Abstract
OBJECTIVE
Spinal cord blood flow (SCBF) after sacrifice of thoracoabdominal aortic segmental arteries (TAASA) during thoracoabdominal aortic aneurysm (TAAA) repair remains poorly understood. This study explored SCBF for 72h after sacrifice of all TAASA.
METHODS
Fourteen juvenile Yorkshire pigs underwent complete serial TAASA sacrifice (T4-L5). Six control pigs underwent anesthesia and cooling to 32C with no TAASA sacrifice. In the experimental animals, spinal cord function was continuously monitored using motor evoked potentials (MEP) until 1 hour (h) after clamping the last TAASA. Fluorescent microspheres enabled segmental measurement of SCBF along the entire spinal cord before, and 5 min, 1h, 5h, 24h and 72h after complete TAASA sacrifice. A modified Tarlov score was obtained for 3 days after surgery.
RESULTS
All the pigs with complete TAASA sacrifice retained normal cord function (MEP) until 1h after TAASA ligation. 7 pigs (50%) with complete TAASA sacrifice recovered after 72h; 7 pigs suffered paraparesis or paraplegia. Intraoperatively— and until 1h postoperatively— SCBF was similar among the three groups along the entire cord. Postoperatively, SCBF did not decrease in any group, but significant hyperemia occurred at 5 hours in controls and recovery animals, but did not occur in pigs that developed paraparesis or paraplegia in the T8-L2 segments (p=.0002) and L3-S segments (p=.0007). At 24h, SCBF remained marginally lower from T8 caudally; at 72h, SCBF was similar among all groups along the entire cord. SCBF in the segments T8-L2 at 5h predicted functional recovery (p=.003).
CONCLUSIONS
This study suggests that critical spinal cord ischemia after complete TAASA sacrifice does not occur immediately (intraoperatively), but is delayed 1–5 hours or longer after clamping, and represents failure to mount a hyperemic response to rewarming and awakening. The short duration of low SCBF associated with spinal cord injury suggests that hemodynamic and metabolic manipulation lasting only 24–72 hours may allow routine preservation of normal cord function despite sacrifice of all TAASA secondary to surgical or endovascular repair of large TAAA.
Keywords: Spinal Cord Perfusion / Protection, Paraparesis / Paraplegia, Segmental Artery Sacrifice, Thoracoabdominal Aortic Aneurysm Repair (TAA/A)
INTRODUCTION
The mortality and morbidity of even extensive thoracoabdominal replacement has improved markedly in recent years.[1] However, postoperative paraplegia remains a devastating albeit rare complication—often associated with significantly reduced long-term survival— and its occurrence is still somewhat unpredictable.[2–6]
Most often, neurologic injury becomes apparent immediately postoperatively and is therefore assumed to be a consequence of ischemic injury during intraoperative aortic cross clamping, or sacrifice of vessels critical to spinal cord function during operative repair. In recent years, various strategies to support the spinal cord circulation during aneurysm resection, often accompanied by intraoperative motor evoked potential (MEP) and somatosensory evoked potential (SSEP) monitoring, have become important features of aortic aneurysm surgery and have contributed to a reduction of permanent postoperative spinal cord injury to below 3% in experienced centers.[7]
The studies described in this report were undertaken to try to gain a better understanding of the impact of extensive sacrifice of segmental arteries (SA) arising from the thoracic and abdominal aorta (TAASA) on spinal cord blood flow (SCBF) intraoperatively and in the early postoperative period under experimental conditions which approximate the circumstances prevailing during clinical thoracoabdominal aortic surgery. Previous studies with this model have established the feasibility of routine extensive SA sacrifice without loss of function, revealing the presence of a dense and complex collateral arterial network feeding the spinal cord.[8–10] Recent studies have revealed that spinal cord perfusion pressure (SCPP) is significantly but transiently reduced for at least several hours after SA sacrifice in this model, potentially causing a critical albeit temporary reduction in spinal cord blood flow (SCBF).[11] Further investigation of the physiological and functional response of the collateral spinal cord perfusion network to the sacrifice of important contributors to its blood supply—and especially of the time course of this response—should help to elucidate how best to prevent even the rare occurrence of paraplegia after extensive SA sacrifice. Avoiding spinal cord injury despite occlusion of most SAs is critical not only for surgical repair, but for the eventual successful endovascular treatment of large thoracoabdominal aneurysms.
MATERIALS AND METHODS
Study design
Twenty female juvenile Yorkshire pigs (Animal Biotech Industries, Allentown, NJ, U.S.A.), 4–5 months of age, weighing 28–32 kg, were used for this experiment.
Fourteen pigs underwent complete serial TAASA sacrifice. In all animals, the descending thoracic and abdominal aorta were exposed, and all TAASA (T4–13, L1–4/5) carefully dissected. Thereafter, all thoracic and abdominal segmental arteries were sequentially clamped along the craniocaudal axis during mild hypothermia (32°C), allowing a three-minute interval between clamping of successive arteries. Spinal cord function was continuously monitored using motor evoked potentials (MEP) until 1 hour (h) after clamping the last TAASA. Six control pigs underwent anesthesia and cooling to 32°C with exposure of the segmental vessels but no TAASA sacrifice. Fluorescent microspheres enabled segmental measurement of SCBF along the entire spinal cord before, and 5 min, 1h, 5h, 24h and 72h after complete aortic TAASA sacrifice. Functional recovery was evaluated for 5 days using a modification of the Tarlov score, and histopathological examination was carried out after sacrifice. Pigs that died before completion of the protocol were replaced.
