee1 with anti-Xwee1 antibodies and then added excess His6-Xchk1 along with either His6-GST-Xwee1-WT or His6-GST-Xwee1-S549A. We observed that the wild-type His6-GST-Xwee1 caused a much more pronounced delay of the cell cycle than the S549A mutant of His6-GST-Xwee1. The shorter delay that was caused by S549A mutant is most probably due to the fact that this mutant possesses a basal activity approximately one-fifth that of the wild-type Xwee1 protein. A similar difference in activity between His6-GST-Xwee1-WT and His6-GST-Xwee1-S549A was observed in Xwee1-depleted extracts containing aphidicolin, indicating that the S549A mutant is also deficient in mediating a cell cycle delay during a replication checkpoint response (our unpublished results). Collectively, these findings suggest that binding of Xwee1 to 14-3-3 is required for Xchk1 to function to its fullest extent in controlling the timing of mitotic entry.
DISCUSSION
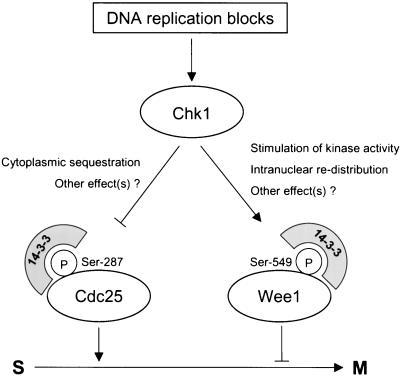
In this study, we have investigated the regulation of Xwee1 by 14-3-3 proteins and the role of this process in cell cycle control in Xenopus egg extracts. Xwee1 associates with 14-3-3 proteins in a cell cycle-dependent manner. This interaction occurs during interphase, when Xwee1 is most active. The binding of 14-3-3 is strongly reduced during M-phase, at which time the activity of Xwee1 is down-regulated (Mueller et al., 1995a). Binding of 14-3-3 proteins requires phosphorylation of Xwee1 on Ser-549. Xchk1 can phosphorylate Ser-549 of Xwee1 well in vitro, and recombinant Xwee1 becomes phosphorylated on this site in Xenopus egg extracts. The binding of 14-3-3 to Xwee1 significantly enhances the kinase activity of Xwee1 toward Cdc2 and also affects the distribution of Xwee1 throughout the nucleus (Figure 7). The interaction between Xwee1 and 14-3-3 appears to be biologically important in Xenopus egg extracts. For example, a recombinant Xwee1 mutant (S549A), which is deficient for 14-3-3 binding, has significantly reduced potency for delaying mitotic entry in cycling egg extracts.
Figure 7.
DNA replication checkpoint in Xenopus egg extracts. See DISCUSSION for details. Note that Xcds1 and at least one other kinase phosphorylate Cdc25 on Ser-287 in egg extracts (Guo and Dunphy, 2000). Likewise, at least one additional kinase phosphorylates Xwee1 on Ser-549.
The Xenopus checkpoint kinases Xchk1 and Xcds1 both can phosphorylate the critical serine (residue 287) in the 14-3-3 binding site of Xenopus Cdc25 (Kumagai et al., 1998a; Guo and Dunphy, 2000). Because Xwee1 can also bind 14-3-3 proteins, we asked whether these kinases could phosphorylate Xwee1. We initially focused on Xchk1, which our laboratory has previously established is important for cell cycle regulation in response to unreplicated or UV-damaged DNA (Kumagai et al., 1998a). Xcds1, which is activated by the presence of double-stranded DNA ends in egg extracts (Guo and Dunphy, 2000), can also phosphorylate Xwee1 in vitro (our unpublished results). However, at this time, further analysis of the relationship between Xcds1 and Xwee1 is complicated by the fact that immunodepletion of Xcds1 does not compromise the cell cycle delay induced by double-stranded DNA ends (Guo and Dunphy, 2000).
