Abstract
Chronic HBV infections remain a major public health problem worldwide. According to WHO estimates, more than 300 million people are chronically infected and exposed to the risk of developing severe complications including cirrhosis and hepatocellular carcinoma. Major progress in the treatment of chronic hepatitis B has been made during the last decade with the development of antivirals that inhibit viral polymerase activity. Antiviral drug resistance is a critical factor in determining the success of long-term therapy for chronic hepatitis B. The development of resistance to nucleoside analogues has been associated with exacerbations of liver disease. Sequential therapy increases the risk of the emergence of multi-drug resistance. The selection of a potent antiviral with a high barrier to resistance as a first line therapy provides the best chance of achieving long-term treatment goals and should be used whenever possible. This has led to a significant decrease in drug resistance in countries where this strategy is affordable. However, the barrier to resistance of a given antiviral agent is influenced by the genetic barrier, drug potency, patient adherence, the pharmacological barrier, viral fitness, the mechanisms of action and cross-resistance. Furthermore, because of specific viral kinetics, prolonged treatment with nucleoside analogues does not result in clearance of the viral genome from the infected liver. It is therefore important to continue research to identify new viral and immune targets and develop novel antiviral strategies for controlling viral replication as well as prevent drug resistance and its complications in the long-term.
Keywords: Chronic hepatitis, Antivirals, Drug resistance, entecavir, tenofovir, interferon
Chronic hepatitis B therapy
The ultimate goals of therapy for chronic hepatitis B (CHB) are to prevent disease progression and to prolong patient survival (1–3). These goals can be achieved as long as HBV replication can be suppressed and sustained. Major clinical studies have demonstrated the role of viral replication in the pathogenesis and progression CHB. A large prospective cohort study from Taiwan has shown that elevated HBV DNA (≥104 copies/mL) and its persistence significantly increase the risk of cirrhosis, hepatocellular carcinoma (HCC) and death, regardless of HBeAg status or baseline ALT levels(4). These data have been supported by several other similarly designed studies. Furthermore, one randomized controlled clinical trial of lamivudine in CHB patients with advanced fibrosis or cirrhosis showed the benefit of antiviral therapy on disease progression (5). However, the clinical benefit of reducing disease progression was limited in patients who developed lamivudine resistance (5). Histological improvement has been observed during treatment with lamivudine, adefovir, tenofovir and entecavir, although the development of resistance had a negative impact on the histological improvement observed with lamivudine (6–9).
Thus, a maintained long-term response to therapy or a sustained off-treatment response are necessary to prevent liver damage and hepatic decompensation and to delay the onset of the long-term complications of CHB such as hepatocellular carcinoma (HCC).
Recent advances in antiviral treatment of CHB
Seven drugs have received approval for the treatment of CHB, including interferon-alpha, pegylated interferon-alpha and the nucleoside analogs (NUCs), which belong to one of three structural groups: L-nucleosides (lamivudine and telbivudine), alkyl phosphonates (adefovir dipivoxil and tenofovir disoproxil fumarate) or D-cyclopentanes (entecavir).
The antiviral efficacy of the currently approved therapies, in terms of viral suppression, has been assessed in several registration studies and recently reviewed (1–3). Comparisons between studies are limited due to differences in patient characteristics, baseline HBV DNA levels, study design and methodologies used to quantify HBV DNA. Without head-to-head comparisons it is currently not possible to rank relative efficacy, but the results of these studies and clinical experience have shown that entecavir, telbivudine and tenofovir are the most potent of the currently available NUCs.
The interferons both have direct antiviral activity and immune-stimulatory properties. In clinical trials, pegylated interferon-alpha induced higher rates of sustained response during the 24-week off-treatment follow-up period despite a lower level of viral suppression compared to lamivudine following 1 year of therapy(10, 11). Furthermore, pegylated interferon administration has been associated with improved serological responses such as hepatitis B surface antigen (HBsAg) and hepatitis B e antigen (HBeAg) seroconversion during long-term follow-up. Interferon administration has been shown to reduce resistance to NUCs when combined with lamivudine (10, 11). However, interferon therapy is associated with side effects and its efficacy is limited to a small proportion of highly selected patients (3).
