Abstract
Objective
In the absence of immunodeficiency, only microchimerism (<0.1%) has been achieved in human fetal recipients or nonhuman primates following in utero hematopoietic cell transplantation (IUHCT). We hypothesized that enhanced long-term engraftment might be more reliably achieved in microchimeric systems if higher levels of chimerism existed during development of adaptive immunity. To evaluate this hypothesis, we stimulated the donor cells with vascular endothelial growth factor (VEGF) and stem cell factor (SCF) prior to IUHCT in a chimerism-resistant murine strain combination.
Methods
Donor Balb/c marrow was cultured in media with or without VEGF and SCF supplementation for 12 hours prior to IUHCT into B6 fetuses at 14 days postcoitum (dpc). Donor cell phenotype, homing, and chimerism were assessed at short and long-term time points and transplanted animals received skin allografts at 8 weeks.
Results
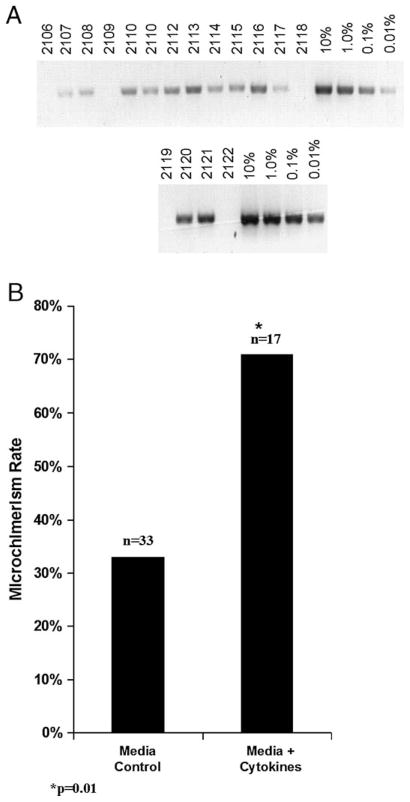
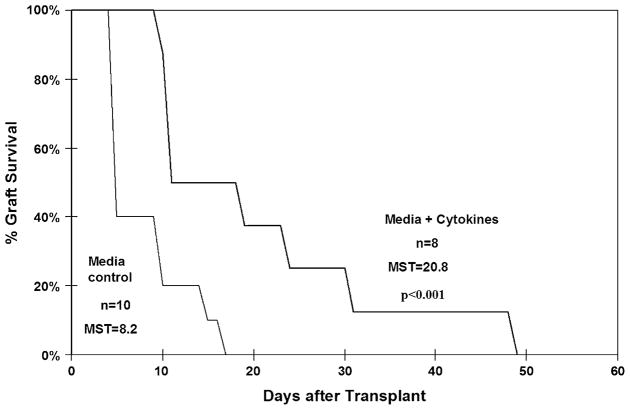
In pretreated allogeneic recipients, early chimerism rates were more than double that of controls (71% vs 33%, p = 0.01). These differences were associated with higher numbers of pretransplant donor cell colony-forming cells without change in donor cell homing. Despite prolonged skin allograft survival for pretreated recipients compared with controls (mean survival = 20.8 vs 8.2 days, p < 0.001), long-term engraftment was unchanged.
Conclusions
These findings demonstrate that higher levels of early chimerism in recipients of cytokine-stimulated marrow result in improved short-term chimerism and tolerance. Future studies are needed to confirm the existence of a “threshold” level of chimerism necessary to sustain long-term engraftment.
In utero hematopoietic cell transplantation (IUHCT) is a promising approach for treatment of a variety of genetic disorders without the need for immunosuppression [1–6]. IUHCT has been successfully applied clinically in the treatment of fetuses with prenatally diagnosed X-linked severe combined immunodeficiency disease [5,7,8], but has not been successful for treatment of other congenital diseases. In the absence of immunosuppression, either no engraftment or only microchimerism (<0.1%) has been achieved in human fetal recipients or nonhuman primate models of IUHCT [3,9–13]. These levels are too low for correction of most diseases and have not been demonstrated to reliably induce tolerance. While recent success in the swine model is encouraging, a better characterization of swine immunology and hematopoietic ontogeny is needed to reconcile the results of these experiments with clinical and nonhuman primate studies [14,15]. Lastly, protocols designed to enhance chimerism, such as postnatal boosting after irradiation or donor lymphocyte infusion [16,17], require the presence of tolerance and have not been successful in non-human primates [9,10].
Similar observations have been made in “chimerism-resistant” murine strain combinations, where even long-term microchimerism has been difficult to achieve with clinically relevant doses of marrow. We have previously shown that durable donor-specific tolerance across major histocompatibility complex (MHC) barriers can be achieved by the mechanism of central thymic deletion when adequate levels of chimerism are present [16–19]. Therefore, we hypothesized that tolerance and long-term engraftment might be more reliably achieved in microchimeric systems by enhancing the levels of prenatal chimerism during development of adaptive immunity. One clinically relevant way to enhance chimerism is by ex vivo stimulation of the donor cells with cytokines that impart a competitive advantage for the homing and engraftment of the transplanted cells.
In this study, we stimulated the donor cells with vascular endothelial growth factor (VEGF) and stem cell factor (SCF) prior to IUHCT. Short-term culture with VEGF and SCF were chosen because of their beneficial and synergistic effects on engraftment. Both VEGF and SCF have been shown to play critical roles in development of early hematopoietic progenitors from putative hemangioblasts and to expand the repopulating pool of hematopoietic progenitors [20–29]. Additionally SCF has well-described positive effects on affinity of various adhesion molecules and chemokine receptors (CD29, CD44, CD49d, CXCR4) critically involved in the homing and retention of hematopoietic progenitors on stroma layers in vitro within the endosteal stem cell niche following transplantation [30–33]. Lastly, short-term [33,34] rather than long-term [35] exposure to SCF has been shown to result in improved repopulating potential of cultured hematopoietic progenitors.
Materials and methods
Mice
Breeding stock for inbred strains of mice, B6 (H2b, CD45.2) and B6Pep3b (H2b, CD45.1) were purchased from Jackson Laboratories (Bar Harbor, ME, USA) and bred in our colony. Balb/c (H2d, CD45.2) mice were purchased from the Charles River Laboratories (Wilmington, MA, USA). Animals were mated and females were checked for introital plugging daily. The day of plugging was defined as gestational day 0 for time dating. All animals were housed in the Laboratory Animal Facilities of the Children’s Hospital of Philadelphia and the University of Wisconsin. All experimental protocols were reviewed and approved by the Institutional Animal Care and Use Committees at the Children’s Hospital of Philadelphia and the University of Wisconsin, and followed guidelines set forth in the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Donor bone marrow harvest
Donor bone marrow was harvested from 4- to 6-month-old mice by flushing the tibias and femurs with phosphate-buffered saline (PBS) using a 26-gauge needle. A single-cell suspension was made by three gentle passes through the needle. Cell suspensions were then filtered through a 70-μm nylon mesh and layered over Ficoll (Histopaque 1077; Sigma-Aldrich, St. Louis, MO, USA). After centrifugation at 600g for 15 minutes, the light density mononuclear cell (LDMC) layer was removed and washed twice with sterile PBS. Cells were counted and >95% viability was ensured by trypan blue exclusion.
