Abstract
Objective
To test the hypothesis of a significant association between resting heart rate (RHR) and coronary artery calcium (CAC).
Methods
This is a cross-sectional study of a subset of women enrolled in the estrogen-alone clinical trial of the Women's Health Initiative (WHI). We used a longitudinal study that enrolled 998 postmenopausal women with a history of hysterectomy between the ages of 50 and 59 at enrollment at 40 different clinical centers. RHR was measured at enrollment and throughout the study, and CAC was determined approximately 7 years after the baseline clinic visit.
Results
The mean (standard deviation [SD]) age was 55 (2.8) years. With adjustment for age and ethnicity, a 10-unit increment in RHR was significantly associated with CAC (SD 1.18, 95% confidence interval [CI] 1.01-1.38), but this was no longer significant after adjustment for body mass index (BMI), income, education, dyslipidemia, diabetes, smoking, and hypertension (SD 1.06, 95% CI 0.90-1.25). In a fully adjusted multivariable model, however, there was a significant interaction (p=0.03) between baseline RHR and systolic blood pressure (SBP) for the presence of any CAC. Compared to women with an RHR < 80 beats per minute (BPM) and an SBP < 140 mm Hg, those who had an RHR ≥ 80 BPM and an SBP ≥ 140 mm Hg had 2.66-fold higher odds (1.08-6.57) for the presence of any CAC.
Conclusions
Compared to those with normal BP and RHR, postmenopausal, hysterectomized women with an elevated SBP and RHR have a significantly higher odds for the presence of calcified coronary artery disease.
Introduction
Heart rate is regulated by the autonomic nervous system (i.e., sympathetic and parasympathetic systems). With aging, the influence of the parasympathetic system diminishes, resulting in a relative increase in sympathetic tone, an increase in resting heart rate (RHR), and reduced heart rate variability.1 Previous studies have found consistent associations between RHR and cardiovascular and noncardiovascular mortality,2–4 and other studies have linked increased RHR with higher levels of hypertension5 and cardiovascular morbidity.4,6,7 Moreover, the increased sympathetic tone (which an increased RHR reflects) has been associated with atherogenesis.8
In a regulated process similar to skeletal bone formation,9 calcium is deposited in atherosclerotic plaques.10 With the advent of computed tomography (CT), these calcified atheromatous plaques can be detected throughout the vasculature,11 including the coronary arteries.12 The extent of coronary artery calcium (CAC) is highly correlated with both the total atheromatous plaque burden13 and the percent stenosis in that vascular bed.14 Moreover, several studies have shown CAC to be a strong and independent predictor of incident coronary heart disease (CHD) events in both men and women.15,16
Use of CAC as a marker of coronary atherosclerosis is increasingly advocated as a component of individual cardiovascular disease (CVD) risk stratification procedures.17,18 Accordingly, the aim of this study was to determine the magnitude of the associations between CAC and RHR in women with a history of hysterectomy who were between the ages of 50 and 59 years at the time of their enrollment in the Women's Health Initiative (WHI). We hypothesize that women with higher RHR would be at increased risk for higher prevalence and levels of CAC. Additionally, as previous work has demonstrated an increased risk for CHD and mortality among those with prehypertension and elevated RHR,19 we hypothesized that women with elevations of both blood pressure (BP) and RHR would have the highest odds for CAC.
Materials and Methods
Subjects
The WHI hormone therapy (HT) clinical trials enrolled 27,347 women at 40 clinical sites located across the United States. Of these, 10,739 were enrolled in the WHI Estrogen Trial (ET) using conjugated equine estrogen (CEE). These women were postmenopausal, aged 50–79 years at randomization, had a history of hysterectomy before enrollment, and were randomized to receive CEE, 0.625 mg/day (Premarin, Wyeth Pharmaceuticals, St Davids, PA) or a matching placebo. Methods for data collection, management, and quality assurance have been published previously.20 The ET was ended after an average of 6.8 years follow-up.
The WHI Coronary Artery Calcium Study (WHI CACS) was a substudy of the WHI ET. All 40 WHI clinical sites were asked to participate in this substudy; 28 agreed. Reasons for site nonparticipation included lack of suitable equipment and logistical concerns. Invitations were mailed to the women at these sites requesting them to undergo a one-time cardiac CT scan to determine CAC by electron beam or multidetector-row CT. Of the women enrolled in the ET, 1,742 were eligible for WHI CACS because they were between 50 and 59 years old at the time of their randomization into the ET and were participants at 1 of the 28 participating sites. Exclusion criteria for study participation included a last measured or reported weight of ≥300 lbs (because of technical and equipment-related restrictions), participant request for no further contact or clinic visits, or participant lost to follow-up or deceased since randomization (30.4% of participants were excluded for one or more of these reasons). As a result of these exclusions, informed consent for WHI CACS was provided by 1,079 women (61.6% of those eligible at the 28 clinical centers) who underwent CAC scanning an average of 1.3 years after the ET trial ended. After specific exclusions for the current study, there were 998 women available for analysis. The Human Subjects Review Committee at each participating institution approved the WHI study protocols.
Data collection
Trial participants provided data on a wide range of factors at the ET baseline clinic visit. Ethnicity was determined by self-report, with the following categories: non-Hispanic white, African American/black (non-Hispanic), Hispanic, Asian/Pacific Islander, American Indian/Alaska Native, or unknown (women who indicated other ethnicity or did not answer the question). Education and income were ascertained by self-report from a range of categories. The presence of hypertension, high cholesterol, or diabetes was identified by self-reported use of a medication for these conditions. Smoking was categorized as current, former, or none. Current smoking was defined as smoking at least 100 cigarettes in their lifetime and reporting smoking at baseline, and former smoking was smoking at least 100 cigarettes but reported not smoking at baseline. Use of postmenopausal HT before WHI CEE trial enrollment was ascertained via an in-person interview at the baseline clinic visit. Total physical activity was assessed by questions on a frequency and duration scale for walking and other types of activity and converted to MET-hours per week.21 Anxiety and depression were assessed using the RAND36 mental health score.22
At baseline, women sat quietly for 5 minutes before heart rate was measured by palpating the radial pulse for 30 seconds and BP was measured twice using a conventional mercury sphygmomanometer and appropriately sized cuffs by a trained observer. Anthropometric measurements were obtained at baseline. Body mass index (BMI) was calculated as weight (kg)/height (m2). Waist and hip circumferences (in cm) were obtained using a standardized measuring tape.
