Abstract
Background:
The role of histology in the targeted management of nonsmall cell lung cancer (NSCLC) has garnered renewed attention in recent years. We provide contemporary population-based estimates of survival and an assessment of important prognostic factors in stage IV NSCLC by major histologic subtype.
Methods:
Using data from the Surveillance, Epidemiology and End Results (SEER) Program, we stratified 51,749 incident stage IV NSCLC patients (1988–2003 with follow-up through 2006) by major histologic subtype. We used Kaplan–Meier and Cox proportional hazards methods to describe overall survival and the prognostic influence of select patient, tumor, and treatment characteristics for each histologic subgroup.
Results:
Survival was highest in patients with bronchioloalveolar adenocarcinoma (1-year survival: 29.1%) and lowest in those with large cell tumors (1-year survival: 12.8%). Diagnosis in later years, female gender, younger age, either Asian/Pacific Islander or Hispanic race/ethnicity, lower tumor grade, and surgery or beam radiation as part of first-line treatment were generally independently associated with a decreased risk of death, but the prognostic significance of some of these factors (age, ethnicity, tumor grade) varied according to histologic subtype.
Conclusion:
Findings demonstrate a poor prognosis across histologic subtypes in stage IV NSCLC patients but highlight differences in both absolute survival and the relative importance of select prognostic factors by histologic subclassification. More research using other sources of population-based data could help clarify the role of histology in the presentation, management, and prognosis of late-stage NSCLC.
Keywords: epidemiology, nonsmall cell lung cancer, histology, survival
Introduction
Lung cancer is the second most commonly diagnosed cancer in the US and accounts for the greatest number of cancer-related deaths in both men and women. In 2010, an estimated 222,520 people were diagnosed with lung cancer and nearly 157,300 died from this disease.1 Representing approximately 85% of lung cancer cases, nonsmall cell lung cancer (NSCLC) presents as metastatic disease in over half of all cases and is associated with a poor prognosis.2 The 5-year relative survival rate is just 17% for patients diagnosed with NSCLC and less than 4% in the subset presenting with distant metastases.2
Several studies have highlighted potentially important prognostic indicators of NSCLC survivorship but with somewhat conflicting conclusions, particularly with respect to demographic factors.3–9 Discordance in past research may partly be explained by relatively small study samples, narrow treatment inclusion criteria (eg, surgically treated patients), varying disease inclusion criteria with respect to tumor histology and stage, and limited geographic or population coverage. To our knowledge, no current or large-scale investigation has reported the relative significance of prognostic factors by NSCLC histologic subtype. In recent years, there has been marked progress in elucidating the molecular origins of NSCLC, better classifying histologic subtypes, and developing targeted therapies, prompting an increased understanding of NSCLC patients by histology.10–13 We undertook this study to describe the distribution of patient, tumor, and treatment characteristics and their influence on survival, overall and by histologic subtype, for patients newly diagnosed with stage IV NSCLC using data from the Surveillance, Epidemiology and End Results (SEER) Program.
Materials and methods
Study population
We conducted this retrospective cohort study using data collected by the National Cancer Institute’s SEER Program, an authoritative, population-based source of information on cancer incidence and survival in the US.14 SEER routinely gathers data on patient demographics, primary tumor site, tumor morphology and stage at diagnosis, first course of treatment, and follow-up for vital status. We limited our study to data collected by the first nine registries supported by SEER (Connecticut, Iowa, New Mexico, Utah, Hawaii, Atlanta, Detroit, San Francisco-Oakland, and Seattle-Puget Sound), representing nearly 10% of the US population, to maximize the time period covered and allow for an assessment of epidemiologic trends over time.
We selected patients with a first and only primary diagnosis of stage IV cancer of the lung and bronchus (C34.0–C34.9, International Classification of Diseases for Oncology, 3rd Edition [ICD-O-3])15 between January 1, 1988 and December 31, 2003. This case ascertainment period was chosen because these are the years for which SEER consistently coded stage of disease according to the third edition of the American Joint Committee on Cancer (AJCC) staging criteria. We restricted our analyses to patients diagnosed with nonsmall cell tumors and categorized them according to ICD-O-3 histologic subtype: squamous and transitional cell (8051–8052, 8070–8084, 8120–8131), adenocarcinoma (bronchioloalveolar adenocarcinoma [BAC]: 8250–8255 and non-BAC: 8050, 8140–8149, 8160–8162, 8190–8221, 8256–8263, 8270–8280, 8290–8337, 8350–8390, 8400–8560, 8570–8576, 8940–8941), large cell (8011–8015), and “other” (8010, 8020–8022, 8030–8040, 8046, 8090–8110, 8150–8156, 8170–8175, 8180, 8230–8231, 8240–8249, 8340–8347, 8561–8562, 8580–8671). We excluded patients who were identified by death certificate or autopsy only and those who died within 30 days after diagnosis (representing less than 8% of the otherwise eligible study population), because SEER reports survival time in months. Because this represents a publicly available dataset in which individual patient identification is not possible, informed consent by participating patients was not necessary.
Statistical analysis
We examined the distribution of incident stage IV NSCLC patients overall and by NSCLC histologic subtype according to time period of diagnosis and patient, tumor, and treatment characteristics. Data on vital status were available through December 31, 2006. The Kaplan–Meier16 method was used to describe overall 1- and 5-year survival for all stage IV NSCLC patients and for each histologic group by variables of interest. To ensure at least 5 years of patient follow-up for these estimates of survival, we limited these analyses to those diagnosed through December 31, 2001. Log-rank tests were used to assess the differences between select survival curves. Cox proportional hazards (PH) modeling17 was used to determine the independent influence of prognostic factors on the risk of death (from all causes) in stage IV NSCLC patients separately for each histologic subtype. Patients were followed from the date of diagnosis (1988–2003) to the date of death and were censored at the date they were last known to be alive (if lost to follow-up) or December 31, 2006, whichever came first. Log(-log) survival plots against time confirmed that proportionality assumptions were met. A two-sided P value <0.05 was considered statistically significant. The statistical software Stata/SE 9.1 for Windows (StataCorp LP, College Station, TX) was used for all analyses.
