Abstract
Functional neuroimaging techniques have allowed for investigations into the mechanisms of age-related deterioration in motor control. This study used functional Magnetic Resonance Imaging (fMRI) to investigate age related differences in the control of grip force magnitude. Using an event-related design, fMRI scans were completed on 13 older adults, and 13 gender matched younger adults, while using their dominant hand to squeeze a rubber bulb for 4s at 10%, 40% or 70% of their maximum voluntary contraction. Both groups were able to match the relative force targets, however the older adults produced significantly lower levels of absolute force. fMRI analysis consisted of a 1) region of interest (ROI) approach to detect differences in selected motor areas within brain and 2) a voxel-wise whole brain comparison to find areas of differential activation that were not defined a priori between the older and younger group. The ROI analysis revealed that despite producing lower levels of absolute force, the older adults showed higher levels of activity predominantly in subcortical structures (putamen, thalamus and cerebellum) when compared to the younger group. The older adults also showed higher levels of activity in the ipsilateral ventral premotor cortex. A total of 19 of the 22 ROIs analyzed showed a significant main effect of the required force-level. In the majority of the ROIs that showed a significant force effect there were no significant differences in the magnitude of the blood-oxygen-level-dependent (BOLD) signal between the 10% and 40% conditions but a significantly higher BOLD signal in the 70% condition, suggesting that the modulation of brain activation with grip force may not be controlled in a linear fashion. It was also found that the older adult group demonstrate higher levels of activation in 7 areas during a force production task at higher force levels using a voxel-wise analysis. The 7 clusters that showed significant differences tended to be areas that are involved in visual-spatial and executive processing. The results of this study revealed that older adults require significantly higher activation of several areas to perform the same motor task as younger adults. Higher magnitudes of the BOLD signal in older adults may represent a compensatory pattern to counter age related deterioration in motor control systems.
Keywords: fMRI, Aging, Force Control, Motor Control
1.0 Introduction
The normal aging process leads to several declines of the motor system that are related to changes within the central nervous system, peripheral nervous system and the musculoskeletal system (Seidler et al., 2010). These changes have the potential to lead to decreased motor performance with aging, which has been observed in the forms of decreased coordination, increased movement variability, slower movements and difficulties with balance and gait (Seidler et al., 2010). Ward (2006) reviewed a number of potential causes for decreased motor performance in older adults, including changes in the brain, such as a loss of grey matter, decreased dendritic density (Anderson and Rutledge, 1996) and reduced cerebral blood flow (Leenders et al., 1990). Other possible reasons for decreased motor function with aging include reduced proprioception (Shaffer and Harrison, 2007), slower peripheral nerve conduction (Gilmore, 1995) and age-related changes in the structure and function of skeletal muscle (Dutta et al., 1997; Lang et al., 2010).
With the recent advances in neuroimaging, it is possible to examine whether functional declines with aging are associated with specific changes in brain activation. A large number of studies using functional magnetic resonance imaging (fMRI) in cognitive and perceptual tasks have found that older adults show activity in a greater number of areas to accomplish the same task as younger control subjects, despite not observing any changes in task performance. For example, neuroimaging research in cognition has demonstrated that activation in the prefrontal cortex is more bilateral in older adults compared to younger adults during memory and word recognition tasks, despite not observing any age-related differences in task performance (Cabeza, 2001). Studies focusing on the motor system have also demonstrated age-related increases in the areas activated during motor tasks. A number of studies have found a greater reliance on the ipsilateral primary sensorimotor cortex (SMC) in older adults when compared to young adults (Hutchinson et al., 2002; Kim et al., 2010; Mattay et al., 2002; Naccarato et al., 2006; Ward et al., 2008). These tasks have included a paced thumb opposition task (Naccarato et al., 2006), a visually guided gripping task at a moderate force level of approximately 45% of the participants maximum voluntary contraction (MVC) (Ward et al., 2008), a continuous abduction/adduction task with the index finger and a wrist flexion/extension task (Hutchinson et al., 2002) and an elbow flexion and extension task (Kim et al., 2010). Taken together these data suggest that the motor control demands for these tasks require additional neural resources in older adults to achieve the same performance as younger adults. These age-related changes in neural activity have been shown by the recruitment of additional areas to complete the task as well as showing higher levels of activity in selected areas that were also activated by younger adults. Age-related increases in brain activation have been found in areas including the contralateral SMC, ipsilateral ventral premotor cortex, supplementary motor area, putamen and cerebellum (Kim et al., 2010; Mattay et al., 2002; Riecker et al., 2006). Interestingly these age-related differences were absent in each case when movements were unresisted or performed at lower force levels and hence motor control demands were diminished, suggesting that these adaptive changes are more pronounced when greater demands are placed on the motor control system.
Most of the motor tasks employed in past studies have been simple finger movements such as a key press (Mattay et al., 2002), a tapping task (Naccarato et al., 2006) or a finger abduction/adduction motion (Hutchinson et al., 2002). These tasks are not necessarily relevant to activities performed in daily life, which commonly require different gradations of muscle force to be produced (eg. lifting a milk jug and handling a delicate object). It is important to study tasks that allow the examination of different levels of force production, as gradation of force is often negatively affected in older adults. For example, as individuals age they can experience a significant loss in the ability to produce muscle force with the upper-limb (Shinohara et al., 2003a) and a larger variance in the production of muscle force (Olafsdottir et al., 2007). Few studies have used fMRI to specifically analyze the effect of aging while varying the level of the target force that is required. Ward et al (2008) had participants perform a gripping task at 15%, 30% and 45% of their MVC. They found that the increase in activation of the contralateral primary motor cortex (M1), the contralateral primary sensory cortex (S1), contralateral posterior cingulate motor areas and contralateral dorsal premotor cortex in response to higher force levels is lower in magnitude for older adults. However, this study used only relatively low levels of force production (< 50% MVC), leaving the effect of aging on brain activation during the production of higher levels of force unclear. Kim et al (2010) had participants perform an elbow flexion-extension task, with or without resistance, in conjunction with fMRI scanning and found that the older adults and younger subjects show similar areas of neural activation when the movement was performed unresisted. When resistance was added, the older adult group showed significantly higher levels of neural activity over a much larger area, including higher activation in the M1, inferior frontal gyrus, putamen, substantia niagra and subthalamic nucleus in the ipsilateral hemisphere and in the orbitofrontal gyrus, the insula and cerebellum in the contralateral hemisphere.
