Abstract
Arsenic is a heavy metal that exhibits a high degree of toxicity to various organ systems. In humans, this compound is associated with an increase risk of skin cancer, and may cause cancers of the lung, liver, bladder, kidney, and colon. The mechanism of arsenic-related carcinogenicity remains to be elucidated. Hence, the aim of the present study was to investigate the cytotoxic effects of arsenic trioxide (As2O3) on adenocarcinoma colorectal cancer (HT-29) cells using the MTT [3-(4,5 dimethylthiazoyl-2-yl)-2,5- diphenyltetrazolium bromide] assay for cell viability. To achieve this objective, HT-29 cells were cultured and exposed to various doses (0, 2, 4, 6, 8, 10, 12, and 14 μg/ml) of arsenic trioxide for 24 h, 48 h, and 72 h respectively, and subsequently assessed for viability following a standard MTT test protocol. Experimental data indicated that arsenic trioxide is cytotoxic to colon cancer cells showing LD50 values of 9.8, 9.4 and 9.0 μg/ml upon 24, 48 and 72 h of exposure, respectively. There was a dose-dependent response with regard to As2O3 toxicity in HT-29 cells. Although there was a reduction in LD50 value with increasing exposure time, this decrease was not statistically significant.
Introduction
Arsenic is a naturally occurring metalloid that has become a worldwide public health concern due to its leading cause of toxicity and its potential to be a human carcinogen. This semi-metal is odorless and tasteless and occurs naturally in rocks and soil, air, and water. It is considered an environmental pollutant and is further released into the environment through natural activities such as volcanic action, erosion of rocks, and forest fires, or through human actions [1, 2]. It enters drinking water supplies from natural deposits in the earth or from agricultural and industrial practices. Environmental Protection Agency (EPA) has set the arsenic standard for drinking water at 10 parts per billion to protect consumers against the effects of long-term, chronic exposure to arsenic [3, 4]. Exposure to arsenic has caused both acute and chronic adverse health effects [5]. The characteristics of severe acute arsenic toxicity in humans include gastrointestinal discomfort, nausea, diarrhea, bloody urine, anuria, shock, convulsions, coma, and even death. Long-term exposure to arsenic has lead to an increase number of diseases. In humans, chronic ingestion of inorganic arsenic (> 500 mg/L) has been associated with cardiovascular, nervous, hepatic and renal diseases, peripheral neuropathy, diabetes, and cancer [5, 6]. However, the trivalent form of arsenic in particular arsenic trioxide (ATO) has been used as an anticancer agent in the treatment of acute promyleocytic leukemia [7, 8]. Studies have shown that arsenic is cytotoxic in liver, skin, lung, and breast cancer cells [8 - 13].
Arsenic exposure has been reported to induce carcinogenesis in humans. The International Agency for Research and Cancer has classified arsenic as a class A human carcinogen [14]. This classification is based on several epidemiological studies. These studies have demonstrated that chronic arsenic exposure causes tumors of the skin, urinary bladder, lung, liver, prostate, kidney, and colon [14, 15]. Studies in arsenic carcinogenesis were first reported in cases of skin cancer after exposure to inorganic arsenic in drinking water [15, 16]. Other studies have shown that exposure to arsenic cause abnormality in cell proliferation leading to carcinogenesis [16]. Studies have also shown that lung and skin cancer may result from exposure of arsenic due to occupational hazards [13]. The mechanism of arsenic-related carcinogenicity is not well understood. Some proposed mechanisms of action may include genotoxicity, oxidative stress, inhibition of DNA repair, tumor promotion, cell proliferation and signal transduction or DNA methylation [13, 17]. The focus of this research was to investigate the cytotoxic effects of arsenic trioxide (As2O3) on adenocarcinoma colorectal cancer (HT-29) cells using the MTT assay. Given the considerable interest in the dose–response relationships for arsenic as a toxin and a carcinogen, evaluating its cytotoxicity on various cell types, is a critical first step in designing subsequent experiments for understanding its mechanisms of toxicity.
