Abstract
Little is known about cell cycle regulation in hypoxic cells, despite its significance. We utilized an experimentally tractable model to study the proliferative responses of rat fibroblasts when rendered hypoxic (0.5% oxygen) or anoxic (<0.01% oxygen). Hypoxic cells underwent G1 arrest, whereas anoxic cells also demonstrated S-phase arrest due to suppression of DNA initiation. Upon reoxygenation, only those cells arrested in G1 were able to resume proliferation. The oncoprotein E1a induced p53-independent apoptosis in anoxic cells, which when suppressed by Bcl-2 permitted proliferation despite anoxia. E1a expression led to marked increases in the transcription factor E2F, and overexpression of E2F-1 allowed proliferation in hypoxic cells, although it had minimal effect on the anoxic suppression of DNA initiation. We thus demonstrate two distinct cell cycle responses to low oxygen and suggest that alterations that lead to increased E2F can overcome hypoxic G1 arrest but that additional alterations, promoted by E1a expression, are necessary for neoplastic cells to proliferate despite anoxia.
Cellular hypoxia is common in many physiological and pathophysiological states, including cancer. Poor and disordered vascularization and rapid tumor cell proliferation lead to areas of significant hypoxia in the tumor microenvironment. Direct measurements of oxygen tension reveal that oxygenation may vary widely in tumors, with some areas approaching anoxia (55). Hypoxic tumors are poorly responsive to radiation and chemotherapy and appear to be more aggressive than nonhypoxic tumors (30). This may be partly related to the observation that some oncogenes, such as c-myc, selectively promote apoptosis in hypoxic p53 wild-type cells; thus, hypoxia can select for cells with mutant p53 (24, 25, 52). The role of hypoxia in tumor proliferation may also have important consequences in therapy resistance and tumor progression. However, the regulation of growth in hypoxia, and how oncogenes may affect this proliferation, is still poorly understood despite its importance in tumor biology and cancer treatment.
Normal cell cycle progression is regulated by the coordinated actions of cyclins, cyclin-dependent kinases (CDKs), cyclin-dependent kinase inhibitors (CDKIs), and the E2F family of transcription factors (22). Exit from G1 is mediated by CDK-mediated phosphorylation of the retinoblastoma protein (Rb) and its related pocket proteins, including p107. This phosphorylation releases members of the E2F family, which may then promote the transcription of genes necessary for S phase, including cyclin E enzymes necessary for deoxynucleotide synthesis and members of the DNA initiation complex, CDC6 and MCM6 (5, 11, 40). CDK2-mediated phosphorylation is important for other steps required for DNA synthesis, including the assembly and activation of the DNA initiation complex (11, 12, 40). CDK2 activity is dependent on the formation of cyclin A or cyclin E complexes and can be inhibited by the CDKIs p27 and p21. Many neoplasms have deregulated cell cycle regulation, including inactivation of Rb, increased free E2F, and increased CDK2 activity (8, 53).
Most normal primary and immortalized cells undergo growth arrest when rendered hypoxic, and several studies have shown that this hypoxia-induced proliferation arrest leads to an increase in the G1 cell population and is associated with many of the molecular events associated with G1 arrest, such as a decrease in CDK2 activity and a concomitant decrease in Rb phosphorylation (3, 20, 23, 25, 26, 35, 52). Many have also noted an induction of the CDK2 inhibitor p27 in hypoxic cells, which appears to be at least partly transcriptionally regulated (10, 20, 23, 35). However, the significance of these molecular events is controversial. Several tumor cell lines deficient for functional Rb appear to proliferate despite moderate hypoxia, although the significance of the absent Rb in the context of multiple other genetic abnormalities is unclear (35, 52). Moderately hyopoxic mouse embryo fibroblasts (MEFs) undergo G1 arrest that is dependent on the presence of Rb and the induction of the CDK2 inhibitor p27 (20). Hence, in p27-null MEFs, moderate hypoxia does not decrease CDK2 activity, Rb remains phosphorylated, and proliferation continues. Separate studies have shown that severely anoxic (<0.01% oxygen) MEFs halt proliferation and have decreased CDK2 activity, though this mechanism does not appear to depend on CDKIs (26). While p27-null MEFs arrest when rendered severely anoxic, they escape from G1 faster than wild-type cells during reoxygenation (26), again suggesting that different regulatory mechanisms of arrest exist, depending on the severity of oxygen deprivation.
Studies of proliferation in hypoxia have been hampered by the fact that different cell lines have varied responses when hypoxic, making the relevance of any cell line to another cell line of different tissue origin or species unclear. Indeed, recent data suggest that hypoxic (1.5% oxygen) hematopoietic stem cells have markedly varied proliferative responses, depending on their degrees of differentiation (13). In addition, as suggested by contradictory data in MEFs, even within the same cell line, small variations in the degrees of hypoxia may elicit varied phenotypic responses (20, 26). We chose to systematically study a well-defined and experimentally tractable model system, the immortalized but nontumorigenic rat fibroblast line REF52. This cell line has the advantages over primary cell lines that it is genetically stable and does not vary with passaging and that it can be readily manipulated (41). We first examined the phenotypic responses of these fibroblasts to moderate hypoxia (0.5% oxygen) and severe anoxia (<0.01% oxygen). We then utilized the oncoprotein E1a as a tool in order to better understand the mechanism of this proliferative arrest in anoxia. E1a cooperates with other oncogenes, such as ras, to transform primary cells and has been frequently utilized to explore the mechanism and significance of dysregulation of the cell cycle (19, 39, 50). E1a binds to Rb and p107 and releases members of the E2F family (29, 32). In addition, other domains of E1a that bind the coactivator p300, as well as the p400/TRRAP complex, are vital for several phenotypes, including stress-induced apoptosis and transformation (1, 49). Recently, E1a has also paradoxically been utilized as an antineoplastic agent in clinical gene therapy experiments, where it induces apoptosis in neoplastic cells, though its exact mechanism and the role the tumor microenvironment plays in this function are unclear (31).
We describe here two separate cell cycle checkpoints generated in low oxygen tensions, as well as the effects of E1a and E2F-1 in abrogating these hypoxia-induced checkpoints. We show that while moderate hypoxia primarily induces G1 arrest in REF52 cells, severe anoxia induces both G1 arrest and S-phase arrest. The S-phase arrest is due primarily to a suppression of the initiation complex of DNA synthesis, while DNA elongation is near normal. The stage of the cell cycle in which the cell arrests when rendered anoxic impacts on its proliferative status upon reoxygenation. Introduction of the E1a protein in anoxic cells induces p53-independent apoptosis, which can be prevented by overexpression of Bcl-2. Cells overexpressing both E1a and Bcl-2 proliferate near normally when hypoxic, suggesting that E1a can overcome the suppression by anoxia of DNA initiation, as well as the G1 block. E2F is markedly increased in E1a-overexpressing cells, even in those cells rendered anoxic. Overexpressing E2F-1 also permits proliferation in hypoxic cells, though it is not sufficient to permit proliferation in anoxic cells.
MATERIALS AND METHODS
Cells and cell culture.
REF52 cells were the kind gift of J. Nevins. MEFs and p53−/− MEFs were the generous gift of T. Jacks. Cells were grown in Dulbecco's modified Eagle's medium with 10% fetal calf serum (FCS), penicillin-streptomycin, and 25 mM HEPES (pH 7.5). All experiments were performed at <30% confluence, and pH was stable throughout hypoxia and anoxia. Normoxic cells were cultured in a 37°C 5% CO2 incubator, and hypoxic and anoxic cells were maintained in a control atmosphere chamber (Plas-Labs, Lansing, Mich.) at 37°C. Oxygen tensions were regulated by a palladium catalyst, which converts environmental oxygen and hydrogen to water. Oxygen was measured with a calibrated Series 200 Percent oxygen analyzer (Alpha Omega Instruments, Cumberland,R.I.).
Retroviral and adenovirus infection.
E2F-1 and Ad-Con control (CMV) adenoviruses were the gift of J. Nevins, E1a retrovirus was the gift of S. Lowe, and LBILy (Bcl-2/CD8) retrovirus was the gift of L. Cheng. Adenovirus was amplified in 293 cells, and titers were determined as described previously (20). REF52 cells were infected with E2F-1 or CMV at a multiplicity of infection of 100, as described in Results and the figure legends. Retroviruses were generated by calcium phosphate cotransfection of an expression plasmid with a Ψ-ecotrophic helper plasmid into 293T cells and collection and filtration of the supernatants. Cells infected with retrovirus were selected by either flow cytometry (Bcl-2) or puromycin at 1 (REF52 cells), 2 (p53+/+ MEFs), or 2.5 (p53−/− MEFs) μg/ml. All MEFs were transfected, and the experiments were done on passage 4 to 9 cells.
Double-labeling DNA synthesis.
