In the Equisetopsida, different wax distribution and composition patterns in the plant organs indicate a close relationship between wax structure and chemistry and the assimilatory function of these organs. Diverging wax compound classes show the two subgenera of Equisetum to be well separated.
Abstract
Background and aims
Only few data on the epicuticular waxes (EWs) of horsetails are available. This contribution therefore focuses on the wax micromorphology and chemical composition of Equisetum species of the subgenera Equisetum and Hippochaete.
Methodology
Distribution patterns and structural details of EW on the shoots were studied by scanning electron microscopy. After extraction with chloroform, the chemical composition of wax isolates was analysed by gas chromatography.
Principal results
Epicuticular wax crystals were non-oriented platelets or membraneous platelets. They were usually located on subsidiary cells of stomata and adjacent cells. Other parts of the shoots were covered mainly with a smooth wax film or small granules only. The chemical constituents found were alkanes, esters, aldehydes, primary alcohols and free fatty acids in a range of C20–C36 (in esters C36–C56). All species of the subgenus Hippochaete showed a similar pattern of fractions with high percentages of alkanes and aldehydes, whereas the subgenus Equisetum species had distinctly different wax compositions. Extracts from the internodes—surfaces without well-developed EW crystals and only few stomata—showed the lowest contents of aldehydes.
Conclusions
The covering with EW crystals will provide unhindered gas exchange and, combined with intracuticular wax, may prevent excess water loss during winter in the evergreen shoots of the subgenus Hippochaete. The results indicate that the Equisetum wax micromorphology and biosynthesis are comparable to EW of other pteridophyte classes and mosses.
Introduction
The horsetails (Equisetopsida) are a group of lower vascular plants with fossil ancestors dating back to the late Devonian. They are characterized by certain special phytochemical features. Equisetum sylvaticum, for example, has the highest accumulation of silicic acid (up to 25 % dry weight) among all vascular plants (Timell 1964). Since lignin is almost absent, silica acts as reinforcement.
The genus Equisetum consists of 15 species grouped in the subgenera Equisetum and Hippochaete. Whereas the distinctness of these two subgroups is well supported, the infrasubgeneric relationships remain uncertain (Hauke 1963, 1978; Des Marais et al. 2003; Guillon 2004, 2007). From a chemotaxonomic view, for example in flavonoid composition, the subgenus Equisetum shows qualitative and quantitative variations between the species: three groups were identified, each accumulating specific glycosides (Veit et al. 1995). These groups proved to be congruent with a classification based on micromorphological characters (Page 1972). In numerous publications, epicuticular waxes (EWs) have also been studied from the taxonomic perspective. For example in the Gramineae, the Cactaceae (Maffei 1996; Maffei et al. 1997), the Plagiochilaceae (Heinrichs et al. 2000; Heinrichs and Rycroft 2001) and in Ficus species (Sonibare et al. 2005), wax composition patterns were used as taxonomic characters to clarify intrageneric relationships.
Ecologically, all species of horsetails are characterized as mesophytes or hygrophytes. Several taxa of subgenus Hippochaete are evergreen and therefore need protection against water stress related to winter drought. Excess water loss in land plants is prevented by the cuticle and cuticular wax (Raven 1984). But little is known about this ‘protective barrier’ (Shepherd and Griffiths 2006) in horsetails, whose EW layer should provide protection against abiotic stresses related to temperature, light and pollution: the only reported wax components in Equisetum are fatty acids with chain lengths between C22 and C30 (Řezanka 1998). Very-long-chain α,ω-dicarboxylic acids such as ‘equisetolic acid’ (octacosan-1,28-dicarboxylic acid; Sosa 1949; Adams et al. 1969) were found exclusively in the spores and strobili of 10 Equisetum species (Víden and Řezanka 1989; Řezanka 1998).
Since almost no information is available on the EW layer of Equisetum spp., the present study provides a survey on EW structure and composition in this group. This should also address taxonomically relevant questions on differences between species and the two subgenera (Equisetum vs. Hippochaete), and further between the fertile and sterile sprouts of heterophyadic species.
Materials and methods
Plant material
The investigation comprised the species (subgenus Equisetum) E. arvense L., E. telmateia Ehrh., E. sylvaticum L., E. fluviatile L. and (subgenus Hippochaete) E. hyemale L. ssp. hyemale, E. hyemale L. ssp. affine (Engelm.) Calder & Roy L. Taylor, E. scirpoides Michx. and E. variegatum ssp. variegatum Schleich. ex Web. et Mohr. The samples were collected in their natural habitats in southwestern Germany or purchased from the Botanical Garden Hohenheim (Table 1).
Table 1.
Plant material and wax yields (whole-sprout extracts include wax from main axis internodes and main axis leaf sheaths).
