Abstract
Recent evidence suggests that urinary F2-isoprostanes (F2-IsoPs) are more accurate markers of oxidative stress than other available biomarkers. Most previous studies used unmetabolized F2-IsoPs as a biomarker. Few previous studies measured 15-F2t-IsoP-M, a metabolite of one of the most common F2-IsoPs, 15-F2t-IsoP. Unlike 15-F2t-IsoP, 15-F2t-IsoP-M is not subject to autoxidation and renal production. To our knowledge, no study has compared the associations of age and body mass index (BMI) with F2-IsoPs to those with 15-F2t-IsoP-M. Urinary levels of F2-IsoPs and 15-F2t-IsoP-M were measured using gas chromatography–mass spectrometry for 845 healthy women aged 40–70 years. Both F2-IsoPs and 15-F2t-IsoP-M were elevated among smokers. The level of 15-F2t-IsoP-M increased with age, particularly after menopause, and with BMI. In contrast, F2-IsoPs decreased with age, regardless of menopausal status, and was not related to BMI. The association of 15-F2t-IsoP-M with age or menopausal status did not differ by BMI category, and the association with BMI was also independent of age or menopausal status. 15-F2t-IsoP-M appears to be a valuable biomarker of oxidative stress in age- and obesity-related diseases. Antioxid. Redox Signal. 14, 2453–2460.
Introduction
Accumulating evidence from in vitro and in vivo studies indicates that normal basal levels of oxygen-derived free radicals or reactive oxygen species (ROS), acting as secondary messengers, play an essential role in the regulation of various normal physiologies (4, 18), including signal transduction, cell proliferation, homeostasis, microorganism defense, senescence, and apoptosis (1, 4, 22). However, overproduction of ROS may play a causative role in the development of numerous human diseases or conditions, such as cancer, neurodegenerative and cardiovascular diseases, and aging process (5, 6, 9, 32).
Since their first discovery in 1990 (24), F2-isoprostanes (F2-IsoPs), a series of free radical-catalyzed lipid peroxidation products of arachidonic acid in situ in phospholipids, have been widely used in animal or human studies to measure in vivo lipid peroxidation, a central feature of free radical damage (18, 31). Studies have shown that F2-IsoPs in human biological fluids can be the most accurate marker in predicting oxidative stress (20, 26, 30). Urinary F2-IsoPs have been used often because the urine is easy to sample and has a high presence of F2-IsoPs (25). However, F2-IsoPs could be artificially produced in vitro in fluids by autoxidation and its in vivo level in the human urine may be affected by local renal isoprostane production, which is age-dependent (20, 25). After β-oxidation, 15-F2t-isoprostane (15-F2t-IsoP), one of the most common F2-IsoPs, converts to 2,3-dinor-5,6-dihydro-15-F2t-IsoP (15-F2t-IsoP-M), a metabolite not subject to autoxidation and renal production (19, 26). A method with both high sensitivity and accuracy has been developed to measure 15-F2t-IsoP-M using gas chromatography–negative ion chemical ionization mass spectrometry (26). Normal levels of plasma or urinary excretion rates of F2-IsoPs and 15-F2t-IsoP-M in humans have been defined previously (19, 24). Although seldom examined in epidemiologic studies (13, 32), 15-F2t-IsoP-M was suggested as another promising marker of in vivo oxidative stress (6).
Recently, we found that the correlation between urinary F2-IsoPs and 15-F2t-IsoP-M was only moderate (r = 0.31) (6). In a nested case–control study, while we observed no significant difference in urinary levels of F2-IsoPs and 15-F2t-IsoP-M between breast cancer cases and their matched controls overall, we found that levels of isoprostanes, particularly 15-F2t-IsoP-M, were associated with a substantially increased risk of breast cancer among overweight/obese women, but not among those of normal weight (6). Several other recent studies have reported that higher levels of body mass index (BMI) were associated with an increased level of F2-IsoPs (7, 14, 15), further suggesting that isoprostanes are promising biomarkers for obesity-related diseases.
Oxidative stress may also play a critical role in age-related diseases (9, 10). Few studies have examined the associations of F2-IsoPs with age (3, 17, 21, 29); however, the results are inconclusive. To the best of our knowledge, no study has compared the associations of age and BMI with F2-IsoPs to those with 15-F2t-IsoP-M. In the current study, we evaluated the associations of urinary F2-IsoPs and 15-F2t-IsoP-M levels with age and BMI using data from a subset of 845 cancer-free midlife women within a large population-based cohort study.
Materials and Methods
Subjects
This cross-sectional study was conducted among 845 women who were free of cancer diagnosis and participants of the Shanghai Women's Health Study (SWHS), a population-based cohort study. Details on the establishment of the cohort have been reported elsewhere (35). In brief, at baseline during March 1997 and May 2000, 74,942 Chinese women between 40 and 70 years of age were recruited in Shanghai, with a 92.7% participation rate. The study was approved by all relevant institutional review boards in the People's Republic of China and the United States. All participants provided informed written consent. Participants of the current study were identified as the control group for a nested case-control study of oxidative stress and breast cancer risk in the SWHS (6).
