Abstract
Purpose:
An effective treatment modality for posterior eye diseases would provide prolonged delivery of therapeutic agents, including macromolecules, to eye tissues using a safe and minimally invasive method. The goal of this study was to assess the ability of a thermosetting gel to deliver a fluorescently labeled protein, Alexa 647 ovalbumin, to the choroid and retina of rats following a single subconjunctival injection of the gel. Additional experiments were performed to compare in vitro to in vivo ovalbumin release rates from the gel.
Methods:
The ovalbumin content of the eye tissues was monitored by spectrophotometric assays of tissue extracts of Alexa 647 ovalbumin from dissected sclera, choroid, and retina at time points ranging from 2 h to 14 days. At the same time points, fluorescence microscopy images of tissue samples were also obtained. Measurement of intact ovalbumin was verified by LDS-PAGE analysis of the tissue extract solutions. In vitro release of Alexa 488 ovalbumin into 37°C PBS solutions from ovalbumin-loaded gel pellets was also monitored over time by spectrophotometric assay. In vivo ovalbumin release rates were determined by measurement of residual ovalbumin extracted from gel pellets removed from rat eyes at various time intervals.
Results:
Our results indicate that ovalbumin concentrations can be maintained at measurable levels in the sclera, choroid, and retina of rats for up to 14 days using the thermosetting gel delivery system. The concentration of ovalbumin exhibited a gradient that decreased from sclera to choroid and to retina. The in vitro release rate profiles were similar to the in vivo release profiles.
Conclusions:
Our findings suggest that the thermosetting gel system may be a feasible method for safe and convenient sustained delivery of proteins to choroidal and retinal tissue in the posterior segments of the eye.
Introduction
Delivering therapeutic agents to the posterior segment of the eye presents many challenges.1 Among the issues that must be addressed in ocular delivery of drugs is the attainment of adequate concentrations of drugs at the affected site, maintenance of these therapeutic levels for prolonged periods of time ranging from weeks to months, and ease and safety of delivery with minimal intervention. Numerous eye diseases, such as diabetic retinopathy, uveitis, cytomegaloviral (CMV) retinitis, and age-related macular degeneration (AMD), would benefit from an effective and safe method of posterior drug delivery. AMD, which accounts for over 50% of the cases of vision loss in the United States,2 is becoming a particularly serious health issue as it affects a growing number of people in our aging population.3,4 A number of drugs are already being tested for treating AMD.5,6 Other drugs also hold promise for treatment. Potential therapeutic agents for ocular diseases vary in molecular size from relatively small synthesized drugs to medium-sized aptamers and polypeptides7 to large proteins and macromolecules. Molecular size is among the many factors that may affect penetration of agents into eye tissue.8–10 Individual treatment methods may have to be specifically tailored to each drug.
At this time, approved treatments for posterior eye diseases, like AMD, involve repeated intravitreal injections of therapeutic solutions. There is a concern that repeated intravitreal injections can cause problems such as infection, retinal detachment, and hemorrhage.11 The notion of using a sustained release method for subconjunctival delivery of drugs, instead, holds attraction if such a method would eliminate these problems and still deliver effective levels of drug. However, subconjunctival methods have so far been shunned, in good part, because of the uncertainty of their ability to deliver ample levels of drug to affected sites in the choroid and retina for long periods of time. Numerous ocular drug transport barriers between the subconjunctival space and the deeper eye tissues have been identified.10 These barriers include scleral diffusion resistance, selective retinal pigment epithelium (RPE) permeability, metabolic degradation of the drug, and clearance mechanics due to conjunctival blood and lymphatic flow and due to choroidal blood flow. The question remains as to whether these barriers are too formidable to allow delivery of certain types of drugs and particularly to maintain sustained delivery for periods of weeks or months.
The goal of our work was to determine if a protein, as a surrogate therapeutic macromolecule, could be delivered to choroidal and retinal tissues for periods of weeks using a single subconjunctival injection of a sustained release thermosetting gel. The model protein that we studied was fluorescently labeled ovalbumin, a serpin protein, with physical characteristics similar to pigment epithelial-derived factor (PEDF), a known antiangiogenic and neurotrophic factor. After injection of the ovalbumin-loaded gel, we monitored the presence of ovalbumin in the sclera, choroid, and retina of rats by fluorescence imaging of histological sections, as well as by spectrofluorimetric analysis of labeled ovalbumin in dissected tissue extracts. As part of the analysis of our gel delivery scheme, we also compared the in vitro and in vivo release rate profiles from the gel. The results of our study provide evidence that proteins can be delivered by a trans-scleral route to the choroid/RPE and retina tissue for prolonged periods. The thermosetting gel delivery system may prove to be a viable, safe, and effective method for sustained delivery of therapeutic agents for treatment of numerous ocular diseases.
Methods
Materials
ReGel™, a biodegradable and thermosensitive triblock copolymer12 consisting of poly(lactic-co-glycolic acid) (PLGA) and polyethylene glycol (PEG), was obtained from Protherics, Salt Lake City Inc., a BTG PLC Company. Ovalbumin (45 kDa), conjugated with either Alexa Fluor 488 or Alexa Fluor 647, was obtained from Molecular Probes (Eugene, OR). All organic solvents were obtained from Fisher Scientific (Suwanee, GA).
Spectrofluorimetry of Alexa Fluor ovalbumin conjugates
Alexa Fluor 488 and Alexa Fluor 647 ovalbumin conjugate concentrations in solution were measured by a Fluoromax-4 photon counting spectrofluorimeter (Horiba Jorbin Yvon, Edison, NJ). The excitation and emission wavelengths used for the Alexa Fluor 488 and Alexa Fluor 647 conjugates were 495 nm/517 nm and 650 nm/668 nm, respectively. All in vitro experiments were performed with Alexa 488 ovalbumin, while Alexa 647 ovalbumin was used in the in vivo experiments due to its lower background interference from autofluorescence.
In vitro release analysis
A series of in vitro release experiments were performed to examine the release characteristics of ovalbumin from ReGel pellets. Ovalbumin-loaded pellets were made by injecting 25 μL of ovalbumin ReGel mixture into the bottom of microcentrifuge tubes and warming the gel at 37°C for 20 min. Three loading doses of ovalbumin in ReGel were studied: 2.0, 1.0, and 0.5 mg ovalbumin/mL ReGel for total loads of 50, 25, and 12.5 μg. Three pellets at each loading dose were tested.
After each pellet had gelled, 1 mL of PBS was added to the microcentrifuge tube. The tube with the PBS-covered pellet was then placed in a constant temperature, quiescent bath maintained at 37°C and protected from light. At various time intervals over a cumulative period of 23 days, the microcentrifuge tube was removed from the bath and the supernatant (∼1 mL) was removed for spectrofluorimetry analysis of the concentration of released ovalbumin. The supernatant was replaced with 1 mL of fresh PBS and the sample tube was then replaced in the bath to continue the release experiment. The cumulative amount of ovalbumin was calculated from our data by successively adding the collected amount from each time interval.