Coronary artery calcified plaque measurements
A standardized protocol was developed based on prior multicenter experience with cardiac CT.23 Phantom scan and test images were obtained from each CT system to verify technical parameters and CT system performance. Analyses of the measurements were performed by certified staff at the central reading center at Wake Forest University who were masked to participants' treatment assignment. The Agatston score was calculated on a computer workstation (TeraRecon Inc, San Mateo, CA) by experienced image analysts using established parameters (lesion size of >1 mm2, adjustment for slice thickness, and threshold of 130 Hounsfield units [HU]).24 After the scan was scored, the participants were provided with a letter documenting their calcium score, which could be reviewed with their healthcare provider if desired.
Women with a history of coronary revascularization before randomization were excluded from the analysis. Also, the reading protocol specified exclusion of coronary stents, pacemakers, metallic clips, and other surgical remnants from the analysis process. Three women with incomplete scans were excluded. Women reporting use of beta-blockers at baseline were excluded, as these drugs may affect heart rate.
Statistical analyses
CAC score was coded as a binary variable (>0 or=0). As some results suggest reduced reproducibility of CAC scores from 0 to 10, we also conducted analyses using a CAC score >10 vs. ≤10 as an outcome. Baseline RHR, the primary exposure variable, was defined as a continuous variable and a categorical variable based on the groupings <60, 60–69, 70–79, and ≥80. These groups were used to provide clinical relevance as well as comparisons to other studies that have been conducted on RHR. Secondary analysis investigated the effect of RHR gathered at baseline and during follow-up.
Baseline characteristics were compared between CAC score (>0 or=0). Differences between the groups were assessed using age-adjusted logistic regression models. For the main analysis, multivariable adjusted logistic regression models were used to evaluate the association between the presence of any CAC and baseline RHR. To control for potential confounding, baseline values of age, race/ethnicity, diabetes, dyslipidemia, smoking, BMI, systolic blood pressure (SBP), diastolic blood pressure (DBP), high BP medication, consumption of alcohol and regular coffee, RAND36 mental health scale, education, income, and CEE randomization assignment were used as covariates. These variables were selected based on previous studies that have shown them to be associated with CAC or theoretically influential of RHR.
Based on previous studies,19 we, a priori, planned interactions between RHR with both BP and high BP medication use. To further quantify the association between CAC and baseline RHR by BP level, we determined the odds for any CAC by different combinations of RHR/SBP group. Specifically, we examined the odds for CAC when the RHR was ≥80 BPM and the SBP was ≥140 mm Hg, as well as when the RHR was ≥70 BPM and the SBP was ≥130 mm Hg. For these analyses, generalized additive models (GAMs) were also used to graphically display the association between CAC and RHR and further quantify their relationship by statistically evaluating their fits. A p value <0.05 was considered statistically significant. No adjustments were made for multiple comparisons, and exact p values are given. All reported p values are two-sided. The main statistical analyses were performed using SAS version 9.1 (SAS Institute Inc, Cary, NC). Graphs of GAMs and goodness of fit were assessed with R version 2.9.2.
Results
The distribution of CAC in the WHI CACS cohort was positively skewed.25,26 Therefore, the characteristics of the 998 women included in this analysis were stratified by the presence and absence of CAC (Table 1). There were 464 (46.5%) women with a CAC score >0. Those who had any CAC were significantly older. After adjustment for age, the CAC-positive group had significantly higher levels of BMI, hip and waist circumference, SBP, hypertension, cholesterol medication use, diabetes medication use, and pack-years cigarette smoking while having significantly lower levels of physical activity, annual income, education, and RAND36 mental health scale score.
Table 1.
Baseline Characteristics by Presence and Absence of Coronary Artery Calcium
| Characteristic | CAC=0 (n=464) | CAC > 0 (n=534) | p valuea |
|---|---|---|---|
| Age at baseline (years)b | 54.7 (2.9) | 55.5 (2.8) | <0.01 |
| Race/ethnicityc | 0.17 | ||
| White | 396 (74.2) | 355 (76.5) | |
| Black | 99 (18.5) | 67 (14.4) | |
| Hispanic | 32 (6) | 28 (6) | |
| American Indian | 1 (0.2) | 7 (1.5) | |
| Asian/Pacific Islander | 1 (0.2) | 2 (0.4) | |
| Unknown | 5 (0.9) | 5 (1.1) | |
| Body mass index (kg/m2)b | 29.7 (6) | 31.3 (6) | <0.01 |
| Waist circumference (cm)b | 88.5 (13.7) | 94.5 (14.2) | <0.01 |
| Hip circumference (cm)b | 110.3 (12.7) | 113 (12.6) | <0.01 |
| SBP (mm Hg)b | 122.4 (14.7) | 126.4 (16.2) | <0.01 |
| DBP (mm Hg)b | 77.5 (8.9) | 77.7 (8.8) | 0.50 |
| Baseline hypertensionc,d | <0.01 | ||
| Normotensive | 184 (34.5) | 124 (26.7) | |
| Prehypertensive | 205 (38.4) | 159 (34.3) | |
| Hypertensive | 145 (27.2) | 181 (39) | |
| Pack-years of smokingb | 7.3 (14.7) | 13.6 (19.9) | <0.01 |
| Smoking statusc | <0.01 | ||
| Never | 288 (54.3) | 195 (42.2) | |
| Past | 202 (38.1) | 186 (40.3) | |
| Current | 40 (7.5) | 81 (17.5) | |
| Cholesterol medication usec | 32 (6) | 54 (11.6) | <0.01 |
| Diabetes medication usec | 16 (3) | 29 (6.3) | 0.02 |
| Randomization status (E-alone arm)c | 280 (52.4) | 222 (47.8) | 0.13 |
| Hormone therapy usec | 0.84 | ||
| Never | 252 (47.2) | 220 (47.4) | |
| Past | 165 (30.9) | 149 (32.1) | |
| Current | 117 (21.9) | 95 (20.5) | |
| Total energy expenditure/week (MET-hours)b | 11.1 (13.9) | 9.2 (12.5) | 0.09 |
| Annual income <$35Kc | 176 (34.7) | 194 (43.1) | 0.01 |
| Educationc | <0.01 | ||
| ≤High school/GED or less | 113 (21.4) | 136 (29.6) | |
| School after high school | 237 (44.8) | 205 (44.6) | |
| College degree or higher | 179 (33.8) | 119 (25.9) | |
| RAND mental health (quartiles)c | 0.02 | ||
| <68 | 93 (17.8) | 106 (23.2) | |
| 68–79 | 102 (19.5) | 105 (23) | |
| 80–87 | 151 (28.9) | 116 (25.4) | |
| ≥88 | 177 (33.8) | 129 (28.3) |
Adjusted for age. **Freq (%)
Mean (standard deviation [SD]).
