Abstract
The highly pathogenic avian influenza H5N1 virus still cause devastating effects to humans, agricultural poultry flocks, and wild birds. Wild birds are also detected to carry H5N1 over long distances and are able to introduce it into new areas during migration. In this article, our objective is to provide lists of bird species potentially involved in the introduction of highly pathogenic avian influenza H5N1 in Qinghai Lake, which is an important breeding and stopover site for aquatic birds along the Central Asian Flyway. Bird species were classified according to the following behavioral and ecological factors: migratory status, abundance, degree of mixing species and gregariousness, and the prevalence rate of H5N1 virus. Most of the high-risk species were from the family Anatidae, order Anseriformes (9/14 in spring, 11/15 in fall). We also estimated the relative risk of bird species involved by using a semi-quantitative method; species from family Anatidae accounted for over 39% and over 91% of the total risk at spring and fall migration periods, respectively. Results also show the relative risk for each bird aggregating site in helping to identify high-risk areas. This work may also be instructive and meaningful to the avian influenza surveillance in the breeding, stopover, and wintering sites besides Qinghai Lake along the Central Asian Flyway.
Key Words: Birds, Field studies, Modeling, Surveillance, Transmission
Introduction
Wild aquatic birds, especially species of Anseriformes and Charadriiformes, are considered to be the natural reservoir for influenza A viruses (Webster et al. 1992, Suss et al. 1994, Olsen et al. 2006, Munster et al. 2007). Wild aquatic birds are exposed to water that may be contaminated with infected fecal matter, especially at specific sites and during specific seasons when these birds densely congregate at relatively confined and shallow water bodies (Webster et al. 1992, Webster and Govorkova 2006). Intensive studies have been performed on the ecology and virology of influenza A viruses as well as on the host ecology, life history, and behavior that can affect virus prevalence in wild bird populations (Alexander 2000, Fouchier et al. 2007, Stallknecht and Brown 2007, Weber and Stilianakis 2007, Capua and Alexander 2008). In 2005, an unprecedented outbreak of highly pathogenic avian influenza (HPAI) H5N1 occurred in Qinghai Lake, China, causing the death of thousands of wild birds (Chen et al. 2005, Liu et al. 2005). The HPAI H5N1 then spread from eastern Asia to Europe, the Middle East, and Africa, raising concern that it will be spread worldwide with bird migration (Simonite 2005). Further, wetlands and lakes are important predictive environmental variables for the risk of HPAI H5N1, because they act as important stopover, breeding, or wintering sites for migratory waterbirds (Xiao et al. 2007, Fang et al. 2008, Lei et al. 2008). Up to now, no direct evidence exists to suggest that bird migration plays an important role in the dispersion of HPAI H5N1 into and out of Qinghai Lake. However, several studies have shown that HPAI H5N1 can be potentially transported by migratory birds of Qinghai Lake (Lei et al. 2008, Wang et al. 2008, Prosser et al. 2009).
Qinghai Lake is one of the most important breeding and stopover sites for migratory birds along the Central Asian Flyway (Zhang and Yang 1997, Mundkur 2006). It harbors thousands of breeding birds in summer, including the bar-headed goose (Anser indicus), the great black-headed gull (Larus ichthyaetus), and the brown-headed gull (Larus brunnicephalus). There are also a few species that winter in the lake, such as the whooper swan (Cygnus cygnus), the common goldeneye (Bucephala clangyla), and the tufted duck (Aythya fuligula) (Liu et al. 2005, Hou et al. 2009). Satellite tracking of migratory birds in Qinghai Lake has revealed a migratory connection between epidemic sites in Mongolia and south Asia (Muzaffar et al. 2008, Prosser et al. 2009). In view of the lake's important geographical location in bird migration, migratory birds in this lake may act as the carrier or vector in the circulation of H5N1 virus between southern and northern areas along the flyway (Wang et al. 2008). During yearly migrations, birds have the potential to disperse avian influenza virus into and out of Qinghai Lake. However, large gaps in the knowledge of wild bird migratory patterns in the lake and in the ecology of wild bird populations have limited our understanding of how this disease spreads (Alexander 2007).
The ability to efficiently control the spread of highly infectious, exotic diseases, such as HPAI H5N1, is dependent on the capacity to rapidly detect the pathogen if introduced. To assess the risk of HPAI H5N1 introducing into Qinghai Lake via bird migration, we investigated the species diversity, abundance, and behavior of gregariousness of aquatic birds in Qinghai Lake in an attempt to provide general advice on avian influenza surveillance. Therefore, in this study, we aim at (1) identifying the potential high-risk species that are most likely to introduce HPAI H5N1 into Qinghai Lake and (2) identifying the optimal sites for monitoring and surveillance of avian influenza at Qinghai Lake.
Materials and Methods
Study area
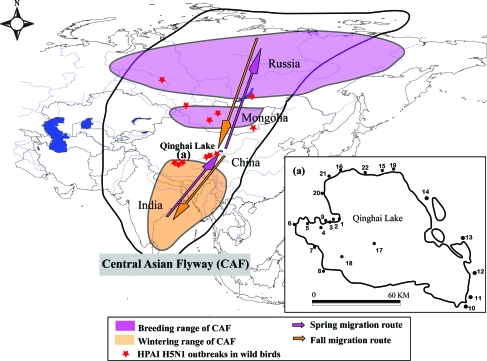
The study was conducted in Qinghai Lake National Nature Reserve, Qinghai Province, China (Fig. 1). Qinghai Lake is located at the north-eastern end of the Qinghai-Tibetan Plateau and is about 280 km west of the city of Xining in Qinghai Province. It is situated at an elevation of 3193 m and is the largest saltwater lake in China, with an area of c.4200 km2 (Shen and Kuang 2003). Qinghai Lake is in an endorheic (closed) basin that is surrounded by five rivers, for example, the Buha River and Shaliu River, which are the source of its water. Egg Island, Sankuaishi Island, and Haixinshan Island are important breeding areas for bar-headed goose, brown-headed gull, great black-headed gull, and great cormorant (Phalacrocorax carbo) (Zhang et al. 2007, Hou et al. 2009). The average annual temperature is 1.1°C–0.3°C, and the lowest temperature is between November and January. The lake is frozen for much of the year (average annual frozen days is 108–116 days), and it opens during the summer and early autumn (April–October) when there is rapid growth of vegetation providing food and a breeding habitat for a large number of waterbirds.
FIG. 1.
Location map of Qinghai Lake, including the range of the Central Asian Flyway. 1, Egg Island (EI); 2, Cormorant Island (CI); 3, Buhahekou (BHK); 4, Shenhekou (SHK); 5,Tiebujiahekou (TBK); 6, Quanwan wetland (QW); 7, Garila (GRL); 8, Heimahe (HMH); 9, Buhahe Delta (BHD); 10, Erhai (EH); 11, Daotanghe (DTH); 12, Xiaopohu (XBH); 13, Sha Island (SI); 14, Ganzihe (GZH); 15, Shaliuhe (SLH); 16, Quanjihekou (QJK); 17, Haixinshan (HXS); 18, Sankuaishi (SKS); 19, Shaliuhe2 (SLH2); 20, Hadatan (HDT); 21, Wushidalaiquan (WSQ); 22, Qinghaihunongchang wetland (QNC) www.liebertonline.com/vbz.
