Abstract
Objectives: We present the first known case in the English-language literature of a myxoma arising in the sphenoid sinus. By describing the patient's clinical course and the salient features of this rare neoplasm, we seek to increase the awareness of the presentation, histological features, and treatment considerations for myxomas of the head and neck. In the process, we intend to describe the work-up of isolated sphenoid sinus lesions and focus on the varying and evolving techniques for surgical access to the sphenoid sinus.
Study Design and Methods: Case report and literature review.
Results: We describe the clinical course of a patient with a myxoma of the sphenoid sinus. The patient underwent an external sphenoethmoidectomy through a lateral rhinotomy approach with medial maxillectomy under MRI-guidance. He remains without evidence of recurrent disease after 8 months.
Conclusions: Myxomas of the head and neck are rare neoplasms. Their infiltrative nature and tendency to recur demand an aggressive surgical approach that may be accomplished with minimal morbidity using currently available image-guided techniques.
Keywords: Myxoma, neoplasms, sphenoid sinus
Introduction
Myxomas are benign neoplasms derived from primitive mesenchyme. These lesions occur most frequently in the atria, but they can also be found in bone, skin, subcutaneous tissue, and skeletal muscle. Initially coined by Virchow in 1871, the term myxoma traditionally has been used to describe tumors that resemble the mucinous appearance of the umbilical cord.1 However, other tumors, particularly certain soft tissue sarcomas, also exhibit myxomatous features, prompting Stout to further characterize myxomas as nonmetastasizing tumors of mesenchymal origin without histological evidence of other recognizable elements such as chondroblasts, lipoblasts, and myoblasts.2
In general, myxomas of the head and neck are rare neoplasms, infrequently reported in the literature. The majority of these tumors arise from the mandible and maxilla, with only scattered reports of myxomas of the temporal bone, pharynx, larynx, soft tissues of the face and neck, and the paranasal sinuses.3–5 Of the paranasal sinus myxomas, the only traceable reports describe lesions predominately arising in the maxillary sinus. We describe the first known case of a myxoma arising in the sphenoid sinus with aggressive extension into surrounding structures.
Case Report
A 45-year-old male presented with a 1-year history of progressive left-sided nasal obstruction without rhinorrhea or epistaxis. Although he had a history of wood dust exposure, he denied exposure to chromium and nickel, as well as prior trauma to the head and neck. This history is pertinent since exposure to these products has been associated with the diagnosis of squamous cell carcinoma of the paranasal sinuses. His past medical and surgical histories were essentially unremarkable, and routine physical examination, including anterior rhinoscopy, was unrevealing. However, flexible fiberoptic nasopharyngoscopy demonstrated a pearly white mass with vascular markings just posterior to the middle turbinate that almost completely obstructed the left nasal cavity.
Due to the patient's sinonasal complaints, and the concerning findings on endoscopy, radiographic evaluation was undertaken with both computed tomography (CT) and magnetic resonance imaging (MRI). The CT scan exhibited a non-enhancing, 3 × 5 cm soft-tissue mass centered in the sphenoid sinus and extending into the left ethmoid sinuses and the pterygopalatine fossa, as well as the middle cranial fossa (Figure 1). The lesion appeared to have caused both erosion of the pterygoid plates and remodeling of the posterior maxillary sinus wall, suggesting an indolent growth pattern. Enhancement with contrast and central necrosis were evident on post-contrast T1-weighted images, which also demonstrated encroachment on the carotid arteries and pituitary gland, with anterior extension along the planum sphenidale (Figure 2). The mass extended laterally into the middle cranial fossa and filled the pterygopalatine fossa in the region of foramen rotundum (Figure 3). Inferiorly, it eroded the anterior sphenoid floor as well as the posterior aspect of the left middle turbinate. An endoscopic biopsy was performed at an outside institution, demonstrating myxoid fragments, fibrous tissue, and sinonasal mucosa with varying degrees of cellularity, as well as stellate neoplastic cells that suggested the diagnosis of myxoma. The patient was then referred to our institution for definitive care.