In vivo studies of subconjunctival injections
Male Brown Norway rats 5 to 6 weeks old were used and were treated according to NIH Office of Animal Care and Use Regulations and Standards. The rats were anesthetized with 150 μL of a 1:1 combination of ketamine (40–80 mg/kg) and xylazine (10–12 mg/kg) by intraperitoneal injection. In some cases, additional anesthetic was needed to maintain appropriate levels of anesthesia. Using a repeat, handheld automatic microsyringe dispenser (Hamilton syringe; Hamilton Co., Reno, NV), 20 μL of sterile ReGel containing 2 mg Alexa 647 ovalbumin/mL ReGel (40 μg Alexa ovalbumin total) were injected into the temporal subconjunctival space of each eye using a half inch long, 30-gauge needle. The vial of ReGel solution was held in an ice bath prior to injection to maintain its liquid form. At various time points from 2 h to 14 days, euthanasia was performed and the eyes were enucleated. Two separate in vivo experiments were performed, each with 2 rats (4 eyes) per time point. In total, therefore, 4 rats were injected for each time point providing 8 eyes for analysis at each time point. Of the 8 eyes, 2 different rats provided 1 eye each for histologic analysis at each time point. Four of the rats provided the remaining 6 eyes for tissue extraction analysis at each time point. Except for the 2 eyes per time point that were used for histology, each of the 6 eyes at each time point provided pellets that were recovered and used to assay for the amount of residual ovalbumin for eventual calculation of the amount of ovalbumin released from that pellet during the experimental time interval (procedure described below).
In vivo release rate determination
At euthanasia times from 2 h to 14 days, residual ReGel pellets were recovered from 6 of the enucleated eyes per time point by blunt dissection around the conjunctival pocket. Location of the ReGel pellets at the time of enucleation was visualized by the residual faint blue color of the Alexa 647 in the depot. In some cases, the conjunctival tissue and ReGel pellet were interlaced. Either a cleanly dissected ReGel pellet or the entire conjunctival pocket with adherent ReGel was placed in a centrifuge tube with 1 mL of PBS and sonicated gently 10 times in 1 s pulses. The tube with pellet in 1 mL PBS was then placed on a hand-motion shaker and maintained at 4°C for up to 2 weeks to allow the ReGel to transition back to liquid form and to permit the remaining ovalbumin to diffuse out of the ReGel into the surrounding PBS. The tubes were then centrifuged in a precooled centrifuge at 10,000g for 15 min at 4°C and kept on ice from that point forward. The supernatant, containing the ovalbumin that was extracted from the ReGel pellet, was analyzed for the Alexa 647-conjugated ovalbumin with a spectrofluorimeter. The amount released by the pellet over the experimental time period was determined by the difference between the amount recovered in the extract and the known initial amount in the fresh pellet. A total of 6 pellets were retrieved for each time point. Control pellets loaded with known amounts of ovalbumin were subjected to the extraction procedure and assayed for ovalbumin to determine the efficiency of extraction and recovery.
Histology and fluorescence microscopy analysis
At each of the time points for tissue dissection, 2 of the 8 harvested eyes were prepared for microscopy by fixation in 4% paraformaldehyde for 4 h and then transferred to PBS. The conjunctiva and pellet were removed, along with the anterior segment, which included the cornea and lens. The vitreous was carefully removed, taking care to ensure that the retina remained attached. The remaining posterior cup was then cut in half to produce a nasal section and a temporal section, each of which was set in an 8% agarose (low-gelling temperature; Invitrogen, Carlsbad, CA) gel and 100 μm sections were cut using a vibratory microtome (Vibratome, St. Louis, MO). The sections were stained with 4′,6-diamidino-2-phenylindole (DAPI) and imaged on an optical sectioning fluorescent microscope (Axio Imager with Apotome system; Carl Zeiss, Gottingen, Germany). The images were analyzed with 2 fluorescent channels, DAPI and Cy5 (for Alexa 647), and by differential interference contrast (DIC). The DIC allowed for more detailed visualization of anatomical structures and the different layers of the eye. At any given time point, the nasal and temporal sections received the same exposure time and contrast for each channel to allow for comparability between these 2 locations. Exposure times were adjusted from the early to the late time points for optimum imaging since lesser amounts of Alexa-labeled ovalbumin were present at the later times.
Quantification of tissue ovalbumin by dissection and extraction
To calculate the amounts of ovalbumin in individual eye tissues (retina, choroid/RPE, and sclera) at different time points, enucleated eyes were dissected and the labeled ovalbumin was extracted from the tissues and analyzed. For each eye, the retina, choroid/RPE, and sclera were separated from each other during the dissection and each tissue placed separately into 1 mL of a tissue protein extraction reagent (T-Per; Pierce, Rockford, IL) with protease inhibitors (including EDTA) (Halt Protease Inhibitor; Thermo Scientific, Rockford, IL) in a microcentrifuge tube and allowed to sit at 4°C overnight. The next morning, each sample was homogenized with a mechanical homogenizer (PRO200; PRO Scientific Inc., Oxford, CT) at 10,000–13,000 rpm and then centrifuged at 10,000g for 15 min to remove debris. The supernatant was removed from each tube and placed in a conical centrifugal filter (concentrator) (Amicon Ultra; Millipore, Billerica, MA) with a molecular weight cutoff of 10,000. The samples were then concentrated at a speed of 4,000g for ∼8 min. The retentate (containing full-length, 45-kDa ovalbumin) was removed and its final volume was determined by weight (assuming a density of 1 g/mL). In some cases, a small volume of T-Per solution was added back to the sample to bring the final volume to approximately 150–200 μL to fill a sample cuvette. The amount of ovalbumin in the samples was then analyzed by the fluorescence label. For each time point, extracts of retina, choroid/RPE, and sclera from 6 separate eyes were analyzed and the average amount of ovalbumin in each tissue type was determined.
Calibration curves were developed for fluorescence assays of the Alexa 647 ovalbumin that was extracted from each tissue (retina, choroid, and sclera). Instead of using PBS to create calibration samples of known ovalbumin concentrations, we used extract fluid from fresh, noninjected eyes. The blank tissue extraction solutions that were used for the calibration were processed with the same dissection and extraction procedure as used for the unknown sample tissues. To the extraction milieu from the noninjected eyes, various known amounts of Alex 647 ovalbumin were serially added to generate calibration samples of increasing known concentration. The fluorescence was analyzed after each addition. Standard curves were generated for retina, sclera, and choroid/RPE tissue extract solutions and these curves were used to calculate the concentration of ovalbumin in the unknown tissue extracts.
LDS-PAGE analysis of protein extracts
After spectrofluorimetric analysis of the experimental tissue extraction solutions, these extract solutions were then analyzed by LDS-PAGE. For each tissue type, retina, choroid/RPE, and sclera and at each time point, one representative extract sample was chosen for LDS-PAGE assay. The protein in the sample was precipitated using trichloroacetic acid (TCA; MG Scientific, Inc., Pleasant Prairie, WI). In brief, an equal volume of cold 30% TCA was added to the sample, which was between 150 and 200 μL, and the sample was left on ice for 15 min. The precipitated protein was collected by centrifuging the sample at 10,000g for 15 min at 4°C. The supernatant was removed and the remaining pellet was washed with acetone. The sample was then centrifuged at 10,000g at room temperature. The acetone was removed and the pellet was allowed to air dry.
