Abstract
Policosanol, a well-defined mixture of very long chain primary alcohols that is available as a nutraceutical product, has been reported to lower blood cholesterol levels. The present studies demonstrate that policosanol promotes the phosphorylation of AMP-kinase and HMG-CoA reductase in hepatoma cells and in mouse liver after intragastric administration, providing a possible means by which policosanol might lower blood cholesterol levels. Treatment of hepatoma cells with policosanol produced a 2.5-fold or greater increase in the phosphorylation of AMP-kinase and HMG-CoA reductase, and increased the phosphorylation of Ca++/calmodulin-dependent kinase kinase (CaMKK), an upstream AMP-kinase kinase. Intra-gastric administration of policosanol to mice similarly increased the phosphorylation of hepatic HMG-CoA reductase and AMP-kinase by greater than 2-fold. siRNA-mediated suppression of fatty aldehyde dehydrogenase, fatty acyl-CoA synthetase 4, and acyl-CoA acetyltransferase expression in hepatoma cells prevented the phosphorylation of AMP-kinase and HMG-CoA reductase by policosanol, indicating that metabolism of these very long chain alcohols to activated fatty acids is necessary for the suppression of cholesterol synthesis, presumably by increasing cellular AMP levels. Subsequent peroxisomal β-oxidation probably augments this effect.
Keywords: Policosanol, AMP-kinase, Peroxisomes, HMG-CoA reductase, Hepatoma cells, Ca++/calmodulin-dependent kinase kinase
Introduction
Policosanol is a well-defined mixture of very long-chain alcohols (C26-C32) derived most commonly from the wax of processed sugar cane [1]. A number of early clinical studies indicated that policosanol at 5–20 mg/day could lower blood cholesterol levels [2–4], although several more recent, larger studies have been unable to replicate this benefit, even with doses up to 80 mg/day [5–7]. Our previous studies [8] showed that policosanol decreases cholesterol synthesis in rat hepatoma cells by up to 30% at concentrations relevant to clinical dosing regimens, providing a possible mechanism to lower blood cholesterol. This inhibition is mediated at or above HMG-CoA reductase, the regulatory step in cholesterol synthesis, but policosanol does not directly inhibit this enzyme nor does it decrease enzyme levels as measured by immunoquantitation [8]. Similar results were obtained with fibroblasts in vitro [9, 10], and two studies with whole animals similarly showed that policosanol treatment decreases hepatic cholesterol synthesis [11, 12]. However, two studies with hamsters were unable to demonstrate an effect of policosanol on cholesterol synthesis in vivo [13, 14].
Policosanol treatment of hepatoma cells increases the phosphorylation of AMP-kinase at Thr172 [8, 15], a modification known to activate this kinase, providing a possible mechanism by which policosanol might down-regulate HMG-CoA reductase activity and decrease cholesterol synthesis without directly inhibiting the enzyme or reducing its expression: AMP-kinase is the principal regulatory kinase for HMG-CoA reductase, catalyzing the phosphorylation of HMG-CoA reductase at Ser872 [16]. This phosphorylation decreases HMG-CoA reductase activity by 70–80% [17] and is thought to provide a rapid means to modulate cholesterol synthesis in response to cellular ATP levels and other stimuli. The present studies were undertaken to test this hypothesis that policosanol treatment promotes the phosphorylation of HMG-CoA reductase and to identify the mechanism by which policosanol activates AMP-kinase.
Materials and Methods
Chemicals
Lesstanol Brand Natural Policosanol OCTA-60 was kindly provided by Garuda International, Inc. (Lemon Cove, CA). Alcohol content, determined by gas chromatography/flame ionization detection (average of two independent determinations), yielded 66% octacosanol (C28), 17% triacontanol (C30), 6% hexacosanol (C26), 5% dotriacontanol (C32), 2% tetracosanol (C24), and 1% tetratriacontanol (C34). Tricosanol (C23) and heptacosanol (C27) each constituted less than 1% of the mixture, and eicosanol (C20), docosanol (C22), and nonacosanol (C29) constituted less than 0.1%; total alcohol content was 98%. Dulbecco’s modified Eagle’s medium (DMEM), penicillin-streptomycin-glutamine (PSG), fetal bovine serum (FBS), and trypsin were purchased from Invitrogen (Carlsbad, CA). Acadesine (AICAR), 1,1-dimethylbiguanide hydrochloride (metformin), ionomycin, sodium acetate, and protease inhibitor cocktail were obtained from Sigma (St. Louis, MO). HALT phosphatase inhibitor and the BCA protein assay kit were purchased from Roche Diagnostics (Indianapolis, IN) and Pierce/Thermo Scientific (Rockford, IL) respectively. McA-RH7777 rat hepatoma cells were obtained from American Type Culture Collection (Manassas, VA) and used between passages 12 and 22.
Cell Culture and Preparation of Lysates
McA-RH7777 rat hepatoma cells were cultured in DMEM supplemented with 10% FBS and 1× PSG in six-well plates at 37 °C under a humidified atmosphere of 5% CO2. After 48 h the medium was replaced with fresh medium with the addition of policosanol (10–25 μg/ml in 50% ethanol) and incubation was continued for 3 h. Control cells received an equal volume of 50% ethanol, which did not exceed 1% final concentration in the medium. Cells were washed once with phosphate-buffered saline (pH 7.4), scraped from the plates, pelleted by low-speed centrifugation, and lysed by two cycles of freeze-thawing (dry ice/ethanol and 37 °C water bath) in 0.25 M Tris–HCl buffer (pH 7.5) containing protease and phosphatase inhibitors at 2× standard concentration. The lysates were cleared by centrifugation (18,300×g, 10 min, at 4 °C) and the supernatant was stored in aliquots at −80 °C.
Animal Treatments
Experiments involving the use of animals were performed following protocols approved by the Institutional Animal Care and Use Committee of the University of Kentucky and were in accordance with all policies for the use and care of laboratory research animals as stipulated by the NIH. Seven-to-eight week-old female C57BL/6 J mice (~15–17 g) were purchased from Jackson laboratories (Bar Harbor, ME) and maintained in a temperature-, humidity-, and light-controlled facility with free access to water and food for one week prior to experimentation. After an overnight fast, mice in groups of 5 were gavaged with 150 μl of policosanol in 50% ethanol in doses of 25, 50 or 100 mg/kg body weight. Control animals received an equal volume of 50% ethanol only. Access to food was restored, and at 0, 3, 6, 12, and 18 or 24 h the mice were euthanized by CO2 asphyxiation and the liver and intestine were removed and portions promptly frozen in liquid nitrogen and stored at −80 °C until use.
