Abstract
Selenium (Se) is an essential trace element in mammals that has been shown to exert its function through selenoproteins. Whereas optimal levels of Se in the diet have important health benefits, a recent clinical trial has suggested that supplemental intake of Se above the adequate level potentially may raise the risk of type 2 diabetes mellitus. However, the molecular mechanisms for the effect of dietary Se on the development of this disease are not understood. In the present study, we examined the contribution of selenoproteins to increased risk of developing diabetes using animal models. C57BL/6J mice (n=6–7 per group) were fed either Se-deficient Torula yeast-based diet or diets supplemented with 0.1 and 0.4 parts per million Se. Our data show that mice maintained on an Se-supplemented diet develop hyperinsulinemia and have decreased insulin sensitivity. These effects are accompanied by elevated expression of a selective group of selenoproteins. We also observed that reduced synthesis of these selenoproteins caused by overexpression of an i6A− mutant selenocysteine tRNA promotes glucose intolerance and leads to a diabetes-like phenotype. These findings indicate that both high expression of selenoproteins and selenoprotein deficiency may dysregulate glucose homeostasis and suggest a role for selenoproteins in development of diabetes. Antioxid. Redox Signal. 14, 2327–2336.
Introduction
A number of epidemiologic and experimental animal studies, and human clinical trials provided strong evidence that supplementation of the diet with selenium (Se) may have protective effects against cancer (3, 6, 9, 13, 39). Both low-molecular-weight Se compounds and selenoproteins have been implicated in the decreased risk of developing tumors (8, 10, 14, 15, 37). While the majority of the previous reports demonstrated an inverse correlation between Se status and cancer incidence, some of the studies showed no chemopreventive effect, and in some there was evidence of unexpected adverse effects, such as an increased number of adult onset diabetes cases in those receiving Se supplements in the Nutritional Prevention of Cancer (NPC) trial (31). Analysis of the NPC study population showed about 50 percent increase in incidence of type 2 diabetes mellitus in the Se-supplemented group, which was associated with the top tertile of plasma Se levels at baseline. More recently, a plausible association between long-term Se exposure and increased risk of diabetes was suggested by results of the Selenium and Vitamin E Cancer Prevention Trial (SELECT). This largest ever, randomized cancer prevention trial, which involved more than 35,000 participants, demonstrated a marginally increased, but statistically nonsignificant, risk of type 2 diabetes in men taking Se supplements (19). Se content is high in soil and food in the United States and Canada, where the trial was carried out, suggesting, together with results of the NPC trial, that supplemental intake of Se by individuals with high baseline plasma Se may raise the risk of developing diabetes.
Although the potential pro-diabetic effect of long-term Se supplementation has been recognized by the scientific community (2), little is known about its underlying mechanisms. Both NPC and SELECT trials used subtoxic doses of Se (200 μg/day) excluding the possibility that the observed effects are caused by overt Se toxicity. It therefore seems possible that the diabetogenic action of Se is mediated by an increased expression of selenoproteins. Consistent with this notion is the recent observation that transgenic mice overexpressing Se-dependent glutathione peroxidase 1 (GPx1) develop insulin resistance and obesity (22). These mice also show hyperglycemia and hyperinsulinemia, and it was proposed that GPx1-induced insulin overproduction and secretion might play a role in the development of diabetes in these mice (36). However, whether increased insulin secretion is the primary cause of the observed phenotype or just a compensatory response to impaired insulin signaling and hyperglycemia is not known. In turn, decreased GPx1 activity and consequent increased levels of reactive oxygen species (ROS) in mice lacking this selenoprotein improve insulin sensitivity and attenuate development of high-fat-diet induced obesity (20). Also of interest are several reports showing a possible association between endoplasmic reticulum (ER) stress and defective protein folding with impaired function of pancreatic β cells (27, 29, 40). Recently, several selenoproteins (altogether there are 25 selenoprotein genes in humans) have been implicated in oxidative protein folding and quality control in the ER (17, 18, 30). Because expression of these proteins may be affected by long-term Se supplementation, their altered activities also may contribute to the development of diabetes.
In the present study, the roles of dietary Se and selenoproteins in the possible development of diabetes were analyzed using two different mouse models. The first model included mice that were fed diets supplemented with Se at concentrations corresponding to those used in human clinical trials. The effects of dietary Se supplementation on insulin sensitivity and regulation of glucose homeostasis were analyzed. In the second model, expression of a distinct subgroup of selenoproteins was genetically altered by overexpressing an i6A− mutant selenocysteine (Sec) tRNA. Strikingly, our data indicate that both maximal expression of selenoproteins caused by elevation in dietary Se and compromised selenoprotein expression caused by overexpression of an i6A− mutant Sec tRNA lead to a mild diabetic phenotype.
Materials and Methods
Se diets and animal care
All experiments that involved mice were performed at the University of Nebraska Animal Research Facility. The mouse experimental protocols were in accordance with the University of Nebraska and National Institutes of Health institutional guidelines and were approved by the Institutional Animal Care and Use Committee.
Male C57BL/6J mice were fed three different diets (Harland TekLad, Madison, WI) containing 0 parts per million (ppm) Se (Se-deficient), 0.1 ppm Se, and 0.4 ppm Se. The diets were based on the Torula yeast Se-deficient diet that was supplemented with the indicated concentrations of Se in the form of sodium selenite (26). Transgenic mice that encode an i6A− mutant Sec tRNA were generated as described (25). i6A− mutant Sec tRNA transgenic and wild-type mice (FVB/N) were fed standard chow diet (Harland TekLad, Madison, WI). Mouse tissues from transgenic mice overexpressing GPx1 were kindly provided by Dr. Xin Gen Lei (Department of Animal Science, Cornell University, Ithaca, NY). GPx1 transgenic tissues were obtained from 10-month-old male animals that were derived from a B6C3 (C57B1×C3H) hybrid line (Taconic, Germantown, NY) (5, 36).