Results
Demographics by histologic subtype
A total of 51,749 patients diagnosed with stage IV NSCLC during 1988–2003 were included for study (Table 1). Median follow-up time was 4 months (range 1–225). Men predominated in all histologic subtypes (ranging from 56% in non-BAC adenocarcinoma to 69% in squamous) except in BAC tumors, where women represented 52% of the cases. Mean age at diagnosis ranged from 64.2 years in both non-BAC adenocarcinoma and large cell subtypes to 67.1 years in “other” NSCLC tumors. Although White patients accounted for at least 75% of cases in every histologic subtype, a higher proportion of BAC patients were Asian/Pacific Islanders (APIs) compared with the other histologic groups.
Table 1.
Distribution of stage IV nonsmall cell lung cancer (NSCLC) cases according to select variables by histologic subtype based on data from the nine Surveillance, Epidemiology and End Results Program registries
| Characteristica |
NSCLC histologic subtype |
Overall n = 51,749 (100%) | ||||
|---|---|---|---|---|---|---|
| Squamous n = 9370 (18%) |
Adenocarcinoma |
Large cell n = 4689 (9%) | Other n = 15,020 (29%) | |||
| Non-BAC n = 21,869 (42%) | BAC n = 801 (2%) | |||||
| Period of diagnosis | ||||||
| 1988–1992 | 35% | 29% | 24% | 40% | 22% | 29% |
| 1993–1997 | 29% | 31% | 30% | 31% | 27% | 29% |
| 1998–2003 | 36% | 41% | 47% | 29% | 51% | 42% |
| Gender | ||||||
| Male | 69% | 56% | 48% | 63% | 59% | 60% |
| Female | 31% | 44% | 52% | 37% | 41% | 40% |
| Age at diagnosis | ||||||
| <45 years | 2% | 5% | 4% | 4% | 3% | 4% |
| 45–54 years | 10% | 15% | 12% | 15% | 12% | 13% |
| 55–64 years | 25% | 28% | 24% | 30% | 24% | 26% |
| 65–74 years | 38% | 32% | 31% | 32% | 33% | 33% |
| 75–84 years | 21% | 17% | 24% | 17% | 23% | 20% |
| 85+ years | 3% | 2% | 5% | 2% | 5% | 3% |
| Mean (SD), years | 66.9 (10.3) | 64.2 (11.4) | 66.8 (11.9) | 64.2 (11.0) | 67.1 (11.6) | 65.6 (11.3) |
| Ethnicity/race | ||||||
| White | 77% | 79% | 75% | 79% | 78% | 78% |
| Black | 14% | 11% | 9% | 13% | 12% | 12% |
| American Indian/Alaskan Native | <1% | <1% | <1% | <1% | <1% | <1% |
| Asian/Pacific Islander | 5% | 7% | 12% | 4% | 6% | 6% |
| Spanish/Hispanic/Latino | 3% | 3% | 4% | 3% | 3% | 3% |
| Tumor grade at diagnosis | ||||||
| Well differentiated | 2% | 3% | 21% | <1% | <1% | 2% |
| Moderately differentiated | 18% | 11% | 11% | <1% | 1% | 8% |
| Poorly differentiated | 39% | 35% | 7% | 24% | 29% | 33% |
| Undifferentiated | 2% | 2% | 1% | 39% | 7% | 7% |
| Unknown | 40% | 49% | 60% | 37% | 64% | 51% |
| Cancer-directed surgery | ||||||
| None | 90% | 86% | 81% | 89% | 92% | 89% |
| Lobectomy/bilobectomy | 2% | 2% | 6% | 2% | 1% | 2% |
| Pneumonectomy | 1% | <1% | 1% | <1% | <1% | <1% |
| Other cancer-directed surgeryb | 7% | 11% | 12% | 9% | 7% | 9% |
| Radiation | ||||||
| None | 36% | 41% | 69% | 36% | 48% | 42% |
| External beam radiation | 64% | 59% | 31% | 64% | 51% | 57% |
| Other radiationc | <1% | 1% | 0% | 1% | 1% | 1% |
| Median follow-up (range) | 4 months (1–225) | 5 months (1–215) | 6 months (1–198) | 4 months (1–200) | 4 months (1–219) | 4 months (1–225) |
Notes:
Column percentages may not add up to 100 because of rounding;
Other cancer-directed surgery includes local surgical tumor destruction and excision/resection of less than one lobe;
Other radiation includes radioactive implants only, radioisotopes only, and radiation not otherwise specified.
Abbreviations: BAC, bronchioloalveolar adenocarcinoma; SD, standard deviation.
Tumor grade and treatment by histologic subtype
The distribution of stage IV NSCLC tumors by grade varied considerably according to histologic subtype (Table 1). The highest proportion of well-differentiated tumors was observed in BAC (21%), whereas the percentage of undifferentiated tumors was greatest in the large cell group (39%). Unknown tumor grade ranged from 37% in large cell tumors to 64% in “other” NSCLC tumors. Overwhelmingly, the majority (89%) of stage IV NSCLC patients did not undergo site-directed surgery as part of first-line treatment, but over half (57%) received external beam radiation (Table 1). Surgery was most common in those diagnosed with adenocarcinomas, particularly for BAC patients: 6% underwent a lobectomy/bilobectomy, 1% had a pneumonectomy, and 12% had local surgical tumor destruction or excision. Conversely, fewer patients with BAC tumors received beam radiation as part their initial therapy (31%) compared with the other histologic groups, and beam radiation treatment was highest in those with squamous or large cell tumors (64% in each).