Frequency (%).
Hypertension definitions: normotensive, SBP < 120, DBP < 80, and no medications; prehypertension, SBP 120–139, DBP 80–89 and no medications; hypertension: SBP ≥ 140 or SBP ≥ 90 or on medication.
BP, blood pressure; CAC, coronary artery calcium; DBP, diastolic blood pressure; E, estrogen; SBP, systolic blood pressure.
Baseline RHR was approximately normally distributed, with a mean (SD) of 69 (9) beats per minute (BPM) and was not significantly different by history of HT use (past, 69.3; current, 68.6; never, 69.7). Table 2 presents the characteristics of the cohort by increasing increments of RHR measured at the baseline WHI visit. After adjustment for age, there were significant positive trends across RHR levels for BMI, waist circumference, and both SBP and DBP, as well as for the prevalence of hypertension, prehypertension, diabetes, current smoking, and an annual income <$35,000. Conversely, physical activity levels decreased significantly across increasing RHR levels. The proportion of African Americans increased, whereas the proportion of non-Hispanic whites decreased. The prevalence of having a CAC score >0 also increased across increasing levels of RHR, which was of borderline statistical significance (p=0.08).
Table 2.
Baseline Characteristics by Resting Heart Rate
| |
Resting heart rate (BPM) |
|
|||
|---|---|---|---|---|---|
| Characteristic | <60 (n=78) | 60–69 (n=452) | 70–79 (n=320) | >80 (n=145) | p valuea |
| Coronary calcium score >0 | 33 (43.4) | 202 (45) | 151 (47.5) | 75 (51.7) | 0.08 |
| Age at baseline (years)b | 55.4 (3.1) | 55.2 (2.9) | 55.2 (2.8) | 54.6 (2.8) | 0.06 |
| Race/ethnicityc | 0.46 | ||||
| White | 57 (75) | 339 (75.5) | 252 (79.2) | 97 (66.9) | |
| Black | 10 (13.2) | 73 (16.3) | 43 (13.5) | 36 (24.8) | |
| Hispanic | 9 (11.8) | 23 (5.1) | 18 (5.7) | 10 (6.9) | |
| American Indian | 0 (0) | 6 (1.3) | 2 (0.6) | 0 (0) | |
| Asian/Pacific Islander | 0 (0) | 0 (0) | 2 (0.6) | 1 (0.7) | |
| Unknown | 0 (0) | 8 (1.8) | 1 (0.3) | 1 (0.7) | |
| Body mass index (kg/m2)b | 30 (6.8) | 30 (5.7) | 30.5 (6.2) | 31.6 (6.2) | 0.01 |
| Waist circumference (cm)b | 90 (13) | 90.1 (13.5) | 92 (15.2) | 94.1 (14.8) | <0.01 |
| Hip circumference (cm)b | 109.3 (12.1) | 111.3 (12.3) | 111.5 (13) | 113.4 (13.8) | 0.05 |
| SBP (mm Hg)b | 122.3 (16.7) | 123 (15.3) | 124.6 (14.8) | 128.3 (16.8) | <0.01 |
| DBP (mm Hg)b | 73.4 (8.7) | 77 (8.8) | 77.8 (8.3) | 81 (8.9) | <0.01 |
| Baseline hypertensionc | <0.01 | ||||
| Normotensive | 31 (40.8) | 147 (32.7) | 98 (30.8) | 30 (20.7) | |
| Prehypertensive | 23 (30.3) | 164 (36.5) | 125 (39.3) | 48 (33.1) | |
| Hypertensive | 22 (28.9) | 138 (30.7) | 95 (29.9) | 67 (46.2) | |
| Pack-years of smokingb | 6.5 (13.1) | 9.9 (17.1) | 11.1 (18.7) | 11.2 (18.4) | 0.06 |
| Smoking statusc | 0.02 | ||||
| Never | 44 (57.9) | 216 (48.3) | 145 (46.2) | 73 (50.3) | |
| Past | 29 (38.2) | 182 (40.7) | 126 (40.1) | 48 (33.1) | |
| Current | 3 (3.9) | 49 (11) | 43 (13.7) | 24 (16.6) | |
| Cholesterol medication usec | 7 (9.2) | 32 (7.1) | 33 (10.4) | 12 (8.3) | 0.31 |
| Diabetes medication usec | 2 (2.6) | 15 (3.3) | 15 (4.7) | 12 (8.3) | 0.01 |
| Randomization status (E-alone arm)c | 38 (50) | 235 (52.3) | 151 (47.5) | 75 (51.7) | |
| Hormone therapy usec | 0.09 | ||||
| Never | 27 (34.6) | 213 (47.1) | 155 (48.4) | 73 (50.3) | |
| Past | 29 (37.2) | 144 (31.9) | 91 (28.4) | 51 (35.1) | |
| Current | 22 (28.2) | 95 (21.0) | 74 (23.1) | 21 (14.5) | |
| Total energy expenditure/week (MET-hours)b | 13.8 (17.6) | 10.5 (12.5) | 9.3 (11.8) | 9.3 (15.1) | 0.01 |
| Annual income <$35Kc | 19 (26) | 166 (38.3) | 118 (38.9) | 63 (45.3) | |
| Educationc | 0.23 | ||||
| ≤High school/GED or less | 11 (14.5) | 115 (25.9) | 78 (24.8) | 40 (27.6) | |
| School after high school | 41 (53.9) | 184 (41.4) | 149 (47.5) | 65 (44.8) | |
| College degree or higher | 24 (31.6) | 145 (32.7) | 87 (27.7) | 40 (27.6) | |
| RAND mental health (quartiles)c | 0.42 | ||||
| <68 | 12 (15.8) | 87 (19.9) | 60 (19.2) | 35 (24.3) | |
| 68 to 79 | 19 (25) | 92 (21.1) | 65 (20.8) | 31 (21.5) | |
| 80 to 87 | 21 (27.6) | 110 (25.2) | 97 (31.1) | 37 (25.7) | |
| ≥88 | 24 (31.6) | 148 (33.9) | 90 (28.8) | 41 (28.5) | |
Adjusted for age. **Freq (%)
Mean (SD).