| Species | Species abbreviation | Origin | No. of sprouts | Extracted stem length (cm) | Stem diameter (cm) | Total wax yielda (µg) | Wax covering (µg cm−2) |
|---|---|---|---|---|---|---|---|
| Subgenus Equisetum | |||||||
| E. arvense, fertile (whole sprouts) | E. arv | S-Plieningen | 50 | 10 | 0.40 | 5050 | 8.0 |
| E. arvense, fertile (internodes) | E. arv | S-Plieningen | 40 | 10 | 0.40 | 3578 | 7.1 |
| E. arvense, fertile (leaf sheaths) | E. arv | S-Plieningen | 40 | – | – | 1660 | – |
| E. arvense, sterile (whole sprouts) | E. arv | S-Plieningen | 28 | 30 | 0.45 | 3641 | 3.1 |
| E. arvense, sterile (branches) | E. arv | S-Plieningen | 28 | – | – | – | – |
| E. telmateia, fertile (whole sprouts) | E. tel | Eislingen | 17 | 20 | 1.00 | 4897 | 4.6 |
| E. telmateia, fertile (internodes) | E. tel | Eislingen | 30 | 18 | 1.00 | 3448 | 2.0 |
| E. telmateia, fertile (leaf sheaths) | E. tel | Eislingen | 30 | – | – | 4004 | – |
| E. telmateia, sterile (whole sprouts) | E. tel | Eislingen | 30 | 30 | 0.95 | 2932 | 1.1 |
| E. telmateia, sterile (branches) | E. tel | Eislingen | 30 | – | – | – | – |
| E. sylvaticum, fertile (whole sprouts) | E. syl | S-Büsnau | 24 | 18 | 0.40 | 8142 | 15.0 |
| E. sylvaticum, fertile (internodes) | E. syl | S-Büsnau | 23 | 18 | 0.40 | 2439 | 4.7 |
| E. sylvaticum, fertile (leaf sheaths) | E. syl | S-Büsnau | 23 | – | – | 2318 | – |
| E. sylvaticum, sterile (whole sprouts) | E. syl | S-Büsnau | 14 | 25 | 0.45 | 5886 | 11.9 |
| E. sylvaticum, sterile (branches) | E. syl | S-Büsnau | 14 | – | – | – | – |
| E. fluviatile (whole sprouts) | E. fluv | S-Büsnau | 27 | 30 | 0.50 | 4583 | 3.6 |
| E. fluviatile (branches) | E. fluv | S-Büsnau | 27 | – | – | – | – |
| Subgenus Hippochaete (whole sprouts) | |||||||
| E. hyemale ssp. hyemale first year | E. hy h 1st | S-Kaltental | 22 | 30 | 0.60 | 19 548 | 15.7 |
| E. hyemale ssp. hyemale second year | E. hy h 2nd | S-Kaltental | 35 | 27 | 0.60 | 43 855 | 24.6 |
| E. hyemale ssp. Affine | E. hy aff | Cultivated | 10 | 30 | 0.70 | 6693 | 10.2 |
| E. scirpoides | E. scirp | Cultivated | 450 | 10 | 0.05 | 14 412 | 20.4 |
| E. variegatum | E. var | Cultivated | 112 | 23 | 0.15 | 12 996 | 10.7 |
aTotal amount of alkanes, aldehydes, primary alcohols, free fatty acids and alkyl esters. –, values not determined.
Scanning electron microscope examinations
Small pieces of internodes and leaf sheaths of the main axis and the branches of fertile and sterile sprouts were excised, mounted on specimen holders (custom made) using conductive carbon adhesive tabs (Plano G3347) and coated with gold/palladium (30 nm) in a Balzers Union SCD 040 sputter coater. A Zeiss DSM 904 scanning electron microscope (SEM) was used to examine the samples. The terminology of the wax crystal morphology refers to Barthlott et al. (1998).
Chemical analyses
Total EWs were obtained by immersing main axes (including internodes and leaf sheaths), isolated leaf sheaths (from the main axes of the fertile sprouts) or isolated branches (second and third order from sterile sprouts including internodes and leaf sheaths) in hot chloroform (ca. 60 °C) for 20 s. After wax extraction, approximate surface areas were determined by measurements of stem lengths and diameters. The extracts were fractionated into compound classes by preparative thin-layer chromatography on silica gel G (Riedel-deHaen) with the solvents (i) n-hexane and (ii) chloroform/n-hexane (75:25, v:v). The spray reagent for detection (UV 360 nm) was 0.005 % primuline in acetone/water (80:20, v:v). Individual fractions were analysed by gas chromatography (GC) (Haas et al. 2003) with a Shimadzu GC-17A gas chromatograph equipped with a CP-Sil 8 CB capillary column (25 m×0.32 mm, Varian-Chrompack), an on-column injector and a flame ionization detector. Operating conditions of the chromatograph were detector temperature 360 °C and linear velocity of helium carrier gas 30 cm s−1. The column temperature was initially set to 160 °C for 2 min and then increased by 8 °C min−1 to 340 °C (or 360 °C for esters). The fractions of primary alcohols and free fatty acids were analysed as trimethylsilyl ethers/trimethylsilyl esters after derivatization with N,O-bis-(trimethylsilyl)-acetamide/pyridine (1:1, v:v). Appropriate internal standards were employed for each fraction.
Similarity of total waxes, including all fractions and chain lengths with their relative contribution to the total wax amount [see Additional Information], was calculated with the software SPSS 10.1, applying hierarchical cluster analysis (chain lengths were treated as continuous variables; distance measure: squared Euclidean distance; cluster method: Ward's) and the multidimensional scaling function (distance measure: squared Euclidean distance; scaling model: Euclidean distance).
Results
Epicuticular wax distribution and micromorphology
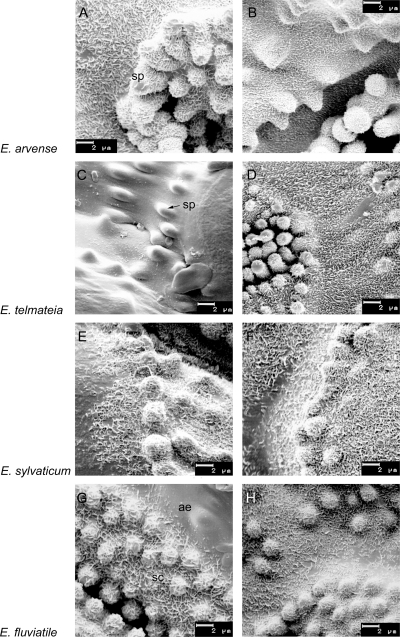
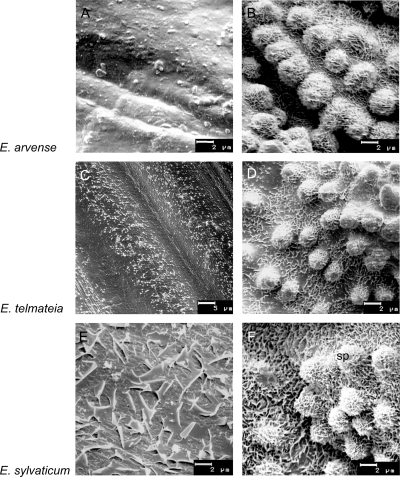
Scanning electron microscopy revealed projections of EW heterogeneously distributed on the Equisetum stem surfaces. The stomatal areas, especially the subsidiary cells, were always covered with well-developed EW crystals, whereas the carinae (ribs) showed a wax film. The presence of wax films or crystal degradation stages in some samples was also visible on the surfaces surrounding the stomata. Different types of EW crystals were present: non-orientated platelets with a size of 0.4–0.6 µm were the most frequent (Figs 1–3). Very thin crystals with filamentous extensions, known as membraneous platelets, were found on leaf sheaths of fertile E. sylvaticum. The comparatively large crystals on internodes of this species (fertile) are probably special forms of the membraneous platelet type (Fig. 3E and F). As a further EW crystal type, granules were present on internodes of fertile E. arvense (Fig. 3A) and on sterile as well as fertile E. telmateia (Figs 1C and 3C).
