Abstract
Aims
‘Functional foods’ supplemented with plant sterol esters (PSE) and plant stanol esters (PSA) are therapeutic options for the management of hypercholesterolaemia. However, their effects on blood monocytes, endothelial function, atherogenesis, and sterol tissue concentrations are poorly understood.
Methods and results
Male apoE−/− mice (n= 30) were randomized to three different diets for 6 weeks (n= 10 per group): high-cholesterol (1.25%) western-type diet (WTD), WTD + 2% PSE, and WTD + 2% PSA. Both supplements reduced serum cholesterol. WTD + PSE resulted in increased plant sterol serum concentrations and increased inflammatory Ly-6C(high) monocyte numbers. WTD + PSA increased plant stanol serum concentrations and Ly-6C-monocyte numbers, but decreased vascular superoxide release, lipid hydroperoxides, and inflammatory cytokines in aortic tissue, in plasma, and in circulating monocytes. Despite reduced serum cholesterol concentrations, both supplements impaired endothelial vasodilation compared with WTD. WTD + PSA reduced the development of atherosclerotic lesions compared with WTD alone (12.7 ± 3.7 vs. 28.3 ± 3.5%), and WTD + PSE was less effective (17.5 ± 3.7%). WTD + PSE and WTD + PSA reduced the cholesterol content in the liver, but not in the brain. However, WTD + PSE and WTD + PSA increased plant sterol and plant stanol concentrations in the liver as well as in the brain.
Conclusion
PSE and PSA supplementation reduced serum cholesterol, but increased plant sterol and plant stanol concentrations. Elevated levels of PSE and PSA were associated with endothelial dysfunction and increased central nervous system depositions. Atherosclerotic lesion retardation was more pronounced in WTD + PSA, coinciding with higher regenerative monocyte numbers, decreased oxidative stress, and decreased inflammatory cytokines compared with WTD + PSE.
Keywords: Cholesterol, Plant sterol, Plant stanol, Monocytes, Cholesterol absorption inhibition
1. Introduction
Hypercholesterolaemia is a major risk factor for cardiovascular diseases.1 An important strategy in the reduction of cardiovascular risk includes a healthy diet and lifestyle.2 ‘Functional foods’ supplemented with plant sterol esters (PSE) and plant stanol esters (PSA) are widely used as a ‘non-prescription’ approach to lower plasma cholesterol levels.2,3 However, recently released guidelines are more critical of food supplementation with phytosterols and draw attention to potential safety issues.4,5 Phytosterols are plant-derived non-nutritive compounds whose chemical structure differs from that of cholesterol only by minor modifications.6 Sitosterol and campesterol are the most frequently occurring phytosterols in food (∼60 and 35%, respectively). Plant stanols, on the other hand, are the less abundant saturated form of plant sterols. Estimates for intestinal absorption of plant sterols range from 0.4 to 5% and from 0.02 to 0.3% for their saturated counterparts. Consequently, plant stanol concentrations in serum are much lower than plant sterol concentrations. In humans, daily phytosterol doses ranging from 0.8 to 4.0 g have been shown to reduce LDL-cholesterol concentrations by 10–15%.3 Their primary mechanism of lowering blood cholesterol levels is the competitive replacement of cholesterol in bile salt micelles, resulting in reduced absorption of unesterified cholesterol from the small intestine.3,6 The precise molecular mechanisms for sterol absorption are not well defined, but absorption of both cholesterol and phytosterol is known to require the NPC1L1 protein. The majority of absorbed phytosterols are excreted by the liver, but small amounts are retained.6 The biological significance of increased plasma and tissue sterol and stanol concentrations induced by phytosterol-enriched food remains a matter of controversial debate.6–9 Moreover, a recently published clinical trial demonstrated a negative effect on the microcirculation induced by a diet supplementation with PSE, but not by PSA in statin-treated humans with mild hyercholsterolaemia.10 On this background and based on differences in chemical structure and metabolism, we hypothesized that inhibition of plasma cholesterol levels in apoE−/− mice by PSE and PSA has differential effects on blood monocytes, lymphocytes, oxidative stress, inflammatory cytokines, endothelial function, and atherogenesis and may differentially affect sterol concentrations in the liver and brain.
2. Methods
2.1. Animals and diets
Male apoE−/− mice (C57/BL6 background, 8–12 weeks of age, 20–25 g) were purchased from Charles River, Sulzfeld, Germany, and maintained in a specific pathogen-free environment on a 12 h light/12 h dark cycle. The investigation conformed with the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Heath (NIH Publication No. 85-23, revised 1996) and was approved by the Ethics Committee of the University of the Saarland. After 1 week of adaptation, 30 mice were randomized to three treatment groups matched for body weight. All three groups were fed a ‘western-type’ diet [WTD; 40 kcal% butterfat, 1.25% (w/w) cholesterol]. One group (n= 10) was fed a diet supplemented with 3.4% PSE (w/w) (WTD + PSE), and the second group was fed a diet supplemented with 3.4% PSA (w/w) (WTD + PSA). Mice fed the standard WTD without supplementation were used as controls (WTD). PSE and PSA were obtained from the RAISIO company (Turku, Finland). Approximately 40% (w/w) of the PSE and PSA were rapeseed oil fatty acids, and to maintain comparable fatty acid profiles and energy contents, 1.4% rapeseed oil was added to control WTD-fed animals. Over the 6-week study period, mice had ad libitum access to water and were subjected to restricted feeding of 3 g per day. Food consumption was monitored throughout the total experimental period. Diets were prepared by the SNIFF company (Soest, Germany). Lipid and sterol content of the diets were controlled by gas–liquid chromatography–mass spectrometry during the course of the study.
