Abstract
The present study investigates tissue-specific prostaglandin secretion and cyclooxygenase-2 (COX-2) induction in full-thickness human gestational membranes. Gestational membranes were collected from healthy, non-laboring cesarean deliveries at 37–39 weeks gestation and cultured in two-chamber Transwell devices. Lipopolysaccharide exposure (100 ng/ml for 8 h) elevated prostaglandin E2 and F2α concentrations in the amniotic chamber medium regardless of whether exposure was to the amniotic, decidual, or both sides of the membranes. However, prostaglandin E2 and F2α concentrations in the decidual chamber medium were elevated compared with controls only if the decidual side was exposed directly to lipopolysaccharide. Whereas prostaglandin F2α concentrations increased to similar extents in the amniotic and decidual chambers regardless of lipopolysaccharide exposure modality, prostaglandin E2 concentrations were 22-fold higher on the amniotic side than the decidual side after lipopolysaccharide stimulation of the amnion. These findings demonstrate the propagation of prostaglandins, prostaglandin precursors or other factors in the direction of the decidua to the amnion, but the reverse situation was not evident. Immunostaining for COX-2 was related to the side of lipopolysaccharide exposure, i.e., exposure to the amnion caused immunostaining in cells of the collagen layers of the amnion and chorion whereas exposure to the decidual side caused staining in decidual cells. These findings suggest that the inflammatory effect of lipopolysaccharide on COX-2 induction occurs within a localized area of exposure and that prostaglandins or their precursors move across the tissues of the gestational membranes by currently undefined transport mechanisms.
Keywords: Prostaglandin, lipopolysaccharide, amnion, chorion, cyclooxygenase-2
Introduction
Extraplacental human gestational membranes are comprised of amnion, chorion laeve and decidual tissues that biosynthesize prostaglandins during human pregnancy.1, 2 Close apposition of the gestational membranes to the myometrium may allow prostaglandins from the gestational membranes to play an important role in parturition. Prostaglandin E2 (PGE2) and prostaglandin F2α (PGF2α) are of particular interest because they are potent stimulators of myometrial contractility and are elevated in women experiencing preterm and term labor.3, 4 Human gestational membranes obtained after vaginal deliveries secrete greater amounts of prostaglandins compared to those from scheduled, non-laboring cesarean deliveries.5 The latter studies indicate that uterine quiescence during pregnancy is associated with low prostaglandin levels and that term physiological and preterm pathological prostaglandin production are associated with uterine contraction and labor. Researchers hypothesize that preterm uterine contractility and parturition result from preterm pathological gestational membrane prostaglandin production caused by exogenous inflammatory stimuli.6, 7
Prostaglandins are derived from arachidonic acid, which is converted to prostaglandin H by cyclooxygenase-2 (COX-2), also known as prostaglandin-endoperoxide synthase 2 (PTGS2). This enzyme is induced in human gestational tissues at the time of parturition and is responsible for the increased prostaglandin formation observed near term.8–10 Under experimental conditions, COX-2 is also induced in response to inflammatory agents.11 Traditionally, PGE2 biosynthesis has been thought to occur throughout the extraplacental gestational membranes with high levels of synthetic activity in the amnion, whereas PGF2α biosynthesis is located in the decidua.12 Expression of the enzymes in the prostaglandin metabolic pathway, including COX-2, are regulated by a variety of hormones, cytokines, and growth factors.13
Intrauterine infection is a significant causative factor in the etiology of preterm labor and birth in humans, accounting for as many as one-third of all preterm deliveries.14 Lipopolysaccharide (LPS) is a cell membrane component of gram negative bacteria that elicits a strong immune response and may be important in cases of intrauterine infection and preterm birth.15 It is commonly used as a model inflammatory agent. In mouse models of preterm labor and birth, injection of LPS into the cervix or peritoneum causes preterm expulsion of the fetuses.16–19 Exposure to inflammatory stimuli such as LPS may activate pathological prostaglandin signaling through induction of enzymes of the prostaglandin synthetic pathway, particularly COX-2.3 Alternatively, LPS may affect prostaglandin production indirectly by stimulating release of inflammatory cytokines (e.g. tumor necrosis factor-α) that subsequently induce COX-2.20
The present study examines the hypothesis that human gestational membranes release prostaglandins in a side-specific manner corresponding to inflammatory-stimulus induction of COX-2. Using LPS as a model inflammatory agent, experiments distinguished the patterns of PGE2 and PGF2α release from the amniotic and decidual sides of full-thickness human gestational membrane explants using a two-chamber Transwell tissue culture system. The Transwell culture system allowed for ex vivo analysis of integrated tissue responses by using full-thickness membranes that maintained physically separated amniotic and decidual media chambers for inflammatory stimulus exposure and measurement of secreted prostaglandins, with immunohistochemical analysis of COX-2 induction. Improved understanding of mechanisms underlying inflammation-stimulated prostaglandin release may contribute to more effective diagnosis and treatment of medical conditions that threaten pregnancy.
Materials and Methods
Chemicals, Reagents and Antibodies
Tissue culture reagents including high glucose Dulbecco's Modified Eagle's Medium (DMEM) with no phenol red, penicillin/streptomycin antibiotic, and heat-inactivated fetal bovine serum were purchased from Invitrogen (Carlsbad, CA). We used LPS from Salmonella typhimurium (Lot #225) purchased from List Biological Laboratory (Campbell, CA) as a model stimulus to elicit a strong inflammatory response. The Vectastain Elite ABC immunohistochemical staining kit, DAB Substrate Kit (3,3'-diaminobenzidine) and hematoxylin were purchased from Vector Laboratories (Burlingame, CA). Anti-cyclooxygenase-2 monoclonal antibody and the PGE2 and PGF2α enzyme immunoassay kits were purchased from Cayman Chemical Co. (Ann Arbor, MI). Transwell frames lacking synthetic membranes were a gift from Corning Corporation (Corning, NY).
