Abstract
STAT (signal transducer and activator of transcription) proteins play a critical role in cellular response to a wide variety of cytokines and growth factors by regulating specific nuclear genes. STAT-dependent gene transcription can be finely tuned through the association with cofactors in the nucleus. We showed previously that STAT5 (including 5a and 5b) specifically interacts with a mitochondrial enzyme PDC-E2 (E2 subunit of pyruvate dehydrogenase complex) in both leukemic T cells and cytokine-stimulated cells. However, the functional significance of this novel association remains largely unknown. Here we report that PDC-E2 may function as a co-activator in STAT5-dependent nuclear gene expression. Subcellular fractionation analysis revealed that a substantial amount of PDC-E2 was constitutively present in the nucleus of BaF3, an interleukin-3 (IL-3)-dependent cell line. IL-3-induced tyrosine-phosphorylated STAT5 associated with nuclear PDC-E2 in co-immunoprecipitation analysis. These findings were confirmed by confocal immunofluorescence microscopy showing constant nuclear localization of PDC-E2 and its co-localization with STAT5 after IL-3 stimulation. Similar to mitochondrial PDC-E2, nuclear PDC-E2 was lipoylated and associated with PDC-E1. Overexpression of PDC-E2 in BaF3 cells augmented IL-3-induced STAT5 activity as measured by reporter assay with consensus STAT5-binding sites. Consistent with the reporter data, PDC-E2 overexpression in BaF3 cells led to elevated mRNA levels of endogenous SOCS3 (suppressor of cytokine signaling 3) gene, a known STAT5 target. We further identified two functional STAT5-binding sites in the SOCS3 gene promoter important for its IL-3-inducibility. The observation that both cis-acting elements were essential to detect the stimulatory effect by PDC-E2 strongly supports the role of PDC-E2 in up-regulating the transactivating ability of STAT5. All together, our results reveal a novel function of PDC-E2 in the nucleus. It also raises the possibility of nuclear-mitochondrial crosstalk through the interaction between STAT5 and PDC-E2.
Keywords: STAT5, PDC-E2, IL-3, SOCS3, gene regulation, co-activator
1. Introduction
Signal transducer and activator of transcription (STAT) proteins are latent cytoplasmic transcription factors that play key roles in cellular responses to cytokines and growth factors [1–3]. Upon binding of a ligand to the receptor, STAT proteins become tyrosine-phosphorylated, dimerize, translocate to the nucleus, and regulate the expression of distinct target genes. Among seven STAT family members, STAT3, STAT5a and STAT5b (collectively called STAT5) exhibit the widest range of target genes, including genes important in cell cycle regulation, resistance to apoptosis, and negative feedback control [4]. Like many transcription factors, STAT activity can be regulated through the association with co-factors in the nucleus [5]. A number of transcriptional co-activators, including p300/CBP (CREB binding protein), Nmi (N-Myc interactor) and NCoA-1, upregulate STAT3- and/or STAT5-dependent gene expression via direct interaction with STAT proteins [6–9]. On the other hand, STAT-mediated gene regulation can be suppressed by binding of co-repressors to STAT proteins. For example, PIAS3 (protein inhibitor of activated STAT 3) and SMRT (silencing mediator for retinoid acid receptor and thyroid hormone receptor) bind to STAT3 and STAT5, respectively, to inhibit their transactivating functions [10,11].
STAT3-mediated gene expression can also be repressed through its association with GRIM-19 (the genes associated with retinoid-interferon-induced mortality-19) [12,13]. GRIM-19 was first identified as a growth suppressive gene in the cell death pathway induced by combined interferon-β and retinoic acid treatment [14]. Subsequent studies showed that GRIM-19 is a subunit of complex I in the electron transport chain (ETC) and is essential for its assembly in the inner membrane of the mitochondrion [15,16]. While GRIM-19 is enriched in the mitochondrion, a small amount of GRIM-19 can be detected in cytoplasm and the nucleus. GRIM-19 outside the mitochondrion inhibits STAT3 signaling through two different mechanisms. Cytosolic GRIM-19 associates with the DNA-binding domain and the linker region of STAT3 to block STAT3 nuclear translocation induced by epidermal growth factor [13]. Nuclear GRIM-19, on the other hand, binds to the transactivation domain of STAT3 and inhibits STAT3-dependent gene transcription [12]. Both reports confirm the specificity of the association between GRIM-19 and STAT3 because GRIM-19 is unable to bind to other STAT family members. These results reveal an unexpected role of a mitochondrial enzyme outside the mitochondrion.
We recently identified a novel STAT5-interacting partner: the E2 subunit of pyruvate dehydrogenase complex (PDC-E2) [17]. Like many mitochondrial proteins, PDC-E2 is encoded by the nuclear genome and translated in the cytoplasm with an amino-terminal targeting presequence [18,19]. Upon translocation into the mitochondrial matrix, PDC-E2’s targeting presequence is cleaved by proteases [20]. Processed PDC-E2 self-assembles with PDC-E1, PDC-E3 and E3 binding protein to form a large complex [21,22]. In addition, the carboxyl group of lipoic acid forms an amide bond with specific lysine residues in the lipoyl domain of PDC-E2 [23]. With thiamine pyrophosphate as the prosthetic group, PDC-E1 transfers acetate from pyruvate to the lipoamide functional groups of PDC-E2. PDC-E2 carries out the subsequent reaction in transferring the acetate to coenzyme A. Through the PDC proteins, pyruvate is converted into acetyl-CoA to be used in the citric acid cycle and subsequent oxidative phosphorylation for mitochondrial respiration [24].
In addition to its essential role in cellular metabolism, PDC-E2 is the key autoantigen in primary biliary cirrhosis (PBC) [25]. PBC is a destructive autoimmune disease of intrahepatic bile ducts, and most patients with PBC have antibodies against PDC-E2 [26]. While abnormal expression of PDC-E2 on the surface of biliary epithelial cells has been reported in PBC patients [27], the precise nature of PDC-E2 as an autoantigen remains unclear. It also raises an important question whether PDC-E2 exhibits other physiological functions outside the mitochondrion in different cell types. In this study, we examined the role of PDC-E2 in the nucleus and, specifically, its contribution in STAT5-dependent gene expression in response to interleukin-3 (IL-3).
2. Materials and methods
2.1. Cell lines and culture conditions
Mouse pro-B cell line BaF3 was maintained in RPMI supplemented with 5% fetal bovine serum (FBS), 5% calf serum and 10% conditioned medium containing IL-3. For cytokine stimulation experiments, BaF3 cells were deprived of IL-3 for 16 h, then either left unstimulated or stimulated with 10 ng/ml of recombinant mouse IL-3 (R&D Systems Inc., Minneapolis, MN) for various lengths of time as indicated.
2.2. Construction and transfection of plasmids
Mouse SOCS3 (suppressor of cytokine signaling 3) promoter region between −388 and +932 was derived from a reporter construct containing SOCS3 promoter region from −6298 to +945 (a generous gift from Dr. Flavia Bazzoni at the University of Verona, Italy) and cloned into the pGL3 luciferase reporter vector (Promega Inc., Madison, WI) using BglII- and KpnI-mediated ligation [28]. Distal and proximal STAT5 site mutants were generated by PCR-mediated mutagenesis. Distal STAT5 site was mutated from 5′-TTCTTAGAA-3′ to 5′-GTCGTCGAC-3′. Proximal STAT5 site was mutated from 5′-TTCCAGGAA-3′ to 5′-GTCAATGAC-3′. Mutated promoter sequences were then ligated into pGL3 vectors. All constructs were verified for accuracy by sequencing analysis.
Firefly luciferase reporter construct pFlash and STAT5-luciferase reporter construct containing six consecutive copies of mammary gland element (6xMGE) were generous gifts from Dr. Bernard Mathey-Prevot (Duke University, Durham, NC) [29]. Human PDC-E2 expression construct was purchased from Origene Technologies (Rockville, MD). Expression vector pcDNA3.1 was purchased from Invitrogen (Carlsbad, CA).
BaF3 cells were transiently transfected by electroporation in a single pulse using the Cell Porator (BRL Life Technologies Inc., Rockville, MD) set at 300 V, 800 μF and low ohms as described previously [30].