Figure 1. Contrasted axial CT scan demonstrating soft tissue mass filling the sphenoid sinus, with extension into the left middle cranial fossa, pterygopalatine fossa, and posterior ethmoid sinus. Note the anterior bowing of the posterior wall of the left maxillary sinus, as well as the destruction of the pterygoid plates.
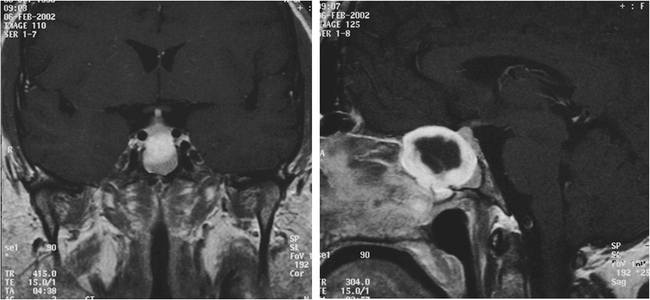
Figure 2. Gadolinium-enhanced T1 weighted MRI images of the sphenoid sinus mass. It appears to encase both carotid arteries and closely approximate the pituitary gland, with anterior extension along the planum sphenoidale.
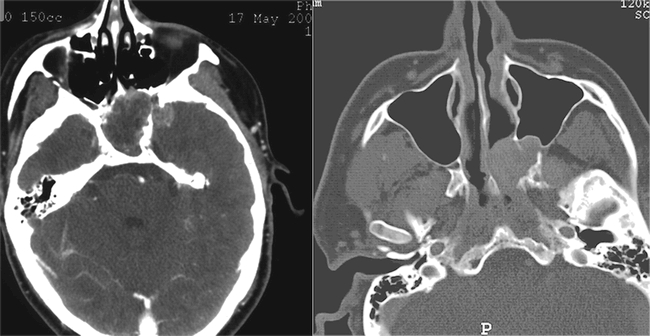
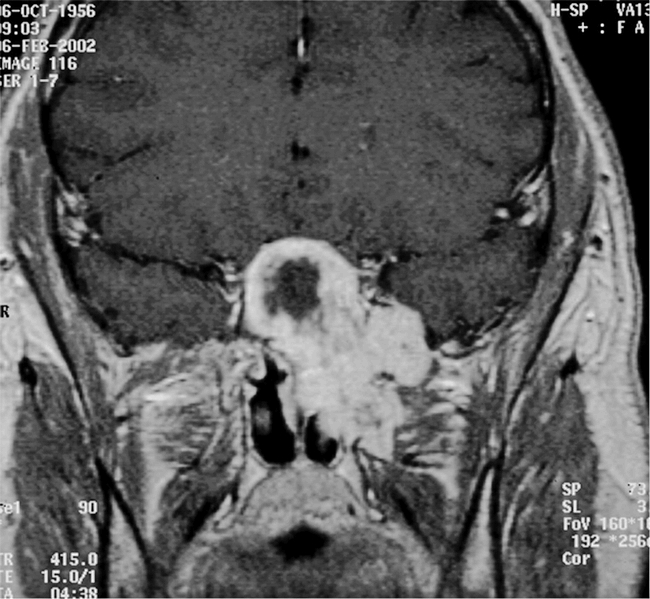
Figure 3. Coronal view of contrasted MRI. The mass spills anteriorly and laterally from the sphenoid sinus, extending throughout the pterygomaxillary space in the region of the vidian canal and foramen rotundum.
After treatment options were discussed and informed consent was obtained, the patient underwent an extradural anterior skull base approach with lateral rhinotomy, medial maxillectomy, external ethmoidectomy, and sphenoidectomy with mini-facial translocation. Because of the complex anatomical relationships of the sphenoid sinus, we employed a 3-D MRI-based navigational system (Stealth®, Medtronic, Memphis, TN) to provide accurate intraoperative anatomic localization. A partially encapsulated mass was noted to have eroded the bone of the superior and lateral sphenoid sinus, with preservation of a thin bony covering over the carotid arteries. Although the dura of the anterior and middle cranial fossae was extensively exposed, it remained intact. The lesion extended grossly into the left pterygopalatine fossa where it eroded portions of both pterygoid plates. Despite the extent of disease, the mass was completely excised without evidence of dural or carotid injury. We utilized the image guidance system frequently during tumor extirpation as an adjunct to known anatomic landmarks of the skull base.