Material and Methods
Cell Line, Medium and Chemicals
The human adenocarcinoma colorectal cancer (HT-29) cells were generously provided by Dr. Stephen Ekunwe, Associate Professor of Biology, Jackson State University; Jackson, MS. Arsenic trioxide with 99.9% purity was purchased from Fisher Scientific (Houston, TX). McCoy's 5A growth medium, phosphate buffered saline (PBS), and trypan blue were purchased from American Type Culture Collection (ATCC) (Manassas, VA). Fetal bovine serum (FBS), penicillin/streptomycin, and 0.25% trypsin-EDTA (w/v) were purchased from Gibco (Grand Island, NY). The thiazolyl blue tetrazolium bromide and dimethyl sulfoxide (DMSO) were obtained from Sigma Chemical Company (St. Louis, MO).
Cell Culture
The HT-29 cells were maintained in McCoy's 5A complete growth medium (CGM) supplemented with 10% FBS and 1% antibiotics (penicillin/streptomycin). HT-29 cells (1 × 106 cells/ml) were plated in T-25 flasks containing 5 mls of CGM and grown in a humidified incubator under an atmosphere of 95% air and 5% CO2 at 37°C to sub-confluence (90 - 95%). The culture medium was replaced every 48 hours. Once the cells reached 90 - 95% confluency, the medium was aspirated, and the cell monolayer was washed three times with sterile phosphate buffered saline. The cell monolayer was treated with 1 ml of 0.25% (w/v) trypsin-EDTA and incubated briefly at 37°C and visualized microscopically to ensure complete cell detachment. Cells were re-suspended in McCoy's 5A complete growth medium. Cells were also stained with trypan blue (100 μl of cell suspension and 100 μl of 0.4% trypan blue), incubated for 2 minutes at room temperature, and counted using a hemacytometer. The cells were seeded at a density of 1.5 × 104 cells/well in 96-well microtiter tissue culture plates prior to arsenic trioxide treatment.
Cytotoxicity Assay
The MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] assay was performed for assessing cell proliferation activity and cytotoxicity in adenocarcinoma colorectal cancer (HT-29) cells exposed to different concentrations of arsenic trioxide. Cell viability was determined using the MTT assay, a colorimetric assay that measures the reduction of MTT by mitochondrial succinate dehydrogenase. Viable cells are able to convert MTT to a water-insoluble formazan dye. The MTT assays were performed according to standard protocols [18]. HT-29 cells were seeded in 96-well plates with 1.5 × 104 cells/well and placed at 37°C in a 5% CO2 humidified incubator until 60% confluency. The CGM was removed and the cells were serum-starved for 24 h prior to treatment. Cells incubated in culture medium alone served as a control for cell viability (untreated cells). The cells were treated with different doses of As2O3: 0, 2, 4, 6, 8, 10, 12, and 14 μg/ml for 24, 48, and 72 h respectively in complete growth medium. Following the As2O3 treatments, the medium was removed and 100 μl of MTT solution (5 mg/mL in sterile H2O) was added to each well. The plates were incubated under 95% atmosphere air and 5% CO2 at 37°C for 4 h. The MTT solution was removed and 200 μl aliquots of DMSO were added to each well to dissolve the formazan crystals followed by incubation for 10 min at 37°C. Treatments were performed in triplicates, and optical densities were read on a Labsystems Mulitskan Accent Plate Reader (Thermo Labsystems, Helsinki, Finland) at a wavelength of 540 nm. A data analysis was performed to determine the chemical doses required to reduce cell viability by 50% (LD50s). Cell viability rate was calculated as the percentage of MTT absorption as follows:
% survival = (mean experimental absorbance/mean control absorbance) × 100.
Statistical Analysis
All assays were set up in triplicates, repeated three times, and the results were expressed as the mean ± standard deviation (SD). The absorbance values obtained per treatment were converted to percentages of cell viability. Regression analysis was performed on cell viability data and the resulting equation was used to compute the lethal dose required to produce a 50% reduction (LD50) in cell viability.