Frequency (%).
BPM, beats per minute.
For a 10-unit increment in baseline RHR, the odds of having a CAC score >0 was 1.17 (95% confidence ratio [CI] 1.00-1.36). The odds ratio (OR) was not appreciably changed with adjustment for age and ethnicity (OR 1.18, 95% CI 1.01-1.38). However, additional adjustment for BMI, income, education, dyslipidemia, diabetes, smoking, and hypertension attenuated the OR to 1.06 (95% CI 0.90-1.25). Of these, addition of BMI or smoking or both, but not the other covariates just listed, to a model containing RHR, age, and ethnicity resulted in the odds for CAC becoming nonsignificant (data not shown). Additional adjustment for alcohol and coffee consumption, RAND36 mental health score, and randomization status did not materially change the OR (1.06, 95% CI 0.90-1.25), nor did further adjustment for SBP and DBP (OR 1.08, 95% CI 0.91-1.28) or physical activity (OR 1.03, 95% CI 08.6-1.24). The results were essentially the same when a CAC score ≥10 (vs. CAC < 10) was used as the outcome.
We also examined the association between baseline RHR groups and the presence of CAC. In unadjusted models and compared to the group with the lowest RHR (<60 BPM), those in the highest group (RHR ≥ 80 BPM) had an OR of 1.77 (95% CI 0.97-3.20) for the presence of CAC, whereas those with an RHR from 70 to 79 BPM and from 60 to 69 BPM had progressively less risk of CAC (OR 1.56, 95% CI 0.91-2.68 and OR 1.38, 95% CI 0.81-2.34, respectively). As before, adjustment for traditional CVD risk factors attenuated the magnitudes of these associations (RHR ≥ 80 BPM: OR 1.49, 95% CI 0.77-2.89; RHR 70–79: OR 1.39, 95% CI 0.76-2.52; RHR 60–69: OR 1.37, 95% CI 0.76-2.44). However, when a CAC score ≥10 vs. <10 was used as the outcome, the associations were more robust. Specifically, compared to those with an RHR < 60 BPM and after adjustment for the traditional CVD risk factors, the odds for a CAC score >10 were 2.19 (95% CI 1.07-4.48), 2.03 (95% CI 1.06-3.88), and 1.93 (95% CI 1.03-3.64) for those with an RHR ≥ 80 BPM, 70–79 BPM, and 60–69 BPM, respectively, with p for trend=0.10. In a post-hoc analysis, combining the categories of RHR > 60 yields an OR (95%CI) of 1.99 (1.07-3.70) and p=0.03.
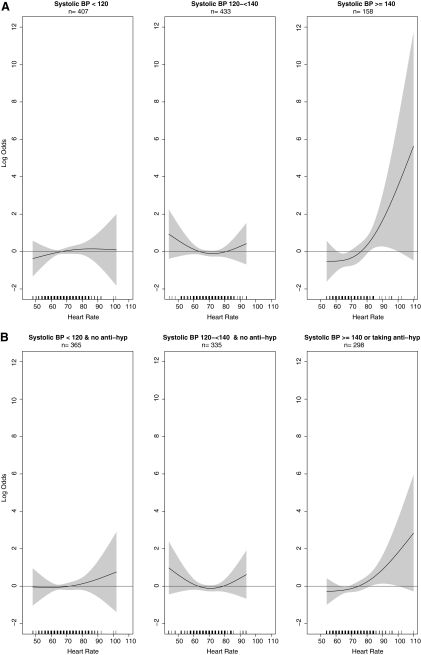
In a fully adjusted multivariable model, there was a significant interaction (p=0.03) between baseline RHR and SBP for the presence of any CAC. Figure 1A shows a multivariable adjusted nonparametric estimate of the log odds of CAC among the entire cohort for increasing RHR by SBP subgroups (i.e. <120, 120–139, and ≥140 mm Hg). The association between RHR and CAC was essentially null in those with SBP of <140 mm Hg. Above this level, the odds for CAC appear to increase significantly as the RHR increases, particularly above 80 BPM. More specifically, among those with an SBP ≥ 140 mm Hg and compared to those with an RHR < 80 BPM, those who had an RHR ≥ 80 BPM had a 2.66-fold higher odds (95% CI 1.08-6.57) for the presence of any CAC (Table 3). Figure 1B shows similar associations where subgroups are defined by both SBP and BP medication use. The association between RHR and CAC was not attenuated when we included women on BP medication who did not have SBP ≥ 140 mm Hg.
