Abstract
The brains of males and females differ anatomically and physiologically, including sex differences in neuron size or number, synapse morphology, and specific patterns of gene expression. Brain sex differences may underlie critical sex differences in physiology or behavior, including several aspects of reproduction, such as the timing of sexual maturation (earlier in females than males) and the ability to generate a preovulatory gonadotropin surge (in females only). The reproductive axis is controlled by afferent pathways that converge upon forebrain gonadotropin-releasing hormone (GnRH) neurons, but GnRH neurons are not sexually dimorphic. Although most reproductive sex differences probably reflect sex differences in the upstream circuits and factors that regulate GnRH secretion, the key sexually-dimorphic factors that influence reproductive status have remained poorly defined. The recently-identified neuropeptide kisspeptin, encoded by the Kiss1 gene, is an important regulator of GnRH secretion, and Kiss1 neurons in rodents are sexually dimorphic in specific hypothalamic populations, including the anteroventral periventricular nucleus—periventricular nucleus continuum (AVPV/PeN) and the arcuate nucleus (ARC). In the adult AVPV/PeN, Kiss1 neurons are more abundant in females than males, a sex difference which is regulated by estradiol signaling during critical periods of postnatal and pubertal development. In contrast, Kiss1 neurons in the ARC are not sexually differentiated in adult rodents, but in mice, the regulation of ARC Kiss1 cells by gonadal hormone-independent factors is sexually dimorphic during prepubertal development. These various sex differences in hypothalamic Kiss1 neurons may relate to known sex differences in reproductive physiology, such as puberty onset and positive feedback.
Keywords: kisspeptin, Kiss1, GPR54, Kiss1r, sexual differentiation, sex differences, development, puberty, hypothalamus, hormone, estrogen
In vertebrates, physiology and behavior, including aspects relating to reproduction and puberty, often vary between animals of the opposite sex. The neuroendocrine reproductive axis is governed by various hormonal and neural pathways that converge upon forebrain gonadotropin-releasing hormone (GnRH) neurons [1]. GnRH neurons direct the activation of the rest of the reproductive axis by stimulating the pituitary to synthesize and secrete gonadotropins [luteinizing hormone (LH) and follicle-stimulating hormone (FSH)] which regulate the gonads. GnRH neurons themselves do not appear to be sexually dimorphic, indicating that sex differences in the control of the reproductive axis likely reflect key sex differences in the afferent neural circuits and factors that regulate the GnRH system. However, many of the sexually-dimorphic factors that influence reproductive status have remained poorly defined. The newly-discovered kisspeptin system has recently been implicated as an important regulator of GnRH neurons, both in development and adulthood, and sex differences in the kisspeptin system may relate to sex differences in reproductive function. This article discusses the recent evidence that supports an important role of kisspeptin signaling in the control of the reproductive axis and how this relates to sexual differentiation of reproductive physiology. The roles of kisspeptin signaling in puberty and adulthood have already been extensively reviewed [2–4] and I will therefore focus primarily on the latest findings connecting sexual differentiation and the kisspeptin system, with a specific focus on rodent animal models.
Sexual Differentiation of Reproductive Physiology and Behavior
In mammals, including humans, males and females often display differences in a variety of physiological and behavioral traits, ranging from sex differences in learning and memory to complex sociosexual and parental behaviors to differential hormonal secretion patterns. Besides sex differences in normal physiology and behavior, sex differences also exist in a variety of human health disorders and diseases, including precocious puberty (more common in girls), depression (more common in women), and autism (more common in boys) [5–10]. It is thought that many of the sex differences in physiology and behavior reflect underlying sex differences in neural mechanisms in the brain. The brain, along with other parts of the central nervous system, possesses many anatomical and physiological sex differences (reviewed in [11–15]). These neural sex differences are present in numerous brain areas, including the amygdala, hippocampus, cortex, bed nucleus of the stria terminalis (BNST), lateral septum, and several nuclei within the hypothalamus [13,16–22]. The nature of sex differences in the brain are wide-ranging and can vary from region to region (and even within a region), depending on the specific trait. For example, neural sex differences may include differences in cell morphology, neuron size, neuron number, axonal fiber projections, synapse morphology, and cellular expression levels of specific genes or proteins. In addition, sex differences in the brain can favor either males over females (male-biased) or females over males (female-biased), depending on the particular trait. For example, in rats, the BNST and the medial preoptic nucleus each contain more neurons and comprise a larger regional volume in males than females [18,22,23]. In contrast, the anteroventral periventricular nucleus (AVPV) of the hypothalamus is not only larger and possesses more cells in females than in males, but the number of AVPV neurons expressing tyrosine hydroxylase (TH) enzyme is greater in females than males [24–27].
Similar to the brain, the neuroendocrine reproductive axis has several aspects that differ between the sexes. In rodents, these reproductive sex differences include earlier sexual maturation in females than males, the presence of neural circuitry that generates preovulatory hormone surges (i.e., positive feedback) in adult females but not males, and sexually differentiated neural circuits that govern gender-specific reproductive behaviors, such as certain female proceptive behaviors (e.g., hopping and darting) and receptive mating behavior (e.g., display of the receptive lordosis posture) [6,10,28–30]. In addition to these sex differences in normal reproductive physiology and behavior, there are several reproductive health disorders and diseases which occur with a differential frequency between males and females, such as idiopathic hypogonadotropic hypogonadism (more common in men), constitutional delayed puberty (more common in boys), and precocious puberty (more common in girls [5,6,31,32].
It is likely that many of the sex differences in reproductive physiology, behavior, and health disorders reflect underlying sex differences in the brain. While studies have identified numerous sex differences in brain structures and neural phenotypes on the one hand, and reproductive physiology and behavior on the other, in most cases the actual link between a specific neural sex difference and sex differences in particular physiology/behaviors have remained elusive. For example, in rodents (and other mammals), sex steroids, particularly estradiol (E2), can feedback during a specific time of the estrous cycle to induce an acute surge in GnRH and LH secretion, thereby triggering ovulation. This positive feedback event is sexually differentiated, occurring only in females but not males. In fact, unlike females, male rodents given exogenous E2 (or other sex steroid treatments) are incapable of generating an LH surge, indicating that the male brain lacks the functional neural circuitry or specific neural factors needed to produce an LH surge. However, while this fact has been known for many decades, the identity of the specific sexually-dimorphic neural population(s) that governs the LH surge has remained elusive.
Sex Differences in Kiss1 Neurons in the AVPV/PeN
As mentioned above, the E2-induced LH surge event (positive feedback) is sexually differentiated in rodents. GnRH neurons are the final common pathway by which the brain controls reproduction, including the LH surge, but GnRH neurons are not themselves sexually dimorphic, suggesting that other neuronal populations influence sex differences in reproductive physiology. Lesion and hormone implant studies in the 1980’s implicated the AVPV region as being a critical part of the LH surge generating mechanism in rodents, findings supported by the observation that the AVPV expresses estrogen receptors, including ERα, and projects to GnRH neurons (reviewed in [33]). Moreover, several aspects of the AVPV are sexually dimorphic, with females possessing more neurons overall than males, as well as greater numbers of neurons containing tyrosine hydroxylase (TH; i.e., dopaminergic cells) and GABA/glutamate (reviewed in [11,18]). Yet, the actual involvement of any specific AVPV population in the sexually-dimorphic LH surge has, until the recent discovery of the kisspeptin system, been equivocal.
The Link Between the Kiss1 System and Reproduction
The Kiss1 gene (KISS1 in humans) encodes kisspeptin, a neuropeptide of 52 or 54 amino acids, depending on the species [34]. In a wide range of mammals, including mice, rats, hamsters, sheep, horses, pigs, and primates, Kiss1 mRNA or kisspeptin protein has been detected in two discrete regions of the hypothalamus, the preoptic area [which in rodents includes the morphological continuum comprising the anteroventral periventricular nucleus and neighboring periventricular nucleus (AVPV/PeN)] and, more caudally, the arcuate nucleus (ARC; analogous to the primate infundibular nucleus) (reviewed in [2,3,35,36]). The Kiss1 gene is also expressed in several peripheral tissues, most notably, the placenta, ovary, testis, pituitary, pancreas, and adipose tissue, but at present, little is known regarding kisspeptin’s role outside the brain. Kisspeptin is a high-affinity ligand for the membrane receptor, GPR54, now renamed the kisspeptin receptor, or Kiss1r [37–39]. The Kiss1r gene is expressed in both peripheral tissues and the brain, most notably the hippocampus, habenula, hypothalamus, and preoptic/septal areas (including GnRH neurons) [40,41].