Univariate analysis of survival by histologic subtype
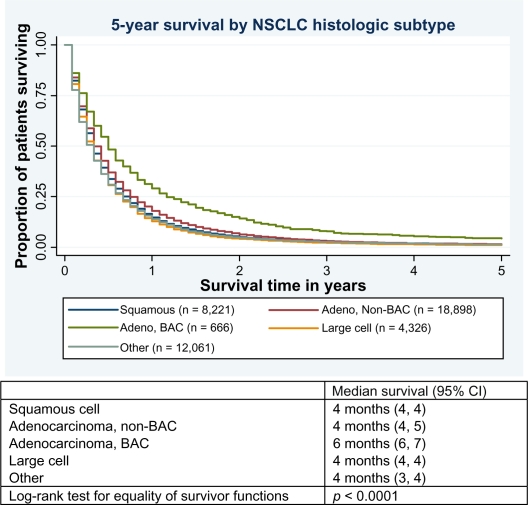
By December 31, 2006, 51,081 patients (99% of the study sample) had died from all causes and 45,347 (88%) had died from lung cancer specifically. Table 2 displays unadjusted 1- and 5-year survival rates for stage IV NSCLC patients as a whole and by histologic subtype according to select variables. Survival was highest for patients with BAC tumors (1-year survival: 29.1% [95% confidence interval (CI): 25.7, 32.6]; 5-year survival: 4.4% [95% CI: 3.0, 6.1]) and lowest in those diagnosed with large cell tumors (1-year survival: 12.8% [95% CI: 11.8, 13.8]; 5-year survival: 1.1% [95% CI: 0.8, 1.4]). Median survival for all stage IV NSCLC patients combined was 4 months (95% CI: 4, 4), with a range of 1–225 months. Survival significantly differed by histologic subgroup, primarily driven by the higher survival observed in patients with BAC (median survival: 6 months [95% CI: 6, 7]) (Figure 1).
Table 2.
One- and 5-year survival (95% confidence interval) in stage IV nonsmall cell lung cancer (NSCLC) patients who survived at least 31 days, overall and by histologic subtype, according to each variable of interest
| Characteristic |
NSCLC histologic subtype |
Overall (n = 44,172) |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Squamous (n = 8221) |
Adenocarcinoma |
Large cell (n = 4326) |
Other (n = 12,061) |
1-year | 5-year | |||||||||
| 1-year | 5-year |
Non-BAC (n = 18,898) |
BAC (n = 666) |
1-year | 5-year | 1-year | 5-year | |||||||
| 1-year | 5-year | 1-year | 5-year | |||||||||||
| Overall | 14.6% (13.9–15.4) |
1.6% (1.3–1.9) |
18.0% (17.5–18.6) |
1.5% (1.4–1.7) |
29.1% (2.7–32.6) |
4.4% (3.0–6.1) |
12.8% (11.8–13.8) |
1.1% (0.8–1.4) |
13.9% (13.3–14.5) |
1.3% (1.2–1.6) |
15.9% (15.6–16.3) |
1.5% (1.4–1.6) |
||
| Period of diagnosis | ||||||||||||||
| 1988–1992 | 13.8% (12.7–15.1) |
1.3% (1.0–1.7) |
16.5% (15.6–17.5) |
1.2% (0.9–1.5) |
25.6% (19.6–32.0) |
4.8% (2.4–8.5) |
10.6% (9.3–12.1) |
0.8% (0.4–1.2) |
11.7% (10.6–12.8) |
1.1% (0.7–1.5) |
14.3% (13.7–14.8) |
1.2% (1.0–1.4) |
||
| 1993–1997 | 14.0% (12.7–15.3) |
1.3% (0.9–1.7) |
16.6% (15.8–17.5) |
1.6% (1.3–1.9) |
28.6% (23.0–34.4) |
2.5% (1.0–5.1) |
12.7% (11.1–14.5) |
0.9% (0.5–1.5) |
13.8% (12.8–14.9) |
1.4% (1.0–1.7) |
15.2% (14.6–15.8) |
1.4% (1.2–1.6) |
||
| 1998–2001 | 16.6% (15.1–18.2) |
2.4% (1.8–3.1) |
21.1% (20.1–22.1) |
1.8% (1.5–2.2) |
32.5% (26.7–38.5) |
5.9% (3.4–9.4) |
17.2% (14.9–19.6) |
2.0% (1.2–3.0) |
15.5% (14.5–16.5) |
1.5% (1.2–1.9) |
18.4% (17.8–19.1) |
1.9% (1.7–2.1) |
||
| Gender | ||||||||||||||
| Male | 14.0% (13.1–14.9) |
1.3% (1.0–1.6) |
16.0% (15.3–16.7) |
1.3% (1.1–1.5) |
22.4% (18.1–27.0) |
3.0% (1.6–5.3) |
11.6% (10.4–12.8) |
1.2% (0.8–1.6) |
12.3% (11.6–13.1) |
1.0% (0.8–1.3) |
14.2% (13.8–14.6) |
1.2% (1.1–1.4) |
||
| Female | 16.2% (14.8–17.7) |
2.2% (1.7–2.9) |
20.6% (19.8–21.5) |
1.9% (1.6–2.2) |
35.9% (30.7–41.0) |
5.7% (3.6–8.6) |
15.0% (13.3–16.7) |
0.9% (0.6–1.5) |
16.1% (15.1–17.2) |
1.8% (1.4–2.2) |
18.5% (17.9–19.1) |
1.9% (1.7–2.1) |
||
| Age at diagnosiss | ||||||||||||||
| <45 years | 16.2% (11.4–21.6) |
4.0% (1.9–7.5) |
25.4% (22.7–28.2) |
2.7% (1.8–3.8) |
25.9% (11.5–43.1) |
- - |
14.4% (9.9–19.9) |
3.2% (1.3–6.5) |
21.2% (17.2–25.4) |
4.0% (2.3–6.3) |
22.3% (20.3–24.2) |
3.1% (2.4–4.0) |
||
| 45–54 years | 15.1% (12.8–17.6) |
2.4% (1.5–3.6) |
23.5% (22.0–25.0) |
2.1% (1.6–2.7) |
35.6% (24.9–46.5) |
6.9% (2.5–14.2) |
18.0% (15.2–21.0) |
2.3% (1.3–3.6) |
19.0% (17.0–21.1) |
2.7% (2.0–3.6) |
20.7% (19.7–21.8) |
2.4% (2.0–2.8) |
||
| 55–64 years | 15.6% (14.0–17.1) |
1.3% (0.9–1.9) |
20.4% (19.3–21.4) |
1.8% (1.4–2.1) |
38.6% (31.3–45.8) |
8.2% (4.7–12.9) |
13.5% (11.7–15.5) |
1.0% (0.5–1.7) |
17.1% (15.8–18.6) |
1.6% (1.2–2.1) |
18.3% (17.6–19.0) |
1.6% (1.4–1.9) |
||
| 65–74 years | 15.8% (14.5–17.1) |
1.7% (1.3–2.2) |
16.2% (15.3–17.1) |