Identification of potential high-risk species to introduce of HPAI H5N1 into Qinghai Lake
To estimate the risk of the introduction of HPAI H5N1 to Qinghai Lake by wild migratory birds through spring and fall migration, we use a modified method that was ever used by European Food Safety Authority to select the potential high-risk species which would introduce HPAI H5N1 into Qinghai Lake (Pfeiffer et al. 2006). The potential high-risk species were selected based on the following criteria: (1) species with population size >100 individuals in Qinghai Lake. We purposely included species that are regularly observed but are present only in small populations, because we believe that diversity also is an important element to take into account while studying bird-associated pathogens; (2) migration from or pass through areas known to have HPAI H5N1; (3) species that had ever been infected by H5N1 in Qinghai Lake or elsewhere; (4) degree of mixing species is high (Supplemental Table S1, available online at www.liebertonline.com); and (5) species that are gregarious. We followed the steps for target species selection described in Figure 2.
FIG. 2.
Decision tree for the selection of migratory species more likely to introduce HPAI H5N1 into Qinghai Lake. HPAI, highly pathogenic avian influenza.
Data of population size of waterbirds were from the survey results during 2007 and 2008. Twenty-two bird aggregating sites around the Lake were selected as the survey sites through the pilot study in 2006. Usually one car with three fieldworkers was in use during the survey. To minimize multiple counting of birds, we selected vantage points from which we would not disturb birds within wetlands and recorded all birds on the ground and those which flew opposite to the counting direction with the aid of binoculars (Olymbus, 8 × 42) and spotting scopes (Kowa TSN-820). Waterbird counts were conducted between 07:00 and 16:00 local time under favorable weather conditions (no rain, wind speed ≤5 on Beaufort scale). Most of the birds were counted within 800 m to the surveyors. During the spring and fall migration periods, some diving ducks were counted with a distant >800 m. The amount of time spent at each survey site varied according to size of survey area, with larger area requiring longer time. Large flocks were counted by dividing them into groups of 10, 20, or 50 individuals and using landmarks to divide flocks into groups that could be more accurately counted. These surveys were conducted over four periods: spring migration period (March to May), summer breeding period (June to August), fall migration period (September and October), and winter period (November to the next February). At least one survey was conducted at each period in 2007 and 2008. On these surveys, we recorded all waterbirds, when possible, to identify all birds to species. Groups of unidentified waterbirds were all various ducks from the family Anatidae. This information was used to create a database of the species regularly observed at the 22 sites selected for monitoring (Fig. 1). In cases of multiple counts from each period, we used the highest count in calculation of population size. The multi-yearly average population sizes should be more representative to map the bird population abundance of Qinghai Lake, The count of population sizes in 2007 and 2008 were averaged when used in the final analyses. We assessed whether bird species migrate from or pass through the areas where HPAI H5N1 viruses were detected. Birds were identified as (1) winters or breeds through areas where poultry or wild birds H5N1 identified; (2) on migration pass through areas where poultry or wild birds H5N1 identified; and (3) no known use of H5N1 infection areas. If bird species were identified as no known use of H5N1 infection areas, then this species did not enter into the next step of selection of potential high-risk species (Supplemental Table S2, available online at www.liebertonline.com). The OIE 2010s Disease outbreak maps were used to locate the HPAI H5N1 epidemic areas (OIE 2010). The information of the bird migration (where these birds are coming from or pass through) was from bird ringing or satellite tracking data that are available in published papers or books (Zhang and Yang 1997, Zhao 2006, Chu et al. 2008, Muzaffar et al. 2008, Prosser et al. 2009, Takekawa et al. 2009, USGS 2010) (Supplemental Table S2). Depending on the species, Qinghai Lake is either a breeding area, a migration stop-over, or a wintering quarter (Hou et al. 2009, Zhang et al. 2007). Many species do not have enough migration information for us to assess whether they migrate from or pass through the areas where HPAI H5N1 were identified, and then we synthesized the distribution information of the species and estimate the migration routes according to the other related species that already have migration information (Zhang and Yang 1997, Mundkur 2006, Zhao 2006, Avidase 2010). The migration routes across Qinghai Lake have the following direction: They start from the Siberia of Russia (main breeding area)- Baikal Lake-wetlands of Mongolia and Inner Mongolia of China-Sichuan, Qinghai and Gansu Province of China-Yunnan and Tibet of China, and end at Bengal Bay and the coastal area of the Indian Ocean (main wintering area) (Zhang and Yang 1997, Mundkur 2006, Zhao 2006). The species were checked with the list of species affected by H5N1 that was established by USGS to make sure whether it was infected by H5N1 earlier (USGS 2007). We recorded the degree of mixing and gregarious of each species during a field survey at Qinghai Lake.
Rank ordering of the high-risk species
We analyzed the relative risk posed by the high-risk species for transmitting of H5N1 into Qinghai Lake by using a semi-quantitative method that combines information on the abundance, infection prevalence, and ecological variable. The risk that a species will infect other birds in the lake can be estimated as
 |
where A is the abundance, P is the H5N1 infection prevalence rate, and Ei (Efficiency of infection per species) is used to assess the probability of a H5N1 infected individual who will transmit virus to other individuals who cohabitat. Vt is the virus titers shed after infection, and Vd is the duration of shedding virus after infection. We used H5N1 surveillance data at Qinghai Lake from 2004 to 2007 to estimate the infection prevalence for each species (Kou et al. 2009). For the species, we have no data available at Qinghai Lake, so we used data published in other lakes that had H5N1 outbreaks earlier. Here, the prevalence data used were from Lake Constance in Europe (Happold et al. 2008). If a species still does not have prevalence rate data, then we use the average prevalence rate of the other species within the same family as the prevalence rate of this species. We used the data of Vt and Vd from experimental studies and collected from published articles (Table 1). Experimental studies clearly demonstrate that responses to HPAI H5N1 vary widely across species of birds (Brown et al. 2006, 2008). If a species does not have Vt or Vd data from experimental studies, then we use the data of the species that has similar ecological habits and within the same family. Otherwise, we use the average Vt or Vd value of the other species within the same family or of the other species with the similar ecological habits as the Vt or Vd value of this species.
Table 1.