Preparation of Tissue Homogenates
Hepatic and intestinal homogenates were prepared as follows: Approximately 25 mg of tissue was transferred into 4 volumes of ice-cold RIPA homogenization buffer (50 mM Tris–HCl, pH 7.4, 150 mM NaCl, 1 mM PMSF, 1 mM EDTA, 5 μg/ml aprotinin, 5 μg/ml leupeptin, 1% Triton X-100, 1% sodium deoxycholate, and 0.1% SDS) and homogenized using a Potter-Elvehjem homogenizer. The samples were then subjected to two cycles of freeze–thawing (dry ice/ethanol and 37 °C water bath) and then cleared by centrifugation (18,300×g, 10 min, 4 °C). The supernatant was stored in aliquots at −80 °C. Protein concentration was determined by BCA assay.
Gel Electrophoresis and Immunoblotting
Thirty micrograms of protein from cell lysate or tissue homogenate was fractionated by SDS-polyacrylamide gel electrophoresis on 8% gels and electroblotted to nitrocellulose (Bio-Rad, Hercules, CA). The membrane was blocked with 0.05% Tween-20 and 5% defatted milk for 1 h at room temperature and then incubated in this same buffer with rabbit antibody to total AMP-kinase (anti-AMPK α-pan, 1:2000; Upstate/Millipore, Billerica, MA) or to phosphorylated AMP-kinase (anti-phospho-AMPKα, 1:500; Upstate/Millipore) overnight at 4 °C with gentle shaking. The immunoblot was developed with a secondary antibody conjugated to horseradish peroxidase for 1 h at room temperature and the chemiluminescent image (Supersignal West Pico Chemiluminescent Substrate, Pierce/Thermo Scientific) captured by autoradiography on a Kodak Image Station or on film and analyzed for mean intensity above background. Band intensity on film was measured with Image J software on the scanned image with background subtraction. The same procedure was followed for detecting total HMG-CoA reductase (anti-HMG-CoA reductase, 1:1000, Millipore) and phosphorylated HMG-CoA reductase (anti-phospho-HMG-CoA reductase, 1:500; Millipore).
Immunoprecipitation
To determine the level of phosphorylation of the kinases LKB1 and calcium-calmodulin-dependent kinase kinase (CaMKK), cell lysates or tissue homogenates were incubated for 1 h at 4 °C with 20 μl of mouse monoclonal antibody to phosphoserine/phosphothreonine/phosphotyrosine (Abcam, Cambridge, MA). Antibody conjugates were precipitated with 35 μl of protein A or G Plus-Aga-rose (Calbiochem/EMD Chemicals, Gibbstown, NJ) for 1 h at 4 °C as follows: After centrifugation at 12,000×g for 20 s at 4 °C, the immunoprecipitated phosphoproteins were released from the agarose beads by heating at 95 °C for 4 min in 25 μl of 2× gel loading buffer [0.5 M Tris–HCl, pH 6.8, 4.4% SDS, 20% (v/v) glycerol, 2% (v/v) 2-mercaptoethanol, and bromophenol blue in deionized water]. After removal of beads by centrifugation at 12,000×g for 20 s at 4 °C, the phosphoproteins were fractionated by SDS-gel electrophoresis and electroblotted to nitrocellulose as described above. Phosphorylated protein content was detected and quantified with an antibody to LKB1 (anti-LKB1, 1:1000; Sigma Chemical) or CaM-KK (anti-CaMKK, 1:1000; BD Transduction, San Jose, CA) followed by a secondary antibody conjugated with horseradish peroxidase as described above.
siRNA Plasmid Transfection and RT-PCR Analysis of Expression
Plasmids for the expression of siRNA (SureSilencing™ shRNA, SABiosciences, Frederick, MD) to fatty aldehyde dehydrogenase 3A2 (ALDH3A2), peroxisomal acyl-CoA synthetase long-chain family member 4 (ACSL4), and peroxisomal acetyl-CoA acyltransferase 1 (β-ketothiolase, ACAA1) were prepared by standard techniques from Escherichia coli DH5α and used to transfect hepatoma cells plated in 6-well plates at a density of 8 × 104 cells per cm2 as follows: On the day following plating, plasmid DNA was mixed with Fugene 6 transfection reagent (Roche Diagnostics) according to the manufacturer’s instructions at a ratio of 1 μg DNA:5 μl reagent and complexes were allowed to form for a minimum of 30 min at room temperature. The cell culture medium then was replaced with antibiotic-free medium containing 10% FBS and the transfection mixture was added drop-wise to the cells with gentle swirling. Four plasmids with unique siRNA sequences were provided for each gene; all four were tested for efficacy by RT-PCR as described below (data not shown), and the plasmid providing the greatest suppression of mRNA expression for each gene was selected for use (Table 1). A plasmid containing a scrambled siRNA sequence served as the negative control.
Table 1.
siRNA plasmid sequences and PCR primers
| Gene | GeneID | siRNA sequence | PCR primers |
|---|---|---|---|
| ALDH3A2 | 65183 | TCACTGATGTTGACCCTAACT | Fw: 5′-GCGAGAGAAGGACATCTTGG-3′ |
| ALDH3A2 | 65183 | GGGAGAGAGTGTTAACAAACT | Rv: 5′-TCGTCCATCATGGTAAGCAG-3′ |
| ACSL4 | 113976 | GAAGGTGGTTATACAGTTCAT | Fw: 5′-CACCATTGCCATTTTCTGTG-3′ Rv: 5′-ATAATGCCGCCTTCAGTTTG-3′ |
| ACAA1 | 24157 | ATCTCTGTGGGTAACGTACTT | Fw: 5′-GGTCCAAGGCTGAAGAACTG-3′ Rw: 5′-CAGTAGAGGGCCTGACTTGC-3′ |
| Scrambled control | GGAATCTCATTCGATGCATAC |
Total RNA was isolated from cells with the use of TRIzol reagent (Invitrogen) at 2, 4, 6, 8, and 10 days post-transfection and the concentration of RNA was determined spectrophotometrically with the use of a Nanodrop instrument (Thermo Scientific). First strand cDNA synthesis was performed using 200 units of Superscript II Reverse Transcriptase (Invitrogen) with 200 ng of random primers, 1 μl RNaseOUT (Invitrogen), 5 μg of total RNA, 2 μl of 0.1 M DTT, and 1 μl of dNTP mix (200 μM each nucleotide) in a total volume of 12 μl in diethylpyrocarbonate-treated water. The reaction was incubated at 25 °C for 10 min, followed by 50 min at 42 °C and then inactivated by incubation at 70 °C for 15 min. Synthesized single-stranded cDNA was quantified by Nanodrop measurement. Gene-specific primers for real-time polymerase chain reaction (PCR) were designed using Primer 3 software (http://frodo.wi.mit.edu/primer3/) and are shown in Table 1. Each 20-μl PCR reaction mixture contained 0.1 μg of single-stranded cDNA or template, 0.5 μl of gene-specific primers (10 μM each), 3 μl of dNTP mix (200 μM each nucleotide) and 1× SYBR Green PCR buffer (Applied Biosystems, Carlsbad, CA). Cycling conditions were: 2 min at 50 °C to activate, initial denaturation for 10 min at 95 °C, followed by 50 cycles of denaturation for 15 s at 95 °C and 1 min of annealing/extension at 55 °C. In order to detect nonspecific amplification, dissociation curves were established for each PCR product. The detection and quantitation of nucleic acid levels was done by using the comparative delta-delta ct method [18].