Histopathologic review of the surgical specimen supported the diagnosis of myxoma, with stellate cells in areas of low to normal cellularity visible on both light and electron microscopy in a background of mucoid material (Figure 4). Additionally, immunohistochemical staining was performed, revealing that the tumor cells were positive for vimentin and negative for S-100, low molecular weight cytokeratin (CAM 5.2), broad spectrum cytokeratin (AE1/AE3), epithelial marker antigen, and carcinoembryonic antigen. The patient has done well postoperatively and remains without evidence of recurrent disease more than 1 year from diagnosis.
Figure 4. Photomicrograph of tumor specimen at 10× magnification with hematoxylin and eosin stain.

Discussion
Despite their description more than 130 years ago, myxomas of the head and neck remain rare neoplasms. Exhaustive reviews of pathologic specimens from major centers have identified myxomas in 0.1% of cases.6 As demonstrated in this review, most myxomas in the head and neck occur in the mandible, followed by the maxilla.6 Other described sites of origin for myxomas of the head and neck are listed in Table 1. Head and neck myxomas have been identified in patients ranging in age from 15 months to the 8th decade, although a peak incidence has been described between the ages of 25 and 35 years. There is a slight female predominance, but there does not appear to be an ethnic predisposition.3
Table 1.
Site of Origin of Head and Neck Myxomas
Paranasal sinus myxomas comprise a minority of the cases in the head and neck, and they may be among the most difficult to diagnose and treat. Heffner describes nine pediatric sinonasal myxomas from a survey of the Armed Forces Institute of Pathology registry of otorhinolaryngologic specimens between 1976 to 1981, eventually adding four more cases to the literature.7,8 Other authors have reported myxomas arising in the paranasal sinuses, nearly all of which involve the maxillary sinus.4,9–12 Although most of these lesions remain well localized, certain tumors have demonstrated more aggressive behavior, as evinced by involvement of the nasal cavity, as well as the ethmoid sinus, frontal sinus, skull base, orbit, and pterygopalatine fossa.4,11 No cases of sphenoid sinus involvement have previously been reported in the English-language literature.
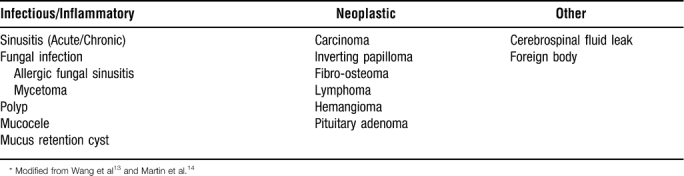
Regardless of etiology, sphenoid sinus disease remains a challenging clinical entity. Numerous recent reports document the inherent difficulties in diagnosing and treating problems in this poorly accessible anatomic site.13,14 Common symptoms, such as headache, visual changes, epistaxis, and cerebrospinal fluid rhinorrhea, are often nonspecific and frequently precipitate a delay in diagnosis. The proximity of the sphenoid sinus to the vital structures of the cavernous sinus and sella turcica at the skull base and middle cranial fossa underscores the importance of early diagnosis and treatment, so that complications such as optic neuritis, cranial nerve palsies, pituitary dysfunction, and intracranial extension do not occur. Fortunately, increased utilization of CT and MRI scans in the work-up of sinonasal complaints has increased detection of isolated sphenoid sinus disease, with diagnosis further aided by endoscopic evaluation and biopsy.14 Table 2 depicts a few of the most frequently encountered causes of isolated sphenoid sinus disease.
Table 2.