Migratory Species More Likely to Introduce Highly Pathogenic Avian Influenza H5N1 into Qinghai Lake Through Spring and Fall Migration and Their Relative Risk
| |
|
|
|
|
|
|
Pathologic response |
|
|
|
|---|---|---|---|---|---|---|---|---|---|---|
| Family/order | English name | Latin name | H5N1 prevalence rate% | Abundance | Ei | Shedding duration (days) | Viral titer sheda | Risk value | Risk% | |
| Spring migration period | Podicipedidae/PODICIPEDIDAE | Great crested grebe | Podiceps cristatus | 5.1b | 3033 | 0.97 | 2.9 | 3.28c | 142720.44 | 2.71 |
| Phalacrocoracidae/PELECANIFORMES | Great cormorant | Phalacrocorax carbo | 3.92d | 10992 | 0.97 | 6.2 | 4.245e | 1100028.42 | 20.90 | |
| Rallidae/GRUIFORMES | Coot | Fulica atra | 2.1b | 530 | 0.90 | 3 | 3.1f | 9315.81 | 0.18 | |
| Laridae/CHARADRIIFORMES | Great black-headed gull | Larus ichthyaetus | 2.34d | 30903 | 0.86 | 6.2 | 4.245e,g | 1636757.48 | 31.10 | |
| Brown-headed gull | Larus brunnicephalus | 3.6d | 3504 | 0.86 | 6.2 | 4.245e,g | 285518.62 | 5.43 | ||
| Anatidae/ANSERIFORMES | Bar-headed goose | Anser indicus | 2.27d | 12695 | 0.83 | 6 | 5.1h | 731910.67 | 13.91 | |
| Ruddy Shelduck | Tadorna ferruginea | 2.17d | 2517 | 0.94 | 3 | 3.1f | 47747.84 | 0.91 | ||
| Common Teal | Anas crecca | 3.11d | 349 | 0.90 | 2 | 3.8i,j | 7424.07 | 0.14 | ||
| Mallard | Anas platyrhynchos | 11.19d | 2906 | 0.90 | 1.5 | 2.1j | 92188.93 | 1.75 | ||
| Eurasian Wigeon | Anas penelope | 6.89k | 3228 | 0.90 | 3 | 3.1f | 186156.50 | 3.54 | ||
| Pintail | Anas acuta | 9.84d | 2263 | 0.90 | 1.5 | 1.5j | 45092.54 | 0.86 | ||
| Red-crested Pochard | Netta rufina | 2.94d | 3220 | 0.87 | 2.5 | 4.0j,l | 82361.16 | 1.57 | ||
| Common Pochard | Aythya ferina | 10.5b | 3326 | 0.97 | 2.5 | 4.0j,l | 338753.10 | 6.44 | ||
| Tufted Duck | Aythya fuligula | 7.09d | 8090 | 0.97 | 2.5 | 4.0j,l | 556373.57 | 10.57 | ||
| Fall migration period | Podicipedidae/PODICIPEDIDAE | Great crested grebe | Podiceps cristatus | 5.1b | 796 | 0.87 | 2.9 | 3.28c | 5262349.14 | 0.63 |
| Phalacrocoracidae/PELECANIFORMES | Great cormorant | Phalacrocorax carbo | 3.92d | 3370 | 0.97 | 6.2 | 4.245 | 33594.98 | 6.30 | |
| Rallidae/GRUIFORMES | Coot | Fulica atra | 2.1b | 2158 | 0.84 | 3 | 3.1f | 337253.98 | 0.66 | |
| Laridae/CHARADRIIFORMES | Great black-headed gull | Larus ichthyaetus | 2.34d | 1552 | 0.70 | 6.2 | 4.245e | 35402.42 | 1.25 | |
| Anatidae/ANSERIFORMES | Bar-headed goose | Anser indicus | 2.27d | 1428 | 0.94 | 6 | 5.1h | 66907.53 | 1.74 | |
| Ruddy Shelduck | Tadorna ferruginea | 2.17d | 5414 | 0.76 | 3 | 3.1f | 93240.23 | 1.55 | ||
| Common Teal | A. crecca | 3.11d | 23183 | 1.00 | 2 | 3.8i,j | 83037.55 | 10.23 | ||
| Mallard | A. platyrhynchos | 11.19 | 3529 | 1.00 | 1.5 | 2.1j | 547953.39 | 2.32 | ||
| Eurasian Wigeon | A. Penelope | 6.89b | 5959 | 1.00 | 3 | 3.1f | 124391.96 | 7.13 | ||
| Gadwall | Anas strepera | 8.3d | 2424 | 0.90 | 3 | 3.1f | 381834.84 | 3.14 | ||
| Pintail | A. acuta | 9.84 | 8194 | 1.00 | 1.5 | 1.5j | 168397.70 | 3.39 | ||
| Red-crested Pochard | Netta rufina | 2.94 | 4548 | 0.97 | 2.5 | 4.0j,l | 181415.16 | 2.42 | ||
| Common goldeneye | Bucephala clangula | 6.89b | 2500 | 1.00 | 3 | 3.1f | 129699.86 | 2.99 | ||
| Common Pochard | Aythya ferina | 10.5d | 25384 | 0.97 | 2.5 | 4.0j,l | 160192.50 | 48.26 | ||
| Tufted Duck | A. fuligula | 7.09 | 6233 | 0.97 | 2.5 | 4.0j,l | 2585360.40 | 8.00 | ||
Average maximum titer (EID50/mL) isolated from the oropharynx or cloaca.
Happold et al. 2008.
Average value of the family Anatidae.
Kou et al. 2009.
Average value of dabbling ducks.
Average value of gulls.
Brown et al. 2008.
Value of Blue-winged teal (Anas discors).
Brown et al. 2006.
Average prevalence rate of the family Anatidae.
Value of redhead (Aythya americana).
HPAI, highly pathogenic avian influenza.
Four variables were included to estimate Ei: (1) degree of mixing species (DM), (2) gregarious group size (GS), (3) gregarious group density (GD), and (4) feeding methods (FM) (Supplemental Table S1). The four variables were selected according to the previous study on behavioral and ecological factors that facilitate cross-species transmission of avian influenza (Garamszegi and Møller 2007, Pfeiffer et al. 2006). The four variables were valued with 0–1 in Supplemental Table S1; the higher the value is, the higher the risk of infection for other wild waterbirds that congregate together. An Analytic Hierarchy Process method was used to assess the weight of each variable (W), with 1–9 scale for making judgments. The matrix was created based on advice from experts. Ei index value was calculated per species as follows:
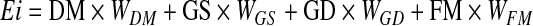 |
Risk of spread of HPAI H5N1 at each bird aggregating site around Qinghai Lake
The analyses of the risk of spread of HPAI H5N1 to local wild bird populations was carried out for each of the 22 wild bird congregate sites around the lake. The risk of spread depends on the chances of contact between possibly infected populations and local susceptible wild waterbirds (Martinez et al. 2009). The risk of spread at each site was calculated as the sum of the risk equation over all high-risk species multiplied by susceptible population abundance and index value of habitat type and temperature:
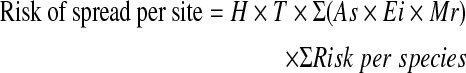 |
Here As was the abundance of susceptible population, and we assume that all the species are susceptible to the newly introduced HPAI H5N1 virus (Olsen et al. 2006, USGS 2007). H was the value of habitat types. Avian influenza virus could be disseminated into water by the infected individuals, and other birds that cohabitated might be infected through drinking the contaminated water (Brown et al. 2007, Stallknecht and Brown 2007). There are four main habitat types for wild waterbirds in the lake: coastal areas with shallow water and mudflat, river mouth, pools, and sand rock (usually used by the breeding colonies). The four habitat types were valued as 1 (pool, virus is easily to congregate in pools with static water), 0.7 (coastal area and river mouth), and 0.3 (sand rock, sand rock is arid and not convenient for the virus spread), respectively, based on the advice from expert. T was temperature. Generally, the avian influenza viruses were most stable at low temperature (<17°C) in natural aquatic habitats, and the persistence of H5 and H7 was inversely proportional to temperature of water (Brown et al. 2007, 2009). The H5N1 virus remained viable for > 100 days at 4°C and for 1 day at 28°C (Shahid et al. 2009). We used average monthly temperature from 1971 to 2000 to calculate the average seasonal temperature (National Meterological Information Center CMA 2005). In this study, the average temperature of all the four seasons was <17°C, so the temperature of all the four seasons was valued as 1. Mr was the morbidity rate of each species. Mr was estimated from the data from experimental studies (Table 2). Mr, which had more data compared with other factors, was included in the equation to vary the differences of susceptibility between the wild waterbird species. ∑(As × Ei × Mr) is the sum of the susceptible bird populations, and ∑ Risk per species is the sum of viruses introduced.