1.3% (1.0–1.6) |
24.5% (18.9–30.4) |
3.8% (1.8–7.0) |
11.1% (9.5–12.8) |
0.7% (0.4–1.3) |
12.5% (11.5–13.6) |
1.1% (0.8–1.5) |
14.8% (14.2–15.3) |
1.3% (1.2–1.5) |
||
| 75–84 years | 11.3% (9.8–12.8) |
1.1% (0.7–1.6) |
11.3% (10.2–12.4) |
0.9% (0.6–1.3) |
24.3% (17.9–31.4) |
0.7% (0.1–3.3) |
10.2% (8.1–12.5) |
0.4% (0.1–1.2) |
10.4% (9.3–11.6) |
0.6% (0.4–1.0) |
11.1% (10.5–11.8) |
0.8% (0.6–1.0) |
||
| 85+ years | 11.8% (7.8–16.7) |
- - |
10.0% (7.4–13.0) |
0.5% (0.1–1.6) |
18.5% (6.8–34.8) |
3.7% (0.3–15.9) |
9.4% (4.6–16.2) |
- - |
6.9% (5.1–9.1) |
0.3% (0.1–1.1) |
9.0% (7.6–10.6) |
0.4% (0.1–0.8) |
||
| Ethnicity/race | ||||||||||||||
| White | 14.7% (13.8–15.6) |
1.5% (1.3–1.9) |
17.6% (17.0–18.2) |
1.4% (1.2–1.6) |
29.7% (25.7–33.7) |
4.6% (3.0–6.8) |
12.9% (11.8–14.1) |
1.0% (0.7–1.4) |
13.6% (12.9–14.3) |
1.2% (1.0–1.4) |
15.7% (15.3–16.1) |
1.4% (1.3–1.5) |
||
| Black | 14.9% (12.9–17.0) |
1.0% (0.6–1.7) |
17.6% (16.0–19.3) |
1.6% (1.2–2.3) |
22.0% (12.5–33.2) |
3.4% (0.6–10.4) |
10.7% (8.4–13.4) |
0.4% (0.1–1.2) |
14.7% (12.9–16.6) |
1.9% (1.3–2.7) |
15.5% (14.5–16.5) |
1.4% (1.1–1.8) |
||
| American Indian/Alaskan Native | 7.5% (1.3–21.3) |
- - |
12.2% (5.0–23.0) |
- - |
- - |
- - |
11.1% (0.6–38.8) |
- - |
8.1% (2.1–19.6) |
2.7% (0.2–12.1) |
9.7% (5.3–15.7) |
0.8% (0.1–4.0) |
||
| Asian/Pacific Islander | 13.5% (10.5–17.0) |
2.9% (1.6–4.8) |
22.1% (19.8–24.4) |
3.1% (2.2–4.2) |
30.4% (20.7–40.7) |
3.8% (1.0–9.7) |
16.7% (11.7–22.5) |
3.9% (1.7–7.5) |
16.1% (13.5–18.9) |
1.1% (0.5–2.1) |
19.0% (17.5–20.5) |
2.6% (2.0–3.3) |
||
| Spanish/Hispanic/Latino | 14.0% (10.0–18.8) |
2.9% (1.3–5.7) |
22.6% (19.2–26.3) |
2.2% (1.1–3.7) |
33.7% (17.0–51.2) |
3.7% (0.3–16.0) |
14.4% (8.8–21.4) |
1.7% (0.3–5.4) |
14.7% (11.3–18.6) |
3.2% (1.7–5.5) |
18.3% (16.2–20.5) |
2.6% (1.8–3.6) |
||
| Tumor grade at diagnosis | ||||||||||||||
| Well differentiated | 20.0% (14.6–26.0) |
0.5% (0.1–2.8) |
24.2% (20.5–28.1) |
2.1% (1.1–3.7) |
32.9% (25.2–40.7) |
4.3% (1.8–8.6) |
20.0% (0.8–58.2) |
- - |
40.0% (16.5–62.8) |
20.0% (4.9–42.4) |
25.0% (22.1–28.0) |
2.4% (1.5–3.7) |
||
| Moderately differentiated | 15.3% (13.5–17.2) |
1.9% (1.3–2.7) |
22.5% (20.7–24.3) |
1.9% (1.4–2.6) |
39.7% (28.1–51.0) |
8.8% (3.6–17.0) |
- - |
- - |
15.5% (7.6–25.9) |
8.6% (3.2–17.5) |
19.8% (18.5–21.1) |
2.1% (1.7–2.7) |
||
| Poorly/undifferentiated | 13.7% (12.6–14.9) |
1.7% (1.3–2.1) |
16.3% (15.5–17.2) |
1.6% (1.4–2.0) |
29.2% (17.9–41.4) |
9.1% (3.4–18.5) |
13.1% (11.9–14.4) |
1.3% (0.9–1.8) |
14.5% (13.5–15.6) |
1.2% (0.9–1.5) |
14.9% (14.4–15.5) |
1.5% (1.3–1.7) |
||
| Unknown | 15.0% (13.8–16.3) |
1.4% (1.0–1.8) |
18.0% (17.2–18.8) |
1.3% (1.1–1.6) |
26.0% (21.8–30.4) |
3.0% (1.6–5.0) |
12.4% (10.8–14.1) |
0.7% (0.3–1.2) |
13.4% (12.7–14.2) |
1.3% (1.1–1.6) |
15.7% (15.2–16.2) |
1.3% (1.2–1.5) |
||
| Cancer-directed surgery | ||||||||||||||
| Yes | 26.1% (23.1–29.1) |
6.6% (5.1–8.5) |
33.1% (31.3–34.9) |
4.4% (3.7–5.3) |
56.5% (47.0–65.0) |
17.4% (11.1–24.8) |
20.2% (16.7–23.8) |
2.9% (1.7–4.7) |
26.6% (23.8–29.4) |
4.9% (3.7–6.4) |
30.0% (28.7–31.3) |
5.0% (4.4–5.6) |
||
| No | 13.4% (12.6–14.2) |
1.0% (0.8–1.3) |
15.5% (15.0–16.1) |
1.1% (0.9–1.2) |
23.4% (19.9–27.0) |
1.6% (0.8–3.0) |
11.9% (10.9–12.9) |
0.8% (0.6–1.2) |
12.8% (12.2–13.4) |
1.0% (0.9–1.2) |
14.1% (13.7–14.4) |
1.0% (0.9–1.1) |
||
| External beam radiation | ||||||||||||||
| Yes | 15.2% (14.3–16.2) |
1.3% (1.1–1.7) |
18.0% (17.3–18.8) |
1.5% (1.3–1.7) |
28.9% (22.9–35.3) |
2.0% (0.7–4.6) |
13.1% (11.9–14.4) |
1.2% (0.8–1.6) |
14.7% (13.8–15.6) |
1.5% (1.3–1.9) |
16.2% (15.8–16.7) |
1.4% (1.3–1.6) |
||
| No | 13.5% (12.3–14.8) |
2.0% (1.6–2.6) |
18.0% (17.1–18.8) |
1.6% (1.4–1.9) |
29.2% (25.1–33.4) |
5.5% (3.6–7.8) |
12.3% (10.8–14.0) |
0.9% (0.5–1.5) |
13.1% (12.2–13.9) |
1.1% (0.9–1.4) |
15.5% (15.0–16.0) |
1.6% (1.4–1.8) |
||
Abbreviation: BAC, bronchioloalveolar adenocarcinoma.