Resistance to NUCs
Resistance to NUCs is a major issue affecting long-term therapy with some of these agents. Cumulative annual incidences of resistance among nucleoside-naïve CHB patients are shown in the Table. The rate of drug resistance has decreased dramatically with the development of the newer generation of NUCs (12). Lamivudine resistance occurs frequently and is observed in up to 80% of patients treated for 5 years (13, 14). Among adefovir-treated patients, the cumulative incidence of resistance over 5 years has been reported to be 29% in HBeAg-negative patients and 42% in HBeAg-positive patients (8, 15). Telbivudine resistance is slower to emerge; however, rates are substantial with 25% of HBeAg-positive and 11% of HBeAg-negative patients experiencing virological breakthrough due to resistance after 2 years of treatment (16). Long-term studies of entecavir monotherapy in nucleoside-naïve patients have demonstrated that resistance remains low (1.2%) after 6 years of therapy at (17). No tenofovir resistance has been observed after 3 years of treatment in the registration studies (7).
Table.
Development of resistance to antiviral therapy (adapted from refernce 12)
| Drug and patient population | Resistance at year of therapy expressed as percentage of patients | |||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | |
| Lamivudine | 23 | 46 | 55 | 71 | 80 | - |
| Telbivudine HBeAg-Pos | 4.4 | 21 | - | - | - | - |
| Telbivudine HBeAg-Neg | 2.7 | 8.6 | - | - | - | - |
| Adefovir HBeAg-Neg | 0 | 3 | 6 | 18 | 29 | - |
| Adefovir (LAM-resistant) | Up to 20% | - | - | - | - | - |
| Tenofovir | 0 | 0 | 0 | - | - | - |
| Entecavir (naïve) | 0.2 | 0.5 | 1.2 | 1.2 | 1.2 | 1.2 |
| Entecavir (LAM resistant) | 6 | 15 | 36 | 46 | 51 | 57 |
Thus, the agents that have demonstrated the highest barrier to resistance in clinical studies in NUC naïve patients are entecavir and tenofovir.
The clinical consequences of developing resistance to NUCs have been well documented. Patients treated with lamivudine or adefovir who develop virological breakthrough due to the presence of resistance mutations frequently experience acute exacerbations of disease (ALT elevations) and more rapid progression to acute liver failure, liver transplant and higher risk of HCC and death (5, 14, 18, 19).
Another important consequence of drug resistance and the subsequent need for rescue therapy is the increased risk of development of multi-drug resistant HBV by sequential accumulation of resistance mutations on the same viral genome (20–22). This risk is particularly high for drugs with low barriers to resistance and with overlapping resistance profiles. In a second or third-line treatment setting, studies have demonstrated that entecavir is effective in patients with adefovir resistance and patients with prior lamivudine treatment who had not developed resistance, but not in patients with proven lamivudine resistance (23). This emphasizes the impact of cross resistance (in this case, between lamivudine and entecavir) on the outcome of rescue therapy. Tenofovir has also been shown to be effective in patients with lamivudine-resistance and an incomplete response to adefovir, but not necessarily in all patients with adefovir resistance (24). Thus, these studies demonstrate that inadequate management of resistant patients increases the risk of developing multi-drug resistance. It has also been shown that variant strains with single point mutations may also exhibit a multi-drug resistant phenotype (25).
Treatment guidelines and resistance management
Improvement in the availability of better therapies and virological monitoring tools have led to a progressive change in treatment guideline recommendations. Besides lowering HBV DNA and ALT thresholds for treatment indications, international guidelines recommend that therapy be initiated with a potent antiviral with a high barrier to resistance, such as entecavir or tenofovir, to reach undetectable HBV DNA as a primary endpoint thus minimizing the risk of selecting resistant variants (2, 3) (Figure).
Figure.
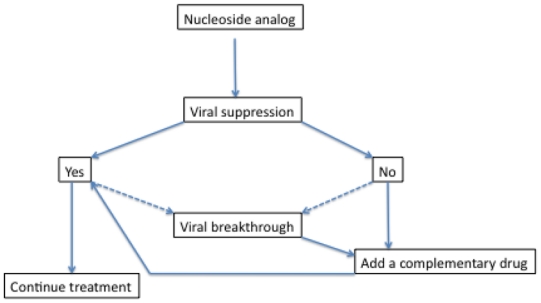
Treatment guidelines for treatment of chronic hepatitis B with nucleoside analogues
Avoid unnecessary treatment
To further minimize the risk of resistance, unnecessary treatment should be avoided and HBV DNA should be carefully monitored to check for primary non-response (<1 log10 drop in HBV DNA at week 12) as well as partial response (detectable HBV DNA at week 24). Treatment decisions for patients with partial response may be influenced by drug potency and barrier to resistance (2). In patients treated with lamivudine, adefovir or telbivudine with a partial response at week 24, treatment should be adapted with a switch to a more potent drug or the addition of a second drug with a non-overlapping cross-resistance profile. In patients treated with entecavir or tenofovir who have a partial response at week 48, some experts suggest adding the other drug (2). Alternatively, as long as the decrease in viral load continues, monotherapy can be continued because of the very low resistance rates. However, if there is no progressive continuous decline in viral load levels and a subsequent plateau occurs, then the regimen should be adapted, preferably with an add-on strategy. With such a strategy, the development of resistance can be prevented in most cases.