2.3. Immunoprecipitation and immunoblotting
Preparation of whole cell lysate as well as the subsequent immunoprecipitation and immunoblotting were performed as described before [30–32]. Cytosolic fractions were prepared by treating cells with cell lysis buffer (5 mM Tris pH8.0, 85 mM KCl, and 0.5% NP-40), followed by 5 min centrifugation at 1,000x g to pellet nuclei. Proteins were extracted from washed nuclear pellets using nuclear lysis buffer as described elsewhere [33]. Antibodies specific for STAT5, STAT5a, STAT5b, PDC-E2, PDC-E1, Eps15 (epidermal growth factor receptor substrate 15), Lamin, VDAC1 (voltage-dependent anion-selective channel protein 1), and GAPDH (glyceraldehyde 3-phosphate dehydrogenase) were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Antibodies specific for lipoic acid and STAT5 phosphorylated on Tyr694/699 were from Abcam (Cambridge, MA) and Cell Signaling Technology (Danvers, MA), respectively. Antibody dilutions for immunoprecipitation and immunoblotting were done as recommended by the manufacturer. Unless specified, signal was detected using the Odyssey Infrared Imaging System (LI-COR Biosciences, Lincoln, NE). Weak signals were detected using the Luminata Forte Western Chemiluminescent System that detects proteins in the femtogram range (Millipore, Billerica, MA).
2.4. Confocal immunofluorescence microscopy
IL-3-deprived and IL-3-stimulated BaF3 cells were adhered to 10-well slides at a concentration of 1.5 × 104 cells/well. Adhered cells were fixed with 4% paraformaldehyde for 10 min, permeabilized with 0.2% Triton X-100 for 10 min, washed, and blocked with 4% bovine serum albumin for 30 min at room temperature. Cells were doubly stained with anti-PDC-E2 polyclonal antibody and anti-STAT5 monoclonal antibody for 1 h at room temperature. Primary antibodies were detected by Alexa Fluor 594-conjugated donkey anti-rabbit antibody and Alexa Fluor 488-conjugated donkey anti-mouse antibody, respectively. Nuclei were visualized by using DAPI as a counterstain. Anti-PDC-E2 and anti-STAT5 antibodies were purchased from Santa Cruz Biotechnology and BD Biosciences (San Jose, CA), respectively. Fluorophore-conjugated antibodies and DAPI were purchased from Invitrogen. Antibodies were diluted following manufacturers’ instructions before staining. Stained cells were viewed with appropriate filters using the Olympus Fluoview 300 fluorescence confocal microscope. Images were analyzed using Fluoview software (Olympus, Melville, NY).
2.5. Dual luciferase assay
BaF3 cells were transiently transfected with 15 μg of firefly luciferase reporter construct and 500 ng of renilla luciferase control using the conditions described above. To examine the effects of PDC-E2, 15 μg of PDC-E2 expression plasmid or vector control were also co-transfected. Transfected cells recovered for 2 h in RPMI supplemented with 5% FBS, 5% calf serum and 10% conditioned medium containing IL-3. Cells were then washed once with RPMI and resuspended in RPMI supplemented with 5% FBS and 5% calf serum for 16 h of IL-3 deprivation. Half of the cells were harvested and the other half were stimulated with 10 ng/ml of IL-3 for another 16 h. Collected cells were subjected to dual luciferase assay (Promega Inc.) according to manufacturer’s protocol.
2.6. Real time PCR analysis
BaF3 cells transfected with 15 μg of PDC-E2 expression construct or vector control were subjected to IL-3 deprivation and stimulation as described above. Total RNA was extracted by TRIzol (Invitrogen Inc.), treated with RQ1 RNase-free DNase (Promega, Inc.), and then reverse transcribed using High Capacity cDNA Reverse Transcription Kit (Applied Biosystems Inc., Foster City, CA) into cDNAs. Real time PCR using SYBR Green chemistry (Applied Biosystems Inc.) was performed according to standard protocol using an annealing temperature of 60°C for all primer sets. Relative fold values were obtained using Δ ΔCT method by normalization to β-actin. Primers for SOCS3 are 5′-CTCTCCTCCAACGTGGC-3′ (forward) and 5′-ACTTTCTCATAGGAGTCCAGGTG-3′ (reverse). Primers for β-actin are 5′-TTCGTTGCCGGTCCACA-3′ (forward) and 5′-ACCAGCGCAGCGATATCG-3′ (reverse).
2.7. Electrophoretic mobility shift assay (EMSA)
Preparation of nuclear extracts, electrophoretic mobility shift assay, competition, and supershift assays were conducted as described previously [30]. The following oligonucleotides were custom synthesized by QIAGEN Operon (Alameda, CA): consensus STAT5 site (5′-AGATTTCTAGGAATTCAATCC-3′), distal STAT5 site (5′-CAGCCTTCTTAGAAGGGAG-3′), AP-1 (activator protein-1) site (5′-AGTAGTGACTAAACATT-3′), STAT5-like site (5′-AAACATTACAAGAAGACCG-3′), proximal STAT5 site (5′-GGCAGTTCCAGGAATCGGG-3′), and proximal STAT5 site mutant (5′-GGCAGGTCAATGACTCGGG-3′). Anti-STAT5a and anti-STAT5b supershifting antibodies were purchased from Santa Cruz Biotechnology. The control rabbit antibody was from Southern Biotechnology Associates (Birmingham, AL).
2.8. Statistical analysis
For quantitative analysis, data are presented as mean ± SD from at least three independent experiments. The significance of differences was analyzed by one-way ANOVA and post hoc analysis with Holm-Sidak test (SigmaPlot 11, Chicago, IL). Differences were considered significant when P < 0.05.
3. Results
3.1. Cytokine-induced interaction between PDC-E2 and STAT5
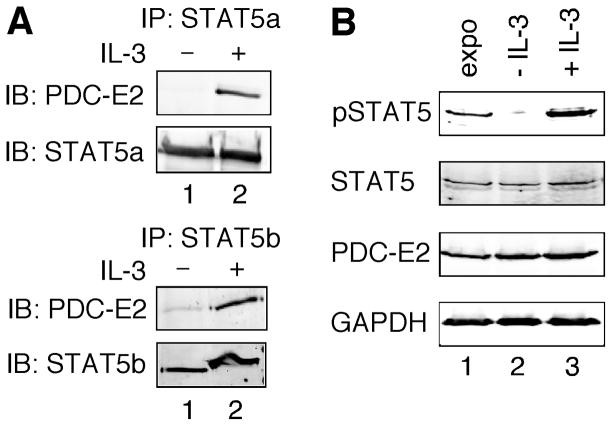
We showed previously that tyrosine-phosphorylated STAT5 interacted with PDC-E2 in a mouse T lymphoma cell line LSTRA, which exhibits constitutive STAT5 activation [17]. This finding suggests that cytokine-induced STAT5 phosphorylation may also facilitate the interaction between STAT5 and PDC-E2. It has been shown that IL-3 strongly activates both STAT5a and STAT5b in BaF3 cells, an IL-3-dependent pro-B cell line [34]. To determine if IL-3 induced the association of PDC-E2 and STAT5, we performed co-immunoprecipitation using antibodies specific for STAT5a and STAT5b. As shown in Fig. 1A, PDC-E2 co-precipitated with STAT5a and STAT5b in IL-3-stimulated (lane 2), but not in IL-3-deprived (lane 1) BaF3 cells. Equal loading of proteins was confirmed by reblotting for STAT5a and STAT5b (lower panels). This result supports a specific interaction between PDC-E2 and STAT5a/b in a cytokine-dependent manner.
Fig. 1. PDC-E2 binds to STAT5a and STAT5b in IL-3-stimulated BaF3 cells.
(A) Whole cell lysates were prepared from IL-3-deprived BaF3 cells either left untreated or stimulated with IL-3 for 15 min. Equal amounts of total proteins were immunoprecipitated (IP) with antibody specific for STAT5a (upper panels) or STAT5b (lower panels). Eluted proteins were resolved by SDS-PAGE and subjected to immunoblotting (IB) using anti-PDC-E2 antibody. Membranes were then stripped and reblotted with antibodies specific for STAT5a or STAT5b to confirm equal amounts of STAT5 proteins in the immunoprecipitates. (B) Whole cell lysates were prepared from exponentially growing (expo), IL-3-deprived (− IL-3), or IL-3-stimulated (+ IL-3) BaF3 cells. Equal amounts of total proteins were subjected to immunoblotting using antibodies specific for tyrosine-phosphorylated STAT5 (pSTAT5), STAT5, PDC-E2 and GAPDH.
