Abstract
The vasodilatory effect of cinnamaldehyde was investigated for its mechanism of action using isolated rings of rat aorta. Cinnamaldehyde relaxed aortic rings precontracted with phenylephrine in a dose-dependent manner, was not affected by either the presence or removal of the endothelium. Pretreatment with NG-nitro-L-arginine methyl ester and 1H-[1,2,4]-oxadiazole-[4,3-a]-quinoxalin-1-one could not block vasodilation by cinnamaldehyde, indicating that nitric oxide signaling is not involved. Potassium channel blockers, such as glibenclamide, tetraethylammonium, and BaCl2, had no effect on the relaxation produced by cinnamaldehyde. In addition, treatment with either indomethacin or propranolol did not affect cinnamaldehyde-induced vasodilatation. On the other hand, pretreatment of endothelium-denuded rings with cinnamaldehyde significantly inhibited vasoconstriction induced by endogenous vasoconstrictors, including angiotensin II, 5-hydroxytryptamine, dopamine, endothelin-1, and phenylephrine. In a Ca2+-free experimental setting, this natural vasodilator not only blocked Ca2+ influx-dependent vasoconstriction by either phenylephrine or KCl, but also inhibited phenylephrine-induced tonic contraction, which relies on intracellular Ca2+ release. This study shows that endothelium-independent, Ca2+ influx and/or an inhibitory release mechanism contributes to the vasodilatory effect of cinnamaldehyde.
Keywords: cinnamaldehyde, vasodilation, endothelium, vascular smooth muscle cell
Introduction
Cinnamomum cassia is a Chinese herbal medicine frequently used for its multiple therapeutic functions, such as enhancing immunity, eliminating the sense of coldness, relieving pain and improving blood circulation.1 In modern pharmacological research, C. cassia has exhibited diverse actions, including antioxidative stress,2 preventing mitochondrial dysfunction,3 antitumor properties,4 and inhibition of tau protein aggregation in Alzheimer’s disease.5 Cinnamaldehyde, one of the main constituents of C. cassia, is an aromatic aldehyde which has been reported to have multiple potential therapeutic activities.6 Ma et al found that cinnamaldehyde could decrease production of prostaglandin E2 stimulated by interleukin-1β and could downregulate the expression of transient receptor potential vanilloid subtype 4 in the cerebral microvascular endothelial cells of the mouse, which may contribute to its antipyretic effects.7 Chao et al has reported that cinnamaldehyde has antioxidant and anti-inflammatory properties. Low concentrations of cinnamaldehyde can inhibit secretion of interleukin-1β, tumor necrosis factor α, and reduce reactive oxygen species in lipopolysaccharide-stimulated J774 A.1 macrophages. The phosphorylation of extracellular signal-regulated kinase 1/2 and c-Jun N-terminal kinase 1/2 induced by lipopolysaccharides was also inhibited.8 In addition, Liao et al found that cinnamaldehyde inhibits adhesion of tumor necrosis factor α-induced monocytes to endothelial cells, and suppresses the expression of vascular cell adhesion molecule-1 and intercellular adhesion molecule-1 at the transcriptional level by suppressing nuclear transcription factor κB activation.9
With regard to the circulation, several studies have shown that cinnamaldehyde has antiplatelet and antithrombotic activity.10,11 In anesthetized rats, cinnamaldehyde decreased blood pressure, left ventricular systolic pressure, and rate of change of left ventricular maximum pressure (dp/dtmax).12 Cinnamaldehyde also showed a dose-dependent relaxation of the rat aorta contraction induced by noradrenaline, potassium, and prostaglandin F2α.12,13 Based on the above observations, we hypothesized that the cardiovascular effect of cinnamaldehyde may be due to signaling beyond the receptor level. In the present study, we systematically evaluated the vasodilatory effects of cinnamaldehyde in isolated rat aorta rings using pharmacological methods and explored its potential mechanism of action.