Cells grown on coverslips were pulse-labeled with 30 μg of 5′-iodo-2′-deoxyuridine (IdU) (Sigma)/ml for 10 min and were then washed twice in medium containing 100 μM thymidine (Sigma). The IdU-labeled cells were then either maintained as normoxic or rendered anoxic for 4 or 8 h in the presence of 10 μM thymidine, pulsed with 30 μg of 5′-chloro-2′-deoxyuridine (CldU)/ml for 20 min, and then quickly fixed. To examine DNA elongation, IdU pulse-labeled cells were incubated in medium containing 5 μg of aphidicolin (Calbiochem-Novabiochem)/ml for 8 h. After the thymidine or aphidicolin medium was removed, the cells were pulse-labeled with CldU for 20 min. Detection of nuclear incorporation of halogenated deoxyuridine residues was performed as described previously (7) with minor modifications. Briefly, cells grown on coverslips were washed with phosphate-buffered saline (PBS) and fixed with 70% ethanol at 4°C and then incubated with 1.5 N HCl for 30 min. The coverslips were rinsed twice with PBS, washed once in PBS containing 0.5% Tween 20 for 5 min, and incubated with PBS containing 5% bovine serum albumin (BSA) and 0.5% Tween 20 for 15 min. CldU was detected with rat anti-BrdU (Accurate) at 1:20 dilution and donkey anti-rat immunoglobulin G (IgG) conjugated with Texas Red (Jackson Laboratories) at 1:25 dilution. IdU was detected with mouse anti-BrdU (Becton Dickinson) at 1:6 dilution and donkey anti-mouse IgG conjugated with fluorescein isothiocyanate (Jackson Laboratories) at 1:100 dilution. Each antibody was incubated for 30 min at 37°C and washed with PBS containing 0.5% Tween 20. High-concentration salt solution (400 mM NaCl, 0.2% Tween 20, 0.2% NP-40, PBS, 0.5% Tween 20) was utilized to prevent cross-reaction of primary antibodies against CldU and IdU. The cells were pulse-labeled with BrdU (30 μg/ml; Sigma) for 10 min and detected with mouse anti-BrdU at 1:10 dilution and goat anti-mouse IgG conjugated with fluorescein isothiocyanate (Jackson Laboratories) at 1:100 dilution. Multiple images of labeled nuclei were collected with a charge-coupled device camera. Patterns were assessed by two different observers, with at least 100 nuclei counted for each condition. Each nucleus was scored for the pattern of IdU incorporation, and then that of CldU incorporation, as described in Results (see Fig. 3A and C). All experiments were repeated at least twice.
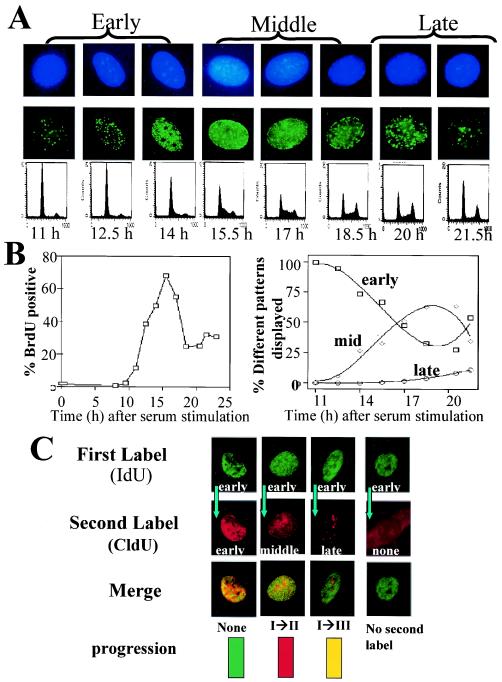
FIG.3.
S-phase progression in normoxic and anoxic cells. (A) Normoxic REF52/Bcl-2 cells were serum starved for 48 h and then stimulated with serum. Every 1.5 h, the cells were pulse-labeled with IdU and then either fixed and stained with anti-IdU antibodies as described in the text or analyzed for DNA content. DAPI staining of nuclei is shown in the top row, and halogenated nucleus contents are shown in the middle row. (B) Incorporation of BrdU and patterns of IdU incorporation during serum starvation and stimulation of normoxic cells. (C) During serum stimulation, cells were initially pulsed with IdU, chased, and subsequently pulsed with CldU. Representative patterns of progression are shown. For demonstration purposes, progression patterns are demonstrated only when the first label was incorporated in an early pattern.
Colony survival.
Cells were plated at various densities from 100 to 10,000 on a 100-cm-diameter dish for ∼8 h. Experimental plates were rendered anoxic for 27 h and then reoxygenated, and the media on both the experimental and control plates were changed. For experiments to determine the survival of G1- and S-phase cells, normoxic or anoxic cells were labeled with Hoechst 3342 (5 μg/ml in Hanks buffered saline solution in 2% FCS) and then sorted with a FACS Calibur system (Becton Dickinson). After 7 to 10 days, the plates were washed twice with PBS, fixed with 4% formaldehyde-PBS, and then stained with 4% crystal violet (Sigma)-PBS. Colonies of more than ∼50 cells were counted. The number of colonies appearing from the normoxic control cells were defined as 100%, as described in the figure legends. All experiments were performed in triplicate and repeated three times.
CFDA labeling.
Cells were washed twice with PBS and then resuspended in PBS- 0.1% BSA with 1 μM 5-(and-6)-carboxyfluorescein diacetate, succinimidyl ester (CFDA; Molecular Probes, Eugene, Oreg.) and incubated for 20 min at 37°C. The cells were then washed twice with Dulbecco's modified Eagle's medium- 10% FCS and plated at 75,000/10-cm-diameter dish for 24 h prior to the experiment. Cell fluorescence was assessed by FACS.
Thymidine labeling.
Cells were grown in 96-well plates in 200 μl of buffered medium. To ensure that the cells were subconfluent, serial dilutions from 10,000 to 500 cells/well were done. Eight replicates at each concentration were performed. Plates that were to be rendered anoxic or normoxic were both seeded from the same aliquot of cells to ensure that equivalent numbers of cells were under experimental and control conditions. After 8 h, the cells were rendered anoxic or hypoxic or maintained as normoxic for 24 h. One hundred microliters of deoxygenated medium containing [3H]thymidine for a final concentration of 1.2 μCi/ml was then added to each well. After 3 h, the plates were removed from their conditions, frozen, and then harvested with a 96-well cell harvester (Packard), and the incorporated counts were determined with a Matrix Direct Beta 96 (Packard). All experiments were repeated at least three times.
Apoptosis and cell cycle analysis.
Cells were plated at low confluence (100,000/10-cm-diameter dish) overnight, and then the medium was changed and the cells were exposed to experimental or control conditions for the appropriate times. The cells were then scraped (for cell cycle analysis) or trypsinized (for apoptosis). Cell cycles were assessed on nuclei after trypsinization, as described previously (20), and apoptosis was determined with an Annexin/PI kit (Biosource International). All experiments were repeated at least three times.
Immunoblotting and EMSA.
For immunoblotting, cells were scraped, boiled in incomplete lamelli, and after quantitation by bicinchoninic acid (Pierce), loaded on a 10% polyacrylamide gel and transferred to a nitrocellulose membrane as described previously (20). E1a and Bcl-2 polycolonal antibodies were obtained from Santa Cruz and PharMingen, respectively. For EMSA, nuclear extracts were prepared as described previously (4). The cells were then incubated for 20 min at room temperature with a 32P-labeled consensus E2F probe (Santa Cruz) in 4.5 mM Tris (pH 8)-32.5 mM KCl- 2 mM dithiothreitol-1 mM EDTA- 10% Ficol-0.3% NP-40-100 μg of BSA/ml and run at 200 V on a 6% polyacrylamide gel containing 0.25× Tris-borate-EDTA and 5% glycerol.
RESULTS
Severe anoxia results in G1 and S-phase arrest in fibroblasts.
To better address the potential differential regulation of proliferation in response to different levels of oxygen, we systematically examined cell proliferation in REF52 fibroblasts rendered either hypoxic (0.5% oxygen) or anoxic (<0.01% oxygen). Serum starvation, which leads to G1 arrest, was used as a control. Cell cycle regulation was examined by utilizing a combination of static (DNA content as an indicator of the cell cycle profile) and dynamic ([3H]thymidine uptake as an indicator of active S phase and CFDA fluorescence as an indicator of active proliferation) assays. CFDA spontaneously and irreversibly couples to the amine groups of intracellular and cell surface proteins. When a cell divides, CFDA labeling is distributed equally between each daughter cell. As a result, each successive generation in a population of proliferating cells is marked by a halving of the cellular fluorescence intensity, which can be followed by florescence-activated cell sorting (FACS).
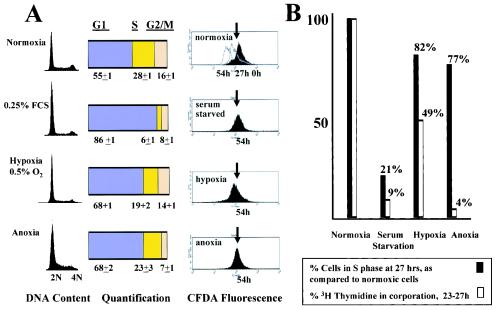
Cells stained with CFDA and then maintained under normoxic conditions revealed a successive halving of fluorescence over 54 h (approximately four doubling times under normoxic conditions), signifying cell division. Moderately hypoxic fibroblasts had a marked decrease in proliferation, as shown by CFDA staining over 54 h (Fig. 1A); fluorescence was not appreciably lower than when these cells were rendered hypoxic. These hypoxic cells demonstrated an increased population fraction in G1 and a decrease in S and G2, suggesting G1 arrest (Fig. 1A), as previously reported in MEFs (20). In addition, compared to normoxic cells, hypoxic cells demonstrated a 50% reduction in [3H]thymidine incorporation over 3 h (Fig. 1B).