Data collection
Information on demographic characteristics, history of chronic diseases (e.g. cardiovascular diseases, diabetes mellitus, hypertension, and hepatitis), reproductive factors, and lifestyle factors was collected during in-person interviews conducted by trained interviewers. Smoking was defined as smoking ≥1 cigarette per day for >6 months continuously at some point during a woman's life. Drinking of alcohol (wine, beer, and/or liquor) or tea ≥3 times per week for >6 months continuously was defined as regular consumption of alcohol or tea, respectively. Use of ginseng was defined as taking ginseng and ginseng supplements at least five times per year in the past 3 years before enrollment. Menopausal status was defined as “cease of menstruation for 12 months or longer, excluding that caused by pregnancy and breastfeeding” (34). Participating in any exercise ≥1 time per week for >3 months continuously over the past 5 years preceding the interview was considered as regularly exercising (yes/no). During the interview, anthropometric parameters, including height, weight, waist circumference, and hip circumference were measured twice. BMI was calculated (weight/height2, kg/m2).
Approximately 88% of the cohort members donated a spot urine sample at baseline or at the first follow-up 2 years later. The urine sample was collected into a sterilized 100-ml cup containing 125 mg of ascorbic acid and kept in a portable, insulated bag with ice packs (at about 0°C–4°C) and transported to the central laboratory for processing. Ascorbic acid was added to the sample container to prevent degradation of unstable compounds. Within 6 h after collection, samples were aliquoted and stored at −70°C until the laboratory analyses. The biologic sample repositories for the study are equipped with appropriate alarm systems and emergency electricity backup to prevent accidental thawing (35).
Measurement of urinary F2-IsoPs and 15-F2t-IsoP-M
Assays were performed for 845 samples in four batches. Urinary excretion of F2-IsoPs (mainly 15-F2t-IsoP or 8-iso-PGF2α) and the major metabolite of 15-F2t-IsoP, 15-F2t-IsoP-M (2,3-dinor-5,6-dihydro-15-F2t-IsoP or 2,3-dinor-5,6-dihydro-8-iso-PGF2α), were measured by gas chromatography–negative ion chemical ionization mass spectrometry at Vanderbilt Eicosanoid Core Laboratory. The method has been reported in detail elsewhere (20, 25, 26). In brief, the metabolite (2,3-dinor-5,6-dihydro-15-F2t-IsoP) was chemically synthesized and converted to an [18O2]-labeled derivative for use as an internal standard. Both urinary F2-IsoPs and 15-F2t-IsoP-M levels were expressed as ng/mg after adjusting for urinary creatinine concentration. Precision of the assay was ± 4% and accuracy was 97%. The lower limit of sensitivity was ∼20 pg/mg (20).
Statistical analysis
Log-transformation was conducted to approach normal distribution for urinary F2-IsoPs and 15-F2t-IsoP-M. Geometric means and 95% confidence intervals (CIs) of F2-IsoPs and 15-F2t-IsoP-M were obtained based on the least square means estimated from general linear regression models. Potential confounding factors adjusted in regression models include age, menopausal status (pre-/post-), education, occupation, physical activity, BMI, tea drinking, cigarette smoking, vitamin supplement use, and batches for assays. Tests for trends were performed by entering the categorical variables as a continuous parameter in the model. Age was classified as six 5-year age groups from 40–45 to 65–70 years, and BMI was categorized according to the WHO cut points for international classification of BMI (i.e., BMI of ≥25 kg/m2 for overweight and ≥30 kg/m2 for obesity) as well as cut points for Asian populations (≥23 kg/m2 for overweight/obesity), recommended by WHO expert consultation (33). We also performed stratified analyses by BMI (<25.0 vs. ≥25.0) or (normal weight vs. overweight/obesity), menopausal status (pre- vs. post-) and age (median, <51 vs. ≥51 years) to evaluate whether the associations of age and BMI with urinary isoprostanes differed according to these factors. Tests for interaction were performed using the likelihood ratio test. All statistical tests were two-sided and were performed using SAS statistical software, version 9.2 (SAS Institute, Cary, NC).
Results
The average age of the study population at study enrollment was 52.9 ± 8.9 standard deviation (SD) years, and the median age was 51 years. The log-transformed mean levels of urinary F2-IsoPs and 15-F2t-IsoP-M were 1.62 (SD 1.51) and 0.56 (SD 0.57) ng/mg of urinary creatinine (cr), respectively. The correlation coefficient was 0.34 between log-transformed F2-IsoPs and log-transformed 15-F2t-IsoP-M in all subjects (p < 0.01, data not shown). Table 1 presents the adjusted geometric means and 95% CI for F2-IsoPs and 15-F2t-IsoP-M levels (ng/mg cr) according to lifestyle factors (adjusted for age and batches for assays). The associations of isoprostanes with education and occupation were significant (p for trend <0.05 for both, data not shown). Smokers had elevated levels of F2-IsoPs (p = 0.007) or 15-F2t-IsoP-M (p = 0.09). Physically active women tended to have lower mean levels of F2-IsoPs and 15-F2t-IsoP-M than women who were inactive, but the associations were not significant. Tea drinking (primarily green tea) was associated with an elevated mean level of 15-F2t-IsoP-M (p = 0.03), but not with F2-IsoPs. Women who took any vitamin supplement regularly had nonsignificantly decreased F2-IsoPs, but significantly reduced 15-F2t-IsoP-M levels (p = 0.008). This significant association was observed mainly with the use of multivitamin or vitamin E supplement, but not of vitamin A, C, or B- complex (data not shown). Women who took ginseng supplements regularly (26% of cohort) had low levels of F2-IsoPs (p = 0.03); however, there was no appreciable difference in 15-F2t-IsoP-M levels between ginseng users and nonusers. Regular alcohol consumption, exposure to environmental tobacco smoke, use of fish oil supplement, and self-reported chronic disease history were not significantly related to either biomarker. Therefore, education, occupation, tea drinking, cigarette smoking, and vitamin supplement use were adjusted in subsequent analyses as potential confounding factors.
Table 1.