2.2. Measurement of sterol concentrations in the plasma, liver, and brain
Blood samples and tissues were immediately centrifuged, and plasma was stored at −70°C. For sterol quantifications, tissue samples were frozen to dryness using a vacuum and subjected to chloroform/methanol extraction for 2 days. Analyses of cholesterol, lathosterol, sitosterol, and campesterol were performed by gas–liquid chromatography–mass spectrometry as described.11
2.3. Evaluation of effects of PSE and PSA on peripheral blood mononuclear cells
Mouse leucocytes were characterized by flow cytometry using anti-CD115 (AbD Serotec, Düsseldorf, Germany), -CD11b, and -Ly-6C staining (BD Biosciences, Heidelberg, Germany). Monocytes were identified in the SSC/CD11b dot blot by granularity and high expression of CD11b. According to this gating strategy, three monocyte subsets were identified: CD115 + CD11b + Ly-6C++ [termed Ly-6C(high)], CD115 + CD11b + Ly-6C+ [termed Ly-6C(low)], and CD115 + CD11b + Ly-6C− (termed Ly-6C−). Although our study was not designed to analyse various lymphocyte subsets, our staining protocol allowed characterization of the CD11b-Ly-6C+ lymphocyte subset, which may be composed of CD4- and CD8-positive lymphocytes. Blood smear analysis was performed to determine absolute cell numbers; to calculate the absolute lymphocyte/monocyte numbers per microlitre of blood, leucocyte frequencies were related to the blood smear analysis. Pappenheim staining was performed followed by microscopic analysis for differentiation of the cellular blood components. For flow cytometric analysis, 50 µL of heparinized blood was first washed with FACS buffer [PBS supplemented with 5% foetal calf serum (Seromed, Berlin, Germany) and 0.5% bovine serum albumin (Serva, Heidelberg, Germany)]. Cells were stained for 20 min at 4°C, followed by a 10 min incubation in lysing buffer (0.83% NH4Cl, 0.1% KCO3, 0.1 mM EDTA-Na4, pH 7.2). After washing the cells, leucocytes were fixed in 1% paraformaldehyde and stored at 4°C until FACS analysis (FACSCalibur, BD Biosciences).
2.4. Measurement of vascular superoxide production and lipid peroxidation
Superoxide release in intact aortic segments was determined by L-012 chemiluminescence and lipid hydroperoxides were determined with Lipid Peroxidation Assay Kit II (Calbiochem, Gibbstown, NJ, USA) and expressed as percentage to controls (WTD) as described.12 NADPH oxidase activity was measured by a lucigenin-enhanced chemiluminescence assay.12
2.5. Measurement of inflammatory cytokines in aortic tissue
mRNA expression in the aorta was assessed by real-time RT–PCR of mmIL-6, mmMCP-1, mmICAM, mmVCAM, and mmTNF-α. Data were analysed in a semi-quantitative fashion and expressed relative to 18S rRNA expression levels.
2.6. Measurement of inflammatory cytokines in plasma
Inflammatory cytokines (TNF-α, MCP1, IL-6, IL-10, IL-12, and interferon-γ) were measured in the plasma of apoE−/− mice by a flow cytometry-based cytokine bead array according to the manufacturer's instructions (BD Biosciences).
2.7. Measurement of inflammatory cytokines in monocytes
Blood was collected and mononuclear cells were isolated using Ficoll® density gradient centrifugation. Real-time RT–PCR was performed for mmIL-6, mmIL1b, and mmMCP-1.
2.8. Aortic ring preparations and tension recording
Two millimetre rings of the descending aorta were mounted in organ baths to record isometric tension and assess endothelial-dependent and -independent function as described previously.12
2.9. Staining of atherosclerotic lesions and morphometric analysis
Hearts with the ascending aorta were embedded in Tissue Tek O.C.T. embedding medium (Miles) as described previously.13 Macrophages were detected by immunostaining with MOMA-2, 1:50 (Serotec MCA519G, Oxford, UK), followed by Alexa Flour, 1:200, 546 (Invitrogen); Ly-6C-positive macrophages were detected by immunostaining with Ly-6C, 1:600 (BD Pharmingen, Franklin Lakes, USA). For smooth muscle cell (SMC) α-actin staining, monoclonal anti-α-smooth muscle actin, 1:500 (Sigma), was applied. For morphometric analysis, haematoxylin staining was performed according to the standard protocols.13 All sections were examined under a Nikon E 600 microscope. Lucia Measurement Version 4.6 software was used to measure the area of histological sections.
2.10. Statistical analysis
Data are reported as mean ± SEM. Differences between experimental groups were analysed by one-way ANOVA followed by application of the Bonferroni test. P-values <0.05 were considered as statistically significant. All statistical tests were performed using SPSS software (Chicago, IL, USA).
3. Results
3.1. Effects of PSE and PSA on plasma sterol concentrations
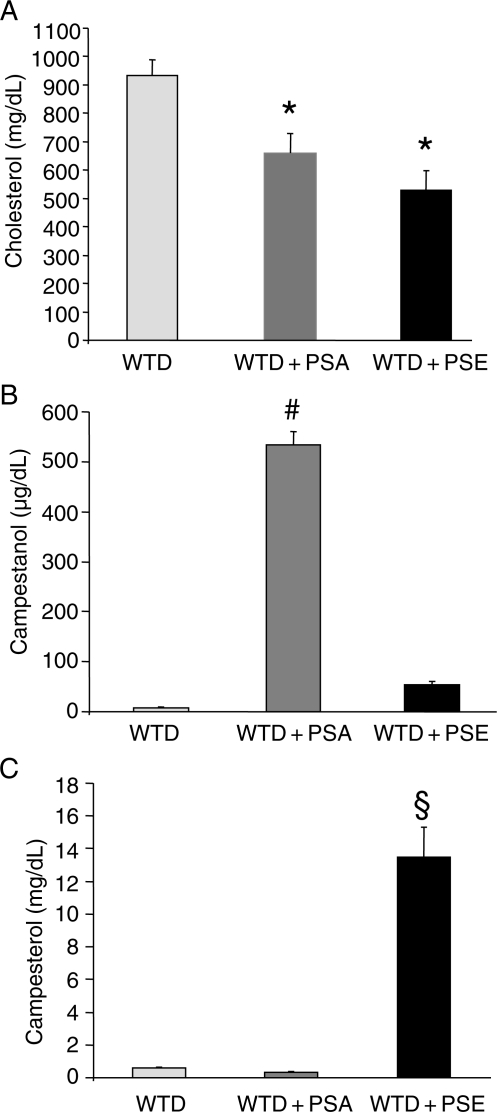
Three groups of apoE−/− mice were each fed specific diets for 6 weeks and plasma lipids and lipoprotein profiles were determined. The average plasma cholesterol level in the group fed the WTD was 932 ± 55 mg/dL. Supplementation with PSA reduced plasma cholesterol levels by 29% (to 661 ± 68 mg/dL), whereas PSE supplementation caused a 43% reduction (to 530 ± 69 mg/dL) (Figure 1A). Dietary supplementation with PSE increased plasma plant sterol concentrations compared with levels in control WTD-fed animals (13.46 ± 1.83 vs. 0.58 ± 0.07 mg/dL for campesterol; P< 0.0005) (Figure 1C). In contrast, supplementation with PSA showed a reduction in plasma plant sterol concentrations; however, this effect did not reach statistical significance (0.33 ± 0.06 vs. 0.58 ± 0.07 mg/dL for campesterol). Dietary supplementation with PSA increased plasma plant stanol concentrations compared with levels detected in control animals fed the WTD (532 ± 28 vs. 7.8 ± 2.0 µg/dL for campestanol; P < 0.0005) (Figure 1B).