FIG. 1.
(A) Association between resting heart rate and coronary artery calcium (CAC) by different systolic blood pressure (SBP) groups. Horizontal line indicates no association between heart rate and CAC. Black curved lines indicate log odds of having any CAC at a given SBP value. Gray shading around black curved lines indicates 95% confidence intervals across the spectrum of SBP values. (B) Association between resting heart rate and CAC by different SBP groups and accounting for blood pressure medication use. Horizontal line indicates no association between heart rate and CAC. Black curved lines indicate log odds of having any CAC at a given SBP value. Gray shading around black curved lines indicates 95% confidence intervals across the spectrum of SBP values. Adjusted for age, ethnicity, body mass index, smoking, SBP, antihypertensive medication, and diastolic blood pressure (for both linear and quadratic terms).
Table 3.
Multivariable Adjusted Odds of Any Coronary Artery Calcium for Resting Heart Rate (≥80 vs. <80 BPM) by Systolic Blood Pressure Subgroups
| Systolic blood pressure subgroupa | Odds ratio | 95% CI | p valueb |
|---|---|---|---|
| 0.03 | |||
| Normotensive (SBP < 120) | 0.89 | (0.44, 1.81) | |
| Prehypertensive (SBP 120–<140) | 0.84 | (0.45, 1.58) | |
| Hypertensive (SBP ≥ 140) | 2.66 | (1.08, 6.57) |
Adjusted for age, race/ethnicity, education, income, diabetes, dyslipidemia, smoking, BMI, systolic blood pressure, diastolic blood pressure, high blood pressure medication, consumption of alcohol and regular coffee. RAND36 mental health scale, and conjugated equine estrogen randomization assignment were used as covariates.
p value for interaction between resting heart rate and systolic blood pressure.
Reference group: RHR < 80 BPM.
CI, confidence interval; BMI, body mass index; RHR, resting heart rate.
Compared to those with an RHR < 80 BPM and an SBP < 120 mm Hg, those with an RHR ≥ 80 BPM and an SBP ≥ 140 mm Hg had nearly a 4-fold (OR 3.91, 95% CI 1.55-9.88) higher odds for the presence of any CAC, and the odds were 0.89 (95% CI 0.44-1.81) among those with an RHR ≥ 80 BPM but an SBP < 120 mm Hg. Similarly, those with an RHR ≥ 70 BPM and an SBP ≥ 130 mm Hg had >2-fold (OR 1.92, 95% CI 1.11-3.32) higher odds for any CAC, while the odds were 0.83 (95% CI 0.52-1.31) among those with an RHR ≥ 70 BPM but an SBP < 120 mm Hg. Similar results were seen for women with persistently high SBP and persistently high RHR over the course of the ET. That is, women with a persistent RHR ≥ 70 BPM and SBP ≥ 130 mm Hg had >2-fold (OR 2.26, 95% CI 1.25-4.09) higher odds for any CAC, while the odds were 1.19 (95% CI 0.74-1.93) among those with an RHR ≥ 70 BPM but an SBP < 120 mm Hg.
Discussion
In this study of women with a history of hysterectomy and between the ages of 50 and 59 at enrollment in the WHI, RHR, in both continuous and categorical distributions and after adjustment for age and ethnicity, was modestly yet significantly associated with the presence of CAC. With further adjustment for the traditional CVD risk factors and other significant covariates, however, this association was no longer statistically significant. Notably, there was a significant interaction between RHR and SBP for the presence of CAC. Specifically, women with higher levels of both RHR (i.e., ≥80 BPM) and SBP (i.e., ≥140 mm Hg) were significantly more likely to have any CAC compared to those with lower levels of these variables. Women with higher levels of RHR but normal/lower levels of SBP did not have higher odds for CAC, suggesting that an elevated BP is necessary for the increased risk to be associated with higher levels of RHR.
The association between RHR and CAC was not independent of the traditional CVD risk factors. This suggests that the association between RHR and CAC may be confounded by these risk factors. We explored this hypothesis by examining the effect of each of the individual risk factors on the association between RHR and CAC. In these analyses, the addition of BMI or smoking to a model containing RHR, age, and ethnicity caused the odds for CAC (per increment in RHR) to become nonsignificant. The other risk factors did not materially affect the association between RHR and CAC. These results are similar to findings of a study that examined the ability of RHR to enhance the ability of the Systematic COronary Risk Evaluation (SCORE) system to discriminate 10-year risk of CVD mortality.27 In this study, the addition of RHR to the full SCORE formula did not improve the area under the curve (AUC) or net reclassification index. However, addition of RHR to a reduced formula that contained age, smoking, gender, and BMI did cause significant improvement in discrimination. Combined with our results, these findings suggest that RHR may be used with easily obtained measures to cost-effectively classify future risk of CVD mortality.
Previous studies have demonstrated an association between cigarette smoking and higher RHR,28,29 and smokers have been shown to have a higher prevalence and levels of CAC.30 Similarly, individuals with higher BMI have been shown to have higher RHR,31 potentially due to increased sympathetic nervous system activation.32,33 Higher BMI has also been associated with a significantly increased odds for having CAC.34 Because it seems more physiologically plausible that smoking and increased body mass result in a higher RHR (instead of vice-versa), and both of these variables were significantly associated with CAC in our study population, we believe smoking and BMI are confounders rather than downstream mediators of the association between RHR and CAC. Thus, RHR could serve as a potential intermediate (and easily measurable) marker of increased risk for coronary artery disease among smokers and those with increased BMI. Notably, as the current study was cross-sectional and not prospective in nature, conclusions about potential mediation effects of these variables can only be made on the basis of existing knowledge of physiology.