FIG. 1.
Effect of hypoxia (0.5% oxygen) and anoxia on proliferation and cell cycle profiles in REF52 cells. (A) Cells were maintained in either 20% oxygen or 0.25% FCS or rendered hypoxic or anoxic. After 27 h, the cells were collected and analyzed for DNA content. FACS profiles of CFDA-labeled normoxic cells at 0, 27, and 54 h and profiles of serum-starved, hypoxic, and anoxic cells at 54 h are displayed. The arrows indicate the fluorescence of the cells at the time they were rendered hypoxic or serum starved. Decay of fluorescence beyond the arrows reflects proliferation. Experiments were repeated at least three times with duplicates each time, and averages and standard errors are shown. (B) Cells were pulse-labeled with [3H]thymidine from 24 to 27 h under the experimental conditions described above, and thymidine incorporation into DNA was measured as described in Materials and methods. The percentages of normoxic cells in S phase, as determined from the DNA contents shown in panel A, were normalized to 100%, and the percentages of serum-starved, hypoxic, and anoxic cells are relative to these normoxic cells. Similarly, [3H]thymidine incorporation by normoxic cells was normalized to 100%, and the rates of [3H]thymidine incorporation by serum-starved, hypoxic, and anoxic cells are relative to this value.
Anoxic cells also ceased proliferation and exhibited an increase in G1 (Fig. 1A). In addition, serum-starved cells that were restimulated while anoxic remained in G1, again confirming the presence of G1 checkpoint activation (data not shown). However, while the percentage of cells in S phase only slightly decreased (by 23%) compared to normoxic cells, thymidine incorporation markedly decreased (96%) (Fig. 1B), suggesting that in addition to the activation of a G1 checkpoint, cells rendered anoxic also undergo significant S-phase arrest. It should be noted that this S-phase arrest is more pronounced in anoxia than in more moderate hypoxia.
To confirm the activation of these two specific checkpoints, cells were serum starved for 48 h and then stimulated with 10% FCS. After 11.5 h of serum stimulation, >80% of this synchronized population entered S phase (Fig. 2A and 3B). When the cells were maintained as normoxic, they demonstrated a dramatic increase in S phase 4.5 h later, an increase in G2 by 6 h, and a return to G1 by 9 h (Fig. 2A). In contrast, when 11.5-h serum-stimulated cells were rendered anoxic, they remained arrested in S phase, again demonstrating activation of an S-phase checkpoint. When cells were rendered hypoxic 11.5 h after serum stimulation, they progressed through S phase at approximately the same rate as normoxic cells (Fig. 2B). However, whereas normoxic cells began to reenter S phase by 13 h, hypoxic cells remained arrested in G1, demonstrating the presence of a hypoxia-induced G1 checkpoint. Thus, severe anoxia not only elicits G1 arrest in REF52 cells but also results in S-phase arrest.
FIG. 2.
Activation of checkpoints in anoxic and hypoxic S-phase cells. (A) Serum-starved cells were stimulated with serum for 11.5 h and then either maintained as normoxic or rendered anoxic. Cells were collected at regular time points, and the DNA content was assessed regularly as described in the text. (B) Serum-starved cells were rendered hypoxic, and the DNA content was assessed regularly as described in the text.
Anoxia-induced S-phase arrest is due to suppression of the DNA initiation complex.
To delineate the kinetics of anoxia-induced S-phase arrest in a well-defined system, we utilized the temporal-spatial relationship of DNA replication. As cells progress through S phase, DNA is replicated in specific and reproducible patterns. For reasons to be discussed later, we utilized REF52 cells overexpressing the antiapoptotic protein Bcl-2; Bcl-2 had no effect on proliferation responses in hypoxic cells (data not shown). To characterize the spatial pattern of DNA replication in REF52/Bcl-2 cells as they progress through S phase, cells were synchronized in G1 by serum starvation for 48 h and then serum stimulated. The cells began to enter S phase 11 h after serum stimulation, as shown by DNA content (Fig. 3A) and BrdU incorporation (Fig. 3B, left). Over the next 11 h of S-phase progression, the cells were pulsed with IdU and then fixed, stained with anti-IdU antibody, and visualized. The cells demonstrated an orderly and specific pattern of halogenated nucleotide incorporation into newly replicated DNA, which could be classified as early, middle, or late DNA replication patterns (Fig. 3A and B, right). In early S phase, cells demonstrate discrete fine foci of labeling within the nucleus, excluding the nucleoli. In mid-S phase, labeling around the periphery of the nucleus, as well as the nucleoli, is noted. During late S phase, larger punctate labeling within the nucleus is evident. If DNA replication is not occurring at all, a cell will not incorporate any halogenated nucleotide. To assess S-phase progression within the same cell, asynchronous cells were pulse-labeled with IdU and, after a chase period, pulse-labeled with CldU. Specific anti-IdU and anti-CldU antibodies will stain in distinct patterns if a cell has progressed through S phase or in a superimposable pattern if minimal or no progression has occurred (Fig. 3C). For example, a cell that was labeled in early S phase with IdU and then later labeled in mid-S phase with CldU was classified as showing stage I progression. A cell initially labeled in mid-S phase and subsequently labeled in late S phase was also classified as showing stage I progression. A cell labeled with IdU in early S phase that later incorporated CldU in a late-S-phase pattern was classified as showing stage II progression. A cell that did not incorporate IdU initially (i.e., that was in G2/M or G1) and later incorporated CldU in a late-S-phase pattern was classified as showing stage III progression. As a final example, a cell that initially incorporated IdU in a mid-S-phase pattern and then incorporated CldU in an early pattern (of the next cell cycle) was also classified as showing stage III progression.
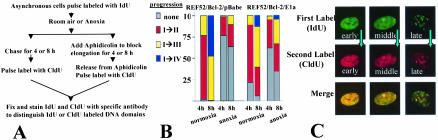
To visualize S-phase progression in normoxic and anoxic REF52/Bcl-2 cells, asynchronous cells were pulsed with IdU and then chased and rapidly rendered anoxic or maintained as normoxic. After either 4 or 8 h of chase, the cells were pulsed with CldU and then rapidly fixed (as shown in Fig. 4A). Normoxic cells revealed normal progression from early to middle to late patterns, with most cells progressing from one pattern to the next by 4 h and progressing further by 8 h (Fig. 4B). Under anoxic conditions, little progression in DNA replication was noted by 4 h. By 8 h of anoxia, only a minority of cells were able to incorporate CldU (17 versus 100% in normoxic cells [Table 1 ]), and those cells that did incorporate CldU did not demonstrate a progression of pattern (Fig. 4B). These findings were similar whether the cells were in early, middle, or late S phase when the first label was incorporated (data not shown).
FIG. 4.
S-phase progression of normoxic and anoxic REF52 and E1a-expressing cells. (A) Schema of experimental design to assess S-phase progression in normoxic and hypoxic cells in the absence (left) or presence (right) of the elongation inhibitor aphidicolin. (B) Progression of S phase at 4 and 8 h in normoxic and anoxic cells. (C) CldU incorporation in cells rendered anoxic for 8 h while in the presence of aphidicolin.
TABLE 1.
S-phase progression in normoxic and anoxic cellsa
| Cell line | Chase time (h) | % Incorporation of second label
|
|||
|---|---|---|---|---|---|
| Normoxia | Anoxia | After release from aphidocolin
|
|||
| Normoxia | Anoxia | ||||
| REF52/Bcl-2/ pBabe | 4 | 100 | 99 ± 2 | 99 ± 3 | 98 ± 3 |
| 8 | 100 | 17 ± 13 | 98 ± 2 | 88 ± 8 | |
| REF52/Bcl-2/ E1a | 4 | 99 ± 1 | 97 ± 4 | ||
| 8 | 90 ± 13 | 58 ± 20 | |||
Normoxic cells were pulse-labeled with IdU and then chased for 4 or 8 h while either normoxic or anoxic. In separate experiments, cells were treated with aphidicolin during the chase period. After the chase period, the cells were washed and then pulsed with CldU. Anoxic cells were then removed from the chamber, and all cells were fixed and later stained as described in the text. The percentages of cells that incorporated CldU are reported.
The slow progression of S phase, as well as the failure to incorporate nucleotides after 8 h of anoxia, could be due to suppression of DNA initiation and/or inhibition of DNA elongation. In order to determine how anoxia affects DNA replication, we determined whether elongation could occur even after 8 h of anoxia, a time at which DNA replication is severely hampered. Asynchronous normoxic cells were pulsed with IdU and then treated with the elongation inhibitor aphidicolin and either maintained as normoxic or rendered anoxic for 8 h. The aphidicolin was then removed, and the cells were pulsed with CIdU and rapidly fixed; throughout this procedure, the cells were maintained as normoxic or anoxic (as shown in Fig. 4A, right). If elongation were affected by anoxia, we would not expect the anoxic cells to incorporate CldU when aphidicolin was removed and elongation was permitted to continue. In fact, after 8 h, 88% of the cells incorporated a second label, despite remaining anoxic (Table 1 and Fig. 4C). These rates of incorporation are not significantly different from those of similarly treated normoxic cells. This is in marked contrast to the first series of experiments, when DNA elongation was not blocked by the addition of aphidicolin and only 17% of the cells incorporated a second label at 8 h and there was little S-phase progression at 8 h. Thus, DNA elongation was essentially normal in anoxic cells, suggesting that poor S-phase progression and failure to incorporate nucleotides is due to a failure of DNA initiation.