Figure 1.
Effects of dietary PSA and PSE on plasma cholesterol, campestanol, and campesterol. ApoE−/− mice were fed a high-cholesterol WTD supplemented with PSA or PSE, and plasma cholesterol (A), campestanol (B), and campesterol (C) concentrations were determined by gas– liquid chromatography–mass spectrometry. Values are mean ± SEM (n= 10 per group). *P< 0.05 for WTD + PSE vs. WTD, WTD + PSA vs. WTD. #P < 0.05 for WTD + PSA vs. WTD and WTD + PSE. §P < 0.05 for WTD + PSE vs. WTD and WTD + PSA.
3.2. Effects of PSE and PSA on peripheral blood mononuclear cells
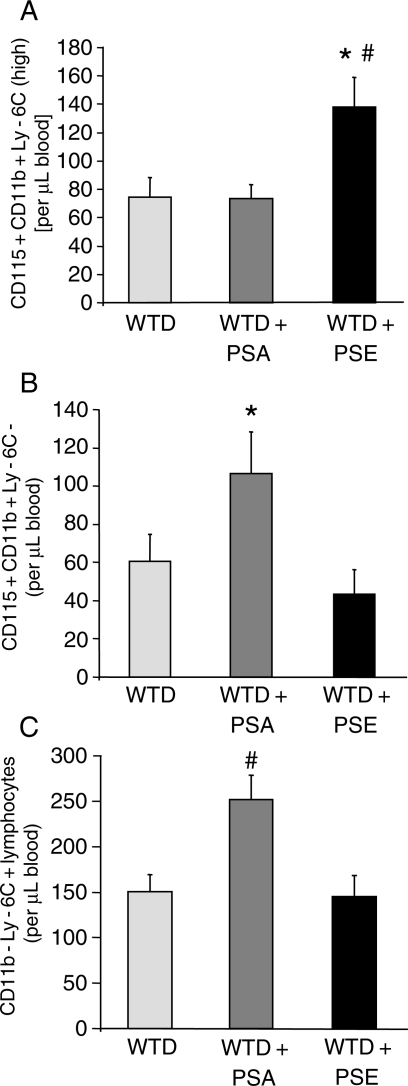
Although no difference in absolute numbers of leucocytes per microlitre blood was observed among the three groups (WTD: 3355.0 ± 215.3, WTD + PSA: 3405.7 ± 278.0, WTD + PSE: 3483.7 ± 345.4; P = 0.95), there was a trend for higher lymphocyte numbers in PSA-fed mice (WTD: 1849.0 ± 161.5, WTD + PSA: 2186.6 ± 176.2, WTD + PSE: 1859.2 ± 215.1; P = 0.36) and higher monocyte numbers in PSE-fed mice (WTD: 311.0 ± 38.9, WTD + PSA: 329.1 ± 30.8, WTD + PSE: 415.6 ± 42.1; P = 0.13). By flow cytometry, the heterogeneous monocytes can be divided into three monocyte subsets (see Section 2): the ‘inflammatory’ subset is defined by high expression of Ly-6C [Ly-6C(high)] and two so-called ‘patrolling’ subpopulations characterized either by weak positive staining [Ly-6C(low)] or by negative staining for the Ly-6C antigen (Ly-6C−) respectively. As depicted in Figure 2A in the apoE knockout model, supplementation with sterol esters increased the inflammatory Ly-6C(high) monocyte subset in comparison to the other diets (WTD: 74.8 ± 13.7, WTD + PSA: 73.6.0 ± 9.9, WTD + PSE: 138.0 ± 21.2; P = 0.01). Elevated numbers of the Ly-6C(low) subpopulation were measured for animals supplemented with PSE, but this difference did not reach statistical significance (WTD: 175.8 ± 38.7, WTD + PSA: 149.0 ± 32.2, WTD + PSE: 234.5 ± 42.0; P = 0.28). In contrast, Ly-6C− monocytes, which may be composed of both ‘patrolling’ monocytes and haematopoietic progenitor cells, were about twice as high in PSA-fed mice in comparison to PSE-fed mice (WTD: 60.4 ± 14.3, WTD + PSA: 106.5 ± 21.7, WTD + PSE: 43.2 ± 13.1; P = 0.04) (Figure 2B). Additionally, the former animal group also showed elevated CD115-CD11b-Ly-6C+ lymphocytes numbers (Figure 2C).
Figure 2.
Effects of dietary PSA and PSE on circulating peripheral blood mononuclear cells. Effects of 6 weeks of treatment of apoE−/− mice with a high-cholesterol WTD supplemented with PSA or PSE. Values are mean ± SEM (n= 10 per group). (A) Effect on circulating inflammatory CD115 + CD11b + Ly6C(high) monocytes. *P < 0.05 for WTD + PSE vs. WTD + PSA, #P < 0.05 for WTD + PSE vs. WTD. (B) Effect on circulating ‘patrolling’ CD115 + CD11b + Ly-6C-monocytes. *P < 0.05 for WTD + PSE vs. WTD + PSA. (C) Effect on circulating lymphocytes. #P < 0.05 for WTD + PSA vs. WTD and WTD + PSE.