Culture of donor bone marrow
Cytokine-stimulated or control donor cells were incubated for 12 hours at 37°C in serum-free α-minimum essential medium (GibcoBRL, Gaithersburg, MD, USA) supplemented with 1% Nutridoma (Boehringer Manheim, Indianapolis, IN, USA) with or without 0.5 μg/mL recombinant mouse VEGF and SCF (R&D Systems, Minneapolis, MN, USA). Cells were then washed twice in PBS and reconstituted into a single-cell suspension.
Clonogenic hematopoietic progenitor assay
Cultures were dispersed into single-cell suspensions by passage through a 21-gauge needle and washed twice in PBS. Viable cells were quantified and plated at a concentration of 5 × 103 cells/mL in 35-mm culture dishes containing serum-free methylcellulose-based medium (M3434; StemCell Technologies) containing SCF (50 ng/mL), erythropoietin (3 U/mL), interleukin-3 (IL-3; 10 ng/mL), and IL-6 (10 ng/mL). Cultures were maintained in triplicate in a humidified chamber at 37°C and 5% CO2. Cultures were scored for burst-forming unit erythroid (BFU-E), colony-forming unit-granulocyte/macrophage (CFU-GM), colony-forming unit granulocyte/erythroid/macrophage/megakaryocyte (CFU-GEMM) and total colony-forming cells (CFC) at 10 to 14 days.
In utero transplantation
Recipient mice were injected on day 14 of gestation. Using isofluorane general anesthesia and sterile technique, a midline laparotomy was made and the uterine horns were delivered from the wound. Each fetus was transplanted by intraperitoneal injection under direct visualization through the intact uterine wall using a 100-μm beveled glass micropipette. Following return of the uterus to the maternal peritoneal cavity, abdominal closure was achieved using two layers of absorbable 5-0 suture.
Assessment of hematopoietic chimerism
Hematopoietic chimerism in recipients of congenic marrow was quantified by dual-color flow cytometry. Blood, liver, or bone marrow from the recipients was individually harvested and dispersed into single-cell suspensions in heparinized PBS. LDMCs were then extracted by Ficoll gradient of the single-cell suspension. Using directly conjugated anti-CD45 phycoerythrin (specific for either CD45 isoform) and anti-CD45.1 FITC (B6Pep3b) mAb (Pharmingen, San Diego, CA, USA), LDMCs were counted by two-color flow cytometry (FACS Calibur; Becton Dickinson, Mansfield, MA, USA). Dead cells were excluded by propidium iodide. Percent donor cell was defined as:
Chimerism was assessed in recipients of allogeneic marrow at 3 weeks of age from a retro-orbital blood sample or at 6 months of age from homogenates of whole hematopoietic organs (liver, spleen, thymus, and bone marrow). Using previously described polymerase chain reaction (PCR) primer sequences specific for donor H-2 loci, a semi-quantitative estimate of chimerism was made [36,37].
Skin grafting
Skin from the ventral trunk of adult Balb/c female mice was cleaned of fatty tissue and cut into 1-cm squares. The B6-recipient graft bed was prepared on the lateral flank by excision of the skin without disturbing the underlying the panniculus carnosus. The donor skin graft was then positioned in the recipient bed, covered with Vaseline gauze and secured in place with a circumferential bandage. After 5 days, bandages were removed and grafts were then inspected daily for the first 3 weeks and weekly thereafter. Grafts were considered rejected when no viable skin remained. Viable skin grafts demonstrated good hair growth and pliability
Statistical analysis
Analysis of chimerism levels and cell phenotype was performed by a two-tailed Student’s t-test for two samples assuming unequal variances. Comparison of chimerism frequency was by the Chi-square statistic. Kaplan-Meier graft survival curves were compared using the log-rank test.
Results
Pretreatment of marrow with VEGF and SCF leads to higher levels of early chimerism in a chimerism-resistant allogeneic strain combination
We measured chimerism in recipients of prenatally transplanted allogeneic marrow using a PCR detection technique that permits semi-quantitative measurement of microchimerism in this system in the range of 0.01% to 10% [36,37]. Figure 1A illustrates the PCR analysis of retro-orbital blood samples in B6 recipients of control or cytokine-stimulated allogeneic Balb/c marrow at 3 weeks of age. Overall, survival to weaning was 74% (17 of 23). As shown, chimerism levels were low but were easily detected in the range of 0.01% to 0.1%. These levels were confirmed to be outside the range of reliable flow cytometric quantification (data not shown). Figure 1B summarizes the prevalence of chimerism among recipients of control or cytokine-stimulated allogeneic marrow. As shown, the levels of microchimerism were in the detectable range more than twice as often in the peripheral blood of 3-week-old recipients of cytokine-stimulated marrow when compared with recipients of control marrow (71% vs 33%, p = 0.01).
Figure 1.
Comparison of peripheral blood microchimerism rates between recipients of control or cytokine-stimulated allogeneic donor marrow. (A) Microchimerism was determined by a polymerase chain reaction (PCR) technique using primer sequences specific for donor H-2 loci, which has a linear sensitivity in the range of 0.01% to 10% chimerism. Sample images are shown for PCR analysis of peripheral blood in 3-week-old allogeneic B6 recipients of control or cytokine stimulated Balb/c marrow. A linear standard of donor Balb/c marrow diluted with host B6 marrow is also shown (B). The microchimerism rates shown reflect the prevalence of microchimerism at any level for either group. Significance values represent analysis by Chi-square statistic.
Prolonged allograft survival in recipients of cytokine-stimulated allogeneic marrow
The impact of cytokine culture on donor allo-specific tolerance was assessed in a separate group of animals. The survival of skin grafts transplanted to 8-week recipients of either control or cytokine-stimulated marrow is illustrated in Figure 2. As shown, recipients of cytokine-stimulated marrow demonstrated significantly prolonged mean survival time when compared with the control group (20.8 days, range 10–49 vs 8.2 days; range 5–17, p < 0.001). Furthermore, the grafts appeared healthier in recipients of cytokine-stimulated marrow prior to undergoing a delayed rejection (data not shown).
Figure 2.
Skin allograft survival. Kaplan-Meier survival curves of skin allografts transplanted to recipients of either control or pretreated allogeneic marrow. Mean survival time (MST) for grafts in each group is shown. As shown MST for allografts applied to recipients of cytokine-stimulated marrow were significantly longer. Significance values represent analysis by the log-rank test.