The protein extract was reconstituted in appropriate amounts of water, lithium dodecyl sulfate (LDS) sample buffer (NuPage; Invitrogen, Carlsbad, CA), and sample reducing agent (NuPage; Invitrogen, Carlsbad, CA). LDS-PAGE chromatography was performed using a 10% acrylamide bis–tris gel (NuPage; Invitrogen). Fluorescent molecular weight markers were also run on the gels (Sigma-Aldrich, Inc., St. Louis, MO). Gels were then scanned for fluorescence using a Typhoon scanner (GE Healthcare, Piscataway, NJ) set to an excitation wavelength of 633 nm and an emission filter of 670 nm with a band pass of 30 nm to detect the Alexa 647. The gels were later stained for total protein loading with a Coomassie blue stain (Brilliant Blue R; Sigma-Aldrich, Inc., St. Louis, MO) for 45 s in the microwave and then for 15 min at room temperature. The gels were destained in a solution of 40% methanol and 7% acetic acid in the microwave for 45 s and then at room temperature for 2 h. The gels were then scanned by a digital scanner to create digital images.
The intensities of the fluorescent bands on the Typhoon scan were analyzed with gel digitizing software (Un-Scan-It; Silk Scientific Corp, Orem, UT) and corrected for protein loading as shown by the Coomassie blue staining. The corrected intensity was then normalized to the highest value, which was seen at 2 h, and plotted on a time scale. Because of variations in the gel assays of the retina samples, analyses were performed on 2 sets of retina samples instead of just 1, and the average normalized value was plotted with respect to time. The plots of fluorescent image intensity versus time obtained from the LDS-PAGE data were then compared to the data obtained from the spectrofluorimetric analysis of the tissue extracts.
Results
In vitro ovalbumin ReGel release study
The results of the in vitro release of ovalbumin from ReGel pellets are depicted in Figure 1. Figure 1A shows that the ovalbumin release had the typical release profile for a biodegradable matrix hydrogel with an initial burst release followed by a longer phase of slower release. Three release curves are given in Figure 1A for 3 different loading doses of ovalbumin in the ReGel. The amount of ovalbumin in the initial burst is proportional to the loading dose. A later portion of the release curve after the burst is nearly linear and we chose that time period from 2 days to 15 days to create a metric of the release rate as a function of the loading dose. To determine this metric, we measured the slope of the quasi-linear phase of each release curve in Figure 1A and considered this to be a measure of the quasi-steady state in vitro release rate, μg/day, for each of the 3 loading doses that were studied. The slopes of the 3 lines are plotted in Figure 1B as a function of the loading dose. We note a reasonably linear relationship between the in vitro release rate and the loading dose as determined from the period between 2 and 15 days. At the longest times of the experiment when the load of ovalbumin is nearly exhausted, we would expect the release rate and the slopes of the lines to eventually and gradually begin to decline to zero.
FIG. 1. .
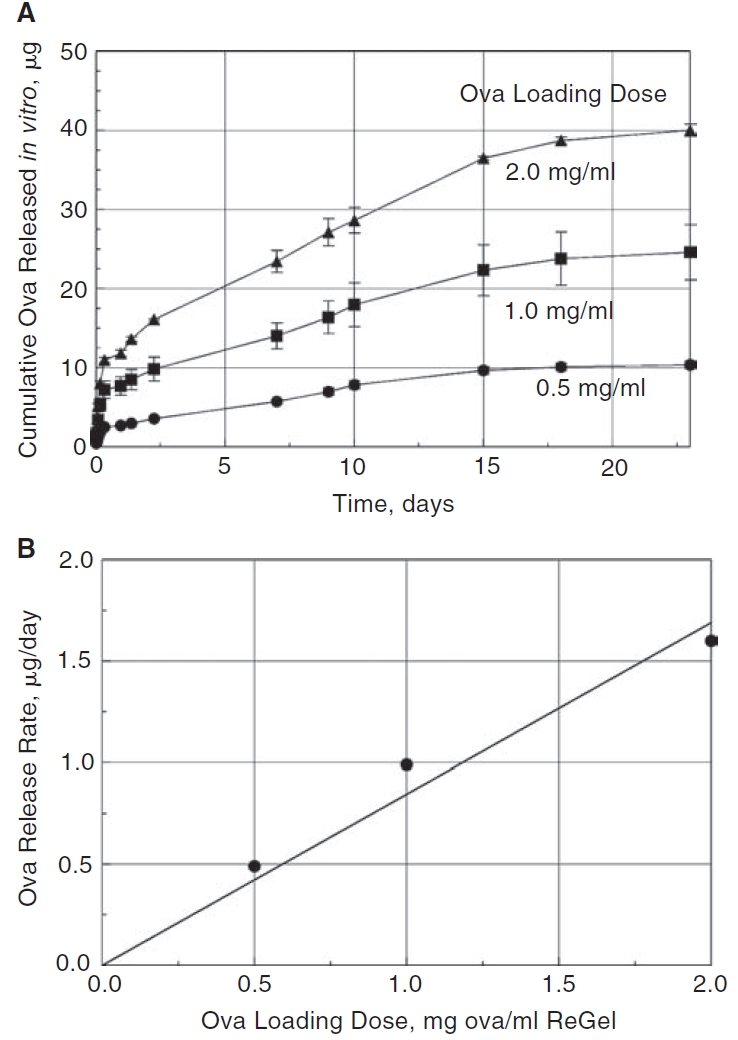
In vitro release of Alexa 488 ovalbumin (Ova) from ReGel pellets into PBS at 37°C. (A) Cumulative mass (μg) of ovalbumin released over time from 25 μL ReGel pellets composed of 3 different loading doses of ovalbumin (2.0, 1.0, and 0.5 mg ovalbumin/mL ReGel yielding total load amounts of 50, 25, and 12.5 μg). Three pellets were analyzed at each loading dose. (B) A linear regression line correlating the initial ReGel loading concentrations of Alexa ovalbumin with a linear release rate phase (R2 = 0.94). From the 3 curves in A, the slopes, μg/day, of the portion of the lines from 2 to 15 days were determined as representative of a phase of quasi-linear release rate.
In vivo ovalbumin ReGel release study
We conducted an extensive set of measurements to determine the in vivo release of ovalbumin from the ReGel pellets during the subconjunctival injection experiments in the rats. The loading dose for these experiments was 2.0 mg ovalbumin/mL ReGel. Figure 2A shows the approximate placement of the 20 μL injection of the ovalbumin-loaded ReGel under the conjunctival tissue in the temporal region of the rat eye. Figure 2B is a schematic representation of the ReGel bleb relative to the eye tissues that were later dissected for determination of ovalbumin amounts. The amount of Alexa 647-labeled ovalbumin that released over time into the rat eyes from the solidified ReGel bleb was determined and this data is plotted in Figure 3. Two separate sets of rats were used to obtain in vivo release data from 2 independent sets of experiments. Figure 3 represents the data from all 6 eyes that were part of these 2 experiments. The total load of ovalbumin in the 20 μL injection (2.0 mg/mL) was 40 μg. After the initial burst release of ovalbumin, there was an interim time from 2 h to 2 days where there was a fast release that gradually slowed to a quasi-linear phase of release from 2 days to 14 days, similar to the in vitro data. The slope in Figure 3 from 2 days to 14 days gives an estimate of the quasi-linear in vivo release rate of 0.9 μg/day. This value can be compared to the in vitro release rate from Figure 1B of 1.6 μg/day over a comparable time period and at the same loading dose of 2.0 mg ovalbumin/mL ReGel.
FIG. 2. .