Plasma glucose and insulin measurements
Fasting (12 h, overnight) or fed state plasma glucose and insulin levels were measured in blood samples collected from tail veins. Glucose concentrations were determined using a glucometer (Bayer, Elkhart, IN) according to the manufacturer's instructions. Insulin levels were assayed using a mouse insulin ELISA kit (Mercodia, Uppsala, Sweden). Glucose tolerance tests were performed in mice that had been fasted overnight (12 h) and then were injected with glucose (1 mg/g) followed by plasma glucose measurements at the indicated time points.
Insulin sensitivity
For insulin sensitivity tests, animals were fasted for 12 h and then were given a single intraperitoneal injection of insulin (0.25 mU/g body weight). Plasma glucose was determined at the indicated time points after insulin injections, and the data were presented as the relative percentage of the initial levels.
Selenoprotein expression and enzyme activity
Stably transfected Chang liver control and GPx1-overexpressing cells were cultured in DMEM supplemented with 10% fetal calf serum as described (12). Cells were passaged in the presence of G418 at 260 μg/ml, and G418 was excluded from the medium for a period of 2 days before experiments. To metabolically label cells with 75Se, the culture medium was replaced with DMEM supplemented with freshly neutralized [75Se]selenious acid (specific activity 1,000 Ci/mmol, final concentration 1 nM) for 48 h. Protein lysates were resolved by SDS-PAGE, followed by immunoblot and autoradiography. To analyze expression of selenoproteins in cell lines and mouse tissues, protein lysates were resolved by SDS-PAGE. Expression of GPx1, MsrB1, selenoprotein S (SelS), selenoprotein T (SelT), thioredoxin reductase 1 (TR1), and thioredoxin reductase 3 (TR3) was analyzed by Western blotting using polyclonal antibodies specific for these proteins. As a loading control, β-actin expression was analyzed using monoclonal β-actin-specific antibody. MsrB1 and SelT-specific antibodies were previously developed in our laboratories, β-actin and SelS-specific antibodies were from Sigma (St. Louis, MO), and GPx1-specific antibodies were from Abcam (Cambridge, MA). Glutathione peroxidase activity was determined in tissue homogenates using a BIOXITECH GPx-340 kit (OxisResearch, Portland, OR) according to the manufacturer's instructions. Methionine-R-sulfoxide reductase activity was determined as described previously (16).
Statistical analysis
Statistical analysis of the data was performed using two-way ANOVA and Student's t-test. All results are represented as means±standard error of the mean (SEM).
Results
Regulation of selenoprotein expression by dietary Se
To study the mechanism by which elevated dietary Se may contribute to increased risk of developing diabetes, we used Mus musculus as a model system. Male C57BL/6J mice (n=6–7 per group) were fed three different diets containing varying levels of dietary Se. In addition to Se-deficient diet (0 ppm Se), we used diets supplemented with 0.1 and 0.4 ppm of sodium selenite. Previous studies indicated that supplementation of the diet with ∼0.1 ppm sodium selenite is sufficient for maximal expression of GPx1 in mice. Since maximal expression of plasma GPx is achieved at ∼55 μg/day selenium in humans (12), the 0.1 ppm Se mouse diet approximately corresponds to the human Recommended Dietary Allowance for adults. By analogy, the 0.4 ppm mouse diet was designed to match ∼200 μg/day in humans, amounts of dietary Se commonly used in clinical trials.
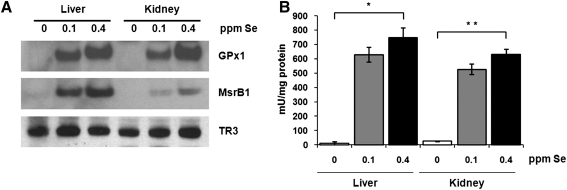
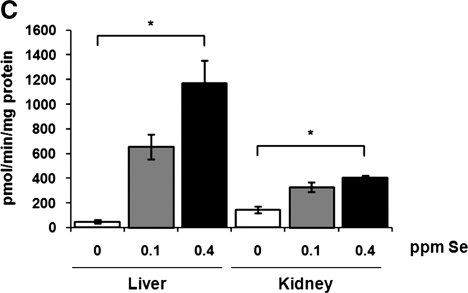
To gauge the degree of regulation of selenoprotein expression by dietary Se, we analyzed expression and catalytic activity of several selenoproteins in livers and kidneys of mice maintained on the three Se diets. Expression of GPx1 and methionine-R-sulfoxide reductase 1 (MsrB1) was extremely low in tissue extracts from mice maintained on the Se-deficient diet, whereas both proteins reached high expression upon supplementation with 0.1 ppm Se (Fig. 1A). Further increase in dietary Se level (to 0.4 ppm) resulted in slight but consistent elevation of GPx1 and MsrB1 protein levels. In contrast, expression of mitochondrial thioredoxin reductase 3 (TR3) was less responsive to changes in dietary Se levels.
FIG. 1.
Regulation of selenoprotein expression and enzymatic activities by dietary Se in mice. (A) Tissue extracts from C57BL/6J mice maintained on diets containing 0, 0.1, or 0.4 parts per million (ppm) dietary Se were analyzed by SDS-PAGE and Western blotting with antibodies specific for GPx1, MsrB1, and TR3. GPx1 (B) and MsrB (C) activities were analyzed in liver and kidney of mice fed three different Se diets. Results are represented as means±SEM (n=3 for each diet; *p<0.01 and **p<0.001 by two-tailed t-test).
Consistent with the expression level of GPx1, which is the most abundant glutathione peroxidase in liver and kidney, the total glutathione peroxidase activity was significantly reduced in these organs in 0 ppm Se diet compared to 0.1 and 0.4 ppm diets (Fig. 1B). Similarly to the glutathione peroxidase activity, activity of methionine-R-sulfoxide reductase was dramatically decreased in livers from mice fed 0 ppm Se diet (Fig. 1C). In kidneys of mice maintained on the 0 ppm Se diet, the level of methionine-R-sulfoxide reductase activity was decreased less significantly, suggesting that residual activity comes from nonselenoprotein MsrB forms.