Figure 1.
Kaplan–Meier survival curves displaying 5-year survival and median survival time by histologic subtype for patients diagnosed with stage IV nonsmall cell lung cancer (NSCLC).
Abbreviations:Adeno,adenocarcinoma;BAC,bronchioloalveolar adenocarcinoma; CI, confidence interval.
Across histologic groups, survival in stage IV NSCLC patients tended to improve for those diagnosed in later years and was more favorable in females than in males (Table 2). Survival for stage IV NSCLC patients generally worsened with increasing age at diagnosis and varied by race/ethnicity, with survival estimates typically highest in Hispanic and API patients. Survival worsened with increasing tumor grade at diagnosis and was better for those who underwent site-directed surgery as part of first-line treatment than for those who did not.
Multivariate analysis of survival by histologic subtype
We performed Cox PH analysis on stage IV NSCLC patients separately for each histologic subtype (Table 3). Mirroring univariate trends in survival, results indicated that across histologic subtypes, diagnosis in later years, female gender, younger age at diagnosis (<65 years), either API or Hispanic race/ethnicity (versus White), lower tumor grade at diagnosis, and cancer-directed surgery or beam radiation as part of first-line treatment were generally all independently associated with a decreased risk of death (all-cause). The nature and strength of these associations did not change when surgery and radiation were excluded from the models (data not shown).
Table 3.
Multivariate regression analysis of factors associated with time to death (all-cause) in patients diagnosed with stage IV nonsmall cell lung cancer (NSCLC) surviving at least 31 days
| Characteristic |
NSCLC histologic subtype |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
Squamous (n = 9370) |
Adenocarcinoma |
Large cell (n = 4689) |
Other (n = 15,020) |
|||||||
|
Non-BAC (n = 21,869) |
BAC (n = 801) |
|||||||||
| aHRa | (95% CI) | aHRa | (95% CI) | aHRa | (95% CI) | |||||
| aHRa | (95% CI) | aHRa | (95% CI) | |||||||
| Period of diagnosis | ||||||||||
| 1988–1992 | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference |
| 1993–1997 | 1.00 | (0.95–1.05) | 0.99 | (0.96–1.03) | 0.95 | (0.78–1.16) | 0.93b | (0.87–1.00) | 0.95b | (0.91–0.99) |
| 1998–2003 | 0.88c | (0.84–0.93) | 0.89c | (0.86–0.92) | 0.82b | (0.68–0.98) | 0.77c | (0.71–0.83) | 0.86c | (0.82–0.89) |
| Gender | ||||||||||
| Male | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference |
| Female | 0.90c | (0.86–0.94) | 0.86c | (0.84–0.89) | 0.78b | (0.68–0.90) | 0.91b | (0.86–0.97) | 0.88c | (0.85–0.90) |
| Age at diagnosis | ||||||||||
| <45 years | 0.79b | (0.69–0.91) | 0.77c | (0.72–0.82) | 1.00 | (0.68–1.46) | 0.80b | (0.69–0.93) | 0.74c | (0.67–0.82) |
| 45–54 years | 0.95 | (0.89–1.02) | 0.83c | (0.79–0.86) | 0.71b | (0.55–0.90) | 0.77c | (0.70–0.84) | 0.78c | (0.74–0.83) |
| 55–64 years | 0.98 | (0.93–1.03) | 0.89c | (0.86–0.92) | 0.74b | (0.61–0.90) | 0.88b | (0.82–0.95) | 0.86c | (0.83–0.90) |
| 65–74 years | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference |
| 75–84 years | 1.17c | (1.10–1.23) | 1.20c | (1.15–1.25) | 1.02 | (0.84–1.23) | 1.14b | (1.05–1.24) | 1.14c | (1.09–1.19) |
| 85+ years | 1.28c | (1.13–1.46) | 1.37c | (1.25–1.50) | 0.85 | (0.60–1.12) | 1.26b | (1.03–1.55) | 1.34c | (1.24–1.45) |
| Ethnicity/race | ||||||||||
| White | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference |
| Black | 1.03 | (0.97–1.09) | 1.05b | (1.00–1.10) | 1.27 | (0.97–1.64) | 1.11b | (1.02–1.21) | 0.98 | (0.93–1.03) |
| Amer Indian/Alaskan Native | 1.58b | (1.12–2.22) | 1.14 | (0.88–1.47) | 3.62 | (0.89–14.65) | 1.05 | (0.54–2.01) | 1.13 | (0.84–1.53) |
| Asian/Pacific Islander | 0.95 | (0.87–1.04) | 0.79c | (0.74–0.83) | 1.09 | (0.87–1.37) | 0.84b | (0.73–0.97) | 0.90b | (0.84–0.96) |
| Spanish/Hispanic/Latino | 1.01 | (0.89–1.14) | 0.91b | (0.84–0.98) | 0.81 | (0.56–1.17) | 1.00 | (0.84–1.19) | 0.93 | (0.85–1.02) |
| Tumor grade at diagnosis | ||||||||||
| Well differentiated | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference |
| Moderately differentiated | 1.08 | (0.93–1.25) | 1.07 | (0.97–1.17) | 0.85 | (0.64–1.12) | 1.35 | (0.52–3.50) | 1.45 | (0.91–2.32) |
| Poorly/undifferentiated | 1.17b | (1.01–1.34) | 1.25c | (1.14–1.36) | 1.05 | (0.77–1.42) | 0.85 | (0.38–1.91) | 1.89b | (1.25–2.88) |
| Unknown | 1.13 | (0.98–1.30) | 1.19c | (1.10–1.30) | 1.14 | (0.96–1.37) | 0.87 | (0.39–1.95) | 1.90b | (1.25–2.90) |
| Cancer-directed surgery | ||||||||||
| Yes | 0.66c | (0.62–0.71) | 0.67c | (0.64–0.70) | 0.46c | (0.37–0.56) | 0.82c | (0.75–0.90) | 0.72c | (0.67–0.76) |
| No | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference |
| External beam radiation | ||||||||||
| Yes | 0.89c | (0.85–0.93) | 0.95b | (0.93–0.98) | 1.04 | (0.89–1.22) | 0.92b | (0.86–0.97) | 0.89c | (0.86–0.92) |
| No | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference |
Notes:
aHR based on Cox proportional hazards model. Model for each NSCLC histologic subtype adjusted for period of diagnosis, gender, age at diagnosis, ethnicity/race, tumor grade at diagnosis, and receipt of cancer-directed surgery and radiation;
P value <0.05 (two-sided);
P value <0.001 (two-sided).
