Abstract
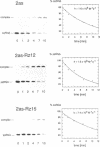
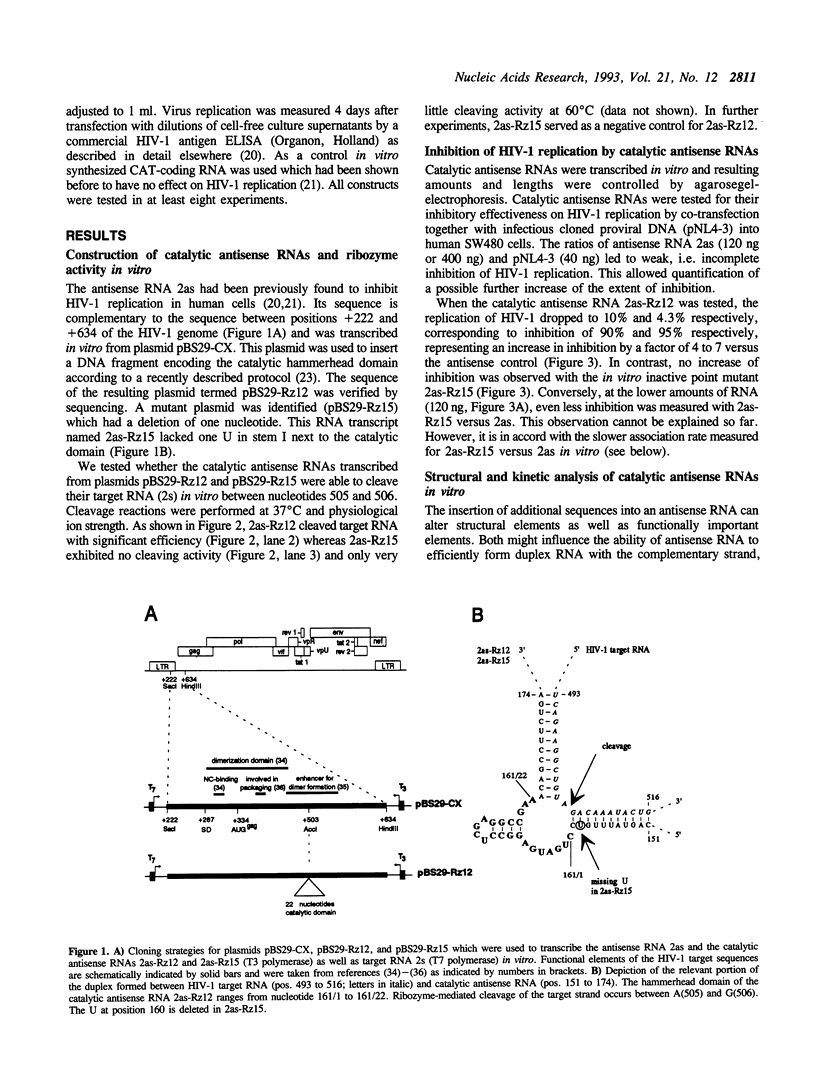
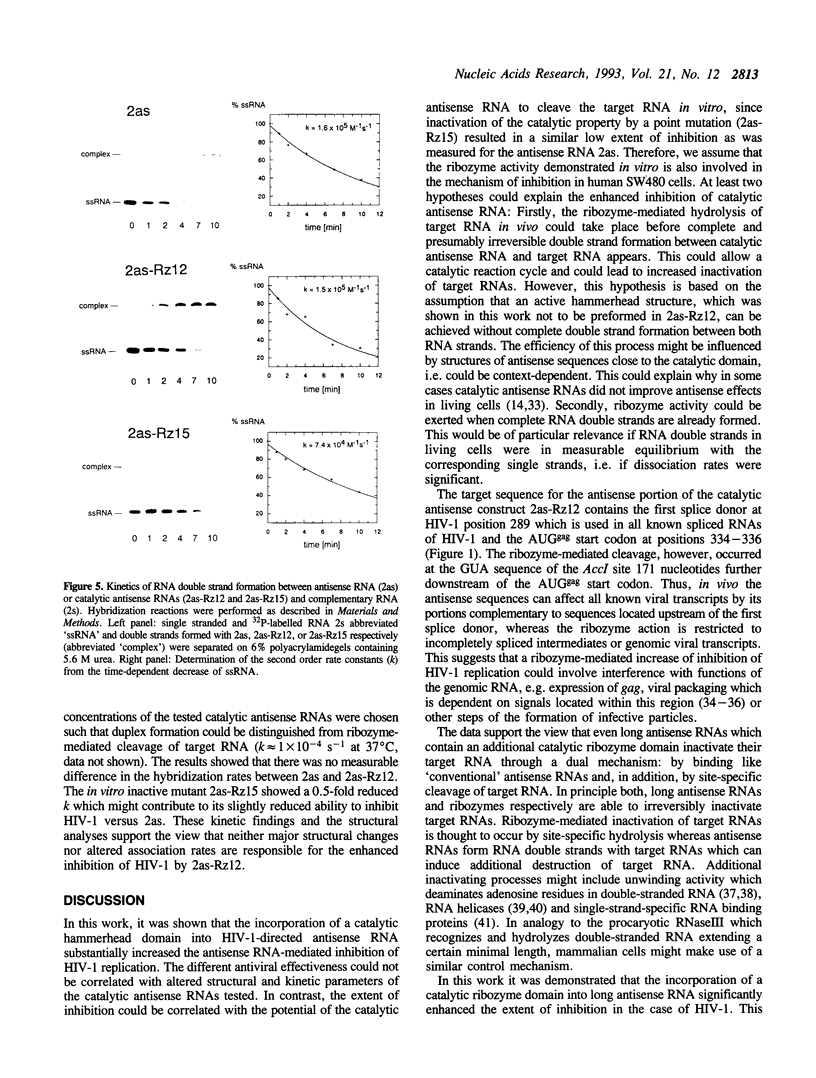
The catalytic domain of a hammerhead ribozyme was incorporated into a 413 nucleotides long antisense RNA directed against the 5'-leader/gag region of the human immunodeficiency virus type 1 (HIV-1) (pos. +222 to +634). The resulting catalytic antisense RNA was shown to cleave its target RNA in vitro specifically at physiological ion strength and temperature. We compared the antiviral effectiveness of this catalytic antisense RNA with that of the corresponding unmodified antisense RNA and with a mutated catalytic antisense RNA, which did not cleave the substrate RNA in vitro. Each of these RNAs was co-transfected into human SW480 cells together with infectious complete proviral HIV-1 DNA, followed by analysis of HIV-1 replication. The presence of the catalytically active domain resulted in 4 to 7 fold stronger inhibition of HIV-1 replication as compared to the parental antisense RNA and the inactive mutant. Kinetic and structural studies performed in vitro indicated that the ability for double strand formation was not changed in catalytic antisense RNA versus parental antisense RNA. Together, these data suggest that the ability to cleave target RNA is a crucial prerequisite for the observed increase of inhibition of the replication of HIV-1.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bass B. L., Weintraub H. An unwinding activity that covalently modifies its double-stranded RNA substrate. Cell. 1988 Dec 23;55(6):1089–1098. doi: 10.1016/0092-8674(88)90253-x. [DOI] [PubMed] [Google Scholar]
- Chang L. J., Stoltzfus C. M. Inhibition of Rous sarcoma virus replication by antisense RNA. J Virol. 1987 Mar;61(3):921–924. doi: 10.1128/jvi.61.3.921-924.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chase J. W., Williams K. R. Single-stranded DNA binding proteins required for DNA replication. Annu Rev Biochem. 1986;55:103–136. doi: 10.1146/annurev.bi.55.070186.000535. [DOI] [PubMed] [Google Scholar]
- Chen C., Okayama H. High-efficiency transformation of mammalian cells by plasmid DNA. Mol Cell Biol. 1987 Aug;7(8):2745–2752. doi: 10.1128/mcb.7.8.2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman J., Hirashima A., Inokuchi Y., Green P. J., Inouye M. A novel immune system against bacteriophage infection using complementary RNA (micRNA). Nature. 1985 Jun 13;315(6020):601–603. doi: 10.1038/315601a0. [DOI] [PubMed] [Google Scholar]