Fig. 1.
Epicuticular wax structures on surfaces (valleculae) of Equisetum subgenus Equisetum species. Scanning electron microscope images of main axis internodes (left) and internodes of the branches (right) of sterile sprouts. (A, B, D–H) Subsidiary cells (sc) with silica papillae (sp), and adjacent epidermis (ae), covered with non-oriented platelets. (C) Smooth wax film with granules on the epidermal surface (sp, silica papilla). (A+B) E. arvense, (C+D) E. telmateia, (E+F) E. sylvaticum, (G+H) E. fluviatile.
Fig. 3.
Epicuticular wax structures on valleculae of main axis internodes (left) and main axis leaf sheaths (right) of fertile sprouts. (A+C) Smooth wax film with granules on the epidermal surface. (B+D) Non-oriented platelets (sp, silica papilla), (E) large membraneous platelets, (F) membraneous platelets. (A+B) E. arvense, (C+D) E. telmateia, (E+F) E. sylvaticum.
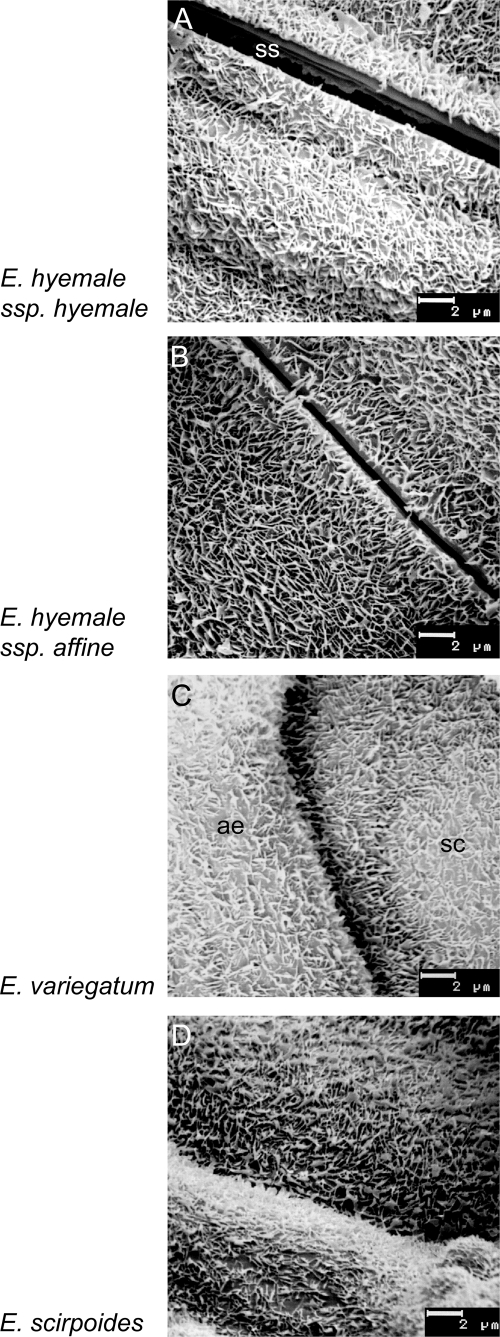
Species of subgenus Equisetum and of subgenus Hippochaete both showed non-oriented platelets as the dominating EW crystal type (Figs 1 and 2). Organ-specific gradual variations, however, became visible as differences in crystal thickness and/or density. The EW platelets on branches of sterile sprouts were usually finer and more densely arranged than those on the internodes of the main shoots (Fig 1A, B and G, H). There was no clear difference between the wax structure of E. hyemale ssp. hyemale first or second years’ sprouts.
Fig. 2.
Epicuticular wax structures on surfaces (valleculae) of Equisetum subgenus Hippochaete species. Scanning electron microscope images of main axis internodes. (A–D) Subsidiary cells (sc), covered with non-oriented platelets (ss,stomatal slit; ae,adjacent epidermis). (A) E. hyemale ssp. hyemale, (B) E. hyemale ssp. affine, (C) E. variegatum, (D) E. scirpoides.
Epicuticular waxes: amounts and composition
Dissolution and removal/extraction of the EW was checked by SEM. Complete removal was only achieved by hot-chloroform treatment. The approximate stem wax covering, based on yields of the whole-sprout extracts (Table 1), ranged between 1 µg cm−2 (sterile E. telmateia) and 25 µg cm−2 (E. hyemale ssp. hyemale second year). With the exception of E. sylvaticum, wax yields of the subgenus Hippochaete species were higher than those of subgenus Equisetum.
Organ-specific differences in wax yields were evident in the fertile sprouts of the heterophyadic species: the leaf sheath wax and internodal wax yields were almost in the same range for E. telmateia and E. sylvaticum; the internodes of fertile E. arvense yielded twice the wax amount of the leaf sheaths. The second years’ sprouts of E. hyemale showed a 1.5-fold higher wax load than the first years’ sprouts of this species (Table 1).
Thin-layer chromatography of the Equisetum EW revealed alkanes, esters, aldehydes, primary alcohols and free fatty acids as the important compound classes. α,ω-Dicarboxylic acids were not found in any of the Equisetum EW extracts.