The Kiss1 system was first implicated in regulating reproduction in 2003, when several groups independently reported that humans and mice with mutations in the kisspeptin receptor exhibit prominent deficits in reproductive function and puberty onset [42,43]. These initial findings were soon echoed by other similar reports in Kiss1r knockout (KO) mice, as well as mice lacking a functional Kiss1 gene [44–49], suggesting that kisspeptin-Kiss1r signaling is critical for proper sexual maturation and fertility. Indeed, early experiments determined that exogenous treatment of rodents and other species, including humans, with kisspeptin robustly increases circulating LH and FSH levels (discussed in [3,50,51]). Subsequent studies from numerous species have now provided a wealth of information supporting the model that hypothalamic-derived kisspeptin directly activates GnRH neurons via Kiss1r to stimulate the reproductive axis (reviewed in [2,4,35,36,52]).
The Role of Kiss1 Neurons in Sexually-Dimorphic LH Secretion (Positive Feedback)
In adulthood, the secretion of GnRH is regulated by positive and negative feedback actions of gonadal sex steroids [i.e., testosterone (T) and estradiol (E2)], but GnRH neurons do not express estrogen receptor α (ERα) or the androgen receptor (AR), the receptors that mediate sex steroid feedback. Thus, other sex steroid-sensitive circuits upstream of GnRH neurons likely relay sex steroid feedback signals to GnRH cells. Recent evidence suggests that hypothalamic Kiss1 neurons are these upstream sex steroid-sensitive neurons. Specifically, it has been proposed that, in rodents, ARC Kiss1 neurons mediate negative feedback effects of sex steroids on reproductive status, whereas AVPV/PeN Kiss1 neurons mediate positive feedback effects of E2 on GnRH secretion, thereby triggering the preovulatory LH surge [3,36,50]. In regards to positive feedback, which is sexually dimorphic, the evidence for a role for AVPV/PeN Kiss1 neurons in governing the LH surge is summarized as follows:
Neuroanatomically, the AVPV/PeN region has been shown to mediate E2-induced positive feedback [17,33].
Kiss1 gene expression in the rodent AVPV/PeN is robustly upregulated by high levels of E2 [53–55], correlating with the occurrence of GnRH/LH surges.
In the absence of adulthood gonadal steroids, such as in gonadectomized animals, Kiss1 levels are decreased in the AVPV/PeN [53–55], correlating with an absence of GnRH/LH surges.
Almost all Kiss1 neurons in the AVPV/PeN express ERα [53,54,56], the receptor subtype that mediates positive feedback.
Fos is induced in Kiss1 neurons in the AVPV/PeN during the LH surge [44,54,56,57].
Kiss1 neurons in the AVPV/PeN of E2-treated female mice display circadian patterns of activation, in synchrony with the circadian timing of the LH surge [57].
Pharmacological or transgenic blockade of kisspeptin signaling prevents the preovulatory LH surge (positive feedback) from occurring [44,58,59], providing functional evidence for the role of kisspeptin signaling in the LH surge event.
Collectively, these findings indicate that kisspeptin signaling arising from neurons in the AVPV/PeN plays an important role in mediating positive feedback effects of estradiol in rodents. (In sheep, and perhaps other non-rodent species, positive feedback may be mediated by Kiss1 neurons in the ARC rather than the preoptic or AVPV/PeN regions [36]).
Sexual Differentiation of AVPV/PeN Kiss1 Neurons
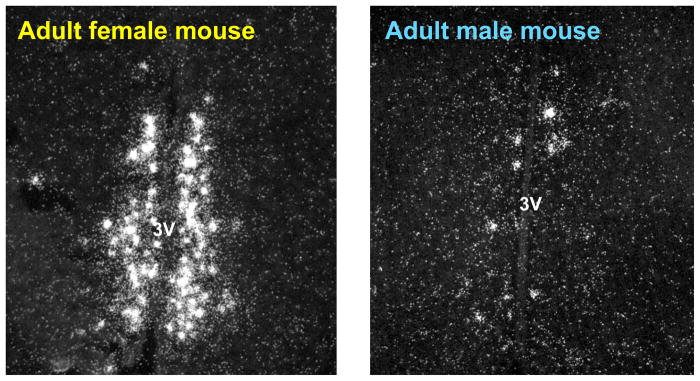
Given the critical role of AVPV/PeN Kiss1 neurons in governing the LH surge, we hypothesized that the sex difference in the LH surge reflects sexual differentiation of the Kiss1 system in the AVPV/PeN. We found that, like both TH and GABA/glutamate neurons, Kiss1 neurons in the rat AVPV/PeN are sexually differentiated, with adult females possessing greater Kiss1 expression in this region than males [55]. More specifically, the number of Kiss1 mRNA-expressing neurons in the AVPV/PeN of adult female rats was 15–25 times greater than in adult males, and the relative amount of Kiss1 mRNA per cell was also higher in this area in females than males [55]. Similar sex differences in kisspeptin protein levels in the AVPV/PeN, as determined by immunohistochemistry, as well as Kiss1 mRNA levels, were reported in intact mice (Figure 1) [60,61]. Thus, similar to the female-biased TH and GABA/glutamate populations in the AVPV/PeN [25,27,62], the number of Kiss1 neurons in the AVPV/PeN correlates with the ability or inability of an animal to generate an LH surge: adult females have high Kiss1 levels and can generate an LH surge, while adult males have little AVPV Kiss1 expression and cannot produce an LH surge, even with E2 treatment. However, in contrast to TH and GABA/glutamate neurons for which the functional role is still unclear, abundant evidence supports a critical involvement of kisspeptin in the LH surge event.
Figure 1.
Sexually dimorphic Kiss1 gene expression in the AVPV/PeN of adult male and female mice. Females have significantly more Kiss1 neurons and Kiss1 mRNA/neuron in the AVPV/PeN than do males, regardless of the adulthood sex steroid milieu. (3V = 3rd ventricle) [For pictures of similar AVPV/PeN sex differences at the protein level, refer to articles by Clarkson and Herbison, 2006 and Adachi et al, 2007.]
In adult rodents, sex steroids dramatically upregulate Kiss1 levels in the AVPV/PeN. To assess whether sex differences in AVPV/PeN Kiss1 neurons are attributable to sex differences in circulating levels of T or E2 in adulthood, we measured Kiss1 expression in adult gonadectomized male and female rats receiving identical sex steroid treatments (i.e., with or without E2 implants). Even when sex steroid levels were similar between the sexes, females had much higher Kiss1 expression in the AVPV/PeN than did males [55]; thus, adult females possess more Kiss1 cells in this region than males, regardless of the circulating adult sex steroid milieu. This finding has since been replicated for Kiss1 mRNA levels in mice, kisspeptin protein levels in mice, and both kisspeptin protein and Kiss1 mRNA levels in rats [61,63–65]. Given the importance of kisspeptin in promoting the LH surge, the sex difference in AVPV/PeN Kiss1 expression in adulthood likely accounts for the sex-specific ability of female rodents, but not males, to produce an LH surge. However, an important role of this AVPV/PeN Kiss1 sex difference in other sexually-dimorphic aspects, such pubertal maturation, cannot be excluded.
The AVPV contains a sexually differentiated population of TH-positive (i.e., dopaminergic) neurons, which, like the Kiss1 population, is greater in adult females than adult males [66,67]. Are the sexually dimorphic Kiss1 and TH cells in the AVPV/PeN the same neuronal population or separate sexually dimorphic systems located in the same brain region? We addressed this issue by performing double-label immunohistochemistry and double-label in situ hybridization assays. In adult female rats, the majority of AVPV/PeN Kiss1 neurons do not co-express TH mRNA, and the few Kiss1 neurons that are co-labeled express only low levels of TH mRNA[55]. Similar findings were obtained with kisspeptin and TH protein co-labeling: only a small number of kisspeptin-immunoreactive cells co-express TH-immunoreactivity in the AVPV/PeN of female rats [55]. Thus, in rats, despite some overlap in the anatomical distribution of these two neuronal populations, the sexually-dimorphic Kiss1 and TH populations in the AVPV/PeN appear to comprise two separate, sexually-differentiated populations with only a minor degree of overlap. Whether this is the case for other species remains to be determined. Unlike kisspeptin, the role of sexually dimorphic AVPV TH neurons is currently ill-defined.