PDC-E2 is susceptible to cleavage by apoptosis-related proteases, such as caspase-3 [35]. To verify whether 16 h of IL-3 deprivation induced BaF3 apoptosis, we examined apoptotic and dead cells by double staining with propidium iodide and annexin V. Flow cytometry analysis showed that the majority of IL-3-deprived cells remained viable (Fig. S1A). There was no significant increase of apoptotic population in IL-3-deprived BaF3 as compared to exponentially growing BaF3 (Fig. S1B). Consistent with the flow cytometry data, the level of PDC-E2 protein was not changed in IL-3-deprived and IL-3-stimulated BaF3 in comparison with the exponentially growing cells (Fig. 1B). Therefore, the absence of PDC-E2 in STAT5 immunoprecipitates of IL-3-deprived BaF3 cells (Fig. 1A) was not due to PDC-E2 degradation. Similarly, IL-3 deprivation and stimulation of BaF3 cells altered STAT5 tyrosine phosphorylation without affecting STAT5 protein levels (Fig. 1B).
3.2. Co-localization of PDC-E2 and STAT5 in the nucleus
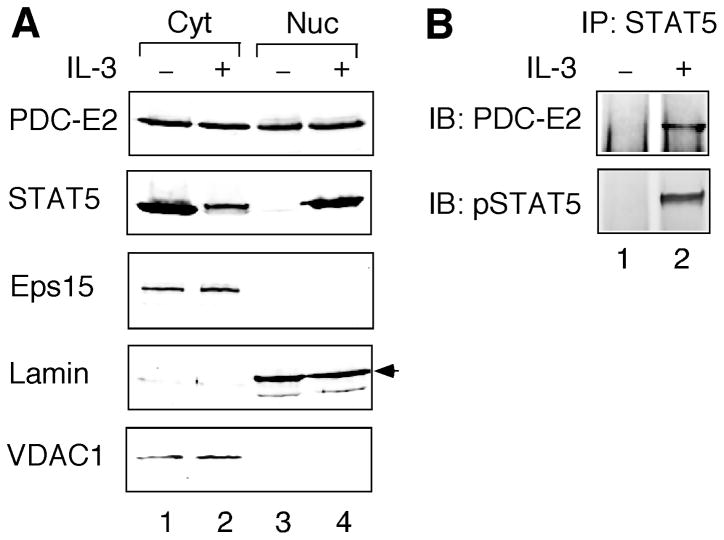
A number of metabolic enzymes exhibit additional functions in the nucleus [36]. However, it is not known whether PDC-E2 is present in the nucleus. To test this hypothesis, we first conducted subcellular fractionation of BaF3 cells without and with IL-3 stimulation. As shown in Fig. 2A, a significant amount of PDC-E2 could be detected in the nuclear fraction of BaF3 cells. In contrast to IL-3-induced STAT5 nuclear import, nuclear localization of PDC-E2 was constitutive and independent of IL-3 stimulation (Fig. 2A). Immunoblotting for Eps15 (a cytoplasmic marker) and Lamin (a nuclear marker) confirmed the absence of cytosolic contamination in the nuclear fraction. The cytosolic fraction did not exclude mitochondrial proteins as shown by the presence of VDAC1, a mitochondrial outer membrane protein [37]. Therefore, a portion of cytosolic PDC-E2 might come from the mitochondrion (Fig. 2A). More importantly, the absence of VDAC1 in the nuclear fraction verified that nuclear PDC-E2 was not from contaminating mitochondria (Fig. 2A). IL-3 induces STAT5 phosphorylation on the highly conserved Tyr694/699 and its subsequent nuclear translocation [34]. Detection of PDC-E2 in the nuclear compartment suggests that nuclear PDC-E2 may interact with tyrosine-phosphorylated STAT5 in IL-3-stimulated BaF3 cells. Indeed, tyrosine-phosphorylated STAT5 co-precipitated with PDC-E2 in the nuclear fraction of IL-3-stimulated BaF3 cells (Fig. 2B).
Fig. 2. PDC-E2 localizes in the nucleus and interacts with tyrosine-phosphorylated STAT5 in IL-3-stimulated BaF3 cells.
BaF3 cells without or with 15 min of IL-3 stimulation were subjected to subcellular fractionation to separate the nuclei (Nuc) from cytosol (Cyt). (A) Normalized total proteins from both fractions were resolved by SDS-PAGE, followed by Western blot analysis with antibodies specific for PDC-E2, STAT5, Eps15, Lamin, and VDAC1. The arrowhead on the right indicates the correct position of Lamin. (B) Normalized nuclear proteins were immunoprecipitated with anti-STAT5 antibody, followed by immunoblotting with antibodies specific for PDC-E2 and tyrosine-phosphorylated STAT5 (pSTAT5).
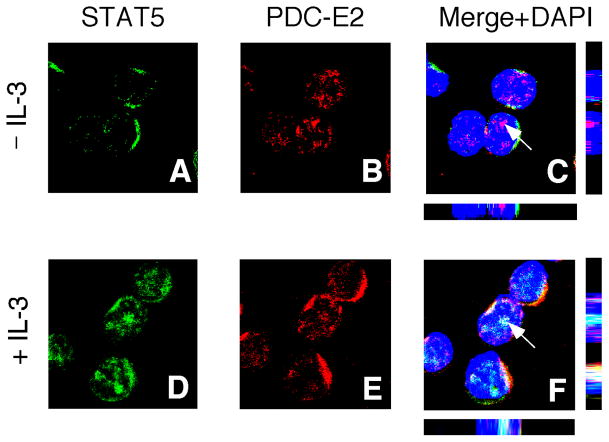
We conducted confocal immunofluorescence microscopy to further confirm subcellular localization of STAT5 and PDC-E2. STAT5 proteins were mostly cytoplasmic in IL-3-deprived BaF3 cells (Fig. 3A) while a substantial amount of nuclear translocation could be detected in IL-3-stimulated cells (Fig. 3D). Consistent with the result from subcellular fractionation (Fig. 2A), a significant amount of PDC-E2 could be found in the nuclei of both IL-3-deprived (Fig. 3B) and IL-3-stimulated (Fig. 3E) BaF3 cells. Three-color merge image analysis revealed co-localization of STAT5 and PDC-E2 inside the nuclei of IL-3-stimulated BaF3 cells (Fig. 3F). The same confocal image also revealed STAT5 and PDC-E2 co-localization outside the nucleus as reported in our previous studies [17]. The Z–sections further demonstrated protein localization on the same focal plane. Consistent with co-immunoprecipitation data (Figs. 1 and 2B), very little co-localization could be observed between STAT5 and PDC-E2 in IL-3-deprived BaF3 cells (Fig. 3C). These results clearly show that IL-3 can induce nuclear translocation of active STAT5 and its co-localization with nuclear PDC-E2 in BaF3 cells.
Fig. 3. Subcellular localization of PDC-E2 and its co-localization with STAT5 in BaF3 cells.
IL-3-deprived BaF3 cells were either left unstimulated (panels A–C) or stimulated with IL-3 for 15 min (panels D–F). Fixed and permeabilized cells were stained with anti-PDC-E2 polyclonal antibody and anti-STAT5 monoclonal antibody, followed by the corresponding secondary antibodies conjugated with different fluorophores (green for STAT5 and red for PDC-E2). Nuclei were visualized using DAPI (blue) as a counterstain. The three-color merge images are shown in panels C and F. The arrowheads denote the points of confocal analysis through the z axes with the bottom and side bars representing the xz and yz axes, respectively. Yellow color indicates co-localization of PDC-E2 and STAT5 outside nuclei. White color shows co-localization of PDC-E2 and STAT5 within nuclei.