In the case of Xchk1, our data support a functional relationship between this checkpoint regulator and Xwee1. First, both recombinant His6-Xchk1 and immunoprecipitated Xchk1 from Xenopus egg extracts phosphorylate Xwee1 readily in vitro on Ser-549, and thereby allow the binding of 14-3-3 proteins. This phosphorylation appears to be specific for Ser-549, because the full-length GST-Xwee1-S549A mutant protein is a poor substrate for Xchk1. Second, immunodepletion experiments also suggest that Xchk1 and Xwee1 lie on a common pathway. Immunodepletion of either Xchk1 or Xwee1 alone significantly compromises the replication checkpoint in egg extracts, although the defect in the Xchk1-depleted extracts is somewhat more severe. This result could have been anticipated, because Xchk1 possesses at least one other target in this system (e.g., Cdc25). Significantly, egg extracts lacking Xchk1 alone or both Xchk1 and Xwee1 display a nearly identical defect in the replication checkpoint. This experiment argues that Xchk1 and Xwee1 act in the same pathway. Finally, immunodepletion of Xwee1 greatly diminishes the cell cycle delay that is caused by addition of excess recombinant His6-Xchk1 to egg extracts, indicating that Xchk1 requires Xwee1 to stall progression of the cell cycle in this context.
Perhaps the central finding of this study is that association of Xwee1 with 14-3-3 proteins greatly enhances its ability to phosphorylate Cdc2. We observed that most, if not all, of the recombinant Xwee1 prepared from baculovirus-infected Sf9 cells is associated with insect 14-3-3 proteins. The initial biochemical characterizations of full-length Wee1 from various species (e.g., fission yeast, human, and Xenopus) were carried out with baculovirus-expressed Wee1 (Featherstone and Russell, 1991; Parker et al., 1991; McGowan et al., 1995; Mueller et al., 1995a; Watanabe et al., 1995). Thus, it appears likely that 14-3-3 proteins might have contributed to the activity of Wee1 that was observed in these experiments. Our first indication that 14-3-3 proteins are important for the function of Wee1 was that the S549A mutant of Xwee1, which cannot bind 14-3-3 proteins, has substantially less kinase activity toward Cdc2 than wild-type Xwee1. As another avenue to assess this issue, we used the detergent Empigen to strip 14-3-3 from baculovirus-expressed Xwee1. This treatment resulted in a significant decrease in the activity of Xwee1, which could be restored by the addition of recombinant 14-3-3 proteins. Similar results were obtained with Xwee1 that was immunoprecipitated from Xenopus egg extracts. Taken together, these results indicate that phosphorylation of Ser-549 by Xchk1 is not by itself sufficient to increase the activity of Xwee1. Instead, this phosphorylation allows the binding of 14-3-3, which in turn results in the stimulation of kinase activity. These results might explain why O'Connell et al. (1997) did not observe activation of baculovirus-expressed fission yeast Wee1 by Chk1. Although Wee1 could be phosphorylated by Chk1 in vitro in this study, it is possible that a large fraction of the Wee1 was already associated with 14-3-3. Moreover, O'Connell et al. (1997) did not add recombinant 14-3-3 to Wee1 after treatment with Chk1.
We have also observed that binding of 14-3-3 proteins affects the localization of Xwee1. It is evident from numerous studies in yeast and vertebrate cells that 14-3-3 proteins have a dramatic effect of the localization of mitotic inducer Cdc25 by restricting it to the cytoplasm (Dalal et al., 1999; Kumagai and Dunphy, 1999; Lopez-Girona et al., 1999; Yang et al., 1999; Zeng and Piwnica-Worms, 1999). Likewise, 14-3-3ς, which is a p53-inducible protein, contributes to the cytoplasmic localization of Cdc2-cyclin B in human cells (Chan et al., 1999). In these two cases, the nuclear membrane is used as a border for compartmentalization. Because Wee1 is a nuclear protein in various species, this type of regulation was not anticipated (McGowan and Russell, 1995; Aligue et al., 1997; Murakami and Vande Woude, 1998). Indeed, we observed that wild-type GFP-Xwee1 expressed in XTC cells is localized evenly throughout the nucleus, provided that an adequate supply of 14-3-3 proteins is available. In contrast, the S549A mutant Xwee1 loses this uniform distribution and associates with punctate structures in the nucleus. The identity of these structures and their role in the regulation of Xwee1 remain to be established, but association with these structures may limit the accessibility of Xwee1 to Cdc2–cyclin complexes in the nucleus. Although reduced kinase activity alone could account for the low biological activity of the S549A mutant that we have observed in egg extracts, aberrant localization also may be a contributing factor.