Statistical Analysis
Image intensity values for each blot were normalized, setting the control (untreated) value at 100 after background subtraction. Results are presented as the mean and standard deviation with significance determined by one-way analysis of variance (ANOVA) with either Dunnett’s or Tukey’s post-hoc test, setting p < 0.05, using Prism (GraphPad Software, La Jolla, CA).
Results
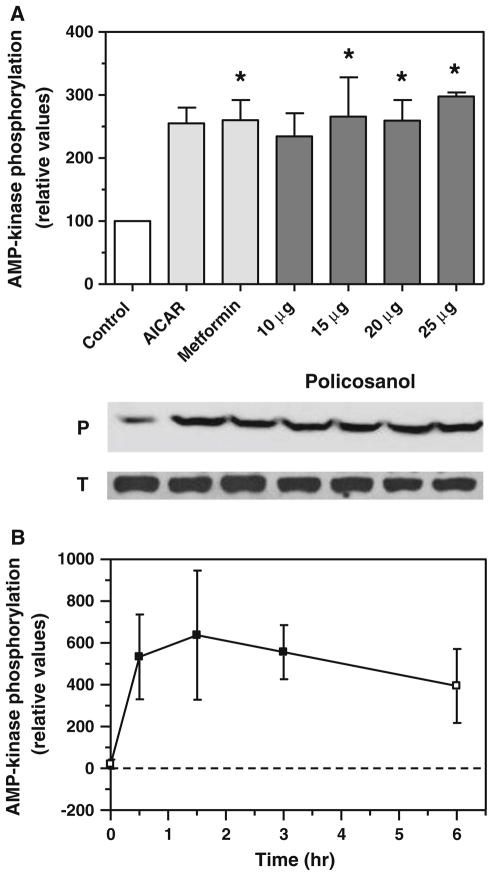
Previous results from our laboratory demonstrated that policosanol activates AMP-kinase in hepatoma cells [8]. To confirm and extend those findings, we carried out dose-response and time-course experiments. Policosanol increased AMP-kinase phosphorylation in hepatoma cells by more than 2.5-fold after a 3-h treatment (Fig. 1a), with maximal stimulation occurring between 15–25 μg/ml. These increases were comparable to those seen with AI-CAR and metformin, both of which are known to promote phosphorylation of AMP-kinase and served as positive controls. No change in the level of total AMP-kinase protein levels were observed over the 3-hr period of the experiment. As shown in Fig. 1b, policosanol treatment rapidly activated AMP-kinase, with an increase in phosphorylation evident at 30 min; phosphorylation peaked at 1.5 h and stayed elevated through 3 h.
Fig. 1.
Policosanol activates AMP-kinase in vitro. a Phosphorylated AMP-kinase in hepatoma cells was measured by immunoquantitation with an antibody specific for the phosphorylated protein after a 3-h treatment with 10–25 μg/ml of policosanol. The AMP-kinase activators AICAR and metformin (1 mM each) served as positive controls. Values represent the mean and standard deviation of three experiments; asterisks indicate values statistically different from control (ANOVA with Dunnett’s post-hoc test, p < 0.05). Representative immunoblots of phosphorylated (P) and total (T) AMP-kinase are shown below the graph. b AMP-kinase phosphorylation in hepatoma cells at various times after treatment with 15 μg/ml of policosanol. Values represent the mean and standard deviation of three experiments; closed symbols are statistically different from the zero-time value
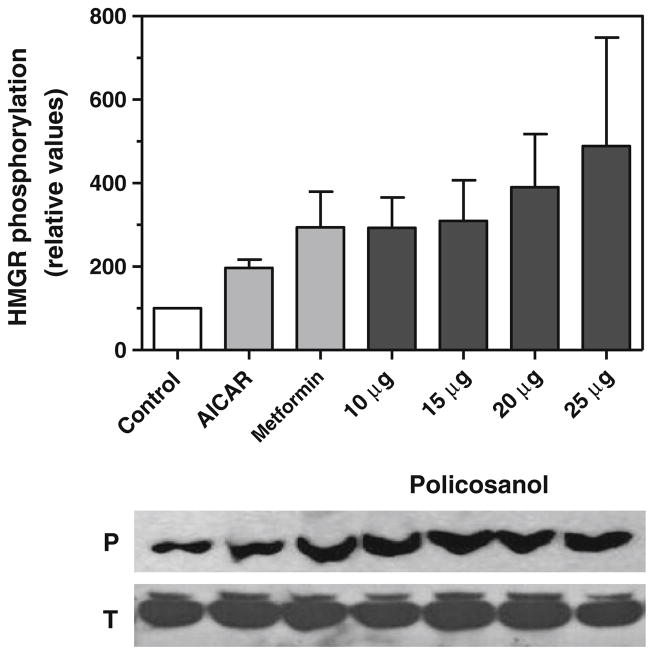
The activation of AMP-kinase by policosanol was concomitant with the phosphorylation of HMG-CoA reductase in hepatoma cells, as shown in Fig. 2. HMG-CoA reductase phosphorylation was increased in a dose-dependent manner with 10–25 μg/ml of policosanol. Treatment of cells with AICAR or metformin yielded a similar increase in phosphorylated HMG-CoA reductase. Total HMG-CoA reductase protein levels did not change over the course of this experiment.
Fig. 2.