This experimental model closely simulates the procedure used for resection of descending thoracic and thoracoabdominal aneurysms clinically at our institution. However, it should be noted that the anatomy of the pig differs from that of humans in having 13 thoracic (and 5 lumbar) segmental arteries which arise together from the descending (abdominal) aorta and subsequently divide. Recent studies suggest the subclavian arteries and the median sacral arteries play a major role in the perfusion of the paraspinous collateral vascular network in both species, although the iliac arteries may provide a greater proportion of direct the blood supply in humans than in pigs.
This experimental model is simplified to allow an uncomplicated focus on the input to the collateral network in the wake of segmental artery sacrifice. Previous experiments with this model have demonstrated that the spinal cord perfusion pressure and the collateral flow in the pig behaves in ways very similar to what is observed under comparable circumstances clinically in humans.
Perioperative management and anesthesia
All animals received humane care in compliance with the guidelines of ‘Principles of Laboratory Animal Care’ formulated by the National Society for Medical Research and the ‘Guide for the Care and Use of Laboratory Animals’ published by the National Institute of Health (NIH Publication No. 88–23, revised 1996). The Mount Sinai Institutional Animal Care and Use Committee approved the protocols for all experiments.
After pre-treatment with intramuscular ketamine (15 mg/kg) and atropine (0.03 mg/kg), an endotracheal tube is placed. The animals are then transferred to the operating room and are mechanically ventilated with a FiO2 of 0.5, and a minute volume adequate for maintenance of a normal pCO2 (35–40mmHg).
Anesthesia is induced via the bolus intravenous administration of propofol (1mg/kg) and fentanyl (50μg/kg) and is maintained with infusions of ketamine (15mg/kg/hr), propofol (7mg/kg/hr) and fentanyl (5μg/kg/hr). This anesthetic regimen has no major effect on MEP responses, and has been described previously [12]. Paralysis for intubation is achieved with intravenous pancuronium (0.1 mg/kg), but no further doses are administered subsequently to avoid interfering with measurement of MEPs.
The ventilator rate and the tidal volume are adjusted to maintain the arterial carbon dioxide tension at 35–40 mmHg. End-expiratory carbon dioxide (PPG Biomedical Systems, Model 2010–200 R, Lenexa, KS, USA) is monitored continuously. Arterial oxygen tension was maintained >90 mm Hg. A bladder catheter (Foley 8–10 F) is inserted for online measurement of urine output. Electrocardiographic measurements are recorded continuously. An arterial line is placed in the right brachial artery for pressure monitoring and blood sampling (pH, oxygen tension, carbon dioxide tension, oxygen saturation, base excess, hematocrit, hemoglobin and glucose, lactate, Blood Gas Analyzer, Ciba Corning 865, Chiron Diagnostics, Norwood, MA, USA).
Body temperature management
After inducing anesthesia, the pigs are cooled to 32ºC rectal temperature by covering them with packs of artificial refrigerants for a period of 30 minutes. In addition, a cooling blanket is used even after the target temperature has been reached to maintain hypothermia and prevent an upward temperature drift during the procedure. The operating room temperature is reduced to 14ºC. No local cooling of the vertebral column is undertaken.
The animals are subsequently warmed using a heating blanket and a heating lamp, usually for 90–100 min, and by raising the operating room temperature to 24ºC. To prevent any intraoperative temperature drift, the small left thoracotomy in the fourth intercostal space is temporarily closed after clamping the thoracic spinal arteries.
Monitoring of intra- and postoperative systemic perfusion pressure (MAP)
Two arterial pressure lines are placed: one in the right brachial artery to monitor intraoperatively and another in the descending aorta, for direct postoperative aortic pressure monitoring. These lines enable systemic perfusion pressure monitoring, blood sampling (pH, oxygen tension, carbon dioxide tension, oxygen saturation, base excess, hematocrit, hemoglobin and glucose, lactate; Blood Gas Analyzer, Ciba Corning 865, Chiron Diagnostics, Norwood, MA, USA) as well as reference sampling for microsphere measurements prior to, during, and after complete TAASA sacrifice.
Spinal cord blood flow (SCBF) measurements – microsphere injections
In all animals, the left pulmonary vein is surgically catheterized distally to the hilus of the left lung allowing for direct microsphere injection into the left atrium while the descending aortic catheter is used for reference sampling.
Fluorescent microspheres were injected to measure SCBF (immediately) prior to TAASA sacrifice (BASELINE, color 1), 5 min after complete TAASA sacrifice (ENDCLAMPING, color 2), at 1h after complete TAASA sacrifice (1h, color 3), 5h complete TAASA sacrifice (5h, color 4), at 24h after complete TAASA sacrifice (24h, color 5) and finally at 72 hours after complete TAASA sacrifice (72h, color 6).
Monitoring technique for motor evoked potentials (MEP)
A 5 cm longitudinal incision is made in the scalp overlying the skull, and the periosteum is removed to expose the sagittal and coronal sutures of the calvarium. Four stainless steel screw electrodes with attached wire leads were screwed into the skull 10 mm lateral to the sagittal suture. Two screws are placed on the left side (8 mm anterior and 8 mm posterior to the coronal suture), and two equally placed on the right. The wire leads are connected to an electrical stimulator (Digitimer Stimulator Model D 180A, Welwyn, Garden City, United Kingdom). Electromyographic recordings are made from sterile stainless steel needle electrodes placed through the skin over the tibialis muscle in the hind leg and the muscles in the foreleg.