3.3. Effects of plant sterols and plant stanols on vascular superoxide production, NADPH activity, and lipid hydroperoxides
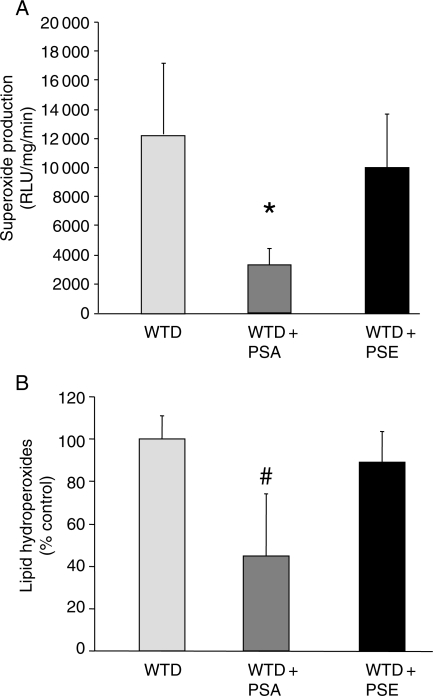
As shown in Figure 3A, vascular superoxide release was significantly decreased in mice on the diet supplemented with PSA compared with those supplemented with PSE. Furthermore, lipid hydroperoxides were significantly decreased in mice on a diet supplemented with PSA compared with WTD and WTD + PSE (Figure 3B). However, no difference in NADPH activity was observed among the WTD (188 ± 26%), WTD + PSA (216 ± 48%), and WTD + PSE (225 ± 65%) groups.
Figure 3.
Effects of dietary PSA and PSE on vascular oxidative stress. Parameters of vascular oxidative stress were assessed in WTD-, WTD + PSA-, and WTD + PSE-fed mice. Values are mean ± SEM (n= 6–7 per group). (A) Superoxide production in intact aortic ring preparations was assessed by L-012 chemiluminescence. *P < 0.05 for WTD + PSA vs. WTD and WTD + PSE. (B) Concentrations of lipid hydroperoxides in aortic homogenates. #P < 0.05 for WTD + PSA vs. WTD and WTD + PSE.
3.4. Effects of plant sterols and plant stanols on inflammatory cytokines in aortic tissue, in plasma, and in monocytes
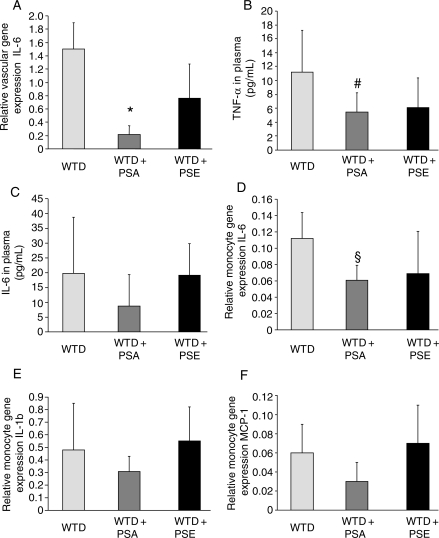
As depicted in Figure 4A, mice on WTD + PSA showed a significant reduction in IL-6 mRNA expression in aortic tissue compared with WTD and WTD + PSE. There were no significant differences in mRNA expression of MCP-1, ICAM, VCAM, and TNF-α between the treatment groups (data not shown). As depicted in Figure 4B, mice on WTD + PSA showed a significant reduction in TNF-α serum levels, whereas a diet supplement with PSE resulted in a less pronounced reduction in TNF-α serum levels. Furthermore, we observed a more pronounced reduction in IL-6 serum levels in animals on a diet supplement with PSA compared with those on PSE (Figure 4C). However, this effect did not reach statistical significance (P = 0.07). We did not observe any differences in regard to serum levels of MCP-1, IL-10, IL-12, and interferon-γ in WTD-, WTD + PSA-, and WTD + PSE-fed animals (data not shown). As depicted in Figure 4D, mice on WTD + PSA showed a reduced expression of IL-6 in circulating monocytes compared with WTD. Again, this reduction was less pronounced in WTD + PSE-fed animals. Furthermore, we observed a more pronounced reduction in IL-1b and MCP-1 in WTD + PSA-fed animals compared with mice on WTD + PSE; however, this effect did not reach statistical significance (Figure 4E and F).
Figure 4.
Effects of dietary PSA and PSE on inflammatory cytokines in aortic tissue, in plasma, and in circulating monocytes. Effect on IL-6 mRNA expression in aortic tissue. *P < 0.05 for WTD + PSA vs. WTD and WTD + PSE. Effect on TNF-α and (C) IL-6 in plasma. #P < 0.05 for WTD + PSA vs. WTD. Effect on IL-6, (E) IL-1b, and (F) MCP-1 in circulating monocytes. §P < 0.05 for WTD + PSA vs. WTD.
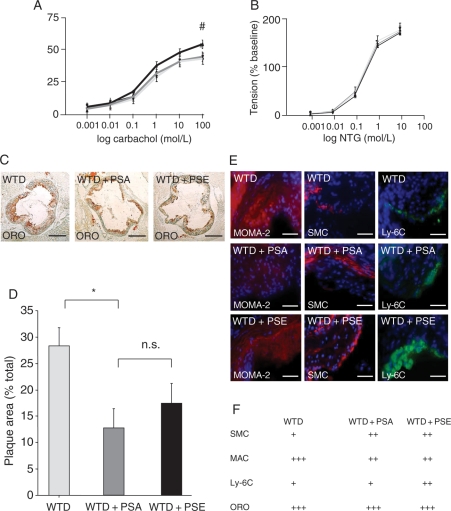
3.5. Effects of plant sterols and plant stanols on endothelial function
Analysis of aortic rings showed that endothelial-dependent vasorelaxation was impaired in mice fed the WTD + PSE and WTD + PSA diets compared with mice fed the WTD (P< 0.05). Endothelial-independent vasorelaxation in response to nitroglycerin was equal in all three groups (Figures 5A and 4B).
Figure 5.
Effects of dietary PSA and PSE on endothelial function and atherosclerosis. Functional performance of isolated aortic segments was assessed in organ chamber experiments. (A) Endothelium-dependent vasorelaxation induced by carbachol and (B) endothelial-independent vasorelaxation induced by nitroglycerin (NTG), expressed as a percent of maximal phenylephrine-induced vasoconstriction. Values are mean ± SEM (n= 10 per group). #P < 0.05 for WTD (black) vs. WTD + PSA (dark grey) and WTD + PSE (bright grey). Atherosclerotic lesion formation in the aortic sinus of apoE−/− mice on a high-cholesterol WTD, WTD with PSA supplementation, and WTD with PSE supplementation. (C) Representative examples (oil-red-O), bars: 500 µm (D) histomorphological analysis, (E) representative atherosclerotic lesions: macrophages (MAC, MOMO-2), vascular SMC (α-actin), and Ly-6C monocytes. Bars: 100 µm. (F) Semi-quantitative grading of stainings: (+) weak staining, (++) moderate staining, and (+++) intense staining. Values are mean ± SEM (n= 10 per group). *P < 0.05 for WTD + PSA vs. WTD.