Abbreviations: aHR, adjusted hazard ratio; BAC, bronchioloalveolar adenocarcinoma; CI, confidence interval.
The prognostic significance of some factors varied according to histologic subtype. A clear pattern of disparate outcomes by age at diagnosis was not apparent in patients with squamous cell or BAC tumors. With respect to race/ethnicity, compared with Whites, the negative prognostic influence of Black race/ethnicity was not observed in patients diagnosed with “other” NSCLC tumors (adjusted hazard ratio [aHR]: 0.98 [95% CI: 0.93–1.03]), and the increased risk of death associated with an American Indian/Alaskan Native background was particularly pronounced in those with squamous (aHR: 1.58 [95% CI: 1.12–2.22]) or BAC (aHR: 3.62 [95% CI: 0.89–14.65]) tumors. Further, the favorable prognostic effect of Hispanic race/ethnicity was not demonstrated in squamous or large cell subgroups. Although survival in stage IV NSCLC tended to improve over time across histologic subtypes, this improvement was evident early on and was strongest in patients with large cell tumors (compared with cases diagnosed in 1988–1992, aHR: 0.93 [95% CI: 0.87–1.00] for 1993–1997 and aHR: 0.77 [95% CI: 0.71–0.83] for 1998–2003). Lastly, although increasing tumor grade was generally associated with poorer survival in stage IV NSCLC patients, no relationship between tumor grade and prognosis was apparent among those diagnosed with BAC or large cell tumors.
Discussion
Findings confirm the poor prognosis in patients diagnosed with stage IV NSCLC, with a median overall survival time of just 4 months and 1- and 5-year survival of less than 16% and 2%, respectively. In this large population-based cohort of newly diagnosed stage IV NSCLC patients, we observed several important trends in survival, some of which differed according to histologic subtype.
Consistent with studies demonstrating a gradual improvement in NSCLC survival over the last few decades,18–20 across histologic subtypes, we observed a moderate improvement in overall survival for patients diagnosed with stage IV NSCLC over the study period. For all patients combined, there was an absolute increase of 0.7% and 4.1% in 1- and 5-year survival, respectively, for patients diagnosed in 1998–2003 versus those diagnosed in 1988–1992. Later years of diagnosis were independently associated with a decreased risk of death after controlling for important patient, tumor, and treatment characteristics for every histologic subtype. Although this may partly be a function of stage migration resulting from more advanced imaging techniques over time, decreased mortality may also be credited to an increased availability of better supportive care measures or systemic therapy regimens. In fact, the timing of improved survival coincides with the adoption of platinum-based chemotherapy as a standard of care in the stage IV NSCLC setting by the American Society of Clinical Oncology,21 and research on trends in chemotherapy utilization and outcomes reflect this guidance. Using the SEER–Medicare linked database, Ramsey et al22 found that between 1994 and 1999, the proportion of stage IIIb and IV NSCLC patients who received chemotherapy increased from 21% to 43%, and survival outcomes were superior among those receiving at least one chemotherapy agent, particularly if that agent was platinum-based. Interestingly, although we found that overall survival was shortest in patients with large cell tumors, survival improvements over time were most pronounced in this group. Large cell NSCLC includes large cell neuroendocrine carcinomas (LCNECs), and our findings are in concordance with research demonstrating that although these patients do not experience superior survival, NSCLC tumors with neuroendocrine features have a higher objective response rate to chemotherapy.21
Gender differences in NSCLC presentation, management, and survival have been investigated extensively, and the majority of studies demonstrate that compared with males, female NSCLC patients are more likely to have adenocarcinoma and be non- or light smokers and younger.3–5,8,9,20,23,24 Several reports also indicate a more favorable prognosis of NSCLC in women versus men,5,9,23,24 but this is less clear in the advanced disease setting. Based on a retrospective analysis of patients undergoing surgery for NSCLC, de Perrot et al5 found that although the protective effect of female gender was present in early-stage NSCLC, it was absent in more advanced disease (stage III and IV). Conversely, Visbal et al9 prospectively followed a larger cohort of NSCLC patients and noted that men were at a significantly increased risk of mortality compared with women, especially for those with stage III/IV disease or adenocarcinoma. Consistent with this latter study, we found that female gender was a significant independent positive prognostic factor across histologic subtypes in our cohort of stage IV NSCLC patients.
Although many investigations have noted that younger NSCLC patients are more likely to be female, have adenocarcinoma, receive more aggressive treatment, and have biologically aggressive tumors compared with those who are elderly, research has not consistently demonstrated a pattern of disparate survival outcomes by age group.4,6,7 In our study of stage IV NSCLC patients, increasing age was generally associated with poorer survival, but there were exceptions to this trend according to histologic subtype. For patients with squamous cell NSCLC, although the elderly (75+ years) and young (<45 years) patients demonstrated poorer and superior survival, respectively, risk of death appeared to be comparable across the three middle age groups. For patients diagnosed with BAC tumors, the prognostic significance of age was unclear, although this most likely reflects the relatively small number of BAC patients in specific age brackets. Taken as a whole, our findings do not support the notion that NSCLC is inherently more aggressive or deadlier in younger patients, at least not among those who present with stage IV disease.