Methods and materials
Reagents
Cinnamaldehyde (C9H8O, Figure 1) was purchased from Aladdinbiotech Company (Shanghai, China). Acetylcholine, phenylephrine, NG-nitro-L-arginine methyl ester (L-NAME), 1H-[1,2,4]-oxadiazole-[4,3-a]-quinoxalin-1-one (ODQ), indomethacin, propranolol, glibenclamide, tetraethylammonium, BaCl2, angiotensin II, 5-hydroxytryptamine, dopamine, and endothelin-1 were purchased from Sigma Chemical Co (St Louis, MO). Ethyleneglycol bis (2-aminoethyl ether) tetra-acetic acid (EGTA) and other inorganic salts were all purchased from Sinopharm Chemical Reagent Co Ltd (Shanghai, China). Acetylcholine, phenylephrine, L-NAME, tetraethylammonium, propranolol, angiotensin II, 5-hydroxytryptamine, dopamine, and endothelin-1 were dissolved in distilled water; indomethacin, glibenclamide, and ODQ were dissolved in dimethyl sulfoxide. Control experiments had demonstrated that the highest dimethyl sulfoxide level (1:400) had no effect on vascular tone.
Figure 1.
A) The Cinnamomum cassia plant. B) The chemical structure of cinnamaldehyde.
Animals and vascular ring preparation
Male Sprague-Dawley rats weighing 250 to 300 g were purchased from the Shanghai Experimental Animal Center of Academia Sinica and used for all experiments. Animals were handled and cared in compliance with the Guide for the Care and Use of Laboratory Animals (Shanghai University of Tradition Chinese Medicine).
The rats were killed by cervical dislocation and their thoracic aortas were rapidly removed and dissected in ice-cold Krebs solution (pH 7.4, containing [mM] NaCl 118, 25, KCl 4.7, MgSO4 1.1, KH2PO4 1.2, CaCl2 1.5, NaHCO3 and glucose 10). The aortas were cut into 3 mm-wide ring segments after removing the surrounding connective tissue and fat. All dissection procedures were done with extreme care to protect the endothelium from inadvertent damage. In some aortic rings, the endothelial layer was mechanically removed by gently rubbing the luminal surface of the aortic ring back and forth several times with a wooden tooth pick. Each ring was suspended with two L-shaped stainless steel wires in a 4 mL organ bath filled with Krebs solution and maintained at 37°C. The upper wire was connected to a force displacement transducer (Grass Instruments, West Warwick, RI) and the lower one fixed at the bottom of the organ bath. The bath solution was continuously bubbled with 95% O2 and 5% CO2. The baseline load placed on the aortic ring was 2.0 g.
Examination of endothelial integrity
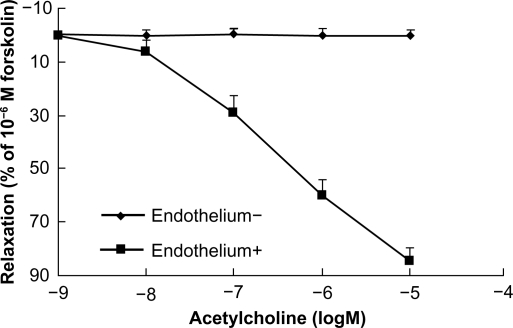
At the beginning of each experiment, the bath solution was replaced every 20 minutes with prewarmed and oxygenated Krebs solution. After equilibrating for 60 minutes, all aortic rings were contracted twice with KCl 60 mM to obtain a maximal response, and the rings were washed three times at 20-minute intervals with Krebs solution. After restoration of vessel tension to baseline levels, the rings were exposed to phenylephrine 10−6 M to test their contractile responses, and subsequently challenged with acetylcholine to verify endothelial integrity or functional removal. Thus, the endothelium was considered intact when 15%–20% relaxation (percentage of 10−6 M forskolin-evoked relaxation) was achieved by acetylcholine 10−7 M, 60% by acetylcholine 10−6 M, and >80% by acetylcholine 10−5 M in aorta rings pre-contracted using phenylephrine. When the endothelium was fully removed, <1% relaxation in response to acetylcholine 10−5 M could be recorded (Figure 2).
Figure 2.
Concentration-response curves showing dilation by acetylcholine of endothelium-intact and endothelium-denuded rings. Acetylcholine induced endothelium-dependent relaxation at the indicated dose in phenylephrine precontracted rat aortic rings (n = 6).