Table 2.
Relative Risk Value at Each Bird Aggregating Site Around Qinghai Lake
| |
|
Spring |
Summer |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Location name | Habitat types | Temperature (°C) | Risk introduced | Abundance of susceptible population ∑(As × Ei × Mr) | Risk% | Temperature (°C) | Risk introduced | Abundance of susceptible population ∑(As × Ei × Mr) | Risk% |
| EI | C, S | 0.4 | 3126.82 | 4508.78 | 34.79 | 9.87 | 2770.68 | 3957.00 | 7.83 |
| CI | S | 0.4 | 637.24 | 1034.89 | 0.70 | 9.87 | 1117.62 | 1799.73 | 0.62 |
| BHK | R | 0.4 | 420.91 | 679.99 | 0.71 | 9.87 | 2808.66 | 5105.20 | 10.25 |
| SHK | R | 0.4 | 2971.38 | 1421.96 | 10.43 | 9.87 | 533.97 | 579.85 | 0.22 |
| TBK | C, R | 0.4 | 2003.15 | 649.30 | 3.21 | 9.87 | 480.51 | 522.14 | 0.18 |
| QW | C | 0.4 | 4205.79 | 2621.87 | 27.22 | 9.87 | 974.17 | 1111.26 | 0.77 |
| GRL | C | 0.4 | 2073.19 | 1034.30 | 5.29 | 9.87 | 979.88 | 838.20 | 0.59 |
| HMH | C, R | 0.4 | 2177.81 | 2324.74 | 12.50 | 9.87 | 819.55 | 1301.82 | 0.76 |
| BHD | C | 0.4 | 33.46 | 498.07 | 0.04 | 9.87 | 588.18 | 1007.70 | 0.42 |
| EH | P | 0.4 | 247.68 | 453.79 | 0.40 | 9.87 | 645.73 | 228.46 | 0.15 |
| DTH | R | 0.4 | 263.45 | 250.33 | 0.16 | 9.87 | 190.46 | 154.90 | 0.02 |
| XBH | P | 0.4 | 839.14 | 157.03 | 0.46 | 9.87 | 9.11 | 20.90 | 0.00 |
| SI | P | 0.4 | 95.06 | 56.29 | 0.02 | 9.87 | 47.32 | 106.96 | 0.01 |
| GZH | P | 0.4 | 134.70 | 289.02 | 0.14 | 9.87 | 173.87 | 89.28 | 0.02 |
| SLH | R | 0.4 | 62.84 | 171.82 | 0.03 | 9.87 | 553.65 | 1055.84 | 0.42 |
| QJK | R | 0.4 | 154.39 | 223.29 | 0.09 | 9.87 | 288.97 | 449.51 | 0.09 |
| HXS | S | 0.4 | 0.00 | 0.00 | 0.00 | 9.87 | 2688.34 | 5344.70 | 4.40 |
| SKS | S | 0.4 | 0.00 | 0.00 | 0.00 | 9.87 | 10050.85 | 23683.94 | 72.89 |
| SLH2 | R | 0.4 | 0.00 | 0.00 | 0.00 | 9.87 | 845.17 | 516.33 | 0.31 |
| HDT | P | 0.4 | 1060.01 | 924.03 | 3.45 | 9.87 | 211.64 | 183.07 | 0.04 |
| WSQ | C | 0.4 | 125.15 | 64.79 | 0.02 | 9.87 | 1.64 | 3.00 | 0.00 |
| QNC | C | 0.4 | 377.80 | 380.13 | 0.35 | 9.87 | 85.86 | 157.40 | 0.01 |
| Fall | winter | ||||||||
| EI | C, S | 3.15 | 1496.28 | 584.45 | 0.22 | −10.25 | 49.11 | 294.70 | 2.72 |
| CI | S | 3.15 | 473.39 | 786.28 | 0.04 | −10.25 | 275.75 | 744.83 | 16.55 |
| BHK | R | 3.15 | 429.33 | 1245.06 | 0.14 | −10.25 | 3.30 | 405.89 | 0.25 |
| SHK | R | 3.15 | 7520.25 | 4995.18 | 9.63 | −10.25 | 0.07 | 16.15 | 0.00 |
| TBK | C, R | 3.15 | 16029.92 | 3706.14 | 15.24 | −10.25 | 37.52 | 1140.39 | 8.04 |
| QW | C | 3.15 | 17433.80 | 6177.68 | 27.62 | −10.25 | 134.07 | 2536.20 | 63.92 |
| GRL | C | 3.15 | 5445.92 | 2225.95 | 3.11 | −10.25 | 18.58 | 131.82 | 0.46 |
| HMH | C, R | 3.15 | 1927.42 | 1616.32 | 0.80 | −10.25 | 4.04 | 35.26 | 0.03 |
| BHD | C | 3.15 | 778.71 | 4436.63 | 0.89 | −10.25 | 0.00 | 0.00 | 0.00 |
| EH | P | 3.15 | 2928.81 | 1289.68 | 1.38 | −10.25 | 0.00 | 0.00 | 0.00 |
| DTH | R | 3.15 | 845.37 | 759.59 | 0.16 | −10.25 | 0.02 | 0.16 | 0.00 |
| XBH | P | 3.15 | 2.19 | 7.56 | 0.00 | −10.25 | 0.00 | 0.00 | 0.00 |
| SI | P | 3.15 | 202.35 | 195.32 | 0.01 | −10.25 | 0.00 | 0.00 | 0.00 |
| GZH | P | 3.15 | 1015.43 | 422.09 | 0.16 | −10.25 | 85.76 | 291.56 | 6.71 |
| SLH | R | 3.15 | 5281.36 | 2936.76 | 3.98 | −10.25 | 20.03 | 183.76 | 0.69 |
| QJK | R | 3.15 | 5734.91 | 3154.56 | 4.64 | −10.25 | 1.06 | 73.98 | 0.01 |
| HXS | S | 3.15 | 1754.89 | 3431.52 | 0.66 | −10.25 | 0.00 | 0.00 | 0.00 |
| SKS | S | 3.15 | 3837.30 | 9744.75 | 4.11 | −10.25 | 0.00 | 0.00 | 0.00 |
| SLH2 | R | 3.15 | 11228.05 | 8364.29 | 24.09 | −10.25 | 13.46 | 239.42 | 0.61 |
| HDT | P | 3.15 | 222.89 | 206.67 | 0.02 | −10.25 | 0.00 | 0.00 | 0.00 |
| WSQ | C | 3.15 | 1953.77 | 960.92 | 0.48 | −10.25 | 3.14 | 10.05 | 0.01 |
| QNC | C | 3.15 | 5363.20 | 1902.10 | 2.62 | −10.25 | 0.00 | 0.00 | 0.00 |
Codes of location name see Figure 1.