Avoid sequential monotherapy
Recommendations for the management of patients who develop antiviral resistance are consistent among treatment guidelines (2, 3, 26). One key principle is that sequential monotherapy should be avoided in mostcases. If initial monotherapy fails, a second drug with a non-overlapping resistance profile should be added or a switch should be made to a more potent combination of drugs (12). Most patients in treatment failure can be controlled with this rescue strategy.
Mechanism of antiviral drug resistance and barrier to resistance
Antiviral drug resistance is a result of adaptive mutations within the HBV genome and reduces the susceptibility of a virus to the inhibitory effects of a drug. The barrier to resistance can be defined as the difficulty with which these resistance mutants are selected (12). Mutations in the viral genome develop at a high frequency during viral replication, resulting in a diverse population of viral variants in infected individuals. Although HBV has a DNA-based genome, its replication cycle relies on an error-prone reverse transcription process, with mutations occurring at an estimated rate of 10−4 substitutions per base, per cycle (27). Thus, drug resistance mutations may preexist in patients who have no previous exposure to antiviral therapy. However, the actual selection of resistance mutations, and hence a drug barrier to resistance, is also influenced by several factors related to the virus (the number of mutations required to confer resistance, and their effect on viral fitness), the drug (mainly its antiviral potency) and the patient (treatment compliance and pharmacodynamics of the drug).
Entecavir and tenofovir are the preferred first-line treatments
The low rates of resistance and reductions in resistance-associated complications are the major benefits of using nucleoside analogs with a high barrier to resistance. Furthermore, since most patients who initiate treatment for CHB therapy, especially HBeAg-negative patients, are likely to require long-term therapy, first-line therapies with a high barrier to resistance offer the greatest chance of successful long-term treatment. Highly potent CHB therapies with the lowest rates of resistance, such as entecavir or tenofovir, are therefore the preferred first-line NUC treatment options in recently updated guidelines (2, 3, 26).
Alternative strategies for the prevention of resistance
A number of practical strategies for the prevention of drug resistance in clinical practice might be considered in view of the practice guidelines recommended by international liver societies (Figure).
Baseline evaluation
Before initiating therapy with a drug that has a low barrier to resistance (especially in countries where the other drugs are not yet available or affordable), an assessment of serum HBV DNA, ALT levels, prior treatment history and genotypic resistance may guide treatment selection in particular clinical situations. Baseline resistance testing may also be considered as drug resistance mutations have been detected in a number of treatment-naïve patients (28). However, at present there is little data to demonstrate the clinical relevance of resistance mutations that are present before treatment. Therefore, such results must be interpreted with caution when making treatment decisions. High levels of serum HBV DNA, ALT and elevated body-mass index have all been linked to increased rates of lamivudine resistance (13, 29). Furthermore, as might be expected, prior treatment with NUCs has been shown to predict drug resistance (18).
On-treatment monitoring and treatment adaptation: HBV DNA at week 24
The roadmap concept includes an algorithm for monitoring HBV DNA levels at weeks 12 and 24, with strategies suggested for patient management based on virological responses at the time points and the genetic barrier of the drug being used (30, 31). The roadmap concept proposes that patients with a complete virological response at week 24 (undetectable HBV DNA by PCR) remain on treatment with regular monitoring while patients with an inadequate virological response (≥2,000 IU/mL at week 24) should receive additional therapy with a more potent drug. Treatment decisions in patients with a partial virological response (≥60 to <2,000 IU/mL at week 24), are based on potency and genetic barrier: patients receiving NUCs with a high genetic barrier can remain on treatment beyond 48 weeks, patients receiving a less potent NUC should continue treatment and be re-assessed at week 48, and patients receiving NUCs with a low genetic barrier should add on a more potent drug because of the high risk of resistance if treatment is not adapted (2). In all cases patients should be monitored regularly for virological breakthrough. The rationale for treatment decisions in the roadmap concept are based on predictors of response from studies of drugs with low resistance barriers (lamivudine, adefovir and telbivudine). These analyses demonstrate that patients with a profound early virological response during treatment with lamivudine (32), adefovir, (8) and telbivudine (16) have a lower chance of developing resistance. However, because of the very low rates of resistance observed with entecavir and tenofovir (7, 33) it has not been possible to accurately assess predictors of resistance in these drugs. The main advantage of the roadmap is that it provides a comprehensive guide to short-term monitoring in patients receiving first-line therapy with a drug that has a low barrier to resistance. However, the clinical outcomes associated with the roadmap concept have not been examined in a prospective study.