Tissue Collection
Methods pertaining to human tissue were reviewed and approved by the local Institutional Review Board prior to initiation of experiments; review and approval were updated yearly. Full-thickness gestational membranes comprised of amnion, chorion laeve and decidua were collected at 37–39 weeks gestation from non-laboring women who underwent elective cesarean deliveries following healthy pregnancies. Exclusion criteria included smoking, multiple fetal gestation, complications of pregnancy such as gestational diabetes or hypertension, use of drugs that may have an effect on arachidonic acid metabolism (e.g., aspirin, montelukast), or any other condition which would require the tissue to be sent to pathology. Within 60 min of delivery, the extraplacental membranes were dissected from the placenta with a scalpel and transported to the laboratory.
Tissue Culture
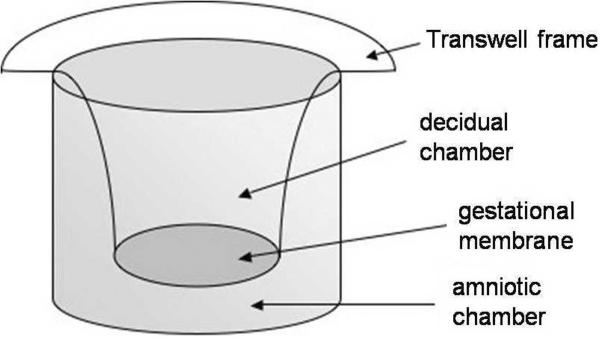
Under sterile laboratory conditions, the membranes were examined to ensure that the amnion and choriodecidua remained attached to one another. Culture medium consisted of DMEM supplemented with 1% (v/v) heat inactivated-fetal bovine serum, 100 units penicillin/ml, and 100 μg streptomycin/ml. Preliminary experiments were conducted with a range of fetal bovine serum (FBS) concentrations to determine optimal FBS concentrations in culture medium. In our protocol of beginning experimental treatment of the membranes within 24 h of collection, we found that 1% FBS was sufficient to maintain the health of the tissue with no additional benefit seen at higher FBS concentrations. Full-thickness gestational membranes were cultured using a Transwell two-chamber tissue culture method.21, 22 The outer 2 cm of the gestational membranes was excluded from experimentation. The membranes were cut into approximately 2 × 2 cm2 pieces, affixed by elastic latex bands onto ethylene oxide sterilized Transwell frames (without a synthetic membrane), and extra tissue was removed with a scalpel. The decidual side of the tissue faced the inner culture chamber (Figure 1). Each Transwell frame with attached tissue was placed in a single well of a 12-well plate with culture medium on both sides of the membranes, creating two distinct chambers on either side of the membrane. Mounted membranes were equilibrated in a 5% (v/v) CO2 tissue culture incubator for 18–24 h, with a medium change after 2–4 h to maintain optimal health of the tissue. The overnight culture medium was exchanged with fresh culture medium 1–2 h prior to beginning the treatment protocol.
Figure 1. Diagram of gestational membrane tissue mounted on a Transwell device.
The gestational membranes form a barrier creating distinct amniotic and decidual chambers for collection of secreted prostaglandins.
Experimental design
Culture wells with mounted gestational membranes were assigned randomly to treatment or control groups. To compensate for potential variability among cultures derived from different parts of the same membranes (e.g., cervix or fundus), three cultures per treatment were established from each specimen. Cultures from one subject comprised an experimental run on a separate day. Treatment triplicates were averaged and used as the result for that subject, with n referring to the number of subjects in each experiment.
Treatment
The LPS was diluted in water to make a primary stock concentration of 100 μg/ml. Immediately prior to an experiment, the LPS primary stock was further diluted in culture medium to achieve the experimental concentration. The medium in one or both chambers of the culture was exchanged with the LPS-containing exposure medium. In cultures exposed on only one side of the membranes to LPS, the medium in the opposite chamber was exchanged with fresh culture medium without LPS. Likewise, the medium of unexposed controls was exchanged with fresh culture medium without LPS. A concentration-response experiment for PGE2 release was conducted with an 8-h exposure to LPS concentrations of 1, 10, 100 and 1000 ng/ml (in both the amniotic and decidual chambers). A time-course experiment for PGE2 release was conducted for 1, 4, 8 and 24 h exposures to 100 ng/ml LPS (in both the amniotic and decidual chambers). Independent observations were made for each time point on gestational membranes collected on different days. Based on the results of the concentration-response and time-course experiments, the side-specific exposure experiment was conducted using 100 ng/ml LPS treatment for 8 h to the amniotic side only, the decidual side only, both sides of the membranes or no LPS treatment to either side of the membranes (control). After treatment, the medium was recovered from the amniotic-facing and decidual-facing chambers of the Transwell cultures and stored at −80°C for subsequent enzyme immunoassay analysis of PGE2 and PGF2α. The tissue was removed from the Transwell device and weighed. Tissue from the side-specific exposure experiment was frozen for subsequent immunohistochemical analysis.
Enzyme Immunoassay
Prostaglandin E2 and PGF2α concentrations in the culture medium were assayed by specific enzyme immunoassays according to the manufacturer's protocol (Cayman Chemical Co., Ann Arbor, MI). Prostaglandin concentrations (pg/ml) were divided by the post-experimental wet weight of the gestational membrane tissue to account for differences in tissue thickness between samples. Overall mean prostaglandin concentrations per gram of tissue (pg/ml/g) and standard error of the mean were calculated from multiple specimens and graphed, with n referring to the number of specimens (subjects) in each experiment.
Immunohistochemistry
Post-treatment gestational membrane tissue was rolled, embedded in freeze medium, frozen in liquid nitrogen-cooled isopentane and stored at −80° C. The tissue was sliced with a cryotome into 8-μm sections, and mounted onto slides. The tissue sections were fixed with 4% paraformaldehyde and immunostained using the Vectastain Elite ABC Kit according to the manufacturer's protocol (Vector Labs Burlingame, CA). Sections were incubated with primary antibody, labeled with a biotinylated secondary antibody and an avidin:biotinylated enzyme complex, stained with 3,3-diaminobenzidine and counterstained with hematoxylin. Sections were visualized with an upright light microscope and recorded digitally.