Multiorgan analysis of chimerism following graft rejection is summarized in Table 1. As shown, long-term chimerism was poor in recipients of both control and cytokine-stimulated marrow (14% vs 0% respectively, p = NS). Long-term thymic microchimerism could be detected more frequently in mice that received cytokine-stimulated marrow when compared with controls (60% vs 29%). This difference may represent the persistence of a subset of T cells or antigen-presenting cells of donor origin within the host thymus; however, this difference was not statistically significant.
Table 1.
Frequency of long-term multi-organ microchimerism in recipients of pretreated allogeneic marrow
| Frequency of microchimerism (%)
|
|||||
|---|---|---|---|---|---|
| Liver | Thymus | Spleen | Marrow | Blood | |
| Media control (n = 7) | 14 | 29 | 14 | 14 | 0 |
| Media+cytokines (n = 5) | 0 | 60 | 20 | 0 | 0 |
At 6 months of age, prenatal recipients of cytokine-stimulated or control marrow were sacrificed and the liver, thymus spleen, marrow, and blood harvested for analysis of chimerism by polymerase chain reaction. Frequency of detectable microchimerism for each tissue type is listed in the table as a percentage of each transplant group. Frequencies between the two groups were compared using Chi-square statistic. Although thymic chimerism was more common in recipients of cytokine-stimulated marrow, the difference was not statistically significant.
Cytokine exposure expands pretransplant CFC frequency but has no impact on the homing of prenatally transplanted marrow to the fetal liver
The enhanced early chimerism and allograft survival observed following prenatal transplantation of cytokine-stimulated marrow suggests the existence of an early competitive advantage for the treated cells. A competitive advantage may result from: 1) expansion of pretransplant progenitor or facilitator cell content of the donor marrow; 2) accelerated proliferation of donor progenitor cells, and 3) enhanced homing of the donor marrow.
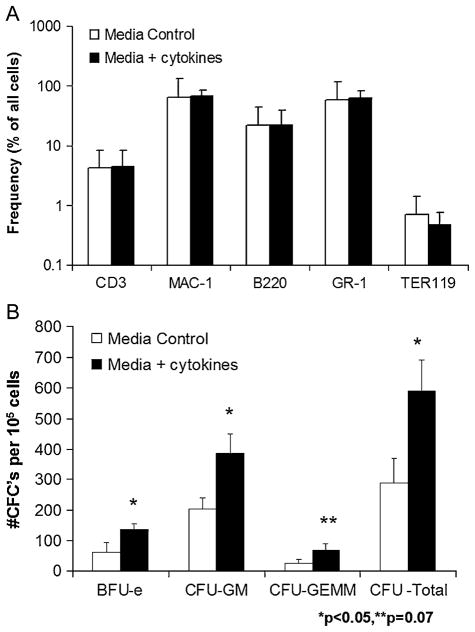
To assess phenotypic alterations that may have occurred as a result of cytokine exposure, we analyzed both groups of donor marrow prior to transplantation. As shown in Figure 3A, the lineage profile of control and cytokine-stimulated marrow was essentially identical. Comparable distributions of granulocyte, monocyte, erythroid, and lymphocyte populations were seen in both groups. Furthermore, cell recovery for either group following incubation was 99% with >95% viability as determined by trypan blue exclusion. To assess progenitor cell content, donor marrow was subjected to clonogenic assay following 12-hour culture in control or cytokine supplemented media. As shown in Figure 3B, significantly higher levels of BFU-E, CFU-GM, and total CFCs were seen in the cytokine-stimulated marrow compared with control. Additionally, higher levels of multipotent CFU-GEMM were seen in the pretreated marrow, but the difference did not reach statistical significance (p = 0.07). Increases in CFCs were not associated with a concomitant increase in the Sca-1+Lin− early progenitor cell frequency between the control and cytokine-stimulated marrow as shown in Table 2 (0.37 ± 0.11 vs 0.34 ± 0.05, p = NS). This finding suggests that marrow exposure to VEGF and SCF led to expansion of lineage-committed progenitors or enhanced differentiation of early progenitors rather than to an increased frequency of early progenitor cells prior to transplantation. Lastly, no differences were seen in frequency of immunoregulatory T (CD4+CD25+) or natural killer T (CD3+DX5+) cell populations in control or cytokine-stimulated marrow, see Table 2.
Figure 3.
Lineage analysis and hematopoietic progenitor content of control or cytokine-stimulated allogeneic marrow. Bone marrow was harvested from 4- to 6-month Balb/c mice and subjected to 12-hour culture in media with or without cytokine supplementation. A phenotypic analysis was performed after culture for each transplant group. (A) Frequency of hematopoietic lineage surface antigen expression is shown for both control and cytokine-stimulated marrow. (B) Results of clonogenic hematopoietic progenitor assay for control or cytokine stimulated marrow. Assays were scored for burst-forming unit erythroid (BFU-E), colony-forming unit granulocyte/macrophage (CFU-GM), colony-forming unit granulocyte/erythroid/macrophage/megakaryocyte (CFU-GEMM) and total CFC at 10 to 14 days. The graph illustrates mean values ± SEM for three separate experiments. Results were compared using a two-tailed Student’s t-test assuming unequal variances.
Table 2.
Analysis of rare-cell populations
| Sca-1+Lin− | CD4+CD25+ | CD3+DX5+ | |
|---|---|---|---|
| Medial control | 0.37 ± 0.11 | 0.22 ± 0.07 | 0.37 ± 0.05 |
| Media+cytokines | 0.34 ± 0.05 | 0.20 ± 0.08 | 0.26 ± 0.12 |
Control and cytokine-stimulated Balb/c marrow were evaluated after 12-hour culture for the frequency of early hematopoietic progenitor cell populations (Sca-1+Lin−) as well as immunoregulatory T (CD4+CD25+) and natural killer T (CD3+DX5+) cell populations. Results were compared using two-tailed Student’s t-test assuming unequal variances. No significant differences were observed between the groups for rare-cell phenotypes included in the analysis.