Policosanol inactivates HMG-CoA reductase in vitro. Phosphorylated HMG-CoA reductase in hepatoma cells was measured by immunoquantitation with an antibody specific for the phosphorylated protein after a 3-h treatment with 10–25 μg/ml of policosanol. AICAR and metformin (1 mM each) served as positive controls. Values represent the mean and standard deviation of three experiments. Representative immunoblots of phosphorylated (P) and total (T) HMG-CoA reductase are shown below the graph
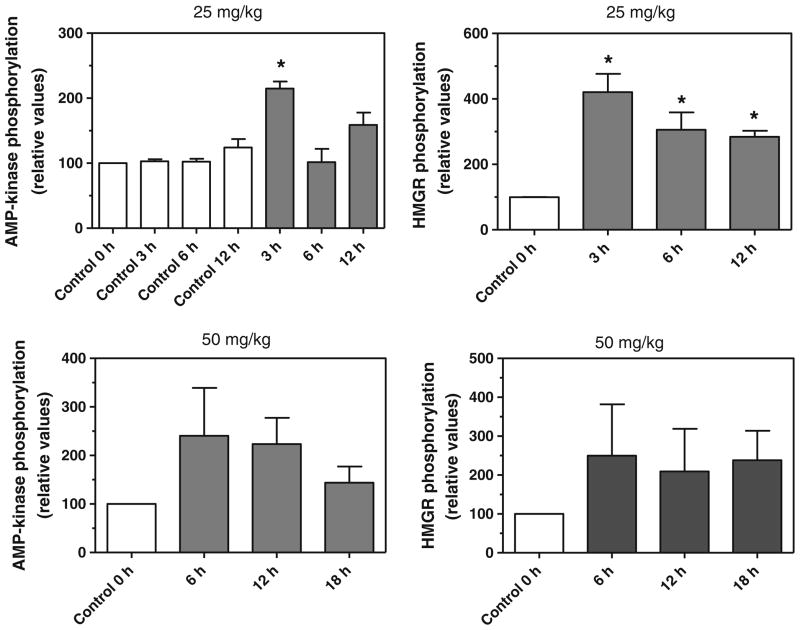
To extend these studies to whole animals in vivo, the phosphorylation of AMP-kinase and HMG-CoA reductase was assessed in the livers of mice gavaged with a single dose of policosanol at 10, 25, 50, and 100 mg/kg. As shown in Fig. 3, policosanol increased the phosphorylation of AMP-kinase and HMG-CoA reductase at 25 and 50 mg/kg by 2 to 4-fold as early as 3 h after dosing. No increase in phosphorylation was seen at lower (10 mg/kg) or higher doses (100 mg/kg), and no change in the total AMP-kinase or HMG-CoA reductase was observed at each of time points and the dosing regimens tested (data not shown). Policosanol had no effect on AMP-kinase in the duodenum of mice gavaged with 25 or 50 mg/kg (data not shown), suggesting that intestinal cholesterol synthesis, which can account for up to 25% of circulating cholesterol, is resistant to suppression by these fatty alcohols.
Fig. 3.
Policosanol increases the phosphorylation of AMP-kinase and HMG-CoA reductase (HMGR) in vivo. Mice were gavaged with policosanol (25 or 50 mg/kg) and phosphorylated AMP-kinase and HMG-CoA reductase were measured in liver homogenates by immunoquantitation. Values represent the mean and standard deviation of 2–3 experiments; values that are statistically different from untreated controls are indicated with asterisks. Statistical significance was determined by ANOVA with Dunnett’s post-hoc test, p < 0.05
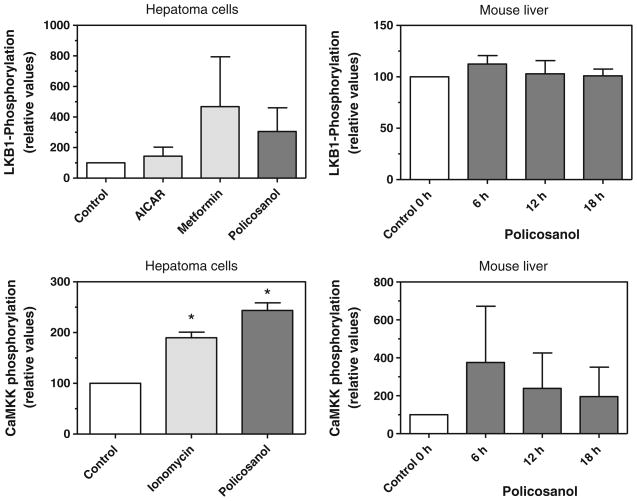
As AMP-kinase is itself activated by upstream kinases, we wanted to determine if policosanol acted on these upstream kinases. The principal AMP-kinase kinase is LKB1, but AMP-kinase can also be activated by CaMKKβ [19]. As both kinases are activated by phosphorylation, we monitored their phosphorylation in hepatoma cells and in mouse liver by immunoblotting after policosanol treatment. LKB1 phosphorylation was increased about 3-fold in hepatoma cells by policosanol (Fig. 4), slightly less than that seen with metformin, a known activator of LKB1, although neither increase was statistically significant. LKB1 phosphorylation was not increased in mouse liver after gavage with policosanol. CaMKK phosphorylation was increased in hepatoma cells about 2.5-fold by policosanol, and to a slightly greater extent than that seen with ionomycin, an activator of CaMKK; both increases were statistically significant. CaMKK phosphorylation also was increased up to 4-fold in mouse liver at 6 h post-dosing, and remained elevated up to 18 h, although these increases did not reach statistical significance. These results support the activation of AMP-kinase by policosanol both in cell culture and in vivo, and suggest that CaMKK contributes to this activation.
Fig. 4.