Statistical Analyses
The prostaglandin enzyme immunoassay results were analyzed by analysis of variance (ANOVA) using Sigma Stat v 3.5 software (Systat Software, Inc., Richmond, CA). Prior to analysis, the data were transformed using log normal transformation to correct for non-normality. Post hoc pair-wise comparisons of means were performed by the Student-Newman-Keuls method. A p-value < 0.05 was considered statistically significant.
Results
Concentration-response of LPS-stimulated prostaglandin E2 release
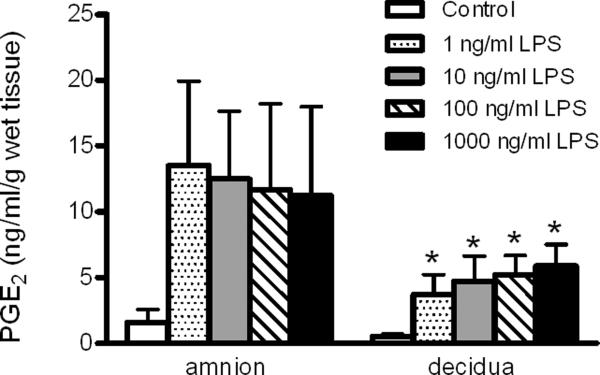
To determine optimal LPS exposure concentrations in Transwell cultures, PGE2 concentrations were measured in culture medium from the amniotic and decidual chambers after an 8-h exposure to 1, 10, 100 or 1000 ng/ml LPS on both sides of the membranes or control (Figure 2). The PGE2 concentrations were significantly affected by LPS treatment (ANOVA treatment effect, p = 0.002). Levels of PGE2 in the decidual chamber medium were significantly elevated for all LPS concentrations compared with the decidual control (p < 0.05). The PGE2 concentrations in the decidual chamber medium ranged from 3.72 ng/ml/g wet tissue weight at 1 ng/ml LPS to 5.86 ng/ml/g wet tissue weight at 1000 ng/ml LPS, but these concentrations were not statistically significant from each other. Although PGE2 concentrations were elevated in the medium from the amniotic versus decidual side of the membranes (ANOVA side-specific effect, p = 0.03), there were no statistically significant differences in pairwise comparison of means from the amniotic and decidual sides at the same LPS concentration. Moreover, the elevated mean PGE2 concentrations in the amniotic chamber medium of cultures exposed to LPS were not significantly increased compared with amniotic controls, possibly related to high variability in the amniotic response in this experiment. For both the decidual and amniotic chambers, near maximal PGE2 concentrations were observed with 1 ng/ml LPS exposure, the lowest concentration of LPS tested, with no further significant increase in response at 10, 100 and 1000 ng/ml LPS. Based on these results and taking into consideration previously published studies that used relatively high LPS concentrations (500–5000 ng/ml) to stimulate full-thickness gestational membranes,23, 24 we chose 100 ng/ml LPS for use in subsequent experiments.
Figure 2. Concentration-response of LPS-stimulated PGE2 release from the amniotic or decidual sides of human gestational membranes.
Concentrations of PGE2 were measured in medium of the amnion- and decidua-facing chambers of Transwell cultures of human gestational membranes after an 8-h exposure to 0 (control), 1, 10, 100 or 1000 ng/ml LPS. The LPS was added to both the amniotic and decidual chambers of the cultures. The x-axis shows the side of the gestational membranes from which the medium was assayed. The legend indicates LPS exposure concentrations. Values are expressed as mean ± SEM (n= 6 subjects). *Significantly different from decidua control (p < 0.05). LPS, lipopolysaccharide; PGE2 prostaglandin E2.
Time-course of LPS-stimulated prostaglandin E2 release
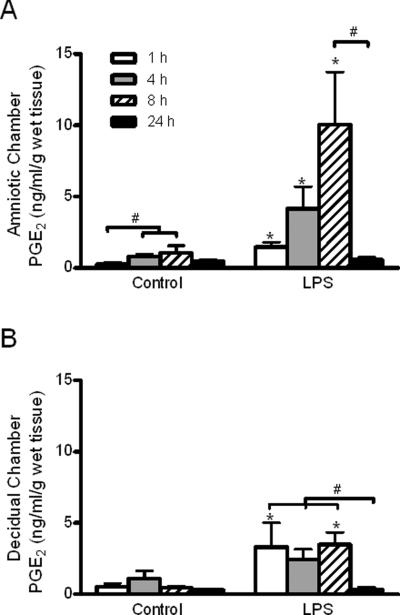
Exposure of both sides of the membranes to 100 ng/ml LPS stimulated PGE2 release in a time-dependent manner (3-way ANOVA treatment and time effects, p < 0.001; treatment-by-time interaction, p = 0.02). Because there were no statistically significant effects (including interactions) related to the side of the membranes (3-way ANOVA, p > 0.2,), the amniotic and decidual responses were subsequently analyzed and presented separately (Figure 3A and B, respectively). In the amniotic chamber medium, LPS treatment increased PGE2 concentrations after 1, 4 and 8 h compared with non-treated controls of the same exposure duration (Figure 2A; p < 0.05). In addition, the mean PGE2 concentrations decreased from a high of 10.0 ng/ml/g wet tissue weight at 8 h to 0.57 ng/ml/g wet tissue weight after 24 h of LPS exposure (p < 0.05). Statistically significant time-dependent increases of PGE2 concentrations in amniotic medium were observed in controls, with higher PGE2 concentrations at 4 h and 8 h compared with the 1-h time point (p < 0.05). In the decidual chamber, treatment with LPS increased mean PGE2 concentrations to a similar extent at 1 h and 8 h (3.30 and 3.48 ng/ml/g wet tissue weight, respectively) compared with respective time-matched controls (Figure 2B; p < 0.05). The increase of PGE2 concentration (2.45 ng/ml/g tissue weight) after a 4-h exposure to LPS was not statistically significantly different from the time-matched control. As was observed for the amniotic chamber medium, PGE2 concentrations markedly decreased to 0.33 ng/ml/g wet tissue weight after 24 h of LPS exposure, a significant reduction compared to concentrations observed with shorter durations of exposure at 1, 4 and 8 h (p < 0.05). In contrast to the amniotic chamber medium, concentrations of PGE2 in the decidual culture medium of controls did not significantly change over time (Figure 2B). The low PGE2 concentrations observed at the 24-h time-point in amniotic and decidual medium from control and LPS-treated cultures suggests that PGE2 ceases to be synthesized and that some mechanism acts to reduce the PGE2 concentrations on both sides of the membrane beyond 8 h in culture. Based on these time-course results, subsequent side-specific experiments were conducted using an 8-h LPS exposure time to capture peak LPS-stimulated prostaglandin release.