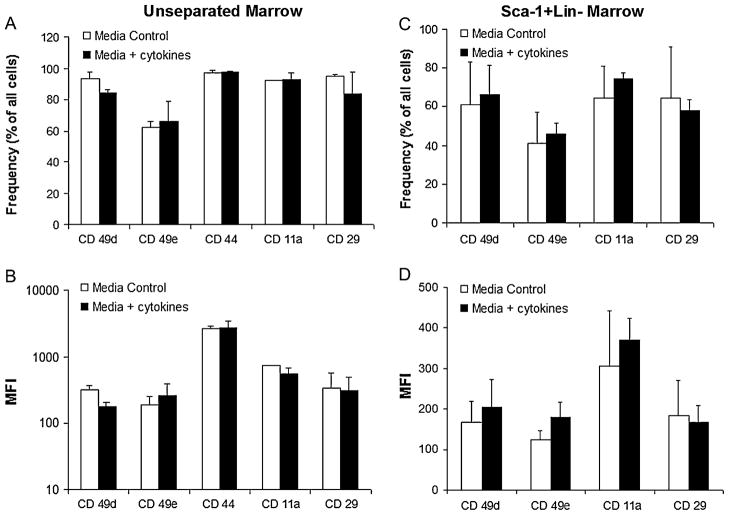
To evaluate the impact of cytokine stimulation on expression of adhesion molecules known to be critically associated with the homing of transplanted marrow, we performed an additional pretransplantation analysis of the marrow groups. Frequency and intensity of expression were compared between cytokine-stimulated and control marrow for the following adhesion receptors: very late antigen (VLA)-4 (CD49d), VLA-5 (CD49e), CD44, lymphocyte function associated antigen-1 (CD11a), or integrin β-1 (CD29). As illustrated in Figures 4A and B, control and cytokine-stimulated marrow cells demonstrated essentially identical frequencies and expression levels of these adhesion receptors. These similarities were also seen among Sca-1+Lin− cell subsets between the groups (Fig. 4C and D). Additionally, Figure 5A illustrates the in vivo homing capacity of either control or cytokine-stimulated marrow to the fetal liver or cord blood 4 hours after injection. As shown, no difference was seen between frequency of donor cells from control or cytokine-stimulated marrow in either the fetal liver (24.1 ± 13.8 vs 24.9 ± 12.9, p = NS) or cord blood (58.8 ± 22.7 vs 57.2 ± 20.2, p = NS) at 4 hours. Lineage analysis based on light scatter characteristics was performed on the homed cells and demonstrates no differences in frequencies of lymphocytes, monocytes, or granulocytes in either transplant group, see Figure 5B and C.
Figure 4.
Adhesion receptor expression of unseparated and Sca-1+Lin− fractions of cultured marrow. (A), (C) Frequency of adhesion receptor-bearing cells for unseparated and Sca-1+Lin− marrow from each culture group. (B), (D) The intensity of adhesion receptor expression for unseparated and Sca-1+Lin− marrow from each culture group is expressed as a function of mean fluorescence intensity (MFI). Values shown represent the mean ± SEM of three separate experiments. Results were compared using a two-tailed Student’s t-test assuming unequal variances. No significant differences were observed between the groups for any of the parameters included in the analysis.
Figure 5.
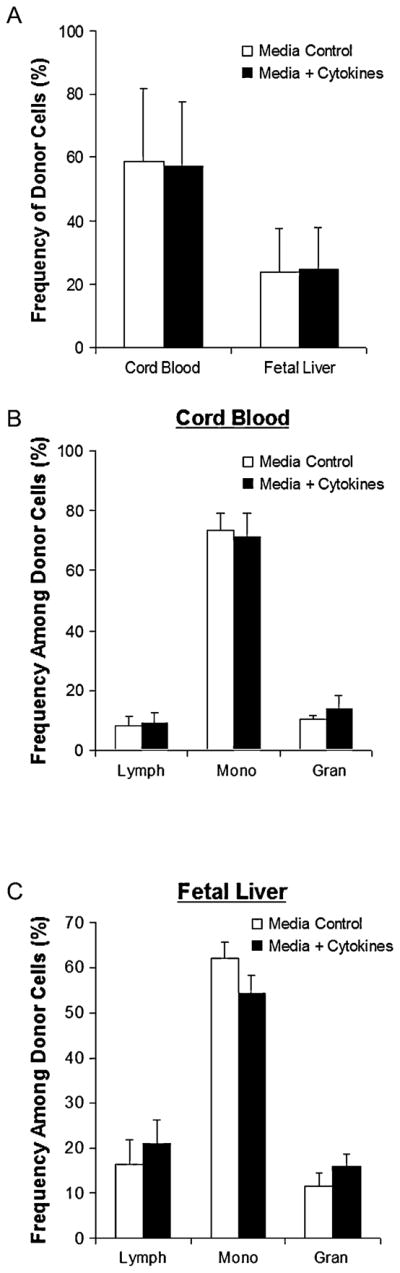
Kinetic homing analysis of transplanted allogeneic marrow. The liver and cord blood were harvested from 14 days postcoitum B6 fetal recipients of Balb/c marrow were harvested 4 hours after transplantation. Frequency of donor cells was measured by dual-color flow cytometry. (A) Donor cell chimerism in fetal liver and cord blood. (B), (C) Lineage analysis of homed cells by light scatter characteristics in cord blood (B) and fetal liver (C). Values shown represent mean ± SEM for fetal recipients of two separate experiments. Results were compared using two-tailed Student’s t-test assuming unequal variances. No significant differences were observed between the groups for any of the parameters included in the analysis.
Pretreatment of marrow with VEGF and SCF leads to higher levels of early chimerism in immunologically matched congenic strain combination
To evaluate the effects of cytokine stimulation on the competitive engraftment capacity of prenatally transplanted marrow independent of immunologic disparity, we studied early chimerism levels in congenic murine recipients. In these experiments, recipient 14 days postcoitum (dpc) B6 fetuses received 0.5 × 106 cytokine-stimulated or control marrow LDMCs from congenic B6Pep3b adult donors. Subsequently, early chimerism was measured at 7 and 14 days after transplantation. Because 7 and 14 day time points bridged the switch from liver to marrow-based hematopoiesis, the primary hematopoietic organ from that developmental stage (liver and bone marrow, respectively) was harvested to assess chimerism [38]. As shown in Table 3, chimerism levels in the recipients of cytokine-stimulated marrow were several times higher at both the liver (2.28 ± 0.71% vs 0.54 ± 0.52%, p = 0.05) and marrow (1.36 ± 0.77% vs 0.15 ± 0.14%, p =0.05) stages of hematopoietic development when compared with recipients of control marrow.
Table 3.
Levels of early chimerism in recipients of control or cytokine-stimulated congenic marrow
| Liver-7 days (n) | Marrow-14 days (n) | |
|---|---|---|
| Media control | 0.54 ± 0.52 (2) | 0.15 ± 0.14 (4) |
| Media+cytokines | 2.28 ± 0.71* (3) | 1.36 ± 0.77* (4) |
Neonatal recipients of control or cytokine-stimulated congenic marrow were sacrificed at 7 and 14 days after transplantation. The dominant hematopoietic organ from that developmental stage (liver, 7 days; marrow, 14 days) was harvested for chimerism analysis. The levels of chimerism shown reflect the frequency of donor CD45.1+ cells among all CD45+ cells in the recipient. Results were compared using two-tailed Student’s t-test assuming unequal variances.
p < 0.05.
