Abstract
Objectives
Lipoprotein-associated phospholipase A2 (Lp-PLA2) levels predict incident coronary heart disease (CHD) in adults without known CHD, independent of heart disease risk factors. We examined whether the independent association was apparent in older adults.
Background
Serum levels of Lp-PLA2, an enzyme that hydrolyzes oxidized phospholipids to yield potentially proatherogenic particles, have been associated with CHD and may help predict cardiovascular risk.
Methods
Participants were 1,077 community-dwelling men and women, median age 72 years, who had no known CHD at baseline (1984 to 1987) when blood samples and risk factor data were collected. Participants were followed for CHD events for a mean of 16 years, through 2002. Cox proportional hazards regression models were used to examine the association of serum Lp-PLA2 with incident CHD (myocardial infarction, angina, or coronary revascularization).
Results
The Lp-PLA2 levels positively correlated with age (r = 0.09), body mass index (r = 0.11), low-density lipoprotein (r = 0.37), triglycerides (r = 0.25), and C-reactive protein (r = 0.10), and negatively correlated with high-density lipoprotein (r = −0.27) (all p < 0.05). During follow-up, 228 participants had incident CHD events. Lipoprotein-associated phospholipase A2 levels in the second, third, and fourth quartiles predicted an increased risk of CHD compared with the lowest quartile (hazard ratios 1.66, 1.80, and 1.89, respectively; p < 0.05 for each). This association persisted after adjusting for C-reactive protein and other CHD risk factors.
Conclusions
Elevated Lp-PLA2 levels predict CHD events in apparently healthy older adults, independent of CHD risk factors.
Lipoprotein-associated phospholipase A2 (Lp-PLA2), also known as platelet-activating factor acetylhydrolase, is a 50-kd calcium-independent enzyme synthesized by macrophages and platelets (1). It is a member of the phospholipase A2 superfamily, and is colocated with and highly expressed by macrophages within the necrotic core and the fibrotic cap of advanced rupture-prone plaques (2,3). The primary enzymatic role of Lp-PLA2 is to hydrolyze oxidized phospholipids, yielding the proinflammatory and proatherogenic products, lysophosphatidylcholine and oxidized nonesterified fatty acids. These bioactive products play critical roles in the induction of chemotactic response, endothelial cell dysfunction, and smooth muscle cell apoptosis (4). As such, Lp-PLA2 appears to be a key mediator of the proatherogenic process.
Several epidemiologic studies support the proatherogenic effects of Lp-PLA2, most of which have included only middle-aged men. Higher Lp-PLA2 levels were strongly associated with an increased risk of incident coronary heart disease (CHD) in a nested case-control analysis of 1,740 men with a mean age of 57 years from WOSCOPS (West of Scotland Coronary Prevention Study) (5) and in a prospective cohort analysis of 934 men ages 45 to 64 years from the MONICA (Monitoring Trends and Determinants in Cardiovascular Diseases) study (6). The significant elevation in risk persisted in these studies after adjustment for traditional risk factors and C-reactive protein (CRP), a marker of systemic inflammation. A case-cohort analysis from the ARIC (Atherosclerosis Risk In Communities) study, which included both men and women (n = 1,652) ages 45 to 64 years, reported a strong positive relationship between Lp-PLA2 levels and CHD outcomes. The association was independent of traditional risk factors and CRP only in those with low-density lipoprotein (LDL) cholesterol <130 mg/dl (7). A nested case-control analysis of 246 low-risk women from the Women’s Health study (mean age 60 years) also reported higher Lp-PLA2 levels in women with increased CHD risk, but adjustment for other cardiovascular risk factors eliminated the association (8), suggesting that Lp-PLA2 may not be as strong a predictor of CHD in women.
Because age is the single most powerful predictor of heart disease, it is possible that Lp-PLA2 levels do not contribute anything further to CHD risk prediction in the elderly. Currently, limited data are available on Lp-PLA2 mass levels and incident CHD in the elderly. In the Rotterdam study, where the mean age of subjects was 70 years and median follow-up was 7 years, those with higher baseline levels of Lp-PLA2 activity were at increased risk for CHD and ischemic stroke, but data on LDL cholesterol levels or Lp-PLA2 mass levels were not reported (9).
We investigated the ability of Lp-PLA2 to predict fatal and nonfatal CHD events independent of traditional risk factors and CRP in community-dwelling older men and women who were in apparently good health and were followed for an average of 16 years.