Figure 3. Time course of LPS-stimulated PGE2 release from the amniotic and decidual sides of human gestational membranes.
Concentrations of PGE2 were measured in medium of the amnion- and decidua-facing chambers of Transwell cultures of human gestational membranes after 1, 4, 8 and 24 h of exposure to 100 ng/ml LPS (added to both the amniotic and decidual chambers of the cultures). Concurrent controls were exposed to culture medium without LPS. A) PGE2 concentrations in amniotic chamber medium. B) PGE2 concentrations in decidual chamber medium. Values are expressed as mean ± SEM from triplicate cultures for each subject (n=6, 8, 11 and 4 subjects at 1, 4, 8 and 24 h, respectively). *Significantly different from time-matched controls (p< 0.05). #Significantly different from time-points indicated within treatment group. LPS, lipopolysaccharide; PGE2 prostaglandin E2.
PGE2 and PGF2α response to side-specific LPS treatment of gestational membranes
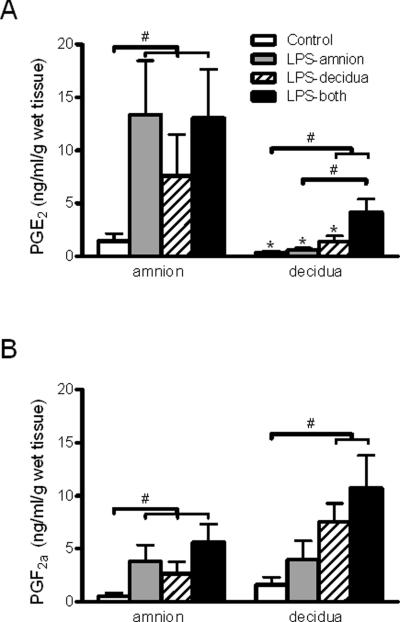
Treatment with LPS stimulated PGE2 release in a side-specific manner (ANOVA treatment and side-specific effects, p < 0.001). All modalities of LPS treatment increased PGE2 concentrations by at least five-fold in the amniotic chamber medium compared to control cultures (p<0.05; Figure 4A, left side). In the decidual chamber medium, PGE2 concentrations increased relative to controls only in response to LPS treatment on the decidual side, either alone or in combination with amniotic side exposure (3.9 and 11.6 fold increase, respectively; p < 0.05; Figure 4A, right side). In addition, the PGE2 concentrations were significantly increased in decidual chamber medium of cultures exposed to LPS on both sides of the membranes compared with cultures treated on only the amniotic side of the membranes (p<0.05). When only the amniotic side of the membranes was treated with LPS (Figure 4A, gray bars), there was a robust PGE2 response detected in the amniotic medium (13.4 ng/ml/g tissue weight) but not the decidual medium (0.6 ng/ml/g tissue weight) (p<0.05). Moreover, when only the decidual side was treated with LPS (Figure 4A, hatched bars), the amniotic PGE2 response (7.58 ng/ml/g tissue weight) remained significantly greater than the decidual response (1.40 ng/ml/g tissue weight) (p<0.05).
Figure 4. Side-specific prostaglandin release from the amniotic and decidual sides of human gestational membranes.
Concentrations of PGE2 and PGF2α were measured in medium of the amnion- and decidua-facing chambers of Transwell cultures of human gestational membranes after an 8-h exposure to 100 ng/ml LPS on the amniotic side only, decidual side only, or both sides of the membranes. Concurrent controls were exposed to culture medium without LPS. A) PGE2 concentrations in culture medium. B) PGF2α concentrations in culture medium. The x-axis shows the side of the gestational membranes assayed. The legend indicates the side of the gestational membranes exposed to LPS. Values are expressed as mean ± SEM (n=6). #Significantly different from time points indicated within treatment group (p< 0.05). *Significantly different from amniotic chamber concentration of the same treatment (p< 0.05). LPS, lipopolysaccharide; PGE2 prostaglandin E2, PGF2α prostaglandin F2α.
Similar to PGE2, LPS stimulated PGF2α release (Fig. 4B; ANOVA treatment effect, p < 0.001). The PGF2α concentrations increased at least five-fold in the amniotic chamber medium with all LPS exposure modalities compared with controls (p < 0.05; Fig. 4B, left side). Significantly-increased release of PGF2α into the medium on the decidual side required LPS treatment on the decidual side, either alone or in combination with amniotic side exposure (4.7 and 6.7 fold increase, respectively) (p < 0.05; Fig. 4B, right side). In contrast to the side-specific PGE2 results, differences in LPS-stimulated increases of PGF2α concentrations from the amniotic and decidual sides the membranes were not statistically significant (Fig. 4B, comparing left and right sides of the graph).