Mechanisms Underlying Sex Differences in AVPV/PeN Kiss1 Neurons
Mechanisms Governing Sexual Differentiation of the Brain
There are several ways that sex differences in the brain can develop, including gonadal sex steroid-dependent mechanisms and sex chromosome gene-dependent mechanisms. To date, most sex differences in the brain and behavior appear to reflect sex differences in the actions of gonadal sex steroid secretion during key stages of development (the “organizational hypothesis”), whereas only a few sexually-dimorphic traits, such as some aggressive and parental behaviors, nociception, and ubiquitin protease mRNA expression in the neocortex have yet to be attributed to differences in sex chromosome gene expression between males and females [68–74]. The fundamental principle of the “organizational hypothesis”, first proposed more than 50 years ago in a landmark study by Phoenix et al. [75], is that the brain is initially bipotential and develops to be male-like or female-like under the direction of gonadal sex steroids during the perinatal “critical period” of development, the duration and timing of which is species-specific (e.g., in rats, it encompasses the first 10 days of postnatal life). During the critical period, the acute secretion of gonadal T in neonatal males, but not neonatal females, induces sexually dimorphic brain regions to differentiate to be masculinized (and defeminized). This effect of perinatal T on guiding the brain’s sexual differentiation occurs via activation of either AR, or more commonly, ER pathways (after aromatization of T to E2 in neural target tissues) [17,76,77]. In contrast to males, females do not normally secrete significant levels of circulating gonadal sex steroids during the perinatal critical period; the absence of high circulating sex steroids in perinatal females results in their brains differentiating to be feminized (and demasculinized) [13,22,78,79]. Supporting this model, experimental manipulation of the postnatal steroid milieu alters the development of sexually dimorphic traits. Specifically, acute sex steroid treatment (T or E2) to newborn female rodents causes the development of a male-like brain, whereas castration of newborn male rodents (thereby removing postnatal T secretion) induces the development of female-like brains. For example, postnatal T or E2 treatment to newborn female rodents masculinizes the development of the AVPV TH population such that these females have a male-like TH phenotype in adulthood; in contrast, neonatal castration of newborn males results in a female pattern of TH expression in adulthood [25,27,67].
Like the brain, sexually-dimorphic reproductive physiology and behavior have also been demonstrated to be sexually differentiated by perinatal sex steroid signaling. For example, the ability of adult female rodents, but not adult males, to display an E2-induced preovulatory LH surge (i.e., “positive feedback”) is sexually differentiated [29,80,81] by differential exposure to gonadal sex steroids during early postnatal life. That is, in developing females, the absence of significant circulating postnatal gonadal steroids allows the neural mechanisms necessary for generating the LH surge to develop, whereas this is prevented in males by postnatal exposure to sex steroids. In support of this model, female rats or mice treated with a single injection of T or E2 during the postnatal critical period (to mimic male’s postnatal T secretion) fail to develop the LH surge-generating circuitry whereas newborn male rats that are castrated at the time of birth (to prevent gonadal T secretion) can generate an E2-induced LH surge in adulthood, similar to normal adult females [29,63,80,81].
Hormonal Induction of Sex Differences in AVPV/PeN Kiss1 Neurons
What mechanisms underlie the development of sex difference in Kiss1 neurons in the AVPV/PeN? Like TH expression in the AVPV, as well as most other sex differences in the brain, the sex difference in Kiss1 neurons is organized early in postnatal development by the actions of gonadal sex steroids. That is, newborn female rats treated with a single injection of T (to mimic the acute T secretion in newborn males) possess very few Kiss1 neurons in the AVPV/PeN as adults, similar to normal adult males [55]. Furthermore, newborn male rats castrated on the day of birth (to remove circulating gonadal T) have high Kiss1 levels in the AVPV/PeN in adulthood [63], indicating that the AVPV/PeN Kiss1 system is sexually-differentiated under the influence of postnatal gonadal sex steroids. The developmental effects of postnatal sex steroids on the sexual differentiation of Kiss1 neurons in the AVPV/PeN are likely mediated specifically by ER rather than AR pathways, based on the following evidence. Kisspeptin immunoreactivity in the AVPV/PeN of adult females is reduced in transgenic mice that were perinatally exposed to E2 due to knockout of the alpha-fetoprotein, which normally prevents E2 from acting in the female brain during early development [65]. Importantly, these alpha-fetoprotein KO females were incapable of mounting an LH surge in response to sex steroid treatment in adulthood, linking the reduced kisspeptin levels to impaired positive feedback [65]. Additionally, female rats treated with a single E2 injection during the postnatal critical period display male-like levels (i.e., low levels) of Kiss1 mRNA and kisspeptin-immunorecativity in the AVPV/PeN in adulthood (and cannot display an LH surge) [63]. Lastly, female newborn rats exposed to E2 or estrogen agonists display masculinized levels of kisspeptin-immunoreactivity in the AVPV/PeN in adulthood [82,83]. Thus, masculinization of the AVPV Kiss1 system is likely mediated via aromatization of T to E2 during the postnatal critical period (as is also the case for the TH system).
In addition to the important role of postnatal E2 signaling in organizing the developmental trajectory of Kiss1 neurons in the AVPV/PeN, recent evidence suggests that E2 may also act sometime between the postnatal period and adulthood to further regulate proper Kiss1 development in the AVPV/PeN. This conjecture is based on recent findings that female mice lacking E2 either permanently (i.e., aromatase KO mice or hpg mice) or during just the peripubertal period (gonadectomized from PND 22–30) express very low levels of kisspeptin-immunoreactivity in the AVPV/PeN as adults [84], even when given supplemental E2 in adulthood [64,85]. These findings suggest that E2 may be necessary after the postnatal critical period sometime during peripubertal life for promoting normal female-like kisspeptin levels in the AVPV/PeN. This conjecture is supported indirectly by recent observations that E2 can act during puberty to regulate the development of certain neuronal populations[86–90]. Whether or not E2 during puberty influences AVPV/PeN Kiss1 development in other species besides mice requires more investigation.
While the development of the sexually dimorphic Kiss1 system is dependent on the postnatal sex steroid milieu, it is unclear exactly how postnatal sex steroids, primarily E2, direct the sexual differentiation of this system. That is, how does E2 organize the sexual differentiation of the Kiss1 population within the AVPV/PeN? Several hormone-dependent mechanisms, such as differential cell migration, neurogenesis, programmed cell death (apoptosis), and epigenetic modifications have been implicated in the sexual differentiation and development of different neural populations [18,19,91]. For example, in the developing rat hippocampus, gonadal sex steroids, which are normally higher in postnatal males than females, increase the number of new cells (i.e., neurogenesis), leading to more neurons present in this region in males than females [20]. Conversely, many sexually dimorphic populations in the brain arise via apoptotic mechanisms. In fact, sex differences in the overall size and total cell number of both the AVPV and BNST are induced by apoptosis during early development [18,21,22,24,92]. Most of these apoptotic-induced sex differences are dependent on the pro-apoptotic gene, Bax, which encodes Bax (Bcl-2–associated X protein), an inducer of an intracellular signaling cascade that culminates in cell death. In the developing rat AVPV, postnatal males have higher Bax expression than females, which correlates with the presence of fewer AVPV cells present in adult males than females [22]. Moreover, adult sex difference in the total number of AVPV neurons is eliminated in Bax KO mice [24], indicating that this sexually dimorphic trait is sexually differentiated via Bax-dependent apoptotic mechanisms. Despite these findings, the sexual differentiation of TH neurons in the AVPV does not occur via Bax-dependent mechanisms, because Bax KO mice do not have altered sexual differentiation of AVPV TH neurons [24]. Thus, different sexually dimorphic traits appear to be organized in development via a variety of mechanisms. The possibility that the sex difference in the AVPV Kiss1 system is caused by E2-induced changes in either neurogenesis, apoptosis, or other key mechanisms has not yet been tested.