C, coastal areas with shallow water and mudflat; R, river mouth; P, pool; S, sandy rock.
Results
High-risk species and their relative risk value
Fourteen and 15 species from 5 orders were selected as the high-risk species during spring and fall migration periods, respectively (Table 1, Supplemental Table S2). Most of them were from the family Anatidae, order Anseriformes (9/14 in spring, 11/15 in fall). Species from family Anatidae accounted for over 39% and over 91% of the total risk at the spring and fall migration periods, respectively (Table 1). Except species from family Anatidae, the other five high-risk species during spring migration period include four summer breeders. The total risk of the four summer breeding species, bar-headed goose, brown-headed gull, great black-headed gull, and great cormorant, accounts for 71.34% of the total risk of all high-risk species during spring migration period. The difference of risk value between the high-risk species is more likely a result of the difference of the rate of virus prevalence and abundance of the high-risk species.
Risk of spread of HPAI H5N1 at each bird aggregating site around Qinghai Lake
During spring and fall migration periods, the risk is high at the important stopover sites that were used by the migratory passengers. In general, the northwestern part of the lake involving Quanwan wetland, Shenhekou, and Tiebujiahekou has higher risk than other parts during spring and fall migration periods (Table 2). Heimahe has high risk during spring migration period, and Shaliuhekou2 has high risk during the fall migration period. During summer breeding period, the risk is high at the sites with breeding colonies, such as Sankuaishi, Haixinshan, and Egg Island. Buhakekou also has high risk for being the most important feeding site of breeding birds. At winter, the risk is high at the unfrozen parts of the lake that serve as feeding habitat for the wintering birds. Quanwan wetland and Cormorant Island had the highest risk at winter, these 2 sites account for >80.47% of the total risk.
Discussion
We developed a qualitative method to select the wild migratory species more likely to introduce HPAI H5N1 virus into Qinghai Lake through spring and fall migration, and then we analyzed their relative risk by using a semi-quantitative method. Also, the risk of spread of HPAI H5N1 within local wild bird populations at each bird aggregating site was evaluated by using a semi-quantitative method. The results of this study indicate that ducks and geese are the predominant birds more likely to introduce HPAI H5N1 into the lake at both spring and fall migration periods. Results also show the risk of spread within local wild bird populations for each bird aggregating site in helping to identify high-risk areas at each season.
Several studies have evaluated the risk for HPAI H5N1 transmission via bird migration in North America, Europe, and Africa (Rappole and Hubalek 2006, Goutard et al. 2007, Martinez et al. 2007, Peterson et al. 2007, Winker et al. 2007). Most of these studies emphasized the movement of virus at the continental or country scale, based on knowledge of large-scale migratory patterns of wild birds, and these studies do provide efficient information on the design of the monitoring program (Pfeiffer et al. 2006, Peterson et al. 2007, Defra 2008). However, risk assessment work at a more fine scale was less (Jourdain et al. 2007). There were large gaps on the quantitative analyses of effect of wild birds in the spread of HPAI H5N1 virus (Feare 2010). Qinghai Lake has become very important in avian influenza surveillance since 2005 when the unprecedented H5N1 epidemic occurred in waterfowl population (Chen et al. 2005, Liu et al. 2005). Therefore, through comprehensive investigation on waterbird species distribution and ecological behavior at the lake and review on the data of virus' pathology responses to different species, we estimated the relative value of risk of high-risk species and risk of specific sites around Qinghai Lake. The species and sites with high-risk scores should be focused on in the surveillance program of avian influenza at Qinghai Lake. In this study, we followed two steps in identifying the high-risk species. First, a qualitative method was used to integrate data of bird distribution, abundance, and intermixing capacity with other species, gregarious habits, feeding habits, and migration flyway to identify the potential high-risk species; and second, a quantitative method was used to integrate data on birds' abundance, prevalence rate of H5N1 virus of bird populations, and the index of ability to spread of H5N1 virus by each high-risk species (Ei) to estimate the relative risk of these high-risk species during each period. The two steps lead to more precise identification of the high-risk species encountered around the lake.
The validity of our conclusions rests on the assumptions we have made and the data on which they are based. Of primary importance is the using of prevalence rate and Ei in calculating the relative risk of the high-risk species and difference of titers duration of shedding virus between species. Wild species could be suspected as the potential long- or short-distance vectors of HPAI H5N1 if they have the ability to excrete virus in the absence of debilitating disease, even for only a few days (Brown et al. 2008, Keawcharoen et al. 2008). At first, the viremia levels should be observed on the bird species, and then they can be regarded as potential vectors in the spread of virus. In this study, we used prevalence rate of each species to assess the potential bird population of each species that would be involved in the H5N1 transmission. However, with a high prevalence rate of HPAI H5N1, most wild birds had no signs of diseases or death in Qinghai Lake (Kou et al. 2009). Ei index in this study mainly indicated the contact rate between the infected birds and the potential susceptible birds. From what we know about the low pathogenic avian influenza virus, avian influenza virus is easily transmitted among wild waterbirds via the fecal-oral route, thereby infecting other animals that drink the contaminated water (Webster et al. 1978). Although there are limited data related to the environmental tenacity of HPAI viruses, HPAI H5N1 had been detected in the environmental samples (Vong et al. 2008). The conclusion that oropharyngeal excretion is the main source of transmissible virus is supported by experimental studies of captive swans and ducks (Brown et al. 2008, Kalthoff et al. 2008). However, virus shedding from the oral cavity may promote transmission between poultry in a wet market or in a crowded breeding colony, but it is unlikely to be as efficient as fecal shedding in dispersing viral particles in the aquatic habitats occupied by waterfowl (Takekawa et al. 2010). Feeding habit is closely related with the fecal-oral route of transmission, so it was included in the calculation of Ei. Contact transmission can be affected not only by the contact rate between infected and the susceptible species but also by the amount of environmental contamination and quantity of virus shedding (Swayne and Slemons, 2008). Quantity of virus shedding is highly strain and species specific in the experimental test (Brown et al. 2006, 2008, Swayne 2007, Swayne and Slemons 2008). Two important factors: titers and duration of shedding virus were included in the equation to differentiate the susceptibility of wild bird species (Takekawa et al. 2010).