Anoxic cells in G1 resume proliferation upon reoxygenation, while those arrested in S phase do not.
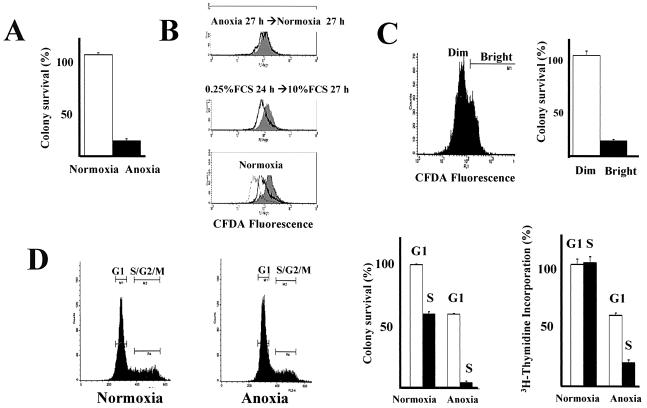
Several in vivo and in vitro studies have suggested that reoxygenation of a variety of hypoxic cells leads to death through apoptotic and nonapoptotic mechanisms (48). This may be a clinically relevant event in cancer, since regions of the tumor microenvironment may be intermittently oxygenated and deoxygenated. While reoxygenation of hypoxic cells reduces clonal survival, some cells are able to proliferate and form colonies. The reason why some cells can proliferate after reoxygenation while others do not is not known. When REF52 cells rendered anoxic for 24 h were reexposed to 20% oxygen, a majority of the cells (83%) did not survive to form colonies (Fig. 5A). CFDA-stained cells that were serum starved for 27 h and then serum stimulated proliferated as a single population (Fig. 5B). In contrast, when cells were rendered anoxic and then reoxygenated, two populations were readily apparent after 24 h: a bright population, which represented arrested cells, and a second population, which proliferated near normally (Fig. 5B). The bright population showed minimal proliferation even after 54 h (data not shown). When the bright (nonproliferating) and dim (proliferating) cells were sorted, the bright cells did not form colonies (10% versus normoxic cells) whereas the dim, proliferating cells formed colonies nearly as well as control cells that were never rendered anoxic (74% versus normoxic cells) (Fig. 5C). This process was not due to inherent genetic differences within the cell population; when dim cells were isolated and again rendered anoxic, two populations were again seen (data not shown).
FIG. 5.
Effects of reoxygenation on 27-h anoxic REF52 cells. (A) REF52 cells were rendered anoxic for 27 h and reexposed to 20% oxygen. After 7 to 10 days, the plates were stained and colonies were counted. The plating efficiency for normoxic unselected REF52 cells was 95%. The error bars indicate standard errors.(B) Cells were stained with CFDA, cultured for 1 day, and then either maintained as normoxic, serum starved for 27 h and then serum stimulated, or rendered anoxic for 27 h and then maintained as normoxic. At various time points, cells were collected and analyzed by FACS. Discrete peaks in the anoxic→ normoxic cells suggest different proliferative capabilities. (C) Cells were rendered anoxic for 27 h and then normoxic for 27 h. The bright (nonproliferating) and dim (proliferating) cells were sorted by FACS and plated, and colony formation was assessed. (D) After 27 h, normoxic or anoxic cells were sorted for G1 or S and G2. The cells were plated, and colony formation and thymidine incorporation over 24 h were assessed.
Cell cycle analysis revealed that 27 h after reoxygenation, most of the bright, nonproliferating cells were in S phase, whereas the proliferating, dim cells were in all stages of the cell cycle (data not shown). To determine whether the phase in which a cell arrests when rendered anoxic affects its proliferative status when reoxygenated, normoxic and anoxic cells were labeled with a vital DNA stain and sorted by their DNA contents. G1 and S cells were then plated and assessed for colony formation. When normoxic cells were sorted, 60% of the S-phase cells later formed colonies compared to sorted normoxic G1 cells (an unexpected decrease, perhaps due to differences in plating efficiency, damage to S-phase cells during the sorting process, or poor specificity of the assay) (Fig. 5D). Rates of thymidine incorporation by the sorted normoxic G1 and S cells were equivalent over 24 h (Fig. 5D). In contrast, colony formation by the anoxic S-phase cells was diminished to 5% compared to that of the normoxic S cells and to only 12% of that of the anoxic G1 population (Fig. 5D). Thymidine incorporation was similarly decreased. Survival of the anoxic G1 population, however, was ∼60% of that of the normoxic G1 population (Fig. 5D). These data suggest that the G1 arrest in anoxia is protective upon reoxygenation.
E1a induces anoxic p53-independent apoptosis.
Several studies have shown that under hypoxic conditions a variety of neoplastic cell lines with inactive Rb do not undergo G1 arrest over short periods (35, 52). The multiple abnormalities found in tumor cells make the significance of the Rb mutations difficult to interpret. To underscore the importance of adequate controls, we have found that even a variety of nontransformed cell lines have varied oxygen thresholds for proliferation arrest (data not shown). The oncoprotein E1a is a well-established tool to explore the effects of cellular deregulation on tumorigenesis and proliferation (19). Many of the cellular phenotypes promoted by E1a expression, such as inactivation of Rb, are seen in a majority of tumors. E1a alone does not transform REF52 cells, but it does cooperate with ras to transform these cells (18, 41, 44).
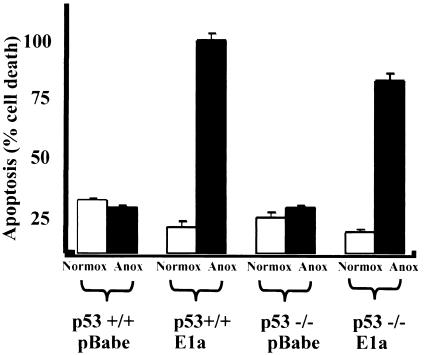
Stable REF52/E1a cell lines were created by retroviral infection and antibiotic selection. REF52/E1a cells underwent apoptosis under anoxic conditions. This apoptosis was associated with annexin positivity, an increased sub-2 N fraction of DNA (Table 2), and caspase-mediated cleavage of PARP, NUMA, and SK-70 (data not shown) and was suppressed by overexpression of the antiapoptotic protein Bcl-2 (as described below). E1a is well known to sensitize cells to apoptosis in response to radiation and chemotherapy in a p53-dependent manner (49). In addition, p53 is also necessary for anoxia-induced apoptosis in c-myc-overexpressing cells (24, 25). Intriguingly, however, we found that E1a promoted apoptosis even in p53−/− MEFs rendered anoxic for 24 h only slightly less than in wild-type MEFs when E1a was expressed to similar degrees in both cell types (Fig. 6 and data not shown). The similar rates of apoptosis in p53−/− and wild-type MEFs were also seen at 12 h (data not shown). c-myc overexpression in these primary cells led to ∼50% apoptosis in 24 h and was dependent on p53 expression (data not shown).
TABLE 2.
E1a-induced apoptosis in anoxic REF52/pBabe and REF52/Bcl-2 cellsa
| Cell line | Apoptosis (% of total cells)
|
|||||||
|---|---|---|---|---|---|---|---|---|
| 24 h
|
48 h
|
|||||||
| Normoxia
|
Anoxia
|
Normoxia
|
Anoxia
|
|||||
| Annexin+ | <2 N | Annexin+ | <2 N | Annexin+ | <2 N | Annexin+ | <2 N | |
| pBabe | ||||||||
| pBabe | 3.3 | 1.0 | 10.2 | 1.4 | 6.4 | 0.7 | 17 | 1.7 |
| E1a | 5.0 | 2.2 | 58.5 | 14 | 12.2 | 1.1 | 93 | |
| Bcl-2 | ||||||||
| pBabe | 3.2 | 1.5 | 6.4 | 2.8 | 2.0 | 0.8 | 7.5 | 0.9 |
| E1a | 5.2 | 0.8 | 15.2 | 2.8 | 5.4 | 0.7 | 19.4 | 1.8 |
Cells were plated at low density and rendered anoxic for 24 or 48 h and then apoptosis was assessed by annexin-propidium iodide staining or by sub-2 N DNA content.
FIG. 6.
p53 dependence of apoptosis in anoxic MEFs. p53+/+ and p53−/− MEFs were stably transfected with E1a or control. They were then rendered anoxic or normoxic for 24 h, and apoptosis was assessed as described in the text. Experiments were repeated three times, and averages and standard errors are shown.
E1a and Bcl-2 promote proliferation despite anoxia.