Urinary F2-IsoPs and 15-F2t-IsoP-M Levels (ng/mg cr) in Middle-Aged and Older Women According to Lifestyle-Related Factors, the Shanghai Women's Health Study
| |
|
F2-IsoPs |
15-F2t-IsoP-M |
||
|---|---|---|---|---|---|
| n = 845 | Geometric mean (95% CI)a | p-valueb | Geometric mean (95% CI)a | p-valueb | |
| Cigarette smoking regularly | |||||
| No | 822 | 1.60 (1.54–1.66) | 0.56 (0.54–0.58) | ||
| Yes | 23 | 2.25 (1.77–2.89) | 0.007 | 0.68 (0.54–0.86) | 0.09 |
| Environmental tobacco smoke | |||||
| No | 146 | 1.68 (1.52–1.86) | 0.54 (0.54–0.59) | ||
| Yes | 699 | 1.62 (1.54–168) | 0.46 | 0.55 (0.50–0.60) | 0.44 |
| Alcohol consumption regularly | |||||
| No | 819 | 1.63 (1.57–1.70) | 0.57 (0.54–0.68) | ||
| Yes | 26 | 1.49 (1.18–1.88) | 0.45 | 0.56 (0.54–0.70) | 0.94 |
| Physically active past 10 years | |||||
| No | 564 | 1.64 (1.57–1.73) | 0.58 (0.54–0.60) | ||
| Yes | 281 | 1.58 (1.48–1.70) | 0.42 | 0.54 (0.51–0.58) | 0.11 |
| Tea drink regularly | |||||
| Never | 605 | 1.62 (1.54–1.70) | 0.55 (0.53–0.58) | ||
| Ever | 240 | 1.63 (1.52–1.77) | 0.77 | 0.60 (0.56–0.64) | 0.03 |
| Fish oil supplement use | |||||
| Never | 633 | 1.60 (1.51–1.68) | 0.57 (0.54–0.59) | ||
| Ever | 165 | 1.66 (1.52–1.82) | 0.47 | 0.56 (0.52–0.61) | 0.72 |
| Ginseng product use | |||||
| Never | 624 | 1.66 (1.58–1.75) | 0.56 (0.53–0.58) | ||
| Ever | 221 | 1.51 (1.39–1.63) | 0.03 | 0.58 (0.54–0.63) | 0.09 |
| Vitamin supplement use | |||||
| Never | 716 | 1.65 (1.57–1.72) | 0.58 (0.55–0.60) | ||
| Ever | 129 | 1.51 (1.36–1.66) | 0.13 | 0.50 (0.45–0.60) | 0.008 |
| History of chronic diseases | |||||
| No | 533 | 1.66 (1.58–1.75) | 0.56 (0.53–0.59) | ||
| Yes | 312 | 1.55 (1.45–1.75) | 0.12 | 0.57 (0.54–0.61) | 0.56 |
Missing value was (<5%) not included in the percentage estimation.
ANOVA estimates (geometric means and standard error of the log-transformed isoprostanes [F2-IsoPs, 15-F2t-IsoP-M]) adjusted for age and batches assays for F2-IsoPs or 15-F2t-IsoP-M (categories).
p-value obtained from the ANOVA of the log-transformed values adjusted for the same variables as above.
ANOVA, analysis of variance; CI, confidence interval.
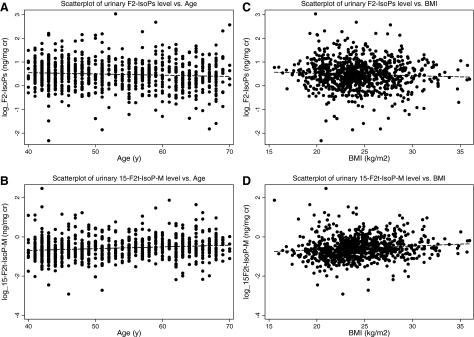
Table 2 presents the association between mean levels of F2-IsoPs or 15-F2t-IsoP-M by age or menopausal status at urine collection, and in analyses stratified by BMI (<25.0 vs. ≥25.0). Overall, there was a statistically significant inverse association between urinary F2-IsoPs levels and age (1.70 ng/mg of creatinine for women aged <45 years and 1.51 for those aged ≥65 years, p for trend = 0.02) independent of menopausal status (p for interaction 0.36, data not shown) and BMI (p for interaction = 0.60). Conversely, urinary level of 15-F2t-IsoP-M was significantly increased with age (0.50 ng/mg of creatinine for women aged <45 years and 0.62 for those aged ≥65 years, p for trend <0.01). This association was seen regardless of BMI status (p for interaction = 0.29). Figure 1A and B shows the univariate associations of age with F2-IsoPs and 15-F2t-IsoP-M, respectively. The mean level of F2-IsoPs did not differ significantly between premenopausal and postmenopausal women (p = 0.46), regardless of BMI status (p for interaction = 0.55). However, 15-F2t-IsoP-M levels were significantly elevated among postmenopausal women (0.62, 95% CI: 0.58–0.65) compared to premenopausal women (0.51, 95% CI: 0.48–0.54) (p < 0.01). This pattern of higher 15-F2t-IsoP-M level in post- than premenopausal women occurred in both normal and overweight/obese women (<25.0 vs. ≥25.0 kg/m2) (p for interaction = 0.48).
Table 2.