If this principle of treatment monitoring and adaptation is followed, most patients can be controlled, whether they start therapy with drugs with a high or low barrier to resistance.
Crucial role of treatment adherence
Poor adherence substantially reduces viral suppression and may increase resistance rates, as previously discussed. Good adherence to regimens with a low barrier to resistance is therefore absolutely necessary. Adherence may be monitored using patient reports, dispensed medication counts or HBV DNA testing. Adherence may be improved by educating patients on the importance of adherence and preventing resistance, providing assistance with treatment management (i.e. reassurance and advice regarding adverse events), by checking and reinforcing the importance of adherence at each appointment and by providing feedback on treatment progress.
Conclusions
Clinical studies have demonstrated that drugs with a high barrier to resistance, such as entecavir and potentially tenofovir, have significantly lower rates of resistance when compared to those with a low barrier to resistance such as lamivudine, adefovir or telbivudine. The barrier to resistance of a drug can be defined as the difficulty with which antiviral resistance is selected during CHB therapy. This can be influenced by a number of factors including the drug’s genetic barrier and intrinsic potency, the level of adherence to the treatment regimen, pharmacological barriers and the replication fitness of any drug-resistant variants that arise during viral replication.
Compelling evidence connects high levels of viral replication to an increased time to HBV DNA undetectability during treatment, and an increased incidence of cirrhosis, HCC and liver-related mortality. Thus, the correct choice of a first line potent therapy to achieve sustained long-term suppression of viral replication provides the best chance of achieving the goals of therapy, which are to prevent the progression of liver disease and to prolong survival. Most patients receiving treatment will require long-term therapy to meet these goals and the development of antiviral resistance is a major concern in these cases. Treatment with a potent drug that has a high barrier to resistance, such as entecavir or tenofovir, will minimize future resistance, preserve future treatment options, protect public health and maximize the chances of long-term treatment success. Furthermore, the correct choice of first-line therapy also provides the best chance of avoiding the need for salvage therapy, which can be significantly affected by NUC antagonism and cross-resistance. In recent years, results of studies and clinical experience have shown that major progress has been made in the management of antiviral drug resistance which has now become a manageable issue provided that adequate virological monitoring and treatment adaptation are performed. In developing countries where HBV infection is endemic programmes are needed for cost-effective delivery of the best drugs and virological monitoring, improving availability of antiviral treatment patient management and the prevention of resistance.
Perspectives
Although chronic HBV infection is not curable because of persistent viral cccDNA and integrated HBV genomes in infected cells, there are very few new drugs in the anti-HBV chemotherapy development pipeline. It is therefore important to continue research in this area to anticipate resistance issues in the vast population of patients who have not yet been treated worldwide. Several viral targets are of potential interest for the development of new drugs with more potent combination strategies to help enhance viral clearance and prevent resistance.
Inhibition of cell entry
The inhibition of virus cell entry is one of these main targets (34, 35). Administration of pre-S1 peptides mimicking the envelope protein domain involved in virus/cell membrane interaction resulted in the prevention of virus entry in the hepatocyte culture and the inhibition of viral infection and spread in a humanized SCID mouse model. Theoretically, the combination of this peptide with NUCs should prevent the infection of new cells while viral load is being suppressed by NUCs, thus increasing the rate of clearance of infected cells. More experimental studies are required for a proof of concept to test this hypothesis in clinical trials.
Targeting cccDNA
Targeting the formation and subsequent processing of viral cccDNA would be the ideal target but currently no candidate drug without cytotoxic effects is available for experimental studies. Epigenetic regulation of cccDNA transcriptional activity is another possible target which needs to be further investigated in experimental models (36).