3.6. Effects of PSE and PSA on the development of atherosclerosis
After the 6 weeks of treatment with specific diets, the mice were sacrificed, aortas were excised, and the aortic lesion area was quantified by histomorphometric analysis. Atherosclerotic lesion formation was most pronounced in mice on the WTD, in which atherosclerotic lesions covered 28.3 ± 3.5% of the luminal area in the aortic root. PSE supplementation reduced atherosclerotic lesions; however, this effect did not reach significance (17.5 ± 3.7 vs. 28.3 ± 3.5% in the WTD + PSE group compared with the WTD group, respectively). The effect on atherosclerotic lesion reduction was more pronounced in response to PSA supplementation (12.7 ± 3.7 vs. 28.3 ± 3.5% in the WTD + PSA group compared with the WTD group, respectively; P < 0.05). However, no significant difference between the two groups with supplemented diets was observed (Figure 5C and D).
3.7. Histological characterization of atherosclerotic lesions in apoE−/− mice on PSE and PSA
In all groups, macrophages localized mostly in the shoulder region of plaques and in the area surrounding the lipid core. Cholesterol lowering reduced macrophage infiltration. Intimal SMC content was less pronounced in WTD animals compared with animals on cholesterol-lowering therapy, and mice on WTD + PSE were characterized by increased infiltration of L6-C(high) macrophages compared with WTD- and WTD + PSA-fed animals. There was no difference in regard to lipid accumulation within atherosclerotic lesions between the groups (Figure 5C, E, and F).
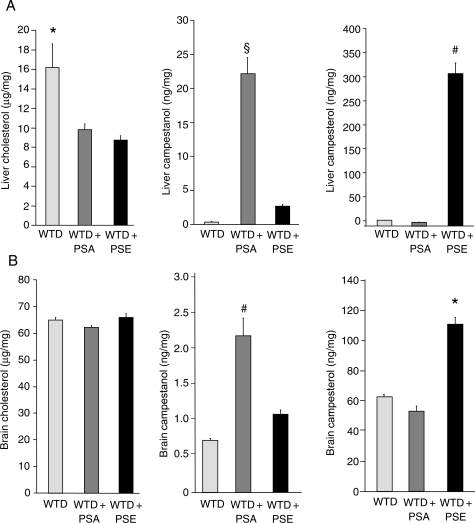
3.8. Effects of PSE and PSA on tissue sterol concentrations
Concentrations of plant stanol and plant sterol in the liver and brain of the three groups of apoE−/− mice were assessed. The average hepatic cholesterol concentration in the group fed the WTD was 16.2 ± 0.8 µg/mg. Diet supplementation with PSA reduced hepatic cholesterol concentrations by 43% (to 9.2 ± 0.8 µg/mg), but increased hepatic plant stanol concentrations 48-fold (0.44 ± 0.06 vs. 21.31 ± 3.37 ng/mg for campestanol; P < 0.0005). PSE supplementation caused a 46% reduction in hepatic cholesterol concentrations (to 8.7 ± 0.6 µg/mg), but increased hepatic plant sterol concentrations 28-fold (11.65 ± 0.85 vs. 315.16 ± 23.80 ng/mg for campesterol; P < 0.0005) (Figure 6A). Supplementation with PSA or PSE had no effect on cholesterol concentrations in the brain (Figure 6B). However, PSA supplementation increased plant stanol concentrations in the brain 2.9-fold (0.73 ± 0.03 vs. 2.16 ± 0.33 ng/mg for campestanol; P < 0.0005), whereas supplementation with PSE increased plant sterol concentrations in the brain 1.8-fold (62.64 ± 1.86 vs. 110.71 ± 3.33 ng/mg for campesterol; P < 0.0005). Of note, supplementation with PSE increased absolute plant sterol concentrations in the brain 51-fold compared with PSA (2.16 ± 0.33 vs. 110.71 ± 4.82 ng/mg), with a more pronounced impact on brain tissue sterol composition (Figure 6B).
Figure 6.
Effects of dietary PSA and PSE on sterol tissue concentrations. Effect of 6-week treatment of apoE−/− mice with a high-cholesterol WTD controls or supplemented with PSA or PSE on liver (A) and brain (B) cholesterol, campestanol, and campesterol concentrations. Values are mean ± SEM (n= 10 per group). (A) *P < 0.05 for WTD vs. WTD + PSA and WTD + PSE; #P < 0.05 for WTD + PSE vs. WTD and WTD + PSA; §P < 0.05 for WTD + PSA vs. WTD and WTD + PSA. (B) *P < 0.05 for WTD + PSE vs. WTD and WTD + PSA; #P < 0.05 for WTD + PSA vs. WTD and WTD + PSE.
4. Discussion
The major and novel finding of this study is an increase in a ‘pro-atherogenic’ monocyte subpopulation and a less pronounced atherosclerotic lesion reduction in mice fed a diet supplemented with PSE compared with PSA, despite a similar reduction in plasma cholesterol. Mice on a diet supplemented with PSA demonstrated a more pronounced reduction in inflammatory cytokines in aortic tissue, in plasma, and in circulating monocytes, vascular superoxide and lipid hydroperoxide production, an increase in ‘regenerative’ monocytes, and, due to their lower absorbability, lower plasma, liver, and brain tissue concentrations of plant stanols.
We previously observed in apoE−/− mice that a diet supplemented with PSE (equivalent to a commercially available spread) induced endothelial dysfunction and led to an increase in ischaemic stroke size in wild-type mice.13 Moreover, we observed that inhibition of cholesterol absorption by a diet supplementation with PSE was associated with twice the amount of atherosclerotic lesion formation compared with ezetimibe treatment (a drug that reduces both plasma cholesterol and plant sterol levels), despite similar plasma cholesterol levels. Thus, our previous study identified a positive correlation between sterol concentrations and the extent of atherosclerotic lesions.
Other groups previously demonstrated the involvement of plant sterols in macrophage apoptosis and suggested a potential mechanism for a possible pro-atherosclerotic effect of plant sterols.14 Furthermore, it has been shown that the Ly-6C(high) subset is dramatically increased among monocytes in hypercholesterolaemic apoE−/− mice consuming a high-cholesterol WTD.15 On the basis of these data, we speculated that a diet supplementation with PSA and PSE would differentially affect the expression of monocyte subpopulations, consequently impacting endothelial function and atherosclerosis.