A stimulation train (3 pulses, 200–300 V, 100 ms pulse duration, and 2 ms interstimulus interval) delivered to the skull electrodes is used to elicit MEPs. MEPs are amplified (gain 2000), bandpass filtered (10–1000 Hz), digitized, and stored on an optical disk for subsequent analysis by a Spectrum 32 neurophysiological recording system (Cadwell Laboratories Inc., Kennewick, WA, USA). MEPs were recorded before clamping, during the three minute interval after clamping of each segmental pair, and after clamping of all thoracic and abdominal segmental arteries for a period of 60 (−90) min. The baseline value was determined just prior to the start of SA clamping. A lack of response to the stimulus is considered evidence of acute spinal cord ischemia.
Data acquisition and analysis were performed on a computer with an AD converter and software (LabVIEW, National Instruments, Austin, TX) as previously published.
Neurobehavioral assessment
All animals were videotaped at the same time daily, and a neuroscientist, blinded to the intraoperative course of events, utilized the coded videotapes to carry out neurological scoring using a modification of the Tarlov score. The modified Tarlov score is scaled as follows: no voluntary movements (0); perceptible movements at joints (1); good movements at joints but inability to stand (2); ability to get up and stand with assistance <1 min (3); ability to get up with assistance and stand unassisted <1 min (4); ability to get up with assistance and stand unassisted >1 min (5); ability to get up and stand unassisted >1 min (6); ability to walk <1 min (7); ability to walk >1 min (8); complete recovery (9).
Regional blood flow assessment (microspheres)
Our regional blood flow assessment strategy involves the use of microspheres – 15 μm polystyrene beads—which fluoresce under ultraviolet light. Use of different colors (yellow, pink, purple, and coral, each of which is available in low, medium, and high varieties), enables multiple measurements in the same tissue; sampling technicalities limit this to about 8 per tissue.
The microsphere bolus consists of 2.5 million spheres which are administered centrally, into a left heart chamber. At the same time as the injection is made, a reference sample of blood is withdrawn from an artery ‘downstream’ in the arterial tree from the injection site. This is withdrawn using a specialized pump at a precise rate, in our experiments 2.91ml/min. This basically allows a ratio calculation: the ratio of microspheres in a given piece of tissue to those in the blood reference sample is the same as the ratio of their blood flows. The reference samples are placed in tubes with EDTA anticoagulant. Multiple injections of different colors are made at relevant stages of the surgical protocol.
At the end of the experiment, the pig is sacrificed by exsanguination under anesthesia and the tissues are harvested for blood flow determination, histological analysis, or both. The spinal cord is removed from the animal through a midline dorsal incision which runs from the skull to the sacrum. The paraspinal muscles are dissected off the vertebral column and the spinal canal is then entered via bilateral laminectomies down the length of the back exposing the spinal cord, which is then removed. The anatomical levels can be identified through the origins of the spinal nerves and the appropriate divisions made for analysis.
The blood and tissue samples are then analyzed at Interactive Medical technologies (IMT) Ltd, Irvine, California. The samples are analyzed in a flow cytometer (fluorescent spectrophotometer) which measures the fluorescence at the various wavelengths. Spinal cord blood flow (SCBF) is determined from the fluorescent intensities (counts) of the tissue and blood reference samples using the formula:
where R = blood reference withdrawal rate (2.91mls/min), It and Ibr are the tissue and blood reference samples’ fluorescent intensities or counts, and Wt is the weight of the tissue sample (g).
Histopathological evaluation
Portions of the spinal cord not used for microsphere analysis were fixed in 10% formalin solution, embedded in paraffin, and then sectioned transverse to the craniocaudal axis, with samples at 0.5 cm intervals. Sections 6 μm in thickness were stained with hematoxylin and eosin, examined and scored blindly by an experienced neuropathologist according to a schematic grading system which was developed to classify the ischemic spinal cord damage (ISCD) at each segmental level: 1 = necrosis of single (motor) neurons only; 2 = necrosis of one posterior horn only; 3 = necrosis of both posterior horns only; 4 = necrosis of both posterior horns + surrounding white matter; 5 = necrosis of both anterior horns only; 6 = central necrosis involving posterior and anterior horns + parts of white matter; 7 = complete necrosis of gray matter only; 8 = complete necrosis of the whole section (Figure 3).
Figure 3.

A schematic grading system was developed to classify the ischemic damage at each segmental level of the spinal cord: 1 = necrosis of single (motor) neurons only; 2 = necrosis of one posterior horn only; 3 = necrosis of both posterior horns only; 4 = necrosis of both posterior horns + surrounding white matter; 5 = necrosis of both anterior horns only; 6 = central necrosis involving posterior and anterior horns + parts of white matter; 7 = complete necrosis of gray matter only; 8 = complete necrosis of the whole section.