Vasodilation by cinnamaldehyde
The vasodilatory effect of cinnamaldehyde was tested in both endothelium-intact and endothelium-denuded rings contracted with phenylephrine 10−6 M. Once a plateau of phenylephrine contraction was obtained, cinnamaldehyde was applied cumulatively at concentrations of 10−7, 10−6, 10−5, and 10−4 g/mL. At the end of experiment, the relaxation induced by forskolin 10−6 M was recorded and used as 100% for blood vessel relaxation.
To understand the mechanisms of relaxation, L-NAME (a nitric oxide synthase inhibitor) and indomethacin (a cyclo-oxygenase inhibitor) were preincubated with an endothelium-intact ring for 25 minutes, and cinnamaldehyde-induced relaxation was observed. Preparations without endothelium were pretreated for 25 minutes with ODQ (a soluble guanylyl cyclase inhibitor), propranolol (an adrenergic β-receptor inhibitor), glibenclamide, (a KATP blocker), tetraethylammonium (a KCa blocker), and BaCl2 (a KIR blocker) prior to addition of phenylephrine 10−6 M. Concentration-dependent vasodilation by cinnamaldehyde was then examined.
Effect of cinnamaldehyde on vasoconstriction
The endothelium-denuded ring was first contracted in a concentration-dependent manner by a series of constrictors, including dopamine, 5-hydroxytryptamine, angiotensin II, K+, endothelin-1, and phenylephrine. After washing, the ring was incubated with cinnamaldehyde 1.3 × 10−5 g/mL or 5 × 10−5 g/mL for 10 minutes, and the contractions induced by the vasoconstrictors were again observed. The response to 60 mM K+ was used as 100% contraction.
Effect of cinnamaldehyde calcium influx
The aorta ring without endothelium was washed and treated with Ca2+-free high-K+ solution (containing 10−4 M EGTA and 60 mM KCl). The Ca2+-free incubated media preparation was then cumulatively contracted with CaCl2 at concentrations in the range 0.5–3.0 mM. The contractions induced by CaCl2 were compared between the group treated with cinnamaldehyde 1.3 × 10−5 g/mL and the controls. Contraction induced by 60 mM K+ in normal Ca2+ media was used as 100%.
Effect of cinnamaldehyde on calcium release
The endothelium-denuded ring was washed and exposed to Ca2+-free Krebs solution (containing 10−4 M EGTA) for 20 minutes. Phenylephrine 10−6 M was added and a small tonic contraction mainly due to the release of intracellular Ca2+ was observed. Comparison between the group treated with cinnamaldehyde 1.3 × 10−5 g/mL and the controls was made, with contraction by 60 mM K+ in normal Ca2+ media used as 100%.
Statistical analysis
All data were expressed as means ± standard error, and analyzed using one-way analysis of variance. P < 0.05 was used as the significance level for statistical tests.
Results
Cinnamaldehyde-induced relaxation
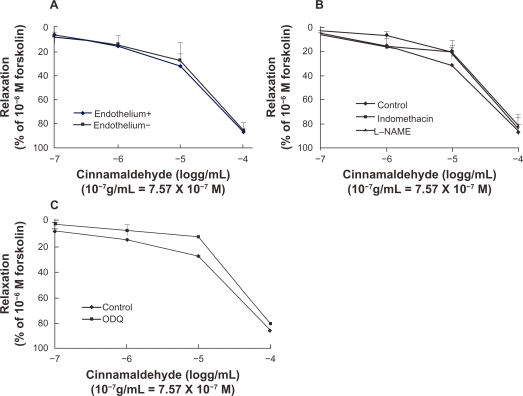
Despite a significant difference in acetylcholine-induced relaxation in aorta ring tissue with or without endothelium (Figure 2), cinnamaldehyde relaxed the blood vessels in an endothelium-independent manner. Maximum relaxation of the vessel with and without endothelium by cinnamaldehyde was 86.78% and 85.71%, respectively, and the EC50 was 1.16 × 10−5 g/mL and 1.32 × 10−5 g/mL, respectively (Figure 3A).
Figure 3.