Association Between Urinary F2-IsoPs and 15-F2t-IsoP-M Levels (ng/mg cr), and Age and Menopausal Status in Middle-Aged and Older Women, the Shanghai Women's Health Study
| |
|
F2-IsoPs |
15-F2t-IsoP-M |
||
|---|---|---|---|---|---|
| n | Geometric mean (95% CI)a | p trendb | Geometric mean (95% CI)a | p trendb | |
| Age, years | All women (n = 845) | ||||
| <45 | 213 | 1.70 (1.57–1.84) | 0.50 (0.45–0.53) | ||
| 45–49 | 159 | 1.72 (1.55–1.88) | 0.55 (0.50–0.59) | ||
| 50–54 | 109 | 1.70 (1.52–1.90) | 0.54 (0.49–0.60) | ||
| 55–59 | 117 | 1.60 (1.43–1.79) | 0.66 (0.60–0.74) | ||
| 60–64 | 121 | 1.48 (1.32–1.65) | 0.59 (0.53–0.64) | ||
| ≥65 | 126 | 1.51 (1.35–1.62) | 0.02 | 0.62 (0.56–0.69) | <0.01 |
| BMI <25 kg/m2 (n = 517) | |||||
| <45 | 164 | 1.70 (1.54–1.86) | 0.48 (0.44–0.53) | ||
| 45–49 | 115 | 1.73 (1.55–1.95) | 0.54 (0.49–0.60) | ||
| 50–54 | 62 | 1.65 (1.42–1.91) | 0.51 (0.44–0.59) | ||
| 55–59 | 63 | 1.57 (1.35–1.84) | 0.62 (0.54–0.73) | ||
| 60–64 | 63 | 1.54 (1.32–1.80) | 0.57 (0.49–0.66) | ||
| ≥65 | 50 | 1.55 (1.31–1.84) | 0.15 | 0.66 (0.56–0.77) | 0.006 |
| BMI ≥25 kg/m2 (n = 328) | |||||
| <45 | 49 | 1.68 (1.42–1.99) | 0.51 (044–0.59) | ||
| 45–49 | 44 | 1.57 (1.32–1.88) | 0.54 (0.47–0.63) | ||
| 50–54 | 47 | 1.79 (1.51–2.12) | 0.59 (0.51–0.68) | ||
| 55–59 | 54 | 1.65 (1.40–1.93) | 0.72 (0.62–0.82) | ||
| 60–64 | 58 | 1.40 (1.21–1.65) | 0.66 (0.54–0.72) | ||
| ≥65 | 76 | 1.49 (1.30–1.65) | 0.14 | 0.62 (0.55–0.70) | 0.02 |
| p for interaction | 0.60 | 0.29 | |||
| Menopausal status | All women (n = 845) | ||||
| Premenopausal | 404 | 1.65 (1.55–1.75) | 0.51 (0.48–0.54) | ||
| Postmenopausal | 441 | 1.60 (1.51–1.70) | 0.46c | 0.62 (0.58–0.65) | <0.01c |
| BMI <25 kg/m2 (n = 517) | |||||
| Premenopausal | 289 | 1.66 (1.55–1.79) | 0.50 (0.46–0.53) | ||
| Postmenopausal | 228 | 1.63 (1.51–1.77) | 0.65c | 0.61 (0.56–0.65) | <0.01c |
| BMI ≥25 kg/m2 (n = 328) | |||||
| Premenopausal | 115 | 1.58 (1.42–1.79) | 0.53 (0.48–0.58) | ||
| Postmenopausal | 213 | 1.57 (1.45–1.72) | 0.90c | 0.64 (0.60–0.69) | 0.002c |
| p for interaction | 0.55 | 0.48 | |||
Age and menopausal status were highly correlated with each other (r = 0.83); thus, they were not included in the same model.
ANOVA estimates (geometric means and standard error of the log-transformed isoprostanes [F2-IsoPs, 15-F2t-IsoP-M]) adjusted for age or menopausal status (pre-/post-), education, occupation, physical activity, BMI, tea drinking, cigarette smoking, vitamin supplement use, and batches assays for F2-IsoPs or 15-F2t-IsoP-M (categories).
p for trend (cp value) obtained from the ANOVA of the log-transformed values adjusted for the same variables as above.
BMI, body mass index.
FIG. 1.
Associations between urinary F2-IsoPs and 15-F2t-IsoP-M, age and BMI.
Further, we examined the levels of F2-IsoPs and 15-F2t-IsoP-M across BMI categories, stratified by age (<51 vs. ≥51 years old) and menopausal status (pre- vs. postmenopause) (Table 3). Overall, the mean levels of F2-IsoPs tended to decrease with increasing BMI, but not statistically significant (p for trend = 0.35). This inverse association was consistent across categories of age and menopausal status (p for interaction = 0.96 and 0.70, respectively). Conversely, 15-F2t-IsoP-M level was significantly positively associated with age (0.53 ng/mg of creatinine, 95% CI: 0.49–0.56 for BMI <23.0 and 0.61 ng/mg of creatinine, 95% CI: 0.53–0.72 for BMI ≥30.0, p for trend = 0.02). This positive association appeared in both younger and older age groups (p for interaction = 0.36) and in pre- and postmenopuasal women (p for interaction = 0.40), although the association reached statistical significance only among younger (p for trend = 0.01) or premenopausal women (p for trend = 0.02). Figure 1C and D shows the univariate correlations of BMI with F2-IsoPs and 15-F2t-IsoP-M, respectively.
Table 3.