An intriguing finding from our study was the significant interaction between RHR and SBP for CAC. As mentioned, women with higher levels for both RHR and SBP had significantly higher odds for the presence of CAC, and this association was dependent on having an SBP > 140 mm Hg. These findings support previous studies that have demonstrated a significantly increased risk for mortality and CHD among those with hypertension35 or prehypertension19 and who also have elevated levels of RHR. Interestingly (and similar to our findings), in the latter study, an RHR ≥ 80 BPM was associated with a significantly higher risk for incident CHD. As increased sympathetic activation can cause higher levels of both RHR and SBP,36 it is possible that this activation may be an important and common contributor to the significantly increased odds for CAC among those who have simultaneously higher levels of RHR and SBP. Indeed, previous studies have demonstrated a significant association between elevated HR and accelerated arterial stiffening among people with hypertension.37
Previous studies on the association between heart rate and CAC are limited. In fact, we were not able to find any reports that primarily studied the association between RHR and CAC. Of those studies that examined heart rate and CAC, most looked at the association between exercise-induced changes in heart rate and prevalent CAC. Findings from these studies indicate a strong inverse association between both the chronotropic response and heart rate recovery with the presence and burden of CAC,38 although this association may be attenuated among young adults.39 Notably, the Heinz Nixdorf Recall study38 tested the association between RHR and CAC within its study of exercise and CAC and found, among women, a significantly higher odds of CAC for each 10-BPM increment in RHR, independent of age, sex, and CVD risk factors (OR 1.11, p=0.02). The results of our study extend these findings and suggest that RHR may be a clinically relevant measure that is easy to perform and can be reliably obtained in a short period of time.
The strengths of this study include prospective and systematic collection of subject characteristics and the breadth of variables available for analysis. Conversely, this study is limited by the sample size of the studied cohort and the relatively low and limited distribution of the coronary calcium scores. Although the WHI CACS study was conducted using women enrolled in a randomized clinical trial, the current analysis was observational and cross-sectional in nature and was conducted using a subset of women in the WHI ET clinical trial. Therefore, there could be residual confounding or bias affecting the results. We have attempted to address this issue by considering as many potential confounding variables as possible in the analysis. Finally, the results of this study are limited to those women who are recently postmenopausal and have a history of hysterectomy.
Acknowledgments
We are indebted to the WHI participants for their extraordinary commitment to women's health research. The list of WHI investigators and affiliated staff follows.
Program Office: National Heart, Lung, and Blood Institute, Bethesda, MD: Jacques Rossouw, Shari Ludlam, Joan McGowan, Leslie Ford, and Nancy Geller.
Clinical Coordinating Center: Fred Hutchinson Cancer Research Center, Seattle, WA: Ross Prentice, Garnet Anderson, Andrea LaCroix, Charles L. Kooperberg; Medical Research Labs, Highland Heights, KY: Evan Stein; University of California at San Francisco, San Francisco, CA: Steven Cummings.
Clinical Centers: Albert Einstein College of Medicine, Bronx, NY: Sylvia Wassertheil-Smoller; Baylor College of Medicine, Houston, TX: Haleh Sangi-Haghpeykar; Brigham and Women's Hospital, Harvard Medical School, Boston, MA: JoAnn E. Manson; Brown University, Providence, RI: Charles B. Eaton; Emory University, Atlanta, GA: Lawrence S. Phillips; Fred Hutchinson Cancer Research Center, Seattle, WA: Shirley Beresford; George Washington University Medical Center, Washington, DC: Lisa Martin; Los Angeles Biomedical Research Institute at Harbor- UCLA Medical Center, Torrance, CA: Rowan Chlebowski; Kaiser Permanente Center for Health Research, Portland, OR: Erin LeBlanc; Kaiser Permanente Division of Research, Oakland, CA: Bette Caan; Medical College of Wisconsin, Milwaukee, WI: Jane Morley Kotchen; MedStar Research Institute/Howard University, Washington, DC: Barbara V. Howard; Northwestern University, Chicago/Evanston, IL: Linda Van Horn; Rush Medical Center, Chicago, IL: Henry Black; Stanford Prevention Research Center, Stanford, CA: Marcia L. Stefanick; State University of New York at Stony Brook, NY: Dorothy Lane; The Ohio State University, Columbus, OH: Rebecca Jackson; University of Alabama at Birmingham, AL: Cora E. Lewis; University of Arizona, Tucson/Phoenix, AZ: Cynthia A. Thomson; University at Buffalo, NY: Jean Wactawski-Wende; University of California at Davis, Sacramento, CA: John Robbins; University of California at Irvine, CA: F. Allan Hubbell; University of California at Los Angeles, CA: Lauren Nathan; University of California at San Diego, LaJolla/Chula Vista, CA: Robert D. Langer; University of Cincinnati, Cincinnati, OH: Margery Gass; University of Florida, Gainesville/Jacksonville, FL: Marian Limacher; University of Hawaii, Honolulu, HI: J. David Curb; University of Iowa, Iowa City/Davenport, IA: Robert Wallace; University of Massachusetts/Fallon Clinic, Worcester, MA: Judith Ockene; University of Medicine and Dentistry of New Jersey, Newark, NJ: Norman Lasser; University of Miami, Miami, FL: Mary Jo O'Sullivan; University of Minnesota, Minneapolis, MN: Karen Margolis; University of Nevada, Reno, NV: Robert Brunner; University of North Carolina, Chapel Hill, NC: Gerardo Heiss; University of Pittsburgh, Pittsburgh, PA: Lewis Kuller; University of Tennessee Health Science Center, Memphis, TN: Karen C. Johnson; University of Texas Health Science Center, San Antonio, TX: Robert Brzyski; University of Wisconsin, Madison, WI: Gloria E. Sarto; Wake Forest University School of Medicine, Winston-Salem, NC: Mara Vitolins; Wayne State University School of Medicine/Hutzel Hospital, Detroit, MI: Michael S. Simon.