Discussion
Following introduction of donor hematopoietic stem cells (HSCs) into the recipient, a number of competitive mechanisms operate to govern engraftment, including homing, lodgment, proliferation, apoptosis, and bidirectional immunologic tolerance [39–49]. In this study, we hypothesized that exposure to VEGF and SCF would affect these mechanisms in favor of the donor marrow, leading to improved engraftment and tolerance in a chimerism-resistant strain combination. However, we found that short-term culture with VEGF and SCF augments only early chimerism levels. This effect was demonstrated in two ways. First, in the chimerism-resistant allogeneic murine strain combination, Balb/c→B6, the rate of detectable peripheral blood microchimerism was more than doubled in the recipients of cytokine-stimulated marrow when compared with control animals. Second, we observed significantly improved donor-specific allograft survival in recipients of cytokine-stimulated marrow when compared with controls. These observations suggest that short-term culture with VEGF and SCF imparts an early competitive advantage to the donor marrow.
A number of explanations exist to explain these early increases in chimerism. One explanation stems from the observation that engraftment of prenatally or postnatally transplanted marrow relies heavily on the homing potential of the transplanted cells [46,50,51]. This process is likely regulated by various adhesion molecules present on the surface of the donor cell and corresponding ligands within the fetal hematopoietic microenvironment. One proposed key interaction is between the VLA-4 receptor and its ligand, the vascular cell adhesion molecule [31,32,50,52]. A number of studies have demonstrated that SCF exposure leads to activation of VLA-4 and enhances the receptor’s affinity for ligand [31,32]. However, in our study, no differences were observed in the level of VLA-4, VLA-5, CD44, LFA-1, CXCR-4, or integrin β-1 by the cytokine-stimulated marrow when compared with control marrow. Additionally, cytokine exposure had no effect on the homing of the donor marrow to the host fetal liver microenvironment. These results suggest that the disparities in early chimerism are not due to alterations in homing. The short incubation time (12 hours) may explain the absence of change in adhesion expression [35,53,54]. Alternatively, it remains possible that cytokine exposure selectively enhanced the homing of early progenitor cell subsets rather than lineage-committed cells within the marrow population leading to higher early chimerism. Although we did not directly assess the homing of early progenitors, the lack of enhanced long-term chimerism lessens the likelihood of this possibility.
Despite the eventual loss of chimerism, improved short-term tolerance may have resulted from higher levels of circulating donor antigen present during late gestation in the recipients of cytokine-stimulated marrow. Long-term decay in overall hematopoietic chimerism may have then lead to loss of donor-specific tolerance and rejection of the skin grafts. Support for this hypothesis comes from studies by Ferber et al., who demonstrated a correlation of T-cell tolerance with the level of peripheral tolerogen [55,56]. Additionally, classic studies by Ramsdell and Fowlkes [57] established the requirement for the persistence of antigen for maintenance of tolerance. Thus, tolerance is related directly to the level of circulating tolerogen and persistence of the tolerogen is necessary for maintenance of that tolerance.
Another potential explanation is that cytokine exposure of the donor marrow leads to expansion of immunoregulatory cell populations capable of facilitating engraftment in an allogeneic microenvironment [10,58,59]. However, as shown in Figure 3 and Table 2, a lineage analysis of the cultured cells revealed a nearly identical phenotype between the cell groups and specifically no differences in the frequencies of CD4+CD25+ or CD3+DX5+ cells. Our analysis was limited to the pretransplantation phenotype and thus does not exclude the possibility that immunoregulatory cell populations could have expanded shortly after transplantation. However, the findings in the immunologically matched strain combination, B6Pep3b→B6, suggest that enhanced early chimerism was likely independent of an immunoregulatory effect.
Taken together, these findings suggest that augmentation of donor progenitor cell proliferation resulting from cytokine exposure led to enhanced early chimerism. Pretransplantation exposure to cytokines likely resulted in cell-cycle activation of various subpopulations of normally quiescent progenitors, leading to an obligatory differentiation shortly after transplantation. The possibility of enhanced post-transplantation progenitor differentiation is further supported by the significantly higher CFC content of the cytokine-stimulated marrow. Subsequently, engraftment of cycling lineage-committed progenitors may have resulted in a rapid expansion of donor cell hematopoiesis and higher levels of early chimerism. Eventual exhaustion of engrafting progenitors may have led to a loss of chimerism. This conclusion is supported by findings from studies in postnatal murine models that demonstrate accelerated cycling and a limited engraftment capacity of resting hematopoietic progenitor populations exposed to SCF [35,60–63].
In previous studies by Sefrioui et al. [64], SCF and granulocyte colony-stimulating factor were used to mobilize donor HSCs in adult mice. The Sca-1+Lin− mobilized B6 HSCs were then enriched from the donor spleen and prenatally transplanted into 13 dpc Balb/c fetal recipients [64]. Transplanted Balb/c recipients demonstrated no evidence for tolerance at 4 and 6 months of age. Instead, they exhibited donor-specific hyper-responsiveness in proliferative and cytolytic T lymphocyte assays when compared to naïve Balb/c mice. The authors concluded that donor-specific alloimmunization had occurred. However, despite performing the transplants in the chimerism-permissive direction (B6→Balb/c rather than Balb/c→B6), the authors were unable to detect chimerism by PCR at any age. This differs from a number of other studies demonstrating a relatively high frequency of microchimerism and tolerance in B6→Balb/c prenatal transplants [16–18,36]. Development of immunity in the study by Serfrioui et al. also contrasts with the improved allograft tolerance seen in the present study and most likely relates to the differences in the donor cell populations (i.e., cytokine-stimulated unseparated marrow vs enriched mobilized splenic HSCs). In the enrichment process, a population of facilitating cells may have been eliminated, as has been described previously [58,59]. This may have resulted in chimerism levels that were insufficient to maintain allogeneic tolerance during development of adaptive immunity. A criticism of our current approach, however, is our inability to accurately quantify the levels of engraftment in the recipients of allogeneic marrow because of the extremely low levels of chimerism. As mentioned previously, engraftment of transplanted marrow in chimerism-resistant allogeneic strain combinations is difficult to achieve in prenatal or postnatal models. Potential explanations for this barrier include a host innate response to class I alloantigens or a lack of strain specific stromal support for the transplanted cells [65–67]. The early proliferative advantage imparted to the cytokine-stimulated marrow may have temporarily overcome this barrier. However, a long-term decay in peripheral chimerism levels may have led to a loss of tolerance. The requirement for persistence of a “threshold” level of chimerism to maintain durable donor-specific tolerance would explain many of the observations of failed engraftment following IUHCT in large animal models and immunocompetent human recipients. A definitive analysis in this area, however, awaits advances in the overall level of chimerism in resistant strain combinations so that a reliable quantification may be performed.