- Cotten M., Schaffner G., Birnstiel M. L. Ribozyme, antisense RNA, and antisense DNA inhibition of U7 small nuclear ribonucleoprotein-mediated histone pre-mRNA processing in vitro. Mol Cell Biol. 1989 Oct;9(10):4479–4487. doi: 10.1128/mcb.9.10.4479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darlix J. L., Gabus C., Nugeyre M. T., Clavel F., Barré-Sinoussi F. Cis elements and trans-acting factors involved in the RNA dimerization of the human immunodeficiency virus HIV-1. J Mol Biol. 1990 Dec 5;216(3):689–699. doi: 10.1016/0022-2836(90)90392-Y. [DOI] [PubMed] [Google Scholar]
- Dropulić B., Lin N. H., Martin M. A., Jeang K. T. Functional characterization of a U5 ribozyme: intracellular suppression of human immunodeficiency virus type 1 expression. J Virol. 1992 Mar;66(3):1432–1441. doi: 10.1128/jvi.66.3.1432-1441.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi T., Shioda T., Iwakura Y., Shibuta H. RNA packaging signal of human immunodeficiency virus type 1. Virology. 1992 Jun;188(2):590–599. doi: 10.1016/0042-6822(92)90513-o. [DOI] [PubMed] [Google Scholar]
- Joshi S., Van Brunschot A., Asad S., van der Elst I., Read S. E., Bernstein A. Inhibition of human immunodeficiency virus type 1 multiplication by antisense and sense RNA expression. J Virol. 1991 Oct;65(10):5524–5530. doi: 10.1128/jvi.65.10.5524-5530.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kittle J. D., Simons R. W., Lee J., Kleckner N. Insertion sequence IS10 anti-sense pairing initiates by an interaction between the 5' end of the target RNA and a loop in the anti-sense RNA. J Mol Biol. 1989 Dec 5;210(3):561–572. doi: 10.1016/0022-2836(89)90132-0. [DOI] [PubMed] [Google Scholar]
- Leibovitz A., Stinson J. C., McCombs W. B., 3rd, McCoy C. E., Mazur K. C., Mabry N. D. Classification of human colorectal adenocarcinoma cell lines. Cancer Res. 1976 Dec;36(12):4562–4569. [PubMed] [Google Scholar]
- Linder P., Lasko P. F., Ashburner M., Leroy P., Nielsen P. J., Nishi K., Schnier J., Slonimski P. P. Birth of the D-E-A-D box. Nature. 1989 Jan 12;337(6203):121–122. doi: 10.1038/337121a0. [DOI] [PubMed] [Google Scholar]
- Lo K. M., Biasolo M. A., Dehni G., Palú G., Haseltine W. A. Inhibition of replication of HIV-1 by retroviral vectors expressing tat-antisense and anti-tat ribozyme RNA. Virology. 1992 Sep;190(1):176–183. doi: 10.1016/0042-6822(92)91203-7. [DOI] [PubMed] [Google Scholar]
- Marquet R., Baudin F., Gabus C., Darlix J. L., Mougel M., Ehresmann C., Ehresmann B. Dimerization of human immunodeficiency virus (type 1) RNA: stimulation by cations and possible mechanism. Nucleic Acids Res. 1991 May 11;19(9):2349–2357. doi: 10.1093/nar/19.9.2349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ojwang J. O., Hampel A., Looney D. J., Wong-Staal F., Rappaport J. Inhibition of human immunodeficiency virus type 1 expression by a hairpin ribozyme. Proc Natl Acad Sci U S A. 1992 Nov 15;89(22):10802–10806. doi: 10.1073/pnas.89.22.10802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persson C., Wagner E. G., Nordström K. Control of replication of plasmid R1: kinetics of in vitro interaction between the antisense RNA, CopA, and its target, CopT. EMBO J. 1988 Oct;7(10):3279–3288. doi: 10.1002/j.1460-2075.1988.tb03195.