Association Between Urinary F2-IsoPs and 15-F2t-IsoP-M Levels (ng/mg cr) and Body Mass Index in Middle-Aged and Older Women, the Shanghai Women's Health Study
| |
|
F2-IsoPs |
15-F2t-IsoP-M |
||
|---|---|---|---|---|---|
| BMI, kg/m2 | n | Geometric mean (95% CI)a | p trendb | Geometric mean (95% CI)a | p trendb |
| All women (n = 845) | |||||
| <23.0 | 292 | 1.66 (1.55–1.79) | 0.53 (0.49–0.56) | ||
| 23.0–24.9 | 225 | 1.62 (1.49–1.73) | 0.59 (0.54–0.63) | ||
| 25.0–29.9 | 278 | 1.60 (1.49–1.72) | 0.58 (0.54–0.62) | ||
| ≥30.0 | 50 | 1.57 (1.34–1.86) | 0.35 | 0.61 (0.53–0.72) | 0.02 |
| Age <51 years (n = 391) | |||||
| <23.0 | 171 | 1.72 (1.58–1.86) | 0.48 (0.44–0.52) | ||
| 23.0–24.9 | 117 | 1.66 (1.51–1.84) | 0.56 (0.51–0.62) | ||
| 25.0–29.9 | 93 | 1.70 (1.51–1.90) | 0.57 (0.50–0.64) | ||
| ≥30.0 | 10 | 1.51 (1.10–2.12) | 0.63 | 0.53 (0.37–0.75) | 0.01 |
| Age ≥51 years (n = 454) | |||||
| <23.0 | 121 | 1.65 (1.48–1.84) | 0.58 (0.53–0.64) | ||
| 23.0–24.9 | 108 | 1.57 (1.39–1.77) | 0.60 (0.54–0.66) | ||
| 25.0–29.9 | 185 | 1.51 (1.38–1.65) | 0.62 (0.56–0.65) | ||
| ≥30.0 | 40 | 1.55 (1.28–1.90) | 0.30 | 0.66 (0.56–0.78) | 0.27 |
| p for interaction | 0.96 | 0.36 | |||
| Premenopausal (n = 404) | |||||
| <23.0 | 173 | 1.68 (1.54–1.82) | 0.47 (0.43–0.51) | ||
| 23.0–24.9 | 116 | 1.63 (1.46–1.80) | 0.56 (0.51–0.62) | ||
| 25.0–29.9 | 102 | 1.68 (1.51–1.90) | 0.55 (0.50–0.62) | ||
| ≥30.0 | 13 | 1.43 (1.06–1.95) | 0.73 | 0.51 (0.37–0.69) | 0.02 |
| Postmenopausal (n = 441) | |||||
| <23.0 | 119 | 1.65 (1.47–1.84) | 0.59 (0.54–0.66) | ||
| 23.0–24.9 | 109 | 1.60 (1.43–1.80) | 0.61 (0.55–0.67) | ||
| 25.0–29.9 | 176 | 1.54 (1.40–1.68) | 0.62 (0.56–0.66) | ||
| ≥30.0 | 37 | 1.62 (1.34–1.97) | 0.48 | 0.68 (0.57–0.80) | 0.30 |
| p for interaction | 0.70 | 0.40 | |||
Age is grouped according to median age (<51 and ≥51 years).
ANOVA estimates (geometric means and standard deviation of the log-transformed isoprostanes [F2-IsoPs, 15-F2t-IsoP-M]) adjusted for age or menopausal, status, education, occupation, physical activity, tea drinking, cigarette smoking, vitamin supplement use, and batches assays for F2-IsoPs or 15-F2t-IsoP-M (categories).
p for trend obtained from ANOVA of the log-transformed values adjusted for the variables as above.
Discussion
In this Chinese population, urinary F2-IsoPs levels were similar to the urinary levels of F2-IsoPs in the U.S. population, as defined previously (1.6 ± 0.6 ng/mg of creatinine) (26). However, urinary isoprostane metabolite (15-F2t-IsoP-M) levels were higher in our population than in the U.S. population (0.39 ± 0.18 ng/mg cr) (26), but race/ethnicity, age, and gender of study subjects were not reported. Although few women smoked in our population, we found that women who smoked had elevated levels of both F2-IsoPs and 15-F2t-IsoP-M than nonsmoking women, a finding that is consistent with the results of previous studies on F2-IsoPs (3, 11, 23), with the exception of the small nested case–control study with 26 pairs of diabetes and controls in which current smokers tended to have a reduced level of 15-F2t-IsoP-M compared with nonsmokers or ex-smokers (13). To our knowledge, no study has compared the associations of age and BMI with F2-IsoPs to those with 15-F2t-IsoP-M. We found the level of F2-IsoPs decreased with age, whereas 15-F2t-IsoP-M significantly increased with age and postmenopausal status. Further, the urinary level of 15-F2t-IsoP-M was significantly positively associated with BMI level, whereas the level of urinary F2-IsoPs was not significantly associated with BMI status. These findings suggest that, in addition to F2-IsoPs, using 15-F2t-IsoP-M as a biomarker will provide independent information, particularly in obesity- and age-related diseases (5, 29, 32).
ROS are continuously formed and degraded in normal cellular processes. It was hypothesized that ROS including oxygen-containing free radicals play a role in the changes associated with aging or in age-induced oxidative stress (9, 10). In this study, we found that a marker of oxidative stress, urinary isoprostane metabolite (15-F2t-IsoP-M), but not F2-IsoPs, was positively associated with age or postmenopausal status. Our finding on F2-IsoPs was consistent with that in two large-scale epidemiologic studies, the Framingham Heart Study (15) and the European Union Study (3), in which urinary concentrations of F2-IsoPs decreased with age, particularly among nonsmokers in the latter study. Other small-scale studies found a positive (23) or no association (17) between age and F2-IsoPs. In addition to smoking status, it is possible that the association between age and urinary F2-IsoPs may be confounded by age-dependent kidney function (29), which might explain in part the inconsistencies in previous studies, whereas 15-F2t-IsoP-M is not affected by local kidney production (12). Further investigations of the associations between age and both parent compound and metabolite isopostanes are needed to clarify these scientific issues.