Capsid formation
Viral pregenome encapsidation and capsid formation represent potential targets. Phenylpropenamide derivatives and heteroaryl-pyrimidines (HAP) have been studied in vitro in hepatoma cell lines and been shown to inhibit replication of both wild type and amivudine resistant mutant genomes (37–40). Unfortunately the development of phenylpropenamides was not continued in the clinical setting because of toxicity. More recently AiCuris Pharmaceuticals has developed the HAP molecules as non-nucleoside inhibitors of HBV core protein dimerization, blocking nucleocapsid formation (39, 40), and demonstrating efficacy in an animal model of HBV infection (39).
Other targets
Finally, viral morphogenesis and egress represent another potential target for inhibition of the viral life cycle. In this respect, it was shown that Iminosugars which modulate the glycosylation status and conformation of envelope proteins may decrease the production of infectious particles in vitro (41), resulting in an antiviral effect in vivo in the woodchuck model of hepadnavirus infection (42).
Other targets needing further experimental investigations are the modulation of the innate response of infected hepatocytes (43) and dendritic cells (44) or the stimulation of the specific adaptive immune response (45–47) to induce a sustained immunological control of HBV infection to allow appropriately timed cessation of NUC administration.
References
- 1.ZOULIM F, PERRILLO R. Hepatitis B: reflections on the current approach to antiviral therapy. J Hepatol. 2008;48 (Suppl 1):S2–19. doi: 10.1016/j.jhep.2008.01.011. [DOI] [PubMed] [Google Scholar]
- 2.EUROPEAN ASSOCIATION FOR THE STUDY OF THE L. EASL Clinical Practice Guidelines: management of chronic hepatitis B. J Hepatol. 2009;50(2):227–42. doi: 10.1016/j.jhep.2008.10.001. [DOI] [PubMed] [Google Scholar]
- 3.LOK AS, MCMAHON BJ. Chronic hepatitis B: update 2009. Hepatology. 2009;50(3):661–2. doi: 10.1002/hep.23190. [DOI] [PubMed] [Google Scholar]
- 4.CHEN CJ, YANG HI, SU J, JEN CL, YOU SL, LU SN, et al. Risk of hepatocellular carcinoma across a biological gradient of serum hepatitis B virus DNA level. Jama. 2006;295(1):65–73. doi: 10.1001/jama.295.1.65. [DOI] [PubMed] [Google Scholar]
- 5.LIAW YF, SUNG JJ, CHOW WC, FARRELL G, LEE CZ, YUEN H, et al. Lamivudine for patients with chronic hepatitis B and advanced liver disease. N Engl J Med. 2004;351(15):1521–31. doi: 10.1056/NEJMoa033364. [DOI] [PubMed] [Google Scholar]
- 6.CHANG TT, LIAW YF, WU SS, SCHIFF E, HAN KH, LAI CL, et al. Long-term entecavir therapy results in the reversal of fibrosis/cirrhosis and continued histological improvement in patients with chronic hepatitis B. Hepatology. doi: 10.1002/hep.23785. [DOI] [PubMed] [Google Scholar]
- 7.MARCELLIN P, HEATHCOTE EJ, BUTI M, GANE E, DE MAN RA, KRASTEV Z, et al. Tenofovir disoproxil fumarate versus adefovir dipivoxil for chronic hepatitis B. N Engl J Med. 2008;359(23):2442–55. doi: 10.1056/NEJMoa0802878. [DOI] [PubMed] [Google Scholar]
- 8.HADZIYANNIS SJ, TASSOPOULOS NC, HEATHCOTE EJ, CHANG TT, KITIS G, RIZZETTO M, et al. Long-term therapy with adefovir dipivoxil for HBeAg-negative chronic hepatitis B for up to 5 years. Gastroenterology. 2006;131(6):1743–51. doi: 10.1053/j.gastro.2006.09.020. [DOI] [PubMed] [Google Scholar]
- 9.DIENSTAG JL, GOLDIN RD, HEATHCOTE EJ, HANN HW, WOESSNER M, STEPHENSON SL, et al. Histological outcome during long-term lamivudine therapy. Gastroenterology. 2003;124(1):105–17. doi: 10.1053/gast.2003.50013. [DOI] [PubMed] [Google Scholar]