3.3. Lipoylation of nuclear PDC-E2
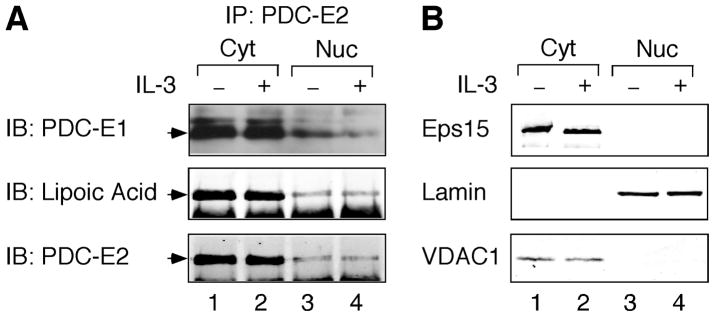
Upon entry into the mitochondrion, PDC-E2 is lipoylated and the lipoamide functional group is essential for PDC-E2 to carry out the enzymatic reaction in transferring acetate [23]. As shown in Fig. 4A, PDC-E2 was constitutively lipoylated in the mitochondrion-containing cytosolic fraction of BaF3 cells (middle panel, lanes 1 and 2). The amount of PDC-E2 in the nuclear immunoprecipitate was significantly less than the cytosolic immunoprecipitate (Fig. 4A, bottom panels). This might be due to lower immunoprecipitating efficiency of PDC-E2 antibody in the nuclear extraction buffer. Nevertheless, lipoic acid could be detected constitutively in the nuclear PDC-E2 (Fig. 4A, middle panel, lanes 3 and 4). The purity of each fraction was confirmed by Eps15, Lamin, and VDAC1 immunoblotting as described for Fig. 2A. Similarly, the nuclear fraction was devoid of contamination from both cytosol and mitochondria (Fig. 4B).
Fig. 4. Nuclear PDC-E2 is lipoylated and binds to PDC-E1 constitutively in BaF3 cells.
Cytosolic and nuclear fractions were prepared from IL-3-deprived and IL-3-stimulated BaF3 cells as described for Fig. 2. (A) Total proteins were immunoprecipitated with anti-PDC-E2 antibody followed by immunoblotting using antibodies specific for PDC-E1, lipoic acid, and PDC-E2. PDC-E1 immunoblotting was performed using Luminata Forte high-sensitivity chemiluminescent system. The arrowheads on the left indicate the correct positions of target proteins. (B) Normalized proteins from cytosolic and nuclear fractions were analyzed by immunoblotting using antibodies specific for Eps15, Lamin, and VDAC1.
In mitochondrial PDC, the E2 lipoamide receives acetate from the associated E1 subunit. As shown in Fig. 4A, PDC-E1 constantly associated with PDC-E2 in mitochondrion-containing cytosol of BaF3 cells (top panel, lanes 1 and 2). PDC-E1 could also be seen in the immunoprecipitate of nuclear PDC-E2 (Fig. 4A, top panel, lanes 3 and 4). It should be noted that high-sensitivity Western detection system was used to detect PDC-E1. Therefore, in comparison with the complex formation in mitochondria, PDC-E1 may associate with PDC-E2 in lower stoichiometry in the nucleus. Nevertheless, we cannot exclude the possibility that PDC-E2 may still work in conjunction with PDC-E1 in conferring transacetylase activity in the nucleus.
3.4. Modulation of STAT5-dependent transcription by PDC-E2
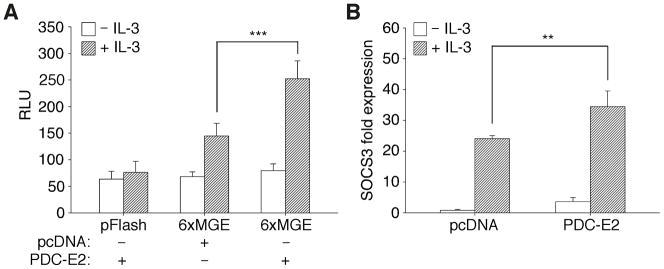
Previous studies showed that GRIM-19 could inhibit STAT3-dependent transcription through direct interaction [12,13]. These reports raise the possibility that PDC-E2 may also modulate STAT5 activity in a similar manner. To test this hypothesis, we examined the effect of exogenous PDC-E2 expression on STAT5 transactivating ability with a reporter construct containing six consecutive mammary gland elements (6xMGE) that specifically bind STAT5. As shown in Fig. 5A, exogenous PDC-E2 had no effect on the STAT5 reporter activity in IL-3-deprived BaF3 cells. In the presence of IL-3, however, exogenous PDC-E2 significantly enhanced STAT5 reporter activity. The elevation of STAT5 reporter activity was not due to an artifact of overexpressed proteins. As shown in Fig. S2A, in the same reporter assay, overexpression of a dominant-negative STAT5b with the critical Tyr699 mutated to Phe abolished reporter activity. The stimulatory effect of wild-type STAT5 and the inhibitory effect of dominant-negative STAT5 on cell proliferation have also been reported [30]. As another negative control, neither IL-3 nor exogenous PDC-E2 activated the basic luciferase reporter pFlash. Expression of exogenous PDC-E2 in transfected BaF3 cells was confirmed by Western blot analysis (data not shown). This finding supports the role of PDC-E2 as a co-activator in STAT5-dependent transcription, which is in contrast to the inhibitory effects observed for GRIM-19.
Fig. 5. IL-3-induced STAT5 reporter activity and endogenous SOCS3 expression is augmented by exogenous PDC-E2.
(A) BaF3 cells were electroporated with firefly luciferase reporter constructs without (pFlash) or with six consecutive mammary gland elements (6xMGE) and co-transfected with either pcDNA vector control or PDC-E2 expression construct. Dual luciferase assay was conducted in transfected cells before and after 16 h of IL-3 stimulation. Data were normalized to renilla luciferase control and expressed as relative luciferase units (RLU). (B) BaF3 cells were transfected with pcDNA vector control or PDC-E2 expression construct as described above. Total RNAs were isolated from transfected cells before and after 30 min of IL-3 stimulation, reverse transcribed, and then subjected to real time PCR using primers specific for mouse SOCS3 and actin mRNAs. Data were normalized to actin and expressed as fold change to vector-transfected cells before IL-3 stimulation. ** P < 0.01, *** P < 0.001.
Next, we wanted to determine whether PDC-E2 overexpression had the same stimulatory effect on STAT5-mediated expression of endogenous genes. We previously reported that IL-3 strongly induced SOCS3 expression in BaF3 cells [38]. Using quantitative real time PCR, we detected significant increase of SOCS3 transcripts in IL-3-stimulated BaF3 cells transfected with exogenous PDC-E2 as compared to the vector control (Fig. 5B). While PDC-E2 overexpression had no effect on the STAT5 reporter without IL-3 (Fig. 5A), exogenous PDC-E2 did elevate the level of SOCS3 transcripts in IL-3-deprived BaF3 cells (Fig. 5B). Expression of exogenous PDC-E2 in transfected BaF3 cells was also confirmed by immunoblotting of protein fractions after RNA extraction (data not shown). Collectively, we have identified a novel function of PDC-E2 in activating STAT5 activity on both STAT5-luciferase reporter and an endogenous nuclear target gene.
3.5. SOCS3 promoter analysis
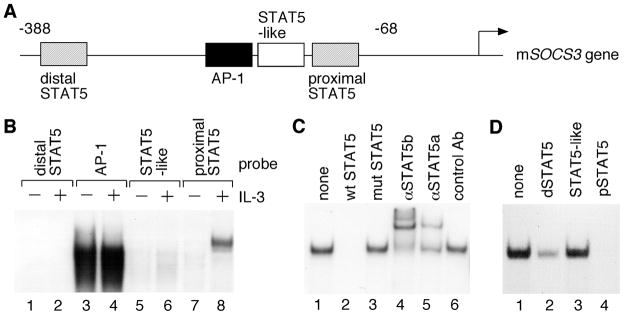
To determine whether PDC-E2 augments IL-3-induced SOCS3 expression through STAT5, it is essential to identify the STAT5-binding sites in the SOCS3 gene promoter [39,40]. Sequence analysis of the mouse SOCS3 promoter region revealed four distinct cis-acting elements: a distal STAT5 site, an AP-1 site, a STAT5-like site, and a proximal STAT5 site (Fig. 6A). The distal and proximal STAT5 sites have the consensus STAT5 binding sequence of TTCN3GAA, while the STAT5-like site has a single nucleotide difference of TTAN3GAA [41]. The ability of each cis-acting element to bind nuclear factors in vitro was analyzed by EMSA with nuclear extracts prepared from IL-3-deprived and IL-3-stimulated BaF3 cells. As shown in Fig. 6B, only the proximal STAT5 site exhibited IL-3-induced DNA-binding activity (compare lanes 7 and 8). This DNA-binding activity was specific because it could be competed out by unlabeled wild-type, but not mutant STAT5 site (Fig. 6C, lanes 1–3). Both STAT5a and STAT5b bound to the proximal STAT5 site as demonstrated by the supershift induced by anti-STAT5a and STAT5b antibodies, but not the control antibody (Fig. 6C, lanes 4–6).