Sex differences in ARC Kiss1 Neurons are Species-Specific and Age-Dependent
Sex Differences Are Absent in ARC Kiss1 Neurons of Adult Rodents
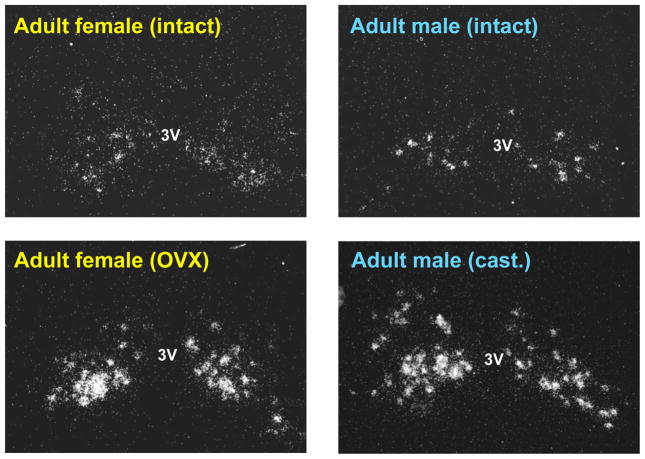
Whereas sex differences in the AVPV/PeN are well-documented, the ARC has received much less attention in terms of sexual differentiation. Unlike the AVPV/PeN, BNST, and mPOA, the rodent ARC does not exhibit noticeable sex differences in overall nucleus size or total number of neurons. However, several more specific parameters of ARC cells are sexually dimorphic. Both the morphology of astroglia and the number of synapses in the ARC differs between male and female rodents, as does growth hormone-releasing hormone gene expression and axonal projections of neurokinin B/dynorphin neurons [93–96]. Despite this, the ARC of adult rodents displays no sex differences in either the total number of Kiss1 neurons or the amount of Kiss1 mRNA per cell [55,61,63]. Sex steroids in adulthood are known to dramatically inhibit Kiss1 gene expression in the ARC. However, the lack of sex difference in ARC Kiss1 expression is not influenced by circulating sex steroids: adult male and female rats and mice display similar high levels of Kiss1 expression or kisspeptin immunoreactivity in the ARC following gonadectomy and similar reduced Kiss1 expression after sex steroid replacement (Figure 2) [55,61,63]. Given that Kiss1 cells in the ARC have been proposed to mediate negative feedback regulation of GnRH secretion (a non-sexually dimorphic process) in both adult males and females (reviewed in [2,36,50]), it is not unexpected that sex differences are absent in ARC Kiss1 neurons, at least in adult rodents.
Figure 2.
Kiss1 expression in the ARC is not sexually dimorphic in adult rodents. Adult male and female mice exhibit similar numbers of Kiss1 neurons in the ARC in both intact and gonadectomized conditions. (3V = 3rd ventricle; OVX = ovariectomized; cast= castrated)
Recent examination of Kiss1 expression in the ARC of adult male and female sheep has yielded a different picture than in rodents. In particular, intact adult female sheep possess greater numbers of Kiss1 neurons in the ARC than do intact males, identifying an important sex difference in the ovine Kiss1 system [97]. Direct comparison of ARC Kiss1 levels in adult ewes and rams treated with equivalent sex steroid levels has yet to be reported. Interestingly, prenatal androgen treatment, which is known to masculinize a number of sexually dimorphic traits in female sheep (such as dynorphin and neurokinin B expression in the ARC), does not reverse the sex difference in ARC kisspeptin cells [97], suggesting that the Kiss1 system of the ovine ARC may not be sexually differentiated by prenatal sex steroids, at least at the specific prenatal times that were tested. The basis for the species difference in sex differences in the ARC Kiss1 system is not entirely known, but may relate to species differences in the neural regions mediating sex steroid positive feedback. In rodents, positive feedback of E2 is mediated by the AVPV/PeN, correlating with a sex difference in Kiss1 in this region, whereas in sheep, the ARC has been implicated in mediating the preovulatory LH surge [36,98], correlating with a Kiss1 sex difference in this region. Whether or not the ARC Kiss1 system of other species, such as primates, displays adult sex differences is not currently known.
Age-dependent Sex differences in ARC Kiss1 Neurons of Mice
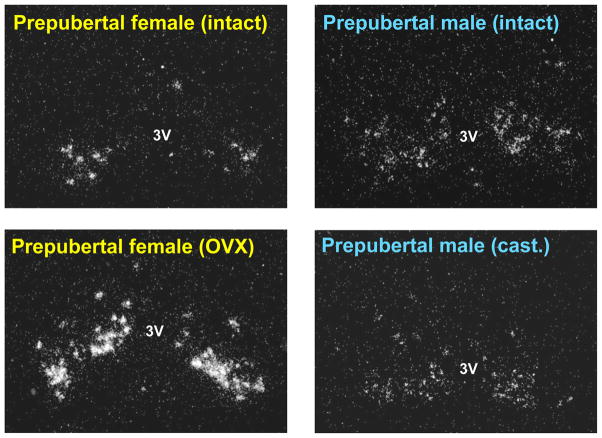
Interestingly, although Kiss1 neurons in the ARC are not sexually dimorphic in adult rodents, this may not be the case in younger animals. Kauffman et al. [61] recently identified a sex difference in ARC Kiss1 neurons in prepubertal mice that is evident when gonadal hormone feedback is removed (Figure 3). Specifically, intact prepubertal male and female mice (postnatal day [PND] 16–18) express similar low levels of Kiss1 mRNA in the ARC. However, whereas gonadectomized PND 16–18 females display a significant increase in ARC Kiss1 levels (reflecting loss of gonadal hormone negative feedback on Kiss1 expression), gonadectomized males of the same age do not have elevated Kiss1 levels in the ARC (Figure 3) [61]. The increased Kiss1 expression in gonadectomized PND 16–18 females suggests that there is little or no gonadal hormone-independent suppression acting on ARC Kiss1 neurons in prepubertal females at this age. Thus, the prepubertal female reproductive axis at this age appears to be kept quiescent predominantly by gonadal hormone negative feedback. In contrast, in prepubertal males, ARC Kiss1 expression did not increase by 2 or 4 days following gonadectomy, and ARC Kiss1 expression in gonadectomized PND 16–18 male mice was similar to that of intact males of the same age [61]. This lack of increased Kiss1 expression after gonadectomy in prepubertal males indicates that some gonadal hormone-independent mechanism(s) suppresses ARC Kiss1 neurons in prepubertal male mice but not females, at least at the ages tested. Adult mice of both sexes exhibit robust increases in ARC Kiss1 expression 4 days following gonadectomy [61], indicating that the sex difference in the gonadal hormone-independent suppression of ARC Kiss1 neurons is present only during prepubertal development. Importantly, prepubertal LH levels followed the same sexually-dimorphic pattern as ARC Kiss1 expression, with elevated LH in gonadectomized prepubertal females but not in gonadectomized prepubertal males [61]. Thus, the regulation of reproductive status during prepubertal development is sexually dimorphic, with PND 16–18 males, but not females, exhibiting non-gonadal suppression of ARC Kiss1 and LH levels. This Kiss1 sex difference may relate to known sex differences in pubertal maturation in rodents, in which males mature later than females. Specifically, later puberty onset in males may reflect sex differences in the gating of peripubertal ARC Kiss1 neuron activation, such that there is greater (or longer-lasting) suppression of Kiss1 circuitry in prepubertal males than prepubertal females.
Figure 3.
Intact prepubertal male and female mice exhibit similar numbers of Kiss1 neurons in the ARC. However, gonadectomized prepubertal females have higher ARC Kiss1 expression than similarly-aged gonadectomized males. The absence of elevated Kiss1 expression in gonadectomized prepubertal males suggests that some non-gonadal factor(s) acts to suppress the ARC Kiss1 system in males at this developmental stage. (3V = 3rd ventricle; OVX = ovariectomized; cast= castrated)
Whereas gonadectomized prepubertal male mice do not demonstrate elevated LH secretion or increased ARC Kiss1 levels after 2 or 4 days (on PND 16–18), they do exhibit increased ARC Kiss1 and LH levels later in adulthood (PND 45) [61]. Importantly, these increases in ARC Kiss1 expression evident in adulthood occurred in the absence of any developmental changes in gonadal hormones (since the males were castrated on PND 14). This indicates that sometime between PND 18 and PND 45 there is a key developmental change in non-gonadal regulation of male reproductive circuits, including ARC Kiss1 neurons. However, the identity of the gonadal hormone-independent factor(s) is currently unknown, as is the specific age in development when this non-gonadal suppression dissipates. Like male mice, in primates, there is gonadal hormone-independent regulation of the developing reproductive axis, and puberty onset in primates appears to include removal of inhibitory input onto reproductive circuits, independent of changes in gonadal hormones [99,100]. However, the precise non-gonadal inhibitory factor(s) involved remains unclear.