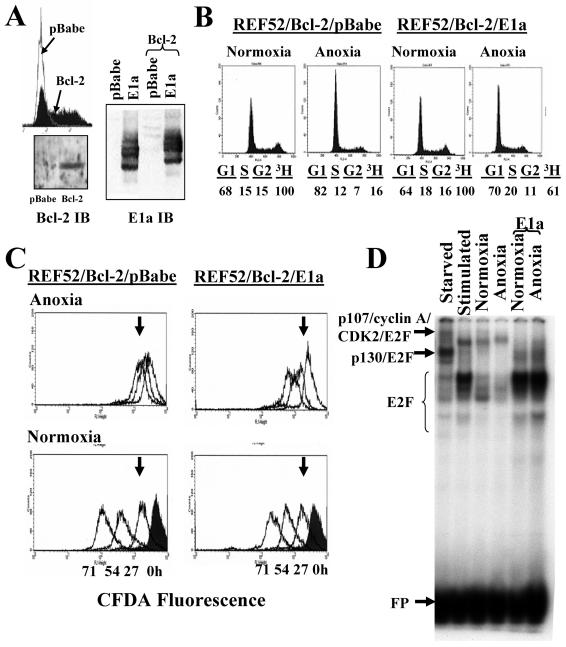
Previous work suggested that E1a/ras-transformed p53−/− MEFs are able to incorporate BrdU after 30 h of anoxia to a greater degree than nontransformed p53−/− MEFs (52). To better explore the effects of E1a expression on the proliferation of genetically stable, nontransformed anoxic cells, we first suppressed apoptosis in anoxic REF52/E1a cells. Hypoxia-induced apoptosis has been shown to be repressed by members of the Bcl-2 antiapoptotic family (51, 54). REF52/Bcl-2 cells were created by infection with a Bcl-2 retrovirus that also expressed a selectable surface marker; high-marker-expressing cells were sorted, and these cells were then infected with E1a or control retrovirus and selected with puromycin. E1a expression levels were similar in control and Bcl-2 cells (Fig. 7A). Bcl-2 overexpression in conjunction with E1a expression is not sufficient to transform primary rat fibroblasts (44).
Bcl-2 potently suppressed apoptosis of anoxic E1a-expressing cells at 24 and 48 h (Table 2). We next compared the proliferation of anoxic REF52/Bcl-2/E1a cells to that of anoxic REF52/Bcl-2/pBabe cells. Bcl-2 overexpression alone slightly altered the DNA profile of normoxic REF52 cells (compare Fig. 7B, left, to Fig. 1A) but did not dramatically change their proliferation, as shown by CFDA fluorescence, and had no affect on anoxia-induced growth arrest (data not shown). However, we cannot rule out the unlikely possibility that when Bcl-2 and E1a are coexpressed, Bcl-2 itself may affect proliferation in anoxic cells. In anoxic REF52/Bcl-2/E1a cells, DNA profiles revealed a less dramatic G1 arrest than in control cells (Fig. 7B). Pulse [3H]thymidine incorporation was 60% of that in normoxia in REF52/Bcl-2/E1a cells compared to 16% in the parental cell lines. Most significantly, CFDA-labeled E1a cells continued to lose fluorescence in anoxia, nearly equaling the proliferation of cells in room air over 71 h (Fig. 7C). In comparison, after 71 h of anoxia, the fluorescence of control cells had barely reached that of 24-h normoxic cells.
FIG. 7.
Effects of E1a on proliferation of anoxic REF52 cells. (A) REF52 cells were infected with either control or Bcl-2 retrovirus. High Bcl-2 expression was assessed by the coexpression of CD8, and high expressers were isolated by FACS. These cells, or control pBabe cells, were then infected with E1a or pBabe as described in Materials and Methods. Immunoblots (IB) of Bcl-2 and E1a reveal appropriate expression. Immunoblots of Bcl-2 in the high expressers and in control-infected cells (left) of and E1a in control-infected cells and Bcl-2-infected cells (right) are shown. (B) Proliferation of control and E1a-expressing cells assessed by DNA content and [3H]thymidine incorporation after being rendered anoxic for 27 h. (C) Proliferation of REF52/Bcl-2/pBabe and REF52/Bcl-2/E1a cells when normoxic or anoxic, as assessed by CFDA fluorescence and FACS. The arrows mark the fluorescence of normoxic cells 27 h after culture. (D) E2F EMSA on REF52/Bcl-2/pBabe cells serum starved for 48 h (Starved), serum starved and then stimulated for 16 h (Stimulated), maintained as normoxic (Normoxia), and rendered anoxic (Anoxia) with and without E1a, as labeled. Complexes were verified with supershifts, and all labeled complexes were successfully competed by unlabeled probe, but not by mutated probe (data not shown). Free probe (FP) is shown.
As revealed previously, initiation of DNA replication is severely hampered in anoxia. As expected, pulse-labeling with IdU and then 4 h later pulsing with CldU in REF52/Bcl-2/E1a cells showed progression of S phase compared to parental cells (Fig. 4B). When cells were pulsed after 8 h of anoxia, further progression had occurred in anoxic REF52/Bcl-2/E1a cells, whereas in anoxic control cells little progression had occurred. In addition, whereas after 8 h of anoxia only 17% of normoxic cells incorporated CldU, 58% of REF52/Bcl-2/E1a cells incorporated the second label (Table 1). In control REF52/Bcl-2/pBabe cells at both 4 and 8 h, very few cells that had not incorporated the first label (i.e., which were in G2/M or G1 when pulsed with IdU) incorporated a second label (2.8% in anoxic cells versus 23.5% in normoxic cells at 4 h [Table 3]), again demonstrating G1 and/or S-phase arrest. In contrast, anoxic REF52/Bcl-2/E1a cells that did not incorporate a first label readily incorporated a second label at nearly the same rate as normoxic cells (Table 3). This confirms that E1a-overexpressing cells were able to escape G1, proceed into S, and initiate DNA replication despite anoxia. Therefore E1a/Bcl-2 expression was able to overcome the anoxia-induced G1 arrest, as well as anoxia's suppression of initiation of DNA replication.
TABLE 3.
G1 escape and active DNA replication in normoxic and anoxic cellsa
| Cell line | Chase time (h) | % IdU−/CIdU+
|
|
|---|---|---|---|
| Normoxia | Anoxia | ||
| REF52/Bcl-2/pBabe | 4 | 23.5 | 2.8 |
| 8 | 30.1 | 6.0 | |
| REF52/Bcl-2/E1a | 4 | 17.0 | 15.2 |
| 8 | 27.5 | 14.9 | |
Cells were labeled and treated as described in the text. The percentages of cells that did not incorporate the first label, IdU, but did incorporate the second label, CIdU, are reported. At least 100 nuclei were counted for each experiment, and each experiment was repeated at least three times.
E1a expression correlates with increased E2F levels, and E2F-1 overexpression permits proliferation in hypoxic, though not anoxic, cells.
Studies have delineated multiple functions of E1a, including the release of free E2F from Rb and p107. Using a consensus site for E2F binding in EMSA, we identified previously described free E2F and E2F complexes in REF52/Bcl-2 cells (Fig. 7D; supershifts with Rb, p107, p130, CDK2, and cyclin A antibodies are not shown). We noted an expected marked increase in E2F in REF52/Bcl-2/E1a cells, similar to that of synchronized cells collected in mid-S phase (Fig. 7D). Interestingly, there was minimal change in the amount of E2F or E2F complexes in anoxic cells compared to normoxic cells, although a slight decrease in free E2F and an increase of E2F/p107 was present in anoxic cells. This is in marked contrast to the significant decrease in E2F and increase in repressive E2F/p130 complexes noted in serum-starved cells.
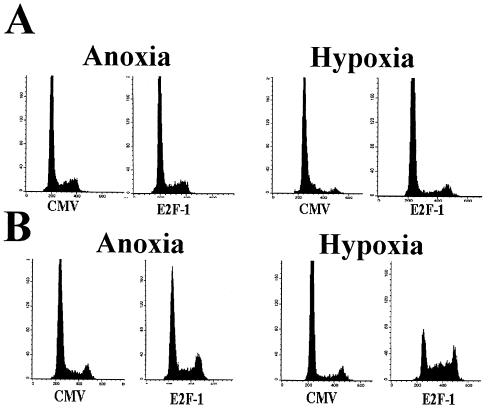
E1a-expressing cells had minimal E2F complexed with the pocket protein p107 or p130, and most of the E2F present was in fast-moving complexes. Normoxic cells showed E2F in both fast-moving complexes and the previously described p107/CDK2/cyclin A complex. E2F-1 overexpression can promote limited proliferation in serum-starved quiescent fibroblasts (34). To determine if overexpression of E2F-1, as found in E1a overexpression and many Rb-null neoplasms, could mimic the proliferative effects of E1a in anoxia, REF52 cells rendered anoxic for 24 h were infected with E2F-1 adenovirus. Infection resulted in E2F-1 protein synthesis within 6 h, and the amount of E2F-1 protein synthesized after 24 h was similar to that seen in E1a-infected cells by EMSA (data not shown). No change in the cell cycle profile was noted after 24 h compared to a CMV control (Fig. 8A, left). As a more sensitive measure, cells were serum starved for 48 h, rendered anoxic, infected, and then stimulated with FCS (Fig. 8B, left). Only small increases in S and G2 were reproducibly seen in E2F-1-overexpressing cells compared to the CMV control cells. In addition, when cells were first infected with E2F-1 for 12 h and then rendered anoxic, after 24 h there was no dramatic change in cell proliferation, as noted by CFDA fluorescence or 24 h of [3H]thymidine incorporation, or in the cell cycle, as noted by DNA content or 3 h of [3H]thymidine incorporation, compared to CMV-infected cells (data not shown). Thus, E2F-1 had a minimal effect on preventing or reversing anoxic cell proliferation arrest.