Gestational membrane prostaglandin-endoperoxide synthase 2 immunohistochemistry
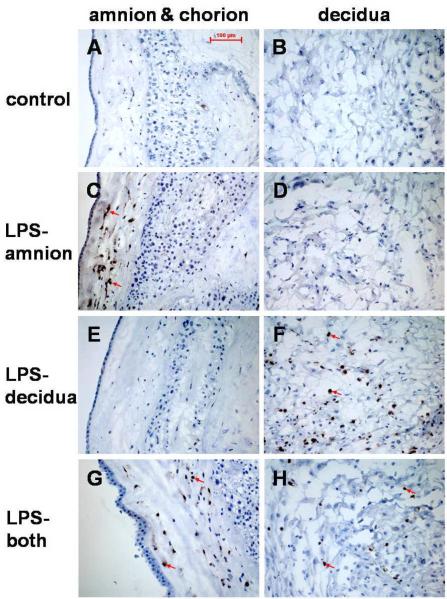
Tissue-specific patterns of anti-COX-2 antibody staining were observed in gestational membranes treated with 100 ng/ml LPS for 8 h in a side-specific manner in Transwell cultures (Fig. 5). No COX-2 immunostaining was seen in non-treated control gestational membrane tissue (Fig. 5A and B). In tissue treated with LPS on the amniotic side only, cells in the amnion and chorion mesodermal regions stained positive for COX-2 (Figure 5C), but COX-2 staining was not seen in the decidua (Fig. 5D). In tissue treated with LPS on the decidual side only, COX-2 immunostaining was detected in cells in the decidua (Fig. 5F), but cells in the amnion and chorion did not show positive COX-2 antibody staining (Fig. 5E). In tissue treated with LPS on both sides of the membranes, cells stained positive for COX-2 in the amnion mesoderm, chorion mesoderm and decidua (Fig. 5G and H).
Figure 5. Immunolocalization of cyclooxygenase-2 (COX-2) in gestational membranes following side-specific LPS exposure.
Frozen sections of full-thickness human gestational membranes were immunostained with anti-COX-2 antibody after an 8-h exposure to 100 ng/ml LPS in Transwell cultures. There was no evidence of COX-2 immunostaining in control samples (A, B). In tissues treated with LPS on the amnion side only (LPS-amnion), amniotic and chorion mesodermal cells stained positive for COX-2 (C) but no immunostaining was detected in the decidua (D). In tissues treated with LPS on the decidual side only (LPS-decidua), cells in the decidua stained positive for COX-2 (F), but cells in the amnion and chorion did not (E). In tissues treated with LPS on both sides of the membranes (LPS-both), positive COX-2 staining was observed in cells in the amniotic and chorion mesoderm region (G), as well as in the decidua (H). The sections were counterstained with hematoxylin. Arrows indicate COX-2 staining (brown). LPS, lipopolysaccharide; COX-2, cyclooxygenase-2.
Discussion
Previous reports of LPS stimulation of amniotic or choriodecidual prostaglandin secretion have been unable to detect the presence or absence of cross-side prostaglandin stimulation in isolated cell cultures6 or floating tissue cultures.3, 25 Nonetheless, distinguishing differences between fetal and maternal inflammatory responses as well as the potential for transmembrane signal transduction is imperative for understanding the risks of intrauterine inflammation during pregnancy.
Taking advantage of the separated amniotic and decidual chambers of the Transwell culture system allowed simultaneous measurement of secreted prostaglandins from the fetal and maternal sides of full-thickness gestational membranes in response to an inflammatory stimulus on one or both sides. Our findings confirm previous reports of transmembrane cross-stimulation from decidua to amnion whereby decidual-only LPS exposure resulted in secretion of PGE2 and PGF2α from the amniotic face of the membranes,23, 26 and show for the first time side-specific COX-2 induction corresponding to the side of LPS exposure.
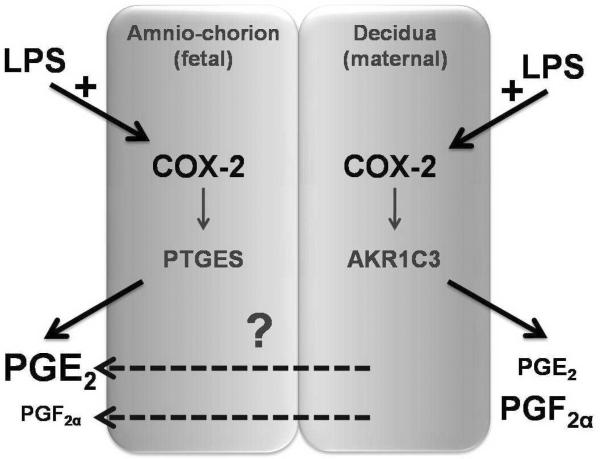
Detection of COX-2 induction in the amniotic and chorionic mesoderm following amniotic LPS treatment and in decidual cells following decidual LPS treatment is consistent with our hypothesis that LPS-stimulated COX-2 induction is responsible for the side-specific prostaglandin response (illustrated in Figure 6). However, because PGE2 increased in the amniotic medium when only the decidual side was exposed to LPS, despite the absence of positive COX-2 immunostaining in the amnion under this exposure condition, additional mechanisms appear to be involved in determination of prostaglandin release from gestational membranes. One possible mechanism could involve the passage of PGE2, PGF2α, or a COX-2 prostaglandin metabolite, originating in the decidua (dashed arrows, Figure 6). Furthermore, the mechanism may involve passage of an inflammatory signal from the decidua. Consistent with the latter suggestion, we previously showed that decidua-only LPS exposure stimulated an increased release of TNF-α but not IL-6, IL-8 or IL-10 on the amniotic side of human gestational membranes cultured on Transwell devices,21 similar to the PGE2 response observed in the present study.
Figure 6. Model of side-specific gestational membrane prostaglandin response to lipopolysaccharide (LPS) stimulation.
LPS induces cyclooxygenase-2 (COX-2) relative to the side of exposure. We propose that relative abundance (denoted by font size) of prostaglandin species secreted from each side is due to tissue-specific expression of prostaglandin synthase enzymes (PTGES and AKR1C3). The mechanism by which prostaglandin E2 (PGE2) and prostaglandin F2α (PGF2α) are released on the amnion side when only the choriodecidual side is treated is not defined (indicated by a question mark).