Results
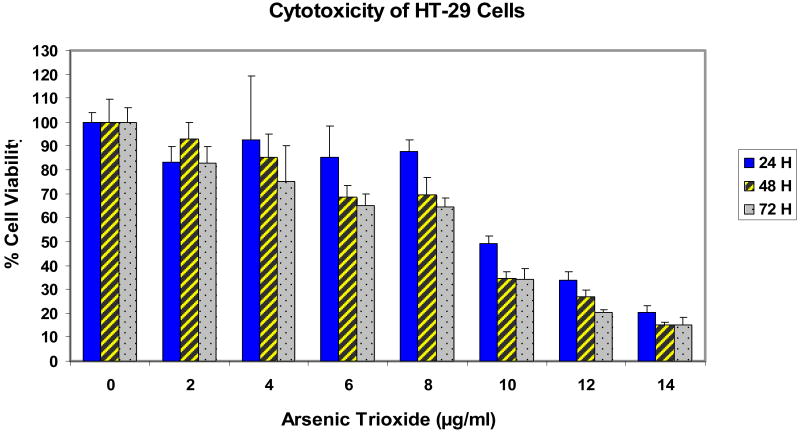
Adenocarcinoma colorectal (HT-29) cells were selected for the study. The cells were treated with different doses of As2O3 for 24, 48, and 72 h exposure, and cytotoxicity was determined by the MTT assay. The results of the cytotoxicity of the HT-29 cells are presented in Figure 1. Data obtained from the bioassays with HT-29 cells revealed a dose response with regard to the cytotoxicity of arsenic trioxide. With the increasing concentrations of As2O3, cell death increased. As indicated in figure 1, there was gradual decrease in the viability of HT-29 cells with increasing doses of arsenic trioxide. The mean percentages at 24 h ranged from 100% ± 4% (control), 83% ± 6% (2 μg/ml), 93% ± 26% (4 μg/ml), 85% ± 13% (6 μg/ml), 88% ± 5% (8 μg/ml), 49% ± 3% (10 μg/ml), 34% ± 4% (12 μg/ml), and 20% ± 3% (14 μg/ml); 48 h ranged from 100% ± 10% (control), 93% ± 7% (2 μg/ml), 85% ± 10% (4 μg/ml), 69% ± 5% (6 μg/ml), 70% ± 7% (8 μg/ml), 35% ± 3% (10 μg/ml), 27% ± 3% (12 μg/ml), and 15% ± 1% (14 μg/ml); and 72 h ranged from 100% ± 6% (control), 83% ± 7% (2 μg/ml), 75% ± 15% (4 μg/ml), 65% ± 5% (6 μg/ml), 65% ± 4% (8 μg/ml), 34% ± 5% (10 μg/ml), 20% ± 1% (12 μg/ml), and 15% ± 2% (14 μg/ml) for 0, 2, 4, 6, 8, 10, 12, and 14 μg/ml of arsenic trioxide. A dose-dependent response was also observed. Arsenic trioxide doses required to reduce cell viability by 50% were computed to be 9.8, 9.4, and 9.0 μg/ml for 24, 48, and 72 h of exposure, respectively.
Figure 1.
Cytotoxicity of HT-29 cells treated with different concentrations of As2O3 (0, 2, 4, 6, 8, 10, 12, and 14 μg/ml). Cytotoxicity was assessed by MTT assay after 24, 48, and 72 h exposure.
Discussion
Cytotoxicity can be defined as the degree to which an agent or chemical compound possesses a specific destructive action on certain cells. In this research, we examined the cytotoxic effect of arsenic trioxide on adenocarcinoma colorectal cancer (HT-29) cells. Finding from our study clearly demonstrated that arsenic trioxide is acutely toxic to adenocarcinoma colorectal cancer (HT-29) cells. Arsenic trioxide reduced cell viability of HT-29 cells as shown in the MTT assay (Figure 1). Although the mechanism by which arsenic trioxide exerts its toxic effect in this cell line has not yet been studied, we have demonstrated that there was a dose-dependent response with regard to arsenic trioxide toxicity to adenocarcinoma colorectal cancer (HT-29) cells. The median lethal doses (LD50) of arsenic trioxide for HT-29 cells were found to be 9.8, 9.4, and 9.0 μg/ml for 24, 48, and 72 h of exposure, respectively.