In studies of young healthy adults, the level of brain activation observed in several motor areas has been shown to be proportional to the amount of force produced in hand muscles (Dai et al., 2001). These motor areas include the M1, S1 and premotor areas in the contralateral hemisphere and the supplementary motor area, prefrontal cortex and cerebellum bilaterally. Cramer et al (2002) specifically analyzed the effect of grip force on the activation of motor areas within the brain across a range of force values. It was found that both the magnitude of activation and the volume of activated voxels within the contralateral SMC increased as the magnitude of the grip force increased. In addition, the ipsilateral supplementary motor area (SMA) showed a greater number of activated voxels as the force increased. Cramer et al (2002) also noted that there was no change in the laterality of the activation of the primary or supplementary motor areas with increasing grip force. Spraker et al (2007) used a region of interest (ROI) analysis to specifically analyze the role of the basal ganglia during a force modulation task, where participants were asked to perform pinch grips ranging between 5% and 80% MVC. It was found that the activity of the globus pallidus internus and the subthalamic nucleus had a positive correlation with the amount of force produced, along with the contralateral SMC.
At the neuromuscular level it has been demonstrated that recruitment of motor units is used during hand force production only up to 40–50% MVC (De Luca et al., 1982; Kukulka and Clamann, 1981), suggesting that rate coding is primarily responsible for increasing force output beyond 50% MVC. Rate coding occurs over a narrower range of forces in older adults (Barry et al., 2007). In addition, the hand muscles of older adults are disproportionately weaker when compared to young groups and therefore higher levels of muscle activation are required to achieve the same force (Shinohara et al., 2003b). These findings indicate some of the potential reasons that older adults could have difficulty with force modulation tasks when compared to young adults.
The goal of this study was to use fMRI to determine how aging affects activation of brain motor areas during a gripping task at different force levels. Specifically, an ROI analysis approach was used to compare areas of neural activity in motor areas between a group of older adults and a control group of young adults. It was hypothesized that the older adults would require greater levels of activation within these motor areas to achieve the same relative force levels as the younger group. It was expected that these differences would be greatest at higher levels of force production due to the extra neural drive that is needed to overcome the neuromuscular changes that occur with aging (Barry et al., 2007; De Luca et al., 1982; Kukulka and Clamann, 1981). A voxel-wise whole brain analysis was also carried out to determine all of the locations of age-related changes in brain activation during this force modulation task.
2.0 Materials and Methods
2.1 Participants
Thirteen healthy young adults (mean age = 26.1 years, range = 22–30 years, 7 Male, 6 Female) and thirteen gender matched older adults (mean age = 67.5, range = 58–78) were recruited from the community. All participants were right hand dominant according to the Edinburgh Handedness Inventory (Oldfield, 1971). Participants completed a brief health screening questionnaire to ensure that there was no history of neurological, psychiatric or cardiovascular disease. Participants also performed a brief questionnaire to ensure there were no contraindications for the use of MR-imaging. The local university and hospital ethical review boards approved all procedures used for this study and informed consent was obtained from all participants prior to participation. The participants’ maximum isometric grip strength was also measured with a standard hydraulic grip strength dynamometer for each hand. Details regarding the participants can be found in Table 1. Group comparisons of the participants’ maximum grip strength, measured with a grip strength dynamometer, were carried out with independent samples t-test to establish any pre-existing group differences in these values.
Table 1.
Participant characteristics. The standard deviation is shown in parentheses. The p-value indicates the result of an independent samples t-test.
| Young Adults | Older Adults | p-value | |
|---|---|---|---|
| N (gender) | 13 - 7 M, 6 F | 13 - 7 M, 6 F | - |
| Age (years) | 26.1 (1.8) | 67.5 (6.4) | - |
| Max. Grip Strength- Right Hand (kg) | 37.5 (9.1) | 30.8 (9.1) | 0.07 |
| Max. Grip Strength- Left Hand (kg) | 36.0 (8.6) | 29.8 (8.8) | 0.08 |
2.2 Protocol
The motor task consisted of a custom-built MR-compatible rubber squeeze bulb with a diameter of 4cm connected to a pressure transducer that measured the pressure of water within the bulb and tubing. Squeezing the rubber bulb caused an increase in the water pressure within the bulb and tubing. Water was used rather than air since it cannot be compressed and the system would be less susceptible to leaks. Before the scanning session, all participants were trained on the task until familiar with its requirements in order to minimize the effect of motor learning during the scanning sessions. In addition, electromyography measurements were taken during this practice session to ensure mirror movements did not occur during task performance.