Moreover, the patterns of PGE2 and PGF2α secretion are similar between the amnion and chorion with relatively more PGE2 than PGF2α in the amniotic medium and relatively more PGF2α than PGE2 in the decidual medium. Induction of COX-2 may be a common mechanism for the increased prostaglandins on both sides of the membranes, with relative differences in tissue-specific expression of PGE synthase enzymes (PTGESI and II) and PGF synthase (aldoketo reductase family 1, member C3, AKR1C3) as a possible explanation for tissue differences of prostaglandin species.12
Lipopolysaccharide caused release of PGE2 and PGF2α from the decidual side regardless of amniotic involvement. Furthermore, decidual-only LPS exposure caused prostaglandin release from the amniotic face of the membranes. This situation mimics ascending infection, the most common type of intrauterine infection whereby microorganisms in the vagina ascend through the cervix and infect the decidua, often leading to premature parturition.14 Elevation of amniotic fluid prostaglandin concentrations is associated with preterm parturition. Although discussions of prostaglandin effects on parturition usually focus on the myometrium, prematurely elevated prostaglandins in the amniotic compartment may cause deleterious effects on the fetus, for example by augmenting an inflammatory response.27, 28
Time-course experiments further defined the differences in amniotic and decidual prostaglandin responses to LPS stimulus. The cellular and tissue responses to LPS occurred rapidly, with PGE2 secretion increased after only 1 h of LPS exposure (Fig. 3A). Secretion of PGE2 into the amniotic culture medium increased between 1 and 8 h of exposure to 100 ng/ml LPS, suggesting a steady rate of biosynthesis over that time period. These results are in agreement with those of Brown et al.,3 in floating intact gestational membrane cultures. The linear release of PGE2 seen in amniotic chamber culture medium from 1–8 h is similar to that observed by Keelen et al. in an Ussing chamber perfusion system; however, at 24 h PGE2 returned to baseline in the present study of Transwell cultures whereas PGE2 concentrations remained elevated at 20 h in the Ussing chamber system.23 We suggest that the decreased PGE2 concentrations observed at 24 h in Transwell cultures is due to degradation or reuptake of secreted prostaglandins. The continued elevation of PGE2 observed by Keelan et al. may be related to continuous perfusion and the greater culture medium volume used with the Ussing chamber. Rajasingam et al. also reported significant increases in amniotic PGE2 production after 6 h of LPS treatment in a dual-chambered culture system.26 However, in contrast to their results, our experiments failed to demonstrate significant increases of decidual PGE2 release after 1, 4 or 8 h of LPS exposure.
Attenuation of the LPS inflammatory response by compensatory anti-inflammatory cytokines could explain the patterns of time-dependent decidual PGE2 release observed in this study. We previously observed that LPS stimulated release of the anti-inflammatory cytokine IL-10 from the decidual side of human gestational membranes in Transwell cultures,21 and decidual-specific increase of interleukin 10 (IL10) may down-regulate the inflammatory response in that tissue. In conjunction with anti-inflammatory cytokines or separately, a shorter duration of prostaglandin synthetic enzyme activation or prostaglandin reuptake and catabolism by hydroxyprostaglandin dehydrogenase 15-(NAD) (HPGD) could thereby have prevented additive accumulation of decidual PGE2 during the 4- and 8-h treatments.
No significant increase of PGE2 concentration was seen in the amniotic or decidual medium with increasing LPS treatment concentrations ranging from 1 to 1000 ng/ml, indicating a threshold effect on PGE2 response to LPS stimulation. Other researchers generally use between 100–1000 ng/ml LPS in cell and tissue culture experiments. Of particular interest, Garcia-Lopez et al.24 observed degradation of the collagen in the mesenchymal layer of gestational membranes exposed to 500 ng/ml LPS for 24 h in Transwell cultures. In the latter study, the effects on the compact collagen layer were proposed as a mechanism for preterm premature rupture of the membranes. However, in the present study, gestational membranes treated with 100 ng/ml LPS treatment showed no evidence of compact layer degradation or other histopathology. We conclude that at least in the case of PGE2, a threshold response is reached at much lower and more physiologically relevant LPS treatment concentrations without evidence of overt tissue damage or cytotoxicity. Differences in LPS potency may be attributed to differences in the bacterial strain source of LPS. We chose LPS from Salmonella typhimurium because of its efficacy in stimulating an immune response.
Ascending infection whereby the decidua is the site of the preliminary immune response is the most common scenario for intrauterine infection. Intrauterine infection originating within the amnion is rare. However, amniotic prostaglandins have been theorized as a source of preterm or term myometrial stimulation. Chorionic HPGD is proposed to serve as a metabolic barrier to the passage of prostaglandins from the amnion to the decidua and myometrium.29 The presence of HPGD in gestational membrane tissues could explain the lack of choriodecidual prostaglandin release when only the amnion side was stimulated, as well as the absence of prostaglandins in choriodecidual medium at the 24-h time point. However, the reverse situation reported here whereby decidual exposure to LPS caused amniotic prostaglandin release complicates the understanding of chorionic HPGD as a metabolic barrier.
In recent years the existence of prostaglandin transporters has become known,30 but no prostaglandin transporters have been identified in human gestational membranes to date. Prostaglandin transporters facilitate passage of prostaglandins into cells in conjunction with lactate exchange.31 Characterization of prostaglandin transporters in gestational membranes may further explain the source and signaling dynamics of prostaglandins in gestational membranes and myometrium.
In vivo, the presence of bacteria, LPS and other bacterial products may augment the inflammatory response and change the dynamics of prostaglandin secretion in the membranes. Therefore, lipopolysaccharide alone may not fully elicit effects on membrane integrity or passage of the inflammatory signal from one side of the membranes to the other. Presumably, the inflammatory response would be prolonged and augmented by mobilization of inflammatory cells to the gestational membranes. However, in the ex vivo experimental model utilized here, the membranes were isolated from such body-wide immunological responses.