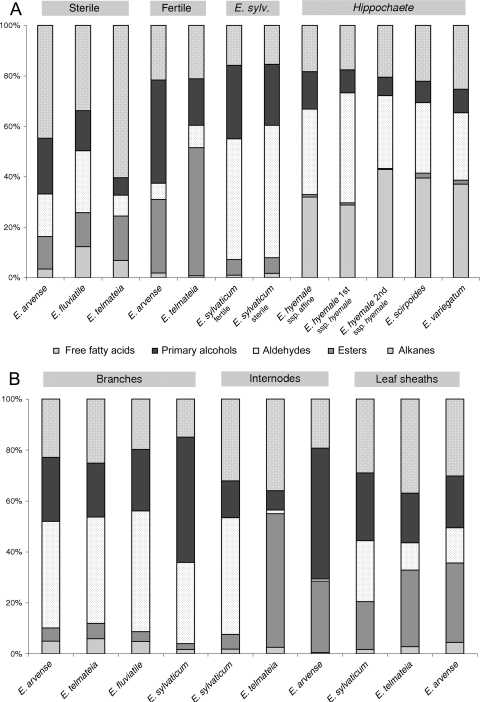
The relative proportions of compound classes in the whole-sprout extracts differed significantly between (i) the two subgenera and (ii) the sterile and fertile sprouts from the heterophyadic species of subgenus Equisetum (Fig. 4A). All species of subgenus Hippochaete showed a nearly identical composition with characteristic high amounts of alkanes and aldehydes (∼30–45 %) and very low proportions of alkyl esters (maximum 2 %).
Fig. 4.
Epicuticular wax composition by compound class (% class). (A) Epicuticular wax from whole sprouts (main axis internodes and main axis leaf sheaths). (B) Epicuticular wax from specific Equisetum organs (fertile main axis internodes, fertile main axis leaf sheaths, sterile branches).
The typical EW composition pattern of sterile sprouts of the subgenus Equisetum species comprised a high content of free fatty acids (34–60 %) and moderate amounts of alkyl esters (maximum 18 %), whereas the fertile sprouts showed up to 50 % alkyl esters (E. telmateia), medium contents of free fatty acids (maximum 22 %) and very low alkane percentages (maximum 2 %). The EW of the semi-heterophyadic species E. sylvaticum, however, did not fit in a fertile/sterile composition scheme, but was characterized by a very high aldehyde content (48 and 53 %), at least 24 % primary alcohols and 2 % alkanes (Fig. 4A).
We also detected organ specificity in the EW composition of the subgenus Equisetum (Fig. 4B): EW extracts from the branches of sterile sprouts were composed of high amounts of aldehydes (>40 %), <6 % of esters and alkanes, and ∼20 % primary alcohols and free fatty acids. Epicuticular wax from the leaf sheaths of fertile sprouts (heterophyadic species) showed less alkanes (maximum 4.5 %) and similar proportions of free fatty acids, primary alcohols, aldehydes and esters. The internodal EW of fertile sprouts contained an extremely low amount of aldehydes. The corresponding values of E. sylvaticum, however, differed markedly from E. arvense and E. telmateia: they showed the same composition pattern as in the EW from branches of the other analysed subgenus Equisetum species (Fig. 4B).
Chain-length distributions of individual fractions were present in clearly alternating profiles with dominating odd-numbered homologues in alkanes and even-numbered ones in the other compound classes. In some samples, however, odd/even ratios were less distinct. Chain lengths ranged from C20 to C36 (in alkyl esters from C36 to C56). In some fractions (e.g. alkanes in E. telmateia), bimodal distribution profiles were found (Table 2).
Table 2.
Epicuticular wax composition in Equisetum species: relative amounts of dominant chain lengths (% of compound class). White lines: whole-sprout waxes (ws,main axis internodes and main axis leaf sheaths); f,fertile sprouts; s, sterile sprouts. Grey lines: epicuticular waxes from isolated plant organs; int,main axis internodes; lsh,main axis leaf sheaths; bch,branches. For species abbreviations, see Table 1.
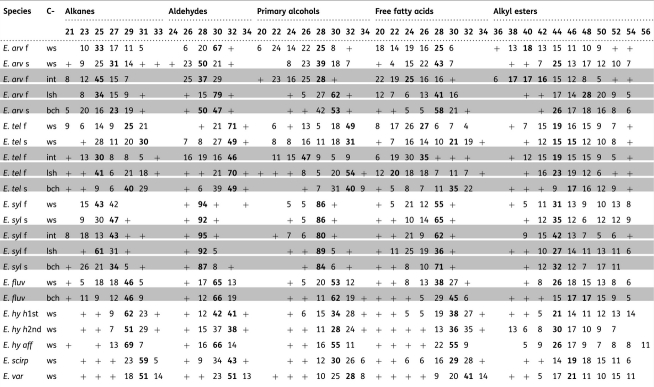 |
+, minor amounts (<5 %); empty fields, chain length not detected; bold values, main homologue(s).
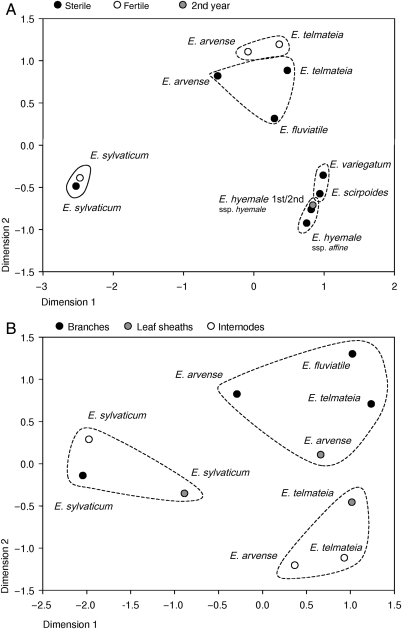
Comparison of the EWs by cluster analysis and multidimensional scaling (MDS), including chain lengths of all compound classes with their actual relevance (% of the total wax), revealed the following relationships (whole-sprout EWs, Fig. 5A). Equisetum sylvaticum showed the most divergent wax composition; the EWs of fertile and sterile sprouts of this species were highly similar. Taxa of subgenus Hippochaete were also clearly separated from the remaining subgenus Equisetum species as a comparatively homogeneous complex in the MDS/cluster analysis. Nonetheless, two groups were distinguishable: E. variegatum/E. scirpoides and E. hyemale ssp. hyemale/E. hyemale ssp. affine. Differences in the EW of first and second years’ sprouts were minimal. The EW of sterile and fertile sprouts of the subgenus Equisetum species formed two distinct clusters.
Fig. 5.