Data analysis
For the purposes of data analysis, pigs were classified into one of 3 groups: controls that had no segmental arteries resected, pigs that had all possible TAASA resected and subsequently recovered spinal cord function, and pigs that had all TAASA resected but were left with either paraplegia or paraparesis. All analyses were implemented with SAS software for the PC version 9.1.3, SAS Institute Inc, Cary, NC. Physiologic data are described by means and standard deviations in each of these groups. Hierarchical linear models, using Proc Mixed, were used to analyze the association of flows with groups and segments. In separate analyses of the 3 segment regions at the 5 hour and 24 hour time points, flows were modeled as a function of group, segments, and interaction, with repeated observations (over segments) and an unstructured or autoregressive covariance matrix. Pairwise comparisons between groups were obtained as solutions to the fixed effects. The Chi-square test for trend was used for predicting the binary outcome, recovery of spinal cord function, on the basis of the 5 hour flows.
RESULTS
Comparability of experimental groups
The results of this experiment were analyzed by comparing three groups: pigs which were operated on to expose the entire aorta but had no interruption of any segmental arteries (n= 6; weight: 30.2 ± 2.4 kg); pigs that had all possible TAASA resected and subsequently recovered spinal cord function (n=7; weight: 26.1 ± 2.0 kg), and pigs that had all TAASA resected but were left with either paraparesis or paraplegia (n=7; weight: 27.2 ± 2.8 kg). The physiological data with regard to each of the groups are shown in Table 1.
Table 1. Intra- and postoperative data.
Mean aortic pressures (MAP) and blood gas analyses for control (N=6), paraparetic / paraplegic (N=7) and recovered (N=7) animals.
| Variable / Group | Baseline prior to ligation |
After TAASA ligation | ||||
|---|---|---|---|---|---|---|
| 5 min | 1h | 5h | 24h | 72h | ||
| MAP (mmHg) | ||||||
| Control group | 90 ± 2 | 90 ± 1 | 96 ± 8 | 93 ± 7 | 91 ± 2 | 90 ± 1 |
| Paraparetic / paraplegic group | 90 ± 8 | 93 ± 15 | 90 ± 9 | 96 ± 15 | 78 ± 15 | 92 ± 16 |
| Recovery group | 91 ± 9 | 91 ± 8 | 89 ± 7 | 96 ± 16 | 99 ± 21 | 108 ± 17 |
| pH | ||||||
| Control group | 7.52 ± 0.04 | 7.51 ± 0.05 | 7.44 ± 0.05 | 7.38 ± 0.03 | 7.53 ± 0.01 | 7.49 ± 0.02 |
| Paraparetic / paraplegic group | 7.48 ± 0.06 | 7.44 ± 0.05 | 7.40 ± 0.05 | 7.39 ± 0.07 | 7.45 ± 0.05 | 7.47 ± 0.04 |
| Recovery group | 7.50 ± 0.04 | 7.48 ± 0.05 | 7.50 ± 0.04 | 7.36 ± 0.08 | 7.47 ± 0.04 | 7.47 ± 0.03 |
| PCO2 | ||||||
| Control group | 33.0 ± 3.8 | 33.2 ± 5.5 | 39.7 ± 8.0 | 43.7 ± 3.9 | 35.8 ± 2.0 | 38.0 ± 2.8 |
| Paraparetic / paraplegic group | 40.0 ± 7.9 | 41.6 ± 6.3 | 44.0 ± 7.4 | 40.4 ± 5.1 | 33.3 ± 2.0 | 33.9 ± 4.1 |
| Recovery group | 37.7 ± 3.6 | 40.2 ± 7.6 | 37.4 ± 3.5 | 37.5 ± 4.7 | 32.4 ± 2.9 | 33.6 ± 1.9 |
| PO2 | ||||||
| Control group | 364 ± 59 | 362 ± 54 | 367 ± 23 | 76 ± 18 | 84 ± 5 | 85 ± 8 |
| Paraparetic / paraplegic group | 390 ± 54 | 388 ± 47 | 382 ± 54 | 74 ± 8 | 78 ± 18 | 73 ± 18 |
| Recovery group | 381 ± 46 | 357 ± 36 | 364 ± 58 | 72 ± 18 | 87 ± 14 | 76 ± 8 |
| Hb | ||||||
| Control group | 10.9 ± 0.6 | 10.1 ± 0.6 | 9.9 ± 0.8 | 10.3 ± 0.8 | 9.8 ± 1.2 | 9.9 ± 1.3 |
| Paraparetic / paraplegic group | 10.4 ± 1.4 | 10.8 ± 2.1 | 9.7 ± 1.3 | 10.5 ± 1.7 | 10.8 ± 2.6 | 9.6 ± 2.2 |
| Recovery group | 11.4 ± 0.7 | 11.9 ± 0.9 | 11.3 ± 1.5 | 13.7 ± 1.1 | 11.1 ± 1.6 | 9.3 ± 1.5 |
| O2 – saturation (%) | ||||||
| Control group | 99.8 ± 0.06 | 99.8 ± 0.0 | 99.8 ± 0.0 | 94.6 ± 4.7 | 97.3 ± 0.5 | 97.0 ± 0.8 |
| Paraparetic / paraplegic group | 99.8 ± 0.05 | 99.8 ± 0.04 | 99.8 ± 0.1 | 94.7 ± 0.7 | 94.9 ± 2.5 | 94.7 ± 3.1 |
| Recovery group | 99.8 ± 0.05 | 99.8 ± 0.06 | 99.8 ± 0.1 | 92.3 ± 5.5 | 93.8 ± 8.0 | 93.4 ± 6.6 |
| Glucose | ||||||
| Control group | 89 ± 42 | 78 ± 35 | 83 ± 21 | 119 ± 18 | 94 ± 12 | 94 ± 12 |
| Paraparetic / paraplegic group | 115 ± 38 | 133 ± 49 | 118 ± 33 | 159 ± 102 | 91 ± 19 | 72 ± 12 |
| Recovery group | 97 ± 32 | 92 ± 42 | 80 ± 39 | 103 ± 32 | 110 ± 36 | 94 ± 12 |
| Lactate | ||||||
| Control group | 1.6 ± 0.9 | 1.6 ± 1.1 | 1.3 ± 0.8 | 2.3 ± 2.1 | 0.6 ± 0.2 | 0.7 ± 0.5 |
| Paraparetic / paraplegic group | 1.4 ± 0.7 | 1.5 ± 1.1 | 1.4 ± 0.6 | 2.7 ± 1.3 | 3.0 ± 1.3 | 1.9 ± 1.8 |
| Recovery group | 2.5 ± 1.7 | 2.0 ± 1.3 | 1.6 ± 0.9 | 5.7 ± 2.4 | 4.1 ± 5.0 | 2.2 ± 1.9 |
Target aortic mean aortic pressure was 90 mmHg intraoperatively. Only volume infusions but no pharmaceuticals were used to maintain aortic pressures. The mean aortic pressures intraoperatively were similar between the spinal cord injury group and the pigs that recovered. No clinically significant differences in intraoperative physiological parameters were evident.