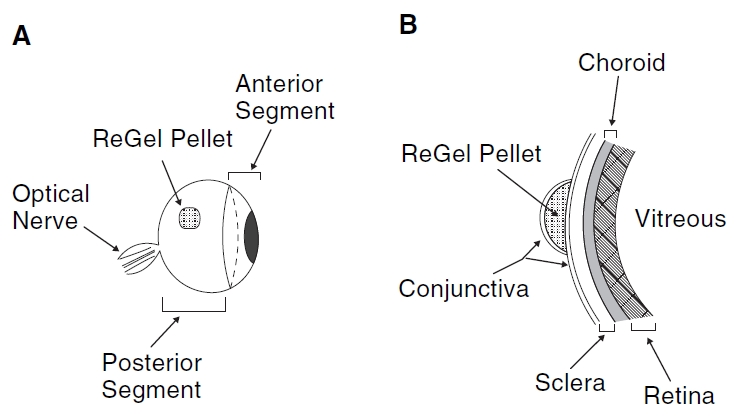
Schematic diagram of the in vivo delivery procedure. ReGel solutions containing protein were injected under the temporal conjunctival surface of rat eyes and the ReGel solidified to form pellets. At sequential time points, the animals were euthanized and the eyes were dissected. (A) Side view of an eye with ReGel pellet in a temporal location. (B) Enlarged cross-sectional view of the posterior region of an eye illustrating the ocular tissue layers that were dissected for this study, that is, the retina, choroid, and sclera. Anterior segments (lens and cornea) and the optic nerve were removed during tissue dissection.
FIG. 3. .
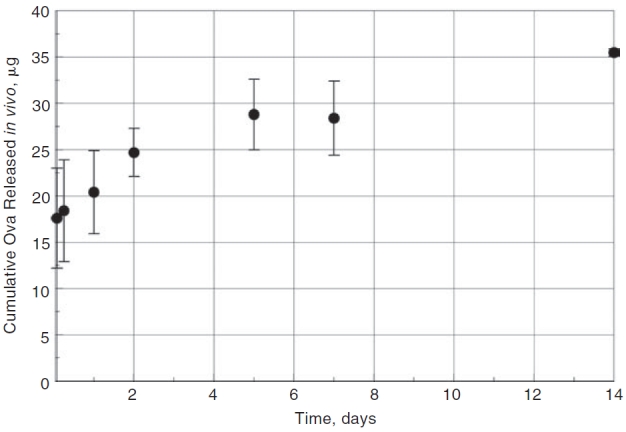
The cumulative in vivo release of Alexa 647 ovalbumin (Ova) from subconjunctival injections of ReGel into rat eyes. Two duplicate experimental sets were conducted providing a total of 6 eyes for release analysis (n = 6). Each experiment consisted of 20 μL injections of ReGel containing 2 mg Alexa ovalbumin/mL ReGel (40 μg ovalbumin total) into each eye. Pellets were recovered from eyes at sequential time points and the amount remaining in the pellet was extracted and measured by spectrofluorimetry. The amount of ovalbumin released during that time interval was estimated by the difference between the extracted amount and the loading amount. The slope of the linear portion of the line from 2 to 14 days, which serves as a metric of release rate, is 0.9 μg/day.
Histology and fluorescence microscopy
Histological examination by fluorescence microscopy was performed with 2 eyes from 2 separate rats at each time point. Representative photomicrographs of such eyes from 6 h and 24 h are shown in Figure 4A and 4B, respectively. These photos show a significant Cy5 signal (rose color) at 6 h postinjection in the temporal aspect of the eye, but not on the nasal side. At 6 h, the signal was strongest in the sclera that was nearest to the ReGel bleb. The signal gradually diminished toward the retina. By 24 h, the temporal gradient of Cy5 had diminished. Concomitantly, the Cy5 signal became visible in the nasal side of the section indicating that by 24 h the protein had moved throughout the eye.
FIG. 4. .
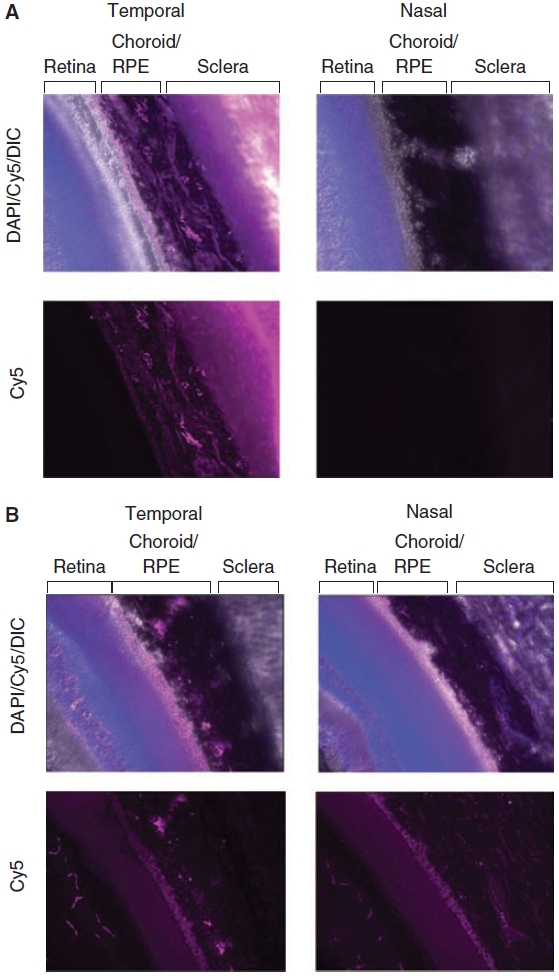
Fluorescence photomicrographs of representative tissue sections collected at 6 h (A) and 24 h (B) after temporal subconjunctival injections of Alexa ovalbumin in ReGel. Images show temporal and nasal sections of retina, choroid/retinal pigment epithelium (RPE), and sclera tissue from the same eye. Both DAPI (blue) and Cy5 (pink) fluorescent channels and DIC (white) for contrast are shown superimposed. Images with the Cy5 channel only, which indicate the location of Alexa 647 ovalbumin, are also shown for each tissue section. For each respective time point, both the nasal and the temporal images were taken with the same Cy5 exposure settings to ensure comparability.
The paragraphs that follow will discuss the quantitative aspects of both the spectrofluorimetric analysis of the tissue extracts and the LDS-PAGE assay of tissue dissections up to 14 days. The data from these assays support the notion that the Cy5 signal in our photomicrographs is related to intact Alexa 647 ovalbumin.
Tissue extraction data for measurement of Alexa 647 ovalbumin
Figure 5 shows the amount (ng) of Alexa 647 ovalbumin measured in the retina (A), choroid/RPE (B), and sclera (C), after tissue dissection, extraction, and spectrofluorimetric analysis for times up to 14 days from 2 separate experiments. Both experimental sets followed the same procedure and consisted of 3 eyes in each set at each time point. As described in the Methods section, the ovalbumin amounts reported in Figure 5 are calculated from the fluorescence values measured in the retentate solution obtained after the tissue extract was filtered with a 10-kDa cutoff filter. This cutoff value would retain the ovalbumin, which has a molecular size of 45 kDa. Further analysis of this retentate by LDS-PAGE is reported in the next section. Both sets of duplicate experiments representing a total of 6 eyes are plotted in Figure 5 and the 2 sets of data are consistent with each other. The ordinate scale (y-axis) of the 3 plots is different in order to reflect the significant differences in the amount of ovalbumin measured in each tissue. Sclera had the largest value and retina had the lowest. In each tissue, there was a substantial rise in the tissue levels of ovalbumin between 2 and 6 h after ReGel ovalbumin injection, followed by a rapid decline. Measurable levels of ovalbumin were seen in all tissues, including the retina, up to 14 days. The inserts in Figure 5 show an expanded scale for the smaller ovalbumin amounts at the longer times from 4 to 14 days. Since the actual values are difficult to read from the graph in Figure 5 at these lower values, these average ovalbumin amounts for 3 tissues at 14 days for both experiment sets #1 and #2 are given here as: sclera (2.3 ± 2.1 ng and 1.7 ± 0.05 ng); choroid (0.5 ± 0.7 ng and 0.3 ± 0.1 ng); and retina (0.13 ± 0.15 ng and 0.23 ± 0.05 ng).