Although our data support a relationship between Xwee1, Xchk1, and 14-3-3 proteins in Xenopus egg extracts, a number of questions remain. Immunodepletion of Xchk1 from egg extracts does not abolish the binding of 14-3-3 proteins to Xwee1, suggesting the existence of at least one other kinase that phosphorylates Ser-549 of Xwee1 (our unpublished results). A comparable situation exists in the case of Xenopus Cdc25, which can be phosphorylated by Xchk1, Xcds1, and at least one other kinase (Kumagai et al., 1998a; Guo and Dunphy, 2000). Thus far, we have not been able to detect an increase in kinase activity or binding of 14-3-3 proteins in the case of Xwee1 that has been immunoprecipitated from aphidicolin-treated extracts, which is consistent with previously published results (Kumagai and Dunphy, 1995; Mueller et al., 1995a). However, only a small fraction of Xwee1 (∼5%) is associated with 14-3-3 proteins in egg extracts, according to our immunoprecipitation studies, suggesting that a subpopulation of Xwee1 might be involved in this interaction. Approximately 10% or less of the Xwee1 becomes incorporated into the nuclei in cycling egg extracts, depending upon the experimental conditions. This portion of Xwee1 would presumably be the most accessible to checkpoint regulators. Moreover, as shown here, Xwee1 is differentially localized within the nucleus depending on whether it is associated with 14-3-3. These considerations suggest that there may be technical limitations in how Xwee1 can be assayed in checkpoint-activated extracts.
Currently, numerous studies point to a role for Xwee1 and/or its relatives in the unreplicated DNA and damaged DNA checkpoints in various organisms. In fission yeast, several observations have implicated Wee1 and/or Mik1 in these DNA-regulated checkpoints (O'Connell et al., 1997; Boddy et al., 1998; Baber-Furnari et al., 2000; Christensen et al., 2000; Raleigh and O'Connell, 2000). The best characterized molecular response in these studies is the stabilization of Mik1 during a checkpoint arrest (Baber-Furnari et al., 2000; Christensen et al., 2000). Likewise, it has been reported that Xwee1 is more stable in aphidicolin-treated Xenopus egg extracts (Michael and Newport, 1998). The Drosophila Wee1 homologue (Dwee1) was shown to be necessary for nuclear division during early embryogenesis (Price et al., 2000). Intriguingly, the phenotype of Dwee1 mutant embryos is very similar to the phenotypes of embryos with mutations in the mei-41 or grp genes, which encode homologues of fission yeast Rad3 and Chk1, respectively. In budding yeast, Swe1 is not required for cell cycle arrest in response to unreplicated or damaged DNA, but stabilization of this kinase is critical for a spindle morphogenesis checkpoint (Lew, 2000). As shown here, 14-3-3 proteins contribute to the positive regulation of Xwee1. We have not been able to observe any difference in the stabilities of wild-type and S549A mutant Xwee1 in egg extracts. However, it is possible that stabilization of Xwee1 could act to reinforce the increased kinase activity of Xwee1 that occurs following the binding of 14-3-3 proteins.
In conclusion, our findings indicate that positive regulation of Xwee1 by 14-3-3 proteins is an additional, and previously unappreciated, mechanism for cell cycle control. Collectively, 14-3-3 proteins play multiple roles in inhibiting mitotic entry by keeping inactive Cdc25 in the cytoplasm and activating Wee1 in the nucleus. Because Chk1 acts upon both Cdc25 and Wee1 to recruit 14-3-3 proteins, this kinase would be able to down-regulate Cdc25 and up-regulate Wee1 in a concerted manner.
ACKNOWLEDGMENTS
We are thankful to our colleagues in the Dunphy lab for helpful comments on this work. We are indebted to Charlotte Pham for assistance with plasmid manipulation. This work was supported in part by a grant from the National Institutes of Health. J.L. is an associate and W.G.D. is an investigator of the Howard Hughes Medical Institute.