It has been previously demonstrated that a diet supplementation with plant sterols compared with plant stanols in mice results in a slightly, however, not statistically significant more pronounced reduction in plasma cholesterol levels.16 The data of this study are in line with these findings and demonstrate that even though plasma cholesterol lowering with PSE in mice was slightly more effective compared with PSA, PSE-fed mice exhibited a significant increase in inflammatory Ly-6C(high) monocyte numbers. In contrast, the Ly-6C− population, which appears to have immunomodulatory tasks,17 is significantly elevated in animals fed with PSA. Therefore, it can be speculated that compared with stanols, plant sterols have an additive inflammatory impact that may contribute to atherosclerosis. This hypothesis is further supported by a more pronounced reduction in vascular superoxide and lipid hydroperoxide production by a diet supplementation with PSA as well as by a more pronounced reduction in inflammatory cytokines in aortic tissue, in plasma, and in circulating monocytes. In previous studies, we and others have observed a prominent role of the NADPH oxidase system for lipid-mediated changes of vascular oxidative stress; therefore, NADPH oxidase activity was assessed here. However, the data suggest that NADPH oxidase is not the most important regulator in this specific context. Other potential sources of superoxide include mitochondrial respiration, as well as enzymes such as xanthine oxidase. In addition, the quantity of O2− is determined by superoxide-scavenging enzymes, most importantly superoxide dismutase. In the present study, the important finding is the differential regulation of superoxide and lipid hydroperoxides by plant sterols and plant stanols. Clearly, these data set the stage for future studies to clarify the effects of sterols and stanols on vascular oxidative stress in more detail.
Only recently, it has been demonstrated that monocytes are cellular protagonists of tissue repair and their specific subtypes are involved in the healing programme after myocardial infarction.18 Panizzi et al. found in apoE−/− mice that Ly-6C(high) monocytes dominate on days 1–4 and digest damaged tissue, whereas reparative Ly-6C− monocytes dominate on days 5–10 and promote angiogenesis and scar formation. Moreover, Panizzi et al. demonstrated that Ly-6C(high) monocytes impair infarct healing through prolonged presence in the infarct and de-regulated resolution of inflammation. Of note, statin treatment of atherosclerotic apoE−/−mice has been shown to reduce Ly-6C(high) monocyte counts.17 These findings underscore the potential importance of monocyte subpopulations in apoE−/−mice.
Although both supplementations reduced atherosclerotic lesion development in the aortic root in apoE−/− mice, plant stanols had a more pronounced effect. This may result from the two-fold higher numbers of Ly-6C− monocytes found in PSA-fed mice. However, higher numbers of this monocyte subtype apparently do not have enough regenerative power to overcome the enormous atherosclerotic burden in apoE knockout mice. Interestingly, despite the reduction in plasma cholesterol concentrations by plant stanols and plant sterols, endothelial function was impaired in these groups compared with controls. This finding is consistent with previous data obtained in wild-type mice on a diet supplemented with PSE,13 but so far unexplained for mice on a diet supplemented by PSA. A possible, although speculative, notion is that increased lymphocyte numbers are associated with impairment in endothelial function under these conditions. However, as our study did not include lymphocyte analysis, no detailed information is available regarding these different lymphocyte subsets in animals fed on PSE and PSA supplemented diets. Therefore, further research will be required.
Our data contribute to the notion that phytosterols may not confer positive effects during atherogenesis. In fact, patients with phytosterolaemia, a rare autosomal-recessive disease, show an increase in plant sterol plasma concentrations by more than 30-fold, caused by a defect in the ABCG5 or ABCG8 transporter genes. In these individuals, elevated plant sterol levels are associated with a malignant premature atherosclerosis.19,20 The second line of evidence stems from several concurrent epidemiological studies, suggesting that upper normal plasma concentrations of plant sterols are associated with increased risk of cardiovascular events.21–26 Only recently, data from the KORA study revealed that common variants in ABCG8 in the normal population were strongly related to elevated serum plant sterol levels and increased risk for coronary artery disease, whereas ABCG8 genotypes associated with decreased serum plant sterol levels were associated with a reduced risk of coronary artery disease.27 These findings have stimulated the discussion that plant sterols, which are also used as markers of cholesterol absorption, might be involved in the pathogenesis of atherosclerosis.6,7,28 Regarding potential molecular mechanisms, one possibility is that plant sterols are more readily oxidized than cholesterol and that especially oxysterols affect the cardiovascular risk.29,30 Data presented in this study demonstrating a difference between PSA and PSE on vascular superoxide production and lipid hydroperoxides further add to this notion.
We previously showed that a diet supplementation with PSE in humans leads to increased plant sterol concentrations in plasma and in aortic valve cusps.13 Therefore, another aim of this study was to determine the deposition of plant stanols and plant sterols in various organ tissues. Our data demonstrate for the first time that in apoE−/− mice, plant stanols and plant sterols are incorporated in both hepatic and cerebral tissues. Interestingly, in a similar study in apoE*3-Leiden mice, Plat et al.31 reported that a diet supplementation with plant stanols and plant sterols increased concentrations in various tissues; however, no increase was observed in liver and in brain tissue. Similarly, Jansen et al.32 reported that diet supplementation of apoE−/−mice with plant sterols did not increase plant sterol concentrations in the brain tissues. Since plant sterols accumulate in the brain of wild-type mice, the authors speculated that apoE is required as a chaperone protein to facilitate sterol transport over the blood–brain barrier. The findings of our study do not support this hypothesis. One potential explanation for these contradictory findings is the difference in the experimental diets. Both Plat et al. and Jansen et al. used a low-cholesterol diet [0.25 and 0.12% (w/w) respectively], whereas in our study, mice were fed a 1.25% (w/w) cholesterol-enriched diet. Therefore, our findings suggest that in the absence of apoE, an increase in cholesterol facilitates both plant stanol and plant sterol accumulation in the brain. Drevets et al. previously demonstrated that Ly-6C monocytes function in the transport of Listeria monocytogenes over the blood–brain barrier, establishing their role as Trojan horses in vivo.33 Thus, another possible explanation is that an increase in Ly-6C(high) monocytes facilitates plant sterol transport over the blood–brain barrier. Further research is required to fully address this hypothesis.