The prognostic role of race/ethnicity in stage IV NSCLC survival varied between histologic subgroups. For most, API ethnicity conferred an independent survival advantage. Although it is widely recognized that among Asian NSCLC patients there is a preponderance of never smokers and that never smokers demonstrate superior survival outcomes,25 recent research suggests that the favorable prognostic effect of Asian ethnicity in NSCLC survival is independent of smoking status.18 High levels of epidermal growth factor receptor (EGFR) protein have been associated with poorer outcomes in NSCLC, and polymorphisms within the EGFR gene that lead to lower EGFR expression are more common in Asians compared with in other ethnic groups.18 Also consistent with previous research,19,26 we found that Black versus White race/ethnicity was generally associated with an increased risk of death for stage IV NSCLC patients. Additionally, Native Americans/Alaskan Natives had reduced survival, particularly among patients with squamous cell or BAC tumors, and Hispanics tended to demonstrate relatively favorable survival in those with adenocarcinomas or “other” NSCLC tumors. Whether these ethnic disparities in stage IV NSCLC mortality according to histologic subtype are tied to important genetic differences (eg, pharmacogenomic or tumor genetic differences associated with anticancer treatment response) among races/ethnicities or simply reflect differential access to care is an area of active research.27
Although the management of advanced NSCLC remains a challenge, our study demonstrates a modest improvement in survival over the last few decades, which may partly be attributed to the adoption of platinum-based chemotherapy. Because histology is increasingly being viewed as an important factor in determining appropriate choice of treatment regimen,10,11,13 it is concerning that nearly 30% of newly diagnosed stage IV NSCLC tumors in our sample were not further classified into one of the main histologic subtypes (squamous cell, adenocarcinoma, BAC, and large cell). Although some of these tumors were of mixed/other NSCLC histology, over 94% (representing >27% of the entire sample) were carcinomas not otherwise specified (NOS). This is in agreement with a recent study of data from the California Cancer Registry (1989–2006), demonstrating that carcinoma NOS is a common NSCLC histologic diagnosis (especially in stage IV disease and among the elderly) and has been increasing over time.28 Further, the authors indicated that carcinoma NOS patients had the poorest survival among the major NSCLC histologies and derived less benefit from chemotherapy than patients with adenocarcinoma. Although definitive histologic diagnosis can be difficult, it provides important data in tailoring treatment, particularly as new targeted agents become available.12
The SEER Program provides population-based data on cancer incidence and survival in the US and is considered the gold standard for quality among cancer registries worldwide.29 Nonetheless, our study has important limitations. Tumors included for study did not undergo central or independent pathology review. Although this makes our findings more generalizable, it introduces heterogeneity in disease classification and increases the potential for misclassification. However, the accuracy of NSCLC histologic reporting in SEER has been assessed favorably in comparison with independent histologic review.30 An additional limitation of this study is that minor changes in lung cancer histological classification (eg, LCNEC was previously considered small cell lung cancer and introduced as a new subtype of NSCLC under large cell carcinoma in 199931) during the study period may have slightly influenced some of the survival estimates or observed trends. Lastly, we were unable to directly evaluate the effects of tobacco use, chemotherapy utilization, treatments throughout the disease course, performance status, and comorbid conditions on survival outcomes, as information on these variables is either incomplete or unavailable in SEER.
This large population-based study provides a recent and comprehensive description of survival by major histologic subtype in stage IV NSCLC in the US. Although we detected moderate improvements in survival across the study period, more progress is needed. We also found that survival and the influence of some important prognostic factors vary according to NSCLC histology and that carcinoma NOS represents a common histologic diagnosis in stage IV disease. Given that data from recent trials of various chemotherapy regimens suggest that NSCLC histology may be prognostic or predictive of clinical efficacy outcomes, our findings highlight the need for continued refinement of NSCLC histologic classifications and definitive tumor subclassification at diagnosis to help realize the promise of target-specific chemotherapeutics and ultimately prolong survival in patients with advanced NSCLC. More research using other sources of population-based data could help clarify the role of histology in the presentation, management, and prognosis of late-stage NSCLC.
Footnotes
Disclosure
This research was funded in part by Amgen Inc.
References
- 1.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 2.Horner MJ, Ries LAG, Krapcho M, et al., editors. National Cancer Institute; 1975–2006. SEER cancer statistics review. http://seer.cancer.gov/csr/1975_2006. Accessed March 30, 2011. [Google Scholar]
- 3.Caldarella A, Crocetti E, Comin CE, et al. Gender differences in non-small cell lung cancer: a population-based study. Eur J Surg Oncol. 2007;33(6):763–768. doi: 10.1016/j.ejso.2007.01.001. [DOI] [PubMed] [Google Scholar]
- 4.Chen KY, Chang CH, Yu CJ, et al. Distribution according to histologic type and outcome by gender and age group in Taiwanese patients with lung carcinoma. Cancer. 2005;103(12):2566–2574. doi: 10.1002/cncr.21087. [DOI] [PubMed] [Google Scholar]
- 5.de Perrot M, Licker M, Bouchardy C, et al. Sex differences in presentation, management, and prognosis of patients with non-small cell lung carcinoma. J Thorac Cardiovasc Surg. 2000;119(1):21–26. doi: 10.1016/s0022-5223(00)70213-3. [DOI] [PubMed] [Google Scholar]
- 6.Gadgeel SM, Ramalingam S, Cummings G, et al. Lung cancer in patients <50 years of age: the experience of an academic multidisciplinary program. Chest. 1999;115(5):1232–1236. doi: 10.1378/chest.115.5.1232. [DOI] [PubMed] [Google Scholar]
- 7.Radzikowska E, Roszkowski K, Glaz P. Lung cancer in patients under 50 years old. Lung Cancer. 2001;33(2–3):203–211. doi: 10.1016/s0169-5002(01)00199-4. [DOI] [PubMed] [Google Scholar]
- 8.Radzikowska E, Glaz P, Roszkowski K. Lung cancer in women: age, smoking, histology, performance status, stage, initial treatment and survival. Population-based study of 20 561 cases. Ann Oncol. 2002;13(7):1087–1093. doi: 10.1093/annonc/mdf187. [DOI] [PubMed] [Google Scholar]
- 9.Visbal AL, Williams BA, Nichols FC, et al. Gender differences in non-small-cell lung cancer survival: an analysis of 4,618 patients diagnosed between 1997 and 2002. Ann Thorac Surg. 2004;78(1):209–215. doi: 10.1016/j.athoracsur.2003.11.021. [DOI] [PubMed] [Google Scholar]
- 10.Bennouna J, Senellart H, Douillard JY. Non-small-cell lung cancer: should histology guide chemotherapy treatment? Ann Oncol. 2009;20(9):1608–1609. doi: 10.1093/annonc/mdp376. [DOI] [PubMed] [Google Scholar]
- 11.Einhorn LH. First-line chemotherapy for non-small-cell lung cancer: is there a superior regimen based on histology? J Clin Oncol. 2008;26(21):3485–3486. doi: 10.1200/JCO.2008.17.2056. [DOI] [PubMed] [Google Scholar]
- 12.Herbst RS, Heymach JV, Lippman SM. Lung cancer. N Engl J Med. 2008;359(13):1367–1380. doi: 10.1056/NEJMra0802714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hirsch FR, Spreafico A, Novello S, et al. The prognostic and predictive role of histology in advanced non-small cell lung cancer: a literature review. J Thorac Oncol. 2008;3(12):1468–1481. doi: 10.1097/JTO.0b013e318189f551. [DOI] [PubMed] [Google Scholar]
- 14.Surveillance, Epidemiology, and End Results (SEER) Program limited-use data (1973–2006) National Cancer Institute, DCCPS, Surveillance Research Program, Cancer Statistics Branch, released April 2009, based on the November 2007 submission. http://www.seer.cancer.gov. Accessed March 30, 2011.