FIG. 8.
Effects of E2F expression on proliferation of anoxic and hypoxic cells. (A) REF52 cells were rendered anoxic (left) or hypoxic (right) for 24 h and then infected with either CMV or E2F-1 adenovirus. After an additional 24 h, the cells were collected and analyzed for DNA content. (B) Cells were serum starved for 48 h and then rendered hypoxic (right) or anoxic (left). The cells were then stimulated with serum and infected with either CMV or E2F-1; 24 h later, the cells were collected and analyzed for DNA content.
Because our data suggest that alternative checkpoints may be more dominant in hypoxia and anoxia, we examined the effect of E2F-1 overexpression in hypoxic cells. Overexpression of E2F-1 in cells rendered hypoxic for 24 h led to a small, though reproducible, decrease in G1 and increase in S and G2 in hypoxic cells after 24 h of infection compared to a CMV control (Fig. 8A, right). This escape was more obvious when cells were synchronized by serum starvation and then infected and restimulated when hypoxic (Fig. 8B, right). Based on our previous studies, these data suggest that E2F can overcome the hypoxia-induced G1 arrest, though not the anoxia-induced S arrest. It also indicates that other biological effects of E1a, in addition to increases in E2F, are necessary to promote proliferation in anoxia.
DISCUSSION
Little is known about the molecular mechanisms that regulate proliferation in hypoxia, despite their likely importance for many clinical phenomena in cancer biology and treatment, including tumor dormancy, genomic instability, and resistance to chemotherapy, radiation, and angiogenesis inhibitors. In addition, little is known about how these regulatory mechanisms may be altered with neoplastic transformation. Contradictions in previous studies may be due to the investigation of cells with complex, unclear genetic backgrounds and oxygen tensions that have varied between 5% and near anoxia. Mechanistic insight from the comparison of the proliferation of different hypoxic cell lines has also been difficult to attain. In addition, even minimally altered cell lines may display markedly different proliferative responses when hypoxic (13). Using a defined system amenable to molecular manipulation, we have provided insight into cell cycle regulation in both hypoxic and anoxic cells. We have shown that a suppression of DNA initiation leads to S-phase arrest that is most apparent at lower oxygen tensions, that this S-phase arrest is primarily due to suppression of DNA initiation, and that cells arrested in S phase cannot proliferate when reoxygenated. Furthermore, we have shown that increased E2F-1 can overcome the G1 checkpoint activated by moderate hypoxia but not the S-phase checkpoint activated in anoxic cells. E1a expression, when apoptosis is suppressed, can overcome both checkpoints. While the results we obtained in REF52 cells, with and without Bcl-2/E1a expression, may not be applicable to other primary or transformed cells, these data provide insight into checkpoint activation under various degrees of low oxygen.
Several studies using a variety of methodologies have examined the kinetics of DNA replication in hypoxia and have arrived at divergent interpretations. Some studies have suggested that DNA initiation is affected by hypoxia and is most apparent at low oxygen tensions (21, 42, 43, 46, 47). Alternatively, deoxynucleotide depletion, which would be expected to affect DNA initiation, as well as elongation, has also been suggested to occur in hypoxic cells (9, 37). Data suggesting that deoxynucleotides can reverse the arrest of some neoplastic cell lines in moderate hypoxia support the theory that depletion of DNA precursors is most affected by hypoxia (2). These studies have primarily utilized indirect approaches involving velocity sedimentation of pulse-labeled nascent DNA. While informative, these assays are difficult to interpret when DNA damage and repair and/or G1 arrest is also occurring. In addition, these studies have utilized neoplastic cell lines with known abnormalities in cell cycle regulation and metabolism. The absence of adequate control cell lines has also made it difficult to directly compare the regulation of DNA replication in normal and deregulated cells. Our finding that DNA initiation is more severely affected than elongation in anoxic cells is consistent with previous findings in neoplastic cell lines. These results suggest that any possible decrease in the deoxynucleotide pool is not sufficient to hamper elongation and that more complex mechanisms are responsible for the suppression of DNA replication in anoxic cells. The complete pathway of replication arrest is unknown. The kinase ATR plays an important role in the regulation of DNA initiation (17), and it is intriguing that anoxia has been reported to activate ATR (27, 28). The significance of anoxic activation of ATR, however, is still unclear and is beyond the scope of our study.
Others have shown that neoplastic cells with disrupted Rb can proliferate despite moderate hypoxia (35, 52). Although the disruption of Rb is expected to increase free E2F, the unclear genetic background of these cells and the absence of adequate controls make interpretation of these data difficult. We have demonstrated that E2F-1 overexpression is sufficient to promote proliferation in moderate hypoxia. This confirms a previous finding that Rb-null MEFs can proliferate when rendered moderately hypoxic (20). We have also noted, however, that some Rb-inactivated neoplastic cell lines can proliferate despite severe anoxia (I. Cheong and L. B. Gardner, unpublished data). The observation that overexpression of E2F-1 is not sufficient for anoxic-cell proliferation suggests that multiple genetic alterations are necessary for the proliferation of an anoxic cancer cell. Since anoxic cells undergo more prominent S-phase arrest than moderately hypoxic cells, we surmise that E2F overexpression alone is sufficient to overcome a hypoxia-induced G1 checkpoint but that events in addition to E2F release are necessary to promote DNA replication in anoxic cells.
The additional events necessary for anoxic-cell proliferation are unknown, but there are several possibilities. It may be that in the absence of additional alteration or activation events, E2F is only minimally transcriptionally active in anoxic REF52 cells. Indeed, p53 is induced but not transcriptionally active in anoxia (33). While previous data have suggested that transformed p53−/− MEFs proliferate when anoxic (52), our observation that E1a is sufficient to promote anoxic proliferation when apoptosis is suppressed may provide further insight into these additional events. E1a expression markedly increased E2F even in anoxic cells, and while even marked E2F-1 overexpression did not promote proliferation of anoxic cells, it is possible that E1a augments the transcriptional activity of E2F in anoxic cells. Although E2F targets include enzymes necessary for maintaining the deoxynucleotide pool (5), our finding that only DNA initiation is hampered in anoxia suggests that this is not the major role of E1a. Other E2F targets include critical components of the replication initiation complex, including Orc1, MCM6, and CDC6, and the effects of anoxia on these components will need to be evaluated (11, 14, 40).
An alternate hypothesis is that induction of E2F targets is not sufficient to promote proliferation in anoxic cells and additional events are necessary. We have noted that E1a not only increases E2F expression but also increases CDK2 activity, even in anoxic cells (L. B. Gardner, unpublished data). It is intriguing that S-phase progression is severely diminished after both 4 and 8 h of anoxia, but after 8 h of anoxia, only a fraction of cells were able to incorporate deoxynucleotides at all. This suggests that DNA initiation is more hampered after 8 h of anoxia than after 4 h. In vitro experiments show that the sequential effects of CDK2-cyclin E and CDK2-cyclin A are necessary for programming and activating initiation complexes (12, 15). We speculate that after 4 h of anoxia, when CDK2 activity is marginally diminished, cells in S phase can activate more DNA initiation complexes than after 8 h of anoxia, when CDK2 activity is greatly decreased (data not shown).
The ability of E1a to promote apoptosis in anoxic cells may be based on several of its features. The E1a carboxy terminus contains a domain which binds the coactivator p300, which is important for transactivation by the hypoxia-induced transcription factor HIF-1 (6, 16). Disruption of p300 binding to HIF-1 has been shown to diminish tumor growth, primarily through apoptosis (16, 36). Further insight will come with study of E1a deletional mutants. E1a's ability to sensitize fibroblasts to chemotherapy or radiation, but not azide treatment, is dependent on p53 induction (49). While p53 is not necessary for hypoxia-induced G1 arrest, it is important for hypoxia-induced apoptosis in myc-overexpressing cells (25). Previous work has also suggested that anoxic E1a/ras-transformed MEFs also undergo apoptosis in a p53-dependent manner (52). Consistent with these studies, angiogenesis inhibitors were found to preferentially destroy wild-type p53 tumors in an animal model (56). We observed that myc-induced apoptosis was p53 dependent in primary MEFs. However, E1a-induced apoptosis in anoxic cells, as verified by multiple assays, as well as suppression by Bcl-2, occurred via a p53-independent mechanism. The finding that hypoxia-induced apoptosis is not universally p53 dependent may have therapeutic implications, both for the utilization of angiogenesis inhibitors and for the use of E1a as gene therapy to induce cell death.