Isolated Sphenoid Sinus Disease*
Although the histologic appearance of myxomas is well described, theories regarding the etiology of this tumor abound. The nomenclature of myxomas, although not universally employed, seeks to delineate the potential histiogenesis of these lesions. Myxomas are regarded as either bone-derived or soft tissue-derived, depending on their location, with the bone-derived forms further subdivided into odontogenic and non-odontogenic forms.15–17 Historically, myxomas were thought to arise from primitive mesenchyme, due to their similarity to the primitive connective tissue in the embryo.2
Myxomas may arise from rests of odontogenic mesenchyme or from modified fibroblasts associated with tooth germ; these altered fibroblasts may hypersecrete mucin.18–20 An association between myxoma formation and the presence of unerupted teeth has provided support for this position.9 However, evidence exists against the odontogenic theory as well; Slootweg and Wittkampf have demonstrated that the extracellular matrix of myxomas differs histologically from the extracellular matrix of dental and peridental tissues.6 The simple fact that a myxoma may arise in the non-odontogenic tissues of the sphenoid sinus challenges the odontogenic hypothesis, but no definitive answer has emerged.
On evaluation, a myxoma appears as a glistening, grey-white, gelatinous mass with discrete surface nodularity and a variable consistency.20 The lesions demonstrate varying degrees of encapsulation, and a pseudocapsule may even develop as a result of compression of surrounding normal structures. The tumor discussed in our case exhibited intratumoral variation in the amount and presence of a capsule, with a well-formed capsule adjacent to the anterior cranial fossa, but no evidence of a capsule in the middle fossa and pterygopalatine fossa extensions. The amorphous, gelatinous texture of myxomas complicates their complete removal and has been identified as a prime contributor to tumor recurrence, which occurs in up to 25% of cases. The lack of a true capsule and the potential for secretion of bioactive enzymes such as hyaluronic acid and acid phosphatase contribute to the locally infiltrative nature of this tumor.11
Myxomas are composed of stellate-shaped cells with slender cytoplasmic processes and small nuclei embedded in a mucoid matrix rich in polysaccharides.21,22 Reticulin fibers are evident, although the amount of collagen is variable, potentially due to the production of mucopolysaccharides by myofibroblasts.23 The cellularity of a myxoma is typically minimal, secondary to the vast mucoid stroma, although some variants with increased cellularity and enhanced spindle cell morphology have been documented.7 Ultrastructurally, myxomas appear as scattered tumor cells containing abundant rough endoplasmic reticulum, well-developed Golgi apparatus, numerous filaments, and pinocytotic vesicles within a background of low electron density.17
The myxoid appearance of these tumors resembles several other lesions, both benign and malignant, which are summarized in Table 3. Ultimately, the diagnosis of a myxoma is a diagnosis of exclusion. Increased cellularity should raise suspicions for one of the malignant myxoid tumors, as a true myxoma is considered a relatively hypocellular tumor. Special staining techniques and immunohistochemistry are helpful adjuncts to clinical cues in identifying and differentiating these lesions. The mucoid matrix of a myxoma stains strongly with Alcian blue and mucicarmine, as well as periodic acid–Schiff, although these are not specific. Immunostaining reveals that myxomas stain positive for vimentin and actin, but negative for desmin, S-100, and cytokeratin.9,23
Table 3.
Differential Diagnosis of Myxoid Tumors
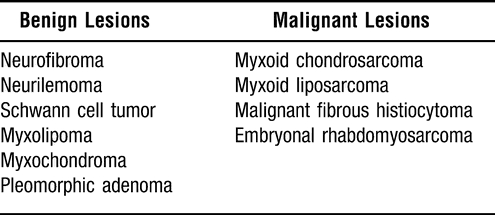
Table 4.