Methods
Study population
The Rancho Bernardo study is an ongoing population-based study of healthy aging in Caucasian residents of a Southern California community, which was established in 1972. Between 1984 and 1987, 82% of surviving community-dwelling older participants attended a research clinic visit, which served as the baseline visit for the present report. The subjects of this report are the 1,077 participants age >40 years who at baseline had no history of cardiovascular disease (myocardial infarction, coronary revascularization, angina, stroke, or transient ischemic attack), who were not current users of hormone therapy, and who had sufficient archived serum for measurement of Lp-PLA2. The study protocol was approved by the Institutional Review Board at the University of California, San Diego; all participants provided written, informed consent.
Medical history and incident CHD information were obtained using standardized questionnaires at baseline, at clinic visits approximately every 4 years thereafter, and from periodic mailings. Follow-up continued through 2002, an 18-year follow-up (mean follow-up 16 years). Study participants were classified as incident CHD cases if at any time during the follow-up period they had a fatal or nonfatal myocardial infarction, coronary revascularization, or angina (grades 1 or 2 by Rose criteria) (10). Myocardial infarction was diagnosed based on a history of physician-diagnosed myocardial infarction or evidence of myocardial infarction on resting 12-lead electrocardiogram using the Whitehall criteria as applied in the World Health Organization’s Multinational Study of Diabetes and Vascular Disease (Minnesota codes 1.1 to 1.2 [major Q-wave] or 7.1.1 [left bundle branch block]) (11,12). At another study visit, diagnosis of a heart attack had been validated in 85% of the cases by a panel of cardiologists who reviewed the medical records of a 30% sample of this cohort.
Vital status was known for 96% of participants. Death certificates were obtained for 95% of all decedents and coded by a certified nosologist using the International Classification of Disease-9th Revision criteria; decedents whose death certificates were unavailable were excluded from this analysis. Fatal CHD included deaths assigned codes 410 through 414. Participants who were alive and event-free were censored at the end of follow-up (December 31, 2002), or at the date of their last follow-up for the 4% whose status was unknown. Subjects who died of non-CHD causes were censored at time of death.
Measurements
Baseline data for these analyses were obtained in 1984 to 1987. Information on medical history, medication use, physical activity (exercise 3+ times per week, yes/no), alcohol consumption (1+ drinks per day vs. less or none), and current smoking (yes/no) was obtained using standard questionnaires. Current medication use was validated by examination of pills and prescriptions brought to the clinic for that purpose. Blood pressure was measured by standard protocol as the mean of 2 readings after the participant had been sitting for 5 min. Body mass index (BMI) (kg/m2) was derived from height and weight measured in the clinic with participants wearing light clothing and no shoes.
Blood samples were obtained by venipuncture between 7:30 AM and 11:00 AM after a requested 12-h fast. Serum and plasma were separated and stored at −70°C. Lipids and lipoproteins were measured on fresh samples in a Lipid Research Clinic laboratory at the University of California at San Diego (San Diego, Calfornia). Total cholesterol and triglyceride levels were measured by enzymatic techniques using an ABA-200 biochromatic analyzer (Abbott Laboratories, Irving, Texas). High-density lipoprotein (HDL) cholesterol was measured by precipitating other circulating lipoproteins with heparin and manganese chloride according to the standard Lipid Research Clinics protocol (13). Low-density lipoprotein cholesterol was estimated using the Friedewald formula (14). Plasma glucose levels were measured by the glucose oxidase method. In 2000, plasma levels of high-sensitivity CRP were measured in a university hospital laboratory using an automated high-sensitivity assay (N Latex CRP mono, Dade Behring, Marburg, Germany; sensitivity 0.2 mg/l) on previously unthawed plasma that had been stored at −70°C.
Lipoprotein-associated phospholipase A2 mass levels were measured in 2005 using the PLAC test (diaDexus, Inc., South San Francisco, California) on frozen sera from the same visit, which had been thawed 2 times previously. The manufacturer reports that samples can be frozen and thawed up to 6 times without affecting Lp-PLA2 quantification. The assay is a microplate-based enzyme-linked immunoadsorbent assay, employing 2 monoclonal antibodies specific for Lp-PLA2, and calibrated to a recombinant enzyme standard. The assay sensitivity was 1.3 ng/ml and intra- and interassay coefficients of variation were <6% and <9%, respectively.
Subjects were classified as persons with diabetes according to 1999 World Health Organization criteria if the fasting plasma glucose was ≥126 mg/dl, the 2-h glucose after oral glucose tolerance test was ≥200 mg/dl, if they were taking insulin or oral diabetes medication, or if they were diagnosed as a patient with diabetes by a physician (15).
Statistical methods
Means are expressed ± standard deviation. High-density lipoprotein, triglycerides, CRP, and Lp-PLA2 were not normally distributed and were log transformed for analyses; geometric means are reported. Baseline characteristics were compared between cases and noncases using general linear models adjusted for age for continuous variables and chi-square analysis for categorical variables; categorical variables were adjusted using the adjusted proportions module in STATA, which calculates adjusted probabilities from logistic regression estimates (16). Associations between Lp-PLA2 and other risk factors were examined using Spearman rank-order correlation.