The findings from our experimental study are of potential clinical importance, as plant stanols and plant sterols incorporated in tissues may potentially cause adverse effects. For example, sitosterolaemic patients are known to have anaemic episodes, probably related to disturbed red blood cell characteristics.19 In fact, in SPHR rats, which are characterized by increased plant sterol concentrations from a defect in ABCG5, high plant sterols were associated with increased stroke risk, which was ascribed to reduced erythrocyte deformability.34 Furthermore, Yang et al.35 demonstrated that a severe accumulation of plant sterols in the adrenals of ABCG5/8−/− mice was associated with depletion of cholesteryl esters, further adding to the notion that a diet supplementation with plant sterols potentially interferes with the hormonal system.
In summary, dietary supplementation with both PSA and PSE reduced plasma cholesterol concentrations in apoE−/− mice. Mice on a diet supplemented with PSE demonstrated an increase in a ‘pro-atherogenic’ monocyte subpopulation and a less pronounced atherosclerotic lesion reduction. Furthermore, mice on a diet supplemented with PSE showed increased vascular superoxide and lipid hydroperoxide production and, due to enhanced absorbability, higher plasma concentrations and increased plant sterol tissue deposition in major organ systems.
These findings underline the need for clinical studies that evaluate not only the effectiveness of serum cholesterol reduction, but also the clinical effects and safety of a diet supplementation with PSE.
Funding
This study was supported by the University of the Saarland (HOMFOR). Funding to pay the Open Access publication charges was supported by a research award for the study ‘Vascular effects of diet supplementation with plant sterols’ to O.W. (Alois-Lauer Förderpreis für Medizin, Dillingen/Germany).
Acknowledgements
We are grateful to Simone Jäger, Ellen Becker, Lena Brachmann, Andrey Kasakov, and Anja Kerksiek for excellent technical assistance.
Conflict of interest: none declared.
References
- 1.Wald NJ, Law MR. Serum cholesterol and ischaemic heart disease. Atherosclerosis. 1995;118(Suppl.):S1–S5. [PubMed] [Google Scholar]
- 2.Lichtenstein AH, Appel LJ, Brands M, Carnethon M, Daniels S, Franch HA, et al. Summary of American Heart Association Diet and Lifestyle Recommendations revision 2006. Arterioscler Thromb Vasc Biol. 2006;26:2186–2191. doi: 10.1161/01.ATV.0000238352.25222.5e. [DOI] [PubMed] [Google Scholar]
- 3.Katan MB, Grundy SM, Jones P, Law M, Miettinen T, Paoletti R. Efficacy and safety of plant stanols and sterols in the management of blood cholesterol levels. Mayo Clin Proc. 2003;78:965–978. doi: 10.4065/78.8.965. [DOI] [PubMed] [Google Scholar]
- 4.Arzneimittelkommission der deutschen Ärzteschaft (Drug Commission of the German Medical Profession) Newsletter 2004–2045 , 15 January 2004. [Google Scholar]
- 5.National Institute for Health and Clinical Excellence. LIPID MODIFICATION. Cardiovascular risk assessment and the modification of blood lipids for the primary and secondary prevention of cardiovascular diseases. www.nice.org.uk/nicemedia/pdf/CG67guideline.pdf. (3 January 2011) [PubMed] [Google Scholar]
- 6.Patel MD, Thompson PD. Phytosterols and vascular disease. Atherosclerosis. 2006;186:12–19. doi: 10.1016/j.atherosclerosis.2005.10.026. [DOI] [PubMed] [Google Scholar]
- 7.Weingärtner O, Böhm M, Laufs U. Controversial role of plant sterol esters in the management of hypercholesterolaemia. Eur Heart J. 2009;30:404–409. doi: 10.1093/eurheartj/ehn580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sudhop T, von Bergmann K. Sitosterolemia—a rare disease. Are elevated plant sterols an additional risk factor. Z Kardiol. 2004;93:921–928. doi: 10.1007/s00392-004-0165-2. [DOI] [PubMed] [Google Scholar]
- 9.Weingärtner O, Pinsdorf T, Rogacev KS, Blömer L, Grenner Y, Gräber S, et al. The relationships of markers of cholesterol homeostasis with carotid intima–media thickness. PLoS One. 2010;5:e13467. doi: 10.1371/journal.pone.0013467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kelly ER, Plat J, Mensink RP, Berendschot TT. Effects of long term plant sterol and -stanol consumption on the retinal vasculature: a randomized controlled trial in statin users. Atherosclerosis. 2011;214:225–230. doi: 10.1016/j.atherosclerosis.2010.10.038. [DOI] [PubMed] [Google Scholar]
- 11.Thelen KM, Rentsch KM, Gutteck U, Heverin M, Olin M, Andersson U, et al. Brain cholesterol synthesis in mice is affected by high dose of simvastatin but not of pravastatin. J Pharmacol Exp Ther. 2006;316:1146–1152. doi: 10.1124/jpet.105.094136. [DOI] [PubMed] [Google Scholar]
- 12.Laufs U, Wassmann S, Czech T, Münzel T, Eisenhauer M, Böhm M, et al. Physical inactivity increases oxidative stress, endothelial dysfunction, and atherosclerosis. Arterioscler Thromb Vasc Biol. 2005;25:809–814. doi: 10.1161/01.ATV.0000158311.24443.af. [DOI] [PubMed] [Google Scholar]
- 13.Weingärtner O, Lütjohann D, Ji S, Weisshoff N, List F, Sudhop T, et al. Vascular effects of diet supplementation with plant sterols. J Am Coll Cardiol. 2008;51:1553–1561. doi: 10.1016/j.jacc.2007.09.074. [DOI] [PubMed] [Google Scholar]
- 14.Bao L, Li Y, Deng SX, Landry D, Tabas I. Sitosterol-containing lipoprotein trigger free sterol-induced caspase-independent death in ACAT-competent macrophages. J Biol Chem. 2006;281:336–349. doi: 10.1074/jbc.M606339200. [DOI] [PubMed] [Google Scholar]