FIG. 5. .
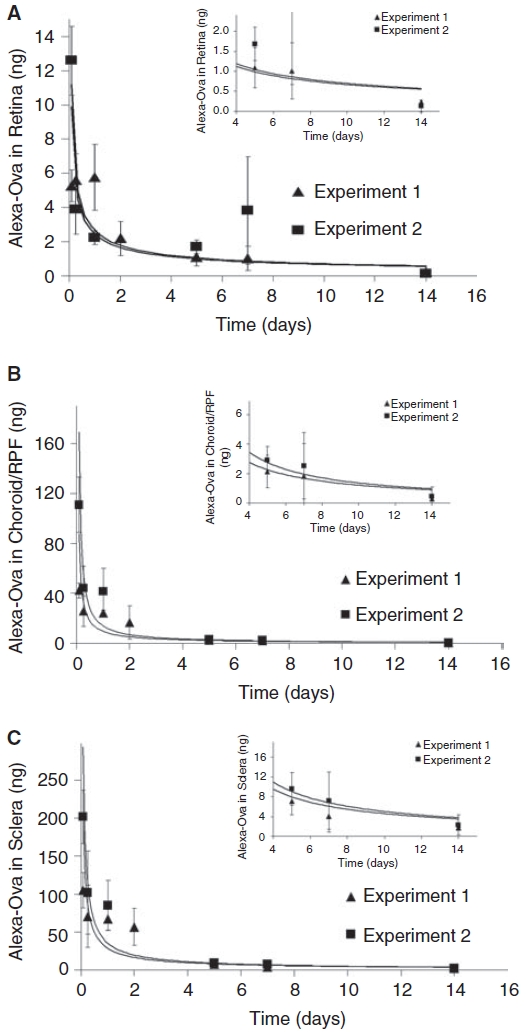
Quantification by spectrofluorimetric analysis of Alexa ovalbumin (Ova) in the tissue extracts at various times following subconjunctival ReGel injections in rat eyes. Two independent, identical experiments were conducted and both sets consisted of 20 μL injections of ReGel at 2 mg ovalbumin/mL ReGel (40 μg ovalbumin total). Three eyes from 2 rats were used in both experimental sets, providing a total of 6 eyes for tissue analysis at each time point. Tissues were dissected from the eyes at several time points and the ovalbumin in the tissue was extracted and measured by spectrofluorimetry. The amount of ovalbumin (ng) extracted from tissue homogenates of retina (A), choroid/RPE (B), and sclera (C) per eye as a function of time is shown. Each data point represents the mean ± SD from 3 eyes (n = 3) per experimental set. Two duplicate sets of experiments were performed and are reported separately giving a total of n = 6 at each time point.
LDS-PAGE analysis of the tissue extract retentate
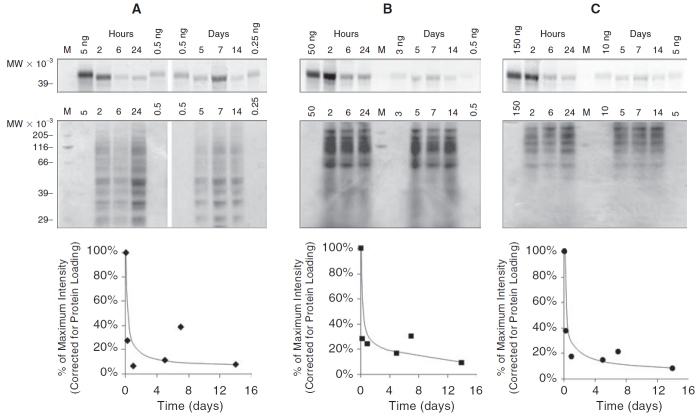
To further verify that the spectrofluorimetric measurements of the 10-kDa cutoff retentate solution of the tissue extracts are associated with the intact Alexa 647 ovalbumin, LDS-PAGE analysis was performed on aliquots of each retentate solution for each tissue at each experimental time point. Figure 6 shows images of the gels obtained from electrophoretic analysis of tissue extracts in the following order: retina (panel A); choroid/RPE (panel B); and sclera (panel C). The upper narrow horizontal gel images in each panel are the Typhoon images generated from the fluorescent-labeled extract solutions from 2 h to 14 days. The lanes are labeled at the top. The Typhoon fluorescence scanner was set to the Cy5 wavelength, so that these images represent the detection of the Alexa 647. The signal bands in the upper strips represent the fluorescently label ovalbumin since they occur vertically at an elution point in the gels that are consistent with a 45-kDa protein, as indicated by the Alexa-labeled 39 kDa reference on the left of the upper strip. Using the blank (no Alexa 647 ovalbumin) tissue extract milieu from our spectrofluorimetric calibrations, we determined that negligible autofluorescence background signal is generated from the other proteins in the extract solution. Therefore, we associate the Typhoon intensity signal in the upper strips with intact Alexa 647-labeled ovalbumin. The upper Typhoon strips are labeled on top with the time of the tissue sample from 2 h to 14 days. Also labeled on the top of the gel strips on the left, center, and right are reference samples of Alexa 647 ovalbumin of known amounts. These reference ovalbumin solutions range from a low of 0.25 ng for retina to a high of 150 ng for the sclera. The label “M” marks the lanes for a standard reference molecular weight ladder. This ladder does not contain a fluorescent marker and only shows up faintly in the Coomassie-stained lower gels strips.
FIG. 6. .
LDS-PAGE of eye tissue extracts from in vivo experiments. In brief, 20 μL of ReGel at 2 mg Alexa 647 ovalbumin/mL ReGel (40 μg Alexa ovalbumin total) were injected into rat eyes and after indicated periods of time, tissues were dissected from eyes, protein was extracted, resolved by LDS-PAGE, and compared to Alexa 647 ovalbumin gel standards. For each tissue type, retina (A), choroid/RPE (B), and sclera (C), the gels were prepared and scanned for fluorescence (Typhoon) to indicate the migration of ovalbumin that was extracted from each tissue (top narrow row of gel images). The gels were later stained with Coomassie Blue to determine total protein loading on the gels for each lane (larger middle row of gel images). The intensity of the Typhoon fluorescent signal was determined, corrected for protein loading, normalized to the maximum value and plotted over time from 2 h to 14 days (bottom row of graphs).