Although only a limited effect was observed, future studies employing refined cytokine culture protocols with larger quantities of enriched cells or early postnatal booster transplantations prior to a significant decay in chimerism may lead to durable engraftment in these resistant strain combinations. The achievement of predictable success in such a rigorous small animal model could provide the basis for future preclinical studies in larger species.
Acknowledgments
This work was supported in part from a grant to the University of Wisconsin Medical School under the Howard Hughes Medical Institute Research Resources Program for Medical Schools (A.F.S.), a Faculty Research Fellowship Award from the American College of Surgeons (A.F.S.), Public Health Service grants AI069882 (A.F.S.), HL64715 (A.W.F.), and HL070596 (A.W.F.), and funds from the Ruth and Tristam C. Colket, Jr. Chair of Pediatric Surgery (A.W.F.).
References
- 1.Shaaban AF, Flake AW. Fetal hematopoietic stem cell transplantation. Semin Perinatol. 1999;23:515–523. doi: 10.1016/s0146-0005(99)80030-5. [DOI] [PubMed] [Google Scholar]
- 2.Flake AW. In utero stem cell transplantation. Best Pract Res Clin Obstet Gynaecol. 2004;18:941–958. doi: 10.1016/j.bpobgyn.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 3.Flake AW. In utero transplantation of haemopoietic stem cells. Best Pract Res Clin Haematol. 2001;14:671–683. doi: 10.1053/beha.2001.0166. [DOI] [PubMed] [Google Scholar]
- 4.Flake AW, Zanjani ED. In utero hematopoietic stem cell transplantation. A status report. JAMA. 1997;278:932–937. [PubMed] [Google Scholar]
- 5.Flake AW, Roncarolo MG, Puck JM, et al. Treatment of X-linked severe combined immunodeficiency by in utero transplantation of paternal bone marrow. N Engl J Med. 1996;335:1806–1810. doi: 10.1056/NEJM199612123352404. [DOI] [PubMed] [Google Scholar]
- 6.Hayashi S, Flake AW. In utero hematopoietic stem cell therapy. Yonsei Med J. 2001;42:615–629. doi: 10.3349/ymj.2001.42.6.615. [DOI] [PubMed] [Google Scholar]
- 7.Westgren M, Ringden O, Bartmann P, et al. Prenatal T-cell reconstitution after in utero transplantation with fetal liver cells in a patient with X-linked severe combined immunodeficiency. Am J Obstet Gynecol. 2002;187:475–482. doi: 10.1067/mob.2002.123602. [DOI] [PubMed] [Google Scholar]
- 8.Wengler GS, Lanfranchi A, Frusca T, et al. In-utero transplantation of parental CD34 haematopoietic progenitor cells in a patient with X-linked severe combined immunodeficiency (SCIDXI) Lancet. 1996;348:1484–1487. doi: 10.1016/s0140-6736(96)09392-0. [DOI] [PubMed] [Google Scholar]
- 9.Shields LE, Gaur L, Delio P, Potter J, Sieverkropp A, Andrews RG. Fetal immune suppression as adjunctive therapy for in utero hematopoietic stem cell transplantation in nonhuman primates. Stem Cells. 2004;22:759–769. doi: 10.1634/stemcells.22-5-759. [DOI] [PubMed] [Google Scholar]
- 10.Shields LE, Gaur LK, Gough M, Potter J, Sieverkropp A, Andrews RG. In utero hematopoietic stem cell transplantation in nonhuman primates: the role of T cells. Stem Cells. 2003;21:304–314. doi: 10.1634/stemcells.21-3-304. [DOI] [PubMed] [Google Scholar]
- 11.Crombleholme TM, Langer JC, Harrison MR, Zanjani ED. Transplantation of fetal cells. Am J Obstet Gynecol. 1991;164:218–230. doi: 10.1016/0002-9378(91)90656-c. [DOI] [PubMed] [Google Scholar]
- 12.Andreoletti M, LePercq J, Loux N, et al. In utero allotransplantation of retrovirally transduced fetal hepatocytes in primates: feasibility and short-term follow-up. J Matern Fetal Med. 1998;7:296–303. doi: 10.1002/(SICI)1520-6661(199811/12)7:6<296::AID-MFM8>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 13.Muench MO, Barcena A. Stem cell transplantation in the fetus. Cancer Control. 2004;11:105–118. doi: 10.1177/107327480401100217. [DOI] [PubMed] [Google Scholar]
- 14.Lee PW, Cina RA, Randolph MA, et al. In utero bone marrow transplantation induces kidney allograft tolerance across a full major histocompatibility complex barrier in Swine. Transplantation. 2005;79:1084–1090. doi: 10.1097/01.tp.0000161247.61727.67. [DOI] [PubMed] [Google Scholar]
- 15.Lee PW, Cina RA, Randolph MA, et al. Stable multilineage chimerism across full MHC barriers without graft-versus-host disease following in utero bone marrow transplantation in pigs. Exp Hematol. 2005;33:371–379. doi: 10.1016/j.exphem.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 16.Hayashi S, Peranteau WH, Shaaban AF, Flake AW. Complete allogeneic hematopoietic chimerism achieved by a combined strategy of in utero hematopoietic stem cell transplantation and postnatal donor lymphocyte infusion. Blood. 2002;100:804–812. doi: 10.1182/blood-2002-01-0016. [DOI] [PubMed] [Google Scholar]
- 17.Peranteau WH, Hayashi S, Hsieh M, Shaaban AF, Flake AW. High-level allogeneic chimerism achieved by prenatal tolerance induction and postnatal nonmyeloablative bone marrow transplantation. Blood. 2002;100:2225–2234. doi: 10.1182/blood-2002-01-0166. [DOI] [PubMed] [Google Scholar]
- 18.Hayashi S, Hsieh M, Peranteau WH, Ashizuka S, Flake AW. Complete allogeneic hematopoietic chimerism achieved by in utero hematopoietic cell transplantation and cotransplantation of LLME-treated, MHC-sensitized donor lymphocytes. Exp Hematol. 32:290–299. doi: 10.1016/j.exphem.2003.12.008. [DOI] [PubMed] [Google Scholar]
- 19.Hayashi S, Abdulmalik O, Peranteau WH, et al. Mixed chimerism following in utero hematopoietic stem cell transplantation in murine models of hemoglobinopathy. Exp Hematol. 2003;31:176–184. doi: 10.1016/s0301-472x(02)01024-x. [DOI] [PubMed] [Google Scholar]