Multidimensional scaling (Euclidean distance model) of the different EWs. (A) Epicuticular wax from whole sprouts (main axis internodes and main axis leaf sheaths). (B) Epicuticular wax from specific Equisetum organs (fertile main axis internodes, fertile main axis leaf sheaths, sterile branches). All detected substances are included with their relative amounts (chain lengths in % of total wax). Lines represent groups obtained by hierarchical cluster analysis (Ward's method) [see Additional Information].
Organ-specific differences in wax composition are also evident in the MDS/cluster analysis (Fig. 5B). The EW of branches and internodes as well as leaf sheaths of E. sylvaticum poorly matched those of the other species. The EWs from branches of E. arvense, E. telmateia and E. fluviatile formed a cluster of high similarity. The internodal EWs of E. arvense and E. telmateia were also positioned in a distinct group. Epicuticular waxes of the leaf sheaths in the cluster analysis were assigned to the ‘branches’ group (E. arvense) and the ‘internodes’ group (E. telmateia), respectively. In the MDS diagram, however, both are positioned near each other, indicating a higher degree of similarity.
Discussion
Epicuticular wax structure
The micromorphology of EWs is very variable, and different types of wax crystals were present on the surfaces of Equisetum. The literature on Equisetum wax structure is scarce, the only reports being from E. arvense surfaces: acicular crystals, 0.2–1.0 µm long and 0.05–0.1 µm wide (Baker and Holloway 1971). Barthlott and Wollenweber (1981) described wax crystals in the same species as scale-like, measuring 0.4 µm. This latter size is in agreement with the present observations, but in some cases the crystals (membraneous platelet type) reached sizes up to 1 µm. The dominating crystal type is non-oriented platelets. This type is very common in plants and therefore insignificant in a taxonomic sense (Barthlott et al. 1998). Apparently, there is no phylogenetic trend towards a special crystal type, and the occurrence of EW crystals is possible in land plants in general. Even liverworts and mosses (both gametophytes and sporophytes) show a comparatively broad spectrum of crystal types: granules, plates, platelets, membraneous platelets, rodlets, tubules and threads (Schönherr and Ziegler 1975; Neinhuis and Jetter 1995; Haas 1999; Heinrichs et al. 2000; Heinrichs and Rycroft 2001). Among the pteridophytes, wax crusts and long, thin, interwoven rodlets were found on the surfaces of Psilotum nudum (Psilotopsida; Geiger 2003). The surfaces of several examined ferns (Pteridopsida) also showed a wide variety of wax crystals, e.g. very thin threads, plates, platelets and diverse kinds of rodlets (Barthlott and Wollenweber 1981). In conclusion, the EW crystals in Equisetum fit in the spectrum found in pteridophytes and land plants in general.
The micromorphology of wax crystals is related to their chemical composition (Barthlott et al. 1998). Crystals (e.g. tubules) produced in vitro by recrystallization experiments of isolated compounds showed shapes almost identical to the corresponding crystals on the plant surface (Jeffree et al. 1975). The platelet type is frequently related to the dominance of the primary alcohols in the EW. This was true in species of Eucalyptus, many Poaceae and several other taxa of the Angiospermae such as Fabaceae. In contrast, platelets may even contain high amounts of triterpenes or not be relatable to any of the wax compound classes (Jeffree 1986; Barthlott et al. 1998).
Chemical composition of EWs
The chemical analysis of EWs from Equisetum species revealed no general dominance of primary alcohols. In many of the extracts, this fraction did not exceed 25 % of the total wax and was not the dominating compound (Fig. 4). One explanation for a possible under-representation of the primary alcohols could be the applied extraction method: it dissolved not only the EW crystals but also the basal wax film and even some intracuticular wax (Baker 1982). Only the EW on the surfaces of the branches of E. sylvaticum consisted of more than 49 % primary alcohols; this could be related to the platelet type. The highest amounts (>51 % primary alcohols) were found on internodes of fertile E. arvense, where a wax film but no crystals were observed.
The compound classes of Equisetum EW are alkanes, esters, aldehydes, primary alcohols and free fatty acids. Thus, the Equisetum EW chemically corresponds to the EW reported for seed plants and ferns (Bianchi 1995), the surface wax of mosses (Haas 1999) and the EW of P. nudum (Geiger 2003). This also holds true for the chain-length distributions, indicating that the elongation systems and other pathways of biosynthesis (Post-Beittenmiller 1996) active in Equisetum should be comparable with those of other plants.
Secondary alcohols and ketones, along with triterpenes and sterols, were detected in the EW of Osmunda regalis (Jetter and Riederer 1999). These compounds were not found in Equisetum. Chiu et al. (1988) reported sterols in the wax of E. arvense, but they applied an extraction method in which the sterols could be derived from the inner tissues of the stem and may not be part of the cuticular wax.
In this study, the homologues of all compound classes were present with chain lengths above C20. The dominating chain lengths of free fatty acids, aldehydes and primary alcohols were C28 and C30. The main n-alkanes were C25 and C29, the dominating alkyl ester homologues C44 and C46. Compared with the results obtained from EW of Pteridium subspecies (Baker and Gaskin 1987), the chain-length distributions of ester moieties in our material are more broad-ranged [see Additional Information: ester alcohols C20–C32; ester fatty acids C18–C32].
In spore extracts of 10 different Equisetum species, a series of long-chain α,ω-dicarboxylic acids (C10–C34) was detected (Řezanka 1998). In the present study, no dicarboxylic acids were found, indicating that synthesis of these compounds may be restricted to the spores.
Organ specificities and functional aspects of the Equisetum EW
The EW composition (Fig. 4A and B) showed organ-specific differences: compared with EW from whole sprouts (sterile, main shoots), the EW from the branches contained lower amounts of free fatty acids and alkyl esters but higher percentages of aldehydes (except E. sylvaticum).
Epicuticular waxes from fertile and sterile sprouts were analysed separately in the heterophyadic species: E. arvense, E. telmateia, E. sylvaticum. Fertile sprouts of E. arvense and E. telmateia both showed considerably higher proportions of alkyl esters and alcohols than the sterile ones, but much lower amounts of free fatty acids. In contrast, the differences in the EW of fertile vs. sterile sprouts of E. sylvaticum were minimal. This could reflect the semi-heterophyadic state of E. sylvaticum: after spore distribution, most of the fertile sprouts become green and develop into ‘normal’ sterile sprouts (Dostál 1984).