Prior studies reported that higher plasma or urinary levels of F2-IsoPs were associated with higher BMI (7, 14, 15). Both BMI and waist circumference were positively related to urinary F2-IsoPs in women and men in Japan (7). Our inverse, but not statistically significant, association between F2-IsoPs and BMI did not support these previous findings. One possible explanation for this inconsistency is that previous studies mainly used the immunoassay method to quantify F2-IsoPs, in which a cross-reactivity was found between the immunoassay antibody of F2-IsoPs and structurally related isoprostane isomers (28), including 15-F2t-IsoP, the major precursor of 15-F2t-IsoP-M (27). Thus, it is possible that 15-F2t-IsoP-M is the underlying factor for the positive association between urinary F2-IsoPs and BMI in most previous studies. Our results of a significant positive association of 15-F2t-IsoP-M with BMI support this possibility. In addition, findings of this study is consistent with our recent report that an increased risk of breast cancer was strongly related to urinary 15-F2t-IsoP-M level than F2-IsoPs level, among overweight/obese women (6). It is possible that the positive association between BMI and isoprostane metabolite may be due to their links to estrogen. Still, replication is needed for our findings on the association between urinary 15-F2t-IsoP-M and BMI.
The present study has a number of notable strengths. Urinary levels of F2-IsoPs were measured together with its major metabolite 15-F2t-IsoP-M using a newly developed, more sensitive method. Another major strength of our study was its relatively large sample size. The parent population-based cohort study had remarkably high rates for baseline participation and follow-up, which minimized selection bias. We were able to adjust for many potential confounding factors, including smoking and vitamin supplement use.
There are several limitations of the present study. One limitation is that a single urine sample was used. However, reliability studies found that at a group level, F2-IsoPs measured in a single urine sample were comparable to that measured using multiple samples or a 24-h urine sample (2). Previous studies generated inconsistent results on the inter-day variation of urinary isoprostanes levels (2), whereas our previous data in the same study population suggest that the major contributor to intra-person variation is seasonal fluctuation (16). Since inter-day variation is random, any residual inter-day variation may lead to nondifferential misclassification, which usually biases the result to the null. To the extent that residual inter-day variation levels exist in our data, the true associations could be stronger than those we observed. Moreover, urinary isoprostanes, age, and BMI were measured at the same time. The temporal sequence thus is unclear for the observed associations. However, it is unlikely that the dose–response associations between age and BMI with urinary concentrations of F2-IsoPs and 15-F2t-IsoP-M are a result of selection biases, as measurement of biomarkers was blinded to subject characteristics. Another limitation is that we did not collect information on plasma lipids and estradiol, and we were therefore unable to investigate the effects of these biomarkers on levels of urinary isoprostanes. However, we have calculated the dietary intake of lipids and found no apparent associations between dietary intakes of arachidonic acid and other lipids and F2-IsoPs or 15-F2t-IsoP-M. Further, we have investigated the associations between urinary isoprostane levels and erythrocyte membrane phospholipid polyunsaturated fatty acid concentrations in our validation study among 48 Chinese men with measurements of blood lipids and urinary isoprostanes for each season over a period of year, and found no significant associations. Further investigations are needed to study how tissue levels of lipids affect isoprostane levels (8).
One of the most critical needs in free radical research in humans has been the development of a reliable noninvasive method to assess oxidative stress (25, 30). High levels of F2-IsoPs have been linked to a number of obesity- or age-related diseases (9, 15, 29). Our findings suggest that in addition to F2-IsoPs, 15-F2t-IsoP-M is another valuable biomarker of oxidative stress in age- and obesity-related diseases. Further studies are warranted to confirm our findings.
Abbreviations Used
- 15-F2t-IsoP
isoprostane 8-iso-prostaglandin F2α
- 15-F2t-IsoP-M
isoprostane 8-iso-prostaglandin F2α metabolite
- BMI
body mass index
- CI
confidence interval
- F2-IsoPs
F2-isoprostanes
- ROS
reactive oxygen species
- SD
standard deviation
- SWHS
Shanghai Women's Health Study
- WHO
World Health Organization
Acknowledgments