Multivariate Cox proportional hazards regression models were developed to determine the association of Lp-PLA2 with incident CHD. The primary null hypothesis was that Lp-PLA2 was not predictive of CHD events over and above various demographic variables and traditional risk factors. Results were expressed as hazard ratios (HRs) with 95% confidence intervals (CIs). Study participants were categorized into quartiles of Lp-PLA2, and 4 sequential Cox regression models were generated. Model 1 adjusted for age and gender. For Model 2, life-style, risk factor, and laboratory covariates from Table 1 were analyzed, and univariate predictors of CHD events were identified if significant at p < 0.25. This process yielded age, gender, HDL, triglycerides, LDL, fasting plasma glucose, systolic blood pressure, diabetes, hypertension, and Lp-PLA2 as potential predictors. Cox regression analysis was performed using these 10 covariates (Model 2), and those that were significant at p < 0.05 (age, gender, fasting plasma glucose, systolic blood pressure, and Lp-PLA2) were retained in the final Model 3. No 2-way interactions were identified. Model 4 incorporated the same covariates as Model 3 but included only the subset of participants who had CRP measurements available (n = 815) so that the effect of adding CRP to the model could be distinguished from any effect due to loss of power. Model 5 was the same as Model 4, but with CRP added as a covariate. All variables were treated as continuous variables except gender, diabetes, history of hypertension, smoking status, physical activity, and alcohol consumption, which were treated as dichotomous variables. The lowest quartile was the reference group for all regressions.
Table 1.
Age-Adjusted Mean ± SD or Prevalence of Risk Factors and Medications at Baseline (1984 to 1987)
| Variable | Cases (n = 228) |
Noncases (n = 849) |
p Value (Cases Vs. Noncases) |
|---|---|---|---|
| Age, yrs* | 72.2 ± 8.8 | 69.5 ± 10.6 | <0.001 |
| Women, %* | 42.5 | 47.5 | 0.19 |
| Lifestyle factors, % | |||
| Current smoker | 12.2 | 12.2 | 0.97 |
| Exercise >30×/week | 80.3 | 81.5 | 0.69 |
| Alcohol daily | 44.9 | 44.2 | 0.84 |
| Medication use, % | |||
| Aspirin | 17.2 | 18.6 | 0.65 |
| Lipid-lowering drugs | 0.4 | 0.5 | 0.88 |
| Beta-blockers | 14.3 | 12.2 | 0.41 |
| Risk factors | |||
| Diabetes, % | 15.9 | 12.2 | 0.13 |
| Hypertension, % | 67.0 | 60.6 | 0.093 |
| BMI | 25.6 ± 3.6 | 25.0 ± 3.6 | 0.041 |
| Systolic BP, mm Hg | 143 ± 19 | 139 ± 19 | 0.005 |
| Diastolic BP, mm Hg | 78 ±9 | 77 ± 9 | 0.22 |
| Laboratory values | |||
| Total cholesterol, mg/dl | 223 ± 40 | 218 ± 40 | 0.078 |
| HDL, mg/dl† | 55 ± 16 | 59 ± 17 | 0.006 |
| LDL, mg/dl | 141 ± 36 | 135 ± 36 | 0.013 |
| Triglycerides, mg/dl† | 106 ± 57 | 99 ± 54 | 0.10 |
| Fasting plasma glucose, g/dl | 106 ± 21 | 100 ± 21 | <0.001 |
| CRP, mg/l† | 1.8 ± 1.7 | 1.6 ± 1.6 | 0.18 |
| Lp-PLA2, ng/ml† | 538 ± 180 | 494 ± 167 | <0.001 |
Unadjusted mean; all others are age-adjusted
geometric mean.
BMI = body mass index; BP = blood pressure; CRP = C-reactive protein; HDL = high-density lipoprotein; LDL = low-density lipoprotein; Lp-PLA2 = lipoprotein-associated phospholipase A2; SD = standard deviation.
Age-adjusted survival curves were generated by the average covariate method, computing a survival curve for each group with age set to the average value for the study sample (17). Receiver-operating characteristic curves were constructed after adjustment for covariates, and the area under the curve with its 95% CI was calculated. Significance of differences between receiver-operating characteristic curves were tested using a nonparametric approach to the analysis of areas under correlated curves (18). A 2-tailed p < 0.05 was considered statistically significant. Data were analyzed using SAS, version 9.1 (SAS Institute, Cary, North Carolina) and STATA version 9 (Stata Corp., College Station, Texas).