The detailed analysis of EW from organs of the fertile shoots revealed a markedly low percentage of aldehydes in the internodes of E. arvense and E. telmateia. This corresponds with the lack of clear EW structures on these parts of the sprouts (Fig. 3A and C), whereas well-developed EW crystals (platelets) on the leaf sheaths (Fig. 3B and D) are paralleled by a more balanced EW composition.
Aldehydes are an important constituent in most EW samples from the Equisetum plants. A considerable part of the aldehydes is present in polymeric form. Increasing evidence shows a stabilizing effect of polymeric aldehydes on EW crystals. Unlike monomeric constituents, polymeric aldehydes are insoluble in chloroform at room temperature but readily dissolve in hot solvents (Lamberton and Redcliffe 1960; Lamberton 1965; Haas et al. 2001; Riedel et al. 2003; Gorb et al. 2005). Epicuticular wax structures containing polymeric aldehydes are therefore degraded only partly by the action of the solvent at room temperature. Other substance classes of EW such as primary alcohols, alkanes and alkyl esters are preferably dissolved. In a subsequent extraction using hot chloroform, the aldehydes are the main fraction, with a significant portion of primary alcohols admixed. Such different results of subsequent extraction steps indicate the presence of aldehydes. Polymeric aldehydes reinforce EW crystals against mechanical stress and weathering (Haas et al. 2001; Gorb et al. 2005). Structural integrity of wax crystals, however, is a prerequisite for water repellency. Non-wettable surfaces would prevent water from covering plant parts and especially the stomata with a continuous film. This feature of the EW therefore provides unhindered gas exchange through the stomata (Smith and McClean 1989; Brewer and Smith 1995).
Aldehydes were the dominating compound class in eight of 22 Equisetum EW extracts, and in six additional extracts they were an important fraction (Fig. 4A and B). The EW extracts with the lowest aldehyde content were those from surfaces without clearly structured EW: fertile sprouts of E. telmateia and E. arvense, as well as sterile sprouts of E. telmateia, show only few to no stomata and a smooth wax film on the internodes. In contrast, the stomatal areas (in Equisetum: subsidiary cell areas) are always covered with well-developed EW crystals, whereas wax on the adjacent regions often showed stages of degradation. Thus, a main function of the surface coverage consisting of intact EW crystals in Equisetum species should be water repellency; this ensures the normal function of stomata. One would expect that the greater the number of stomatal per surface area, the higher the amount of aldehydes in the total wax. In agreement with this, the branches of E. arvense, E. telmateia and E. fluviatile show high stomatal densities and high proportions of aldehydes in their EW. Because the branches contain most of the chlorenchyma in the plant, they are probably the sites where gas exchange preferably occurs. Equisetum sylvaticum had considerable proportions of aldehydes in EW extracts of all plant parts (Fig. 4A and B), emphasizing the special status of this species.
High aldehyde contents were also found in EW from whole sprouts of the unbranched species in subgenus Hippochaete (E. hyemale, E. variegatum and E. scirpoides, Fig. 4A). From an ecological point of view, all species of horsetails are characterized as mesophytes or hygrophytes. In the species of subgenus Hippochaete, however, the stomata are positioned below the level of the epidermis, forming a stomatal crypt. This was interpreted as a xerophytic adaptation (e.g. Riebner 1925) because several taxa of this subgenus are evergreen and therefore need protection against water stress related to winter drought (e.g. E. hyemale) or to very high insolation in mountain regions (e.g. E. variegatum and E. scirpoides). A further factor in drought resistance could be the reduced cuticular transpiration due to increased EW covering. Approximate wax yields are clearly higher (10–25 µg cm−2, Table 1) in species of subgenus Hippochaete compared with those in the deciduous and hardly drought-resistant species of the subgenus Equisetum.
Taxonomic implications
According to Hauke (1963, 1978), the genus Equisetum consists of 15 species grouped in the subgenera Equisetum and Hippochaete. Molecular phylogenetic studies support the integrity and distinctness of these two subgroups (Des Marais et al. 2003; Guillon 2004), although the infrasubgeneric relationships remain uncertain (see also Guillon 2007). A sister relationship of E. arvense and E. fluviatile is assumed in several studies (Page 1972; Duckett 1979; Veit et al. 1995; Des Marais et al. 2003; Guillon 2004). The EW composition, analysed in the present study, does not reflect this. Cluster analysis presents E. arvense, E. telmateia and E. fluviatile in one group [see Additional Information], and the MDS graph shows more or less equal distances between these three species (Fig. 5A). Based on the very low alkyl ester and high alkane contents, species of subgenus Hippochaete are positioned clearly apart from those of subgenus Equisetum, in agreement with the accepted classification. The cluster analysis revealed a higher similarity of E. variegatum and E. scirpoides EWs on the one hand and the two E. hyemale subspecies on the other (Fig. 5A) [see Additional Information]. The relative amounts of the compound classes are very similar (Fig. 4A) and, therefore, group differences are due to differences in chain-length distributions: for example, C31 is the dominating chain length in E. variegatum and E. scirpoides EW, but C29 is predominant in E. hyemale ssp. hyemale and E. hyemale ssp. affine EW (Table 2). The relationship found here is incongruent with the molecular phylogenies (Des Marais et al. 2003; Guillon 2004, 2007), but in agreement with the classification of Hauke (1963) where these taxa are placed in different subsections (‘Perennantia/Homocormia’). The EW composition of E. sylvaticum, undoubtedly a species of the subgenus Equisetum, should be more similar to those of E. arvense/E. telmateia and E. fluviatile than to the subgenus Hippochaete. This, however, is not the case here. On the contrary, its wax composition is the most different to all other samples (Figs 4A and 5A), indicating that phylogenetic relationships are not generally reflected by the EW composition. This problem is well known. The biosynthesis of secondary metabolites has turned out to be much more variable than previously assumed. For example, environmental factors may influence both the kind and the amounts of metabolites produced. The genetic code for enzymes that catalyse steps of the biosynthetic pathway could be present without being expressed (e.g. Waterman 1998).