While we have demonstrated that E1a promotes both proliferation and apoptosis in anoxic cells, further studies are necessary to determine if these two processes are linked. Premature entry into S phase, such as with enforced expression of CDKs, under adverse conditions has been associated with apoptosis. Apoptosis has also been linked to E2F generation, as seen in E1a-expressing cells, through direct caspase activation (38). However, E2F-1 overexpression alone was unable to promote apoptosis in our anoxic cells and only minimally increased apoptosis in hypoxic cells (data not shown). Unrepaired DNA damage also serves as a potent stimulus for apoptosis and clonal deletion. Careful studies have suggested that DNA damage is minimal in anoxic, arrested cells and is at much higher levels when these cells are reoxygenated (28, 45). It is unclear whether this damage occurs from the generation of reactive oxygen species and/or resumption of DNA replication under adverse conditions. Further studies will determine whether E1a-induced proliferation of anoxic cells leads to DNA damage and whether this damage is related to E1a-induced apoptosis in anoxic cells. We also observed that reoxygenated G1-arrested cells could proliferate nearly normally, whereas reoxygenated S-arrested cells could not. While this may again be a mechanism to limit the proliferation of damaged cells, additional studies will also be necessary to determine the relationship of stalled replication forks, DNA damage, and the proliferative fate of S- and G1-phase-arrested cells upon reoxygenation.
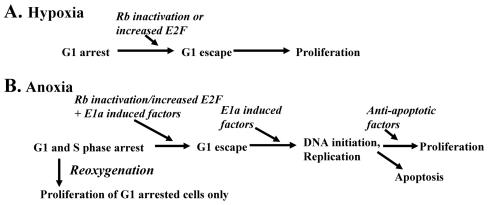
By systematically studying and manipulating a system, we can propose a model of the proliferative responses of normal cells to hypoxia and anoxia (Fig. 9). Our model suggests that inactivation of Rb and increases in E2F are not sufficient to overcome replication arrest in anoxic cells and that other events, as found in E1a/Bcl-2 cells, are required for neoplastic cells to proliferate when anoxic. The observation that distinguishable checkpoints are activated in anoxic and hypoxic cells not only provides a conceptual basis for understanding cell growth arrest responses to oxygen deprivation and how these responses may be altered in neoplastic cells with deficient cell cycle checkpoints but helps to explain some contradictions in previous published reports (20, 23, 26). Further understanding of the genetic alterations necessary for anoxic proliferation will provide insight into the processes of neoplastic progression.
FIG. 9.
Proposed model of checkpoint activation in hypoxic (A) and anoxic (B) cells.
Acknowledgments
We acknowledge the generous gifts of reagents from J. Nevins, S. Lowe, and L. Cheng. We appreciate comments on the manuscript from R. Osthus, K. O'Donnell, and L. Lee, and we thank members of the Dang laboratory and M. Horton for support and assistance. This work was greatly assisted by insightful advice and suggestions from Jonathan Powell. We thank anonymous reviewers for suggestions of additional experiments that are reported in this paper.
This work was supported by a Johns Hopkins Clinician Scientist Award and NIH grants K08CA89265 (L.B.G.) and R37CA51497 (C.V.D.) and NHLBI contract N01-HV-28180 (C.V.D.).
REFERENCES
- 1.Alevizopoulos, K., B. Catarin, J. Vlach, and B. Amati. 1998. A novel function of adenovirus E1A is required to overcome growth arrest by the CDK2 inhibitor p27(Kip1). EMBO J. 17:5987-5997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Amellem, O., M. Loffler, and E. O. Pettersen. 1994. Regulation of cell proliferation under extreme and moderate hypoxia: the role of pyrimidine (deoxy)nucleotides. Br. J. Cancer 70:857-866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Amellem, O., J. A. Sandvik, T. Stokke, and E. O. Pettersen. 1998. The retinoblastoma protein-associated cell cycle arrest in S-phase under moderate hypoxia is disrupted in cells expressing HPV18 E7 oncoprotein. Br. J. Cancer 77:862-872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Andrews, N. C., and D. V. Faller. 1991. A rapid micropreparation technique for extraction of DNA-binding proteins from limiting numbers of mammalian cells. Nucleic Acids Res. 19:2499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Angus, S. P., L. J. Wheeler, S. A. Ranmal, X. Zhang, M. P. Markey, C. K. Mathews, and E. S. Knudsen. 2002. Retinoblastoma tumor suppressor targets dNTP metabolism to regulate DNA replication. J. Biol. Chem. 277:44376-44384. [DOI] [PubMed] [Google Scholar]
- 6.Arany, Z., L. E. Huang, R. Eckner, S. Bhattacharya, C. Jiang, M. A. Goldberg, H. F. Bunn, and D. M. Livingston. 1996. An essential role for p300/CBP in the cellular response to hypoxia. Proc. Natl. Acad. Sci. USA 93:12969-12973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aten, J. A., P. J. Bakker, J. Stap, G. A. Boschman, and C. H. Veenhof. 1992. DNA double labelling with IdUrd and CIdUrd for spatial and temporal analysis of cell proliferation and DNA replication. Histochem. J. 24:251-259. [DOI] [PubMed] [Google Scholar]
- 8.Bloom, J., and M. Pagano. 2003. Deregulated degradation of the cdk inhibitor p27 and malignant transformation. Semin. Cancer Biol. 13:41-47. [DOI] [PubMed] [Google Scholar]
- 9.Brischwein, K., M. Engelcke, H. J. Riedinger, and H. Probst. 1997. Role of ribonucleotide reductase and deoxynucleotide pools in the oxygen-dependent control of DNA replication in Ehrlich ascites cells. Eur. J. Biochem. 244:286-293. [DOI] [PubMed] [Google Scholar]
- 10.Carmeliet, P., Y. Dor, J. M. Herbert, D. Fukumura, K. Brusselmans, M. Dewerchin, M. Neeman, F. Bono, R. Abramovitch, P. Maxwell, C. J. Koch, P. Ratcliffe, L. Moons, R. K. Jain, D. Collen, E. Keshert, and E. Keshet. 1998. Role of HIF-1alpha in hypoxia-mediated apoptosis, cell proliferation and tumour angiogenesis. Nature 394:485-490. [DOI] [PubMed] [Google Scholar]
- 11.Cook, J. G., C. H. Park, T. W. Burke, G. Leone, J. DeGregori, A. Engel, and J. R. Nevins. 2002. Analysis of Cdc6 function in the assembly of mammalian prereplication complexes. Proc. Natl. Acad. Sci. USA 99:1347-1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Coverley, D., H. Laman, and R. A. Laskey. 2002. Distinct roles for cyclins E and A during DNA replication complex assembly and activation. Nat. Cell Biol. 4:523-528. [DOI] [PubMed] [Google Scholar]
- 13.Danet, G. H., Y. Pan, J. L. Luongo, D. A. Bonnet, and M. C. Simon. 2003. Expansion of human SCID-repopulating cells under hypoxic conditions. J. Clin. Investig. 112:126-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.DeGregori, J., T. Kowalik, and J. R. Nevins. 1995. Cellular targets for activation by the E2F1 transcription factor include DNA synthesis- and G1/S-regulatory genes. Mol. Cell Biol. 15:4215-4224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dimitrova, D. S., T. A. Prokhorova, J. J. Blow, I. T. Todorov, and D. M. Gilbert. 2002. Mammalian nuclei become licensed for DNA replication during late telophase. J. Cell Sci. 115:51-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ebert, B. L., and H. F. Bunn. 1998. Regulation of transcription by hypoxia requires a multiprotein complex that includes hypoxia-inducible factor 1, an adjacent transcription factor, and p300/CREB binding protein. Mol. Cell. Biol. 18:4089-4096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Feijoo, C., C. Hall-Jackson, R. Wu, D. Jenkins, J. Leitch, D. M. Gilbert, and C. Smythe. 2001. Activation of mammalian Chk1 during DNA replication arrest: a role for Chk1 in the intra-S phase checkpoint monitoring replication origin firing. J. Cell Biol. 154:913-923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Franza, B. R., Jr., K. Maruyama, J. I. Garrels, and H. E. Ruley. 1986. In vitro establishment is not a sufficient prerequisite for transformation by activated ras oncogenes. Cell 44:409-418. [DOI] [PubMed] [Google Scholar]
- 19.Frisch, S. M., and J. S. Mymryk. 2002. Adenovirus-5 E1A: paradox and paradigm. Nat. Rev. Mol. Cell Biol. 3:441-452. [DOI] [PubMed] [Google Scholar]
- 20.Gardner, L. B., Q. Li, M. S. Park, W. M. Flanagan, G. L. Semenza, and C. V. Dang. 2001. Hypoxia inhibits G1/S transition through regulation of p27 expression. J. Biol. Chem. 276:7919-7926. [DOI] [PubMed] [Google Scholar]