Effect of dietary Se supplementation on insulin sensitivity and glycemic control in C57BL/6J mice
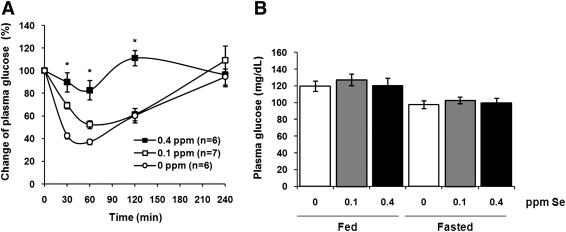
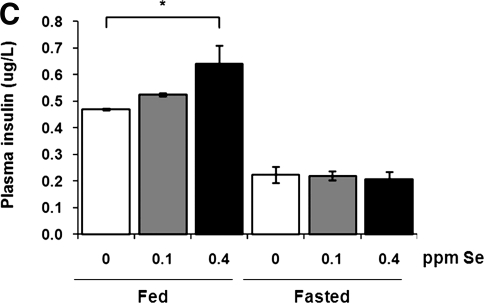
The onset of type 2 diabetes mellitus is usually preceded by insulin resistance, which is thought to be a major contributing factor in the pathogenesis of this disease. To test if Se supplementation results in insulin resistance, we measured relative changes in plasma glucose levels in mice after insulin challenge. Following 3 months of dietary Se supplementation, mice in the 0.4 ppm Se group showed decreased insulin sensitivity (p<0.001) compared to mice maintained on Se-deficient and 0.1 ppm Se-supplemented diets (Fig. 2A). We further analyzed steady-state plasma glucose and insulin levels in fasted and fed mice. The plasma glucose levels were not changed upon Se supplementation (Fig. 2B). However, there was a positive correlation between concentration of Se in the diet and plasma insulin levels in fed mice (Fig. 2C). These observations are consistent with previous reports showing impaired insulin sensitivity and hyperinsulinemia in transgenic mice overexpressing GPx1 (22, 36), and suggest a possible role for GPx1 and/or other selenoproteins in regulation of glucose homeostasis.
FIG. 2.
Impaired insulin sensitivity and hyperinsulinemia in mice fed high Se diet. (A) Impact of dietary Se supplementation on insulin sensitivity in C57BL/6J mice. Male C57BL/6J mice maintained on different Se diets for 3 months were fasted overnight, intraperitoneally injected with insulin (0.25 mU/g body weight), and then plasma glucose levels were measured at the indicated time points. Results are expressed as the percentage of the initial glucose levels. Shown are the mean and SEM in each group (n=6–7; *p<0.001 by two-way ANOVA for 0 ppm vs. 0.4 ppm groups). Steady-state plasma glucose (B) and insulin (C) levels were analyzed in mice maintained on different Se diets during fasting (fasted) or in fed state (fed). Results are represented as means±SEM (n=3–10, *p<0.01 by two-tailed t-test).
Development of insulin resistance in GPx1-overexpressing mice is accompanied by elevated expression of several other selenoproteins
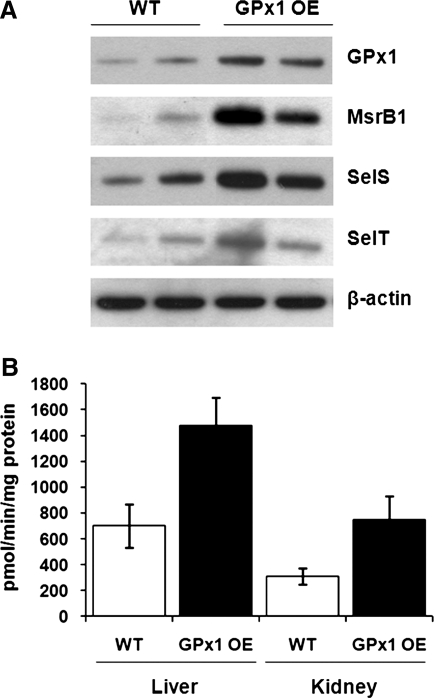
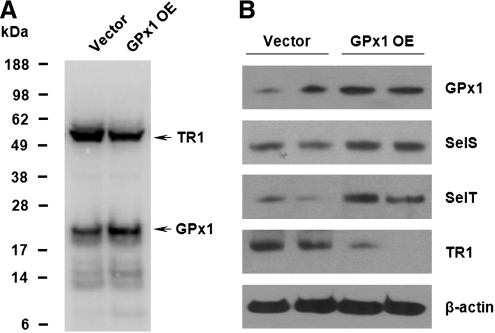
It was proposed that overproduction of GPx1 in pancreatic β cells of GPx1 transgenic mice leads to chronic insulin resistance through redox-regulated upregulation of insulin production and secretion (36). It is possible that increased dietary Se supplementation exerts its effect on insulin sensitivity by solely increasing levels of GPx1 in pancreatic islets of Langerhans. Another possibility is that GPx1 may serve as a storage pool of Se for other selenoproteins (32–34). Because GPx1 protein has a short half-life, mice overproducing GPx1 have Sec readily available for expression of other selenoproteins, which may affect insulin secretion and production. Increased levels of GPx1 may also affect redox homeostasis resulting in altered expression of other selenoproteins. To test these possibilities, we measured expression levels of selenoproteins in livers of GPx1 transgenic mice. When GPx1 protein was overexpressed, we observed elevated levels of MsrB1, SelS, and SelT selenoproteins (Fig. 3A). Moreover, increased levels of MsrB1 expression correlated with methionine-R-sulfoxide activity measurements in livers and kidneys of GPx1 transgenic mice, suggesting production of functionally competent Sec-containing MsrB1 (Fig. 3B). These results further reveal a possible and previously unanticipated role of other selenoproteins in the development of insulin resistance in mice that overexpress GPx1. To obtain further mechanistic insights, we analyzed the selenoprotein expression pattern in a GPx1-overexpressing cell line using metabolic labeling with 75Se (Fig. 4A). Remarkably, almost all detected selenoproteins showed increased levels in GPx1-overexpressing cells, except TR1. By contrast, TR1 expression was significantly reduced in GPx1-overexpressing cells compared to control cells. The contrasting pattern of regulation of selenoproteins by GPx1 overexpression was further confirmed by Western blotting (Fig. 4B). Consistent with the results obtained in GPx1-overexpressing mice, levels of selenoproteins SelS and SelT were increased in cells overexpressing GPx1. However, expression of TR1, a component of the thioredoxin system that is one of the major antioxidant systems in the cell, was significantly decreased. The contrasting pattern of regulation of TR1 selenoprotein provides evidence that GPx1 overexpression may result in reductive stress (decreased ROS levels) as evident by the lower expression of TR1.