Conclusions and forward look
The EW biosynthesis in the subgenus Hippochaete seems to be comparatively homogeneous, with only little variability, whereas the subgenus Equisetum exhibits a much higher wax diversity and functional plasticity. Further studies designed to clarify these aspects in more detail should include more species, different developmental stages and different plant organs.
Additional information
The following additional information is available in the online version of this article –
Table: Lists the complete dataset of cuticular wax analysis with all detected chain lengths (even and odd).
Diagram: Shows the detailed results of hierarchical cluster analysis (Ward's method) of the different EWs in the form of dendrograms (see groups in lines in Fig. 5). (a) Epicuticular wax from whole sprouts (main axis internodes and main axis leaf sheaths). (b) Epicuticular wax from specific Equisetum organs (fertile main axis internodes, fertile main axis leaf sheaths, sterile branches). Scale: rescaled distance.
Contributions by the authors
Both authors contributed to a similar extent overall.
Conflict of interest statement
None declared.
References
- Adams KR, Bonnet R, Hall J, Kutney JP. Long-chain α,ω-dicarboxylic acids from the spores of Equisetum spp. Chemical Communications. 1969;350:456–457. [Google Scholar]
- Baker EA. Chemistry and morphology of plant epicuticular waxes. In: Cutler DF, Alvin KL, Price CE, editors. The plant cuticle. London: Academic Press; 1982. pp. 139–165. [Google Scholar]
- Baker EA, Gaskin RE. Composition of leaf epicuticular waxes of Pteridium subspecies. Phytochemistry. 1987;26:2847–2848. doi:10.1016/S0031-9422(00)83602-X. [Google Scholar]
- Baker EA, Holloway PJ. Scanning electron microscopy of waxes on plant surfaces. Micron. 1971;2:364–380. doi: [Google Scholar]
- Barthlott W, Wollenweber E. Zur Feinstruktur, Chemie und taxonomischen Signifikanz epicuticularer Wachse und ähnlicher Sekrete. Tropische und Subtropische Pflanzenwelt. 1981;32:7–67. doi: [Google Scholar]
- Barthlott W, Neinhuis C, Cutler D, Ditsch F, Meusel I, Theisen I, Wilhelmi H. Classification and terminology of plant epicuticular waxes. Botanical Journal of the Linnean Society. 1998;126:237–260. doi:10.1111/j.1095-8339.1998.tb02529.x. [Google Scholar]
- Bianchi G. Plant waxes. In: Hamilton RJ, editor. Waxes: chemistry, molecular biology and functions. Dundee: The Oily Press; 1995. pp. 174–222. [Google Scholar]
- Brewer CA, Smith WK. Leaf surface wetness and gas exchange in the pond lily Nuphar polysepalum (Nymphaeaceae) American Journal of Botany. 1995;82:1271–1277. doi:10.2307/2446250. [Google Scholar]
- Chiu P-L, Patterson GW, Salt TA. Sterol composition of pteridophytes. Phytochemistry. 1988;27:819–822. doi:10.1016/0031-9422(88)84099-8. [Google Scholar]
- Des Marais DL, Smith AR, Britton DM, Pryer KM. Phylogenetic relationships and evolution of extant horsetails, Equisetum, based on chloroplast DNA sequence data (rbcL and trnL-F) International Journal of Plant Sciences. 2003;164:737–751. doi:10.1086/376817. [Google Scholar]
- Dostál J. Sphenopsida. In: Hegi G, editor. Flora von Mitteleuropa. Band 1 Pteridophyta Teil 1. Berlin: Parey; 1984. pp. 54–79. [Google Scholar]
- Duckett JG. Comparative morphology of the gametophytes of Equisetum subgenus Hippochaete and the sexual behaviour of Equisetum ramosissimum subsp. debile, (Roxb.) Hauke, Equisetum hyemale var. affine (Engelm.) A.A., and Equisetum laevigatum A. Br. Botanical Journal of the Linnean Society. 1979;79:179–203. doi:10.1111/j.1095-8339.1979.tb01513.x. [Google Scholar]
- Geiger K. Germany: University of Hohenheim; 2003. Struktur und chemische Zusammensetzung des Epicuticularwachses von Psilotum nudum (L.) P. BEAUV. Diploma Thesis. [Google Scholar]
- Gorb E, Haas K, Henrich A, Enders S, Barbakadze N, Gorb S. Composite structure of the crystalline epicuticular wax layer of the slippery zone in the pitchers of the carnivorous plant Nepenthes alata and its effect on insect attachment. The Journal of Experimental Biology. 2005;208:4651–4662. doi: 10.1242/jeb.01939. doi:10.1242/jeb.01939. [DOI] [PubMed] [Google Scholar]
- Guillon J.-M. Phylogeny of horsetails (Equisetum) based on the chloroplast rps4 gene and adjacent noncoding sequences. Systematic Botany. 2004;29:251–259. doi:10.1600/036364404774195467. [Google Scholar]
- Guillon J.-M. Molecular phylogeny of horsetails (Equisetum) including chloroplast atpB sequences. Journal of Plant Research. 2007;120:569–574. doi: 10.1007/s10265-007-0088-x. doi:10.1007/s10265-007-0088-x. [DOI] [PubMed] [Google Scholar]
- Haas K. Phytochemische und rasterelektronenmikroskopische Untersuchungen zum Oberflächenwachs von Laubmoosen (Bryatae) Stuttgart: Grauer; 1999. [Google Scholar]
- Haas K, Brune T, Rücker E. Epicuticular wax crystalloids in rice and sugar cane are reinforced by polymeric aldehydes. Journal of Applied Botany. 2001;75:178–187. doi: [Google Scholar]
- Haas K, Bauer M, Wollenweber E. Epicuticular waxes and flavonol aglycones of mistletoes. Zeitschrift für Naturforschung. 2003;58c:464–470. doi: 10.1515/znc-2003-7-803. doi: [DOI] [PubMed] [Google Scholar]
- Hauke RL. A taxonomic monograph of the genus Equisetum subgenus. Hippochaete. Nova Hedwigia Beihefte. 1963;8:123 pp. doi: [Google Scholar]