Our analyses show that the greatest known threat of H5N1 virus introduction to Qinghai Lake is through ducks and geese. The ducks and geese are the most abundant groups in the lake, and the virus prevalence is relatively high in ducks (Hou et al. 2009, Kou et al. 2009). Previous studies have reported that several duck species carry the H5N1 virus without showing clinical signs (Chen et al. 2006, Keawcharoen et al. 2008). Thus, considering the low disease and mortality rate of ducks and geese to HPAI H5N1 virus in an experimental study, we suggest that the risk of introduction of HPAI H5N1 into Qinghai Lake by wild migratory birds is still most possibly persistent (Perkins and Swayne 2003, Brown et al. 2006, 2008).
A semi-quantitative approach was used to analyze the relative risk of spread per site around the lake. The main purpose of our study was to find the sites with highest risk of spread of H5N1 within different seasons. Risk of spread per site around the lake mostly depends on the following aspects: amount of H5N1 virus introduced by migratory birds, species of wild birds at each site, susceptibility of local wild birds to H5N1, exposure of local wild birds to H5N1, and virus survival in the environment (Pfeiffer et al. 2006, Kasemsuwan et al. 2009). Susceptibility of wild birds to HPAI H5N1 virus was quite different between species and isolates (Swayne 2007, Brown et al. 2008). On the circumstance that we do not know the genotype of the newly introduced H5N1 isolate into Qinghai Lake, it is difficult to assess the susceptibility of different species, so we used the morbidity rate data from experimental studies to estimate the difference of susceptibility between species. Based on experimental exposure trials on wild bird species, there were a series of differences in exposure responses ranging from asymptomatic periods, shedding periods, and development of clinical signs, morbidity rates and mortality rates (Takekawa et al. 2010). However, most of the experimental studies of the exposure responses were conducted on the species from order of Anseriformes (ducks and geese), Galliformes (chickens), and Passeriformes (songbirds) (Perkins and Swayne 2003, Brown et al. 2006, Boon et al. 2007). There were very few studies that focused on the shorebirds from order Charadriiformes. There were large information gaps on the exposure responses of the wild waterbird species in this study; only the morbidity rate that had more data compared with other factors was included in the equation to vary the differences of susceptibility between the wild waterbird species. The Ei value was also included to differentiate the variation of behavior between the species. We included environmental factors such as temperature and habitat types in the analyses, as the survival of avian influenza virus is closely correlated with the temperature (Stallknecht 2003, Shahid et al. 2009). Also, the water-borne transmission is important in the epidemiology of avian influenza virus (Roche et al. 2009). Many other aspects of climate factors such as temperature drops and dust storms would also exacerbate the spread of H5N1 virus by causing the birds' physiological stress to suppress the immune system (Liu et al. 2007). These factors should be considered in future risk evaluation, when we have a full understanding of the effects of these factors on the spread of avian influenza virus. Isolation of H5N1 from wild pikas (Ochotona curzoniae) by Zhou et al. (2009) called to great attention for monitoring the possible across species (mammals vs. birds) transmission of the virus in the Qinghai Lake ecosystem, as we have observed the great black-headed gull preying on the wild pikas at Qinghai Lake.
In summary, the paper provided a list of high-risk species and high-risk sites in that we may target the ecological security and avian influenza surveillance activities around Qinghai Lake. This list may also prove a useful tool in determining the target species for a further study on H5N1 ecology. The merit of our study is that we make the risk value comparable for the high-risk species and comparable at spatiotemporal scales. The study may also be instructive and meaningful to the avian influenza monitoring in the breeding, stopover, and wintering sites besides Qinghai Lake along the Central Asian Flyway.
Supplementary Material
Acknowledgments
We thank the staff of Qinghai Lake National Nature Reserve for field and logistical support. The research was supported by CAS Innovation Program (KSCX2-YW-N-063), grants from the National Science Fund for Distinguished Young Scientists (No. 30925008), and an NSFC program grant (No. J0930004) to Cui P. Funding was also partially provided by IDRC-APEIR, NIH, and USDA.
Disclosure Statement
No competing financial interests exist.
References
- Alexander DJ. A review of avian influenza in different bird species. Vet Microbiol. 2000;74:3–13. doi: 10.1016/s0378-1135(00)00160-7. [DOI] [PubMed] [Google Scholar]
- Alexander DJ. An overview of the epidemiology of avian influenza. Vaccine. 2007;25:5637–5644. doi: 10.1016/j.vaccine.2006.10.051. [DOI] [PubMed] [Google Scholar]
- Avibase. The world bird database. http://avibase.bsc-eoc.org. 2010. http://avibase.bsc-eoc.org
- Boon AC. Sandbulte MR. Seiler P. Webby RJ. Songserm T, et al. Role of terrestrial wild birds in ecology of influenza A virus (H5N1) Emerg Infect Dis. 2007;13:1720–1724. doi: 10.3201/eid1311.070114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown JD. Stallknecht DE. Beck JR. Suarez DL, et al. Susceptibility of North American ducks and gulls to H5N1 highly pathogenic avian influenza viruses. Emerg Infect Dis. 2006;12:1663–1670. doi: 10.3201/eid1211.060652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown JD. Stallknecht DE. Swayne DE. Experimental infection of swans and geese with highly pathogenic avian influenza virus (H5N1) of Asian lineage. Emerg Infect Dis. 2008;14:136–142. doi: 10.3201/eid1401.070740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown JD. Swayne DE. Cooper RJ. Burns RE, et al. Persistence of H5 and H7 avian influenza viruses in water. Avian Dis. 2007;51:285–289. doi: 10.1637/7636-042806R.1. [DOI] [PubMed] [Google Scholar]
- Brown JD. Goekjian G. Poulson R. Valeika S. Stallknecht DE. Avian influenza virus in water: Infectivity is dependent of pH, salinity and temperature. Vet Microboil. 2009;136:20–26. doi: 10.1016/j.vetmic.2008.10.027. [DOI] [PubMed] [Google Scholar]