FIG. 3.
Effect of GPx1 overexpression on expression levels of other selenoproteins in mice. (A) Liver extracts from wild-type (WT) and GPx1-overexpressing (GPx1 OE) mice, matched for sex and age, were probed by Western blotting with polyclonal antibodies specific for GPx1, MsrB1, SelS, SelT, or monoclonal β-actin-specific antibody as a loading control. Expression of proteins is shown for two WT and two GPx1 OE mice. (B) MsrB activity was analyzed in liver and kidney of WT mice and mice overexpressing GPx1. Results are represented as means±SEM (n=3).
FIG. 4.
Contrasting expression patterns of selenoproteins in GPx1-overexpressing and control cell lines. (A), Control cells and cells overexpressing GPx1 were labeled with 75Se, and selenoprotein expression patterns were analyzed by SDS-PAGE followed by autoradiography. Migration of GPx1 and TR1 is shown with arrows. (B) Expression patterns of GPx1, SelS, SelT, and TR1 were analyzed in control and GPx1-overexpressing cell lines by Western blotting. Cell lysates were also probed with monoclonal β-actin-specific antibody as a loading control. The results shown are from two independent experiments.
Selenoprotein deficiency leads to dysregulation of glucose homeostasis in i6A− mutant Sec tRNA transgenic mice
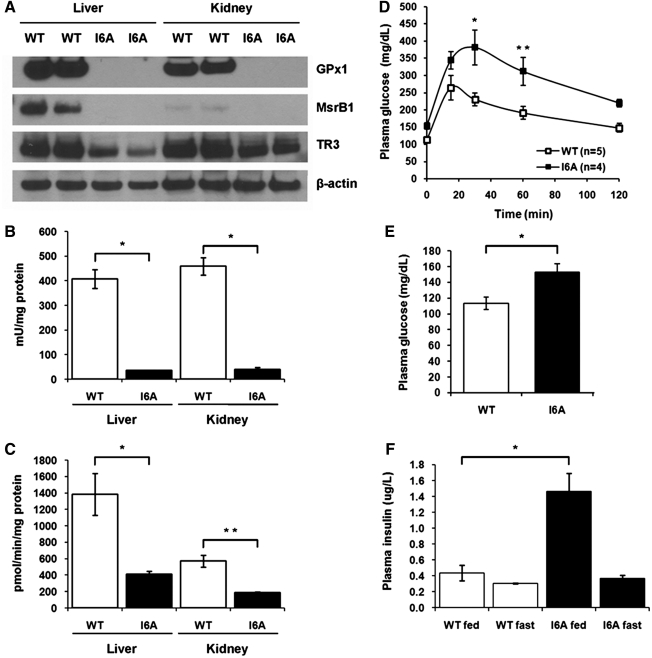
The above experiments used dietary interventions to regulate expression of selenoproteins in mice, and we observed a mild diabetic phenotype associated with increased expression and activities of GPx1 and MsrB1 selenoproteins. As mice were exposed to Se diets only for 3 months starting from weaning, we examined whether constitutive alteration of selenoprotein expression would result in a more pronounced phenotype. We therefore utilized a transgenic mouse model that overexpresses an i6A− mutant Sec tRNA, thereby genetically changing expression of selenoproteins. When these mice are fed an Se-adequate diet, the efficiency of Sec insertion is decreased by the mutant tRNA, resulting in lower expression of selenoproteins. As expected, we found that expression levels of several selenoproteins, including GPx1 and MsrB1, were dramatically decreased in i6A− mutant Sec tRNA transgenic mice compared to wild-type mice matched for sex and age (Fig. 5A). In addition, decrease in GPx1 and MsrB1 protein expression was associated with a concomitant drop in their enzymatic activities (Fig. 5B and C, respectively). By contrast, expression of selenoprotein TR3 was decreased in i6A− mutant Sec tRNA transgenic mice only slightly.
FIG. 5.
Selenoprotein deficiency leads to a dysregulation of glucose homeostasis in i6A− mutant Sec tRNA transgenic mice. (A) Tissue extracts from WT or i6A− mutant Sec tRNA transgenic mice (I6A) were analyzed by SDS-PAGE and Western blotting with polyclonal antibodies specific for GPx1, MsrB1, TR3, or monoclonal β-actin-specific antibody as a loading control. Expression of proteins is shown for two WT and two I6A transgenic mice. (B) GPx1 activity was analyzed in liver and kidney of WT and I6A transgenic mice. Results are represented as means±SEM (n=3; *p<0.001 by two-tailed t-test). (C) MsrB activity was analyzed in liver and kidney of WT and I6A transgenic mice. Results are represented as means±SEM (n=3; *p<0.05; and **p<0.01 by two-tailed t-test). (D) Effect of an i6A− mutant Sec tRNA overexpression on glucose tolerance. 10-month-old WT and I6A males were given a single intraperitoneal injection of glucose (1 mg/g), and plasma glucose levels were measured at the indicated time points. Data are shown as means±SEM in each group (n=4–5; *p<0.01; and **p<0.05 by two-way ANOVA). (E) Fasting plasma glucose levels in WT and I6A mice. Results are represented as means±SEM (n=4–5; *p<0.05 by two-tailed t-test). (F) Steady-state plasma insulin levels in WT and I6A mice during fasting (fast) or in fed state (fed). Results are represented as means±SEM (n=3; *p<0.05 by two-tailed t-test).