Previous studies have shown that As2O3 has significant cytotoxic effects on various cell lines [8 - 13]. The degree of cytotoxicity to various cells is quite different in which some cells lines are more sensitive than others [8 - 13]. Arsenic trioxide has been shown to be highly toxic to human leukemia (HL-60) showing a 24 h-LD50 of 6.4 ± 0.6 μg/mL [13]. It has also been reported that arsenic trioxide is cytotoxic to human liver carcinoma (HepG2) cells, showing a 48 h-LD50 of 8.55 ± 0.58 μg/mL [8]. Studies in our laboratory have shown that arsenic significantly reduced the viability of MCF-7 and A549 cells. Upon 48 h of exposure, the LD50 values from arsenic trioxide treatment were 11.5 and 14.1 μg/ml for A549 cells and MCF-7 cells, respectively [10]. Graham-Evans et al. studied the cytotoxicity effect of arsenic trioxide on several established human cell lines such as keratinocytes (HaCat), dermal fibroblast (CRL 1904), monocytes (THP-1/A23187), and melanocytes (1675). These authors reported that arsenic was toxic at high doses to keratinocytes (6 μg/ml), fibroblasts (1.5 μg/ml), monocytes (0.19 μg/ml) and toxic at lower doses in melanocytes (0.19 μg/ml) upon 72 h of exposure [11]. Chow et al. reported that the arsenic trioxide exhibited inhibitory effects on the proliferation of MCF-7 cells in a dose and time dependent manner, and found the LD50 values to be 8, 1.8 and 1.2 μM upon 1, 2, and 3 day treatments, respectively [19]. Epidemiological studies have shown that As2O3 induces cytotoxicity in prostate carcinoma cell lines (DU145 and PC-3) and in the ovarian carcinoma cell line (MDAH 2774) [20]. Using the MTT assay, we found a dose-dependent relationship between the degree of toxicity in colon cancer cells exposed to arsenic trioxide. This data serves as a foundation to further study the mechanisms of cytotoxicity and carcinogenesis in colon cancer cells.
Acknowledgments
This research was financially supported in part by a grant from the National Institutes of Health (Grant # 1G2RR13459) through the NCRR-RCMI Center for Environmental Health, and in part by a grant from the National Institutes of Health/MBRS-Research Initiative Research Enhancement (RISE) Program (Grant # R25GM067122-03) at Jackson State University. The authors thank Dr. Abdul K. Mohamed, Dean Emeritus of the College of Science, Engineering and Technology for his technical support in this research.
References
- 1.Hughes MF. Arsenic toxicity and potential mechanisms of action. Toxicology Letters. 2002;122(1):1–16. doi: 10.1016/s0378-4274(02)00084-x. [DOI] [PubMed] [Google Scholar]
- 2.Miller WH, Schipper HM, Lee JS, Waxman S. Mechanisms of action of arsenic trioxide. Cancer Research. 2002;62:3893–3903. [PubMed] [Google Scholar]
- 3.Frumkin H, Thun MJ. Arsenic. CA J Clin. 2001;51:254–262. doi: 10.3322/canjclin.51.4.254. [DOI] [PubMed] [Google Scholar]
- 4.Guo H. Cancer Risks Associated with Arsenic in Drinking Water. Environmental Health Perspectives. 2007;115(7):A339–A340. doi: 10.1289/ehp.9927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hughes MF. Biomarkers of exposure: A case study with inorganic arsenic. Environmental Health Perspectives. 2006;114(11):1–8. doi: 10.1289/ehp.9058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Patrick L. Toxic Metal and antioxidants: Part II. The Role of antioxidants in arsenic and cadmium toxicity. Alternative Medicine Review. 2003;8(2):106–128. [PubMed] [Google Scholar]