REFERENCES
- Aligue R, Wu L, Russell P. Regulation of Schizosaccharomyces pombe Wee1 tyrosine kinase. J Biol Chem. 1997;272:13320–13325. doi: 10.1074/jbc.272.20.13320. [DOI] [PubMed] [Google Scholar]
- Baber-Furnari BA, Rhind N, Boddy MN, Shanahan P, Lopez-Girona A, Russell P. Regulation of mitotic inhibitor Mik1 helps to enforce the DNA damage checkpoint. Mol Biol Cell. 2000;11:1–11. doi: 10.1091/mbc.11.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blasina A, de Weyer IV, Laus M, Luyten W, Parker A, McGowan C. A human homologue of the checkpoint kinase Cds1 directly inhibit Cdc25 phosphatase. Curr Biol. 1999;9:1–10. doi: 10.1016/s0960-9822(99)80041-4. [DOI] [PubMed] [Google Scholar]
- Boddy MN, Furnari B, Mondesert O, Russell P. Replication checkpoint enforced by kinases Cds1 and Chk1. Science. 1998;280:909–912. doi: 10.1126/science.280.5365.909. [DOI] [PubMed] [Google Scholar]
- Boyle WJ, van der Geer O, Hunter T. Phosphopeptide mapping and phosphoamino acid analysis by two-dimensional separation on thin-layer cellulose plates. Methods Enzymol. 1991;201:110–149. doi: 10.1016/0076-6879(91)01013-r. [DOI] [PubMed] [Google Scholar]
- Brown AL, Lee C-H, Schwarz JK, Mitiku N, Piwnica-Worms H, Chung JH. A human Cds1-related kinase that functions downstream of ATM protein in the cellular response to DNA damage. Proc Natl Acad Sci USA. 1999;96:3745–3750. doi: 10.1073/pnas.96.7.3745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan TA, Hermeking H, Lengauer C, Kinzler KW, Vogelstein B. 14-3-3ς is required to prevent mitotic catastrophe after DNA damage. Nature. 1999;401:616–620. doi: 10.1038/44188. [DOI] [PubMed] [Google Scholar]
- Christensen PU, Bentley NJ, Martinho RG, Nielsen O, Carr AM. Mik1 levels accumulate in S phase and may mediate an intrinsic link between S phase and mitosis. Proc Natl Acad Sci USA. 2000;14:2579–2584. doi: 10.1073/pnas.97.6.2579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman TR, Dunphy WG. Cdc2 regulatory factors. Curr Opin Cell Biol. 1994;6:877–882. doi: 10.1016/0955-0674(94)90060-4. [DOI] [PubMed] [Google Scholar]
- Dalal SN, Schweitzer CM, Gan J, DeCaprio JA. Cytoplasmic localization of human cdc25C during interphase requires an intact 14-3-3 binding site. Mol Cell Biol. 1999;19:4465–4479. doi: 10.1128/mcb.19.6.4465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunphy WG, Kumagai A. The cdc25 protein contains an intrinsic phosphatase activity. Cell. 1991;61:189–196. doi: 10.1016/0092-8674(91)90582-j. [DOI] [PubMed] [Google Scholar]
- Elledge SJ. Cell cycle checkpoint: preventing an identity crisis. Science. 1996;274:1664–1672. doi: 10.1126/science.274.5293.1664. [DOI] [PubMed] [Google Scholar]
- Featherstone C, Russell P. Fission yeast p107wee1 mitotic inhibitor is a tyrosine/serine kinase. Nature. 1991;349:808–811. doi: 10.1038/349808a0. [DOI] [PubMed] [Google Scholar]
- Gautier J, Solomon MJ, Booher RN, Bazan JF, Kirschner MW. Cdc25 is a specific tyrosine phosphatase that directly activates p34cdc2. Cell. 1991;67:197–211. doi: 10.1016/0092-8674(91)90583-k. [DOI] [PubMed] [Google Scholar]
- Guo Z, Dunphy WG. Response of Xenopus Cds1 in cell-free extracts to DNA templates with double-stranded ends. Mol Biol Cell. 2000;11:1535–1546. doi: 10.1091/mbc.11.5.1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honda R, Ohba Y, Yasuda H. 14-3-3ζ protein binds to the carboxyl half of mouse Wee1 kinase. Biochem Biophys Res Commun. 1997;230:262–265. doi: 10.1006/bbrc.1996.5933. [DOI] [PubMed] [Google Scholar]
- Izumi T, Walker DH, Maller JL. Periodic changes in phosphorylation of the Xenopus cdc25 phosphatase regulate its activity. Mol Biol Cell. 1992;3:927–939. doi: 10.1091/mbc.3.8.927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumagai A, Dunphy WG. Regulation of the cdc25 protein during the cell cycle in Xenopus extracts. Cell. 1992;70:139–151. doi: 10.1016/0092-8674(92)90540-s. [DOI] [PubMed] [Google Scholar]