- 10.MARCELLIN P, LAU GK, BONINO F, FARCI P, HADZIYANNIS S, JIN R, et al. Peginterferon alfa-2a alone, lamivudine alone, and the two in combination in patients with HBeAg-negative chronic hepatitis B. N Engl J Med. 2004;351(12):1206–17. doi: 10.1056/NEJMoa040431. [DOI] [PubMed] [Google Scholar]
- 11.LAU GK, PIRATVISUTH T, LUO KX, MARCELLIN P, THONGSAWAT S, COOKSLEY G, et al. Peginterferon Alfa-2a, lamivudine, and the combination for HBeAg-positive chronic hepatitis B. N Engl J Med. 2005;352(26):2682–95. doi: 10.1056/NEJMoa043470. [DOI] [PubMed] [Google Scholar]
- 12.ZOULIM F, LOCARNINI S. Hepatitis B Virus Resistance to Nucleos(t)ide Analogues. Gastroenterology. 2009 doi: 10.1053/j.gastro.2009.08.063. [DOI] [PubMed] [Google Scholar]
- 13.LAI CL, DIENSTAG J, SCHIFF E, LEUNG NW, ATKINS M, HUNT C, et al. Prevalence and clinical correlates of YMDD variants during lamivudine therapy for patients with chronic hepatitis B. Clin Infect Dis. 2003;36(6):687–96. doi: 10.1086/368083. [DOI] [PubMed] [Google Scholar]
- 14.LOK AS, LAI CL, LEUNG N, YAO GB, CUI ZY, SCHIFF ER, et al. Long-term safety of lamivudine treatment in patients with chronic hepatitis B. Gastroenterology. 2003;125(6):1714–22. doi: 10.1053/j.gastro.2003.09.033. [DOI] [PubMed] [Google Scholar]
- 15.MARCELLIN P, CHANG TT, LIM SG, SIEVERT W, TONG M, ARTERBURN S, et al. Long-term efficacy and safety of adefovir dipivoxil for the treatment of hepatitis B e antigen-positive chronic hepatitis B. Hepatology. 2008;48(3):750–8. doi: 10.1002/hep.22414. [DOI] [PubMed] [Google Scholar]
- 16.LIAW YF, GANE E, LEUNG N, ZEUZEM S, WANG Y, LAI CL, et al. 2-Year GLOBE Trial Results: Telbivudine Is Superior to Lamivudine in Patients With Chronic Hepatitis B. Gastroenterology. 2009;136(2):486–95. doi: 10.1053/j.gastro.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 17.CHANG TT, LAI CL, KEW YOON S, LEE SS, COELHO HS, CARRILHO FJ, et al. Entecavir treatment for up to 5 years in patients with hepatitis B e antigen-positive chronic hepatitis B. Hepatology. 51(2):422–30. doi: 10.1002/hep.23327. [DOI] [PubMed] [Google Scholar]
- 18.NAFA S, AHMED S, TAVAN D, PICHOUD C, BERBY F, STUYVER L, et al. Early Detection of Viral Resistance by Determination of Hepatitis B Virus Polymerase Mutations in Patients Treated by Lamivudine for Chronic Hepatitis B. Hepatology. 2000;32(5):1078–88. doi: 10.1053/jhep.2000.19619. [DOI] [PubMed] [Google Scholar]
- 19.DI MARCO V, MARZANO A, LAMPERTICO P, ANDREONE P, SANTANTONIO T, ALMASIO PL, et al. Clinical outcome of HBeAg-negative chronic hepatitis B in relation to virological response to lamivudine. Hepatology. 2004;40(4):883–91. doi: 10.1002/hep.20381. [DOI] [PubMed] [Google Scholar]
- 20.YIM HJ, HUSSAIN M, LIU Y, WONG SN, FUNG SK, LOK AS. Evolution of multi-drug resistant hepatitis B virus during sequential therapy. Hepatology. 2006;44(3):703–12. doi: 10.1002/hep.21290. [DOI] [PubMed] [Google Scholar]
- 21.VILLET S, PICHOUD C, VILLENEUVE JP, TREPO C, ZOULIM F. Selection of a multiple drug-resistant hepatitis B virus strain in a liver-transplanted patient. Gastroenterology. 2006;131(4):1253–61. doi: 10.1053/j.gastro.2006.08.013. [DOI] [PubMed] [Google Scholar]
- 22.VILLET S, OLLIVET A, PICHOUD C, BARRAUD L, VILLENEUVE JP, TREPO C, et al. Stepwise process for the development of entecavir resistance in a chronic hepatitis B virus infected patient. J Hepatol. 2007;46(3):531–8. doi: 10.1016/j.jhep.2006.11.016. [DOI] [PubMed] [Google Scholar]