Conclusions
Recent findings from several rodent species have provided exciting information about the role of kisspeptin neurons in the regulation of the reproductive axis, including sexually dimorphic processes such as the preovulatory LH surge. In addition, recent evidence has emerged demonstrating sex differences in various Kiss1 populations in both rodents and sheep, perhaps identifying the specific neuroanatomical basis underlying various sexually dimorphic reproductive physiological processes. We now appreciate that Kiss1 neurons in the rodent AVPV/PeN are sexually differentiated under the direction of sex steroid signaling, primarily E2, during early postnatal development (and again later during pubertal development), and that these sexually-dimorphic AVPV/PeN Kiss1 neurons comprise a key element of the neural mechanism underlying the sexually-dimorphic LH surge that occurs in adult females but not adult males. Moreover, while not sexually dimorphic in adulthood, the Kiss1 population in the rodent ARC displays a sex difference in its regulation during prepubertal development, such that Kiss1 neurons of prepubertal male mice experience greater non-gonadal suppression than do prepubertal female mice. This intriguing sex difference in the prepubertal ARC Kiss1 system, or more precisely, the “upstream” systems that regulate prepubertal ARC Kiss1 neurons, may contribute to differences in puberty onset between males and females, although this remains to be tested.
Acknowledgments
Dr. Kauffman’s research is supported by NICHD grants R00 HD056157 and U54 HD012303.
References
- 1.Herbison AE. Physiology of the Gonadotropin-Releasing Hormone Neuronal Network. In: Neill JD, editor. Knobil and Neill’s Physiology of Reproduction. 3. Elsevier; 2006. pp. 1415–1482. [Google Scholar]
- 2.Kauffman AS. Coming of age in the Kisspeptin Era: Sex differences, development, and puberty. Mol Cell Endocrinol. 2010 doi: 10.1016/j.mce.2010.01.017. (In Press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Oakley AE, Clifton DK, Steiner RA. Kisspeptin Signaling in the Brain. Endocr Rev. 2009;30:713–743. doi: 10.1210/er.2009-0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Roa J, Aguilar E, Dieguez C, Pinilla L, Tena-Sempere M. New frontiers in kisspeptin/GPR54 physiology as fundamental gatekeepers of reproductive function. Front Neuroendocrinol. 2008;29:48–69. doi: 10.1016/j.yfrne.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 5.Cesario SK, Hughes LA. Precocious puberty: a comprehensive review of literature. J Obstet Gynecol Neonatal Nurs. 2007;36:263–274. doi: 10.1111/j.1552-6909.2007.00145.x. [DOI] [PubMed] [Google Scholar]
- 6.Fechner PY. Gender differences in puberty. J Adolesc Health. 2002;30:44–48. doi: 10.1016/s1054-139x(02)00357-9. [DOI] [PubMed] [Google Scholar]
- 7.Weissman MM, Klerman GL. Sex differences and the epidemiology of depression. Arch Gen Psychiatry. 1977;34:98–111. doi: 10.1001/archpsyc.1977.01770130100011. [DOI] [PubMed] [Google Scholar]
- 8.Weissman MM, Leaf PJ, Holzer CE, 3rd, Myers JK, Tischler GL. The epidemiology of depression. An update on sex differences in rates. J Affect Disord. 1984;7:179–188. doi: 10.1016/0165-0327(84)90039-9. [DOI] [PubMed] [Google Scholar]
- 9.Wing L. Sex ratios in early childhood autism and related conditions. Psychiatry Res. 1981;5:129–137. doi: 10.1016/0165-1781(81)90043-3. [DOI] [PubMed] [Google Scholar]
- 10.Grumbach MM. The neuroendocrinology of human puberty revisited. Horm Res. 2002;57 (Suppl 2):2–14. doi: 10.1159/000058094. [DOI] [PubMed] [Google Scholar]
- 11.Semaan SJ, Kauffman AS. Sexual differentiation and development of forebrain reproductive circuits. Current Opinion in Neurobiology. 2010 doi: 10.1016/j.conb.2010.04.004. (In press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schwarz JM, McCarthy MM. Cellular mechanisms of estradiol-mediated masculinization of the brain. J Steroid Biochem Mol Biol. 2008;109:300–306. doi: 10.1016/j.jsbmb.2008.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cooke B, Hegstrom CD, Villeneuve LS, Breedlove SM. Sexual differentiation of the vertebrate brain: principles and mechanisms. Front Neuroendocrinol. 1998;19:323–362. doi: 10.1006/frne.1998.0171. [DOI] [PubMed] [Google Scholar]
- 14.Breedlove SM. Sexual differentiation of the human nervous system. Annu Rev Psychol. 1994;45:389–418. doi: 10.1146/annurev.ps.45.020194.002133. [DOI] [PubMed] [Google Scholar]
- 15.Morris JA, Jordan CL, Breedlove SM. Sexual differentiation of the vertebrate nervous system. Nat Neurosci. 2004;7:1034–1039. doi: 10.1038/nn1325. [DOI] [PubMed] [Google Scholar]
- 16.Shah NM, Pisapia DJ, Maniatis S, Mendelsohn MM, Nemes A, Axel R. Visualizing sexual dimorphism in the brain. Neuron. 2004;43:313–319. doi: 10.1016/j.neuron.2004.07.008. [DOI] [PubMed] [Google Scholar]
- 17.Simerly RB. Wired for reproduction: organization and development of sexually dimorphic circuits in the mammalian forebrain. Annu Rev Neurosci. 2002;25:507–536. doi: 10.1146/annurev.neuro.25.112701.142745. [DOI] [PubMed] [Google Scholar]
- 18.Forger NG. Control of cell number in the sexually dimorphic brain and spinal cord. J Neuroendocrinol. 2009;21:393–399. doi: 10.1111/j.1365-2826.2009.01825.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McCarthy MM, Schwarz JM, Wright CL, Dean SL. Mechanisms mediating oestradiol modulation of the developing brain. J Neuroendocrinol. 2008;20:777–783. doi: 10.1111/j.1365-2826.2008.01723.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang JM, Konkle AT, Zup SL, McCarthy MM. Impact of sex and hormones on new cells in the developing rat hippocampus: a novel source of sex dimorphism? Eur J Neurosci. 2008;27:791–800. doi: 10.1111/j.1460-9568.2008.06073.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tsukahara S, Inami K, Maekawa F, Kakeyama M, Yokoyama T, Yuji M, Kitagawa H, Kannan Y, Yamanouchi K. Postnatal apoptosis, development, and sex difference in the lateral septum of rats. J Comp Neurol. 2004;475:177–187. doi: 10.1002/cne.20184. [DOI] [PubMed] [Google Scholar]
- 22.Tsukahara S. Sex differences and the roles of sex steroids in apoptosis of sexually dimorphic nuclei of the preoptic area in postnatal rats. J Neuroendocrinol. 2009;21:370–376. doi: 10.1111/j.1365-2826.2009.01855.x. [DOI] [PubMed] [Google Scholar]
- 23.Murray EK, Hien A, de Vries GJ, Forger NG. Epigenetic control of sexual differentiation of the bed nucleus of the stria terminalis. Endocrinology. 2009;150:4241–4247. doi: 10.1210/en.2009-0458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Forger NG, Rosen GJ, Waters EM, Jacob D, Simerly RB, de Vries GJ. Deletion of Bax eliminates sex differences in the mouse forebrain. Proc Natl Acad Sci U S A. 2004;101:13666–13671. doi: 10.1073/pnas.0404644101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Simerly RB, Zee MC, Pendleton JW, Lubahn DB, Korach KS. Estrogen receptor-dependent sexual differentiation of dopaminergic neurons in the preoptic region of the mouse. Proc Natl Acad Sci U S A. 1997;94:14077–14082. doi: 10.1073/pnas.94.25.14077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Simerly RB, Swanson LW. The distribution of neurotransmitter-specific cells and fibers in the anteroventral periventricular nucleus: implications for the control of gonadotropin secretion in the rat. Brain Res. 1987;400:11–34. doi: 10.1016/0006-8993(87)90649-4. [DOI] [PubMed] [Google Scholar]