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray B. K., Lawson T. G., Kramer J. C., Cladaras M. H., Grifo J. A., Abramson R. D., Merrick W. C., Thach R. E. ATP-dependent unwinding of messenger RNA structure by eukaryotic initiation factors. J Biol Chem. 1985 Jun 25;260(12):7651–7658. [PubMed] [Google Scholar]
- Rhodes A., James W. Inhibition of heterologous strains of HIV by antisense RNA. AIDS. 1991 Feb;5(2):145–151. doi: 10.1097/00002030-199102000-00003. [DOI] [PubMed] [Google Scholar]
- Rhodes A., James W. Inhibition of human immunodeficiency virus replication in cell culture by endogenously synthesized antisense RNA. J Gen Virol. 1990 Sep;71(Pt 9):1965–1974. doi: 10.1099/0022-1317-71-9-1965. [DOI] [PubMed] [Google Scholar]
- Rittner K., Burmester C., Sczakiel G. In vitro selection of fast-hybridizing and effective antisense RNAs directed against the human immunodeficiency virus type 1. Nucleic Acids Res. 1993 Mar 25;21(6):1381–1387. doi: 10.1093/nar/21.6.1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rittner K., Sczakiel G. Identification and analysis of antisense RNA target regions of the human immunodeficiency virus type 1. Nucleic Acids Res. 1991 Apr 11;19(7):1421–1426. doi: 10.1093/nar/19.7.1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarver N., Cantin E. M., Chang P. S., Zaia J. A., Ladne P. A., Stephens D. A., Rossi J. J. Ribozymes as potential anti-HIV-1 therapeutic agents. Science. 1990 Mar 9;247(4947):1222–1225. doi: 10.1126/science.2107573. [DOI] [PubMed] [Google Scholar]
- Sczakiel G., Oppenländer M., Rittner K., Pawlita M. Tat- and Rev-directed antisense RNA expression inhibits and abolishes replication of human immunodeficiency virus type 1: a temporal analysis. J Virol. 1992 Sep;66(9):5576–5581. doi: 10.1128/jvi.66.9.5576-5581.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sczakiel G., Pawlita M., Kleinheinz A. Specific inhibition of human immunodeficiency virus type 1 replication by RNA transcribed in sense and antisense orientation from the 5'-leader/gag region. Biochem Biophys Res Commun. 1990 Jun 15;169(2):643–651. doi: 10.1016/0006-291x(90)90379-2. [DOI] [PubMed] [Google Scholar]
- Simons R. W. Naturally occurring antisense RNA control--a brief review. Gene. 1988 Dec 10;72(1-2):35–44. doi: 10.1016/0378-1119(88)90125-4. [DOI] [PubMed] [Google Scholar]