Relationship between functional recovery and spinal cord blood flow (SCBF)
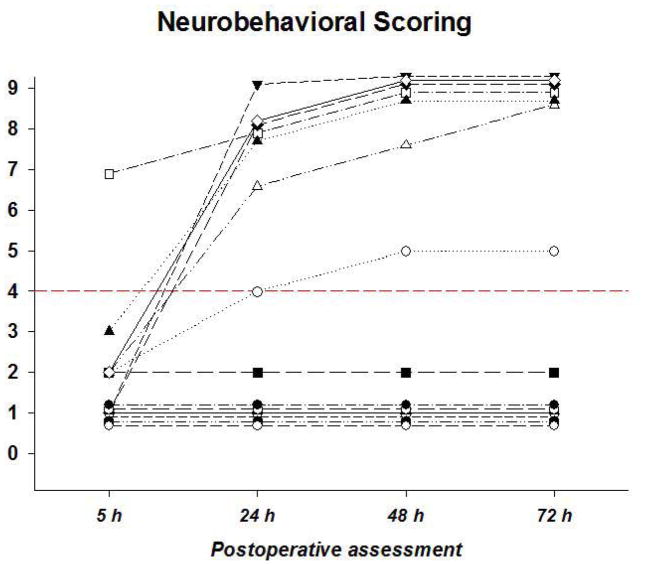
All the pigs retained normal cord function (MEP) until 1h after complete TAASA ligation. Since our main interest was in the relationship between flow parameters and spinal cord integrity, the pigs with TAASA ligation were divided for subsequent analysis into two groups depending upon the degree of functional recovery postoperatively. Those animals that regained normal function within the five days of postoperative observation—with a modified Tarlov score ≥4, indicating that they were able to stand without assistance—were considered to have recovered. Those with a score < 4 by the fifth day postoperatively were considered to have sustained spinal cord injury (paraparesis / paraplegia; Figure 1).
Figure 1.
Postoperative neurobehavioral recovery after complete TAASA ligation (N=14 animals) using a modified Tarlov score, as detailed in the text. Recovery was defined as a score ≥4, which requires the pig to get up with assistance and stand unassisted <1 min.
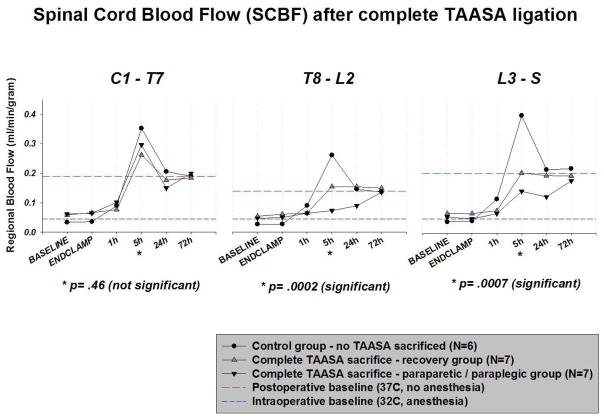
Seven pigs (50%) with complete TAASA sacrifice recovered after 72 hours, but 7 suffered paraparesis / paraplegia. SCBF was similar in the two groups with TAASA sacrifice and in the controls intraoperatively, and until 1h postoperatively, as shown in Figure 2.
Figure 2.
Regional spinal cord blood flow (SCBF) was determined using fluorescent microspheres (as detailed in the text) at various time points following serial ligation of all segmental arteries (TAASA). No segmental artery sacrifice was carried out in the controls. Pigs in the recovery group subsequently regained hind limb function, but those in the paraparetic/paraplegia group suffered permanent spinal cord injury. The dashed lines represent baseline flows at 32ºC during anesthesia, and after awakening, at 37ºC. The three graphs represent cervical and upper thoracic, lower thoracic and upper lumbar, and lumbosacral regions of the spinal cord, as indicated. The first baseline was recorded at 32ºC under anesthesia, and the second at 72 hrs. It is notable that there were no significant differences among the groups in the cervical region of the cord. Below this level, however, there was a significant difference between the paraparetic animals and the controls at five hours, with the controls exhibiting a marked hyperemia. There were no significant differences among the groups at the later time points.