Fig. 6. Characterization of the mouse SOCS3 promoter.
(A) Schematic diagram of four potential cis-regulatory elements in the mouse SOCS3 promoter. (B) Nuclear extracts were prepared from IL-3-deprived BaF3 cells either left untreated or stimulated with IL-3 for 30 min. Equal amounts of nuclear proteins were subjected to EMSA with 32P-labeled oligonucleotides containing the four cis-acting elements as described in A. (C) Nuclear extracts from IL-3-stimulated BaF3 cells were incubated with a 32P-labeled probe containing the proximal STAT5 site (lane 1). Competition assays were conducted in the presence of 100-fold molar excess of unlabeled oligonucleotides that contain either the wild-type (lane 2) or the mutated proximal STAT5 site (lane 3). Supershift assays were performed with control antibody or antibodies specific for STAT5a and STAT5b (lanes 4–6). (D) Nuclear extracts from IL-3-stimulated BaF3 cells were incubated with a 32P-labeled consensus STAT5 probe derived from the sheep β-casein gene promoter (lane 1). Competition assays were carried out with 100-fold molar excess of unlabeled oligonucleotides derived from the distal STAT5 site (lane 2), the STAT5-like site (lane 3) or the proximal STAT5 site (lane 4) in the mouse SOCS3 gene promoter.
We could not detect IL-3-induced DNA-binding activity toward the distal STAT5 site with the consensus sequence (Fig. 6B, lanes 1 and 2). To determine whether STAT5 bound to distal and proximal STAT5 sites with different affinity, we performed competition assays using the mammary gland element (MGE) as the probe. Unlabeled proximal STAT5 site in 100-fold molar excess completely abolished STAT5 binding to MGE suggesting that it had the highest affinity in STAT5 binding (Fig. 6D, lane 4). Unlabeled distal STAT5 site in 100-fold molar excess partially reduced STAT5 binding to MGE suggesting its lower affinity in STAT5 binding (Fig. 6D, lane 2). On the other hand, STAT5-like site had the lowest affinity in STAT5 binding due to its inability to compete with MGE (Fig. 6D, lane 3). Similar to the distal STAT5 site, no DNA-binding activity could be detected at the STAT5-like site (Fig. 6B, compare lanes 5 and 6). In contrast to all STAT5 sites, the AP-1 site exhibited constitutive DNA binding activity (Fig. 6B, compare lanes 3 and 4). The specificity was confirmed by competition assay, and antibody supershift assay verified the presence of c-Fos and c-Jun proteins in the DNA-protein complex (data not shown).
3.6. Effect of mutant STAT5 binding sites on SOCS3 promoter activity
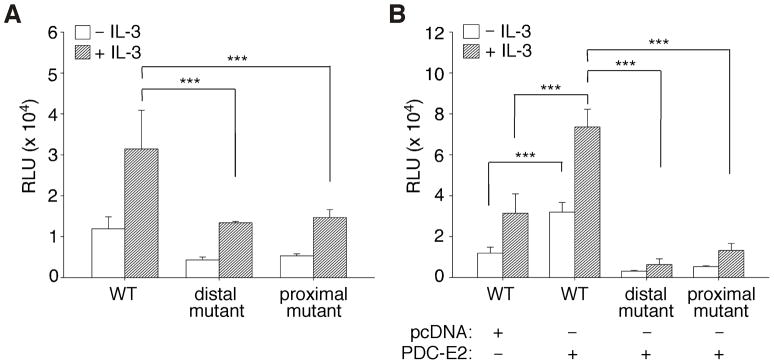
To further examine if the two consensus STAT5-binding sites were functional in the SOCS3 promoter, we constructed a firefly luciferase reporter driven by the SOCS3 promoter sequence spanning from 388 bp upstream to 932 bp downstream of the transcription start site. Mutations were introduced into the distal or the proximal STAT5 site that rendered them unable to bind STAT5. Dual luciferase assay was then performed as described for Fig. 5A. Consistent with the EMSA data (Fig. 6), mutation of the proximal STAT5 site significantly impaired SOCS3 promoter activity in response to IL-3 stimulation (Fig. 7A). While the distal STAT5 site exhibited lower STAT5-binding affinity (Fig. 6D), mutation of the distal site reduced SOCS3 promoter activity to a level similar to the proximal site mutation (Fig. 7A). These results suggest that sequences flanking the distal STAT5 site in the SOCS3 promoter may affect STAT5 binding. Therefore, both the distal and proximal STAT5 sites are required for IL-3-induced SOCS3 promoter activity.
Fig. 7. Combined effects of IL-3 and PDC-E2 on the SOCS3 promoter are mediated through both proximal and distal STAT5 sites.
Dual-luciferase reporter assays were conducted in IL-3-deprived and IL-3-stimulated BaF3 cells as described for Fig. 5A. (A) BaF3 cells were transfected with firefly luciferase reporter driven by the wild-type mSOCS3 promoter (WT), the promoter with mutated distal STAT5 site, or the promoter with mutated proximal STAT5 site. (B) BaF3 cells were co-transfected with PDC-E2 expression construct or pcDNA vector control in addition to the reporter plasmids as described in A. *** P < 0.001.
To determine if the SOCS3 promoter region (− 388 to +932) conferred the same response to exogenous PDC-E2 as the endogenous SOCS3 gene (Fig. 5B), dual luciferase assay was conducted using the same SOCS3 promoter constructs in the presence of exogenous PDC-E2. As shown in Fig. 7B, PDC-E2 overexpression activated SOCS3 promoter activity both before and after IL-3 stimulation of BaF3 cells. This was not due to an artifact of overexpressed proteins. Similar to the inhibitory effect on STAT5 reporter (Fig. S2A), overexpression of dominant-negative STAT5 also greatly diminished IL-3-induced mSOCS3 promoter activity (Fig. S2B). Furthermore, PDC-E2-induced activation of SOCS3 promoter was greatly reduced when either the distal or the proximal STAT5 site was mutated. As described for Fig. 5, expression of exogenous PDC-E2 was confirmed by Western blot (not shown). These results clearly demonstrate the importance of both the distal and proximal consensus STAT5-binding sites in IL-3-mediated SOCS3 gene transcription. Their involvement in PDC-E2 responsiveness further illustrates the potential role of PDC-E2 as a co-activator in STAT5-dependent gene expression.
4. Discussion
Cumulative evidence suggests that metabolic enzymes can have dual functions in the nucleus dependent or independent of their previously characterized enzymatic activity [36]. Arg5,6 is a mitochondrial enzyme involved in arginine biosynthesis, but also functions as a transcription factor in regulating specific nuclear genes [42]. On the other hand, a short chain dehydrogenase/reductase hRoDH-E2 was reported to function as a transcriptional repressor in the nucleus [43]. Pyruvate kinase is a cytosolic enzyme in the glycolytic pathway which produces pyruvate molecules, and has been shown to translocate into the nucleus after IL-3 stimulation [44]. Pyruvate kinase in the nucleus enhances cell proliferation, but has no effect on STAT5-dependent gene expression. Fumarase is a well-known citric acid cycle enzyme in the mitochondrial matrix. However, a recent report indicated that, upon DNA damage induction, cytosolic fumarase was recruited to the nucleus and protected cells from DNA damage [45]. While PDC-E2 is well known for its metabolic function in the mitochondrion and its role as an autoantigen in PBC, we demonstrate here that PDC-E2 can also be found in the nucleus. More importantly, PDC-E2 can augment STAT5-dependent nuclear gene transcription.
Enhanced histone acetylation has been linked to active gene transcription and many transcriptional co-activators exhibit the enzymatic activity of histone acetyltransferase (HAT) [46]. Acetyl-CoA is the source of the acetyl group in histone acetylation. PDC-E2 is the key mitochondrial enzyme in producing acetyl-CoA to be used by the citric acid cycle. Acetyl-CoA synthetases represent another pathway in generating acetyl-CoA and have been detected in three different subcellular compartments: the mitochondrion, the nucleus, and cytosol [47]. These findings raise an intriguing possibility that nuclear PDC-E2 may contribute to the production of acetyl-CoA to be used for histone acetylation. Its interaction with STAT5 may create a microenvironment with higher levels of acetyl-CoA as the substrate for nearby histone acetyltransferase. This hypothesis is supported by our observation that nuclear PDC-E2 is lipoylated (Fig. 4A), which is essential for its dihydrolipoyl transacetylase activity.