Results
Baseline characteristics
Of the 1,077 participants in the study population, 228 (131 men, 97 women) experienced a CHD event during follow-up (mean 16 years) and were categorized as cases. The events included 86 (37.7%) fatal myocardial infarction, 70 (30.7%) nonfatal myocardial infarction, 43 (18.9%) angina, and 29 (12.7%) revascularization procedures, with mean time to event of 7.6 years. Noncases included 446 men and 403 women.
Baseline characteristics of the study population by CHD status are shown in Table 1. In an analysis of baseline risk factors and medication use, the study population was similar to the overall cohort from which it was derived except that those without Lp-PLA2 measurements (n = 494) were slightly younger (69 vs. 70 years, p < 0.01), more likely to be women (59% vs. 46%, p < 0.01), slightly more likely to have diabetes (17% vs. 13%, p = 0.02), and had lower diastolic blood pressure (76 mm Hg vs. 78 mm Hg, p < 0.001).
As shown in Table 1, at baseline individuals who later experienced CHD events were significantly older (mean age 72.2 ± 8.8 years vs. 69.5 ± 10.6 years) and had significantly higher BMI, systolic blood pressure, LDL, and Lp-PLA2 (538 ng/ml vs. 494 ng/ml, p < 0.001) and significantly lower HDL levels than noncases. No significant differences by CHD status were observed for CRP, diastolic blood pressure, total cholesterol, triglycerides, or interleukin 6, nor were there differences in gender distribution or in the prevalence of smoking, diabetes, or hypertension in cases versus noncases.
Lp-PLA2 values and associations with other risk factors
Median Lp-PLA2 levels were similar in participants who were older than 70 compared with those who were younger and did not differ by gender, diabetes, or smoking status (Table 2). Lipoprotein-associated phospholipase A2 levels were significantly lower in participants who drank alcohol daily compared with less frequent drinkers. Lipoprotein-associated phospholipase A2 levels positively correlated (all p < 0.05) with total cholesterol (r = 0.29), LDL cholesterol (r = 0.37), and triglycerides (r = 0.25) and negatively correlated with HDL cholesterol (r = −0.27) (Table 3). Weak, but significant, positive associations were also observed between Lp-PLA2 and systolic blood pressure (r = 0.08), CRP (r = 0.10), and BMI (r = 0.11) (Table 3). Lipoprotein-associated phospholipase A2 levels did not differ by use of aspirin or beta-blockers (data not shown).
Table 2.
Median Lp-PLA2 Levels by Baseline Characteristics
| Variable | n | Lp-PLA2 (ng/ml) |
IQR | p Value |
|---|---|---|---|---|
| Gender | ||||
| Male | 577 | 496 | 234 | 0.12 |
| Female | 500 | 477 | 202 | |
| Age, yrs | ||||
| <70 | 471 | 478 | 223 | 0.15 |
| ≥70 | 606 | 496 | 209 | |
| Diabetes | ||||
| Diabetic patients | 144 | 514 | 235 | 0.14 |
| Nondiabetic patients | 933 | 487 | 216 | |
| Smoking | ||||
| Current smokers | 136 | 510 | 246 | 0.23 |
| Nonsmokers | 939 | 487 | 214 | |
| Exercise | ||||
| Exercise ≥3× per week | 875 | 485 | 215 | 0.086 |
| Exercise <3× per week | 202 | 517 | 227 | |
| Alcohol | ||||
| Daily alcohol drinkers | 477 | 476 | 216 | 0.048 |
| Less than daily drinkers | 599 | 498 | 216 | |
IQR = interquartile range; Lp-PLA2 = lipoprotein-associated phospholipase A2.
Table 3.
Correlation Between Lp-PLA2 and Other Risk Factors
| Risk Factor | Spearman Rho | p Value |
|---|---|---|
| Age | 0.07 | 0.018 |
| BMI | 0.11 | <0.001 |
| Systolic BP | 0.08 | 0.010 |
| Diastolic BP | 0.01 | 0.070 |
| Total cholesterol | 0.29 | <0.001 |
| HDL | −0.27 | <0.001 |
| LDL | 0.37 | <0.001 |
| Triglycerides | 0.25 | <0.001 |
| Fasting plasma glucose | 0.06 | 0.054 |
| CRP | 0.10 | 0.003 |
Abbreviations as in Table 1.