- 15.International Classification of Diseases for Oncology. Geneva, Switzerland: World Health Organization; 2000. [Google Scholar]
- 16.Kaplan EL, Meier P. Non parametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 17.Cox DR. Regression models and life tables. J R Stat Soc B. 1972;34:187–220. [Google Scholar]
- 18.Ou SH, Ziogas A, Zell JA. Asian ethnicity is a favorable prognostic factor for overall survival in non-small cell lung cancer (NSCLC) and is independent of smoking status. J Thorac Oncol. 2009;4(9):1083–1093. doi: 10.1097/JTO.0b013e3181b27b15. [DOI] [PubMed] [Google Scholar]
- 19.Gadgeel SM, Severson RK, Kau Y, et al. Impact of race in lung cancer: analysis of temporal trends from a surveillance, epidemiology, and end results database. Chest. 2001;120(1):55–63. doi: 10.1378/chest.120.1.55. [DOI] [PubMed] [Google Scholar]
- 20.Fu JB, Kau TY, Severson RK, Kalemkerian GP. Lung cancer in women: analysis of the national Surveillance, Epidemiology, and End Results database. Chest. 2005;127(3):768–777. doi: 10.1378/chest.127.3.768. [DOI] [PubMed] [Google Scholar]
- 21.Clinical practice guidelines for the treatment of unresectable non-small-cell lung cancer. Adopted on May 16, 1997 by the American Society of Clinical Oncology. J Clin Oncol. 1997;15(8):2996–3018. doi: 10.1200/JCO.1997.15.8.2996. [DOI] [PubMed] [Google Scholar]
- 22.Ramsey SD, Howlader N, Etzioni RD, Donato B. Chemotherapy use, outcomes, and costs for older persons with advanced non-small-cell lung cancer: evidence from surveillance, epidemiology and end results-Medicare. J Clin Oncol. 2004;22(24):4971–4978. doi: 10.1200/JCO.2004.05.031. [DOI] [PubMed] [Google Scholar]
- 23.Cerfolio RJ, Bryant AS, Scott E, et al. Women with pathologic stage I, II, and III non-small cell lung cancer have better survival than men. Chest. 2006;130(6):1796–1802. doi: 10.1378/chest.130.6.1796. [DOI] [PubMed] [Google Scholar]
- 24.Wisnivesky JP, Halm EA. Sex differences in lung cancer survival: do tumors behave differently in elderly women? J Clin Oncol. 2007;25(13):1705–1712. doi: 10.1200/JCO.2006.08.1455. [DOI] [PubMed] [Google Scholar]
- 25.Subramanian J, Govindan R. Lung cancer in never smokers: a review. J Clin Oncol. 2007;25(5):561–570. doi: 10.1200/JCO.2006.06.8015. [DOI] [PubMed] [Google Scholar]
- 26.Wang SJ, Fuller CD, Thomas CR., Jr Ethnic disparities in conditional survival of patients with non-small cell lung cancer. J Thorac Oncol. 2007;2(3):180–190. doi: 10.1097/JTO.0b013e318031cd4e. [DOI] [PubMed] [Google Scholar]
- 27.Sekine I, Yamamoto N, Nishio K, Saijo N. Emerging ethnic differences in lung cancer therapy. Br J Cancer. 2008;99:1757–1762. doi: 10.1038/sj.bjc.6604721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ou SH, Zell JA. Carcinoma NOS is a common histologic diagnosis and is increasing in proportion among non-small cell lung cancer histologies. J Thorac Oncol. 2009;4(10):1202–1211. doi: 10.1097/JTO.0b013e3181b28fb9. [DOI] [PubMed] [Google Scholar]
- 29.Zippin C, Lum D, Hankey BF. Completeness of hospital cancer case reporting from the SEER Program of the National Cancer Institute. Cancer. 1995;76(11):2343–2350. doi: 10.1002/1097-0142(19951201)76:11<2343::aid-cncr2820761124>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 30.Field RW, Smith BJ, Platz CE, et al. Lung cancer histologic type in the surveillance, epidemiology, and end results registry versus independent review. J Natl Cancer Inst. 2004;96(14):1105–1107. doi: 10.1093/jnci/djh189. [DOI] [PubMed] [Google Scholar]
- 31.Hann CL, Ettinger DS. The change in pattern and pathology of small cell lung cancer. In: Govindan R, editor. American Society of Clinical Oncology 2009 Educational Book. Alexandria, VA: American Society of Clinical Oncology; 2009. pp. 451–454. [Google Scholar]