Postoperatively, however, SCBF in pigs with ischemic cord injury was significantly lower at 5h in the T8-L2 segments (p=.0001) and L3-S segments (p=.02) than in contemporaneous controls without TAASA ligation. At 24h, SCBF remained marginally lower from T8 caudally in the pigs that subsequently developed paraparesis or paraplegia. By 72 hours postoperatively, SCBF was similar among all groups at all levels of the spinal cord.
Outcome was predictable on the basis of SCBF at 5 hours post TAASA ligation (Table 2). SCBF 5 hours after complete TAASA ligation, averaged over these 4 central segments (T8-L2) showed a statistically significant association (p=.003, chi-square test for trend) with outcome (paraparetic / paraplegic vs. recovery).
Table 2.
Predicting outcome (paraparetic / paraplegic vs. recovery) on the basis of SCBF @ 5 hours post TAASA ligation.
The table shows the segmental flows @ 5h after complete TAASA ligation, averaged over the 4 segments (T8 to L2) divided into equal size groups, and their relation to the chance of paraplegia. This association is statistically significant (p=.003, chi-square test for trend).
| SCBF (ml/min/g) in T8 – L2 @ 5h after complete TAASA ligation | # of animals | # with paraplegia | % with paraplegia |
|---|---|---|---|
| < 0.08 | 5 | 4 | 80% |
| 0.08 - 0.125 | 5 | 3 | 60% |
| 0.125 – 0.24 | 5 | 0 | 0 |
| ≥ 0.24 | 4* | 0 | 0 |
In examining the results, the pattern of SCBF in the control group is notable in several respects. As indicated by the dotted lines in Figure 2, the blood flow in the cool, anesthetized pigs was much lower than in the postoperative state, awake at 37ºC. More surprisingly, there was a marked hyperemia at 5 hours in the control pigs at all levels of the spinal cord. This hyperemia was also present in the pigs with TAASA ligation in the cervical region, but was not seen to the same extent in the pigs with TAASA ligation in the central and—to a lesser extent—in the lumbosacral region of the cord, and was significantly absent—as noted above—in those pigs that did not recover spinal cord function.
Statistical analyses of regional SCBF at 5 and 24 hours
SCBF was similar among the three groups prior to TAASA clamping along the entire cord, but differed significantly at 5 hours in the T8-L2 segments (p=.0002) and the L3-S segments (p=.0007). Later differences (at 24h and 72h) did not reach statistical significance. Lower mean SCBF values in T8-L2 at 5 hours were associated with higher proportions of pigs with paraparesis / paraplegia, as shown in Table 2.
Looking at the different regions of the spinal cord at 5 hours, in the upper spinal cord (segments C1 to T7) there was no significant overall difference in SCBF between recovered, paraparetic / paraplegic and control animals (p=.46). In the middle portion of the cord (segments T8 to L2) regional SCBF at 5 hours was significantly different among the three groups (p=.0002). However, between paraparetic / paraplegic and recovered animals there was only a trend (p=0.1) whereas paraparetic / paraplegic and control animals were highly statistically significantly different (p<.0001) in this area.
In the caudal spinal cord (segments L3 to S) regional SCBF was statistically significantly different among the three groups (p=.0007). However, again paraparetic / paraplegic and recovered animals were similar (p=.40), whereas paraparetic / paraplegic and control animals were significantly different (p=.02).
By 24 hours after complete TAASA sacrifice, there were no longer any statistically significant differences in SCBF among the groups: segments C1 to T7, p=.62; segments T8 to L2, p=.17, and segments L3 to S, p=.08.
MEP monitoring
MEP monitoring was carried out to assess the impact on function as the TAASAs were being serially sacrificed, as described in detail previously, and until one hour after the last of them had been sacrificed: later monitoring is not possible without prolonging the duration of anesthesia. As was seen in earlier studies, MEPs after extensive SA sacrifice were intact at the end of the procedure even in those pigs that subsequently suffered a loss of spinal cord function. This suggests that critical ischemia occurred in these pigs after the last measurement of MEPs had been carried out (one hour after the last of the TAASAs had been occluded), at a time when spinal cord perfusion pressure is still very low, but MEPs can no longer be recorded.