- 23.REIJNDERS JG, DETERDING K, PETERSEN J, ZOULIM F, SANTANTONIO T, BUTI M, et al. Antiviral effect of entecavir in chronic hepatitis B: influence of prior exposure to nucleos(t)ide analogues. J Hepatol. 52(4):493–500. doi: 10.1016/j.jhep.2010.01.012. [DOI] [PubMed] [Google Scholar]
- 24.VAN BOMMEL F, DE MAN RA, WEDEMEYER H, DETERDING K, PETERSEN J, BUGGISCH P, et al. Long-term efficacy of tenofovir monotherapy for hepatitis B virus-monoinfected patients after failure of nucleoside/nucleotide analogues. Hepatology. 51(1):73–80. doi: 10.1002/hep.23246. [DOI] [PubMed] [Google Scholar]
- 25.VILLET S, PICHOUD C, BILLIOUD G, BARRAUD L, DURANTEL S, TREPO C, et al. Impact of hepatitis B virus rtA181V/T mutants on hepatitis B treatment failure. J Hepatol. 2008;48(5):747–55. doi: 10.1016/j.jhep.2008.01.027. [DOI] [PubMed] [Google Scholar]
- 26.LIAW YF, LEUNG N, KAO JH, PIRATVISUTH T, GANE E, HAN KH, et al. Asian-Pacific consensus statement on the management of chronic hepatitis B: a 2008 update. Hepatol Int. 2008;2(3):263–83. doi: 10.1007/s12072-008-9080-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.GIRONES R, MILLER RH. Mutation rate of the hepadnavirus genome. Virology. 1989;170(2):595–7. doi: 10.1016/0042-6822(89)90455-8. [DOI] [PubMed] [Google Scholar]
- 28.SOLMONE M, VINCENTI D, PROSPERI MC, BRUSELLES A, IPPOLITO G, CAPOBIANCHI MR. Use of massively parallel ultradeep pyrosequencing to characterize the genetic diversity of hepatitis B virus in drug-resistant and drug-naive patients and to detect minor variants in reverse transcriptase and hepatitis B S antigen. J Virol. 2009;83(4):1718–26. doi: 10.1128/JVI.02011-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.YUEN MF, SABLON E, HUI CK, YUAN HJ, DECRAEMER H, LAI CL. Factors associated with hepatitis B virus DNA breakthrough in patients receiving prolonged lamivudine therapy. Hepatology. 2001;34(4 Pt 1):785–91. doi: 10.1053/jhep.2001.27563. [DOI] [PubMed] [Google Scholar]
- 30.KEEFFE EB, DIETERICH DT, HAN SH, JACOBSON IM, MARTIN P, SCHIFF ER, et al. A treatment algorithm for the management of chronic hepatitis B virus infection in the United States: an update. Clin Gastroenterol Hepatol. 2006;4(8):936–62. doi: 10.1016/j.cgh.2006.05.016. [DOI] [PubMed] [Google Scholar]
- 31.KEEFFE EB, ZEUZEM S, KOFF RS, DIETERICH DT, ESTEBAN-MUR R, GANE EJ, et al. Report of an international workshop: Roadmap for management of patients receiving oral therapy for chronic hepatitis B. Clin Gastroenterol Hepatol. 2007;5(8):890–7. doi: 10.1016/j.cgh.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 32.YUEN MF, FONG DY, WONG DK, YUEN JC, FUNG J, LAI CL. Hepatitis B virus DNA levels at week 4 of lamivudine treatment predict the 5-year ideal response. Hepatology. 2007 doi: 10.1002/hep.21939. [DOI] [PubMed] [Google Scholar]
- 33.TENNEY DJ, ROSE RE, BALDICK CJ, POKORNOWSKI KA, EGGERS BJ, FANG J, et al. Long-term monitoring shows hepatitis B virus resistance to entecavir in nucleoside-naive patients is rare through 5 years of therapy. Hepatology. 2009;49(5):1503–14. doi: 10.1002/hep.22841. [DOI] [PubMed] [Google Scholar]
- 34.GRIPON P, CANNIE I, URBAN S. Efficient inhibition of hepatitis B virus infection by acylated peptides derived from the large viral surface protein. J Virol. 2005;79(3):1613–22. doi: 10.1128/JVI.79.3.1613-1622.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.PETERSEN J, DANDRI M, MIER W, LUTGEHETMANN M, VOLZ T, VON WEIZSACKER F, et al. Prevention of hepatitis B virus infection in vivo by entry inhibitors derived from the large envelope protein. Nat Biotechnol. 2008;26(3):335–41. doi: 10.1038/nbt1389. [DOI] [PubMed] [Google Scholar]