In conclusion, the present study demonstrated that LPS caused secretion of uterotonic prostaglandins from both the amnion and decidual sides of intact, non-laboring human gestational membranes in vitro. Immunohistochemistry revealed a regional induction of COX-2 that corresponded with secreted prostaglandins in side-specific exposure experiments. Lipopolysaccharide stimulation of uterotonic prostaglandins from the decidual side of non-laboring gestational membranes may demonstrate a pathological induction of COX-2 and subsequent prostaglandin biosynthesis that could explain some aspects of preterm parturition. However, it remains possible that gestational membranes may respond differently at earlier gestational ages compared with the term tissues used in the present experiments.
Acknowledgements
We thank Sarah Thiel for help with tissue culture experiments and Mark Miller for drawing the Transwell device diagram. We are grateful to the Labor & Delivery staff of the University of Michigan Birth Center for assistance in acquisition of extra-placental tissues. We thank Vasantha Padmanabhan, Dawn Misra and Peter Mancuso for helpful discussions.
Funding This research was completed at The University of Michigan and was support by grants from the National Institute of Environmental Health Sciences (NIEHS), NIH to RLC (P42 ES04911 and ES014860), a University of Michigan Elizabeth H. Crosby Award, and a Society of Toxicology Colgate-Palmolive Grant for Alternative Research. Additional support for NWT was provided by Institutional Training Grants from the NIEHS (T32-ES07062) and the National Institute of Child Health and Human Development (T32 HD007048).
Footnotes
Declaration of interests The authors declare that they have no competing interests.
References
- 1.Mitchell MD, Bibby J, Hicks BR, Turnbull AC. Specific production of prostaglandin E by human amnion in vitro. Prostaglandins. 1978 Feb;15(2):377–382. doi: 10.1016/0090-6980(78)90177-6. [DOI] [PubMed] [Google Scholar]
- 2.Mitchell MD, Trautman MS. Molecular mechanisms regulating prostaglandin action. Mol Cell Endocrinol. 1993 Jun;93(2):C7–10. doi: 10.1016/0303-7207(93)90112-w. [DOI] [PubMed] [Google Scholar]
- 3.Brown NL, Alvi SA, Elder MG, Bennett PR, Sullivan MH. A spontaneous induction of fetal membrane prostaglandin production precedes clinical labour. J Endocrinol. 1998 May;157(2):R1–6. doi: 10.1677/joe.0.157r001. [DOI] [PubMed] [Google Scholar]
- 4.Munns MJ, King RG, Rice GE. Regulation of prostaglandin F2alpha by the specific inhibition of secretory type II phospholipase A2: implications for the management of premature labour. Gynecol Obstet Invest. 1999;48(1):22–27. doi: 10.1159/000010128. [DOI] [PubMed] [Google Scholar]
- 5.Mijovic JE, Zakar T, Nairn TK, Olson DM. Prostaglandin-endoperoxide H synthase-2 expression and activity increases with term labor in human chorion. Am J Physiol. 1997 May;272(5 Pt 1):E832–840. doi: 10.1152/ajpendo.1997.272.5.E832. [DOI] [PubMed] [Google Scholar]
- 6.Romero R, Hobbins JC, Mitchell MD. Endotoxin stimulates prostaglandin E2 production by human amnion. Obstet Gynecol. 1988 Feb;71(2):227–228. [PubMed] [Google Scholar]
- 7.Van Meir CA, Sangha RK, Walton JC, Matthews SG, Keirse MJ, Challis JR. Immunoreactive 15-hydroxyprostaglandin dehydrogenase (PGDH) is reduced in fetal membranes from patients at preterm delivery in the presence of infection. Placenta. 1996 Jul–Aug;17(5–6):291–297. doi: 10.1016/s0143-4004(96)90052-1. [DOI] [PubMed] [Google Scholar]
- 8.Teixeira FJ, Zakar T, Hirst JJ, et al. Prostaglandin endoperoxide-H synthase (PGHS) activity and immunoreactive PGHS-1 and PGHS-2 levels in human amnion throughout gestation, at term, and during labor. J Clin Endocrinol Metab. 1994 Jun;78(6):1396–1402. doi: 10.1210/jcem.78.6.8200943. [DOI] [PubMed] [Google Scholar]
- 9.Hirst JJ, Teixeira FJ, Zakar T, Olson DM. Prostaglandin endoperoxide-H synthase-1 and -2 messenger ribonucleic acid levels in human amnion with spontaneous labor onset. J Clin Endocrinol Metab. 1995 Feb;80(2):517–523. doi: 10.1210/jcem.80.2.7852513. [DOI] [PubMed] [Google Scholar]
- 10.Gibb W, Sun M. Localization of prostaglandin H synthase type 2 protein and mRNA in term human fetal membranes and decidua. J Endocrinol. 1996 Sep;150(3):497–503. doi: 10.1677/joe.0.1500497. [DOI] [PubMed] [Google Scholar]
- 11.Norwitz ER, Lopez Bernal A, Starkey PM. Tumor necrosis factor-alpha selectively stimulates prostaglandin F2 alpha production by macrophages in human term decidua. Am J Obstet Gynecol. 1992 Sep;167(3):815–820. doi: 10.1016/s0002-9378(11)91595-6. [DOI] [PubMed] [Google Scholar]
- 12.Okazaki T, Casey ML, Okita JR, MacDonald PC, Johnston JM. Initiation of human parturition. XII. Biosynthesis and metabolism of prostaglandins in human fetal membranes and uterine decidua. Am J Obstet Gynecol. 1981 Feb 15;139(4):373–381. [PubMed] [Google Scholar]
- 13.Khan AH, Carson RJ, Nelson SM. Prostaglandins in labor--a translational approach. Front Biosci. 2008;13:5794–5809. doi: 10.2741/3117. [DOI] [PubMed] [Google Scholar]