As increased expression of GPx1 and MsrB1 selenoproteins correlated with increased insulin production and insulin intolerance in mice fed the Se-supplemented diet, we expected improved insulin signaling in i6A− mutant Sec tRNA transgenic mice, which have lower expression of these selenoproteins compared to wild-type mice. Surprisingly, these mice also demonstrated a diabetic phenotype. When i6A− mutant Sec tRNA transgenic mice were challenged with a high glucose load, their blood glucose levels were increased more dramatically (p<0.01) compared to wild-type mice, and were restored to background level at a later time (Fig. 5D). In addition, these mice had elevated (p<0.05) fasting plasma glucose levels (Fig. 5E) and hyperinsulinemia in the fed state (Fig. 5F). However, we were not able to detect any changes in morphological organization or insulin immunostaining intensity in the Langerhans islets of i6A− mutant Sec tRNA transgenic mice (Supplementary Fig. S1; Supplementary Data are available online at www.liebertonline.com/ars), and their mild diabetic phenotype did not progress with age (Supplementary Figs. S2 and S3).
Taken together, these data indicate that both maximal expression of selenoproteins caused by chronic supranutritional Se and selenoprotein deficiency caused by overexpression of an i6A− mutant Sec tRNA lead to dysregulation of glucose homeostasis in mice.
Discussion
We used two different mouse models to assess the role of Se and selenoproteins in the adverse effects of long-term Se supplementation. In the first model, dietary intervention was used to regulate expression of selenoproteins, whereas in the second model selenoprotein expression was genetically reduced by overproducing mutant Sec tRNA. Our data show that availability of Se in the diet regulates expression and activity of various selenoproteins. In particular, expression of antioxidant selenoproteins GPx1 and MsrB1, which are involved in degradation of intracellular hydrogen peroxide and repair of oxidized methionine residues (methionine-R-sulfoxide) in proteins, respectively, was dramatically increased in liver and kidney of mice by changing dietary Se levels from 0 to 0.4 ppm. In contrast, expression levels of selenoprotein TR3 remained unchanged regardless of Se concentration. These data are consistent with the known hierarchy of selenoprotein synthesis. Previous studies have demonstrated that under conditions of selenium deficiency selenoproteins respond differently to Se availability, and different tissues are able to retain Se to a different degree (1, 21). Particularly, selenoprotein levels more rapidly decrease in liver, kidney, and blood, whereas endocrine tissues and brain tend to retain Se for a longer time after Se depletion. Together, these observations are consistent with the idea that two major groups of selenoproteins exist: one group is easily regulated by Se levels in the diet, which we have referred to as stress-related selenoproteins (among the best characterized stress-related selenoproteins are GPx1, GPx3, and MsrB1; other proteins in this group include SelW, SelS and SelP), whereas another group—housekeeping selenoproteins (e.g., TR3 and GPx4)—is less regulated by Se availability (4). One possibility is that the former group may be involved in adverse effects of long-term Se supplementation in humans, including increased risk of developing diabetes.
Consistent with the results of NPC trial, we observed impaired insulin sensitivity and hyperinsulinemia in mice supplemented with 0.4 ppm Se, which approximately corresponds to the supplemented amounts of Se used in human studies (200 μg/day). However, an increase in dietary Se from 0 to 0.1 ppm (approximately corresponding to ∼55 μg/day in humans) resulted in only small changes in insulin sensitivity and plasma insulin levels. Although our studies cannot be directly applied to interpret the results of the human trials due to different forms of Se used as the intervention agent (i.e., NPC trial utilized selenium-enriched baker's yeast and SELECT used selenomethionine) and different baseline Se intakes and optimal Se requirements in humans, these data suggest the possibility that prolonged exposure of human subjects to Se above adequate levels may predispose them to the development of diabetes. Moreover, there is a clear correlation between expression of stress-related selenoproteins and decreased insulin sensitivity in mice suggesting that these selenoproteins may, at least in part, mediate a pro-diabetic effect of the supranutritional dose Se in humans.
The possible link between expression of stress-related selenoproteins and diabetes is also supported by the recent reports showing altered expression of SelS selenoprotein in P. obesus, an animal model of metabolic syndrome and diabetes (7, 35), and selenoprotein SelP in patients with type 2 diabetes mellitus (24). Additionally, McClung et al. reported that overexpression of GPx1 leads to development of insulin resistance and obesity in mice (22). It has been proposed that decreased levels of intracellular ROS caused by GPx1 overexpression positively regulates expression and secretion of insulin in pancreatic β cells (36). It is likely that overproduction of insulin in response to glucose would result in decreased insulin sensitivity in peripheral tissues. Our findings are consistent with this hypothesis as dietary Se supplementation increased expression of GPx1. However, it cannot be excluded that other selenoproteins may also contribute to the development of insulin resistance by a different mechanism. To test this possibility, we analyzed expression of other selenoproteins in mice overexpressing GPx1. Strikingly, expression of other selenoproteins, including MsrB1, SelS, and SelT, was also increased in GPx1 transgenic mice. Together, these findings suggest that, in addition to decreasing hydrogen peroxide levels, GPx1 overexpression selectively upregulates other selenoproteins, which may account for the observed diabetic phenotype.
Interestingly, another group reported that increased ROS levels caused by GPx1 deficiency led to improved insulin sensitivity and attenuation of high-fat-diet-induced obesity in GPx1 knockout mice (20). In this study, decreased levels of GPx1 did not alter insulin secretion, but rather improved insulin signaling by ROS-mediated inhibition of PTEN protein tyrosine phosphatase selectively in muscle tissue.
To further test the role of selenoproteins in regulation of glucose homeostasis, we genetically reduced selenoprotein expression by overproducing mutant Sec tRNA. As expected, we found decreased expression of GPx1 and other stress-related selenoproteins in i6A− mutant Sec tRNA transgenic mice fed normal chow diet, but expression of housekeeping selenoproteins did not dramatically change. Since these mice also have decreased expression of GPx1 protein, we expected that GPx1 deficiency caused by mutant tRNA overexpression would improve insulin signaling. In contrast, i6A− mutant Sec tRNA transgenic mice also developed glucose intolerance, hyperglycemia, and hyperinsulinemia, hallmarks of type 2 diabetes mellitus.