Dr. Jason Morrow, our beloved colleague, long-term collaborator, and key co-investigator of the project, passed away. Without him, our research work would have been impossible. This article is dedicated to his memory. The authors thank the Shanghai residents who participated in the study and the research staff of the SWHS for their dedication and contributions to the study. This work was supported by research grant R01CA106591 as well as parent studies R37CA70867 and N02 CP1101066 from the National Institutes of Health. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute or the National Institutes of Health.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Basu S. F2-isoprostanes in human health and diseases: from molecular mechanisms to clinical implications. Antioxid Redox Signal. 2008;10:1405–1434. doi: 10.1089/ars.2007.1956. [DOI] [PubMed] [Google Scholar]
- 2.Basu S. Helmersson J. Factors regulating isoprostane formation in vivo. Antioxid Redox Signal. 2005;7:221–235. doi: 10.1089/ars.2005.7.221. [DOI] [PubMed] [Google Scholar]
- 3.Basu S. Helmersson J. Jarosinska D. Sallsten G. Mazzolai B. Barregard L. Regulatory factors of basal F(2)-isoprostane formation: population, age, gender and smoking habits in humans. Free Radic Res. 2009;43:85–91. doi: 10.1080/10715760802610851. [DOI] [PubMed] [Google Scholar]
- 4.Comporti M. Signorini C. Arezzini B. Vecchio D. Monaco B. Gardi C. F2-isoprostanes are not just markers of oxidative stress. Free Radic Biol Med. 2008;44:247–256. doi: 10.1016/j.freeradbiomed.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 5.Cracowski JL. Kom GD. Salvat-Melis M. Renversez JC. McCord G. Boignard A. Carpentier PH. Schwedhelm E. Postocclusive reactive hyperemia inversely correlates with urinary 15-F2t-isoprostane levels in systemic sclerosis. Free Radic Biol Med. 2006;40:1732–1737. doi: 10.1016/j.freeradbiomed.2006.01.014. [DOI] [PubMed] [Google Scholar]
- 6.Dai Q. Gao YT. Shu XO. Yang G. Milne G. Cai Q. Wen W. Rothman N. Cai H. Li H. Xiang Y. Chow WH. Zheng W. Oxidative stress, obesity, and breast cancer risk: results from the Shanghai Women's Health Study. J Clin Oncol. 2009;27:2482–2488. doi: 10.1200/JCO.2008.19.7970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Furukawa S. Fujita T. Shimabukuro M. Iwaki M. Yamada Y. Nakajima Y. Nakayama O. Makishima M. Matsuda M. Shimomura I. Increased oxidative stress in obesity and its impact on metabolic syndrome. J Clin Invest. 2004;114:1752–1761. doi: 10.1172/JCI21625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Halliwell B. Lee CY. Using isoprostanes as biomarkers of oxidative stress: some rarely considered issues. Antioxid Redox Signal. 2010;13:145–156. doi: 10.1089/ars.2009.2934. [DOI] [PubMed] [Google Scholar]
- 9.Harman D. Free radical theory of aging: an update: increasing the functional life span. Ann N Y Acad Sci. 2006;1067:10–21. doi: 10.1196/annals.1354.003. [DOI] [PubMed] [Google Scholar]
- 10.Harper ME. Bevilacqua L. Hagopian K. Weindruch R. Ramsey JJ. Ageing, oxidative stress, and mitochondrial uncoupling. Acta Physiol Scand. 2004;182:321–331. doi: 10.1111/j.1365-201X.2004.01370.x. [DOI] [PubMed] [Google Scholar]
- 11.Helmersson J. Larsson A. Vessby B. Basu S. Active smoking and a history of smoking are associated with enhanced prostaglandin F(2alpha), interleukin-6 and F2-isoprostane formation in elderly men. Atherosclerosis. 2005;181:201–207. doi: 10.1016/j.atherosclerosis.2004.11.026. [DOI] [PubMed] [Google Scholar]
- 12.Huang HY. Caballero B. Chang S. Alberg AJ. Semba RD. Schneyer CR. Wilson RF. Cheng TY. Vassy J. Prokopowicz G. Barnes GJ. Bass EB. The efficacy and safety of multivitamin and mineral supplement use to prevent cancer and chronic disease in adults: a systematic review for a National Institutes of Health state-of-the-science conference. Ann Intern Med. 2006;145:372–385. doi: 10.7326/0003-4819-145-5-200609050-00135. [DOI] [PubMed] [Google Scholar]
- 13.Il'yasova D. Morrow JD. Wagenknecht LE. Urinary F2-isoprostanes are not associated with increased risk of type 2 diabetes. Obes Res. 2005;13:1638–1644. doi: 10.1038/oby.2005.201. [DOI] [PubMed] [Google Scholar]
- 14.Kauffman LD. Sokol RJ. Jones RH. Awad JA. Rewers MJ. Norris JM. Urinary F2-isoprostanes in young healthy children at risk for type 1 diabetes mellitus. Free Radic Biol Med. 2003;35:551–557. doi: 10.1016/s0891-5849(03)00333-2. [DOI] [PubMed] [Google Scholar]
- 15.Keaney JF., Jr. Larson MG. Vasan RS. Wilson PW. Lipinska I. Corey D. Massaro JM. Sutherland P. Vita JA. Benjamin EJ. Obesity and systemic oxidative stress: clinical correlates of oxidative stress in the Framingham Study. Arterioscler Thromb Vasc Biol. 2003;23:434–439. doi: 10.1161/01.ATV.0000058402.34138.11. [DOI] [PubMed] [Google Scholar]
- 16.Luo J. Gao YT. Chow WH. Shu XO. Li H. Yang G. Cai Q. Rothman N. Cai H. Shrubsole MJ. Franke AA. Zheng W. Dai Q. Urinary polyphenols and breast cancer risk: results from the Shanghai Women's Health Study. Breast Cancer Res Treat. 2010;120:693–702. doi: 10.1007/s10549-009-0487-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Meghdadi S. Rodrigues M. Oguogho A. Santler R. Sinzinger H. 8-Epi-PGF2alpha and 6-oxo-PGF1alpha in human (varicose) veins: influence of age, sex, and risk factors. Angiology. 2003;54:317–324. doi: 10.1177/000331970305400307. [DOI] [PubMed] [Google Scholar]