Mitochondrial PDC is a large complex containing multiple copies of E1, E2 and E3 subunits as well as the E3 binding proteins [21,22]. PDC-E1 carries out the first reaction on pyruvate and transfers the acetate to the lipoyl group attached on PDC-E2. Cryoelectron microscopy further demonstrates an organized structure of multiple PDC-E1 surrounding the inner core of multiple PDC-E2 [22]. While a small amount of PDC-E1 can be detected in association with nuclear PDC-E2 (Fig. 4A), it is unlikely that the E1 and E2 subunits form a nuclear complex similar to the mitochondrial complex. It is not known whether coordinated enzymatic reactions occur between PDC-E1 and PDC-E2 in the nucleus. It also remains to be determined whether PDC-E3 and E3 binding protein associate with nuclear PDC-E2 in a way similar to mitochondrial PDC-E2. We cannot rule out the possibility that nuclear PDC-E2 may exert functions independent of its dihydrolipoyl transacetylase activity. Alternatively, but not mutually exclusive, cytoplasmic and/or mitochondrial PDC-E2 may indirectly affect STAT5-dependent gene transcription.
Like many other mitochondrial proteins, PDC-E2 is synthesized in the cytoplasm with presequence for mitochondrial targeting. Our previous studies showed that the STAT5-interacting PDC-E2 lacked the presequence in its amino terminus [17]. Similarly, we only detected processed PDC-E2 in the nucleus (Fig. 2). It is not clear whether cleavage of PDC-E2 presequence in the mitochondrion is required for its mitochondrial export and subsequent nuclear import. Endonuclease G, another mitochondrial protein, has been shown to be released from mitochondria and then translocate to the nucleus during apoptosis [48]. However, our confocal immunofluorescence microscopy (Fig. 3) and flow cytometry (Fig. S1) data did not support the role of apoptosis in PDC-E2 nuclear translocation. As a component of mitochondrial ETC complex I, GRIM-19 also has distinct functions in the nucleus [12]. Nevertheless, the underlying mechanism of how GRIM-19 mitochondrial and nuclear translocation are coordinated has not been elucidated. Compared to mitochondrial import machinery, much less is known on the mechanisms of mitochondrial export [49]. It remains to be determined how PDC-E2 translocates to the nucleus under normal physiological conditions.
Other than STAT5, STAT3 is the only other STAT family member known to have functional interaction with a mitochondrial enzyme. Compared to GRIM-19, PDC-E2 exhibits many unique features in regulating STAT activity. First, PDC-E2 and GRIM-19 specifically interact with STAT5 and STAT3, respectively [12,13,17]. Zhang et al. proposed that phosphorylation of the conserved Ser727 in STAT3 is important for its binding to GRIM-19 [12]. We showed previously that BaF3 cells exhibit constitutive phosphorylation of the conserved Ser725 in STAT5a and Ser730 in STAT5b even under the condition of IL-3 deprivation [34]. IL-3 stimulation strongly induces phosphorylation of STAT5 on the conserved tyrosine residue next to the SH2 domain. Our co-immunoprecipitation (Figs. 1 and 2B) and immunofluorescence (Fig. 3) results clearly showed PDC-E2 interaction with STAT5 in IL-3 stimulated, but not in IL-3-deprived BaF3 cells. It is, therefore, unlikely that phosphorylation of the conserved serine residue of STAT5 plays a key role in its binding to PDC-E2. Instead, our data suggest that phosphorylation of the conserved tyrosine residue in STAT5 may be critical in its association with PDC-E2. We cannot rule out the possibility, however, that tyrosine phosphorylation-induced conformational change of STAT5 proteins, such as dimerization, may be important in the interaction with PDC-E2. It is also plausible that other cellular protein(s) mediate the interaction between STAT5 and PDC-E2.
Second, our data demonstrated that a significant amount of PDC-E2 localized in the nucleus and augmented STAT5-dependent transcription of nuclear target genes. This is in sharp contrast to the inhibitory effects of GRIM-19 on STAT3-mediated gene expression [12,13]. Lufei et al. concluded that GRIM-19 in the perinuclear region bound to STAT3 and inhibited nuclear translocation of STAT3 in response to growth factor [13]. However, we could not detect any significant PDC-E2 translocation into the nuclei upon IL-3 stimulation (Fig. 2A). Therefore, it is unlikely that PDC-E2 acts as a carrier to facilitate cytokine-induced STAT5 nuclear translocation. It is equally implausible that STAT5 dimer functions as a carrier for importing PDC-E2 into the nucleus. PDC-E2 is composed of the lipoyl domain, the E1-binding domain, and the inner catalytic domain [24]. There is no discernible nuclear localization signal, DNA binding domain, or transcriptional activation domain in PDC-E2. It remains to be determined how PDC-E2 translocates to the nucleus and collaborates with the transcriptional machinery in up-regulating STAT5 activity.
Third, our confocal immunofluorescence microscopy showed that endogenous STAT5 and PDC-E2 also co-localized outside the nuclei in IL-3-stimulated, but not IL-3-deprived BaF3 cells (Fig. 3, compare panels C and F). This is consistent with our previous finding that cytokines induced translocation of tyrosine-phosphorylated STAT5 into mitochondria [17]. By transfection of epitope-tagged GRIM-19 and STAT3, Lufei et al. showed that exogenous GRIM-19 and STAT3 constitutively co-localized outside the nucleus in cells without ligand stimulation [13]. Indeed, a recent study reported the constant presence of STAT3 in mitochondria where GRIM-19 is most concentrated [50]. Therefore, the functional interaction between STAT5 and PDC-E2 is distinctly different from the association between STAT3 and GRIM-19 based on cytokine responsiveness.
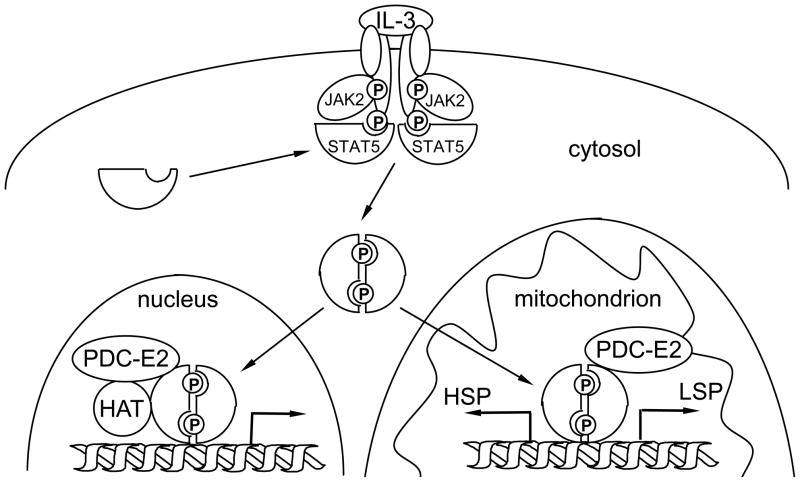
In summary, we report here a novel function of PDC-E2 outside the mitochondrion in promoting STAT5-dependent nuclear gene transcription. Results from our studies provide an intriguing mechanistic insight of how a metabolic enzyme may alter nuclear gene expression through its association with distinct transcription factors. Increasing evidence points to the role of STAT signaling in regulating cellular metabolism and mitochondrial functions. Mitochondrial STAT3 participates in oxidative phosphorylation [50] and contributes to altered metabolism in Ras-transformed cells [51]. On the other hand, nuclear STAT3 also functions as a metabolic switch by regulating distinct target genes [52]. It is important to note that the mitochondrion has its own circular genome encoding important ETC proteins [53,54]. Our previous studies showed that, in cytokine-stimulated cells, tyrosine-phosphorylated STAT5 translocated to mitochondria and bound to a putative STAT5 site located within the transcriptional control region of mitochondrial DNA [17]. All together, these data add another level of complexity in the network of metabolic pathways and nuclear gene expression as well as nuclear-mitochondrial crosstalk that regulates cellular responses to environmental cues (Fig. 8).
Fig. 8. A hypothetical model of nuclear-mitochondrial crosstalk through cytokine-induced STAT5 and PDC-E2 interaction.