- 27.Simerly RB, Swanson LW, Handa RJ, Gorski RA. Influence of perinatal androgen on the sexually dimorphic distribution of tyrosine hydroxylase-immunoreactive cells and fibers in the anteroventral periventricular nucleus of the rat. Neuroendocrinology. 1985;40:501–510. doi: 10.1159/000124122. [DOI] [PubMed] [Google Scholar]
- 28.Corbier P, Roffi J, Rhoda J. Female sexual behavior in male rats: effect of hour of castration at birth. Physiol Behav. 1983;30:613–616. doi: 10.1016/0031-9384(83)90229-9. [DOI] [PubMed] [Google Scholar]
- 29.Corbier P. Sexual differentiation of positive feedback: effect of hour of castration at birth on estradiol-induced luteinizing hormone secretion in immature male rats. Endocrinology. 1985;116:142–147. doi: 10.1210/endo-116-1-142. [DOI] [PubMed] [Google Scholar]
- 30.Karsch FJ, Foster DL. Sexual differentiation of the mechanism controlling the preovulatory discharge of luteinizing hormone in sheep. Endocrinology. 1975;97:373–379. doi: 10.1210/endo-97-2-373. [DOI] [PubMed] [Google Scholar]
- 31.Fechner A, Fong S, McGovern P. A review of Kallmann syndrome: genetics, pathophysiology, and clinical management. Obstet Gynecol Surv. 2008;63:189–194. doi: 10.1097/OGX.0b013e3181641278. [DOI] [PubMed] [Google Scholar]
- 32.Bianco SD, Kaiser UB. The genetic and molecular basis of idiopathic hypogonadotropic hypogonadism. Nat Rev Endocrinol. 2009;5:569–576. doi: 10.1038/nrendo.2009.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Herbison AE. Estrogen positive feedback to gonadotropin-releasing hormone (GnRH) neurons in the rodent: the case for the rostral periventricular area of the third ventricle (RP3V) Brain Res Rev. 2008;57:277–287. doi: 10.1016/j.brainresrev.2007.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee YR, Tsunekawa K, Moon MJ, Um HN, Hwang JI, Osugi T, Otaki N, Sunakawa Y, Kim K, Vaudry H, Kwon HB, Seong JY, Tsutsui K. Molecular evolution of multiple forms of kisspeptins and GPR54 receptors in vertebrates. Endocrinology. 2009;150:2837–2846. doi: 10.1210/en.2008-1679. [DOI] [PubMed] [Google Scholar]
- 35.Colledge WH. Transgenic mouse models to study Gpr54/kisspeptin physiology. Peptides. 2009;30:34–41. doi: 10.1016/j.peptides.2008.05.006. [DOI] [PubMed] [Google Scholar]
- 36.Smith JT. Sex steroid control of hypothalamic Kiss1 expression in sheep and rodents: Comparative aspects. Peptides. 2009;30:94–102. doi: 10.1016/j.peptides.2008.08.013. [DOI] [PubMed] [Google Scholar]
- 37.Kotani M, Detheux M, Vandenbogaerde A, Communi D, Vanderwinden JM, Le Poul E, Brezillon S, Tyldesley R, Suarez-Huerta N, Vandeput F, Blanpain C, Schiffmann SN, Vassart G, Parmentier M. The metastasis suppressor gene KiSS-1 encodes kisspeptins, the natural ligands of the orphan G protein-coupled receptor GPR54. J Biol Chem. 2001;276:34631–34636. doi: 10.1074/jbc.M104847200. [DOI] [PubMed] [Google Scholar]
- 38.Muir AI, Chamberlain L, Elshourbagy NA, Michalovich D, Moore DJ, Calamari A, Szekeres PG, Sarau HM, Chambers JK, Murdock P, Steplewski K, Shabon U, Miller JE, Middleton SE, Darker JG, Larminie CG, Wilson S, Bergsma DJ, Emson P, Faull R, Philpott KL, Harrison DC. AXOR12, a novel human G protein-coupled receptor, activated by the peptide KiSS-1. J Biol Chem. 2001;276:28969–28975. doi: 10.1074/jbc.M102743200. [DOI] [PubMed] [Google Scholar]
- 39.Ohtaki T, Shintani Y, Honda S, Matsumoto H, Hori A, Kanehashi K, Terao Y, Kumano S, Takatsu Y, Masuda Y, Ishibashi Y, Watanabe T, Asada M, Yamada T, Suenaga M, Kitada C, Usuki S, Kurokawa T, Onda H, Nishimura O, Fujino M. Metastasis suppressor gene KiSS-1 encodes peptide ligand of a G-protein-coupled receptor. Nature. 2001;411:613–617. doi: 10.1038/35079135. [DOI] [PubMed] [Google Scholar]
- 40.Herbison AE, de Tassigny X, Doran J, Colledge WH. Distribution and postnatal development of Gpr54 gene expression in mouse brain and gonadotropin-releasing hormone neurons. Endocrinology. 2010;151:312–321. doi: 10.1210/en.2009-0552. [DOI] [PubMed] [Google Scholar]
- 41.Irwig MS, Fraley GS, Smith JT, Acohido BV, Popa SM, Cunningham MJ, Gottsch ML, Clifton DK, Steiner RA. Kisspeptin activation of gonadotropin releasing hormone neurons and regulation of KiSS-1 mRNA in the male rat. Neuroendocrinology. 2004;80:264–272. doi: 10.1159/000083140. [DOI] [PubMed] [Google Scholar]
- 42.de Roux N, Genin E, Carel JC, Matsuda F, Chaussain JL, Milgrom E. Hypogonadotropic hypogonadism due to loss of function of the KiSS1-derived peptide receptor GPR54. Proc Natl Acad Sci U S A. 2003;100:10972–10976. doi: 10.1073/pnas.1834399100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Seminara SB, Messager S, Chatzidaki EE, Thresher RR, Acierno JS, Jr, Shagoury JK, Bo-Abbas Y, Kuohung W, Schwinof KM, Hendrick AG, Zahn D, Dixon J, Kaiser UB, Slaugenhaupt SA, Gusella JF, O’Rahilly S, Carlton MB, Crowley WF, Jr, Aparicio SA, Colledge WH. The GPR54 gene as a regulator of puberty. N Engl J Med. 2003;349:1614–1627. doi: 10.1056/NEJMoa035322. [DOI] [PubMed] [Google Scholar]
- 44.Clarkson J, d’Anglemont de Tassigny X, Moreno AS, Colledge WH, Herbison AE. Kisspeptin-GPR54 signaling is essential for preovulatory gonadotropin-releasing hormone neuron activation and the luteinizing hormone surge. J Neurosci. 2008;28:8691–8697. doi: 10.1523/JNEUROSCI.1775-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.d’Anglemont de Tassigny X, Fagg LA, Dixon JP, Day K, Leitch HG, Hendrick AG, Zahn D, Franceschini I, Caraty A, Carlton MB, Aparicio SA, Colledge WH. Hypogonadotropic hypogonadism in mice lacking a functional Kiss1 gene. Proc Natl Acad Sci U S A. 2007;104:10714–10719. doi: 10.1073/pnas.0704114104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kauffman AS, Park JH, McPhie-Lalmansingh AA, Gottsch ML, Bodo C, Hohmann JG, Pavlova MN, Rohde AD, Clifton DK, Steiner RA, Rissman EF. The kisspeptin receptor GPR54 is required for sexual differentiation of the brain and behavior. J Neurosci. 2007;27:8826–8835. doi: 10.1523/JNEUROSCI.2099-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lapatto R, Pallais JC, Zhang D, Chan YM, Mahan A, Cerrato F, Le WW, Hoffman GE, Seminara SB. Kiss1−/− mice exhibit more variable hypogonadism than Gpr54−/− mice. Endocrinology. 2007;148:4927–4936. doi: 10.1210/en.2007-0078. [DOI] [PubMed] [Google Scholar]