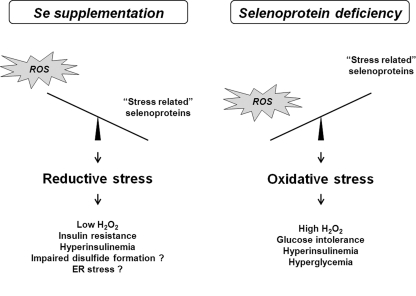
Taken together, our data suggest that both high levels of selenoprotein expression caused by dietary Se supplementation and selenoprotein deficiency caused by overexpression of mutated Sec tRNA may lead to diabetes. To our knowledge, this is the first report showing that decreased levels of selenoproteins can lead to dysregulation of glucose homeostasis and promote a diabetes-like phenotype. On the surface, the results from the mutant Sec tRNA transgenic model may appear to contradict our mouse dietary model data and the results of NPC and SELECT human clinical trials. However, it cannot be excluded that selenoproteins may have opposing roles in promoting diabetes. This possibility is indirectly supported by our recent observations that selenoprotein deficiency as well as high selenoprotein expression can inhibit tumorigenesis and by contrasting roles of several selenoproteins in both cancer prevention and promotion of cancer (26). What may account for the observations involving different mouse models? Elevated levels of stress-related selenoproteins as well as their deficiency may have influenced glucose metabolism by disrupting redox homeostasis. Reduced levels of GPx1 and possibly other selenoproteins in mice overexpressing mutant Sec tRNA may lead to an increased production of hydrogen peroxide and oxidative stress promoting insulin resistance. Although GPx1 can protect cells against oxidative stress, excess of GPx1 expression caused by supranutritional Se in the diet may, in turn, cause diabetes by inducing reductive stress and eliminating hydrogen peroxide pools involved in normal oxidative signaling (12, 28). The concept of reductive stress is further supported by a contrasting pattern of TR1 regulation in GPx1-overexpressing cells, which have lower levels of TR1 protein, a marker of reductive stress (11). Thus, an appropriate redox balance/ROS flux must be maintained to limit negative effects of either oxidative or reductive stresses on glucose homeostasis and β cell function (Fig. 6).
FIG. 6.
Model for the diabetogenic effect of dietary Se supplementation and selenoprotein deficiency in mice. The effect of supranutritional selenium on the development of diabetes in our Se diet mouse model can be mediated by elevated expression of antioxidant selenoproteins, including GPx1. High GPx1 stimulates reductive stress and prevents normal hydrogen peroxide signaling leading to hyperinsulinemia and decreased insulin sensitivity. However, decreased expression of GPx1 and possibly other selenoproteins in mice overexpressing mutant Sec tRNA may result in high levels of reactive oxygen species (ROS) production and lead to oxidative stress-induced insulin resistance.
Recent studies suggest that redox homeostasis in the cell is closely interlinked with protein folding, modification, and quality control systems in the ER (23), and ROS produced by mitochondria are actively used to facilitate disulfide formation (38). It is an attractive possibility that oxidative protein folding of insulin and/or insulin receptors may be suppressed at either end of the selenoprotein spectrum (during both high selenoprotein expression and selenoprotein deficiency) by changed expression level of GPx1 required to maintain the optimal ROS levels for disulfide formation.
Supplementary Material
Abbreviations Used
- ER
endoplasmic reticulum
- GPx1
glutathione peroxidase 1
- MsrB1
methionine-R-sulfoxide reductase B1
- NPC
Nutritional Prevention of Cancer
- ppm
parts per million
- ROS
reactive oxygen species
- Se
selenium
- Sec
selenocysteine
- SELECT
selenium and vitamin E cancer prevention trial
- SelP
selenoprotein P
- SelS
selenoprotein T
- SelT
selenoprotein T
- SEM
standard error of the mean
- TR1
thioredoxin reductase 1
- TR3
thioredoxin reductase 3
Acknowledgments
We would like to thank Xin Gen Lei for providing mouse tissues from transgenic mice overexpressing GPx1 and Sergey Novoselov for help with glucose tolerance tests. This work was supported by National Institutes of Health grants CA080946 and AG021518 (to VNG), HL61795, HL81587, HL70819, and HL48743 (to JL), and the Intramural Research Program of the National Institutes of Health, National Cancer Institute, Center for Cancer Research (to DLH).
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Behne D. Hilmert H. Scheid S. Gessner H. Elger W. Evidence for specific selenium target tissues and new biologically important selenoproteins. Biochim Biophys Acta. 1988;966:12–21. doi: 10.1016/0304-4165(88)90123-7. [DOI] [PubMed] [Google Scholar]
- 2.Bleys J. Navas-Acien A. Guallar E. Selenium and diabetes: more bad news for supplements. Ann Intern Med. 2007;147:271–272. doi: 10.7326/0003-4819-147-4-200708210-00177. [DOI] [PubMed] [Google Scholar]
- 3.Blot WJ. Li JY. Taylor PR. Guo W. Dawsey S. Wang GQ. Yang CS. Zheng SF. Gail M. Li GY. Nutrition intervention trials in Linxian, China: supplementation with specific vitamin/mineral combinations, cancer incidence, and disease-specific mortality in the general population. J Natl Cancer Inst. 1993;85:1483–1492. doi: 10.1093/jnci/85.18.1483. [DOI] [PubMed] [Google Scholar]
- 4.Carlson BA. Xu XM. Shrimali R. Sengupta A. Yoo MH. Zhong N. Hatfield DL. Irons R. Davis CD. Lee BJ. Novoselov SV. Gladyshev VN. Mouse models for assessing the role of selenoproteins in health and development. In: Hatfield DL, editor; Berry MJ, editor; Gladyshev VN, editor. Selenium: Its Molecular Biology and Role in Human Health. New York, NY: Springer; 2006. pp. 333–341. [Google Scholar]