During the fMRI scanning sessions, participants lay supine with their elbow flexed at 90° and the forearm in a resting position across their stomach with their hand pronated, gripping the squeeze bulb. Participants were instructed to squeeze the bulb using a power grip and to not change their grip during the scanning session. The MVC of each participant was measured with the water-based pressure system prior to starting the task and all subsequent squeezes were scaled to this value. During fMRI scanning, participants viewed a computer screen via a back projection-mirror system that had a cross at the center of the screen for fixation. Each trial began with an increase in size of the fixation cross, which indicated that a squeeze was about to be required. After 2s, a vertical bar appeared on the screen to cue the subject to squeeze the bulb and illustrate the required force. Participants were required to squeeze until the pressure level matched the target level and then release when the target disappeared, allowing the pressure level to return to baseline, while receiving visual feedback on the force they were producing on the screen. Three target force levels (10%, 40% and 70% of MVC) were presented in each trial in a pseudo-random order.
An event related design was used to analyze the fMRI data for this study. Four runs were completed during the MRI scanning session with 24 squeeze trials occurring per run. Each squeeze trial lasted 4s to allow the hemodynamic response to stabilize. It was decided not to use a squeeze duration greater than 4s in order to reduce the chance of developing muscular fatigue. Blank (resting) scan intervals were included as a baseline control and randomly inserted at the end of each squeeze trial; 60% of total scan time consisted of these blank trials (Burock et al., 1998). These blank runs were jittered to last between 10s and 16s. This procedure was employed to differentiate activation trials from baseline activation (Burock et al., 1998). In total, each run lasted 6.8 min. The hand required to squeeze the bulb was alternated between each of the 4 runs with the dominant hand being tested first. Only data concerning the dominant hand were analyzed for this study. Following the fMRI runs, a high resolution T1-weight anatomical scan was carried out for each participant.
2.3 fMRI data acquisition
A Philips Gyroscan Intera 3.0 T scanner (Philips, Best, the Netherlands), equipped with a head-coil, was used to acquire both high resolution T1-weighted anatomical images (170 axial slices, matrix size = 256 × 256, voxel size = 1.0 × 1.0 × 1.0 mm) and T2*-weighted echo-planar (EPI) images (matrix size = 128 × 128, pixel size = 1.9 × 1.9 mm, TR=2000 ms, TE = 3.7). A total of 4 functional runs (each with 24 squeezes) took place, with each run lasting 6.8 minutes. A total of 206 interleaved volumes were acquired during each run. Each volume acquired during the fMRI analysis consisted of 36 axial slices of 3mm thickness, with a gap thickness of 1mm.
2.4 Behavioral data analysis
Custom Matlab software (Mathworks, Natwick MA) and the Psychtoolbox (Brainard, 1997) were used to design and present stimuli and to collect behavioral data from the squeeze-bulb device. Pressure data, in pounds per square inch (PSI), were collected during the fMRI scanning sessions with a sampling rate of 60Hz. The pressure values were then normalized to the participant’s MVC, which was collected at the beginning of the scanning session. The average force magnitude applied over the middle second of each 4-second squeeze was recorded for statistical analysis.
The effects of age (old versus young) and target force level (10%, 40% or 70% MVC) on the absolute and relative force produced by the participants were analyzed with a two-way mixed factor analysis of variance (ANOVA), with age as a between subject factor and target force level as a within-subject factor.
2.5 fMRI Data Processing
Functional images were analyzed using publically available open-source software -Analysis of Functional NeuroImaging (AFNI) (Cox, 1996). The following describes the data processing procedures that were undertaken for the data for each individual participant. To begin the fMRI data analysis the functional data were spatially aligned to remove head motion artifacts, then coregistered to the same coordinate system and the four functional runs were concatenated into a single file for data analysis. No subjects had head motion that exceeded 2mm in any direction. The skull was then stripped from the anatomical image and this anatomical image was aligned to the concatenated functional data. The functional data were then analyzed with a random effects General Linear Model (GLM) to produce impulse response functions (IRFs) on a voxel-wise level, for each participant. The regression matrix for the GLM consisted of three vectors, which served as predictors of the timing of the stimuli to squeeze for each of the three force levels. This analysis was carried out with delays between the blood-oxygen-level-dependent (BOLD) signal and the force-level predictors ranging between 0TRs to 5 TRs, for a total of 6 TRs. Varying the delay between the BOLD signal and the force-level predictors allowed for the determination of the ideal delay which would allow the identification of the maximum percent signal change (PSC) for each force level. The GLM analysis produced an estimate of the hemodynamic response for each of the three force levels relative to a baseline state (rest) without making a priori assumptions about the shape, delay, or magnitude of the IRF and provided regression coefficients for each TR that defined the hemodynamic activity for each force level. The GLM analysis also provided a baseline coefficient for each functional run, which defined the variation in baseline activity during the resting periods, for each participant. The percent signal change (PSC) was estimated on a voxel-wise level across the whole brain, for each participant, by dividing the regression coefficient for each force level by the average of the baseline coefficients and multiplying by 100. The PSC value was calculated on a voxel-wise basis, for each of the delay values, for each participant.
All data was transformed to Talairach-Tournoux space by the AFNI software package. In order to do this, relevant landmarks were selected on the anatomical image and the transformation matrix was determined by the software package and applied to both the processed functional images and the anatomical image. Gaussian blur with a kernel width of 4-mm full-width-half-maximum was then applied to each of the processed functional images containing the PSC values, to account for anatomical variability and allow for the application of statistical tests between participants. The processed functional images were then analyzed using an ROI approach and a voxel-wise whole brain analysis, which will be detailed in the following sections.