- Hauke RL. A taxonomic monograph of Equisetum subgenus Equisetum. Nova Hedwigia. 1978;30:385–455. doi: [Google Scholar]
- Heinrichs J, Rycroft DS. Leaf surface waxes and lipophilic secondary metabolites place the endemic European liverwort Plagiochila atlantica F. Rose in the Neotropical Plagiochila sect. Bursatae Carl. Cryptogamie Bryologie. 2001;22:95–103. doi:10.1016/S1290-0796(01)01061-6. [Google Scholar]
- Heinrichs J, Anton H, Gradstein SR, Mues R, Holz I. Surface wax, a new taxonomic feature in Plagiochilaceae. Plant Systematics and Evolution. 2000;225:225–233. doi:10.1007/BF00985469. [Google Scholar]
- Jeffree CE. The cuticle, epicuticular waxes and trichomes of plants, with reference to their structure, functions and evolution. In: Juniper B, Southwood TRE, editors. Insects and the plant surface. London: Edward Arnold; 1986. pp. 23–64. [Google Scholar]
- Jeffree CE, Baker EA, Holloway PJ. Ultrastructure and recrystallisation of plant epicuticular waxes. New Phytologist. 1975;75:539–549. doi:10.1111/j.1469-8137.1975.tb01417.x. [Google Scholar]
- Jetter R, Riederer M. Long-chain alkanediols, ketoaldehydes, ketoalcohols and ketoalkylesters in the epicuticular waxes of Osmunda regalis fronds. Phytochemistry. 1999;52:907–915. doi:10.1016/S0031-9422(99)00309-X. [Google Scholar]
- Lamberton JA. The long-chain aldehydes of sugar-cane wax. Australian Journal of Botany. 1965;18:911–913. doi: [Google Scholar]
- Lamberton JA, Redcliffe AH. The chemistry of sugar-cane wax I. The nature of sugar-cane wax. Australian Journal of Botany. 1960;13:261–268. doi: [Google Scholar]
- Maffei M. Chemotaxonomic significance of leaf wax alkanes in the Gramineae. Biochemical Systematics and Ecology. 1996;24:53–64. doi:10.1016/0305-1978(95)00102-6. [Google Scholar]
- Maffei M, Meregalli M, Scannerini S. Chemotaxonomic significance of surface wax n-alkanes in the Cactaceae. Biochemical Systematics and Ecology. 1997;25:241–253. doi:10.1016/S0305-1978(96)00102-0. [Google Scholar]
- Neinhuis C, Jetter R. Ultrastructure and chemistry of epicuticular wax crystals in Polytrichales sporophytes. Journal of Bryology. 1995;18:399–406. doi: [Google Scholar]
- Page CN. An assessment of inter-specific relationships in Equisetum subgenus Equisetum. New Phytologist. 1972;71:355–369. doi:10.1111/j.1469-8137.1972.tb04082.x. [Google Scholar]
- Post-Beittenmiller D. Biochemistry and molecular biology of wax production in plants. Annual Review of Plant Physiology and Plant Molecular Biology. 1996;47:405–430. doi: 10.1146/annurev.arplant.47.1.405. doi:10.1146/annurev.arplant.47.1.405. [DOI] [PubMed] [Google Scholar]
- Raven JA. Physiological correlates of the morphology of early vascular plants. Botanical Journal of the Linnean Society. 1984;88:105–126. doi:10.1111/j.1095-8339.1984.tb01566.x. [Google Scholar]
- Řezanka T. Branched and very long-chain dicarboxylic acids from Equisetum species. Phytochemistry. 1998;47:1539–1543. doi:10.1016/S0031-9422(97)00774-7. [Google Scholar]
- Riebner F. Über Bau und Funktion der Spaltöffnungsapparate bei den Equisetinae und Lycopodiinae. Planta. 1925;1:260–300. doi:10.1007/BF02038171. [Google Scholar]
- Riedel M, Eichner A, Jetter R. Slippery surfaces of carnivorous plants: composition of epicuticular wax crystals in Nepenthes alata Blanco pitchers. Planta. 2003;218:87–97. doi: 10.1007/s00425-003-1075-7. doi:10.1007/s00425-003-1075-7. [DOI] [PubMed] [Google Scholar]
- Schönherr J, Ziegler H. Hydrophobic epicuticular ledges prevent water entering the air pores of liverwort thalli. Planta. 1975;124:51–60. doi: 10.1007/BF00390067. doi:10.1007/BF00390067. [DOI] [PubMed] [Google Scholar]
- Shepherd T, Griffiths DW. The effects of stress on plant epicuticular waxes. New Phytologist. 2006;171:469–499. doi: 10.1111/j.1469-8137.2006.01826.x. doi:10.1111/j.1469-8137.2006.01826.x. [DOI] [PubMed] [Google Scholar]
- Smith WK, McClean TM. Adaptive relationship between leaf water repellency, stomatal distribution, and gas exchange. American Journal of Botany. 1989;76:465–469. doi:10.2307/2444617. [Google Scholar]
- Sonibare MA, Adeniyi A, Jayeolab AA, Egunyomi A. Chemotaxonomic significance of leaf alkanes in species of Ficus (Moraceae) Biochemical Systematics and Ecology. 2005;33:79–86. doi:10.1016/j.bse.2004.05.010. [Google Scholar]
- Sosa A. Sur quelques constituants nouveaux des spores d'une prêle, l'Equisetum maximum Lam. (Equisétacées) Bulletin de la Société de chimie biologique. 1949;31:57–69. doi: [Google Scholar]
- Timell TE. Studies on some ancient plants. Svensk Papperstidning. 1964;67:356–363. doi: [Google Scholar]
- Veit M, Beckert C, Höhne C, Bauer K, Geiger H. Interspecific and intraspecific variation of phenolics in the genus Equisetum subgenus Equisetum. Phytochemistry. 1995;38:881–891. doi:10.1016/0031-9422(94)00658-G. [Google Scholar]
- Víden I, Řezanka T. Capillary gas chromatography–mass spectrometry of very-long-chain α,ω-dicarboxylic acid dimethyl esters from Equisetum (horsetail) Journal of Chromatography. 1989;465:390–394. doi:10.1016/S0021-9673(01)92677-9. [Google Scholar]
- Waterman PG. Chemosystematics—current status. Phytochemistry. 1998;49:1175–1178. doi:10.1016/S0031-9422(98)00055-7. [Google Scholar]