Dose-dependent vasodilatory effect of cinnamaldehyde on rat aorta rings. Cinnamaldehyde dilated both endothelium-intact and endothelium-denuded rings precontracted with phenylephrine, in a dose-dependent manner. The effect of cinnamaldehyde on this dilation was not different between the two groups (A, n = 6). Endothelium-intact rings preincubated with NG-nitro-L-arginine methyl ester (L-NAME) 10−4 M or indomethacin 10−5 M did not affect cinnamaldehyde function (B, n = 6). Endothelium-denuded rings preincubated with 1H-[1,2,4]-oxadiazole-[4,3-a]-quinoxalin-1-one (ODQ) 10−5 M did not inhibit the effects of cinnamaldehyde (C, n = 6).
To verify further the involvement of nitric oxide/cyclic guanosine monophosphate signaling pathway, we pretreated endothelium-intact aortic rings with L-NAME 10−4 M or ODQ 10−5 M. Neither the nitric oxide synthase inhibitor nor the soluble guanylyl cyclase blocker affected cinnamaldehyde-induced vasodilation (Figures 3B and 3C).
To understand the involvement of the cyclooxygenase/prostaglandin I2 pathway, indomethacin 10−5 M was used. The relaxation curve for cinnamaldehyde was not affected by indomethacin (Figure 3B).
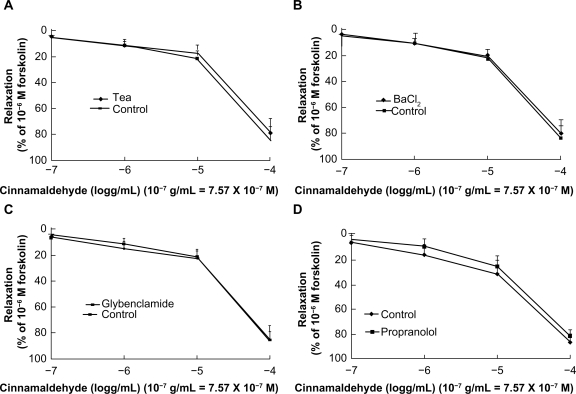
Effect of cinnamaldehyde on potassium channels and β-receptors
To test for possible involvement of K+ channels in cinnamaldehyde-induced relaxation, we preincubated endothelium-denuded rings with tetraethylammonium 3 × 10−3 M, BaCl2 10−4 M, and glibenclamide 10−5 M, each for 25 minutes. Tetraethylammonium (Figure 4A), BaCl2 (Figure 4B), and glibenclamide (Figure 4C) did not inhibit vascular relaxation by cinnamaldehyde. We also used propranolol 10−5 M to preincubate the endothelium-denuded rings, which did not inhibit vascular relaxation induced by cinnamaldehyde (Figure 4D).
Figure 4.
Effect of cinnamaldehyde on K+ channels and the β-receptor. After preincubation with tetraethylammonium 3 × 10−3 M, BaCl2 10−4 M, or glibenclamide 10−5 M for 25 minutes, the effects of cinnamaldehyde were not inhibited by tetraethylammonium (A, n = 6), BaCl2 (B, n = 6), or glibenclamide (C, n = 6). After preincubation with propranolol 10−5 M, the vasodilatory effect of cinnamaldehyde was not inhibited (D, n = 6).
Effect of cinnamaldehyde on endogenous vasoconstrictors
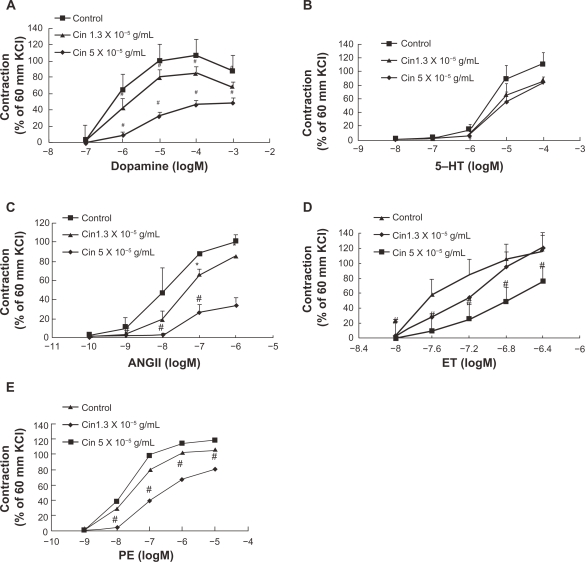
Dopamine, 5-hydroxytryptamine, angiotensin II, endothelin-1, and phenylephrine are all endogenous vasoconstrictors and play key roles in vascular tone. We wondered whether cinnamaldehyde relaxes blood vessel by blocking one of the above vasoconstrictors. We pretreated endothelium-denuded aorta rings with cinnamaldehyde at 1.3 × 10−5 g/mL (EC50 relaxation by cinnamaldehyde) and 5.0 × 10−5 g/mL, and found that cinnamaldehyde exerted inhibitory effects on the contraction curves of dopamine (Figure 5A), 5-hydroxytryptamine (Figure 5B), angiotensin II (Figure 5C), and endothelin-1 (Figure 5D), and phenylephrine (Figure 5E).