Prediction of incident CHD
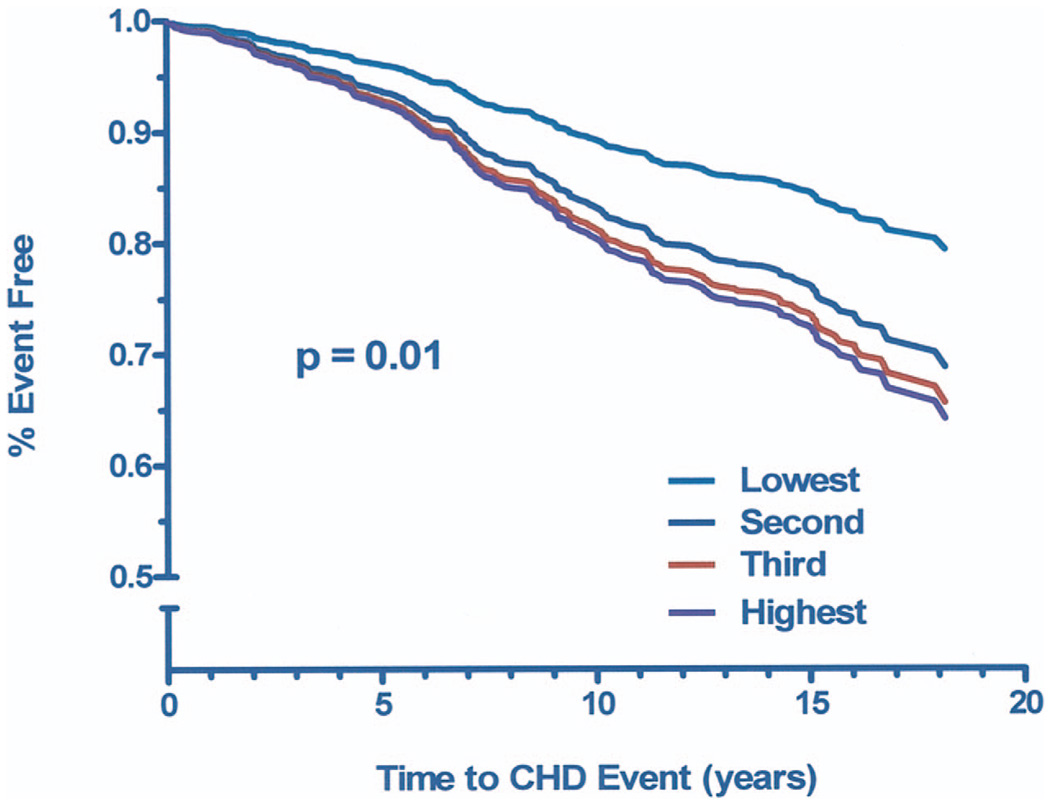
In an age- and gender-adjusted Cox proportional hazards regression model, participants with Lp-PLA2 levels in the second, third, and fourth quartiles had 66%, 80%, and 89% increased risk of incident CHD compared with those in the lowest quartile (all p < 0.05) (Table 4, Model 1). Results remained significant after further adjustment for cardiovascular risk factors (Model 2); only fasting plasma glucose levels and systolic blood pressure remained in the final model (Model 3). When analysis was limited to those who had CRP measurements, results were significant for the third and fourth quartiles of Lp-PLA2 (Model 4), with risk estimates similar to those for the entire study group. Additional adjustment for CRP levels had minimal effect on the Lp-PLA2-incident CHD association (Model 5). Results were also similar when those with LDL levels above the median were compared with those with LDL levels below the median (data not shown). There was no interaction of gender and Lp-PLA2 in any of these models. Figure 1 shows graded age-adjusted CHD event-free survival by Lp-PLA2 tertile (p < 0.01).
Table 4.
HRs (95% CIs) for Incident CHD by Lp-PLA2 Quartile*
| Lp-PLA2 Quartiles | |||
|---|---|---|---|
| 2nd Quartile | 3rd Quartile | 4th Quartile | |
| (409.9–488.4 ng/ml) | (488.5–625.2 ng/ml) | (> 625.2 ng/ml) | |
| Model 1 | 1.66 (1.09–2.54) [269] |
1.80 (1.19–2.72) [270] |
1.89 (1.26–2.86) [269] |
| Model 2 | 1.54 (1.01–2.37) [269] |
1.71 (1.12–2.61) [270] |
1.61 (1.03–2.51) [269] |
| Model 3 | 1.59 (1.04–2.44) [269] |
1.83 (1.21–2.77) [270] |
1.81 (1.20–2.73) [269] |
| Model 4 | 1.41 (0.87–2.26) [211] |
1.93 (1.22–3.07) [198] |
1.75 (1.10–2.77) [199] |
| Model 5 | 1.43 (0.89–2.31) [211] |
1.96 (1.23–3.10) [198] |
1.75 (1.10–2.78) [199] |
Lowest quartile (<409.9 ng/ml) is reference. The number in brackets is the n for each quartile. Quartile cutpoints were calculated based on the sample of 1,077. The n for Q1 is [269] for Models 1, 2, and 3 and [209] for Models 4 and 5. Model 1 (n = 1,077): adjusted for age and gender; Model 2 (n = 1,077): adjusted for age, gender, fasting plasma glucose, systolic blood pressure, hypertension, diabetes, low-density lipoprotein, lnhigh-density lipoprotein, and lntriglycerides; Model 3 (n = 1,077): adjusted for age, gender, fasting plasma glucose, systolic blood pressure; Model 4 (n = 815): Model 3, but includes only those subjects who had C-reactive protein measured (C-reactive protein not in the model); Model 5 (n = 815): Model 4 + C-reactive protein.