- 7.Chen GQ, Zhu J, Shi XG, Ni HU, Zhong HJ, Si GY, Jin XL, Tang W, Li XS, Xong SM, Shen ZX, Sun GL, MA J, Zhang P, Zhang TD, Gazin C, Naoe T, Chen ST, Wang ZY, Chen Z. In vitro studies on cellular and molecular mechanisms of arsenic trioxide (As2O3) in the treatment of acute promyelocytic leukemia: arsenic trioxide induces NB 4 cell apoptosis with down-regulation of bcl-2 expression and modulation of PML-RAR αPML proteins. Blood. 88:1052–1061. [PubMed] [Google Scholar]
- 8.Yedjou CG, Tchouwou PB. In-vitro cytotoxic and genotoxic effects of arsenic trioxide on human leukemia (HL-60) cells using the MTT and alkaline single cell electrophoresis (Comet) assays. Mole Cell Biochem. 2007;301:123–130. doi: 10.1007/s11010-006-9403-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yedjou CG, Tchouwou PB. Oxidative stress in human leukemia (HL-60), human liver carcinoma (HepG2), and human (Jurkat-T) cells exposed to arsenic trioxide. Metal Ions in Biology and Medicine. 2006;9:298–303. [PMC free article] [PubMed] [Google Scholar]
- 10.Walker AM, Stevens JJ, Tchounwou PB. Arsenic trioxide mediated cytotoxicity and cell proliferation in breast and lung carcinoma cell lines. Metal Ions in Biology and Medicine. 2006;9:287–292. [Google Scholar]
- 11.Graham-Evans B, Tchounwou PB, Cohly HHP. Cytotoxicity and proliferation studies with arsenic in established human cell lines: Keratinocytes, melanocytes, dendritic cells, dermal fibroblasts, microvascular endothethial cells, monocytes and T-cells. Int J Mol Sci. 2003;4:13–21. [Google Scholar]
- 12.Tchounwou PB, Wilson BA, Abdelghani AA, Ishaque AB, Patlolla AK. Differential cytotoxicity and gene expression in human liver carcinoma (HepG2) cells exposed to arsenic trioxide, and monosodium acid methanearsonate (MSMA) Int J Mol Sci. 2002;3:1117–1132. [Google Scholar]
- 13.Lee T, Ho I. Differential cytotoxic effects on arsenic on human and animal cells. Environmental Health Perspectives. 1994;102(3):101–105. doi: 10.1289/ehp.94102s3101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Roy P, Saha A. Metabolism and toxicity of arsenic: A human carcinogen. Current Science. 2002;82(1):38–45. [Google Scholar]
- 15.Tchounwou PB, Patlolla AK, Centeno JA. Carcinogenic and systemic health effects associated with arsenic exposure – a critical review. Toxicol Pathol. 2003;31(6):575–588. doi: 10.1080/01926230390242007. [DOI] [PubMed] [Google Scholar]
- 16.Abernathy C, Liu Y, Longfellow D, Aposhian V, Beck B, Fowler B, Goyer R, Menzer R, Rossman T, Thompson C, Waalkes M. Arsenic: Health Effects, Mechanism of Actions, and Research Issues. Environmental Health Perspectives. 1999;107(7):1–8. doi: 10.1289/ehp.99107593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hartwig A, Asmuss M, Ehleben I, Herzer H, Kostelac D, Pelzer A, Schwerdtle T, Burkle A. Interference by Toxic Metal Ions with DNA repair processes and cell cycle control: molecular mechanisms. Environmental Health Perspectives. 2002;110(5):797–799. doi: 10.1289/ehp.02110s5797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mosmann T. Rapid colorimetric assay for cellular growth and survival: applications to proliferation and cytotoxicity assays. J Immunol Methods. 1988;65(1-2):55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 19.Chow SKY, Chan JYW, Fung KP. Inhibition of cell proliferation and the action mechanisms of arsenic trioxide As2O3 on human breast cancer cells. J Cell Biochem. 2004;93(1):173–187. doi: 10.1002/jcb.20102. [DOI] [PubMed] [Google Scholar]
- 20.Usla R, Sanli UA, Sezgin C, Karabulant B, Terzioglu E, Omay SB, Goker E. Arsenic trioxide-mediated cytotoxicity and apoptosis in prostate and ovarian carcinoma cell lines. Clinical Cancer Research. 2000;6:4957–4964. [PubMed] [Google Scholar]