- 5.Cheng WH. Ho YS. Ross DA. Han Y. Combs GF., Jr. Lei XG. Overexpression of cellular glutathione peroxidase does not affect expression of plasma glutathione peroxidase or phospholipid hydroperoxide glutathione peroxidase in mice offered diets adequate or deficient in selenium. J Nutr. 1997;127:675–680. doi: 10.1093/jn/127.5.675. [DOI] [PubMed] [Google Scholar]
- 6.Clark LC. Combs GF. Turnbull BW. Slate EH. Chalker DK. Chow J. Davis LS. Glover RA. Graham GF. Gross EG. Krongrad A. Lesher JL. Park HK. Sanders BB. Smith CL. Taylor JR. Effects of selenium supplementation for cancer prevention in patients with carcinoma of the skin. A randomized controlled trial. Nutritional Prevention of Cancer Study Group. JAMA. 1996;276:1957–1963. [PubMed] [Google Scholar]
- 7.Curran JE. Jowett JB. Elliott KS. Gao Y. Gluschenko K. Wang J. Abel Azim DM. Cai G. Mahaney MC. Comuzzie AG. Dyer TD. Walder KR. Zimmet P. MacCluer JW. Collier GR. Kissebah AH. Blangero J. Genetic variation in selenoprotein S influences inflammatory response. Nat Genet. 2005;37:1234–1241. doi: 10.1038/ng1655. [DOI] [PubMed] [Google Scholar]
- 8.Diwadkar-Navsariwala V. Prins GS. Swanson SM. Birch LA. Ray VH. Hedayat S. Lantvit DL. Diamond AM. Selenoprotein deficiency accelerates prostate carcinogenesis in a transgenic model. Proc Natl Acad Sci U S A. 2006;103:8179–8184. doi: 10.1073/pnas.0508218103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Duffield-Lillico AJ. Dalkin BL. Reid ME. Turnbull BW. Slate EH. Jacobs ET. Marshall JR. Clark LC. Selenium supplementation, baseline plasma selenium status and incidence of prostate cancer: an analysis of the complete treatment period of the Nutritional Prevention of Cancer Trial. BJU Int. 2003;91:608–612. doi: 10.1046/j.1464-410x.2003.04167.x. [DOI] [PubMed] [Google Scholar]
- 10.Ganther HE. Selenium metabolism, selenoproteins and mechanisms of cancer prevention: complexities with thioredoxin reductase. Carcinogenesis. 1999;20:1657–1666. doi: 10.1093/carcin/20.9.1657. [DOI] [PubMed] [Google Scholar]
- 11.Gladyshev VN. Factor VM. Housseau F. Hatfield DL. Contrasting patterns of regulation of the antioxidant selenoproteins, thioredoxin reductase, and glutathione peroxidase, in cancer cells. Biochem Biophys Res Commun. 1998;251:488–493. doi: 10.1006/bbrc.1998.9495. [DOI] [PubMed] [Google Scholar]
- 12.Handy DE. Lubos E. Yang Y. Galbraith JD. Kelly N. Zhang YY. Leopold JA. Loscalzo J. Glutathione peroxidase-1 regulates mitochondrial function to modulate redox-dependent cellular responses. J Biol Chem. 2009;284:11913–11921. doi: 10.1074/jbc.M900392200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ip C. Dong Y. Ganther HE. New concepts in selenium chemoprevention. Cancer Metastasis Rev. 2002;21:281–289. doi: 10.1023/a:1021263027659. [DOI] [PubMed] [Google Scholar]
- 14.Ip C. Thompson HJ. Zhu Z. Ganther HE. In vitro and in vivo studies of methylseleninic acid: evidence that a monomethylated selenium metabolite is critical for cancer chemoprevention. Cancer Res. 2000;60:2882–2886. [PubMed] [Google Scholar]
- 15.Irons R. Carlson BA. Hatfield DL. Davis CD. Both selenoproteins and low molecular weight selenocompounds reduce colon cancer risk in mice with genetically impaired selenoprotein expression. J Nutr. 2006;136:1311–1317. doi: 10.1093/jn/136.5.1311. [DOI] [PubMed] [Google Scholar]
- 16.Kim HY. Gladyshev VN. Methionine sulfoxide reduction in mammals: characterization of methionine-R-sulfoxide reductases. Mol Biol Cell. 2004;15:1055–1064. doi: 10.1091/mbc.E03-08-0629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kryukov GV. Castellano S. Novoselov SV. Lobanov AV. Zehtab O. Guigo R. Gladyshev VN. Characterization of mammalian selenoproteomes. Science. 2003;300:1439–1443. doi: 10.1126/science.1083516. [DOI] [PubMed] [Google Scholar]
- 18.Labunskyy VM. Hatfield DL. Gladyshev VN. The Sep15 protein family: roles in disulfide bond formation and quality control in the endoplasmic reticulum. IUBMB Life. 2007;59:1–5. doi: 10.1080/15216540601126694. [DOI] [PubMed] [Google Scholar]
- 19.Lippman SM. Klein EA. Goodman PJ. Lucia MS. Thompson IM. Ford LG. Parnes HL. Minasian LM. Gaziano JM. Hartline JA. Parsons JK. Bearden JD., 3rd Crawford ED. Goodman GE. Claudio J. Winquist E. Cook ED. Karp DD. Walther P. Lieber MM. Kristal AR. Darke AK. Arnold KB. Ganz PA. Santella RM. Albanes D. Taylor PR. Probstfield JL. Jagpal TJ. Crowley JJ. Meyskens FL., Jr. Baker LH. Coltman CA., Jr. Effect of selenium and vitamin E on risk of prostate cancer and other cancers: the Selenium and Vitamin E Cancer Prevention Trial (SELECT) JAMA. 2009;301:39–51. doi: 10.1001/jama.2008.864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Loh K. Deng H. Fukushima A. Cai X. Boivin B. Galic S. Bruce C. Shields BJ. Skiba B. Ooms LM. Stepto N. Wu B. Mitchell CA. Tonks NK. Watt MJ. Febbraio MA. Crack PJ. Andrikopoulos S. Tiganis T. Reactive oxygen species enhance insulin sensitivity. Cell Metab. 2009;10:260–272. doi: 10.1016/j.cmet.2009.