The WHI program is funded by the National Heart, Lung, and Blood Institute, National Institutes of Health, U.S. Department of Health and Human Services through contracts N01WH22110, 24152, 32100–2, 32105–6, 32108–9, 32111–13, 32115, 32118–19, 32122, 42107–26, 42129–32, and 44221. Wyeth provided study pills (active and placebo) for the WHI CEE trial but had no other role in the study. This work was also supported in part by a grant from the American Heart Association (M.A.A).
Disclosure Statement
The authors have no financial or other conflicts of interest to report.
References
- 1.Monahan KD. Effect of aging on baroreflex function in humans. Am J Physiol Regul Integr Comp Physiol. 2007;293:R3–12. doi: 10.1152/ajpregu.00031.2007. [DOI] [PubMed] [Google Scholar]
- 2.Futterman LG. Lemberg L. Heart rate is a simplistic marker of mortality in acute myocardial infarction. Am J Crit Care. 1999;8:197–199. [PubMed] [Google Scholar]
- 3.Palatini P. Julius S. Heart rate and the cardiovascular risk. J Hypertens. 1997;15:3–17. doi: 10.1097/00004872-199715010-00001. [DOI] [PubMed] [Google Scholar]
- 4.Hsia J. Larson JC. Ockene JK, et al. Resting heart rate as a low tech predictor of coronary events in women: Prospective cohort study. BMJ. 2009;338:b219. doi: 10.1136/bmj.b219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Greenland P. Daviglus ML. Dyer AR, et al. Resting heart rate is a risk factor for cardiovascular and noncardiovascular mortality: The Chicago Heart Association Detection Project in Industry. Am J Epidemiol. 1999;149:853–862. doi: 10.1093/oxfordjournals.aje.a009901. [DOI] [PubMed] [Google Scholar]
- 6.Aboyans V. Criqui MH. Can we improve cardiovascular risk prediction beyond risk equations in the physician's office? J Clin Epidemiol. 2006;59:547–558. doi: 10.1016/j.jclinepi.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 7.Dyer AR. Persky V. Stamler J, et al. Heart rate as a prognostic factor for coronary heart disease and mortality: Findings in three Chicago epidemiologic studies. Am J Epidemiol. 1980;112:736–749. doi: 10.1093/oxfordjournals.aje.a113046. [DOI] [PubMed] [Google Scholar]
- 8.Kaplan JR. Manuck SB. Clarkson TB. The influence of heart rate on coronary artery atherosclerosis. J Cardiovasc Pharmacol. 1987;10(Suppl 2):S100–102. [PubMed] [Google Scholar]
- 9.Demer LL. Tintut Y. Mineral exploration: Search for the mechanism of vascular calcification and beyond: The 2003 Jeffrey M. Hoeg Award lecture. Arterioscler Thromb Vasc Biol. 2003;23:1739–1743. doi: 10.1161/01.ATV.0000093547.63630.0F. [DOI] [PubMed] [Google Scholar]
- 10.Stary HC. Natural history of calcium deposits in atherosclerosis progression and regression. Z Kardiol. 2000;89(Suppl 2):28–35. doi: 10.1007/s003920070097. [DOI] [PubMed] [Google Scholar]
- 11.Allison MA. Criqui MH. Wright CM. Patterns and risk factors for systemic calcified atherosclerosis. Arterioscler Thromb Vasc Biol. 2004;24:331–336. doi: 10.1161/01.ATV.0000110786.02097.0c. [DOI] [PubMed] [Google Scholar]
- 12.Budoff M. Comparison of spiral and electron beam tomography in the evaluation of coronary calcification in asymptomatic persons. Int J Cardiol. 2002;82:299. doi: 10.1016/s0167-5273(00)00449-6. [DOI] [PubMed] [Google Scholar]
- 13.Sangiorgi G. Rumberger JA. Severson A, et al. Arterial calcification and not lumen stenosis is highly correlated with atherosclerotic plaque burden in humans: A histologic study of 723 coronary artery segments using nondecalcifying methodology. J Am Coll Cardiol. 1998;31:126–133. doi: 10.1016/s0735-1097(97)00443-9. [DOI] [PubMed] [Google Scholar]
- 14.Haberl R. Becker A. Leber A, et al. Correlation of coronary calcification and angiographically documented stenoses in patients with suspected coronary artery disease: Results of 1,764 patients. J Am Coll Cardiol. 2001;37:451–457. doi: 10.1016/s0735-1097(00)01119-0. [DOI] [PubMed] [Google Scholar]
- 15.Budoff MJ. Shaw LJ. Liu ST, et al. Long-term prognosis associated with coronary calcification: Observations from a registry of 25,253 patients. J Am Coll Cardiol. 2007;49:1860–1870. doi: 10.1016/j.jacc.2006.10.079. [DOI] [PubMed] [Google Scholar]
- 16.Kondos GT. Hoff JA. Sevrukov A, et al. Electron-beam tomography coronary artery calcium and cardiac events: A 37-month follow-up of 5635 initially asymptomatic low- to intermediate-risk adults. Circulation. 2003;107:2571–2576. doi: 10.1161/01.CIR.0000068341.61180.55. [DOI] [PubMed] [Google Scholar]
- 17.Polonsky TS. McClelland RL. Jorgensen NW, et al. Coronary artery calcium score and risk classification for coronary heart disease prediction. JAMA. 2010;303:1610–1616. doi: 10.1001/jama.2010.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Erbel R. Mohlenkamp S. Moebus S, et al. Coronary risk stratification, discrimination, and reclassification improvement based on quantification of subclinical coronary atherosclerosis: The Heinz Nixdorf Recall Study. J Am Coll Cardiol. 2010;56:1397–1406. doi: 10.1016/j.jacc.2010.06.030. [DOI] [PubMed] [Google Scholar]