- 21.Gekeler, V., J. Epple, G. Kleymann, and H. Probst. 1993. Selective and synchronous activation of early-S-phase replicons of Ehrlich ascites cells. Mol. Cell. Biol. 13:5020-5033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gitig, D. M., and A. Koff. 2001. Cdk pathway: cyclin-dependent kinases and cyclin-dependent kinase inhibitors. Mol. Biotechnol. 19:179-188. [DOI] [PubMed] [Google Scholar]
- 23.Goda, N., H. E. Ryan, B. Khadivi, W. McNulty, R. C. Rickert, and R. S. Johnson. 2003. Hypoxia-inducible factor 1α is essential for cell cycle arrest during hypoxia. Mol. Cell. Biol. 23:359-369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Graeber, T. G., C. Osmanian, T. Jacks, D. E. Housman, C. J. Koch, S. W. Lowe, and A. J. Giaccia. 1996. Hypoxia-mediated selection of cells with diminished apoptotic potential in solid tumours. Nature 379:88-91. [DOI] [PubMed] [Google Scholar]
- 25.Graeber, T. G., J. F. Peterson, M. Tsai, K. Monica, A. J. Fornace, Jr., and A. J. Giaccia. 1994. Hypoxia induces accumulation of p53 protein, but activation of a G1-phase checkpoint by low-oxygen conditions is independent of p53 status. Mol. Cell. Biol. 14:6264-6277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Green, S. L., R. A. Freiberg, and A. J. Giaccia. 2001. p21(Cip1) and p27(Kip1) regulate cell cycle reentry after hypoxic stress but are not necessary for hypoxia-induced arrest. Mol. Cell. Biol. 21:1196-1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hammond, E. M., N. C. Denko, M. J. Dorie, R. T. Abraham, and A. J. Giaccia. 2002. Hypoxia links ATR and p53 through replication arrest. Mol. Cell. Biol. 22:1834-1843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hammond, E. M., M. J. Dorie, and A. J. Giaccia. 2003. ATR/ATM targets are phosphorylated by ATR in response to hypoxia and ATM in response to reoxygenation. J. Biol. Chem. 278:12207-12213. [DOI] [PubMed] [Google Scholar]
- 29.Hiebert, S. W., S. P. Chellappan, J. M. Horowitz, and J. R. Nevins. 1992. The interaction of RB with E2F coincides with an inhibition of the transcriptional activity of E2F. Genes Dev. 6:177-185. [DOI] [PubMed] [Google Scholar]
- 30.Hockel, M., K. Schlenger, B. Aral, M. Mitze, U. Schaffer, and P. Vaupel. 1996. Association between tumor hypoxia and malignant progression in advanced cancer of the uterine cervix. Cancer Res. 56:4509-4515. [PubMed] [Google Scholar]
- 31.Hung, M. C., G. N. Hortobagyi, and N. T. Ueno. 2000. Development of clinical trial of E1A gene therapy targeting HER-2/neu-overexpressing breast and ovarian cancer. Adv. Exp. Med. Biol. 465:171-180. [DOI] [PubMed] [Google Scholar]
- 32.Kaelin, W. G., Jr., D. C. Pallas, J. A. DeCaprio, F. J. Kaye, and D. M. Livingston. 1991. Identification of cellular proteins that can interact specifically with the T/E1A-binding region of the retinoblastoma gene product. Cell 64:521-532. [DOI] [PubMed] [Google Scholar]
- 33.Koumenis, C., R. Alarcon, E. Hammond, P. Sutphin, W. Hoffman, M. Murphy, J. Derr, Y. Taya, S. W. Lowe, M. Kastan, and A. Giaccia. 2001. Regulation of p53 by hypoxia: dissociation of transcriptional repression and apoptosis from p53-dependent transactivation. Mol. Cell. Biol. 21:1297-1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kowalik, T. F., J. DeGregori, J. K. Schwarz, and J. R. Nevins. 1995. E2F1 overexpression in quiescent fibroblasts leads to induction of cellular DNA synthesis and apoptosis. J. Virol. 69:2491-2500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Krtolica, A., N. A. Krucher, and J. W. Ludlow. 1998. Hypoxia-induced pRB hypophosphorylation results from downregulation of CDK and upregulation of PP1 activities. Oncogene 17:2295-2304. [DOI] [PubMed] [Google Scholar]
- 36.Kung, A. L., S. Wang, J. M. Klco, W. G. Kaelin, and D. M. Livingston. 2000. Suppression of tumor growth through disruption of hypoxia-inducible transcription. Nat. Med. 6:1335-1340. [DOI] [PubMed] [Google Scholar]
- 37.Loffler, M. 1989. The biosynthetic pathway of pyrimidine (deoxy)nucleotides: a sensor of oxygen tension necessary for maintaining cell proliferation? Exp. Cell Res. 182:673-680. [DOI] [PubMed] [Google Scholar]
- 38.Nahle, Z., J. Polakoff, R. V. Davuluri, M. E. McCurrach, M. D. Jacobson, M. Narita, M. Q. Zhang, Y. Lazebnik, D. Bar-Sagi, and S. W. Lowe. 2002. Direct coupling of the cell cycle and cell death machinery by E2F. Nat. Cell Biol. 4:859-864. [DOI] [PubMed] [Google Scholar]
- 39.Nevins, J. R. 1993. Disruption of cell-cycle control by viral oncoproteins. Biochem. Soc. Trans. 21:935-938. [DOI] [PubMed] [Google Scholar]
- 40.Ohtani, K., A. Tsujimoto, M. Ikeda, and M. Nakamura. 1998. Regulation of cell growth-dependent expression of mammalian CDC6 gene by the cell cycle transcription factor E2F. Oncogene 17:1777-1785. [DOI] [PubMed] [Google Scholar]
- 41.Perry, M. E., M. Commane, and G. R. Stark. 1992. Simian virus 40 large tumor antigen alone or two cooperating oncogenes convert REF52 cells to a state permissive for gene amplification. Proc. Natl. Acad. Sci. USA 89:8112-8116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Probst, H., H. Schiffer, V. Gekeler, H. Kienzle-Pfeilsticker, U. Stropp, K. E. Stotzer, and I. Frenzel-Stotzer. 1988. Oxygen dependent regulation of DNA synthesis and growth of Ehrlich ascites tumor cells in vitro and in vivo. Cancer Res. 48:2053-2060. [PubMed] [Google Scholar]
- 43.Probst, H., H. Schiffer, V. Gekeler, and K. Scheffler. 1989. Oxygen dependent regulation of mammalian ribonucleotide reductase in vivo and possible significance for replicon initiation. Biochem. Biophys. Res. Commun. 163:334-340. [DOI] [PubMed] [Google Scholar]
- 44.Reed, J. C., S. Haldar, C. M. Croce, and M. P. Cuddy. 1990. Complementation by BCL2 and C-HA-RAS oncogenes in malignant transformation of rat embryo fibroblasts. Mol. Cell. Biol. 10:4370-4374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Reynolds, T. Y., S. Rockwell, and P. M. Glazer. 1996. Genetic instability induced by the tumor microenvironment. Cancer Res. 56:5754-5757. [PubMed] [Google Scholar]
- 46.Riedinger, H. J., V. Gekeler, and H. Probst. 1992. Reversible shutdown of replicon initiation by transient hypoxia in Ehrlich ascites cells. Dependence of initiation on short-lived protein. Eur. J. Biochem. 210:389-398. [DOI] [PubMed] [Google Scholar]
- 47.Riedinger, H. J., M. van Betteraey, and H. Probst. 1999. Hypoxia blocks in vivo initiation of simian virus 40 replication at a stage preceding origin unwinding. J. Virol. 73:2243-2252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Saikumar, P., Z. Dong, J. M. Weinberg, and M. A. Venkatachalam. 1998. Mechanisms of cell death in hypoxia/reoxygenation injury. Oncogene 17:3341-3349. [DOI] [PubMed] [Google Scholar]
- 49.Samuelson, A. V., and S. W. Lowe. 1997. Selective induction of p53 and chemosensitivity in RB-deficient cells by E1A mutants unable to bind the RB-related proteins. Proc. Natl. Acad. Sci. USA 94:12094-12099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sang, N., J. Caro, and A. Giordano. 2002. Adenoviral E1A: everlasting tool, versatile applications, continuous contributions and new hypotheses. Front. Biosci. 7:d407-d413. [DOI] [PubMed] [Google Scholar]
- 51.Santore, M. T., D. S. McClintock, V. Y. Lee, G. R. Budinger, and N. S. Chandel. 2002. Anoxia-induced apoptosis occurs through a mitochondria-dependent pathway in lung epithelial cells. Am. J. Physiol. Lung Cell Mol. Physiol. 282:L727-L734. [DOI] [PubMed] [Google Scholar]
- 52.Schmaltz, C., P. H. Hardenbergh, A. Wells, and D. E. Fisher. 1998. Regulation of proliferation-survival decisions during tumor cell hypoxia. Mol. Cell. Biol. 18:2845-2854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sherr, C. J., and J. M. Roberts. 1999. CDK inhibitors: positive and negative regulators of G1-phase progression. Genes Dev. 13:1501-1512. [DOI] [PubMed] [Google Scholar]
- 54.Shimizu, S., Y. Eguchi, H. Kosaka, W. Kamiike, H. Matsuda, and Y. Tsujimoto. 1995. Prevention of hypoxia-induced cell death by Bcl-2 and Bcl-xL. Nature 374:811-813. [DOI] [PubMed] [Google Scholar]
- 55.Vaupel, P., K. Schlenger, C. Knoop, and M. Hockel. 1991. Oxygenation of human tumors: evaluation of tissue oxygen distribution in breast cancers by computerized O2 tension measurements. Cancer Res. 51:3316-3322. [PubMed] [Google Scholar]
- 56.Yu, J. L., J. W. Rak, B. L. Coomber, D. J. Hicklin, and R. S. Kerbel. 2002. Effect of p53 status on tumor response to antiangiogenic therapy. Science 295:1526-1528. [DOI] [PubMed] [Google Scholar]