- 48.Messager S, Chatzidaki EE, Ma D, Hendrick AG, Zahn D, Dixon J, Thresher RR, Malinge I, Lomet D, Carlton MB, Colledge WH, Caraty A, Aparicio SA. Kisspeptin directly stimulates gonadotropin-releasing hormone release via G protein-coupled receptor 54. Proc Natl Acad Sci U S A. 2005;102:1761–1766. doi: 10.1073/pnas.0409330102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chan YM, Broder-Fingert S, Wong KM, Seminara SB. Kisspeptin/Gpr54-independent gonadotrophin-releasing hormone activity in Kiss1 and Gpr54 mutant mice. J Neuroendocrinol. 2009;21:1015–1023. doi: 10.1111/j.1365-2826.2009.01926.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kauffman AS, Clifton DK, Steiner RA. Emerging ideas about kisspeptin- GPR54 signaling in the neuroendocrine regulation of reproduction. Trends Neurosci. 2007;30:504–511. doi: 10.1016/j.tins.2007.08.001. [DOI] [PubMed] [Google Scholar]
- 51.Tena-Sempere M. GPR54 and kisspeptin in reproduction. Hum Reprod Update. 2006;12:631–639. doi: 10.1093/humupd/dml023. [DOI] [PubMed] [Google Scholar]
- 52.Plant TM, Ramaswamy S. Kisspeptin and the regulation of the hypothalamic-pituitary-gonadal axis in the rhesus monkey (Macaca mulatta) Peptides. 2009;30:67–75. doi: 10.1016/j.peptides.2008.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Smith JT, Cunningham MJ, Rissman EF, Clifton DK, Steiner RA. Regulation of Kiss1 gene expression in the brain of the female mouse. Endocrinology. 2005;146:3686–3692. doi: 10.1210/en.2005-0488. [DOI] [PubMed] [Google Scholar]
- 54.Smith JT, Popa SM, Clifton DK, Hoffman GE, Steiner RA. Kiss1 neurons in the forebrain as central processors for generating the preovulatory luteinizing hormone surge. J Neurosci. 2006;26:6687–6694. doi: 10.1523/JNEUROSCI.1618-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kauffman AS, Gottsch ML, Roa J, Byquist AC, Crown A, Clifton DK, Hoffman GE, Steiner RA, Tena-Sempere M. Sexual differentiation of Kiss1 gene expression in the brain of the rat. Endocrinology. 2007;148:1774–1783. doi: 10.1210/en.2006-1540. [DOI] [PubMed] [Google Scholar]
- 56.Adachi S, Yamada S, Takatsu Y, Matsui H, Kinoshita M, Takase K, Sugiura H, Ohtaki T, Matsumoto H, Uenoyama Y, Tsukamura H, Inoue K, Maeda K. Involvement of anteroventral periventricular metastin/kisspeptin neurons in estrogen positive feedback action on luteinizing hormone release in female rats. J Reprod Dev. 2007;53:367–378. doi: 10.1262/jrd.18146. [DOI] [PubMed] [Google Scholar]
- 57.Robertson JL, Clifton DK, de la Iglesia HO, Steiner RA, Kauffman AS. Circadian Regulation of Kiss1 Neurons: Implications for Timing the Preovulatory GnRH/LH Surge. Endocrinology. 2009;150:3664–3671. doi: 10.1210/en.2009-0247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kinoshita M, Tsukamura H, Adachi S, Matsui H, Uenoyama Y, Iwata K, Yamada S, Inoue K, Ohtaki T, Matsumoto H, Maeda K. Involvement of central metastin in the regulation of preovulatory luteinizing hormone surge and estrous cyclicity in female rats. Endocrinology. 2005;146:4431–4436. doi: 10.1210/en.2005-0195. [DOI] [PubMed] [Google Scholar]
- 59.Pineda R, Garcia-Galiano D, Roseweir A, Romero M, Sanchez-Garrido MA, Ruiz-Pino F, Morgan K, Pinilla L, Millar RP, Tena-Sempere M. Critical Roles of Kisspeptins in Female Puberty and Preovulatory Gonadotropin Surges as Revealed by a Novel Antagonist. Endocrinology. 2010 doi: 10.1210/en.2009-0803. (In Press) [DOI] [PubMed] [Google Scholar]
- 60.Clarkson J, Herbison AE. Postnatal development of kisspeptin neurons in mouse hypothalamus; sexual dimorphism and projections to gonadotropin-releasing hormone neurons. Endocrinology. 2006;147:5817–5825. doi: 10.1210/en.2006-0787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kauffman AS, Navarro VM, Kim J, Clifton DK, Steiner RA. Sex Differences in the Regulation of Kiss1/NKB Neurons in Juvenile Mice: Implications for the Timing of Puberty. Am J Physiol: Endocrinol and Metab. 2009;297:1212–1221. doi: 10.1152/ajpendo.00461.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ottem EN, Godwin JG, Krishnan S, Petersen SL. Dual-phenotype GABA/glutamate neurons in adult preoptic area: sexual dimorphism and function. J Neurosci. 2004;24:8097–8105. doi: 10.1523/JNEUROSCI.2267-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Homma T, Sakakibara M, Yamada S, Kinoshita M, Iwata K, Tomikawa J, Kanazawa T, Matsui H, Takatsu Y, Ohtaki T, Matsumoto H, Uenoyama Y, Maeda KI, Tsukamura H. Significance of Neonatal Testicular Sex Steroids to Defeminize Anteroventral Periventricular Kisspeptin Neurons and the GnRH/LH Surge System in Male Rats. Biol Reprod. 2009 doi: 10.1095/biolreprod.109.078311. [DOI] [PubMed] [Google Scholar]
- 64.Bakker J, Pierman S, Gonzalez-Martinez D. Effects of aromatase mutation (ArKO) on the sexual differentiation of kisspeptin neuronal numbers and their activation by same versus opposite sex urinary pheromones. Horm Behav. 2010;57:390–395. doi: 10.1016/j.yhbeh.2009.11.005. [DOI] [PubMed] [Google Scholar]
- 65.Gonzalez-Martinez D, De Mees C, Douhard Q, Szpirer C, Bakker J. Absence of gonadotropin-releasing hormone 1 and Kiss1 activation in alpha-fetoprotein knockout mice: prenatal estrogens defeminize the potential to show preovulatory luteinizing hormone surges. Endocrinology. 2008;149:2333–2340. doi: 10.1210/en.2007-1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Simerly RB, Swanson LW, Gorski RA. The distribution of monoaminergic cells and fibers in a periventricular preoptic nucleus involved in the control of gonadotropin release: immunohistochemical evidence for a dopaminergic sexual dimorphism. Brain Res. 1985;330:55–64. doi: 10.1016/0006-8993(85)90007-1. [DOI] [PubMed] [Google Scholar]
- 67.Simerly RB. Hormonal control of the development and regulation of tyrosine hydroxylase expression within a sexually dimorphic population of dopaminergic cells in the hypothalamus. Brain Res Mol Brain Res. 1989;6:297–310. doi: 10.1016/0169-328x(89)90075-2. [DOI] [PubMed] [Google Scholar]
- 68.De Vries GJ, Rissman EF, Simerly RB, Yang LY, Scordalakes EM, Auger CJ, Swain A, Lovell-Badge R, Burgoyne PS, Arnold AP. A model system for study of sex chromosome effects on sexually dimorphic neural and behavioral traits. J Neurosci. 2002;22:9005–9014. doi: 10.1523/JNEUROSCI.22-20-09005.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Arnold AP, Rissman EF, De Vries GJ. Two perspectives on the origin of sex differences in the brain. Ann N Y Acad Sci. 2003;1007:176–188. doi: 10.1196/annals.1286.018. [DOI] [PubMed] [Google Scholar]
- 70.Gatewood JD, Wills A, Shetty S, Xu J, Arnold AP, Burgoyne PS, Rissman EF. Sex chromosome complement and gonadal sex influence aggressive and parental behaviors in mice. J Neurosci. 2006;26:2335–2342. doi: 10.1523/JNEUROSCI.3743-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Arnold AP, Xu J, Grisham W, Chen X, Kim YH, Itoh Y. Minireview: Sex chromosomes and brain sexual differentiation. Endocrinology. 2004;145:1057–1062. doi: 10.1210/en.2003-1491. [DOI] [PubMed] [Google Scholar]
- 72.Xu J, Taya S, Kaibuchi K, Arnold AP. Sexually dimorphic expression of Usp9x is related to sex chromosome complement in adult mouse brain. Eur J Neurosci. 2005;21:3017–3022. doi: 10.1111/j.1460-9568.2005.04134.x. [DOI] [PubMed] [Google Scholar]
- 73.Chen X, Watkins R, Delot E, Reliene R, Schiestl RH, Burgoyne PS, Arnold AP. Sex difference in neural tube defects in p53-null mice is caused by differences in the complement of X not Y genes. Dev Neurobiol. 2008;68:265–273. doi: 10.1002/dneu.20581. [DOI] [PubMed] [Google Scholar]