Policosanol activates CaMKK and LKB1. Phosphorylated LKB1 and CaMKK were immunoprecipitated from hepatoma cell lysates or mouse liver homogenates with an antibody to phosphoserine/phosphothreonine/phosphotyrosine and then quantified by immunoblotting with an antibody to LKB1 or CaMKK. Metformin (1 mM) served as a positive control and AICAR (1 mM) as a negative control for the LKB1 assays; ionomycin (1 μg/ml) was a positive control for the CaMKK assay. Hepatoma cells were incubated with 15 μg/ml of policosanol for 3 h; mice were gavaged with 50 mg/kg body weight of policosanol and the livers removed at 6, 12, and 18 h post-dosing. Values represent the mean and standard deviation of 2–3 experiments; values that are statistically different from controls are indicated with asterisks. Statistical significance was determined by ANOVA with Dunnett’s post-hoc test, p < 0.05
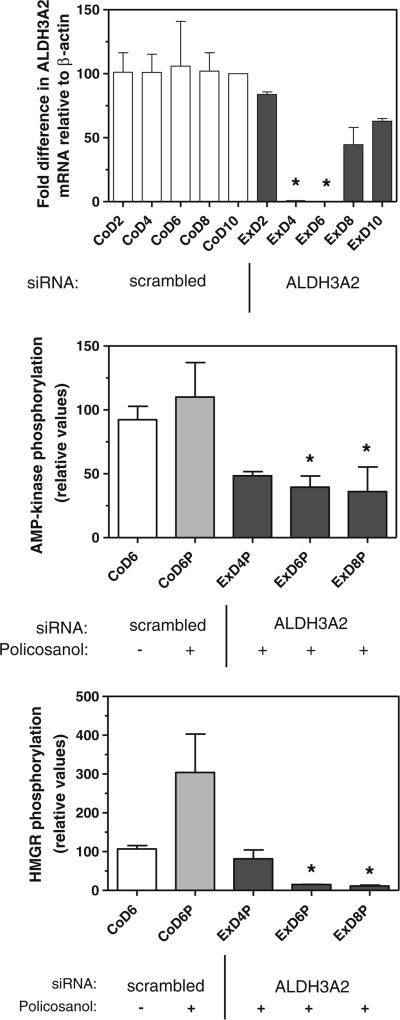
Long-chain fatty alcohols are substrates for the ‘fatty alcohol cycle’ which converts these alcohols to their corresponding fatty acids [20]. To determine if metabolism of policosanol by this pathway was necessary for the activation of AMP-kinase, we used siRNA to suppress the expression the second enzyme in this pathway, fatty aldehyde dehydrogenase 3A2, in hepatoma cells. As shown in Fig. 5, the siRNA plasmid suppressed ALDH3A2 mRNA expression nearly completely at 4 and 6 days post-transfection. The corresponding ability of policosanol to promote AMP-kinase phosphorylation at 4, 6, and 8 days post-transfection was reduced by greater than 50% in these cells, and phosphorylation of HMG-CoA reductase was nearly extinguished. These results reveal that the very long chain alcohols that make up policosanol must be converted to fatty acids in order to activate AMP-kinase.
Fig. 5.
siRNA-mediated suppression of fatty aldehyde dehydrogenase 3A2 (ALDH3A2) prevents the increase in phosphorylation of AMP-kinase and HMG-CoA reductase by policosanol. ALDH3A2 mRNA was quantified by RT-PCR with the primers listed in Table 1; control cells contained a plasmid with a scrambled siRNA sequence. Phosphorylated AMP-kinase and HMG-CoA reductase were measured by immunoquantitation. Cells were incubated with 15 μg/ml of policosanol for 3 h as indicated. Values represent the mean and standard deviation of 2–3 experiments; values that are statistically different from the corresponding day control (mRNA levels) or the policosanol-treated control are indicated with asterisks, as determined by one-way ANOVA with Tukey’s post-hoc test, p < 0.05. Co control cells, Ex cells transfected with ALDH3A2 siRNA plasmid; ‘D’ indicates day post-transfection; ‘P’ indicates policosanol treatment
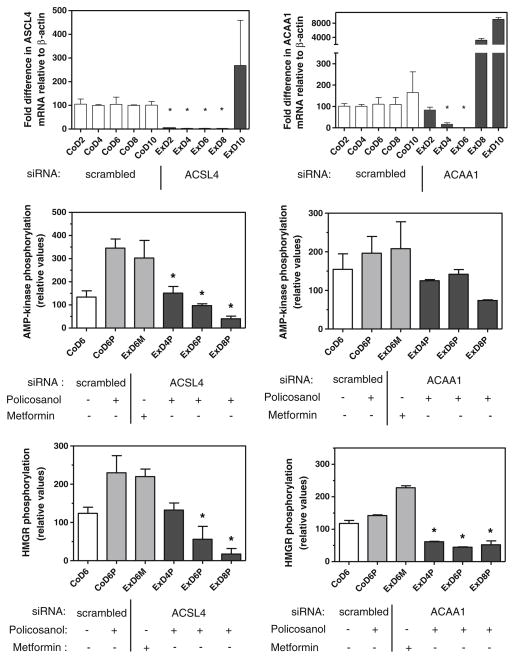
To determine if peroxisomal metabolism of policosanol was also required for activation of AMP-kinase, we used siRNA to suppress the expression of peroxisomal acyl-CoA synthetase long-chain family member 4 (ACSL4) and peroxisomal acetyl-CoA acyltransferase 1 (β-ketothiolase, ACAA1) in hepatoma cells; these enzymes catalyze the activation of long-chain fatty acids and the last step in peroxisomal β-oxidation, respectively. As shown in Fig. 6, mRNA levels for both enzymes were suppressed by greater than 80% on days 4 and 6 post-transfection, similar to that seen with ALDH3A2. The ability of policosanol to stimulate the phosphorylation of AMP-kinase and HMG-CoA reductase was fully prevented in cells transfected with either siRNA plasmid. The ability of metformin to activate AMP-kinase and promote HMG-CoA reductase phosphorylation was not affected by these siRNAs, demonstrating that the AMP-kinase pathway itself was not impaired by the suppression of peroxisomal fatty acid catabolism.
Fig. 6.