- 20.Holyoake TL, Freshney MG, McNair L, et al. Ex vivo expansion with stem cell factor and interleukin-11 augments both short-term recovery posttransplant and the ability to serially transplant marrow. Blood. 1996;87:4589–4595. [PubMed] [Google Scholar]
- 21.Ziegler BL, Valtieri M, Porada GA, et al. KDR receptor: a key marker defining hematopoietic stem cells. Science. 1999;285:1553–1558. doi: 10.1126/science.285.5433.1553. [DOI] [PubMed] [Google Scholar]
- 22.Umeda K, Heike T, Yoshimoto M, et al. Development of primitive and definitive hematopoiesis from nonhuman primate embryonic stem cells in vitro. Development. 2004;131:1869–1879. doi: 10.1242/dev.01065. [DOI] [PubMed] [Google Scholar]
- 23.Perlingeiro RC, Kyba M, Bodie S, Daley GQ. A role for thrombopoietin in hemangioblast development. Stem Cells. 2003;21:272–280. doi: 10.1634/stemcells.21-3-272. [DOI] [PubMed] [Google Scholar]
- 24.Nakayama N, Lee J, Chiu L. Vascular endothelial growth factor synergistically enhances bone morphogenetic protein-4-dependent lymphohematopoietic cell generation from embryonic stem cells in vitro. Blood. 2000;95:2275–2283. [PubMed] [Google Scholar]
- 25.Miyagi T, Takeno M, Nagafuchi H, Takahashi M, Suzuki N. Flk1+ cells derived from mouse embryonic stem cells reconstitute hematopoiesis in vivo in SCID mice. Exp Hematol. 2002;30:1444–1453. doi: 10.1016/s0301-472x(02)00961-x. [DOI] [PubMed] [Google Scholar]
- 26.Hidaka M, Stanford WL, Bernstein A. Conditional requirement for the Flk-1 receptor in the in vitro generation of early hematopoietic cells. Proc Natl Acad Sci U S A. 1999;96:7370–7375. doi: 10.1073/pnas.96.13.7370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ema M, Faloon P, Zhang WJ, et al. Combinatorial effects of Flk1 and Tal1 on vascular and hematopoietic development in the mouse. Genes Dev. 2003;17:380–393. doi: 10.1101/gad.1049803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cortes F, Debacker C, Peault B, Labastie MC. Differential expression of KDR/VEGFR-2 and CD34 during mesoderm development of the early human embryo. Mech Dev. 1999;83:161–164. doi: 10.1016/s0925-4773(99)00030-1. [DOI] [PubMed] [Google Scholar]
- 29.Briddell RA, Hartley CA, Smith KA, McNiece IK. Recombinant rat stem cell factor synergizes with recombinant human granulocyte colony-stimulating factor in vivo in mice to mobilize peripheral blood progenitor cells that have enhanced repopulating potential. Blood. 1993;82:1720–1723. [PubMed] [Google Scholar]
- 30.Yednock TA, Cannon C, Vandevert C, et al. Alpha 4 beta 1 integrin-dependent cell adhesion is regulated by a low affinity receptor pool that is conformationally responsive to ligand. J Biol Chem. 1995;270:28740–28750. doi: 10.1074/jbc.270.48.28740. [DOI] [PubMed] [Google Scholar]
- 31.Kovach NL, Lin N, Yednock T, Harlan JM, Broudy VC. Stem cell factor modulates avidity of alpha 4 beta 1 and alpha 5 beta 1 integrins expressed on hematopoietic cell lines. Blood. 1995;85:159–167. [PubMed] [Google Scholar]
- 32.Legras S, Levesque JP, Charrad R, et al. CD44-mediated adhesiveness of human hematopoietic progenitors to hyaluronan is modulated by cytokines. Blood. 1997;89:1905–1914. [PubMed] [Google Scholar]
- 33.Peled A, Petit I, Kollet O, et al. Dependence of human stem cell engraftment and repopulation of NOD/SCID mice on CXCR4. Science. 1999;283:845–848. doi: 10.1126/science.283.5403.845. [DOI] [PubMed] [Google Scholar]
- 34.Hart C, Drewel D, Mueller G, et al. Expression and function of homing-essential molecules and enhanced in vivo homing ability of human peripheral blood-derived hematopoietic progenitor cells after stimulation with stem cell factor. Stem Cells. 2004;22:580–589. doi: 10.1634/stemcells.22-4-580. [DOI] [PubMed] [Google Scholar]
- 35.Peters SO, Kittler EL, Ramshaw HS, Quesenberry PJ. Ex vivo expansion of murine marrow cells with interleukin-3 (IL-3), IL-6, IL-11, and stem cell factor leads to impaired engraftment in irradiated hosts. Blood. 1996;87:30–37. [PubMed] [Google Scholar]
- 36.Kim HB, Shaaban AF, Yang EY, Liechty KW, Flake AW. Microchimerism and tolerance after in utero bone marrow transplantation in mice. J Surg Res. 1998;77:1–5. doi: 10.1006/jsre.1997.5255. [DOI] [PubMed] [Google Scholar]
- 37.Kim HB, Shaaban AF, Milner R, Fichter C, Flake AW. In utero bone marrow transplantation induces donor-specific tolerance by a combination of clonal deletion and clonal anergy. J Pediatr Surgery. 1998;34:726–729. doi: 10.1016/s0022-3468(99)90364-0. discussion 729–730. [DOI] [PubMed] [Google Scholar]
- 38.Tavassoli M. Embryonic and fetal hemopoiesis: an overview. Blood Cells. 1991;17:269–281. discussion 282–286. [PubMed] [Google Scholar]
- 39.Cashman J, Clark-Lewis I, Eaves A, Eaves C. Stromal-derived factor 1 inhibits the cycling of very primitive human hematopoietic cells in vitro and in NOD/SCID mice. Blood. 2002;99:792–799. doi: 10.1182/blood.v99.3.792. [DOI] [PubMed] [Google Scholar]
- 40.Craddock C, Nakamoto B, Elices M, Papayannopoulou T. The role of CS1 moiety of fibronectin in VLA mediated haemopoietic progenitor trafficking. Br J Haematol. 1997;97:15–21. doi: 10.1046/j.1365-2141.1997.d01-2120.x. [DOI] [PubMed] [Google Scholar]
- 41.Deguchi T, Komada Y. Homing-associated cell adhesion molecule (H-CAM/CD44) on human CD34+ hematopoietic progenitor cells. Leuk Lymphoma. 2000;40:25–37. doi: 10.3109/10428190009054878. [DOI] [PubMed] [Google Scholar]
- 42.Hendrikx PJ, Martens CM, Hagenbeek A, Keij JF, Visser JW. Homing of fluorescently labeled murine hematopoietic stem cells. Exp Hematol. 1996;24:129–140. [PubMed] [Google Scholar]
- 43.Hidalgo A, Sanz-Rodriguez F, Rodriguez-Fernandez JL, et al. Chemokine stromal cell-derived factor-1alpha modulates VLA-4 integrin-dependent adhesion to fibronectin and VCAM-1 on bone marrow hematopoietic progenitor cells. Exp Hematol. 2001;29:345–355. doi: 10.1016/s0301-472x(00)00668-8. [DOI] [PubMed] [Google Scholar]