2.6 ROI Analysis
A total of 22 (11 each in each hemisphere) ROIs were included in the analysis. The first 12 ROIs were specified using the Human Motor Areas Template (HMAT), which specifies cortical areas that are involved in human motor control based on a meta-analysis of 126 studies (Mayka et al., 2006). The areas included in the HMAT are the primary motor cortex (M1), the primary somatosensory cortex (S1), supplementary motor area (SMA), pre-supplementary motor area (pSMA), dorsal premotor cortex (PMd) and ventral premotor cortex (PMv). These regions were analyzed in both the left and right hemispheres. The remaining 10 ROIs were manually drawn on the anatomical image in Talairach space for each participant and represented subcortical structures involved in human motor control: caudate (CAU), putamen (PUT), thalamus (THAL), anterior cerebellum (ACER) and posterior cerebellum (PCER). These areas were chosen to represent some of the sub-cortical structures that are expected to be active during a force modulation task (Spraker et al., 2007; Ward et al., 2008). The caudate, putamen and thalamus were manually drawn by selecting the grey matter corresponding to the anatomical regions, guided by a neuroanatomical atlas (Talairach and Tournoux, 1988) and were verified in both the axial and sagittal planes. The cerebellum was divided into the anterior and posterior lobe; the division between these two lobes was selected to be the primary fissure (Kimberley et al., 2008; Schmahmann et al., 1999).
The average PSC across all of the voxels contained within each ROI was determined at each of the 6 TRs that were used as a delay between the force level predictors and the BOLD signal. The maximum average PSC across these 6 TRs for each ROI was recorded for statistical analysis. The maximum average PSC tended to be found in the third TR. This process was completed for each participant.
The effect of age and target force level on the peak PSC values across all ROIs were analyzed using a two-way mixed multivariate analysis of variance (MANOVA), with age as a between subject factor and target force level as a within subject factor. The peak PSC values were then analyzed with a two-way univariate ANOVA for each ROI, with age as a between subject factor and target force level as a within subject factor to determine which ROIs contributed to any significant effects found in the MANOVA. The global alpha level was set at 0.05 for all analyses (α = 0.05). Post-hoc analysis of any significant interaction or main effects was carried out through a pair-wise comparison of Bonferroni corrected means.
2.7 Voxel-wise fMRI analysis
A whole brain voxel-wise analysis was carried out to determine which regions that were not identified a priori demonstrated a group-by-force interaction effect during the force modulation task used in this study. To examine regions associated age-related differences in brain activation during a force production task, we conducted a mixed-model analysis of variance (ANOVA) on the PSC data at the third TR following the onset of the stimulus to initiate the squeeze; this particular TR was chosen as it represented the peak signal intensity for the majority of squeezes and represented the BOLD signal from the middle of the actual squeeze. In the ANOVA each subject was treated as a random factor, nested within a group (older versus younger adults) and with force condition (10%, 40% or 70% MVC) as a repeated factor. To illustrate the overall effect of our experimental manipulations we tested the group-by-condition interaction. A clustering algorithm was applied to find all regions with a group by force interaction with a p-value of less than 0.002; the minimum threshold for the volume of the cluster was set to 200 μL (Forman et al., 1995). The anatomical regions that were related to the peak activation of each cluster were determined with the use of the Talairach daemon (an online neuroanatomical atlas) (Lancaster et al., 2000). Clusters that were found to be outside the analysis volume or were within white matter were excluded from further analysis. The average PSC was then determined for each of the remaining clusters for each group and force-level. The PSC for each group and force-level was then plotted to determine the nature of the interaction effect within each cluster. The mean PSC values within the clusters were also analyzed with a post-hoc analysis that consisted of a comparison of Bonferroni corrected means.
3.0 Results
3.1 Participants
All of the participants were able to complete the force-matching task without difficulty. The details regarding the participants are shown in Table 1. Although the grip strength of the older adults was 18% less in the right hand and 20% lower in the left hand when compared to the younger group, there was no statistically significant group difference in the grip strength in either the right (t(12) = 1.90; P = 0.07) or left (t(12) = 1.90; P = 0.08) hands.
3.2 Behavioral Results
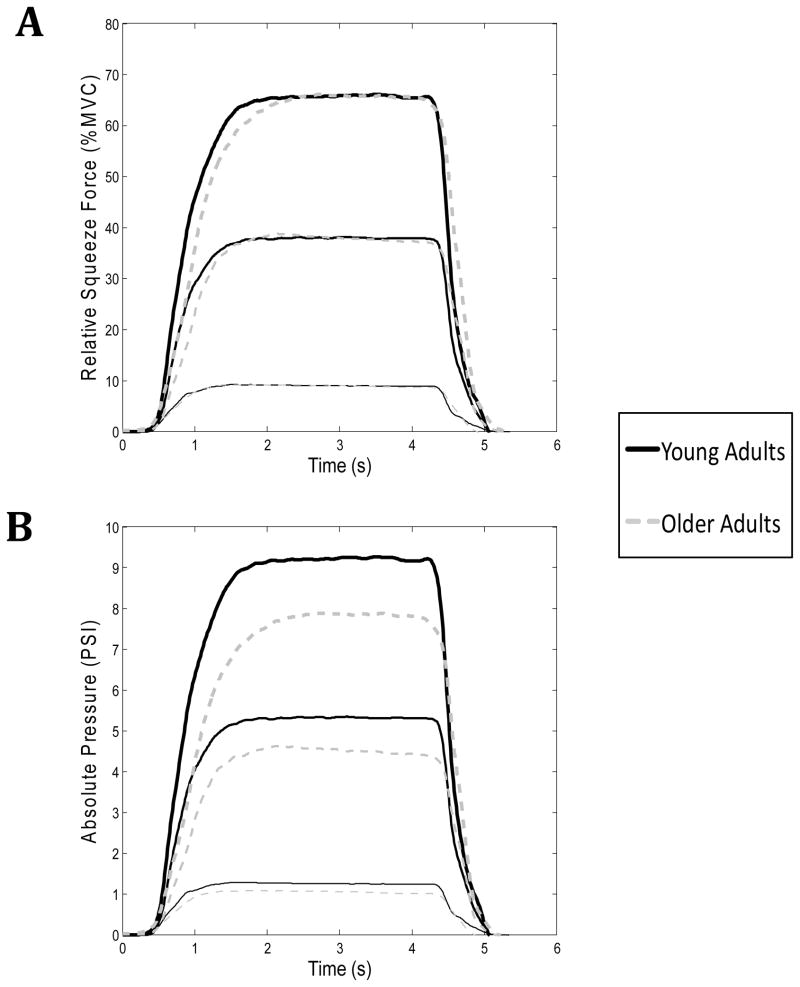
The mean and standard deviation of the force level produced, in absolute and relative terms, over the middle 1s of each squeeze were calculated for each force level and group (Table 2). A plot showing the group differences in the ensemble averaged time-series of the absolute and relative pressure at each force level is shown in Figure 1. While not analyzed statistically in this experiment, it can be observed that the older adults took a longer period of time to match the prescribed force level. No significant group difference was found in the pressure when it was calculated as a percentage of the participants’ MVC (F(1,72) = 0.851; P = 0.359). In contrast, a significant group difference was found when the absolute force levels were compared (F(1,72) = 8.166; P = 0.006), with the older group producing significantly lower levels of force when compared to the younger group at the 40% and 70% MVC levels. A significant force-level effect was found for both the absolute and relative force values (P < 0.001), and the post-hoc analysis confirmed that the participants produced three distinct levels of force.