- Kumagai A, Dunphy WG. Control of the Cdc2/Cyclin B complex in Xenopus extracts arrested at a G2/M checkpoint with DNA synthesis inhibitors. Mol Biol Cell. 1995;6:199–213. doi: 10.1091/mbc.6.2.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumagai A, Dunphy WG. Regulation of Xenopus Cdc25 protein. Methods Enzymol. 1997;283:564–571. doi: 10.1016/s0076-6879(97)83044-3. [DOI] [PubMed] [Google Scholar]
- Kumagai A, Dunphy WG. Binding of 14-3-3 proteins and nuclear export control the intracellular localization of the mitotic inducer Cdc25. Genes Dev. 1999;13:1067–1072. doi: 10.1101/gad.13.9.1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumagai A, Dunphy WG. Claspin, a novel protein required for the activation of Chk1 during a DNA replication checkpoint response in Xenopus egg extracts. Mol Cell. 2000;6:839–849. doi: 10.1016/s1097-2765(05)00092-4. [DOI] [PubMed] [Google Scholar]
- Kumagai A, Guo Z, Emami KH, Wang SX, Dunphy WG. The Xenopus Chk1 protein kinase mediates a caffeine-sensitive pathway of checkpoint control in cell-free extracts. J Cell Biol. 1998a;142:1559–1569. doi: 10.1083/jcb.142.6.1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumagai A, Yakowec P, Dunphy WG. 14-3-3 proteins act as negative regulators of the mitotic inducer Cdc25 in Xenopus egg extracts. Mol Biol Cell. 1998b;9:345–354. doi: 10.1091/mbc.9.2.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lew DJ. Cell-cycle checkpoints that ensure coordination between nuclear and cytoplasmic events in Sacchromyces cerevisiae. Curr Opin Genet Dev. 2000;10:47–53. doi: 10.1016/s0959-437x(99)00051-9. [DOI] [PubMed] [Google Scholar]
- Lopez-Girona A, Furnari B, Mondesert O, Russell P. Nuclear localization of Cdc25 is regulated by DNA damage and a 14-3-3 protein. Nature. 1999;397:172–175. doi: 10.1038/16488. [DOI] [PubMed] [Google Scholar]
- Matsuoka S, Huang M, Elledge SJ. Linkage of ATM to cell cycle regulation by the Chk2 protein kinase. Science. 1998;282:1893–1897. doi: 10.1126/science.282.5395.1893. [DOI] [PubMed] [Google Scholar]
- McGowan CH, Russell P. Cell cycle regulation of human WEE1. EMBO J. 1995;14:2166–2175. doi: 10.1002/j.1460-2075.1995.tb07210.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michael WM, Newport J. Coupling of mitosis to the completion of S phase through Cdc34-mediated degradation of Wee1. Science. 1998;282:1886–1889. doi: 10.1126/science.282.5395.1886. [DOI] [PubMed] [Google Scholar]
- Morgan DO. Cyclin-dependent kinases: engines, clocks, and microprocessors. Annu Rev Cell Dev Biol. 1997;13:261–291. doi: 10.1146/annurev.cellbio.13.1.261. [DOI] [PubMed] [Google Scholar]
- Mueller PR, Coleman TR, Dunphy WG. Cell cycle regulation of a Xenopus Wee1-like kinase. Mol Biol Cell. 1995a;6:119–134. doi: 10.1091/mbc.6.1.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller PR, Coleman TR, Kumagai A, Dunphy WG. Myt1: a membrane-associated inhibitory kinase that phosphorylates Cdc2 on both threonine-14 and tyrosine-15. Science. 1995b;270:86–90. doi: 10.1126/science.270.5233.86. [DOI] [PubMed] [Google Scholar]
- Murakami MS, Vande Woude GF. Analysis of the early embryonic cell cycles of Xenopus; regulation of cell cycle length by Xe-wee1 and Mos. Development. 1998;125:237–248. doi: 10.1242/dev.125.2.237. [DOI] [PubMed] [Google Scholar]
- Murray AW. Cell cycle extracts. Methods Cell Biol. 1991;36:581–605. [PubMed] [Google Scholar]
- Muslin AJ, Tanner JW, Allen PM, Shaw AS. Interaction of 14-3-3 with signaling proteins is mediated by the recognition of phosphoserine. Cell. 1996;84:889–897. doi: 10.1016/s0092-8674(00)81067-3. [DOI] [PubMed] [Google Scholar]
- O'Connell MJ, Walworth NC, Carr AM. The G2-phase DNA-damage checkpoint. Trends Cell Biol. 2000;10:296–303. doi: 10.1016/s0962-8924(00)01773-6. [DOI] [PubMed] [Google Scholar]