- 36.POLLICINO T, BELLONI L, RAFFA G, PEDICONI N, SQUADRITO G, RAIMONDO G, et al. Hepatitis B virus replication is regulated by the acetylation status of hepatitis B virus cccDNA-bound H3 and H4 histones. Gastroenterology. 2006;130(3):823–37. doi: 10.1053/j.gastro.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 37.KING RW, LADNER SK, MILLER TJ, ZAIFERT K, PERNI RB, CONWAY SC, et al. Inhibition of human hepatitis B virus replication by AT-61, a phenylpropenamide derivative, alone and in combination with (-)b-L-2′,3′-dideoxy-3′-thiacytidine. Antimicrob Agents Chemother. 1998;42:3179–86. doi: 10.1128/aac.42.12.3179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.DELANEY WET, EDWARDS R, COLLEDGE D, SHAW T, FURMAN P, PAINTER G, et al. Phenylpropenamide derivatives AT-61 and AT-130 inhibit replication of wild-type and lamivudine-resistant strains of hepatitis B virus in vitro. Antimicrob Agents Chemother. 2002;46(9):3057–60. doi: 10.1128/AAC.46.9.3057-3060.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.WEBER O, SCHLEMMER KH, HARTMANN E, HAGELSCHUER I, PAESSENS A, GRAEF E, et al. Inhibition of human hepatitis B virus (HBV) by a novel non-nucleosidic compound in a transgenic mouse model. Antiviral Res. 2002;54(2):69–78. doi: 10.1016/s0166-3542(01)00216-9. [DOI] [PubMed] [Google Scholar]
- 40.DERES K, SCHRODER CH, PAESSENS A, GOLDMANN S, HACKER HJ, WEBER O, et al. Inhibition of hepatitis B virus replication by drug-induced depletion of nucleocapsids. Science. 2003;299(5608):893–6. doi: 10.1126/science.1077215. [DOI] [PubMed] [Google Scholar]
- 41.LAZAR C, DURANTEL D, MACOVEI A, ZITZMANN N, ZOULIM F, DWEK RA, et al. Treatment of hepatitis B virus-infected cells with alpha-glucosidase inhibitors results in production of virions with altered molecular composition and infectivity. Antiviral Res. 2007 doi: 10.1016/j.antiviral.2007.04.004. [DOI] [PubMed] [Google Scholar]
- 42.BLOCK TM, LU X, MEHTA AS, BLUMBERG BS, TENNANT B, EBLING M, et al. Treatment of chronic hepadnavirus infection in a woodchuck animal model with an inhibitor of protein folding and trafficking. Nature Medicine. 1998;4:610–14. doi: 10.1038/nm0598-610. [DOI] [PubMed] [Google Scholar]
- 43.THOMPSON AJ, COLLEDGE D, RODGERS S, WILSON R, REVILL P, DESMOND P, et al. Stimulation of the interleukin-1 receptor and Toll-like receptor 2 inhibits hepatitis B virus replication in hepatoma cell lines in vitro. Antiviral Therapy. 2009;14 doi: 10.3851/IMP1294. in press. [DOI] [PubMed] [Google Scholar]
- 44.VINCENT IE, LUCIFORA J, DURANTEL D, HANTZ O, CHEMIN I, ZOULIM F, et al. Inhibitory effect of the combination of CpG-induced cytokines with lamivudine against hepatitis B virus replication in vitro. Antivir Ther. 2009;14(1):131–5. [PubMed] [Google Scholar]
- 45.DAS A, HOARE M, DAVIES N, LOPES AR, DUNN C, KENNEDY PT, et al. Functional skewing of the global CD8 T cell population in chronic hepatitis B virus infection. J Exp Med. 2008;205(9):2111–24. doi: 10.1084/jem.20072076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.LOPES AR, KELLAM P, DAS A, DUNN C, KWAN A, TURNER J, et al. Bim-mediated deletion of antigen-specific CD8 T cells in patients unable to control HBV infection. J Clin Invest. 2008;118(5):1835–45. doi: 10.1172/JCI33402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.DUNN C, BRUNETTO M, REYNOLDS G, CHRISTOPHIDES T, KENNEDY PT, LAMPERTICO P, et al. Cytokines induced during chronic hepatitis B virus infection promote a pathway for NK cell-mediated liver damage. J Exp Med. 2007;204(3):667–80. doi: 10.1084/jem.20061287. [DOI] [PMC free article] [PubMed] [Google Scholar]