Table 2.
Mean relative and absolute squeeze forces produced by the dominant hand at each target force level, for each group. The standard deviation is shown in parenthesis.
| Target Squeeze Force (%MVC) | ||||
|---|---|---|---|---|
| 10% | 40% | 70% | ||
| Relative Force Produced (% MVC) | Young | 9.19 (1.07) | 37.73 (1.32) | 65.23 (2.51) |
| Old | 9.27 (1.44) | 38.12 (1.34) | 63.31 (4.42) | |
|
| ||||
| Absolute Force Produced (PSI) | Young | 1.28 (0.29) | 5.28 (1.13) | 9.13 (1.96) |
| Old | 1.09 (0.26) | 4.55 (1.14) | 7.54 (1.87) | |
Figure 1.
Average time series plots of the squeeze force, at each target force level, relative to the participants’ maximum voluntary contraction (A) and in terms of absolute pressure units (B). The means for the older adult group are shown in dashed grey lines, while the means from the younger group are shown with solid black lines.
3.3 ROI Results
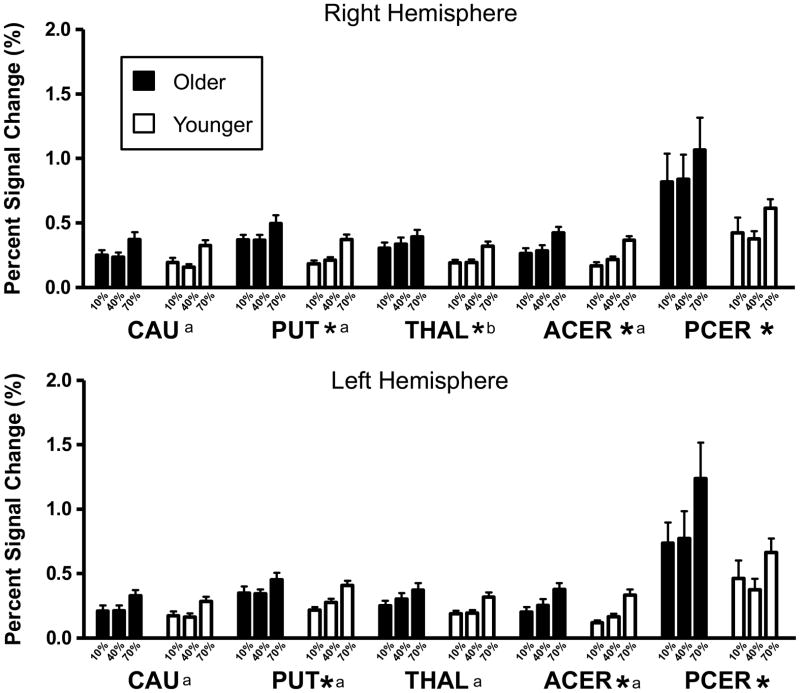
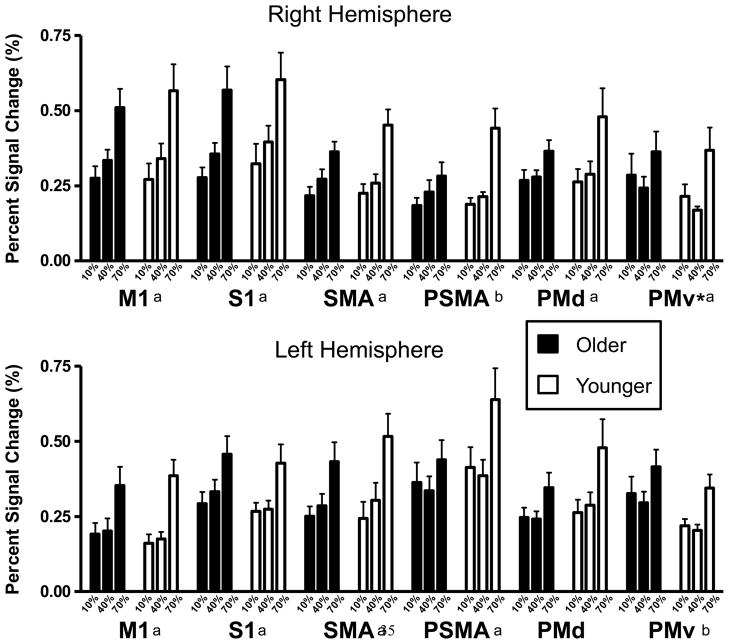
The MANOVA that was carried out on the peak PSC values across all the ROIs revealed that there were significant effects of both age (Wilk’s Lambda = 0.426; P < 0.001) and force-level (Wilk’s Lambda = 0.247; P = 0.001). The age-by-force-level interaction effect was not significant (Wilk’s Lambda = 0.621; P = 0.960). The univariate ANOVAs that were carried out on the peak PSC values for each ROI revealed significant group effects for the putamen bilaterally, the right thalamus, all cerebellar ROIs and the right PMv. In all cases where a significant age effect was found the older adult group showed a higher peak PSC value when compared to the younger group. A significant force-level effect was found on the peak PSC values for all of the ROIs with the exception of the posterior cerebellar ROIs bilaterally, and PMd in the left hemisphere. The results of these univariate ANOVAs are presented in Table 3, while the mean PSC values for each group in each force condition are shown in Figures 2 and 3.