Figure 5.
Concentration-response curves showing the vasoconstriction of dopamine A), 5-hydroxytryptamine B), angiotensin II C), endothelin-1 D), and phenylephrine E) in the absence or presence of cinnamaldehyde (Cin). The contraction curves of all the vasoconstrictors can be inhibited by cinnamaldehyde at the indicated concentration.
Notes: *P < 0.05 versus controls, #P < 0.01 versus controls, n = 6.
Effect of cinnamaldehyde on calcium influx or release
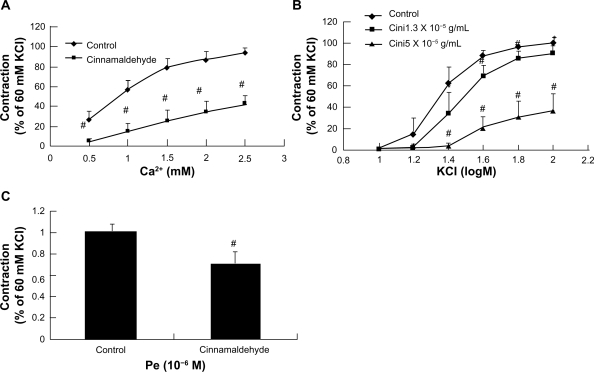
Phenylephrine contracts vascular smooth muscle mainly by activation of receptor-operated Ca2+ channels, while KC1 mainly activates potential-dependent Ca2+ channels, all of which result in calcium influx.14 In this study, cinnamaldehyde pretreatment significantly reduced vasoconstriction by phenylephrine (Figure 5E) and KC1 (Figure 6B).
Figure 6.
Effect of cinnamaldehyde on the Ca2+ channel. Rat aortic rings were preincubated with or without cinnamaldehyde for 10 minutes, and the curves of CaCl2 in Ca2+-free solution containing 10−4 M EGTA and 60 mM KCl were inhibited by cinnamaldehyde (A, #P < 0.01 versus controls, n = 6). Comparison of contraction percentage between the cinnamaldehyde-preincubated and control groups on cumulative concentration-response curves of KCl. The constriction curve of KCl was inhibited by cinnamaldehyde (B, *P < 0.05 versus control, #P < 0.01 versus control, n = 6). Effect of cinnamaldehyde on the transient contraction effect of phenylephrine in Ca2+-free solution containing 10−4 M EGTA. With cinnamaldehyde preincubation for 10 minutes, the contraction effect of phenylephrine in Ca2+-free solution was inhibited by cinnamaldehyde (C, #P < 0.01 versus controls, n = 6).
To confirm the aforementioned observations, we tested the inhibitory effect of cinnamaldehyde on K+ -stimulated voltage-dependent Ca2+ influx during a Ca2+ -free experiment.14 As demonstrated in Figure 6A, Ca2+-induced vasoconstrictions stimulated by K+ 60 mM were significantly suppressed by pretreatment with cinnamaldehyde 1.3 × 10−5 g/mL.
When aorta rings were exposed to Ca2+-free media, addition of phenylephrine 10−6 M elicited a small tonic contraction induced mainly by intracellular Ca2+ release from endoplasmic reticulum stores.15 Pretreatment of endothelium-denuded rings with cinnamaldehyde 5.0 × 10−5 g/mL significantly reduced phenylephrine-induced contraction under extracellular Ca2+-free conditions (Figure 6C).