Histopathological findings
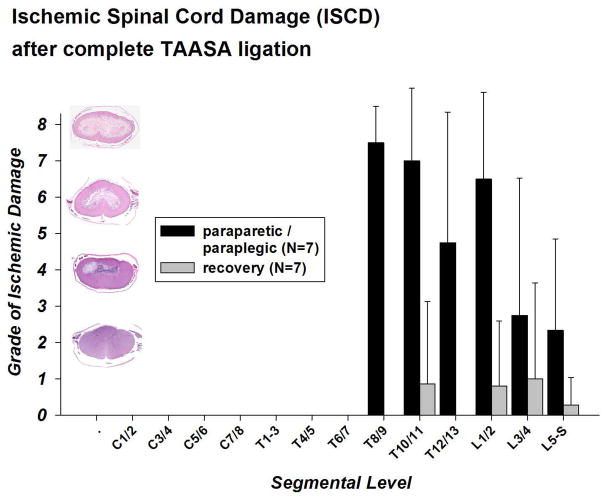
Despite complete TAASA sacrifice, there was no evidence of necrosis in the cervical and higher thoracic segments in either paraparetic / paraplegic or recovered animals to the level of T7 in all animals (and T9 in recovered animals; Figure 4). In the lower thoracic and lumbar segments, however, paraparetic / paraplegic animals showed distinctive necrosis of the gray matter involving the posterior and anterior horns and surrounding white matter, sparing a thin peripheral rim of white matter below the pia (Figures 3 and 4) with the center of ischemic damage between T8 and L2. Although the necrosis had an abrupt onset between segments T8 and T9 in most of the paraparetic / paraplegic animals, in other respects the areas in which necrosis was found correspond to the vascular territory of the anterior spinal artery.
Figure 4. Regional ischemic damage of the spinal cord (ISCD) after complete TAASA sacrifice – paraparetic / paraplegic vs recovery group.
The Y-axis shows the grade of the ischemic damage and a corresponding low power (2x) transverse section of the spinal cord, stained with hematoxylin and eosin, from pigs three days after extensive sacrifice of segmental arteries as examples. The cord segments from which the sections are taken are indicated at the X-axis.
In pigs that regained function, all segments showed a normal population of neurons and intact gray matter at all levels at lower magnification. There was no evidence of comprehensive segmental necrosis, although higher power evaluation showed rare minute areas with necrotic cells in sections from the lower thoracic (T10/11) and lumbar levels (L1-S; see Figure 4).
DISCUSSION
The blood flow studies described in this report have led to several new observations which provide valuable insight into the response of the spinal cord circulation to extensive segmental artery sacrifice (TAASA). This has led to a better understanding of the gradual adaptation of the spinal cord to loss of much of its usual vascular input, and an increase in the likelihood that elimination of postoperative spinal cord injury following surgery for extensive thoracic and thoracoabdominal aneurysms is an achievable goal.
To begin with, SCBF under mild hypothermia and anesthesia, as seen in Figure 2, is considerably less than the flow when the pig is warm and awake. This relatively reduced flow is not surprising—assuming that autoregulation is present—given the expectation of a reduced metabolic rate in the spinal cord under conditions of hypothermia and muscle paralysis. But it is surprising that SCBF does not diminish below the intraoperative baseline value after all TAASA have been sacrificed, and even one hour later. This implies that complete TAASA sacrifice does not itself cause spinal cord injury, an observation which is confirmed by the presence of intact MEPs in all pigs—including those who subsequently develop paraparesis or paraplegia—at the one hour time point.
The ischemia which leads to spinal cord injury seems to occur between one and 5 hours after complete TAASA sacrifice. But even at 5 hours, SCBF does not fall below intraoperative baseline values, even though we know—from previous studies in the same model—that spinal cord perfusion pressures are very low.[12] Rather, as seen from SCBF measurements in the control animals—who did not undergo any TAASA ligation—there seems to be a requirement for increased blood flow during rewarming and recovery from anesthesia: the control levels are above those seen after the pigs are completely recovered at normothermia. At the 5 hour time point, pigs that subsequently develop spinal cord injury have levels of SCBF that are higher than the anesthetized, hypothermic baseline, but below the baseline level for awake normothermic pigs. In contrast, the pigs that recover function after TAASA ligation have levels of SCBF at 5 hours in the same range as the normothermic awake baseline, but—except in the cervical regions of the cord—they do not exhibit the hyperemia seen in the controls.
Why should there be a requirement for spinal cord hyperemia during recovery from anesthesia and return to normothermia? Again assuming some degree of autoregulation, spinal cord hyperemia implies the presence of an increase in demand and thus a higher level of metabolism. This could be a consequence of enhanced neural activity associated with recovery from anesthesia, including spontaneous movement and shivering.
Beginning at about 24 hours, SCBF returns to normal baseline levels—as defined by the controls in whom no TAASA were ligated—within 72 hours, even in pigs that subsequently show evidence of spinal cord injury. It is intriguing to speculate on how this is achieved after TAASA ligation—whether by an increase in diameter and change in structure of pre-existing collateral vessels (which has been termed arteriogenesis), or by formation of entirely new vessels by endothelial sprouting (angiogenesis).
The implications arising from these observations are heartening. First of all, whatever strategies are required to prevent spinal cord injury need to be put in place for only 72 hours. The most direct approach is maintenance of a high perfusion pressure not only intraoperatively, but also for the entire vulnerable postoperative recovery period. Another possibility, however, is to prevent the putative increase in spinal cord metabolic rate from occurring during the period before cord flow can return to normothermic awake baseline levels. A temporary reduction in metabolic rate could possibly be achieved by prolonging hypothermia, or by transient pharmacological paralysis of muscles usually activated upon awakening from anesthesia. The optimal approach may turn out to be a combination of techniques to enhance blood flow and to decrease metabolic rate in the spinal cord for at least 24 hours, and possibly as long as 72 hours.
The histological evidence of minute areas of necrosis even in the pigs that recover function after extensive TAASA sacrifice, and the failure of the levels of SCBF in recovered animals to reach the same levels as the controls at 5 and 24 hours both suggest that the margin of safety (in terms of SCBF) for preservation of spinal cord integrity after TAASA sacrifice is currently very small.