- 14.Goldenberg RL, Culhane JF, Iams JD, Romero R. Epidemiology and causes of preterm birth. Lancet. 2008 Jan 5;371(9606):75–84. doi: 10.1016/S0140-6736(08)60074-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ghidini A, Sirtori M, Romero R, Hobbins JC. Prenatal diagnosis of pentalogy of Cantrell. J Ultrasound Med. 1988 Oct;7(10):567–572. doi: 10.7863/jum.1988.7.10.567. [DOI] [PubMed] [Google Scholar]
- 16.Fidel PL, Jr., Romero R, Wolf N, et al. Systemic and local cytokine profiles in endotoxin-induced preterm parturition in mice. Am J Obstet Gynecol. 1994 May;170(5 Pt 1):1467–1475. doi: 10.1016/s0002-9378(94)70180-6. [DOI] [PubMed] [Google Scholar]
- 17.Kaga N, Katsuki Y, Obata M, Shibutani Y. Repeated administration of low-dose lipopolysaccharide induces preterm delivery in mice: a model for human preterm parturition and for assessment of the therapeutic ability of drugs against preterm delivery. Am J Obstet Gynecol. 1996 Feb;174(2):754–759. doi: 10.1016/s0002-9378(96)70460-x. [DOI] [PubMed] [Google Scholar]
- 18.Reznikov LL, Fantuzzi G, Selzman CH, et al. Utilization of endoscopic inoculation in a mouse model of intrauterine infection-induced preterm birth: role of interleukin 1beta. Biol Reprod. 1999 May;60(5):1231–1238. doi: 10.1095/biolreprod60.5.1231. [DOI] [PubMed] [Google Scholar]
- 19.Buhimschi IA, Buhimschi CS, Weiner CP. Protective effect of N-acetylcysteine against fetal death and preterm labor induced by maternal inflammation. Am J Obstet Gynecol. 2003 Jan;188(1):203–208. doi: 10.1067/mob.2003.112. [DOI] [PubMed] [Google Scholar]
- 20.Sato TA, Keelan JA, Mitchell MD. Critical paracrine interactions between TNF-alpha and IL-10 regulate lipopolysaccharide-stimulated human choriodecidual cytokine and prostaglandin E2 production. J Immunol. 2003 Jan 1;170(1):158–166. doi: 10.4049/jimmunol.170.1.158. [DOI] [PubMed] [Google Scholar]
- 21.Thiex N, Chames M, Loch-Caruso R. Tissue-specific cytokine release from human extra-placental membranes stimulated by lipopolysaccharide in a two-compartment tissue culture system. Reproductive Biology and Endocrinology. 2009;7(1):117. doi: 10.1186/1477-7827-7-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zaga V, Estrada-Gutierrez G, Beltran-Montoya J, Maida-Claros R, Lopez-Vancell R, Vadillo-Ortega F. Secretions of interleukin-1beta and tumor necrosis factor alpha by whole fetal membranes depend on initial interactions of amnion or choriodecidua with lipopolysaccharides or group B streptococci. Biol Reprod. 2004 Oct;71(4):1296–1302. doi: 10.1095/biolreprod.104.028621. [DOI] [PubMed] [Google Scholar]
- 23.Keelan JA, Khan S, Yosaatmadja F, Mitchell MD. Prevention of inflammatory activation of human gestational membranes in an ex vivo model using a pharmacological NF-{kappa}B inhibitor. J Immunol. 2009 October 15;183(8):5270–5278. doi: 10.4049/jimmunol.0802660. 2009. [DOI] [PubMed] [Google Scholar]
- 24.Garcia-Lopez G, Vadillo-Ortega F, Merchant-Larios H, et al. Evidence of in vitro differential secretion of 72 and 92 kDa type IV collagenases after selective exposure to lipopolysaccharide in human fetal membranes. Mol. Hum. Reprod. 2007 June 1;13(6):409–418. doi: 10.1093/molehr/gam025. 2007. [DOI] [PubMed] [Google Scholar]
- 25.Lappas M, Permezel M, Georgiou HM, Rice GE. Nuclear factor kappa B regulation of proinflammatory cytokines in human gestational tissues in vitro. Biol Reprod. 2002 Aug;67(2):668–673. doi: 10.1095/biolreprod67.2.668. [DOI] [PubMed] [Google Scholar]
- 26.Rajasingam D, Bennett PR, Alvi SA, Elder MG, Sullivan MH. Stimulation of prostaglandin production from intact human fetal membranes by bacteria and bacterial products. Placenta. 1998 May;19(4):301–306. doi: 10.1016/s0143-4004(98)90062-5. [DOI] [PubMed] [Google Scholar]
- 27.Malaeb S, Dammann O. Fetal Inflammatory Response and Brain Injury in the Preterm Newborn. Journal of Child Neurology. 2009 Sep;24(9):1119–1126. doi: 10.1177/0883073809338066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Romero R, Gotsch F, Pineles B, Kusanovic JP. Inflammation in pregnancy: its roles in reproductive physiology, obstetrical complications, and fetal injury. Nutr Rev. 2007 Dec;65(12 Pt 2):S194–202. doi: 10.1111/j.1753-4887.2007.tb00362.x. [DOI] [PubMed] [Google Scholar]
- 29.Challis JR, Patel FA, Pomini F. Prostaglandin dehydrogenase and the initiation of labor. J Perinat Med. 1999;27(1):26–34. doi: 10.1515/JPM.1999.003. [DOI] [PubMed] [Google Scholar]
- 30.Kanai N, Lu R, Satriano JA, Bao Y, Wolkoff AW, Schuster VL. Identification and characterization of a prostaglandin transporter. Science. 1995 May 12;268(5212):866–869. doi: 10.1126/science.7754369. [DOI] [PubMed] [Google Scholar]
- 31.Chan BS, Endo S, Kanai N, Schuster VL. Identification of lactate as a driving force for prostanoid transport by prostaglandin transporter PGT. Am J Physiol Renal Physiol. 2002 Jun;282(6):F1097–1102. doi: 10.1152/ajprenal.00151.2001. [DOI] [PubMed] [Google Scholar]