- 44.Hinds KA, Hill JM, Shapiro EM, et al. Highly efficient endosomal labeling of progenitor and stem cells with large magnetic particles allows magnetic resonance imaging of single cells. Blood. 2003;102:867–872. doi: 10.1182/blood-2002-12-3669. [DOI] [PubMed] [Google Scholar]
- 45.Matsuzaki Y, Kinjo K, Mulligan RC, Okano H. Unexpectedly efficient homing capacity of purified murine hematopoietic stem cells. Immunity. 2004;20:87–93. doi: 10.1016/s1074-7613(03)00354-6. [DOI] [PubMed] [Google Scholar]
- 46.Papayannopoulou T, Craddock C. Homing and trafficking of hemopoietic progenitor cells. Acta Haematol. 1997;97:97–104. doi: 10.1159/000203665. [DOI] [PubMed] [Google Scholar]
- 47.Shaaban AF, Kim HB, Milner R, Flake AW. A kinetic model for the homing and migration of prenatally transplanted marrow. Blood. 1999;94:3251–3257. [PubMed] [Google Scholar]
- 48.Tavassoli M, Minguell JJ. Homing of hemopoietic progenitor cells to the marrow. Proc Soc Exp Biol Med. 1991;196:367–373. doi: 10.3181/00379727-196-43201. [DOI] [PubMed] [Google Scholar]
- 49.Zanjani ED, Flake AW, Almeida-Porada G, Tran N, Papayannopoulou T. Homing of human cells in the fetal sheep model: modulation by antibodies activating or inhibiting very late activation antigen-4-depen dent function. Blood. 1999;94:2515–2522. [PubMed] [Google Scholar]
- 50.Shaaban AF, Kim HB, Liechty KW, Milner R, Flake AW. Blockade of a4 or b1 integrins impairs homing of prenatally transplanted allogeneic marrow to the fetal liver. Blood. 1997;90(suppl 1):488a. abstr 2168. [Google Scholar]
- 51.Tavassoli M, Hardy CL. Molecular basis of homing of intravenously transplanted stem cells to the marrow. Blood. 1990;76:1059–1070. [PubMed] [Google Scholar]
- 52.Papayannopoulou T, Craddock C, Nakamoto B, Priestley GV, Wolf NS. The VLA4/VCAM-1 adhesion pathway defines contrasting mechanisms of lodgement of transplanted murine hemopoietic progenitors between bone marrow and spleen. Proc Natl Acad Sci U S A. 1995;92:9647–9651. doi: 10.1073/pnas.92.21.9647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Oostendorp RA, Dormer P. VLA-4-mediated interactions between normal human hematopoietic progenitors and stromal cells. Leuk Lymphoma. 1997;24:423–535. doi: 10.3109/10428199709055581. [DOI] [PubMed] [Google Scholar]
- 54.Levesque JP, Haylock DN, Simmons PJ. Cytokine regulation of proliferation and cell adhesion are correlated events in human CD34+ hemopoietic progenitors. Blood. 1996;88:1168–1176. [PubMed] [Google Scholar]
- 55.Ferber I, Schönrich G, Schenkel J, Mellor AL, Hämmerling GJ, Arnold B. Levels of peripheral T cell tolerance induced by different doses of tolerogen. Science. 1994;263:674–676. doi: 10.1126/science.8303275. [DOI] [PubMed] [Google Scholar]
- 56.Hammerling GJ, Schonrich G, Ferber I, Arnold B. Peripheral tolerance as a multi-step mechanism. Immunological Rev. 1993;133:93–104. doi: 10.1111/j.1600-065x.1993.tb01511.x. [DOI] [PubMed] [Google Scholar]
- 57.Ramsdell F, Fowlkes BJ. Maintenance of in vivo tolerance by persistence of antigen. Science. 1992;257:1130–1134. doi: 10.1126/science.257.5073.1130. [DOI] [PubMed] [Google Scholar]
- 58.Kaufman CL, Colson YL, Wren SM, Watkins S, Simmons RL, Ildstad ST. Phenotypic characterization of a novel bone marrow-derived cell that facilitates engraftment of allogeneic bone marrow stem cells. Blood. 1994;84:2436–2446. [PubMed] [Google Scholar]
- 59.Gaines BA, Colson YL, Kaufman CL, Ildstad S. Facilitating cells enable engraftment of purified fetal liver stem cells in allogeneic recipients. Exp Hematol. 1996;24:902–913. [PubMed] [Google Scholar]
- 60.Li CL, Johnson GR. Stem cell factor enhances the survival but not the self-renewal of murine hematopoietic long-term repopulating cells. Blood. 1994;84:408–414. [PubMed] [Google Scholar]
- 61.Trevisan M, Yan XQ, Iscove NN. Cycle initiation and colony formation in culture by murine marrow cells with long-term reconstituting potential in vivo. Blood. 1996;88:4149–4158. [PubMed] [Google Scholar]
- 62.Traycoff CM, Cornetta K, Yoder MC, Davidson A, Srour EF. Ex vivo expansion of murine hematopoietic progenitor cells generates classes of expanded cells possessing different levels of bone marrow repopulating potential. Exp Hematol. 1996;24:299–306. [PubMed] [Google Scholar]
- 63.Peters SO, Kittler EL, Ramshaw HS, Quesenberry PJ. Murine marrow cells expanded in culture with IL-3, IL-6, IL-11, and SCF acquire an engraftment defect in normal hosts. Exp Hematol. 1995;23:461–469. [PubMed] [Google Scholar]
- 64.Sefrioui H, Donahue J, Srivastava AS, Gilpin E, Lee TH, Carrier E. Alloreactivity following in utero transplantation of cytokine-stimulated hematopoietic stem cells: the role of recipient CD4(−) cells. Exp Hematol. 2002;30:617–624. doi: 10.1016/s0301-472x(02)00803-2. [DOI] [PubMed] [Google Scholar]
- 65.Sugiura K, Yasumizu R, Iwai H, et al. Long-term immunologic tolerance induction in chimeric mice after bone marrow transplantation across major histocompatibility barriers: persistent or redeveloping immunologic responsiveness after prolonged survival. Thymus. 1991;18:137–153. [PubMed] [Google Scholar]
- 66.Hashimoto F, Sugiura K, Inoue K, Ikehara S. Major histocompatibility complex restriction between hematopoietic stem cells and stromal cells in vivo. Blood. 1997;89:49–54. [PubMed] [Google Scholar]
- 67.Hisha H, Nishino T, Kawamura M, Adachi S, Ikehara S. Successful bone marrow transplantation by bone grafts in chimeric-resistant combination. Exp Hematol. 1995;23:347–352. [PubMed] [Google Scholar]