Discussion
Vascular endothelium plays a key role in maintaining normal function of the vasculature.15 Endothelial cells release endothelium-dependent vasodilators, such as nitric oxide and prostacyclin (prostaglandin I2), upon stimulation of various factors in the blood stream and as a result of physiological stress.15,16 Nitric oxide is mainly formed by nitric oxide synthase using L-arginine as a substrate. Diffusible nitric oxide gas penetrates vascular smooth muscle and activates soluble guanylyl cyclase which catalyzes guanosine triphosphate to form cyclic guanosine monophosphate. Cyclic guanosine monophosphate-activated protein kinase G inhibits the Ca2+ influx, reduces sensitivity of contractile elements to Ca2+, and relaxes the blood vessel.14 In our study, cinnamaldehyde-induced vasodilation was neither affected by removal of endothelium nor by treatment with L-NAME (Figure 3B) or ODQ (Figure 3C). These results suggest that the vasodilatory effect of cinnamaldehyde is not mediated through the nitric oxide/cyclic guanosine monophosphate pathway. The cyclo-oxygenase in either endothelial or smooth muscle cells can catalyze arachidonic acid to endoperoxide prostaglandin H2, which is finally converted into prostacyclin, which activates adenylyl cyclase and elevates cyclic adenosine monophosphate levels to relax the blood vessel.18 In the current study, relaxation by cinnamaldehyde in endothelium-intact aortic rings was not affected by indomethacin (Figure 3B), which rules out the involvement of the cyclo-oxygenase pathway.
Potassium channels regulate vascular smooth muscle tone by interfering with the cellular membrane potential.19 When the K+ channel is activated, an efflux of K+ causes membrane hyperpolarization, which reduces calcium influx and attenuates vascular tone.19 At least four types of K+ channels were identified in arterial smooth muscle cells, ie, the voltage-dependent K+ (KV) channel, activated by depolarizing stimuli; the Ca2+-activated K+ (KCa) channel which responds to intracellular Ca2+; the inward rectifier K+ (KIR) channel which may be responsible for external K+-induced dilation; and the adenosine triphosphate-sensitive K+ (KATP) channel which responds to changes in adenosine triphosphate levels. It is known that Ba2+ and tetraethylammonium antagonize a broad range of K+ channels, and glibenclamide can block KATP.20 To test for possible involvement of K+ channels in cinnamaldehyde-induced relaxation, we preincubated vessel preparations with tetraethylammonium 3 × 10−3 M (Figure 4A), BaCl2 10−4 M (Figure 4B), and glibenclamide 10−5 M (Figure 4C). We found that potassium channel blockers did not affect cinnamaldehyde-induced vasorelaxation.
The adrenergic β-receptor is an important contributor to vasodilation by increasing intracellular cyclic adenosine monophosphate and activating protein kinase A.21 However, in our study, propranolol 10−5 M had no effect on cinnamaldehyde (Figure 4D), suggesting that vasodilation is not mediated via the adrenergic β-receptor.
Endogenous vasoconstrictors not only play a key role in maintaining vascular tension, but also serve as therapeutic targets in many pathological conditions, such as hypertension. Blood vessels pretreated with cinnamaldehyde attenuated vasoconstriction by dopamine (Figure 5A), 5-hydroxytryptamine (Figure 5B), angiotensin II (Figure 5C), endothelin-1 (Figure 5D), and phenylephrine (Figure 5E) in a concentration-dependent manner. Thus, the involvement of a secondary signaling pathway beyond specific receptor levels is speculated. It has been shown that adrenalin, angiotensin, endothelin-1, and 5-hydroxytryptamine all have G protein-coupled receptors which can manipulate the intracellular Ca2+ concentration by interference with either Ca2+ influx or intracellular Ca2+release.22 Indeed, calcium channels appear to play a crucial role in contraction of vascular smooth muscle.20,23
Intracellular Ca2+ controls smooth muscle contraction through binding with calmodulin to form a calcium-calmodulin complex. This complex further activates myosin light chain kinase to phosphorylate myosin light chains and cause muscle contraction.24 The intracellular Ca2+ concentration can be regulated by extracellular Ca2+ influx through both the voltage-dependent Ca2+ channel and the receptor-operated Ca2+ channel, or by intracellular Ca2+ release from endoplasmic reticulum Ca2+ stores.15 KCl mainly activates potential-dependent Ca2+ channels to promote calcium influx and vasoconstriction. In our study, cinnamaldehyde markedly inhibited CaCl2-induced contraction of vessels treated with Ca2+-free high-K+ media (Figure 6A). In addition, cinnamaldehyde also inhibited concentration-dependent contraction by K+ (Figure 6B). Thus, at least, Ca2+ influx through voltage-sensitive Ca2+ channels is affected by cinnamaldehyde.