- 19.King DE. Everett CJ. Mainous AG., 3rd Liszka HA. Long-term prognostic value of resting heart rate in subjects with prehypertension. Am J Hypertens. 2006;19:796–800. doi: 10.1016/j.amjhyper.2006.01.019. [DOI] [PubMed] [Google Scholar]
- 20.Anderson GL. Manson J. Wallace R, et al. Implementation of the Women's Health Initiative study design. Ann Epidemiol. 2003;13:S5–17. doi: 10.1016/s1047-2797(03)00043-7. [DOI] [PubMed] [Google Scholar]
- 21.Curb JD. McTiernan A. Heckbert SR, et al. Outcomes ascertainment and adjudication methods in the Women's Health Initiative. Ann Epidemiol. 2003;13:S122–128. doi: 10.1016/s1047-2797(03)00048-6. [DOI] [PubMed] [Google Scholar]
- 22.Hsia J. Wu L. Allen C, et al. Physical activity and diabetes risk in postmenopausal women. Am J Prev Med. 2005;28:19–25. doi: 10.1016/j.amepre.2004.09.012. [DOI] [PubMed] [Google Scholar]
- 23.Carr JJ. Nelson JC. Wong ND, et al. Calcified coronary artery plaque measurement with cardiac CT in population-based studies: Standardized protocol of Multi-Ethnic Study of Atherosclerosis (MESA) and Coronary Artery Risk Development in Young Adults (CARDIA) study. Radiology. 2005;234:35–43. doi: 10.1148/radiol.2341040439. [DOI] [PubMed] [Google Scholar]
- 24.Agatston AS. Janowitz WR. Hildner FJ. Zusmer NR. Viamonte M., Jr Detrano R. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol. 1990;15:827–832. doi: 10.1016/0735-1097(90)90282-t. [DOI] [PubMed] [Google Scholar]
- 25.Allison MA. Manson JE. Langer RD, et al. Oophorectomy, hormone therapy, and subclinical coronary artery disease in women with hysterectomy: The Women's Health Initiative coronary artery calcium study. Menopause. 2008;15:639–647. doi: 10.1097/gme.0b013e31816d5b1c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Manson JE. Allison MA. Rossouw JE, et al. Estrogen therapy and coronary-artery calcification. N Engl J Med. 2007;356:2591–2602. doi: 10.1056/NEJMoa071513. [DOI] [PubMed] [Google Scholar]
- 27.Cooney MT. Vartiainen E. Laatikainen T. Joulevi A. Dudina A. Graham I. Simplifying cardiovascular risk estimation using resting heart rate. Eur Heart J. 2010;31:2141–2147. doi: 10.1093/eurheartj/ehq164. [DOI] [PubMed] [Google Scholar]
- 28.Gilbert DG. Spielberger CD. Effects of smoking on heart rate, anxiety, and feelings of success during social interaction. J Behav Med. 1987;10:629–638. doi: 10.1007/BF00846659. [DOI] [PubMed] [Google Scholar]
- 29.Parrott AC. Winder G. Nicotine chewing gum (2 mg, 4 mg) and cigarette smoking: Comparative effects upon vigilance and heart rate. Psychopharmacology. 1989;97:257–261. doi: 10.1007/BF00442260. [DOI] [PubMed] [Google Scholar]
- 30.Bild DE. Detrano R. Peterson D, et al. Ethnic differences in coronary calcification: The Multi-Ethnic Study of Atherosclerosis (MESA) Circulation. 2005;111:1313–1320. doi: 10.1161/01.CIR.0000157730.94423.4B. [DOI] [PubMed] [Google Scholar]
- 31.Valentini M. Parati G. Variables influencing heart rate. Prog Cardiovasc Dis. 2009;52:11–19. doi: 10.1016/j.pcad.2009.05.004. [DOI] [PubMed] [Google Scholar]
- 32.Julius S. Valentini M. Palatini P. Overweight and hypertension: A 2-way street? Hypertension. 2000;35:807–813. doi: 10.1161/01.hyp.35.3.807. [DOI] [PubMed] [Google Scholar]
- 33.Sztajzel J. Golay A. Makoundou V, et al. Impact of body fat mass extent on cardiac autonomic alterations in women. Eur J Clin Invest. 2009;39:649–656. doi: 10.1111/j.1365-2362.2009.02158.x. [DOI] [PubMed] [Google Scholar]
- 34.Allison MA. Michael Wright C. Body morphology differentially predicts coronary calcium. Int J Obes Relat Metab Disord. 2004;28:396–401. doi: 10.1038/sj.ijo.0802571. [DOI] [PubMed] [Google Scholar]
- 35.Tierney WM. Brunt M. Kesterson J. Zhou XH. L'Italien G. Lapuerta P. Quantifying risk of adverse clinical events with one set of vital signs among primary care patients with hypertension. Ann Fam Med. 2004;2:209–217. doi: 10.1370/afm.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Grassi G. Assessment of sympathetic cardiovascular drive in human hypertension: Achievements and perspectives. Hypertension. 2009;54:690–697. doi: 10.1161/HYPERTENSIONAHA.108.119883. [DOI] [PubMed] [Google Scholar]
- 37.Benetos A. Adamopoulos C. Bureau J-M, et al. Determinants of accelerated progression of arterial stiffness in normotensive subjects and in treated hypertensive subjects over a 6-year period. Circulation. 2002;105:1202–1207. doi: 10.1161/hc1002.105135. [DOI] [PubMed] [Google Scholar]
- 38.Möhlenkamp S. Lehmann N. Schmermund A, et al. Association of exercise capacity and the heart rate profile during exercise stress testing with subclinical coronary atherosclerosis: Data from the Heinz Nixdorf Recall study. Clin Res Cardiol. 2009;98:665–676. doi: 10.1007/s00392-009-0054-9. [DOI] [PubMed] [Google Scholar]
- 39.Kizilbash M. Carnethon M. Chan C, et al. The association of heart rate recovery immediately after exercise with coronary artery calcium: The Coronary Artery Risk Development in Young Adults study. Clin Autonom Res. 2007;17:46–49. doi: 10.1007/s10286-006-0391-y. [DOI] [PMC free article] [PubMed] [Google Scholar]