Approaches to the Sphenoid Sinus

After an accurate diagnosis is achieved, complete surgical excision is the treatment of choice. Intranasal, transantral, transseptal, transethmoidal, and various external approaches have all been utilized to perform a complete surgical excision.3,4,8,13 With recurrence rates often quoted at 25% and usually attributed to inadequate primary excision, radical primary resection has been advocated by some authors, with en bloc removal when possible.4 Furthermore, aggressive variants of myxomas have been reported that have exhibited marked infiltration and erosion into surrounding structures, resulting in significant morbidity and even death.11 Citing the risk of recurrence and the potential for the myxoma to endanger surrounding structures, Deron et al. advocate “radical primary resection in spite of significant functional and aesthetic sequellae.”11 However, such radical resection is not always possible or practical, particularly at the skull base. Resection in these areas, as in our patient, requires excellent exposure and meticulous surgical technique to ensure complete tumor removal. The challenges inherent in skull base resection of myxomas have been previously described.24 Despite these limitations, complete surgical resection provides the best chance for cure, as these tumors do not respond appreciably to radiation therapy or chemotherapy.11
Advances in surgical technique, particularly the use of image-guided systems, may allow complete resection of myxomas while minimizing the functional and aesthetic consequences. Although numerous options exist for the extirpation of sphenoid sinus lesions, we opted to perform a lateral rhinotomy, medial maxillectomy, ethmoidectomy, and sphenoidectomy with mini-facial translocation in our patient to maximize the exposure of the tumor and its finger-like projections. Frozen section analysis provided an assessment of tumor margins in an area where en bloc resection is not reasonable for benign disease. Exposure provided by the lateral rhinotomy facilitated adequate visualization of the pterygopalatine fossa extension of disease. This approach provided an excellent view of the skull base with, ultimately, an acceptable oncologic and cosmetic result. Our position could be readily determined with the image-guided system, particularly as we approached the superior and lateral recesses of the sphenoid sinus with underlying carotid arteries and optic nerves. Although numerous other approaches to sphenoid sinus could have been employed, including endoscopic, sublabial, and craniofacial techniques (Figure 4), we maintain that our method provides excellent exposure while minimizing intraoperative risk and morbidity.
Conclusions
Myxomas of the head and neck are rare neoplasms, commonly involving the mandible and maxilla. We describe the first known case of an aggressive myxoma arising from the sphenoid sinus and extending to the middle and anterior cranial fossae, the maxillary sinus, and the pterygopalatine fossa. Because myxomas are infiltrative lesions with a propensity to recur locally if not completely excised, we aggressively resected the lesion with frozen section control of margins through a lateral rhinotomy, medial maxillectomy, and external sphenoethmoidectomy approach. The risks of radical excision must be balanced with the risks of tumor recurrence in determining the appropriate surgical technique. The use of image-guided surgery may allow for more precise and thorough tumor extirpation while minimizing cosmetic and functional sequelae.
Footnotes
Presented at The Southern Section of the Triological Society January 10–12, 2003, Naples, FL.
References
- Virchow R. Die cellularpathologie in ihrer Begrundung auf physiologische und pathologische Gewebelehre. Berlin, Germany: Verlag von August Hirschwald; 1871. p. 563. [Google Scholar]
- Stout A. P. Myxoma: the tumor of primitive mesenchyme. Ann Surg. 1948;127:706–719. [PubMed] [Google Scholar]
- Allphin A. L., Manigilia A. J., Gregor R. T., Sawyer R. Myxomas of the mandible and maxilla. Ear Nose Throat J. 1993;72:280–284. [PubMed] [Google Scholar]