On the other hand, phenylephrine regulates intracellular Ca2+ through both receptor-operated calcium channels and intracellular Ca2+ release. As showed in Figure 5E, pretreatment with cinnamaldehyde significantly reduced phenylephrine contraction, suggesting possible interference with Ca2+ influx through receptor-operated calcium channels. Furthermore, cinnamaldehyde also markedly inhibited small tonic contractions elicited by phenylephrine in Ca2+-free media-treated rings (Figure 6C), indicating a blockage of intracellular Ca2+ release. Taken together, we propose that cinnamaldehyde relaxes the isolated rat aorta by inhibiting both Ca2+ influx and Ca2+ release. However, further experimentation with patch clamp methods is needed to confirm this mechanism in detail.
In conclusion, the present study demonstrates that cinnamaldehyde dilates vascular smooth muscle in an endothelium-independent manner. The vasodilatory effect of cinnamaldehyde may be related to its ability to interfere with both Ca2+ influx and Ca2+ release.
Acknowledgments
This study was supported by the Shanghai Committee of Science & Technology, E-Institutes for Nitric Oxide and Inflammatory Medicine, National Science and Technology Major Project of the Ministry of Science, and Technology of China.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Teng Jia-lin T, Li Xing-Guang, Le Yin-Min. Chinese Materia Medica. Beijing, China: People’s Medical Publishing House; 2007. Chinese–English bilingual textbooks for international students of Chinese TCM institutions. [Google Scholar]
- 2.Amin KA, Abd El-Twab TM. Oxidative markers, nitric oxide and homocysteine alteration in hypercholesterolemic rats: Role of atorvastatin and cinnamon. Int J Clin Exp Med. 2009;2(3):254–265. [PMC free article] [PubMed] [Google Scholar]
- 3.Panickar KS, Polansky MM, Anderson RA. Cinnamon polyphenols attenuate cell swelling and mitochondrial dysfunction following oxygen-glucose deprivation in glial cells. Exp Neurol. 2009;216(2):420–427. doi: 10.1016/j.expneurol.2008.12.024. [DOI] [PubMed] [Google Scholar]
- 4.Kwon HK, Jeon WK, Hwang JS, et al. Cinnamon extract suppresses tumor progression by modulating angiogenesis and the effector function of CD8+ T cells. Cancer Lett. 2009;278(2):174–182. doi: 10.1016/j.canlet.2009.01.015. [DOI] [PubMed] [Google Scholar]
- 5.Peterson DW, George RC, Scaramozzino F, et al. Cinnamon extract inhibits tau aggregation associated with Alzheimer’s disease in vitro. J Alzheimers Dis. 2009;17(3):585–597. doi: 10.3233/JAD-2009-1083. [DOI] [PubMed] [Google Scholar]
- 6.Wei RP, Huang YF, Hu DH, Zheng YG. Status and trends of researches on Cinnamomum cassia Presl. Nonwood Forest Research. 2006;24(3):65–70. [Google Scholar]
- 7.Ma YY, Huo HR, Li CH, et al. Effects of cinnamaldehyde on PGE2 release and TRPV4 expression in mouse cerebral microvascular endothelial cells induced by interleukin-1β. Biol Pharm Bull. 2008;31(3):426–430. doi: 10.1248/bpb.31.426. [DOI] [PubMed] [Google Scholar]
- 8.Chao LK, Hua KF, Hsu HY, et al. Cinnamaldehyde inhibits pro-inflammatory cytokine secretion from monocytes/macrophages through suppression of intracellular signaling. Food Chem Toxicol. 2008;46:220–231. doi: 10.1016/j.fct.2007.07.016. [DOI] [PubMed] [Google Scholar]