- Steinecke P., Herget T., Schreier P. H. Expression of a chimeric ribozyme gene results in endonucleolytic cleavage of target mRNA and a concomitant reduction of gene expression in vivo. EMBO J. 1992 Apr;11(4):1525–1530. doi: 10.1002/j.1460-2075.1992.tb05197.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabler M., Tsagris M. Catalytic antisense RNAs produced by incorporating ribozyme cassettes into cDNA. Gene. 1991 Dec 15;108(2):175–183. doi: 10.1016/0378-1119(91)90432-b. [DOI] [PubMed] [Google Scholar]
- Takayama K. M., Inouye M. Antisense RNA. Crit Rev Biochem Mol Biol. 1990;25(3):155–184. doi: 10.3109/10409239009090608. [DOI] [PubMed] [Google Scholar]
- Tamm J., Polisky B. Characterization of the ColE1 primer-RNA1 complex: analysis of a domain of ColE1 RNA1 necessary for its interaction with primer RNA. Proc Natl Acad Sci U S A. 1985 Apr;82(8):2257–2261. doi: 10.1073/pnas.82.8.2257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- To R. Y., Booth S. C., Neiman P. E. Inhibition of retroviral replication by anti-sense RNA. Mol Cell Biol. 1986 Dec;6(12):4758–4762. doi: 10.1128/mcb.6.12.4758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomizawa J., Som T. Control of ColE1 plasmid replication: enhancement of binding of RNA I to the primer transcript by the Rom protein. Cell. 1984 Oct;38(3):871–878. doi: 10.1016/0092-8674(84)90282-4. [DOI] [PubMed] [Google Scholar]
- Wagner R. W., Smith J. E., Cooperman B. S., Nishikura K. A double-stranded RNA unwinding activity introduces structural alterations by means of adenosine to inosine conversions in mammalian cells and Xenopus eggs. Proc Natl Acad Sci U S A. 1989 Apr;86(8):2647–2651. doi: 10.1073/pnas.86.8.2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walder J. Antisense DNA and RNA: progress and prospects. Genes Dev. 1988 May;2(5):502–504. doi: 10.1101/gad.2.5.502. [DOI] [PubMed] [Google Scholar]
- Weerasinghe M., Liem S. E., Asad S., Read S. E., Joshi S. Resistance to human immunodeficiency virus type 1 (HIV-1) infection in human CD4+ lymphocyte-derived cell lines conferred by using retroviral vectors expressing an HIV-1 RNA-specific ribozyme. J Virol. 1991 Oct;65(10):5531–5534. doi: 10.1128/jvi.65.10.5531-5534.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weintraub H. M. Antisense RNA and DNA. Sci Am. 1990 Jan;262(1):40–46. doi: 10.1038/scientificamerican0190-40. [DOI] [PubMed] [Google Scholar]
- Zuker M., Stiegler P. Optimal computer folding of large RNA sequences using thermodynamics and auxiliary information. Nucleic Acids Res. 1981 Jan 10;9(1):133–148. doi: 10.1093/nar/9.1.133. [DOI] [PMC free article] [PubMed] [Google Scholar]