- 15.Swirski FK, Libby P, Aikawa E, Alcaide P, Luscinskas FW, Weissleder P, et al. Ly-6Chi monocytes dominate hypercholesterolemia-associated monocytosis and give rise to macrophages in atheromata. J Clin Invest. 2007;117:195–205. doi: 10.1172/JCI29950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Plat J, Beugels I, Gijbels MJ, de Winther MP, Mensink RP. Plant sterol or stanol esters retard lesion formation in LDL receptor-deficient mice independent of changes in serum plant sterols. J Lipid Res. 2006;47:2762–2771. doi: 10.1194/jlr.M600346-JLR200. [DOI] [PubMed] [Google Scholar]
- 17.Geissmann F, Jung S, Littman DR. Blood monocytes consist of two principal subsets with distinct migratory properties. Immunity. 2003;19:71–82. doi: 10.1016/s1074-7613(03)00174-2. [DOI] [PubMed] [Google Scholar]
- 18.Panizzi P, Swirski FK, Figueiredo JL, Waterman P, Sosnovik DE, Aikawa E, et al. Impaired infarct healing in atherosclerotic mice with Ly-6C(hi) monocytosis. J Am Coll Cardiol. 2010;55:1629–1638. doi: 10.1016/j.jacc.2009.08.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bhattacharyya AK, Connor WE. Beta-sitosterolemia and xanthomatosis. A newly described lipid storage disease in two sisters. J Clin Invest. 1974;53:1033–1043. doi: 10.1172/JCI107640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kidambi S, Patel SB. Sitosterolaemia: pathophysiology, clinical presentation and laboratory diagnosis. J Clin Pathol. 2008;61:588–594. doi: 10.1136/jcp.2007.049775. [DOI] [PubMed] [Google Scholar]
- 21.Glueck CJ, Speirs J, Tracy T, Streicher P, Illig E, Vandegrift J. Relationships of serum plant sterols (phytosterols) and cholesterol in 595 hypercholesterolemic subjects, and familial aggregation of phytosterols, cholesterol, and premature coronary heart disease in hyperphytosterolemic probands and their first-degree relatives. Metabolism. 1991;40:842–848. doi: 10.1016/0026-0495(91)90013-m. [DOI] [PubMed] [Google Scholar]
- 22.Rajaratnam RA, Gylling H, Miettinen TA. Independent association of serum squalene and noncholesterol sterols with coronary artery disease in postmenopausal women. J Am Coll Cardiol. 2000;35:1185–1191. doi: 10.1016/s0735-1097(00)00527-1. [DOI] [PubMed] [Google Scholar]
- 23.Sudhop T, Gottwald BM, von Bergmann K. Serum plant sterols as a potential risk factor for coronary heart disease. Metabolism. 2002;51:1519–1521. doi: 10.1053/meta.2002.36298. [DOI] [PubMed] [Google Scholar]
- 24.Assmann G, Cullen P, Erbey J, Ramey DR, Kannenberg F, Schulte H. Plasma sitosterol elevations are associated with an increased incidence of coronary events in men: results of a nested case–control analysis of the Prospective Cardiovascular Munster (PROCAM) study. Nutr Metab Cardiovasc Dis. 2006;16:13–21. doi: 10.1016/j.numecd.2005.04.001. [DOI] [PubMed] [Google Scholar]
- 25.Weingärtner O, Weingärtner N, Scheller B, Lütjohann D, Gräber S, Schäfers HJ, et al. Alterations in cholesterol homeostasis are associated with coronary heart disease in patients with aortic stenosis. Coron Artery Dis. 2009;20:376–382. doi: 10.1097/MCA.0b013e32832fa947. [DOI] [PubMed] [Google Scholar]
- 26.Matthan NR, Pencina M, Larocque JM, Jacques PF, D'Agostino RB, Schäfer EJ, et al. Alterations in cholesterol absorption and synthesis characterize Framingham offspring study participants with coronary heart disease. J Lipid Res. 2009;50:1927–1935. doi: 10.1194/jlr.P900039-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Teupser D, Baber R, Ceglarek U, Scholz M, Gieger C, Holdt LM, et al. Genetic Regulation of serum phytosterol levels and risk of coronary artery disease. Circ Cardiovasc Genet. 2010;3:331–339. doi: 10.1161/CIRCGENETICS.109.907873. [DOI] [PubMed] [Google Scholar]
- 28.Weingärtner O, Lütjohann D, Böhm M, Laufs U. Relationship between cholesterol synthesis and intestinal absorption is associated with cardiovascular risk. Atherosclerosis. 2010;210:362–365. doi: 10.1016/j.atherosclerosis.2010.01.003. [DOI] [PubMed] [Google Scholar]
- 29.Plat J, Brzezinka H, Lütjohann D, Mensink RP, von Bergmann K. Oxidized plant sterols in human serum and lipid infusions as measured by combined gas-liquid chromatography-mass spectrometry. J Lipid Res. 2001;42:2030–2038. [PubMed] [Google Scholar]
- 30.Weingärtner O, Laufs U, Böhm M, Lütjohann D. An alternative pathway of reverse cholesterol transport: the oxysterol 27-hydroxycholesterol. Atherosclerosis. 2010;209:39–41. doi: 10.1016/j.atherosclerosis.2009.09.015. [DOI] [PubMed] [Google Scholar]
- 31.Plat J, de Jong A, Volger OL, Princen HM, Mensink RP. Preferential campesterol incorporation into various tissues in apolipoprotein E*3-Leiden mice consuming plant sterols or stanols. Metabolism. 2008;57:1241–1247. doi: 10.1016/j.metabol.2008.04.018. [DOI] [PubMed] [Google Scholar]
- 32.Jansen PJ, Lütjohann D, Abildayeva K, Vanmirlo T, Plösch T, Plat J, et al. Dietary plant sterols accumulate in brain. Biochem Biophys Acta. 2006;1761:445–453. doi: 10.1016/j.bbalip.2006.03.015. [DOI] [PubMed] [Google Scholar]
- 33.Drevets DA, Dillon MJ, Schawang JS, Van Rooijen N, Ehrchen J, Sunderkötter C, et al. The Ly-6Chigh monocyte subpopulation transports Listeria monocytogenes into the brain during systemic infection of mice. J Immunol. 2004;172:4418–4424. doi: 10.4049/jimmunol.172.7.4418. [DOI] [PubMed] [Google Scholar]
- 34.Ratnayake WM, L'Abbe MR, Mueller R, Hayward S, Plouffe L, Hollywood R, et al. Vegetable oils high in phytosterols make erythrocytes less deformable and shorten the life span of stroke-prone spontaneously hypertensive rats. J Nutr. 2000;130:1166–1178. doi: 10.1093/jn/130.5.1166. [DOI] [PubMed] [Google Scholar]
- 35.Yang C, Yu L, Li W, Xu F, Cohen JC, Hobbs HH. Disruption of cholesterol homeostasis by plant sterols. J Clin Invest. 2004;114:813–822. doi: 10.1172/JCI22186. [DOI] [PMC free article] [PubMed] [Google Scholar]