Table 3.
Results for univariate two-way ANOVA on peak PSC values for each ROI. Values that are significant at P < 0.05 have been bolded and italicized.
| Age Effect (df = 1) | Force Effect (df = 2) | Interaction Effect | |||||
|---|---|---|---|---|---|---|---|
|
|
|||||||
| ROI | Hemisphere | F | P | F | P | F | P |
| CAU | Right | 3.55 | 0.06 | 8.37 | 0.00 | 0.08 | 0.93 |
| Left | 1.89 | 0.17 | 6.34 | 0.00 | 0.01 | 0.99 | |
| PUT | Right | 20.63 | 0.00 | 8.88 | 0.00 | 0.30 | 0.74 |
| Left | 6.39 | 0.01 | 7.44 | 0.00 | 0.62 | 0.54 | |
| THAL | Right | 10.80 | 0.00 | 4.09 | 0.02 | 0.37 | 0.69 |
| Left | 0.99 | 0.32 | 8.40 | 0.00 | 1.02 | 0.37 | |
| ACER | Right | 5.67 | 0.02 | 12.92 | 0.00 | 0.12 | 0.88 |
| Left | 5.13 | 0.03 | 13.69 | 0.00 | 0.18 | 0.83 | |
| PCER | Right | 10.12 | 0.00 | 1.22 | 0.30 | 0.02 | 0.98 |
| Left | 8.34 | 0.01 | 2.88 | 0.06 | 0.37 | 0.69 | |
| M1 | Right | 0.05 | 0.82 | 12.27 | 0.00 | 0.33 | 0.72 |
| Left | 0.17 | 0.68 | 11.63 | 0.00 | 0.16 | 0.85 | |
| S1 | Right | 1.08 | 0.30 | 7.64 | 0.00 | 0.08 | 0.92 |
| Left | 0.62 | 0.43 | 11.06 | 0.00 | 0.00 | 1.00 | |
| SMA | Right | 0.47 | 0.49 | 9.22 | 0.00 | 0.35 | 0.71 |
| Left | 0.91 | 0.34 | 14.91 | 0.00 | 1.13 | 0.33 | |
| PSMA | Right | 3.06 | 0.08 | 3.79 | 0.03 | 0.77 | 0.47 |
| Left | 2.37 | 0.13 | 11.34 | 0.00 | 3.02 | 0.06 | |
| PMd | Right | 2.26 | 0.14 | 5.53 | 0.01 | 0.65 | 0.53 |
| Left | 2.02 | 0.16 | 2.70 | 0.07 | 0.60 | 0.55 | |
| PMv | Right | 6.85 | 0.01 | 5.41 | 0.01 | 0.09 | 0.91 |
| Left | 1.03 | 0.31 | 4.43 | 0.02 | 0.32 | 0.72 | |
CAU = Caudate Nucleus, PUT = Putamen Nucleus, THAL = Thalamus, ACER = Anterior Cerebellum, PCER = Posterior Cerebellum, M1 = Primary Motor Cortex, S1 = Primary Sensory Cortex, SMA = Supplementary Motor Area, PSMA = Pre-supplementary Motor Area, PMd = Dorsal Premotor Cortex, PMv = Ventral Premotor Cortex.
Figure 2.
Mean peak PSC values for the subcortical ROIs at each force level. The black bars represent the means for the older adult group while the white bars represent the means for the younger group. The asterisks represent a significant age effect exists for that ROI. Significant force-level effects are indicated with an ‘a’ or a ‘b’; ‘a’ indicates that the PSC in the 70% condition is significantly greater than the PSC in the 10% and 40% conditions, while ‘b’ represents a significant difference exists only between the 70% and 10% MVC conditions. The error bars represent the standard error of the mean. CAU = Caudate, PUT = Putamen, THAL = Thalamus, ACER = Anterior Cerebellum, PCER = Posterior Cerebellum.
Figure 3.
Mean peak PSC values for the cortical ROIs at each force level. The black bars represent the means for the older adult group while the white bars represent the means for the younger group. The asterisks represent a significant age effect exists for that ROI. Significant force-level effects are indicated with an ‘a’ or a ‘b’; ‘a’ indicates that the PSC in the 70% condition is significantly greater than the PSC in the 10% and 40% conditions, while ‘b’ represents a significant difference exists only between the 70% and 10% MVC conditions. The error bars represent the standard error of the mean. M1 = Primary Motor Cortex, S1 = Primary Sensory Cortex, SMA = Supplementary Motor Area, PSMA = Pre-supplementary Motor Area, PMd = Dorsal Premotor Cortex, PMv = Ventral Premotor Cortex.