CHD = coronary heart disease; CI = confidence interval; HR = hazard ratio; ln = natural logarithm; Lp-PLA2 = lipoprotein-associated phospholipase A2.
Figure 1. Age-Adjusted Event-Free Survival by Lp-PLA2 Tertile.
Age-adjusted survival free of fatal or nonfatal myocardial infarction, coronary revascularization, or angina, by lipoprotein-associated phospholipase A2 (Lp-PLA2) tertile (low 108.1 to 434.4 ng/ml; medium 434.4 to 574.2 ng/ml; high 574.3 to 3,126.8 ng/ml). CHD = coronary heart disease.
Receiver-operating characteristic curves were constructed to evaluate the incremental benefit of Lp-PLA2 in predicting incident CHD. Although the addition of CRP to a model including age, gender, hypertension, diabetes, smoking, and exercise did not change the area under the curve (0.595 [95% CI 0.549 to 0.640] vs. 0.595 [95% CI 0.550 to 0.641], p < 0.93), the further addition of Lp-PLA2 significantly increased the area under the curve to 0.617 (95% CI 0.572 to 0.662) (p < 0.04) (Table 5).
Table 5.
Predictive Accuracy of Various Multivariate Models to Predict Incident CHD as Measured by an Increase in the AUC
| Model Adjusted for | AUC (95% CI) | p Value* |
|---|---|---|
| 1. Age, gender, hypertension, diabetes, smoking, exercise | 0.595 (0.549–0.640) | |
| 2. Above covariates plus CRP | 0.595 (0.550–0.641) | 0.93 |
| 3. Above covariates plus CRP and Lp-PLA2 | 0.617 (0.572–0.662) | 0.037 |
p values are for comparisons between 2 consecutive models.
AUC = area under the receiver-operating characteristic curve; CRP = C-reactive protein; other abbreviations as in Table 4.
The overall findings remained the same when the definition of incident CHD excluded angina or was limited to myocardial infarction or revascularization or myocardial infarction alone.
Discussion
In the present study of older community-dwelling individuals, Lp-PLA2 levels were significantly higher in those who developed CHD compared with those who did not. Associations between Lp-PLA2 and LDL, HDL, total cholesterol, and triglycerides were especially strong, and the magnitude of the correlations was in good agreement with previous studies (7,19). Despite these associations, Lp-PLA2 remained a strong and independent predictor of fatal and nonfatal CHD events, over and above these and other traditional risk factors. Thus, Lp-PLA2 added information to lipid and lipoprotein prediction of future CHD and may identify subpopulations at risk for CHD who would not be identified otherwise.
A similar independent association of Lp-PLA2 with CHD risk was reported in the younger participants from the WOSCOPS (5) and ARIC (7,19) studies and in a nested case-control study from the Rotterdam study (9). We now confirm these results for the first time in a cohort of apparently healthy older men and women followed for a longer period of time. In agreement with the ARIC and Rotterdam studies (7,9,19), the adjusted risk of incident CHD was significantly elevated beginning in the second quartile of Lp-PLA2, suggesting that, as in middle-aged adults, even minimally elevated Lp-PLA2 values are associated with an increased risk of developing CHD in older adults.
The addition of CRP to the fully adjusted model did not diminish the predictive capability of Lp-PLA2, evidence that the 2 markers are not codependent. This is in agreement with previous studies in which Lp-PLA2 maintained significance in models adjusted for CRP and all traditional factors in WOSCOPS (relative risk per 1 standard deviation = 1.18, 95% CI 1.05 to 1.33) (5) and ARIC (HR of highest tertile in the LDL <130 mg/dl population = 2.08, 95% CI 1.20 to 3.62) (7). Similar results were also seen for the 2 highest quartiles of Lp-PLA2 in the Rotterdam study CHD analysis (9).