- 18.Milne GL. Morrow JD. Isoprostanes and related compounds: update 2006. Antioxid Redox Signal. 2006;8:1379–1384. doi: 10.1089/ars.2006.8.1379. [DOI] [PubMed] [Google Scholar]
- 19.Milne GL. Sanchez SC. Musiek ES. Morrow JD. Quantification of F2-isoprostanes as a biomarker of oxidative stress. Nat Protoc. 2007;2:221–226. doi: 10.1038/nprot.2006.375. [DOI] [PubMed] [Google Scholar]
- 20.Milne GL. Yin H. Brooks JD. Sanchez S. Jackson RL. Morrow JD. Quantification of F2-isoprostanes in biological fluids and tissues as a measure of oxidant stress. Methods Enzymol. 2007;433:113–126. doi: 10.1016/S0076-6879(07)33006-1. [DOI] [PubMed] [Google Scholar]
- 21.Montine TJ. Peskind ER. Quinn JF. Wilson AM. Montine KS. Galasko D. Increased cerebrospinal fluid F(2)-isoprostanes are associated with aging and latent Alzheimer's disease as identified by biomarkers. Neuromolecular Med. 2010 Jul 15; doi: 10.1007/s12017-010-8126-6. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Montuschi P. Barnes P. Roberts LJ. Insights into oxidative stress: the isoprostanes. Curr Med Chem. 2007;14:703–717. doi: 10.2174/092986707780059607. [DOI] [PubMed] [Google Scholar]
- 23.Morrow JD. Frei B. Longmire AW. Gaziano JM. Lynch SM. Shyr Y. Strauss WE. Oates JA. Roberts LJ. Increase in circulating products of lipid peroxidation (F2-isoprostanes) in smokers. Smoking as a cause of oxidative damage. N Engl J Med. 1995;332:1198–1203. doi: 10.1056/NEJM199505043321804. [DOI] [PubMed] [Google Scholar]
- 24.Morrow JD. Hill KE. Burk RF. Nammour TM. Badr KF. Roberts LJ. A series of prostaglandin F2-like compounds are produced in vivo in humans by a non-cyclooxygenase, free radical-catalyzed mechanism. Proc Natl Acad Sci U S A. 1990;87:9383–9387. doi: 10.1073/pnas.87.23.9383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Morrow JD. Roberts LJ. Mass spectrometric quantification of F2-isoprostanes in biological fluids and tissues as measure of oxidant stress. Methods Enzymol. 1999;300:3–12. doi: 10.1016/s0076-6879(99)00106-8. [DOI] [PubMed] [Google Scholar]
- 26.Morrow JD. Zackert WE. Yang JP. Kurhts EH. Callewaert D. Dworski R. Kanai K. Taber D. Moore K. Oates JA. Roberts LJ. Quantification of the major urinary metabolite of 15-F2t-isoprostane (8-iso-PGF2alpha) by a stable isotope dilution mass spectrometric assay. Anal Biochem. 1999;269:326–331. doi: 10.1006/abio.1999.4008. [DOI] [PubMed] [Google Scholar]
- 27.Pratico D. Lawson JA. Rokach J. FitzGerald GA. The isoprostanes in biology and medicine. Trends Endocrinol Metab. 2001;12:243–247. doi: 10.1016/s1043-2760(01)00411-8. [DOI] [PubMed] [Google Scholar]
- 28.Proudfoot J. Barden A. Mori TA. Burke V. Croft KD. Beilin LJ. Puddey IB. Measurement of urinary F(2)-isoprostanes as markers of in vivo lipid peroxidation-A comparison of enzyme immunoassay with gas chromatography/mass spectrometry. Anal Biochem. 1999;272:209–215. doi: 10.1006/abio.1999.4187. [DOI] [PubMed] [Google Scholar]
- 29.Reckelhoff JF. Kanji V. Racusen LC. Schmidt AM. Yan SD. Marrow J. Roberts LJ. Salahudeen AK. Vitamin E ameliorates enhanced renal lipid peroxidation and accumulation of F2-isoprostanes in aging kidneys. Am J Physiol. 1998;274:R767–R774. doi: 10.1152/ajpregu.1998.274.3.R767. [DOI] [PubMed] [Google Scholar]
- 30.Roberts LJ. Moore KP. Zackert WE. Oates JA. Morrow JD. Identification of the major urinary metabolite of the F2-isoprostane 8-iso-prostaglandin F2alpha in humans. J Biol Chem. 1996;271:20617–20620. doi: 10.1074/jbc.271.34.20617. [DOI] [PubMed] [Google Scholar]
- 31.Roberts LJ. Morrow JD. Isoprostanes. Novel markers of endogenous lipid peroxidation and potential mediators of oxidant injury. Ann N Y Acad Sci. 1994;744:237–242. doi: 10.1111/j.1749-6632.1994.tb52741.x. [DOI] [PubMed] [Google Scholar]
- 32.Schwedhelm E. Bartling A. Lenzen H. Tsikas D. Maas R. Brummer J. Gutzki FM. Berger J. Frolich JC. Boger RH. Urinary 8-iso-prostaglandin F2alpha as a risk marker in patients with coronary heart disease: a matched case-control study. Circulation. 2004;109:843–848. doi: 10.1161/01.CIR.0000116761.93647.30. [DOI] [PubMed] [Google Scholar]
- 33.WHO. Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet. 2004;363:157–163. doi: 10.1016/S0140-6736(03)15268-3. [DOI] [PubMed] [Google Scholar]
- 34.WHO. Research on the menopause in the 1990s. Report of a WHO Scientific Group. World Health Organ Tech Rep Ser. 1996;866:1–107. [PubMed] [Google Scholar]
- 35.Zheng W. Chow WH. Yang G. Jin F. Rothman N. Blair A. Li HL. Wen W. Ji BT. Li Q. Shu XO. Gao YT. The Shanghai Women's Health Study: rationale, study design, and baseline characteristics. Am J Epidemiol. 2005;162:1123–1131. doi: 10.1093/aje/kwi322. [DOI] [PubMed] [Google Scholar]