siRNA-mediated suppression of peroxisomal fatty acyl-CoA synthetase 4 (ACSL4) or acetyl-CoA acyltransferase 1 (ACAA1) prevents the increase in phosphorylation of AMP-kinase and HMG-CoA reductase by policosanol. ACSL4 and ACAA1 mRNA was quantified by RT-PCR with the primers listed in Table 1; control cells contained a plasmid with a scrambled siRNA sequence. Phosphorylated AMP-kinase and HMG-CoA reductase were measured by immunoquantitation. Cells were incubated with 15 μg/ml of policosanol or 1 mM metformin for 3 h as indicated. Values represent the mean and standard deviation of 2 experiments; values that are statistically different from the policosanol-treated control are indicated with asterisks, as determined by one-way ANOVA with Tukey’s post-hoc test, p < 0.05. Co control cells; Ex cells transfected with ACSL4 or ACAA1 siRNA plasmid; ‘D’ indicates day post-transfection; ‘M’ indicates metformin treatment; ‘P’ indicates policosanol treatment
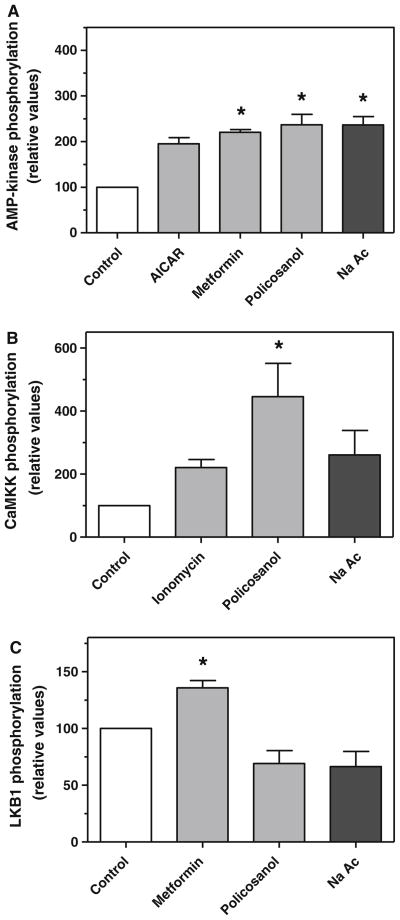
As the principal metabolite of fatty acid β-oxidation is acetyl-CoA, we determined if addition of acetate to cells would similarly increase the phosphorylation of AMP-kinase. As shown in Fig. 7a, addition of sodium acetate (0.12 mM) increased AMP-kinase phosphorylation by 2–3-fold, similar to that seen with AICAR, metformin, and policosanol. To determine if acetate activated AMP-kinase by the same pathway as policosanol, the effect of acetate treatment on CaMKK phosphorylation and LKB1 phosphorylation was measured. As seen with policosanol, acetate increased CaMKK phosphorylation by nearly 3-fold (Fig. 7b), but did not increase LKB1 phosphorylation (Fig. 7c).
Fig. 7.
Treatment of hepatoma cells with sodium acetate promotes the phosphorylation of AMP-kinase through CaMKK. Phosphorylated AMP-kinase (a), CaMKK (b), and LKB1 (c) were measured by immunoquantitation in hepatoma cells three hours after treatment with sodium acetate (0.12 mM), AICAR (1 mM), metformin (1 mM), policosanol (15 μg/ml) or ionomycin (1 μg/ml). Values represent the mean and standard deviation of 2–3 experiments; values that are statistically different from the control are indicated with asterisks, as determined by one-way ANOVA with Dunnett’s post-hoc test, p < 0.05
Discussion
The present studies reveal that the activation of AMP-kinase by policosanol is dependent upon the oxidation and peroxisomal metabolism of these very long-chain alcohols. It was anticipated that these alcohols would be metabolized by this route [21, 22]; moreover, the ability of long chain fatty acids to activate AMP-kinase is well established [23]. These findings thus provide a biochemical mechanism to explain the observed ability of policosanol to decrease cholesterol synthesis, through the activation of AMP-kinase and subsequent inactivation of HMG-CoA reductase [8, 15]. It is worth noting that D-003, a mixture of very long chain fatty acids also derived from sugarcane wax, has similarly been shown to decrease cholesterol synthesis in cultured fibroblasts [24]. These results do not exclude the possibility that policosanol acts through other HMG-CoA reductase kinases as well, such as a protein kinase C or a calmodulin-dependent protein kinase, as both have been shown to be capable of phosphorylating HMG-CoA reductase in vitro [25].
Metformin, a widely used drug in Type II diabetes, is a potent activator of AMP-kinase [26] and is well known to modestly lower blood lipid levels in patients. The present results are consistent with the possibility that policosanol could act via this same mechanism to lower blood cholesterol levels. However, although we show here that policosanol is able to increase the phosphorylation of HMG-CoA reductase in mouse liver after intragastric administration, the ability of therapeutically recommended doses of policosanol to decrease blood cholesterol levels by this pathway remains in doubt. The doses used in our study, ranging from 10 to 100 mg/kg by body weight, are considerably higher than those used in clinical studies, where the typical dose is between 5 to 80 mg per day. Assuming that the typical patient weighs 70 kg, a minimal 10 mg/kg dose would require a 35-fold higher dose than that typically used (up to 1 g/d), and this calculation does not consider differences in intestinal absorption between mice and men. Nonetheless, long-chain alcohols are a common constituent of human diets [27], and no significant adverse effects have been noted for policosanol in animal or clinical studies.
CaMKK is thought to be a principal AMP-kinase kinase in brain, but is considered to be an alternative to LKB1 in the liver [28]. In our studies policosanol produced a statistically significant increase in CaMKK phosphorylation in hepatoma cells, suggesting that activation of this upstream kinase by policosanol contributes to the phosphorylation of AMP-kinase. CaMKK phosphorylation was also increased 4-fold in mouse liver after gavage, although this increase was not statistically significant. CaMKK also appeared to be activated by addition of acetate to hepatoma cells, which would bypass the requirement for peroxisomal metabolism; either acetate per se, or its activation to acetyl-CoA or subsequent metabolism would appear to be sufficient to activate this kinase. Notably, Za’tara et al. [23] have shown that non-metabolizable analogs of long-chain fatty acids are also able to activate AMP-kinase, but that this requires activation with coenzyme A. The CoA synthetases that activate these fatty acids to acyl-CoAs generate AMP; AMP is an allosteric regulator of AMP-kinase that facilitates its phosphorylation by LKB1, but not, interestingly, by CaMKK [19, 28]. Thus, β-oxidation of very long chain fatty acids may not be required if they are present in sufficient abundance to generate elevated levels of AMP by activation with coenzyme A.
Our studies with siRNA-mediated suppression of fatty alcohol cycle (ALDH3A2) and peroxisomal enzymes (ACSL4, ACAA1) demonstrate that peroxisomal metabolism of policosanol is necessary for the activation of AMP-kinase in hepatoma cells. This requirement for β-oxidation, as shown with ACAA1 suppression, suggests that repeated chain-shortening with subsequent activation by ketothiolase is necessary to generate sufficient AMP to increase AMP-kinase phosphorylation. However, the suppression of peroxisomal β-oxidation not only blocked the effect of policosanol, but also reduced the phosphorylation of AMP-kinase and HMG-CoA reductase below the level seen in non-suppressed (control) cells. This suggests that peroxisomal metabolism contributes to the basal level of AMP-kinase activation under normal physiological conditions. It is thus formally possible that the activation of policosanol-derived fatty acids to acyl-CoAs is sufficient to increase AMP-kinase phosphorylation and activity, without further metabolism by β-oxidation, as found by Za’tara et al. [23]. Nonetheless, under normal circumstances the subsequent peroxisomal metabolism of these activated very long chain fatty acids would greatly increase the generation of AMP and amplify this signaling pathway.