- 9.Liao BC, Hsieh CW, Liu YC, Tzeng TT, Sun YW, Wung BS. Cinnamaldehyde inhibits the tumor necrosis factor-α-induced expression of cell adhesion molecules in endothelial cells by suppressing NF-κB activation: Effects upon IκB and Nrf2. Toxicol Appl Pharmacol. 2008;292(2):161–171. doi: 10.1016/j.taap.2008.01.021. [DOI] [PubMed] [Google Scholar]
- 10.Huang J, Wang S, Luo X, et al. Cinnamaldehyde reduction of platelet aggregation and thrombosis in rodents. Thromb Res. 2007;119(3):337–342. doi: 10.1016/j.thromres.2006.03.001. [DOI] [PubMed] [Google Scholar]
- 11.Takenaga M, Hirai A, Terano T, et al. In vitro effect of cinnamic aldehyde, a main component of Cinnamomi cortex, on human platelet aggregation and arachidonic acid metabolism. J Pharmacobiodyn. 1987;10(5):201–208. doi: 10.1248/bpb1978.10.201. [DOI] [PubMed] [Google Scholar]
- 12.Xu Ming, Yu Lu, Ding Yuan-yuan, et al. Experimental study on hypotensive effects of cinnamaldehyde in anesthetized rats. Chin Heart J. 2006;28(3):272–276. [Google Scholar]
- 13.Yanaga A, Goto H, Nakagawa T, et al. Cinnamaldehyde induces endothelium-dependent and -independent vasorelaxant action on isolated rat aorta. Biol Pharm Bull. 2006;29(12):2415–2418. doi: 10.1248/bpb.29.2415. [DOI] [PubMed] [Google Scholar]
- 14.Zhang Tuan-xiao, Niu Cai-qin, Jing Hua-e, et al. Effect of water decoction of the Gastrodia elata BI on vasodiIation of rabbit aorta in vitro. Journal of Clinical Rehabilitative Tissue Engineering Research. 2008;13(1):203988–203991. [Google Scholar]
- 15.Bian K, Toda N. Reoxygenation and calcium-induced cerebroarterial contractions as affected by vasodilator agents. J Cereb Blood Flow Metab. 1988;8(6):808–815. doi: 10.1038/jcbfm.1988.136. [DOI] [PubMed] [Google Scholar]
- 16.Bian K, Doursout MF, Murad F. Vascular system: Role of nitric oxide in cardiovascular diseases. J Clin Hypertens (Greenwich) 2008;10(4):304–310. doi: 10.1111/j.1751-7176.2008.06632.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bian K, Murad F. Nitric oxide (NO) – biogeneration, regulation, and relevance to human diseases. Front Biosci. 2003;8:d264–d278. doi: 10.2741/997. [DOI] [PubMed] [Google Scholar]
- 18.Martin W, Villani GM, JotIlianandan D. Selective blockade of endothelium-dependent and glyceryl trinitrate-induced relaxation by hemoglobin and by methylene blue in the rabbit aorta. J Pharmacol Exp Ther. 1985;232(3):708–7l6. [PubMed] [Google Scholar]
- 19.Jia Hong-jun, Wang Zhong-ling, Yang Qi-dong. Ion Channels and Cardio-Cerebrovascular Diseases Basic Science and Clinical Practice. Beijing, China: People’s Health Press; 2001. [Google Scholar]
- 20.Bian K, Hermsmeyer K. Glyburide actions on the dihydropyridine-sensitive Ca2+ channel in rat vascular muscle. J Vasc Res. 1994;31(5):256–264. doi: 10.1159/000159051. [DOI] [PubMed] [Google Scholar]
- 21.Liu ZX. Practical Cardiovascular Receptorology. Beijing, China: Science Press; 2001. [Google Scholar]
- 22.Berridge MJ, Bootman MD, Roderick HL. Calcium signalling: Dynamics, homeostasis and remodelling. Nat Rev Mol Cell Biol. 2003;4(7):517–529. doi: 10.1038/nrm1155. [DOI] [PubMed] [Google Scholar]
- 23.Bian K, Hermsmeyer K. Ca2+ channel actions of the non-dihydropyridine Ca2+ channel antagonist Ro 40-5967 in vascular muscle cells cultured from dog coronary and saphenous arteries. Naunyn Schmiedebergs Arch Pharmacol. 1993;348(2):191–196. doi: 10.1007/BF00164798. [DOI] [PubMed] [Google Scholar]
- 24.Yao T. Physiology. Beijing, China: People’s Health Press; 2003. [Google Scholar]