Different amounts of total protein were plated on the gel lanes for each time point in order to optimize the signal intensity for the varying levels of Alexa 647 ovalbumin. To normalize our results relative to the total protein load, we used Coommassie blue staining to determine the total protein loading in each sample. The Coommassie blue stained gels are shown for the extracts from each tissue type and for each time point just below the narrow Typhoon-imaged strips in Figure 6A–6C. The upper Typhoon images show that intact ovalbumin is detectable in all tissues, including the retina (panel A), up to 14 days. The graphs at the bottom of Figure 6A–6C provide a semiquantitative representation of ovalbumin levels. The data in these graphs represent the relative Cy5 ovalbumin fluorescence signal intensity from the Typhoon imager for each tissue, normalized for total protein loading, at various times up to 14 days. These gel signal intensity curves at the bottom of Figure 6 are similar in dynamics to the curves in Figure 5 that give the ovalbumin tissue levels obtained by the tissue extraction and spectrofluorimetry.
Discussion
We have demonstrated that a large molecule (ovalbumin, a serpin protein of molecular size 45 kDa) can be delivered across the sclera to the retina of rats for up to 14 days from a sustained release device. The delivery was achieved by a subconjunctival, 30-gauge needle injection of a thermosetting gel solution containing the protein. In our hands, this method of trans-scleral delivery was relatively easy to perform and was shown to be safe. We saw no evidence of inflammation, infection, or irritation to the eyes during the time period of our experiments.
A good correlation was obtained between our in vitro and in vivo release experiments. A validation of the in vitro–in vivo correlation allows a more confident extrapolation of the data from simpler, less labor intensive in vitro experiments to predictions of desired in vivo drug delivery amounts.
The in vitro release profiles that we obtained have the typical shape for a matrix hydrogel release mechanism. These systems usually give an initial burst release followed by a slower, gradually declining release rate.13,14 The release profiles obtained from our in vivo experiments were qualitatively similar to the in vitro release profiles, that is, an initial burst followed eventually by a period of quasi-steady release and then a gradual decline in release rate as the drug was spent.
The in vivo data of Figure 3 from 2 h to 2 days, which follows the burst, provides a short-term in vivo release rate value of 3.5 μg/day. Between 2 and 14 days, the slope of the quasi-linear phase of the in vivo release profile gives a release rate of ∼0.9 μg/day for the loading dose of 2.0 mg/mL ReGel. This longer term rate compares reasonable well with the in vitro release rate of 1.6 μg/day shown in Figure 1B that was calculated from the slope of the in vitro data from 2 to 15 days at the same 2.0 mg/mL ReGel loading dose. Considering the different environments that the pellets experienced in the in vivo and in vitro experiments, we feel that this similarity is significant. Also, the shape of the pellet and the surface-to-volume ratio of the solidified gel pellet in each case would be expected to influence the release rate. In the in vitro release study, fresh PBS was placed over the pellet after each sampling, approximating sink conditions for diffusion. In the in vivo experiment, a concentration gradient from the sclera inward was present. This difference of local environment may help to explain the lower release rate from the in vivo experiment. It also seems plausible that part of the explanation for the different release rates between the in vivo and in vitro experiments could be related to the difference in surface-to-volume ratio. The injected liquid volume of ReGel that dispersed under the conjunctival layer during the in vivo experiments likely varied in shape from the pellet shape created at the bottom of the conical microcentrifuge tube during the in vitro experiments.
One convenient parameter that we examined, in vitro, as a means to regulate the sustained release rate, was the initial loading dose of drug (protein). As a quantifiable measure of a longer term sustained release rate value, we chose a portion of the release curves in Figure 1A between 2 days and 15 days. In our data, this phase followed the burst and the shorter term (2 h and 2 days) faster release phase. The release rate during the 2 days to 15 days time increment correlated well with the loading dose, as seen in Figure 1B. At later times, as is typical of matrix delivery devices, the release rate gradually declined to zero as the payload of drug was depleted. The in vitro correlation of the release rate with loading dose and the similarity of the release profiles between the in vitro and the in vivo experiments suggests that loading dose is one adjustable parameter that can be used to modify the in vivo release rate in a predictable manner. The availability of such in vitro/in vivo correlations for release rates also provides important data that can be used in pharmacokinetic models aimed at predicting drug concentrations at target sites. Kato and colleagues15 did such a correlation for betamethasone. To the best of our knowledge, our study is unique in that it uses a macromolecule to verify a direct correlation between the commonly used in vitro release experiments and actual in vivo release data for a sustained release system.
The use of a 2.0 mg/mL loading dose and a 20 μL injection volume was an arbitrary choice for our in vivo experiments. Higher values of the initial protein loading should be possible. The drug miscibility or solubility in the delivery vehicle and the potential influence that high drug loads may have on the phase transition temperature of a thermosetting gel, that is, its ability to exist as a solution at or below room temperature and to be easily injected, may be a consideration when deciding on a drug load amount. Previously, protein/ReGel formulations with protein loading as high as 70 mg/mL have been successfully prepared and evaluated in vitro and in vivo.16 Higher initial drug loading would enable greater concentrations at the target site, in this case, the choroid and retina, and could maintain therapeutic levels for periods even longer than those of this study. Other limitations to the amount of the loading dose are the cost and availability of the drug. The 20 μL injection volume was deemed adequate in a rat to obtain a discernable bleb under the conjunctival tissue with a reasonable area of scleral surface coverage and minimal distress to the rat. With a larger injection volume, one may expect to have a higher and a more prolonged delivery duration of drug and higher drug concentrations due to the greater drug reservoir and due to a larger surface area of contact between the sclera and the bleb.
The photomicrographic images in Figure 4 provide qualitative evidence of the presence of Alexa marker in the various tissue layers. Tissue protein extractions and LDS-PAGE analysis of the individual ocular layers confirms that the fluorescence in the photomicrographs is associated with tagged ovalbumin. Little fluorescence is seen in the nasal region of the eye at 6 h since the injection was in the temporal area. By 24 h, fluorescence does appear in the nasal area, while the intensity of the fluorescence had diminished in the temporal region as Alexa ovalbumin moves out of that region. The full temporal profiles of the ovalbumin transport in the various tissue layers is shown in the graphs of Figures 5 and 6 that were obtained from the tissue extraction experiments. The ovalbumin levels from these data represent average values of the tissue layers from the entire globe and do not depict spatial resolution. The mechanism of transport of ovalbumin from the temporal area to the nasal region is not fully explained, but may include uveoscleral flow17,18 as well as diffusion.
The spectrofluorimetric assays of the tissue extracts shown in Figure 5 give a quantitative determination of ovalbumin amounts in each tissue layer. By analyzing the fluorescence in the retentate from the microcon filtrations of the tissue extract solution, we are measuring tagged ovalbumin of greater than 10 kDa. We assume that this represents the intact protein. The electrophoresis assay of the retentate shown in Figure 6 confirms that the molecule is in the 45-kDa range, equivalent to ovalbumin. The temporal behavior of the quantitative data in Figure 5 agrees quite well with the semiquantitative analysis of the gel fluorescence intensity measurements (Typhoon) in Figure 6. The data in Figure 5 shows that a concentration gradient of ovalbumin exists from sclera toward the retina. For example, in Figure 5, for experiment #1 and experiment #2 at 14 days, the respective average values of ovalbumin amounts for each tissue are: sclera (2.3 ± 2.1 ng and 1.7 ± 0.05 ng); choroid (0.5 ± 0.7 ng and 0.3 ± 0.1 ng), and retina (0.13 ± 0.15 ng and 0.23 ± 0.05 ng). Though these average values of ovalbumin amounts are low by 14 days, the readings are still measurable and significantly above background.