Upon IL-3 stimulation, receptor dimerization induces tyrosine phosphorylation of the associated JAK2 tyrosine kinase. Active JAK2 phosphorylates the cytoplasmic tails of receptor subunits. Cytoplasmic STAT5 is recruited to the receptor complex and phosphorylated by the JAK2 kinase. Tyrosine-phosphorylated STAT5 proteins dimerize and translocate to the nucleus and the mitochondrion. In the nucleus, tyrosine-phosphorylated STAT5 binds to promoter regions of distinct target genes, such as SOCS3. The associated PDC-E2 may work in concert with histone acetyltransferase (HAT) to enhance STAT5-dependent nuclear gene expression. On the other hand, binding of tyrosine-phosphorylated STAT5 to the control region of mitochondrial DNA may modulate transcription initiated from the heavy strand promoter (HSP) and light strand promoter (LSP). Phosphorylation of key tyrosine residues are represented by “P” in circles.
Supplementary Material
Exponentially growing and IL-3-deprived BaF3 cells were stained with FITC-conjugated annexin V and propidium iodide using the Dead Cell Apoptosis Kit from Invitrogen, Inc. Stained cells were analyzed by FACS Calibur flow cytometer from BD Biosciences. (A) Dot plot analysis was performed using the FlowJo software (Tree Star, Inc., Ashland, OR) on one set of experiments. The lower-left, lower-right, and upper-right quadrants represent live, apoptotic, and dead cells, respectively. (B) Statistical analysis of three independent experiments.
BaF3 cells were electroporated with firefly luciferase reporter constructs with six consecutive MGE as described for Fig. 5A(A), or with wild-type mSOCS3 promoter as described for Fig. 7(B). The reporter constructs were co-transfected with pcDNA without (vector) or with wild-type (WT) STAT5b or STAT5b with Y699F mutation. Dual-luciferase reporter assays were performed in transfected cells before and after 16 h of IL-3 stimulation. Data were normalized to renilla luciferase control and fold induction was calculated as compared to vector-transfected cells without IL-3 stimulation. *** P < 0.001.
Acknowledgments
We thank Drs. Mohanan Veettil and Sathish Sadagopan in Dr. Bala Chandran’s lab as well as Dr. Patricia Loomis at the RFUMS Microscopy and Imaging Facility for their assistance in confocal immunofluorescence microscopy. We thank Robert Dickinson at the RFUMS Flow Cytometry Core Facility for his assistance in flow cytometry analysis. We also thank Drs. Kwang-Poo Chang, Michael Sarras, David Everly, Gulam Waris, Joseph DiMario and Neelam Sharma-Walia for their suggestions and comments on the initial manuscript. This work was supported in part by NIH grant CA107210 and RFUMS H. M. Bligh Cancer Research Fund to C.-L.Y.
Abbreviations
- STAT
signal transducer and activator of transcription
- GRIM-19
genes associated with retinoid-interferon-induced mortality-19
- ETC
electron transport chain
- PDC
pyruvate dehydrogenase complex
- PBC
primary biliary cirrhosis
- IL-3
interleukin-3
- SOCS
suppressor of cytokine signaling
- FBS
fetal bovine serum
- MGE
mammary gland element
- Eps15
epidermal growth factor receptor substrate 15
- VDAC1
voltage-dependent anion-selective channel protein 1
- GAPDH
glyceraldehyde 3-phosphate dehydrogenase
- EMSA
electrophoretic mobility shift assay
- AP-1
activator protein 1
- HAT
histone acetyltransferase
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Levy DE, Darnell JE., Jr Nat Rev Mol Cell Biol. 2002;3:651–662. doi: 10.1038/nrm909. [DOI] [PubMed] [Google Scholar]
- 2.Schindler C, Levy DE, Decker T. J Biol Chem. 2007;282:20059–20063. doi: 10.1074/jbc.R700016200. [DOI] [PubMed] [Google Scholar]
- 3.Lanning NJ, Carter-Su C. Rev Endocr Metab Dis. 2006;7:225–235. doi: 10.1007/s11154-007-9025-5. [DOI] [PubMed] [Google Scholar]
- 4.Yu H, Jove R. Nat Rev Cancer. 2004;4:97–105. doi: 10.1038/nrc1275. [DOI] [PubMed] [Google Scholar]
- 5.Shuai K. Oncogene. 2000;19:2638–2644. doi: 10.1038/sj.onc.1203522. [DOI] [PubMed] [Google Scholar]
- 6.Paulson M, Pisharody S, Pan L, Guadagno S, Mui AL, Levy DE. J Biol Chem. 1999;274:25343–25349. doi: 10.1074/jbc.274.36.25343. [DOI] [PubMed] [Google Scholar]
- 7.Gewinner C, Hart G, Zachara N, Cole R, Beisenherz-Huss C, Groner B. J Biol Chem. 2004;279:3563–3572. doi: 10.1074/jbc.M306449200. [DOI] [PubMed] [Google Scholar]
- 8.Zhu M, John S, Berg M, Leonard WJ. Cell. 1999;96:121–130. doi: 10.1016/s0092-8674(00)80965-4. [DOI] [PubMed] [Google Scholar]
- 9.Litterst CM, Kliem S, Marilley D, Pfitzner E. J Biol Chem. 2003;278:45340–45351. doi: 10.1074/jbc.M303644200. [DOI] [PubMed] [Google Scholar]
- 10.Chung CD, Liao J, Liu B, Rao X, Jay P, Berta P, Shuai K. Science. 1997;278:1803–1805. doi: 10.1126/science.278.5344.1803. [DOI] [PubMed] [Google Scholar]
- 11.Nakajima H, Brindle PK, Handa M, Ihle JN. EMBO J. 2001;20:6836–6844. doi: 10.1093/emboj/20.23.6836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang J, Yang J, Roy SK, Tininini S, Hu J, Bromberg JF, Poli V, Stark GR, Kalvakolanu DV. Proc Natl Acad Sci U S A. 2003;100:9342–9347. doi: 10.1073/pnas.1633516100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lufei C, Ma J, Huang G, Zhang T, Novotny-Diermayr V, Ong CT, Cao X. EMBO J. 2003;22:1325–1335. doi: 10.1093/emboj/cdg135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Angell JE, Lindner DJ, Shapiro PS, Hofmann ER, Kalvakolanu DV. J Biol Chem. 2000;275:33416–33426. doi: 10.1074/jbc.M003929200. [DOI] [PubMed] [Google Scholar]
- 15.Fearnley IM, Carroll J, Shannon RJ, Runswick MJ, Walker JE, Hirst J. J Biol Chem. 2001;276:38345–38348. doi: 10.1074/jbc.C100444200. [DOI] [PubMed] [Google Scholar]
- 16.Huang G, Lu H, Hao A, Ng DCH, Ponniah S, Guo K, Lufei C, Zeng Q, Cao X. Mol Cell Biol. 2004;24:8447–8456. doi: 10.1128/MCB.24.19.8447-8456.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chueh F-Y, Leong K-F, Yu C-L. Biochem Biophys Res Commun. 2010;402:778–783. doi: 10.1016/j.bbrc.2010.10.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thekkumkara TJ, Ho L, Wexler ID, Pons G, Liu TC, Patel MS. FEBS Lett. 1988;240:45–48. doi: 10.1016/0014-5793(88)80337-5. [DOI] [PubMed] [Google Scholar]
- 19.Wang L, Kaneko S, Kagaya M, Ohno H, Honda M, Kobayashi K. J Gastroenterol. 2002;37:449–454. doi: 10.1007/s005350200065. [DOI] [PubMed] [Google Scholar]
- 20.Pfanner N, Geissler A. Nat Rev Mol Cell Biol. 2001;2:339–349. doi: 10.1038/35073006. [DOI] [PubMed] [Google Scholar]