For the 19 ROIs that showed a significant force-level effect, post-hoc analysis revealed that there was not a significant difference between the magnitude of the BOLD signal at the 10% and 40% MVC force levels, however the magnitude of the BOLD signal at the 70% force level was significantly higher than the lower force levels. Three of the 19 ROIs that were analyzed showed exceptions from this pattern. In the right thalamus ROI, the BOLD signal only showed a significant difference between the 10% and 70% MVC conditions. In the right pSMA and the left PMv the BOLD signal was lowest in the 40% MVC condition, which resulted in a significant difference being found between the BOLD signal in the 40% and 70% MVC condition, however there was no significant difference between the 40% and 70% MVC conditions.
3.4 Voxel-wise fMRI results
The clustering algorithm that was applied resulted in a total of 9 clusters being identified that satisfied the clustering criteria (p < 0.002 for group-by-force interaction; min. volume = 200 μL). The coordinates and the anatomical descriptions of the clusters identified are shown in Table 4. Two of the clusters (Clusters 5 and 7) were excluded from further analysis as they were found to be in an area of white matter (Cluster 5) or were outside the analysis volume (Cluster 7). Post-hoc analysis of the Bonferroni corrected mean PSC values within these clusters revealed that the older adults had similar PSC values to the younger group at the 10% and 40% force levels, however the average PSC values of the older adult group were significantly higher at the 70% force level. The PSC values from these clusters for both the older and younger groups are shown in Table 5.
Table 4.
Clusters identified by a voxel-level analysis, which showed significant group-by-force interaction effect (p = 0.002; min cluster size = 200μL). The coordinates of the peak activation are given in Talairach- Tournoux space. The anatomical label associated with the cluster was determined using the Talairach daemon. Results are for the third TR following the onset of the stimulus to initiate the squeeze.
| T-T Coordinate of Peak Activation (RAI) | |||||
|---|---|---|---|---|---|
| Cluster Number | # of Voxels | X (mm) | Y (mm) | Z (mm) | Anatomical Label |
| 1 | 500 | 46 | −28 | 14 | R. Superior Temporal Gyrus |
| 2 | 448 | 1 | 46 | 31 | R. Medial Frontal Gyrus |
| 3 | 366 | −19 | −3 | 9 | L. Lentiform Nucleus |
| 4 | 292 | −9 | −45 | 39 | L. Cingulate Gyrus |
| 5 | 289 | 26 | −27 | 31 | No Gray Matter |
| 6 | 280 | −2 | −14 | 63 | L. Medial Frontal Gyrus |
| 7 | 278 | 49 | −76 | 48 | No Gray Matter |
| 8 | 264 | 33 | −81 | 44 | R. Precuneus |
| 9 | 253 | −44 | 14 | 11 | L. Precentral Gyrus |
Table 5.
Percent signal change values from the clusters identified with the voxel-wise analysis. The PSC data are from the third TR following the stimulus to initiate the squeeze. The standard deviation is indicated in parentheses. An asterisk indicates that the PSC value for the older adults in that particular condition is significantly greater than that of the younger group (P < 0.05).
| Cluster | Region | Group | Easy Force (10% MVC) | Medium Force (40% MVC) | Hard Force (70% MVC) |
|---|---|---|---|---|---|
| Mean (SD) | Mean (SD) | Mean (SD) | |||
| 1 | R. Superior Temporal Gyrus | Young | 0.087 (0.136) | −0.023 (0.152) | −0.168 (0.152) |
| Old | −0.034 (0.265) | 0.026 (0.183) | 0.254 (0.227) * | ||
| 2 | R. Medial Frontal Gyrus | Young | 0.021 (0.113) | −0.069 (0.094) | −0.202 (0.198) |
| Old | −0.185 (0.325) | −0.138 (0.302) | 0.159 (0.422) * | ||
| 3 | L. Lentiform Nucleus | Young | 0.149 (0.132) | 0.119 (0.130) | 0.074 (0.145) |
| Old | −0.129 (0.261) | 0.333 (0.235) | 0.397 (0.297) * | ||
| 4 | L. Cingulate Gyrus | Young | −0.036 (0.105) | −0.124 (0.197) | −0.241 (0.167) |
| Old | −0.171 (0.181) | −0.171 (0.187) | 0.011 (0.145) * | ||
| 6 | L. Medial Frontal Gyrus | Young | 0.228 (0.157) | 0.263 (0.149) | 0.180 (0.220) |
| Old | 0.421 (0.392) | 0.417 (0.316) | 0.727 (0.416) * | ||
| 8 | R. Precuneus | Young | 0.704 (0.976) | 0.704 (0.775) | −0.124 (1.007) |
| Old | 0.524 (0.709) | 0.512 (0.908) | 2.010 (2.073) * | ||
| 9 | L. Precentral Gyrus | Young | 0.077 (0.239) | −0.031 (0.163) | −0.068 (0.229) |
| Old | 0.026 (0.619) | 0.236 (0.504) | 0.519 (0.640) * |
4.0 Discussion
The main hypothesis of this study was that older adults would require higher levels of neural activity, as measured by the BOLD signal, to achieve the same relative force level as a group of younger adults. With the ROI analysis, higher levels of neural activity were found in subcortical motor areas of the older adult group, specifically the putamen, the ipsilateral thalamus and cerebellum. The older adults also demonstrated higher BOLD signals in the ipsilateral ventral premotor cortex. With a voxel-wise whole brain analysis, seven clusters were identified as having a significant group-by-force interaction effect; however, differences between older and younger adults became apparent only at the 70% MVC condition with the older adults showing higher levels of neural activity. The anatomical regions that were identified using the whole-brain analysis revealed age-related differences in activation of areas that have been shown to be involved in visuo-spatial processing and executive function in other fMRI studies (Cavanna and Trimble, 2006; Talati and Hirsch, 2005). The fact that the older adults showed higher levels of neural activity is even more remarkable when it is considered that the older adults produced significantly lower levels of absolute force as compared to the younger group. Thus, older adults required similar, if not higher levels of neural activity to achieve lower absolute force levels.