There are a number of possible sources of error in these experiments. For example, because of the high viscosity of the ReGel solution, the automated microsyringe dispenser can sometimes have small amounts of air trapped in the needle or syringe barrel that can result in slight variations in the small 20 μL volume of ReGel that is dispensed. Also, even though the ReGel transitions from a liquid to a gel relatively quickly, it is possible that small amounts of the ReGel injected into the subconjunctival space can leak out of the needle track when the syringe needle is withdrawn. This leakage can be avoided by compressing the injection site with tweezers for about 30 s. Any of the above mentioned factors could result in small losses of dose to the eye and lead to variations in the amounts delivered, but with care and proper technique, in our hands, we were able to minimize or avoid these losses. The bleb geometry in the rat eye is not controllable after an injection. Even at equal volumes of injection, slightly different bleb shapes are possible. Bleb shape differences may result in differences in the surface area of contact and in surface-to-volume variations that can give different in vivo release rates. Another source of variability in the amount of ovalbumin measured in a given tissue section can arise from the tissue dissection procedure, where complete removal of separate tissues regions may not always be achieved.
Overall, our data supports the notion that this gel system can provide sustained delivery of ovalbumin protein to the retina of rats for up to 14 days. In view of the many hypothesized transport barriers that would inhibit trans-scleral delivery from the subconjuntiva to the retina,11,19 should this result be surprising? Other researchers have shown that various compounds can reach retinal tissue via a trans-scleral route. For example, Amaral and colleagues20 measured both PEDF and ovalbumin up to 24 h after a single injection of fluorescently labeled compounds. The fluorescent signal was very strong by 1 h in choroid/RPE and retina, but by 6 h the signal was barely detectable, indicating a rapid clearance of these proteins. Degradation by matrix metalloproteases is also possible. Amaral and colleagues20 also tested a matrix polymer implant (polyvinyl alcohol) loaded with fluorescent ovalbumin. Their uptake results were similar to our ReGel injections. Their measurements included tissue histology and ovalbumin tissue extraction up to 24 h. Their data showed the presence of ovalbumin by 1 h primarily in the temporal eye tissue near the insertion site with a gradient from the sclera to the retina. By 24 h, the ovalbumin was more uniformly distributed from sclera to retina. The ReGel release system seems less traumatic than insertion of a solid implant and can still give prolonged and ample delivery. Mac Gabhann and colleagues21 measured GFP in rat ocular tissue after a single subconjunctival dose and correlated his data with a one-dimensional distributed mathematical model of the eye tissue. These studies did not attempt long-term trans-scleral delivery with a sustained delivery biodegradable polymer. Ambati and colleagues22 reported on the trans-scleral delivery of IgG to rabbit retina using a miniature osmotic pump with slow infusion via a catheter placed in a subconjuctival pocket for up to 30 days. Demetriades and colleagues23 delivered a variety of proteins to the eye after periocular injections either as the native protein or via an adenoviral vector expressing the protein, including PEDF. They measured the PEDF in the retina out to 24 h and found that the relative amount of PEDF in the retina was greater for the adenovirus-delivered PEDF than for the single PEDF injection. There are reports in the literature on the use of polymeric delivery systems for ocular drug delivery, but most reports to date with these systems do not attempt to deliver a macromolecule like ovalbumin. A number of intravitreal implant devices have been reported that delivered ganciclovir,24 integrin antagonists,25 and fluocinolone.26 There are also reports of trans-scleral delivery of betamethasone from implant devices,15 sodium fluorescein,27 and ganciclovir.28
One of the proposed barriers to trans-scleral delivery is represented by the choroidal blood flow clearance mechanism. Perhaps, the ovalbumin protein is large enough to breach this clearance mechanism and escapes rapid removal by the choroidal blood. Loss of drug may also occur at the site of the injection depot due to uptake by conjunctival blood flow. This loss may be significant for drugs that have large capillary permeability. Loss of drug by lymphatic flow near the conjunctival space has also been postulated. It is our belief, however, that this avenue of loss would be small compared to the loss by conjunctival blood flow for highly permeable (ie, smaller) molecules, since lymphatic flow is thought to be up to 100 times slower than blood flow in that region. For micro or large nanoparticles or large macromolecules, lymphatic clearance may become a route for removal. Another significant barrier to protein transport to the retina could be the RPE layer. Amaral and colleagues20 have reported that PEDF and ovalbumin can cross RPE layers both in vivo and in cell culture. There is no certainty that the permeability of RPE in culture would be the same as that in vivo, but our data would suggest that the RPE permeability in vivo is great enough to allow significant transport of ovalbumin to the retinal layers. The Amaral study required laborious and frequently invasive daily injections to achieve measurable levels in retinal tissue. Our delivery method involved a one-time injection of a liquid thermosetting gel that was capable of delivering measurable ovalbumin levels for up to 14 days. We feel that this method is only minimally invasive and that less frequent subconjunctival injections of a liquid would seem easier and safer than other potential methods such as intravitreal injections or insertion of solid implants. A device with a zero order, constant delivery rate would be most desirable, which may be a minor drawback with the gel system that gives a small burst followed by a declining delivery rate. However, the ReGel system is biodegradable that obviates the need for retrieval of a spent non-biodegradable implant device. The trade-offs on the various trans-scleral delivery methods would have to be assessed as we attempt to obtain the best therapeutic outcome. Studies that report a specific target concentration for the drug of choice are most valuable. Information about a desired target therapeutic concentration would be necessary in order to set a proper delivery rate from a device or vehicle. In addition, other mechanisms in the eye such as metabolism and clearances would have to be known and quantitated in order to compute an appropriate delivery rate that would be needed to obtain a desired tissue concentration. In our system, we feel that the delivery rate can be adjusted by varying the loading dose, among other options.
Acknowledgments
We would like to acknowledge Dr. Ramesh Rathi and Dr. Kirk Fowers of Protherics, Salt Lake City Inc., a BTG PLC Company for making the ReGel available and for their technical consultations. We thank Dr. Maria Campos for her assistance with preparing and analyzing the histological specimens. Our appreciation also goes to Paul Guillod who assisted with the in vitro release rate study. This research was supported by the Intramural Research Program of the NIH, including NEI and NIBIB.
Contributor Information
Erin R. Rieke, Laboratory of Bioengineering and Physical Sciences, National Institute of Biomedical Imaging and Bioengineering, National Eye Institute, National Institutes of Health, Bethesda, Maryland.
Juan Amaral, Section on Protein Structure and Function, Laboratory of Retinal Cell and Molecular Biology, National Eye Institute, National Institutes of Health, Bethesda, Maryland..
S. Patricia Becerra, Section on Protein Structure and Function, Laboratory of Retinal Cell and Molecular Biology, National Eye Institute, National Institutes of Health, Bethesda, Maryland..