This peroxisomal pathway for the activation of AMP-kinase by policosanol in hepatoma cells can be extrapolated to the liver in an intact animal. However, the observation made here and earlier [29] that acetate, the product of β-oxidation, leads to the activation of AMP-kinase, raises the possibility that policosanol does not need to be absorbed intact to activate hepatic AMP-kinase. Although there is good evidence that the very long-chain alcohols of policosanol are absorbed into the systemic circulation, the fractional absorption is very low [22, 30, 31]. It can be considered that a significant portion of these alcohols may be metabolized in the colon by gut bacteria to generate chain-shortened metabolites, including acetate, propionate, and butyrate, which are readily absorbed and transported to the liver [reviewed in 32]. Moreover, a number of studies have reported that propionate produced in the gut can decrease hepatic cholesterol synthesis [32]. These chain-shortened metabolites would bypass peroxisomal metabolism and serve as direct substrates for cytosolic acyl-CoA synthases, thereby generating AMP; the ability of intraperitoneal administration of acetate to increase AMP levels in hepatocytes and in the liver [33, 34] supports this hypothesis. It was also reported that acetate treatment of hepatocytes decreased SREBP-1 mRNA levels [29], which could lead to decreased expression of HMG-CoA reductase. Taken together, these observations suggest that there are multiple pathways by which policosanol might generate elevated hepatic acyl-CoA and AMP levels to activate AMP-kinase and suppress cholesterol synthesis.
Acknowledgments
We thank Dr. Geza Bruckner for helpful insight and advice, and numerous anonymous reviewers for their comments and suggestions. This work was supported by the National Institutes of Health National Center for Complementary and Alternative Medicine [Grant AT003488].
Abbreviation
- CaMKK
Calcium-calmodulin-dependent kinase kinase
Contributor Information
Subhashis Banerjee, Graduate Center for Toxicology, University of Kentucky, Lexington, KY 40536-0305, USA.
Sarbani Ghoshal, Department of Pharmaceutical Sciences, College of Pharmacy, University of Kentucky, Lexington, KY 40536-0596, USA.
Todd D. Porter, Email: tporter@email.uky.edu, Graduate Center for Toxicology, University of Kentucky, Lexington, KY 40536-0305, USA, Department of Pharmaceutical Sciences, College of Pharmacy, University of Kentucky, Lexington, KY 40536-0596, USA
References
- 1.Marinangeli CP, Jones PJ, Kassis AN, Eskin MN. Policosanols as nutraceuticals: fact or fiction. Crit Rev Food Sci Nutr. 2010;50:259–267. doi: 10.1080/10408391003626249. [DOI] [PubMed] [Google Scholar]
- 2.Torres O, Agramonte AJ, Illnait J, Mas Ferreiro R, Fernandez L, Fernandez JC. Treatment of hypercholesterolemia in NIDDM with policosanol. Diabetes Care. 1995;18:393–397. doi: 10.2337/diacare.18.3.393. [DOI] [PubMed] [Google Scholar]
- 3.Arruzazabala ML, Molina V, Mas R, Fernandez L, Carbajal D, Valdes S, Castano G. Antiplatelet effects of policosanol (20 and 40 mg/day) in healthy volunteers and dyslipidaemic patients. Clin Exp Pharmacol Physiol. 2002;29:891–897. doi: 10.1046/j.1440-1681.2002.03746.x. [DOI] [PubMed] [Google Scholar]
- 4.Castano G, Mas R, Fernandez L, Illnait J, Gamez R, Alvarez E. Effects of policosanol 20 versus 40 mg/day in the treatment of patients with type II hypercholesterolemia: a 6-month double-blind study. Int J Clin Pharmacol Res. 2001;21:43–57. [PubMed] [Google Scholar]
- 5.Cubeddu LX, Cubeddu RJ, Heimowitz T, Restrepo B, Lamas GA, Weinberg GB. Comparative lipid-lowering effects of policosanol and atorvastatin: a randomized, parallel, double-blind, placebo-controlled trial. Am Heart J. 2006;152:982, e981–e985. doi: 10.1016/j.ahj.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 6.Berthold HK, Unverdorben S, Degenhardt R, Bulitta M, Gouni-Berthold I. Effect of policosanol on lipid levels among patients with hypercholesterolemia or combined hyperlipidemia: a randomized controlled trial. Jama. 2006;295:2262–2269. doi: 10.1001/jama.295.19.2262. [DOI] [PubMed] [Google Scholar]
- 7.Francini-Pesenti F, Beltramolli D, Dall’acqua S, Brocadello F. Effect of sugar cane policosanol on lipid profile in primary hypercholesterolemia. Phytother Res. 2008;22:318–322. doi: 10.1002/ptr.2315. [DOI] [PubMed] [Google Scholar]
- 8.Singh DK, Li L, Porter TD. Policosanol inhibits cholesterol synthesis in hepatoma cells by activation of AMP-kinase. J Pharmacol Exp Ther. 2006;318:1020–1026. doi: 10.1124/jpet.106.107144. [DOI] [PubMed] [Google Scholar]
- 9.Menendez R, Amor AM, Rodeiro I, Gonzalez RM, Gonzalez PC, Alfonso JL, Mas R. Policosanol modulates HMG-CoA reductase activity in cultured fibroblasts. Arch Med Res. 2001;32:8–12. doi: 10.1016/s0188-4409(00)00265-4. [DOI] [PubMed] [Google Scholar]
- 10.Menendez R, Fernandez SI, Del Rio A, Gonzalez RM, Fraga V, Amor AM, Mas RM. Policosanol inhibits cholesterol biosynthesis and enhances low density lipoprotein processing in cultured human fibroblasts. Biol Res. 1994;27:199–203. [PubMed] [Google Scholar]
- 11.Menendez R, Amor AM, Gonzalez RM, Fraga V, Mas R. Effect of policosanol on the hepatic cholesterol biosynthesis of mormocholesterolemic rats. Biol Res. 1996;29:253–257. [PubMed] [Google Scholar]
- 12.Menendez R, Arruzazabala L, Mas R, Del Rio A, Amor AM, Gonzalez RM, Carbajal D, Fraga V, Molina V, Illnait J. Cholesterol-lowering effect of policosanol on rabbits with hypercholesterolaemia induced by a wheat starch-casein diet. Br J Nutr. 1997;77:923–932. doi: 10.1079/bjn19970090. [DOI] [PubMed] [Google Scholar]