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Low SC. Grundner-Culemann E. Harney JW. Berry MJ. SECIS-SBP2 interactions dictate selenocysteine incorporation efficiency and selenoprotein hierarchy. EMBO J. 2000;19:6882–6890. doi: 10.1093/emboj/19.24.6882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McClung JP. Roneker CA. Mu W. Lisk DJ. Langlais P. Liu F. Lei XG. Development of insulin resistance and obesity in mice overexpressing cellular glutathione peroxidase. Proc Natl Acad Sci U S A. 2004;101:8852–8857. doi: 10.1073/pnas.0308096101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Merksamer PI. Trusina A. Papa FR. Real-time redox measurements during endoplasmic reticulum stress reveal interlinked protein folding functions. Cell. 2008;135:933–947. doi: 10.1016/j.cell.2008.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Misu H. Takamura T. Takayama H. Hayashi H. Matsuzawa-Nagata N. Kurita S. Ishikura K. Ando H. Takeshita Y. Ota T. Sakurai M. Yamashita T. Mizukoshi E. Honda M. Miyamoto K. Kubota T. Kubota N. Kadowaki T. Kim HJ. Lee IK. Minokoshi Y. Saito Y. Takahashi K. Yamada Y. Takakura N. Kaneko S. A liver-derived secretory protein, selenoprotein p, causes insulin resistance. Cell Metab. 2010;12:483–495. doi: 10.1016/j.cmet.2010.09.015. [DOI] [PubMed] [Google Scholar]
- 25.Moustafa ME. Carlson BA. El-Saadani MA. Kryukov GV. Sun QA. Harney JW. Hill KE. Combs GF. Feigenbaum L. Mansur DB. Burk RF. Berry MJ. Diamond AM. Lee BJ. Gladyshev VN. Hatfield DL. Selective inhibition of selenocysteine tRNA maturation and selenoprotein synthesis in transgenic mice expressing isopentenyladenosine-deficient selenocysteine tRNA. Mol Cell Biol. 2001;21:3840–3852. doi: 10.1128/MCB.21.11.3840-3852.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Novoselov SV. Calvisi DF. Labunskyy VM. Factor VM. Carlson BA. Fomenko DE. Moustafa ME. Hatfield DL. Gladyshev VN. Selenoprotein deficiency and high levels of selenium compounds can effectively inhibit hepatocarcinogenesis in transgenic mice. Oncogene. 2005;24:8003–8011. doi: 10.1038/sj.onc.1208940. [DOI] [PubMed] [Google Scholar]
- 27.Oyadomari S. Koizumi A. Takeda K. Gotoh T. Akira S. Araki E. Mori M. Targeted disruption of the Chop gene delays endoplasmic reticulum stress-mediated diabetes. J Clin Invest. 2002;109:525–532. doi: 10.1172/JCI14550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rajasekaran NS. Connell P. Christians ES. Yan LJ. Taylor RP. Orosz A. Zhang XQ. Stevenson TJ. Peshock RM. Leopold JA. Barry WH. Loscalzo J. Odelberg SJ. Benjamin IJ. Human alpha B-crystallin mutation causes oxido-reductive stress and protein aggregation cardiomyopathy in mice. Cell. 2007;130:427–439. doi: 10.1016/j.cell.2007.06.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Scheuner D. Kaufman RJ. The unfolded protein response: a pathway that links insulin demand with beta-cell failure and diabetes. Endocr Rev. 2008;29:317–333. doi: 10.1210/er.2007-0039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shchedrina VA. Zhang Y. Labunskyy VM. Hatfield DL. Gladyshev VN. Structure-function relations, physiological roles, and evolution of mammalian ER-resident selenoproteins. Antioxid Redox Signal. 2010;12:839–849. doi: 10.1089/ars.2009.2865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stranges S. Marshall JR. Natarajan R. Donahue RP. Trevisan M. Combs GF. Cappuccio FP. Ceriello A. Reid ME. Effects of long-term selenium supplementation on the incidence of type 2 diabetes: a randomized trial. Ann Intern Med. 2007;147:217–223. doi: 10.7326/0003-4819-147-4-200708210-00175. [DOI] [PubMed] [Google Scholar]
- 32.Sunde RA. Selenium. In: O'Dell BL, editor; Sunde RA, editor. Handbook of Nutritionally Essential Mineral Elements. New York, NY: Marcel Dekker; 1997. pp. 493–556. [Google Scholar]
- 33.Sunde RA. Selenium. In: Bowman BA, editor; Russell RM, editor. Present Knowledge in Nutrition. Washington, DC: ILSI Press; 2001. pp. 352–365. [Google Scholar]
- 34.Sunde RA. Regulation of glutathione peroxidase-1 expression. In: Hatfield DL, editor; Berry MJ, editor; Gladyshev VN, editor. Selenium: Its Molecular Biology and Role in Human Health. New York, NY: Springer; 2006. pp. 149–160. [Google Scholar]
- 35.Walder K. Kantham L. McMillan JS. Trevaskis J. Kerr L. De Silva A. Sunderland T. Godde N. Gao Y. Bishara N. Windmill K. Tenne-Brown J. Augert G. Zimmet PZ. Collier GR. Tanis: a link between type 2 diabetes and inflammation? Diabetes. 2002;51:1859–1866. doi: 10.2337/diabetes.51.6.1859. [DOI] [PubMed] [Google Scholar]
- 36.Wang XD. Vatamaniuk MZ. Wang SK. Roneker CA. Simmons RA. Lei XG. Molecular mechanisms for hyperinsulinaemia induced by overproduction of selenium-dependent glutathione peroxidase-1 in mice. Diabetologia. 2008;51:1515–1524. doi: 10.1007/s00125-008-1055-3. [DOI] [PubMed] [Google Scholar]
- 37.Whanger PD. Selenium and its relationship to cancer: an update. Br J Nutr. 2004;91:11–28. doi: 10.1079/bjn20031015. [DOI] [PubMed] [Google Scholar]
- 38.Yang Y. Song Y. Loscalzo J. Regulation of the protein disulfide proteome by mitochondria in mammalian cells. Proc Natl Acad Sci U S A. 2007;104:10813–10817. doi: 10.1073/pnas.0702027104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang Z. Kimura M. Itokawa Y. The decrement of carcinogenesis by dietary selenium and expression of placental form of glutathione-S-transferase in rat glioma. Biol Trace Elem Res. 1997;57:147–155. doi: 10.1007/BF02778198. [DOI] [PubMed] [Google Scholar]
- 40.Zito E. Chin KT. Blais J. Harding HP. Ron D. ERO1-beta, a pancreas-specific disulfide oxidase, promotes insulin biogenesis and glucose homeostasis. J Cell Biol. 2010;188:821–832. doi: 10.1083/jcb.200911086. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.