Robert J. Lutz, Laboratory of Bioengineering and Physical Sciences, National Institute of Biomedical Imaging and Bioengineering, National Eye Institute, National Institutes of Health, Bethesda, Maryland.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Duvvuri S, Majumdar S, Mitra A.K. Drug delivery to the retina: challenges and opportunities. Expert Opin. Biol. Ther. 2003;3:45–56. doi: 10.1517/14712598.3.1.45. [DOI] [PubMed] [Google Scholar]
- 2.The Eye Diseases Prevalence Research Group Causes and prevalence of visual impairment among adults in the United States. Arch. Ophthalmol. 2004;122:477–485. doi: 10.1001/archopht.122.4.477. [DOI] [PubMed] [Google Scholar]
- 3.Bressler N.M. Age-related macular degeneration is the leading cause of blindness. JAMA. 2004;291:1900–1901. doi: 10.1001/jama.291.15.1900. [DOI] [PubMed] [Google Scholar]
- 4.Friedman D.S, O’Colmain B.J, Muñoz B, et al. Eye Diseases Prevalence Research Group. Prevalence of age-related macular degeneration in the United States. Arch. Ophthalmol. 2004;122:564–572. doi: 10.1001/archopht.122.4.564. [DOI] [PubMed] [Google Scholar]
- 5.Bressler N.M. Treatment of Age-Related Macular Degeneration with Photodynamic Therapy (TAP) Study Group. Photodynamic therapy of subfoveal choroidal neovascularization in age-related macular degeneration with verteporfin: two-year results of 2 randomized clinical trials-tap report 2. Arch. Ophthalmol. 2001;119:198–207. [PubMed] [Google Scholar]
- 6.Rosenfeld P.J, Brown D.M, Heier J.S, et al. MARINA Study Group. Ranibizumab for neovascular age-related macular degeneration. N. Engl. J. Med. 2006;355:1419–1431. doi: 10.1056/NEJMoa054481. [DOI] [PubMed] [Google Scholar]
- 7.Li H, Tran V.V, Hu Y, et al. A PEDF N-terminal peptide protects the retina from ischemic injury when delivered in PLGA nanospheres. Exp. Eye Res. 2006;83:824–833. doi: 10.1016/j.exer.2006.04.014. [DOI] [PubMed] [Google Scholar]
- 8.Ambati J, Canakis C.S, Miller J.W, et al. Diffusion of high molecular weight compounds through sclera. Invest. Ophthalmol. Vis. Sci. 2000;41:1181–1185. [PubMed] [Google Scholar]
- 9.Pitkänen L, Ranta V.P, Moilanen H, et al. Permeability of retinal pigment epithelium: effects of permeant molecular weight and lipophilicity. Invest. Ophthalmol. Vis. Sci. 2005;46:641–646. doi: 10.1167/iovs.04-1051. [DOI] [PubMed] [Google Scholar]
- 10.Geroski D.H, Edelhauser H.F. Drug delivery for posterior segment eye disease. Invest. Ophthalmol. Vis. Sci. 2000;41:961–964. [PubMed] [Google Scholar]
- 11.Del Amo E.M, Urtti A. Current,and future ophthalmic drug delivery systems. A shift to the posterior segment. Drug Discov. Today. 2008;13:135–143. doi: 10.1016/j.drudis.2007.11.002. [DOI] [PubMed] [Google Scholar]
- 12.Rathi R.C, Zentner G.M, Jeong B. Biodegradable low molecular weight triblock poly(lactide-co-glycolide) polyethylene glycol copolymers having reverse thermal gelation properties. US Patent. 2001;6 [Google Scholar]
- 13.Huang X, Brazel C.S. On the importance and mechanisms of burst release in matrix-controlled drug delivery systems. J. Control. Release. 2001;73:121–136. doi: 10.1016/s0168-3659(01)00248-6. [DOI] [PubMed] [Google Scholar]
- 14.Zhang Y, Chu C.C. Biodegradable dextran–polylactide hydrogel network and its controlled release of albumin. J. Biomed. Mater. Res. 2001;54:1–11. doi: 10.1002/1097-4636(200101)54:1<1::aid-jbm1>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 15.Kato A, Kimura H, Okabe K, et al. Feasibility of drug delivery to the posterior pole of the rabbit eye with an episcleral implant. Invest. Ophthalmol. Vis. Sci. 2004;45:238–244. doi: 10.1167/iovs.02-1258. [DOI] [PubMed] [Google Scholar]
- 16.Zentner G.M, Rathi R, Shih C, et al. Biodegradable block copolymers for delivery of proteins and water-insoluble drugs. J. Control. Release. 2001;72:203–215. doi: 10.1016/s0168-3659(01)00276-0. [DOI] [PubMed] [Google Scholar]
- 17.Bill A. The aqueous humor drainage mechanism in cynamolgous monkeys with evidence of unconventional drainage. Invest. Ophthalmol. 1965;4:911–919. [PubMed] [Google Scholar]
- 18.Wagner J.A, Edwards A, Schuman J.S. Characterization of uveoscleral outflow in enucleated porcine eyes perfused under constant pressure. Invest. Ophthalmol. Vis. Sci. 2004;45:3203–3206. doi: 10.1167/iovs.03-1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim S.H, Lutz R.J, Wang N.S, et al. Transport barriers in transscleral drug delivery for retinal diseases. Ophthalmic Res. 2007;39:244–254. doi: 10.1159/000108117. [DOI] [PubMed] [Google Scholar]
- 20.Amaral J, Fariss R.N, Campos M.M, et al. Transscleral-RPE permeability of PEDF and ovalbumin proteins: implications for subconjunctival protein delivery. Invest. Ophthalmol. Vis. Sci. 2005;46:4383–4392. doi: 10.1167/iovs.05-0492. [DOI] [PubMed] [Google Scholar]
- 21.Mac Gabhann F, Demetriades A.M, Deering T, et al. Protein transport to choroid and retina following periocular injection: theoretical and experimental study. Ann. Biomed. Eng. 2007;35:615–630. doi: 10.1007/s10439-006-9238-x. [DOI] [PubMed] [Google Scholar]
- 22.Ambati J, Gragoudas E.S, Miller J.W, et al. Transscleral delivery of bioactive protein to the choroid and retina. Invest. Ophthalmol. Vis. Sci. 2000;41:1186–1191. [PubMed] [Google Scholar]
- 23.Demetriades A.M, Deering T, Liu H, et al. Trans-scleral delivery of antiangiogenic proteins. J. Ocul. Pharmacol. Ther. 2008;24:70–79. doi: 10.1089/jop.2007.0061. [DOI] [PubMed] [Google Scholar]
- 24.Cantrill H.L, Henry K, Melroe N.H, et al. Treatment of cytomegalovirus retinitis with intravitreal ganciclovir. Long-term results. Ophthalmology. 1989;96:367–374. doi: 10.1016/s0161-6420(89)32900-9. [DOI] [PubMed] [Google Scholar]
- 25.Fu Y, Ponce M.L, Thill M, et al. Angiogenesis inhibition and choroidal neovascularization suppression by sustained delivery of an integrin antagonist, EMD478761. Invest. Ophthalmol. Vis. Sci. 2007;48:5184–5190. doi: 10.1167/iovs.07-0469. [DOI] [PubMed] [Google Scholar]
- 26.Jaffe G.J, Yang C.H, Guo H, et al. Safety and pharmacokinetics of an intraocular fluocinolone acetonide sustained delivery device. Invest. Ophthalmol. Vis. Sci. 2000;41:3569–3575. [PubMed] [Google Scholar]
- 27.Pontes de Carvalho R.A, Krausse M.L, Murphree A.L, et al. Delivery from episcleral exoplants. Invest. Ophthalmol. Vis. Sci. 2006;47:4532–4539. doi: 10.1167/iovs.06-0030. [DOI] [PubMed] [Google Scholar]
- 28.Sakurai E, Matsuda Y, Ozeki H, et al. Scleral plug of biodegradable polymers containing ganciclovir for experimental cytomegalovirus retinitis Invest. Ophthalmol. Vis Sci. 422043–2048.2001 [PubMed] [Google Scholar]