- Capua I. Alexander DJ. Ecology, epidemiology and human health implications of avian influenza viruses: why do we need to share genetic data? Zoonoses Public Health. 2008;55:2–15. doi: 10.1111/j.1863-2378.2007.01081.x. [DOI] [PubMed] [Google Scholar]
- Chen H. Li Y. Li Z. Shi J, et al. Properties and dissemination of H5N1 viruses isolated during an influenza outbreak in migratory waterfowl in western China. J Virol. 2006;80:5976–5983. doi: 10.1128/JVI.00110-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H. Smith GJD. Zhang SY. Qin K, et al. H5N1 virus outbreak in migratory waterfowl. Nature. 2005;436:191–192. doi: 10.1038/nature03974. [DOI] [PubMed] [Google Scholar]
- Chu GZ. Hou YQ. Zhang GG. Liu DP, et al. Satellite tracking of the migration routes of the waterbirds breeding in Qinghai Lake. Chin J Nat. 2008;30:84–89. [Google Scholar]
- Sabirovic M. Clarke G. Highly athogenic Avian Influenza—H5N1: Recent Developments in the EU and the Likelihood of the Introduction into Great Britain by Wild Birds. London, United Kingdom: Department for Environment, Food and Rural Affairs, Food and Farming Group, Veterinary Science Team; 2008. Released 29 October. [Google Scholar]
- Fang LQ. de Vlas SJ. Liang S. Looman CW, et al. Environmental factors contributing to the spread of H5N1 avian influenza in mainland China. PLoS ONE. 2008;3:e2268. doi: 10.1371/journal.pone.0002268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feare CJ. Role of wild birds in the spread of highly pathogenic avian influenza virus H5N1 and implications for global surveillance. Avian Dis. 2010;54:201–212. doi: 10.1637/8766-033109-ResNote.1. [DOI] [PubMed] [Google Scholar]
- Fouchier RAM. Munster VJ. Keawcharoen J. Osterhaus ADME, et al. Virology of avian influenza in relation to wild birds. J Wildlife Dis. 2007;43:S7–S14. [Google Scholar]
- Garamszegi LZ. Moller AP. Prevalence of avian influenza and host ecology. Proc R Soc B. 2007;274:2003–2012. doi: 10.1098/rspb.2007.0124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goutard F. Roger F. Guitian FJ. Balanca G, et al. Conceptual framework for avian influenza risk assessment in Africa: the case of Ethiopia. Avian Dis. 2007;51:504–506. doi: 10.1637/7591-040206R.1. [DOI] [PubMed] [Google Scholar]
- Happold JR. Brunhart I. Schwermer H. Stark KDC. Surveillance of H5 avian influenza virus in wild birds found dead. Avian Dis. 2008;52:100–105. doi: 10.1637/8021-051407-Reg. [DOI] [PubMed] [Google Scholar]
- Hou YS. He YB. Xing Z. Cui P, et al. Distribution and diversity of waterfowl population in Qinghai Lake National Nature Reserve. Acta Zootaxonomica Sinica. 2009;34:184–187. [Google Scholar]
- Jourdain E. Gauthier-Clerc M. Bicout DJ. Sabatier P. Bird migration routes and risk for pathogen dispersion into western Mediterranean wetlands. Emerg Infect Dis. 2007;13:365–372. doi: 10.3201/eid1303.060301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalthoff D. Breithaupt A. Teifke JP. Globig A, et al. Highly pathogenic avian influenza virus (H5N1) in experimentally infected adult mute swans. Emerg Infect Dis. 2008;14:1267–1270. doi: 10.3201/eid1408.080078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasemsuwan S. Poolkhet C. Patanasatienkul T. Buameetoop N, et al. Qualitative risk assessment of the risk of introduction and transmission of H5N1 HPAI virus for 1-km buffer zones surrounding compartmentalised poultry farms in Thailand. The Pro-poor HPAI Risk Reduction Project Report. 2009:9. [Google Scholar]
- Keawcharoen J. van Riel D. van Amerongen G. Bestebroer T, et al. Wild ducks as long-distance vectors of highly pathogenic avian influenza virus (H5N1) Emerg Infect Dis. 2008;14:600–607. doi: 10.3201/eid1404.071016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kou Z. Li Y. Yin Z. Guo S, et al. The survey of H5N1 flu virus in wild birds in 14 provinces of China from 2004 to 2007. PLoS ONE. 2009;4:e6926. doi: 10.1371/journal.pone.0006926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei FM. Cui P. Yin ZH. The spread and risk of highly pathogenic avian influenza. Chin J Nat. 2008;30:74–77. [Google Scholar]
- Liu J. Xiao H. Lei F. Zhu Q, et al. Highly pathogenic H5N1 influenza virus infection in migratory birds. Science. 2005;309:1206. doi: 10.1126/science.1115273. [DOI] [PubMed] [Google Scholar]
- Liu CM. Lin SH. Chen YC. Lin KCM. Wu TSJ, et al. Temperature drops and the onset of severe avian influenza A H5N1 virus outbreaks. PLoS ONE. 2007;2:e191. doi: 10.1371/journal.pone.0000191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez M. Munoz MJ. De La Torre A. Iglesias I, et al. Risk of introduction of H5N1 HPAI from Europe to Spain by wild water birds in autumn. Transbound Emerg Dis. 2009;56:86–98. doi: 10.1111/j.1865-1682.2008.01062.x. [DOI] [PubMed] [Google Scholar]
- Martinez M. Munoz MJ. De la Torre A. Martinez B, et al. Risk assessment applied to Spain's prevention strategy against highly pathogenic avian influenza virus H5N1. Avian Dis. 2007;51:507–511. doi: 10.1637/7622-042606R1.1. [DOI] [PubMed] [Google Scholar]
- Mundkur T. Flyway conservation in the Central Asian Flyway. Workshop Introduction. In: Boere GC, editor; Galbraith CA, editor; Stroud DA, editor. Waterbirds Around the World. Edinburgh: The Stationery Office; 2006. p. 263. [Google Scholar]
- Munster VJ. Baas C. Lexmond P. Waldenstrom J, et al. Spatial, temporal, and species variation in prevalence of influenza A viruses in wild migratory birds. PLoS Pathog. 2007;3:630–638. doi: 10.1371/journal.ppat.0030061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muzaffar SB. Takekawa JY. Prosser DJ. Douglas DC, et al. Seasonal movements and migration of Pallas's Gulls Larus ichthyaetus from Qinghai Lake, China. Forktail. 2008;24:100–107. [Google Scholar]
- National Meterological Information Center CMA. The monthly mean surface climate data of China. http://cdc.cma.gov.en/shuju/index3.jsp?tpcat=SURF&dsid=SURF_CLI_CHN_MUL_MON. 2005. http://cdc.cma.gov.en/shuju/index3.jsp?tpcat=SURF&dsid=SURF_CLI_CHN_MUL_MON
- OIE. Update on highly pathogenic avian influenza in animals (type H5 and H7) www.oie.int/downld/AVIAN%20INFLUENZA/A_AI-Asia.htm. 2010. www.oie.int/downld/AVIAN%20INFLUENZA/A_AI-Asia.htm
- Olsen B. Munster VJ. Wallensten A. Waldenstrom J, et al. Global patterns of influenza A virus in wild birds. Science. 2006;312:384–388. doi: 10.1126/science.1122438. [DOI] [PubMed] [Google Scholar]