- 74.Gioiosa L, Chen X, Watkins R, Klanfer N, Bryant CD, Evans CJ, Arnold AP. Sex chromosome complement affects nociception in tests of acute and chronic exposure to morphine in mice. Horm Behav. 2008;53:124–130. doi: 10.1016/j.yhbeh.2007.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Phoenix CH, Goy RW, Gerall AA, Young WC. Organizing action of prenatally adminstered testosterone propionate on the tissues mediating mating behavior in the female guinea pig. Endocrinology. 1959;65:369–382. doi: 10.1210/endo-65-3-369. [DOI] [PubMed] [Google Scholar]
- 76.Simerly RB. Organization and regulation of sexually dimorphic neuroendocrine pathways. Behav Brain Res. 1998;92:195–203. doi: 10.1016/s0166-4328(97)00191-5. [DOI] [PubMed] [Google Scholar]
- 77.McCarthy MM, Wright CL, Schwarz JM. New tricks by an old dogma: mechanisms of the Organizational/Activational Hypothesis of steroid-mediated sexual differentiation of brain and behavior. Horm Behav. 2009;55:655–665. doi: 10.1016/j.yhbeh.2009.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bakker J, De Mees C, Douhard Q, Balthazart J, Gabant P, Szpirer J, Szpirer C. Alpha-fetoprotein protects the developing female mouse brain from masculinization and defeminization by estrogens. Nat Neurosci. 2006;9:220–226. doi: 10.1038/nn1624. [DOI] [PubMed] [Google Scholar]
- 79.Bakker J. Sexual differentiation of the neuroendocrine mechanisms regulating mate recognition in mammals. J Neuroendocrinol. 2003;15:615–621. doi: 10.1046/j.1365-2826.2003.01036.x. [DOI] [PubMed] [Google Scholar]
- 80.Handa RJ, Corbier P, Shryne JE, Schoonmaker JN, Gorski RA. Differential effects of the perinatal steroid environment on three sexually dimorphic parameters of the rat brain. Biol Reprod. 1985;32:855–864. doi: 10.1095/biolreprod32.4.855. [DOI] [PubMed] [Google Scholar]
- 81.Handa RJ, Gorski RA. Alterations in the onset of ovulatory failure and gonadotropin secretion following steroid administration to lightly androgenized female rats. Biol Reprod. 1985;32:248–256. doi: 10.1095/biolreprod32.2.248. [DOI] [PubMed] [Google Scholar]
- 82.Bateman HL, Patisaul HB. Disrupted female reproductive physiology following neonatal exposure to phytoestrogens or estrogen specific ligands is associated with decreased GnRH activation and kisspeptin fiber density in the hypothalamus. Neurotoxicology. 2008;29:988–997. doi: 10.1016/j.neuro.2008.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Patisaul HB, Todd KL, Mickens JA, Adewale HB. Impact of neonatal exposure to the ERalpha agonist PPT, bisphenol-A or phytoestrogens on hypothalamic kisspeptin fiber density in male and female rats. Neurotoxicology. 2009;30:350–357. doi: 10.1016/j.neuro.2009.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Clarkson J, Boon WC, Simpson ER, Herbison AE. Postnatal development of an estradiol-kisspeptin positive feedback mechanism implicated in puberty onset. Endocrinology. 2009;150:3214–3220. doi: 10.1210/en.2008-1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Gill J, Wang O, Majzoub J, Kaiser U. Altered feedback response to estradiol in GnRH-deficient hpg mice emphasizes differential regulation of AVPV and ARC kisspeptin neurons. The Annual Meeting of the Endocrine Society; Washington D.C. 2009. pp. 2–268. [Google Scholar]
- 86.Ahmed EI, Zehr JL, Schulz KM, Lorenz BH, DonCarlos LL, Sisk CL. Pubertal hormones modulate the addition of new cells to sexually dimorphic brain regions. Nat Neurosci. 2008;11:995–997. doi: 10.1038/nn.2178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Schulz KM, Molenda-Figueira HA, Sisk CL. Back to the future: The organizational-activational hypothesis adapted to puberty and adolescence. Horm Behav. 2009;55:597–604. doi: 10.1016/j.yhbeh.2009.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Schulz KM, Richardson HN, Zehr JL, Osetek AJ, Menard TA, Sisk CL. Gonadal hormones masculinize and defeminize reproductive behaviors during puberty in the male Syrian hamster. Horm Behav. 2004;45:242–249. doi: 10.1016/j.yhbeh.2003.12.007. [DOI] [PubMed] [Google Scholar]
- 89.Schulz KM, Sisk CL. Pubertal hormones, the adolescent brain, and the maturation of social behaviors: Lessons from the Syrian hamster. Mol Cell Endocrinol. 2006;254–255:120–126. doi: 10.1016/j.mce.2006.04.025. [DOI] [PubMed] [Google Scholar]
- 90.Sisk CL, Zehr JL. Pubertal hormones organize the adolescent brain and behavior. Front Neuroendocrinol. 2005;26:163–174. doi: 10.1016/j.yfrne.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 91.McCarthy MM, Auger AP, Bale TL, De Vries GJ, Dunn GA, Forger NG, Murray EK, Nugent BM, Schwarz JM, Wilson ME. The epigenetics of sex differences in the brain. J Neurosci. 2009;29:12815–12823. doi: 10.1523/JNEUROSCI.3331-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Gotsiridze T, Kang N, Jacob D, Forger NG. Development of sex differences in the principal nucleus of the bed nucleus of the stria terminalis of mice: role of Bax-dependent cell death. Dev Neurobiol. 2007;67:355–362. doi: 10.1002/dneu.20353. [DOI] [PubMed] [Google Scholar]
- 93.Ciofi P, Leroy D, Tramu G. Sexual dimorphism in the organization of the rat hypothalamic infundibular area. Neuroscience. 2006;141:1731–1745. doi: 10.1016/j.neuroscience.2006.05.041. [DOI] [PubMed] [Google Scholar]
- 94.Mong JA, Kurzweil RL, Davis AM, Rocca MS, McCarthy MM. Evidence for sexual differentiation of glia in rat brain. Horm Behav. 1996;30:553–562. doi: 10.1006/hbeh.1996.0058. [DOI] [PubMed] [Google Scholar]
- 95.Mong JA, McCarthy MM. Ontogeny of sexually dimorphic astrocytes in the neonatal rat arcuate. Brain Res Dev Brain Res. 2002;139:151–158. doi: 10.1016/s0165-3806(02)00541-2. [DOI] [PubMed] [Google Scholar]
- 96.Nurhidayat, Tsukamoto Y, Sasaki F. Role of the gonads in sex differentiation of growth hormone-releasing hormone and somatostatin neurons in the mouse hypothalamus during postnatal development. Brain Res. 2001;890:154–161. doi: 10.1016/s0006-8993(00)03159-0. [DOI] [PubMed] [Google Scholar]
- 97.Cheng G, Coolen LM, Padmanabhan V, Goodman RL, Lehman MN. The kisspeptin/neurokinin B/dynorphin (KNDy) cell population of the arcuate nucleus: sex differences and effects of prenatal testosterone in the sheep. Endocrinology. 2010;151:301–311. doi: 10.1210/en.2009-0541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Foster DL, Jackson LM, Padmanabhan V. Programming of GnRH feedback controls timing puberty and adult reproductive activity. Mol Cell Endocrinol. 2006;254–255:109–119. doi: 10.1016/j.mce.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 99.Plant TM. The male monkey as a model for the study of the neurobiology of puberty onset in man. Mol Cell Endocrinol. 2006;254–255:97–102. doi: 10.1016/j.mce.2006.04.022. [DOI] [PubMed] [Google Scholar]
- 100.Plant TM, Witchel SM. Puberty in non-human primates and humans. In: Neill JD, editor. Knobil and Neill’s Physiology of Reproduction. Elsevier; 2006. pp. 2177–2230. [Google Scholar]