- 13.Wang Y, Ebine N, Jia X, Jones PJ, Fairow C, Jaeger R. Very long chain fatty acids (policosanols) and phytosterols affect plasma lipid levels and cholesterol biosynthesis in hamsters. Metabolism. 2005;54:508–514. doi: 10.1016/j.metabol.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 14.Wang YW, Jones PJ, Pischel I, Fairow C. Effects of policosanols and phytosterols on lipid levels and cholesterol biosynthesis in hamsters. Lipids. 2003;38:165–170. doi: 10.1007/s11745-003-1047-3. [DOI] [PubMed] [Google Scholar]
- 15.Oliaro-Bosso S, Calcio Gaudino E, Mantegna S, Giraudo E, Meda C, Viola F, Cravotto G. Regulation of HMGCoA reductase activity by policosanol and octacosadienol, a new synthetic analogue of octacosanol. Lipids. 2009;44:907–916. doi: 10.1007/s11745-009-3338-y. [DOI] [PubMed] [Google Scholar]
- 16.Clarke PR, Hardie DG. Regulation of HMG-CoA reductase: identification of the site phosphorylated by the AMP-activated protein kinase in vitro and in intact rat liver. Embo J. 1990;9:2439–2446. doi: 10.1002/j.1460-2075.1990.tb07420.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Beg ZH, Stonik JA, Brewer HB., Jr 3-Hydroxy-3-methyl-glutaryl coenzyme A reductase: regulation of enzymatic activity by phosphorylation and dephosphorylation. Proc Natl Acad Sci USA. 1978;75:3678–3682. doi: 10.1073/pnas.75.8.3678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 19.Carling D, Sanders MJ, Woods A. The regulation of AMP-activated protein kinase by upstream kinases. Int J Obes (Lond) 2008;32(Suppl 4):S55–S59. doi: 10.1038/ijo.2008.124. [DOI] [PubMed] [Google Scholar]
- 20.Rizzo WB, Craft DA, Dammann AL, Phillips MW. Fatty alcohol metabolism in cultured human fibroblasts. Evidence for a fatty alcohol cycle. J Biol Chem. 1987;262:17412–17419. [PubMed] [Google Scholar]
- 21.Kabir Y, Kimura S. Biodistribution and metabolism of orally administered octacosanol in rats. Ann Nutr Metab. 1993;37:33–38. doi: 10.1159/000177746. [DOI] [PubMed] [Google Scholar]
- 22.Menendez R, Marrero D, Mas R, Fernandez I, Gonzalez L, Gonzalez RM. In vitro and in vivo study of octacosanol metabolism. Arch Med Res. 2005;36:113–119. doi: 10.1016/j.arcmed.2004.12.006. [DOI] [PubMed] [Google Scholar]
- 23.Za’tara G, Bar-Tana J, Kalderon B, Suter M, Morad E, Samovski D, Neumann D, Hertz R. AMPK activation by long chain fatty acyl analogs. Biochem Pharmacol. 2008;76:1263–1275. doi: 10.1016/j.bcp.2008.08.028. [DOI] [PubMed] [Google Scholar]
- 24.Menendez R, Mas R, Amor AM, Rodeiros I, Gonzalez RM, Alfonso JL. Inhibition of cholesterol biosynthesis in cultured fibroblasts by D003, a mixture of very long chain saturated fatty acids. Pharmacol Res. 2001;44:299–304. doi: 10.1006/phrs.2001.0851. [DOI] [PubMed] [Google Scholar]
- 25.Beg ZH, Stonik JA, Brewer HB., Jr Modulation of the enzymic activity of 3-hydroxy-3-methylglutaryl coenzyme A reductase by multiple kinase systems involving reversible phosphorylation: a review. Metabolism. 1987;36:900–917. doi: 10.1016/0026-0495(87)90101-6. [DOI] [PubMed] [Google Scholar]
- 26.Shaw RJ, Lamia KA, Vasquez D, Koo SH, Bardeesy N, Depinho RA, Montminy M, Cantley LC. The kinase LKB1 mediates glucose homeostasis in liver and therapeutic effects of metformin. Science. 2005;310:1642–1646. doi: 10.1126/science.1120781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hargrove JL, Greenspan P, Hartle DK. Nutritional significance and metabolism of very long chain fatty alcohols and acids from dietary waxes. Exp Biol Med (Maywood) 2004;229:215–226. doi: 10.1177/153537020422900301. [DOI] [PubMed] [Google Scholar]
- 28.Hawley SA, Pan DA, Mustard KJ, Ross L, Bain J, Edelman AM, Frenguelli BG, Hardie DG. Calmodulin-dependent protein kinase kinase-beta is an alternative upstream kinase for AMP-activated protein kinase. Cell Metab. 2005;2:9–19. doi: 10.1016/j.cmet.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 29.Sakakibara S, Yamauchi T, Oshima Y, Tsukamoto Y, Kadowaki T. Acetic acid activates hepatic AMPK and reduces hyperglycemia in diabetic KK-A(y) mice. Biochem Biophys Res Commun. 2006;344:597–604. doi: 10.1016/j.bbrc.2006.03.176. [DOI] [PubMed] [Google Scholar]
- 30.Haim D, Berrios M, Valenzuela A, Videla LA. Trace quantification of 1-octacosanol and 1-triacontanol and their main metabolites in plasma by liquid-liquid extraction coupled with gas chromatography-mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci. 2009;877:4154–4158. doi: 10.1016/j.jchromb.2009.10.034. [DOI] [PubMed] [Google Scholar]
- 31.Keller S, Gimmler F, Jahreis G. Octacosanol administration to humans decreases neutral sterol and bile acid concentration in feces. Lipids. 2008;43:109–115. doi: 10.1007/s11745-007-3127-4. [DOI] [PubMed] [Google Scholar]
- 32.Wong JM, de Souza R, Kendall CW, Emam A, Jenkins DJ. Colonic health: fermentation and short chain fatty acids. J Clin Gastroenterol. 2006;40:235–243. doi: 10.1097/00004836-200603000-00015. [DOI] [PubMed] [Google Scholar]
- 33.Kawaguchi T, Osatomi K, Yamashita H, Kabashima T, Uyeda K. Mechanism for fatty acid “sparing” effect on glucose-induced transcription: regulation of carbohydrate-responsive element-binding protein by AMP-activated protein kinase. J Biol Chem. 2002;277:3829–3835. doi: 10.1074/jbc.M107895200. [DOI] [PubMed] [Google Scholar]
- 34.Zydowo MM, Smolenski RT, Swierczynski J. Acetate-induced changes of adenine nucleotide levels in rat liver. Metabolism. 1993;42:644–648. doi: 10.1016/0026-0495(93)90225-d. [DOI] [PubMed] [Google Scholar]