One difference of note is the higher distribution of Lp-PLA2 values in the present study compared with that seen in previous studies. Mean values of Lp-PLA2 in young adults in the CARDIA (Coronary Artery Risk Development in Young Adults) study were 296 ng/ml and 267 ng/ml in cases and control subjects, respectively (20). The middle-aged population evaluated in the ARIC study reported Lp-PLA2 mean values of 404 ng/ml and 373 ng/ml in cases and control subjects. The higher values observed in the present study (538 ng/ml for cases, 494 ng/ml for noncases) may reflect the higher risk in an older population, an effect of the decade-long storage of samples, or may result from other as yet undetermined factors. Although we found no difference in Lp-PLA2 values in participants older or younger than the median age of 70 years, Lp-PLA2 values were significantly higher in subjects >55 years of age compared with those seen in subjects age ≤55 years, lending credence to an age-associated increment in Lp-PLA2 at midlife. Although the absolute Lp-PLA2 values in this study are higher than in previous studies, the correlations between Lp-PLA2 levels and other measures such as LDL and HDL are almost identical to those seen in prior studies (6,7,9), supporting the utility if not the optimal cutpoint for our values.
As a corollary to the observed higher Lp-PLA2 levels in this older population, it is likely that nearly all subjects had some degree of cardiovascular disease pathology since atherosclerotic burden increases with age. Thus, HRs for cases versus noncases might be somewhat lower than in a population where the noncases have a lower atherosclerotic burden. However, our observed HR for incident CHD of 1.6 for the highest versus lowest quartile in the present study is only slightly lower than the reported HR of approximately 1.8 from WOSCOPS (though quintiles were used there).
One advantage of our study is that the cohort was recruited in the 1970s. At the time samples were drawn, available pharmacotherapies were limited, and only 5 participants were taking lipid-lowering medications. Also during this time period, few were taking aspirin regularly for cardiovascular prevention. If our subjects’ use of statins, angiotensin-converting enzyme inhibitors, and anti-inflammatory medications was more in line with the present higher levels of use, the perceived utility of Lp-PLA2 might have been altered. It is unknown whether the modern pharmacotherapies would attenuate, accentuate, or have no effect on the predictive capability of Lp-PLA2. Additional studies are required to identify and characterize the magnitude of any such alterations.
Conclusions
In summary, in a cohort of community-dwelling older adults followed for an average of 16 years, measurement of Lp-PLA2 mass demonstrated strong predictive capability for subsequent CHD events. This predictive capability was independent of traditional risk factors of CHD and in accord with previous findings. The present study extends the previous findings into the older population and represents a potential clinical application of Lp-PLA2 measurements in identification of individuals at risk for CHD who would not otherwise be identified by traditional measures.
Acknowledgments
The Rancho Bernardo study was funded by research grant AG07181 from the National Institute on Aging and grant DK31801 from the National Institute of Diabetes and Digestive and Kidney Diseases. The present analysis was supported, in part, by a grant from the American Heart Association (to Dr. Daniels). The Lp-PLA2 levels were measured by diaDexus, Inc. Mr. Sarno and Ms. Bettencourt have received consulting fees from diaDexus, Inc. Dr. Wolfert is employed by and owns stock options in diaDexus, Inc. Steven E. Nissen, MD, MACC, served as Guest Editor for this paper.
Abbreviations and Acronyms
- BMI
body mass index
- CHD
coronary heart disease
- CI
confidence interval
- CRP
C-reactive protein
- HDL
high-density lipoprotein
- HR
hazard ratio
- LDL
low-density lipoprotein
- Lp-PLA2
lipoprotein-associated phospholipase A2
REFERENCES
- 1.Tjoelker LW, Eberhardt C, Unger J, et al. Plasma platelet-activating factor acetylhydrolase is a secreted phospholipase A2 with a catalytic triad. J Biol Chem. 1995;270:25481–25487. doi: 10.1074/jbc.270.43.25481. [DOI] [PubMed] [Google Scholar]
- 2.Hakkinen T, Luoma JS, Hiltunen MO, et al. Lipoprotein-associated phospholipase A(2), platelet-activating factor acetylhydrolase, is expressed by macrophages in human and rabbit atherosclerotic lesions. Arterioscler Thromb Vasc Biol. 1999;19:2909–2917. doi: 10.1161/01.atv.19.12.2909. [DOI] [PubMed] [Google Scholar]