- Fu Y. S., Perzin K. H. Non-epithelial tumors of the nasal cavity, paranasal sinuses and nasopharynx: a clinico-pathologic study. Cancer. 1977;39:195–203. doi: 10.1002/1097-0142(197701)39:1<195::aid-cncr2820390131>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Perzin K. H., Panyu J., Wechter S. Nonepithelial tumors of the nasal cavity, paranasal sinuses, and nasopharynx. Cancer. 1982;50:2193–2202. doi: 10.1002/1097-0142(19821115)50:10<2193::aid-cncr2820501036>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- Slootweg P. J., Wittkampf A. R. M. Myxoma of the jaws: an analysis of 15 cases. J Max Fac Surg. 1986;14:46–52. doi: 10.1016/s0301-0503(86)80258-2. [DOI] [PubMed] [Google Scholar]
- Heffner D. K. Problems in pediatric otorhinolaryngic pathology. I. Sinonasal and nasopharyngeal tumors and masses with myxoid features. Int J Ped Otorhinolaryngol. 1983;5:77–91. doi: 10.1016/s0165-5876(83)80010-x. [DOI] [PubMed] [Google Scholar]
- Heffner D. K. Sinonasal myxomas and fibromyxomas in children. Ear Nose Throat J. 1993;72:365–368. [PubMed] [Google Scholar]
- Gregor R. T., Loftus-Coll B. Myxomas of the paranasal sinuses. J Laryngol Otol. 1994;108:679–681. doi: 10.1017/s0022215100127811. [DOI] [PubMed] [Google Scholar]
- Batti J. S., Zahtz G., O'Reilly B. Pathology forum: quiz case 4. Maxillary myxoma. Arch Otolaryngol Head Neck Surg. 2000;126:676–683. [PubMed] [Google Scholar]
- Deron P. B., Nikolovski N., Den Hollander J. C., Spoelstra H. A., Knegt P. P. Myxoma of the maxilla: a case with extremely aggressive biologic behavior. Head Neck. 1996;18:459–464. doi: 10.1002/(SICI)1097-0347(199609/10)18:5<459::AID-HED10>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- Greenfield S. D., Friedman O. Myxoma of maxillary sinus. NY State J Med. 1951;51:1319–1320. [PubMed] [Google Scholar]
- Wang Z. M., Kanoh N., Dai C. F., et al. Isolated sphenoid sinus disease: an analysis of 122 cases. Ann Otol Rhinol Laryngol. 2002;111:323–327. doi: 10.1177/000348940211100407. [DOI] [PubMed] [Google Scholar]
- Martin T. J., Smith T. L., Smith M. M., Loehrl T. A. Evaluation and surgical management of isolated sphenoid sinus disease. Arch Otolaryngol Head Neck Surg. 2002;128:1413–1419. doi: 10.1001/archotol.128.12.1413. [DOI] [PubMed] [Google Scholar]
- Landa L. E., Hedrick M. H., Nepomuceno-Perez M. C., Sotereanos G. C. Recurrent myxoma of the zygoma: a case report. J Oral Maxillofac Surg. 2002;60:704–708. doi: 10.1053/joms.2002.33126. [DOI] [PubMed] [Google Scholar]
- Osterdock R. J., Greene S., Mascott C. R., Amedee R., Crawford B. E. Primary myxoma of the temporal bone in a 17-year old boy: case report. Neurosurgery. 2001;48:945–947. doi: 10.1097/00006123-200104000-00055. [DOI] [PubMed] [Google Scholar]
- Shugar J. M. A., Som P. M., Meyers R. J., Schaeffer B. T. Intramuscular head and neck myxoma: report of a case and review of the literature. Laryngoscope. 1987;97:105–107. doi: 10.1288/00005537-198701000-00021. [DOI] [PubMed] [Google Scholar]
- White D. K., Chen S., Mohnac A., Miller A. S. Odontogenic myxoma – a clinical and ultrastructural study. Oral Surg. 1975;39:901–917. doi: 10.1016/0030-4220(75)90111-5. [DOI] [PubMed] [Google Scholar]
- Moshiri S., Oda D., Worthington P., Myall R. Odontogenic myxoma: histochemical and ultrastructural study. J Oral Pathol Med. 1992;21:401–403. doi: 10.1111/j.1600-0714.1992.tb01027.x. [DOI] [PubMed] [Google Scholar]
- Canalis R. F., Smith G. A., Konrad H. R. Myxomas of the head and neck. Arch Otolaryngol. 1976;102:300–305. doi: 10.1001/archotol.1976.00780100086012. [DOI] [PubMed] [Google Scholar]
- Ang H. K., Ramani P., Michaels L. Myxoma of the maxillary antrum in children. Histopathology. 1993;23:361–365. doi: 10.1111/j.1365-2559.1993.tb01220.x. [DOI] [PubMed] [Google Scholar]
- Batsakis J. G. Pathology consultation: myxomas of soft tissues and the facial skeleton. Ann Otol Rhinol Laryngol. 1987;96:618–619. doi: 10.1177/000348948709600527. [DOI] [PubMed] [Google Scholar]
- Gourin C. G., Donegan J. O., Gonzalez J. L. Pathologic quiz case 2. Soft tissue myxoma of the parotid gland. Arch Otolaryngol Head Neck Surg. 1999;125 [PubMed] [Google Scholar]
- Charabi S., Engel P., Bonding P. Myxoid tumors in the temporal bone. J Laryngol Otol. 1989;103:1206–1209. doi: 10.1017/s0022215100111351. [DOI] [PubMed] [Google Scholar]