Conclusion
This study of spinal cord perfusion has documented that extensive ligation of TAASA does not diminish SCBF below intraoperative baseline values. What distinguishes pigs that develop spinal cord injury from those who recover normal function is a failure to mount an adequate hyperemic response during awakening from anesthesia and rewarming. But even in animals with spinal cord injury, flow returns to normal within 72 hours, suggesting that a combination of strategies to optimize SCBF and decrease spinal cord metabolism for 72 hours postoperatively could reliably prevent paraplegia following extensive TAASA sacrifice. This would enable endovascular treatment of TAA/A, offering therapy to many patients too old and frail to be considered for open surgical treatment.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Svensson LG. Paralysis after aortic surgery: in search of lost cord function. Surgeon. 2005;3:396–405. doi: 10.1016/s1479-666x(05)80050-2. [DOI] [PubMed] [Google Scholar]
- 2.Coselli JS, LeMaire SA, Miller CC, 3rd, Schmittling ZC, Koksoy C, Pagan J, Curling PE. Mortality and paraplegia after thoracoabdominal aortic aneurysm repair: a risk factor analysis. Ann Thorac Surg. 2000;69:409–414. doi: 10.1016/s0003-4975(99)01478-2. [DOI] [PubMed] [Google Scholar]
- 3.Bachet J, Guilmet D, Rosier J, Cron C, Dreyfus G, Goudot B, Piquois A, Brodaty D, Dubois C, de Lentdecker P. Protection of the spinal cord during surgery of thoraco-abdominal aortic aneurysms. Eur J Cardiothorac Surg. 1996;10:817–825. doi: 10.1016/s1010-7940(96)80305-8. [DOI] [PubMed] [Google Scholar]
- 4.Kouchoukos NT, Rokkas CK. Hypothermic cardiopulmonary bypass for spinal cord protection: rationale and clinical results. Ann Thorac Surg. 1999;67:1940–1942. doi: 10.1016/s0003-4975(99)00442-7. discussion 1953–1948. [DOI] [PubMed] [Google Scholar]
- 5.Svensson LG, Patel V, Robinson MF, Ueda T, Roehm JO, Jr, Crawford ES. Influence of preservation or perfusion of intraoperatively identified spinal cord blood supply on spinal motor evoked potentials and paraplegia after aortic surgery. J Vasc Surg. 1991;13:355–365. doi: 10.1067/mva.1991.26137. [DOI] [PubMed] [Google Scholar]
- 6.Yamauchi T, Takano H, Nishimura M, Matsumiya G, Sawa Y. Paraplegia and paraparesis after descending thoracic aortic aneurysm repair: a risk factor analysis. Ann Thorac Cardiovasc Surg. 2006;12:179–183. [PubMed] [Google Scholar]
- 7.Etz CD, Halstead JC, Spielvogel D, Shahani R, Lazala R, Homann TM, Weisz DJ, Plestis K, Griepp RB. Thoracic and thoracoabdominal aneurysm repair: is reimplantation of spinal cord arteries a waste of time? Ann Thorac Surg. 2006;82:1670–1677. doi: 10.1016/j.athoracsur.2006.05.029. [DOI] [PubMed] [Google Scholar]
- 8.Strauch JT, Spielvogel D, Lauten A, Zhang N, Shiang H, Weisz D, Bodian CA, Griepp RB. Importance of extrasegmental vessels for spinal cord blood supply in a chronic porcine model. Eur J Cardiothorac Surg. 2003;24:817–824. doi: 10.1016/s1010-7940(03)00460-3. [DOI] [PubMed] [Google Scholar]
- 9.Strauch JT, Spielvogel D, Lauten A, Zhang N, Shiang H, Weisz D, Bodian CA, Griepp RB. Importance of extrasegmental vessels for spinal cord blood supply in a chronic porcine model. Rev Port Cir Cardiotorac Vasc. 2003;10:185–191. [PubMed] [Google Scholar]
- 10.Strauch JT, Lauten A, Spielvogel D, Rinke S, Zhang N, Weisz D, Bodian CA, Griepp RB. Mild hypothermia protects the spinal cord from ischemic injury in a chronic porcine model. Eur J Cardiothorac Surg. 2004;25:708–715. doi: 10.1016/j.ejcts.2004.01.007. [DOI] [PubMed] [Google Scholar]
- 11.Etz CD, Homann TM, Plestis KA, Zhang N, Luehr M, Weisz DJ, Kleinman G, Griepp RB. Spinal cord perfusion after extensive segmental artery sacrifice: can paraplegia be prevented? Eur J Cardiothorac Surg. 2007;31:643–648. doi: 10.1016/j.ejcts.2007.01.023. [DOI] [PubMed] [Google Scholar]
- 12.Etz CD, Luehr M, Kari F, Bodian CA, Smego S, Plestis KA, Griepp RB. Paraplegia after Extensive Thoracic and Thoracoabdominal Aortic Aneurysm Repair: Does Critical Spinal Cord Ischemia Occur Postoperatively? The Journal of Thoracic and Cardiovascular Surgery. 2007 doi: 10.1016/j.jtcvs.2007.11.002. in press. [DOI] [PubMed] [Google Scholar]