- Perkins LE. Swayne DE. Comparative susceptibility of selected avian and mammalian species to a Hong Kong-origin H5N1 high-pathogenicity avian influenza virus. Avian Dis. 2003;47:956–967. doi: 10.1637/0005-2086-47.s3.956. [DOI] [PubMed] [Google Scholar]
- Peterson AT. Benz BW. Papes M. Highly pathogenic H5N1 avian influenza: entry pathways into North America via bird migration. PLoS ONE. 2007;2:e261. doi: 10.1371/journal.pone.0000261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeiffer DU. Brown I. Fouchier RAM. Gaidet N, et al. Migratory birds and their possible role in the spread of highly pathogenic avian influenza. EFSA J. 2006;357:46. doi: 10.2903/j.efsa.2006.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prosser DJ. Takekawa JY. Newman SH. Yan BP, et al. Satellite-marked waterfowl reveal migratory connection between H5N1 outbreak areas in China and Mongolia. IBIS. 2009;151:568–576. [Google Scholar]
- Rappole JH. Hubalek Z. Birds and influenza H5NI virus movement to and within North America. Emerg Infect Dis. 2006;12:1486–1492. doi: 10.3201/eid1210.051577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roche B. Lebarbenchon C. Gauthier-Clerc M. Chang CM, et al. Water-borne transmission drives avian influenza dynamics in wild birds: the case of the 2005–2006 epidemics in the Camargue area. Infect Genet Evol. 2009;9:800–805. doi: 10.1016/j.meegid.2009.04.009. [DOI] [PubMed] [Google Scholar]
- Shahid MA. Abubakar M. Hameed S. Hassan S. Avian influenza virus (H5N1); effects of physico-chemical factors on its survival. Virol J. 2009;6:38. doi: 10.1186/1743-422X-6-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen F. Kuang DB. Remote sensing investigation and survey of Qinghai lake in the past 25 years. J Lake Sci. 2003;15:289–296. [Google Scholar]
- Simonite T. Migration threatens to send flu south. Nature. 2005;437:1212–1213. doi: 10.1038/4371212a. [DOI] [PubMed] [Google Scholar]
- Stallknecht DE. Brown JD. Wild birds and the epidemiology of avian influenza. J Wildlife Dis. 2007;43:S15–S20. [Google Scholar]
- Stallknecht DE. Ecology and epidemiology of avian influenza viruses in wild bird populations: waterfowl, shorebirds, pelicans, cormorants, etc. Avian Diseases; Proceedings of Fourth International Symposium on Avian Influenza; 2003. pp. 61–69. [Google Scholar]
- Takekawa JY. Heath SR. Douglas DC. Perry WM. Javed S, et al. Geographic variation in Bar-headed Geese Anser indicus: connectivity of wintering areas and breeding grounds across a broad front. Wildfowl. 2009;59:102–125. [Google Scholar]
- Takekawa JY. Prosser DJ. Newman SH. Muzaffar SB. Hill NJ, et al. Victims and vectors: highly pathogenic avian influenza H5N1 and the ecology of wild birds. Avian Biol Res. 2010;3:1–23. [Google Scholar]
- USGS. List of species affected by H5N1 (avian influenza) www.nwhc.usgs.gov/disease_information/avian_influenza/affected_specieschart.jsp. 2007. www.nwhc.usgs.gov/disease_information/avian_influenza/affected_specieschart.jsp
- USGS. Western Ecological Research Center. Satellite tracking migration birds. www.werc.usgs.gov/ResearchTopicPage.aspx?id = 12. 2010. www.werc.usgs.gov/ResearchTopicPage.aspx?id = 12
- Suss J. Schafer J. Sinnecker H. Webster RG. Influenza-virus subtypes in aquatic birds of eastern Germany. Arch Virol. 1994;135:101–114. doi: 10.1007/BF01309768. [DOI] [PubMed] [Google Scholar]
- Swayne DE. Understanding the complex pathobiology of high pathogenicity avian influenza viruses in birds. Avian Dis. 2007;51:242–249. doi: 10.1637/7763-110706-REGR.1. [DOI] [PubMed] [Google Scholar]
- Swayne DE. Slemons RD. Using mean infectious dose of high- and low-pathogenicity avian influenza viruses originating from wild duck and poultry as one measure of infectivity and adaptation to poultry. Avian Dis. 2008;52:455–460. doi: 10.1637/8229-012508-Reg.1. [DOI] [PubMed] [Google Scholar]
- Vong S. Ly S. Mardy S. Holl D. Buchy P. Environmental contamination during influenza A virus (H5N1) outbreaks, Cambodia, 2006. Emerg Infect Dis. 2008;14:1303–1305. doi: 10.3201/eid1408.070912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang GH. Zhan DW. Li LX. Lei FM, et al. H5N1 avian influenza re-emergence of Lake Communication Qinghai: phylogenetic and antigenic analyses of the newly isolated viruses and roles of migratory birds in virus circulation. J Gen Virol. 2008;89:697–702. doi: 10.1099/vir.0.83419-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber TP. Stilianakis NI. Ecologic immunology of avian influenza (H5N1) in migratory birds. Emerg Infect Dis. 2007;13:1139–1143. doi: 10.3201/eid1308.070319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster RG. Bean WJ. Gorman OT. Chambers TM, et al. Evolution and ecology of influenza-A viruses. Microbiol Rev. 1992;56:152–179. doi: 10.1128/mr.56.1.152-179.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster RG. Govorkova EA. Focus on research: H5N1 influenza—continuing evolution and spread. New Engl J Med. 2006;355:2174–2177. doi: 10.1056/NEJMp068205. [DOI] [PubMed] [Google Scholar]
- Webster RG. Yakhno M. Hinshaw VS. Bean WJ, et al. Intestinal influenza—replication and characterization of influenza-viruses in ducks. Virology. 1978;84:268–278. doi: 10.1016/0042-6822(78)90247-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winker K. McCracken KG. Gibson DD. Pruett CL, et al. Movements of birds and avian influenza from Asia into Alaska. Emerg Infect Dis. 2007;13:547–552. doi: 10.3201/eid1304.061072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao XM. Gilbert M. Slingenbergh J. Lei F, et al. Remote sensing, ecological variables, and wild bird migration related to outbreaks of highly pathogenic H5N1 avian influenza. J Wildlife Dis. 2007;43:S40–S46. [PMC free article] [PubMed] [Google Scholar]
- Zhang FY. Yang RL. Bird Migration Research of China. Beijing: China Forestry Publishing House; 1997. [Google Scholar]
- Zhang GG. Liu DP. Jiang HX. Shan K, et al. Diversity and dynamics of waterbirds in non-wintering season at Qinghai Lake. Scientia Silvae Sinicae. 2007;43:101–105. [Google Scholar]
- Zhao X. Bird Migration and Bird Flu in the Mainland of China. Beijing: China Forestry Publishing House; 2006. [Google Scholar]
- Zhou JY. Sun WB. Wang JH. Guo JQ. Yin W, et al. Characterization of the H5N1 highly pathogenic avian influenza virus derived from wild pikas in China. J Virol. 2009;83:8957–8964. doi: 10.1128/JVI.00793-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.