- 3.Kolodgie FD, Burke AP, Skorija KS, et al. Lipoprotein-associated phospholipase A2 protein expression in the natural progression of human coronary atherosclerosis. Arterioscler Thromb Vasc Biol. 2006;26:2523–2529. doi: 10.1161/01.ATV.0000244681.72738.bc. [DOI] [PubMed] [Google Scholar]
- 4.Yang EH, McConnell JP, Lennon RJ, et al. Lipoprotein-associated phospholipase A2 is an independent marker for coronary endothelial dysfunction in humans. Arterioscler Thromb Vasc Biol. 2006;26:106–111. doi: 10.1161/01.ATV.0000191655.87296.ab. [DOI] [PubMed] [Google Scholar]
- 5.Packard CJ, O’Reilly DS, Caslake MJ, et al. Lipoprotein-associated phospholipase A2 as an independent predictor of coronary heart disease. West of Scotland Coronary Prevention study group. N Engl J Med. 2000;343:1148–1155. doi: 10.1056/NEJM200010193431603. [DOI] [PubMed] [Google Scholar]
- 6.Koenig W, Khuseyinova N, Lowel H, Trischler G, Meisinger C. Lipoprotein-associated phospholipase A2 adds to risk prediction of incident coronary events by C-reactive protein in apparently healthy middle-aged men from the general population: results from the 14-year follow-up of a large cohort from southern Germany. Circulation. 2004;110:1903–1908. doi: 10.1161/01.CIR.0000143377.53389.C8. [DOI] [PubMed] [Google Scholar]
- 7.Ballantyne CM, Hoogeveen RC, Bang H, et al. Lipoprotein-associated phospholipase A2, high-sensitivity C-reactive protein, and risk for incident coronary heart disease in middle-aged men and women in the Atherosclerosis Risk in Communities (ARIC) study. Circulation. 2004;109:837–842. doi: 10.1161/01.CIR.0000116763.91992.F1. [DOI] [PubMed] [Google Scholar]
- 8.Blake GJ, Dada N, Fox JC, Manson JE, Ridker PM. A prospective evaluation of lipoprotein-associated phospholipase A(2) levels and the risk of future cardiovascular events in women. J Am Coll Cardiol. 2001;38:1302–1306. doi: 10.1016/s0735-1097(01)01554-6. [DOI] [PubMed] [Google Scholar]
- 9.Oei HH, van der Meer IM, Hofman A, et al. Lipoprotein-associated phospholipase A2 activity is associated with risk of coronary heart disease and ischemic stroke: the Rotterdam study. Circulation. 2005;111:570–575. doi: 10.1161/01.CIR.0000154553.12214.CD. [DOI] [PubMed] [Google Scholar]
- 10.Rose GA. The diagnosis of ischaemic heart pain and intermittent claudication in field surveys. Bull World Health Organ. 1962;27:645–658. [PMC free article] [PubMed] [Google Scholar]
- 11.Diabetes Drafting Group. Prevalence of small vessel and large vessel disease in diabetic patients from 14 centres. The World Health Organisation Multinational Study of Vascular Disease in Diabetics. Diabetes drafting group. Diabetologia. 1985;28 Suppl:615–640. doi: 10.1007/BF00290267. [DOI] [PubMed] [Google Scholar]
- 12.Reid DD, Hamilton PJ, McCartney P, Rose G, Jarrett RJ, Keen H. Smoking and other risk factors for coronary heart-disease in British civil servants. Lancet. 1976;2:979–984. doi: 10.1016/s0140-6736(76)90830-8. [DOI] [PubMed] [Google Scholar]
- 13.National Heart and Lung Institute Lipid Research Clinics Program Laboratory Methods Committee. 2nd edition. Washington, DC: US Government Printing Office, DHEW publication no. 75–628; 1974. Manual of Laboratory Operations: Lipid and Lipoprotein Analysis. [Google Scholar]
- 14.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 15.World Health Organization. Geneva: World Health Org.; 1999. Definition, Diagnosis and Classification of Diabetes Mellitus and its Complications: Report of a WHO Consultation. Part 1: Diagnosis and Classification of Diabetes Mellitus. [Google Scholar]
- 16.Garrett J. Calculation of Adjusted Means and Proportions. [Accessed January 28, 2007];2001 Available at: http://www.stata.com/stb/stb24/sg33/ [Google Scholar]
- 17.Chang IM, Gelman R, Pagano M. Corrected group prognostic curves and summary statistics. J Chronic Dis. 1982;35:669–674. doi: 10.1016/0021-9681(82)90019-4. [DOI] [PubMed] [Google Scholar]
- 18.DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44:837–845. [PubMed] [Google Scholar]
- 19.Ballantyne CM, Hoogeveen RC, Bang H, et al. Lipoprotein-associated phospholipase A2, high-sensitivity C-reactive protein, and risk for incident ischemic stroke in middle-aged men and women in the Atherosclerosis Risk in Communities (ARIC) study. Arch Intern Med. 2005;165:2479–2484. doi: 10.1001/archinte.165.21.2479. [DOI] [PubMed] [Google Scholar]
- 20.Iribarren C, Gross MD, Darbinian JA, Jacobs DR, Jr, Sidney S, Loria CM. Association of lipoprotein-associated phospholipase A2 mass and activity with calcified coronary plaque in young adults: the CARDIA study. Arterioscler Thromb Vasc Biol. 2005;25:216–221. doi: 10.1161/01.ATV.0000148322.89911.44. [DOI] [PubMed] [Google Scholar]