- O'Connell MJ, Raleigh JM, Verkade HM, Nurse P. Chk1 is a wee1 kinase in the G2 DNA damage checkpoint inhibiting cdc2 by Y15 phosphorylation. EMBO J. 1997;16:545–554. doi: 10.1093/emboj/16.3.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohi R, Gould KL. Regulating the onset of mitosis. Curr Opin Cell Biol. 1999;11:67–273. doi: 10.1016/s0955-0674(99)80036-2. [DOI] [PubMed] [Google Scholar]
- Parker LL, Atherton-Fessler S, Lee MS, Ogg S, Falk JL, Swenson KI, Piwnica-Worms H. Cyclin promotes the tyrosine phosphorylation of p34cdc2 in a wee1+ dependent manner. EMBO J. 1991;10:1255–1263. doi: 10.1002/j.1460-2075.1991.tb08067.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker LL, Piwnica-Worms H. Inactivation of the p34cdc2-cyclin B complex by the human WEE1 tyrosine kinase. Science. 1992;257:1955–1957. doi: 10.1126/science.1384126. [DOI] [PubMed] [Google Scholar]
- Peng C-Y, Graves PR, Thoma RS, Wu Z, Shaw AS, Piwnica-Worms H. Mitotic and G2 checkpoint control: regulation of 14-3-3 protein binding by phosphorylation of Cdc25C on serine-216. Science. 1997;277:1501–1505. doi: 10.1126/science.277.5331.1501. [DOI] [PubMed] [Google Scholar]
- Price D, Rabinovitch S, O'Farrell PH, Campbell SD. Drosophila wee1 has an essential role in the nuclear divisions of early embryogenesis. Genetics. 2000;155:159–166. doi: 10.1093/genetics/155.1.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raleigh JM, O'Connell M. The G2 DNA damage checkpoint targets both Wee1 and Cdc25. J Cell Sci. 2000;113:1727–1736. doi: 10.1242/jcs.113.10.1727. [DOI] [PubMed] [Google Scholar]
- Rowley R, Hudson J, Young PG. The wee1 protein kinase is required for radiation-induced mitotic delay. Nature. 1992;356:353–355. doi: 10.1038/356353a0. [DOI] [PubMed] [Google Scholar]
- Sanchez Y, Wong C, Thoma R, Richman R, Wu Z, Piwnica-Worms H, Elledge S. Conservation of the Chk1 checkpoint pathway in mammals: linkage of DNA damage to Cdk regulation through Cdc25. Science. 1997;277:1497–1501. doi: 10.1126/science.277.5331.1497. [DOI] [PubMed] [Google Scholar]
- Thorson JA, Yu LW, Hsu AL, Shih N-Y, Graves PR, Tanner JM, Allen PM, Piwnica-Worms H, Shaw AS. 14-3-3 proteins are required for maintenance of Raf-1 phosphorylation and kinase activity. Mol Cell Biol. 1998;18:5229–5238. doi: 10.1128/mcb.18.9.5229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Jacobs C, Hook KE, Duan H, Booher RN, Sun Y. Binding of 14-3-3β to the carboxyl terminus of Wee1 increases Wee1 stability, kinase activity, and G2-M cell population. Cell Growth Differ. 2000;11:211–219. [PubMed] [Google Scholar]
- Watanabe N, Broome M, Hunter T. Regulation of the human WEE1Hu CDK tyrosine 15-kinase during the cell cycle. EMBO J. 1995;14:1878–1891. doi: 10.1002/j.1460-2075.1995.tb07180.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaffe MB, Rittinger K, Volinia S, Caron PR, Aitken A, Leffers H, Gamblin SJ, Smerdon SJ, Cantley LC. The structural basis for 14-3-3: phosphopeptide binding specificity. Cell. 1997;91:961–971. doi: 10.1016/s0092-8674(00)80487-0. [DOI] [PubMed] [Google Scholar]
- Yang J, Winkler K, Yoshida M, Kornbluth S. Maintenance of G2 arrest in the Xenopus oocyte: a role for 14-3-3-mediated inhibition of Cdc25 nuclear import. EMBO J. 1999;18:2174–2183. doi: 10.1093/emboj/18.8.2174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng Y, Forbes KC, Wu Z, Moreno S, Piwnica-Worms H, Enoch T. Replication checkpoint requires phosphorylation of the phosphatase Cdc25 by Cds1 or Chk1. Nature. 1998;395:507–510. doi: 10.1038/26766. [DOI] [PubMed] [Google Scholar]
- Zeng Y, Piwnica-Worms H. DNA damage and replication checkpoints in fission yeast require nuclear exclusion of the Cdc25 phosphatase via 14-3-3 binding. Mol Cell Biol. 1999;19:7410–7419. doi: 10.1128/mcb.19.11.7410. [DOI] [PMC free article] [PubMed] [Google Scholar]