- 21.Milne JLS, Wu X, Borgnia MJ, Lengyel JS, Brooks BR, Shi D, Perham RN, Subramaniam S. J Biol Chem. 2006;281:4364–4370. doi: 10.1074/jbc.M504363200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Milne JLS, Shi D, Rosenthal PB, Sunshine JS, Domingo GJ, Wu X, Brooks BR, Perham RN, Henderson R, Subramaniam S. EMBO J. 2002;21:5587–5598. doi: 10.1093/emboj/cdf574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mao TK, Davis PA, Odin JA, Coppel RL, Gershwin ME. Hepatology. 2004;40:1241–1248. doi: 10.1002/hep.20491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thekkumkara TJ, Pons G, Mitroo S, Jentoft JE, Patel MS. Ann N Y Acad Sci. 1989;573:113–129. doi: 10.1111/j.1749-6632.1989.tb14990.x. [DOI] [PubMed] [Google Scholar]
- 25.Migliaccio C, Van de Water J, Ansari AA, Kaplan MM, Coppel RL, Lam KS, Thompson RK, Stevenson F, Gershwin ME. Hepatology. 2001;33:792–801. doi: 10.1053/jhep.2001.23783. [DOI] [PubMed] [Google Scholar]
- 26.Invernizzi P, Selmi C, Gershwin ME. Digest Liver Dis. 2010;42:401–408. doi: 10.1016/j.dld.2010.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Joplin R, Wallace LL, Johnson GD, Lindsay JG, Yeaman SJ, Palmer JM, Strain AJ, Neuberger JM. J Pathol. 1995;176:381–390. doi: 10.1002/path.1711760409. [DOI] [PubMed] [Google Scholar]
- 28.Gatto L, Berlato C, Poli V, Tininini S, Kinjyo I, Yoshimura A, Cassatella MA, Bazzoni F. J Biol Chem. 2004;279:13746–13754. doi: 10.1074/jbc.M308999200. [DOI] [PubMed] [Google Scholar]
- 29.Callus BA, Mathey-Prevot B. J Biol Chem. 2000;275:16954–16962. doi: 10.1074/jbc.M909976199. [DOI] [PubMed] [Google Scholar]
- 30.Shi M, Cooper JC, Yu C-L. Mol Cancer Res. 2006;4:39–45. doi: 10.1158/1541-7786.MCR-05-0202. [DOI] [PubMed] [Google Scholar]
- 31.Yu C-L, Jin YJ, Burakoff SJ. J Biol Chem. 2000;275:599–604. doi: 10.1074/jbc.275.1.599. [DOI] [PubMed] [Google Scholar]
- 32.Venkitachalam S, Chueh F-Y, Leong K-F, Pabich S, Yu C-L. Oncol Rep. 2011;25:677–683. doi: 10.3892/or.2011.1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ye JZ-S, Donigian JR, van Overbeek M, Loayza D, Luo Y, Krutchinsky AN, Chait BT, de Lange T. J Biol Chem. 2004;279:47264–47271. doi: 10.1074/jbc.M409047200. [DOI] [PubMed] [Google Scholar]
- 34.Cooper JC, Boustead JN, Yu C-L. Cell Signal. 2006;18:851–860. doi: 10.1016/j.cellsig.2005.07.013. [DOI] [PubMed] [Google Scholar]
- 35.Matsumura S, Van De Water J, Kita H, Coppel RL, Tsuji T, Yamamoto K, Ansari AA, Gershwin ME. Hepatology. 2002;35:14–22. doi: 10.1053/jhep.2002.30280. [DOI] [PubMed] [Google Scholar]
- 36.Bhardwaj A, Wilkinson MF. BioEssays. 2005;27:467–471. doi: 10.1002/bies.20232. [DOI] [PubMed] [Google Scholar]
- 37.Blachly-Dyson E, Baldini A, Litt M, McCabe ER, Forte M. Genomics. 1994;20:62–67. doi: 10.1006/geno.1994.1127. [DOI] [PubMed] [Google Scholar]
- 38.Cooper JC, Shi M, Chueh F-Y, Venkitachalam S, Yu C-L. Int J Oncol. 2010;36:1201–1208. doi: 10.3892/ijo_00000603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.He B, You L, Uematsu K, Matsangou M, Xu Z, He M, McCormick F, Jablons DM. Biochem Biophys Res Commun. 2003;301:386–391. doi: 10.1016/s0006-291x(02)03071-1. [DOI] [PubMed] [Google Scholar]
- 40.Auernhammer CJ, Bousquet C, Melmed S. Proc Natl Acad Sci U S A. 1999;96:6964–6969. doi: 10.1073/pnas.96.12.6964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ehret GB, Reichenbach P, Schindler U, Horvath CM, Fritz S, Nabholz M, Bucher P. J Biol Chem. 2001;276:6675–6688. doi: 10.1074/jbc.M001748200. [DOI] [PubMed] [Google Scholar]
- 42.Hall DA, Zhu H, Zhu X, Royce T, Gerstein M, Snyder M. Science. 2004;306:482–484. doi: 10.1126/science.1096773. [DOI] [PubMed] [Google Scholar]
- 43.Markova NG, Pinkas-Sarafova A, Simon M. J Invest Dermatol. 2006;126:2019–2031. doi: 10.1038/sj.jid.5700347. [DOI] [PubMed] [Google Scholar]
- 44.Hoshino A, Hirst JA, Fujii H. J Biol Chem. 2007;282:17706–17711. doi: 10.1074/jbc.M700094200. [DOI] [PubMed] [Google Scholar]
- 45.Yogev O, Yogev O, Singer E, Shaulian E, Goldberg M, Fox TD, Pines O. PLoS Biol. 2010;8:e1000328. doi: 10.1371/journal.pbio.1000328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Clayton AL, Hazzalin CA, Mahadevan LC. Mol Cell. 2006;23:289–296. doi: 10.1016/j.molcel.2006.06.017. [DOI] [PubMed] [Google Scholar]
- 47.Takahashi H, McCaffery JM, Irizarry RA, Boeke JD. Mol Cell. 2006;23:207–217. doi: 10.1016/j.molcel.2006.05.040. [DOI] [PubMed] [Google Scholar]
- 48.Li LY, Luo X, Wang X. Nature. 2001;412:95–99. doi: 10.1038/35083620. [DOI] [PubMed] [Google Scholar]
- 49.Poyton RO, Duhl DMJ, Clarkson GHD. Trend Cell Biol. 1992;2:369–375. doi: 10.1016/0962-8924(92)90049-s. [DOI] [PubMed] [Google Scholar]
- 50.Wegrzyn J, Potla R, Chwae YJ, Sepuri NB, Zhang Q, Koeck T, Derecka M, Szczepanek K, Szelag M, Gornicka A, Moh A, Moghaddas S, Chen Q, Bobbili S, Cichy J, Dulak J, Baker DP, Wolfman A, Stuehr D, Hassan MO, Fu XY, Avadhani N, Drake JI, Fawcett P, Lesnefsky EJ, Larner AC. Science. 2009;323:793–797. doi: 10.1126/science.1164551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gough DJ, Corlett A, Schlessinger K, Wegrzyn J, Larner AC, Levy DE. Science. 2009;324:1713–1716. doi: 10.1126/science.1171721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Demaria M, Giorgi C, Lebiedzinska M, Esposito G, D’Angeli L, Bartoli A, Gough DJ, Turkson J, Levy DE, Watson CJ, Wieckowski MR, Provero P, Pinton P, Poli V. Aging. 2010;2:823–842. doi: 10.18632/aging.100232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shoubridge EA. Nat Genet. 2002;31:227–228. doi: 10.1038/ng0702-227. [DOI] [PubMed] [Google Scholar]
- 54.Bonawitz ND, Clayton DA, Shadel GS. Mol Cell. 2006;24:813–825. doi: 10.1016/j.molcel.2006.11.024. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Exponentially growing and IL-3-deprived BaF3 cells were stained with FITC-conjugated annexin V and propidium iodide using the Dead Cell Apoptosis Kit from Invitrogen, Inc. Stained cells were analyzed by FACS Calibur flow cytometer from BD Biosciences. (A) Dot plot analysis was performed using the FlowJo software (Tree Star, Inc., Ashland, OR) on one set of experiments. The lower-left, lower-right, and upper-right quadrants represent live, apoptotic, and dead cells, respectively. (B) Statistical analysis of three independent experiments.
BaF3 cells were electroporated with firefly luciferase reporter constructs with six consecutive MGE as described for Fig. 5A(A), or with wild-type mSOCS3 promoter as described for Fig. 7(B). The reporter constructs were co-transfected with pcDNA without (vector) or with wild-type (WT) STAT5b or STAT5b with Y699F mutation. Dual-luciferase reporter assays were performed in transfected cells before and after 16 h of IL-3 stimulation. Data were normalized to renilla luciferase control and fold induction was calculated as compared to vector-transfected cells without IL-3 stimulation. *** P < 0.001.