Recent studies have shown that subcortical structures are important during force production tasks (Spraker et al., 2007; Vaillancourt et al., 2007; Wasson et al., 2010); the present study builds upon these findings by demonstrating that older adults have a greater reliance on these subcortical structures during a visually guided force production task. This increased level of activity in the older group could be considered a compensatory strategy that is in response to age-related changes in the neurobiology of motor control systems (Seidler et al., 2010; Ward, 2006). Using a motor task similar to that used in this study but at lower force levels, Ward et al (2008) also showed an age-related increase in the activity of the putamen bilaterally. This study also found that older adults show higher activation in the ipsilateral ventral premotor cortex (PMv). This area has been shown to be active in motor tasks involving the hand and the foot and in tasks where the recognition of motor pattern is required (Binkofski and Buccino, 2006). This finding that the older adult group displayed greater activation in this area, could indicate an increased amount of processing by premotor circuitry to produce the same force output as the younger group.
A common finding in fMRI studies of age related differences in motor control tasks is that older adults show higher levels of bilateral activation of the SMC (Kim et al., 2010; Naccarato et al., 2006; Ward et al., 2008). The present study found that there were no significant age related differences in the activity level of the ipsilateral sensorimotor cortex. One potential reason for this discrepancy could be the differences in fMRI analysis techniques. Previous studies used an ROI approach to compare the number of active voxels in the sensorimotor cortex, by setting an activation threshold (Naccarato et al., 2006), whereas the present study compared the peak PSC. However, past work has shown that the use of signal intensity, such as the peak PSC, is more reliable than voxel counting techniques for representing the overall activity in healthy participants (Kimberley et al., 2008).
The modulation of grip force is a complex task that requires coordination of several muscles. The present study shows that there are significant changes in activation in the motor areas of the brain depending on the magnitude of the squeeze force required. It was observed for most of the ROIs analyzed in this study that the level of the BOLD signal in the 10% MVC and 40% MVC conditions were statistically similar, while the BOLD signal for the 70% MVC condition was significantly greater than the other conditions, indicating that there was not a linear relationship between the magnitude of the force and the BOLD signal. Previous studies have shown considerable variation in the relationship between the BOLD signal and the generation of muscle force. Dai et al (2001) found increases in both the average intensity and number of active voxels in cortical areas and the cerebellum as grip force levels were increased. Within the basal ganglia, Spraker et al (2007) found that there was no association between the level of activity within the putamen or caudate and the level of force produced, however regions of the thalamus did have a positive relationship between activity levels and the amount of force produced. The present study’s findings regarding increased activity of the basal ganglia at higher force levels could be due to methodological differences from Spraker et al (2007), who used a block design where participants alternated between 30s of force generation (producing 4s force pulses) using a pinch grip and rest, whereas this study used a power grip and used an event related design to analyze which neural areas were associated with a single squeeze. The differences of the type of grip used and the methods of fMRI analysis could possibly explain some of the differences observed between this study and the work of Spraker et al (2007). The cerebellum is thought to serve as a comparator for the actual movement that is occurring and the intended movement and is also used in the processing of visual feedback (Ghez and Thach, 2001). Previous studies of age related changes in grip force control have shown increased variability of force production (Nagasawa and Demura, 2009) and increased reliance on visual feedback in older adults (Sosnoff and Newell, 2007). These changes in force variability and visual processing could explain the higher levels of activity within the cerebellum for the older group.
The results of the voxel-wise analysis primarily revealed age-related differences in areas that were different from the ROIs that were analyzed for this study. Two clusters were identified that had some overlap with the ROIs that were drawn for this study. Cluster 3, which was identified as the left lentiform nucleus by the Talairach daemon overlapped with the ROI of the left putamen. This finding shows some consistency between the two analysis approaches used in this study. Cluster 6 was identified as the left medial frontal gyrus and overlapped with the left SMA ROI. Once again the voxel-wise analysis showed a group-by-force interaction effect with the older group showing a significantly higher activation in the 70% MVC condition; however the ROI analysis did not reveal any group differences for this area. This is likely due to the fact that our ROI analysis averaged the PSC values over the entire ROI even when a cluster only took up a very small volume of the ROI. Previous studies have reported differences in findings based on whole brain analyses of group data versus individual participant ROI approaches (Johansen-Berg and Matthews, 2002; Szycik et al., 2009). Differences between ROI and whole-brain analyses largely stem from differences in the numbers of multiple comparisons. Most of the areas identified in the voxel-wise analysis were associated with visual-spatial processing and executive function. These findings are consistent with previous studies that have demonstrated that older adults also tend to exhibit higher levels of activation in areas involved in sensorimotor processing when compared to younger adults; this increased activation is often associated with matching their performance to that of younger control subjects, and thus can be seen as compensatory (Seidler et al., 2010). Reasons for compensatory brain activation could be due to the decreased volume of grey and white matter or the decreased ability to recruit the relevant structures to elicit an appropriate motor response (Seidler et al., 2010).
The voxel-wise comparison of the group-by-force interaction effect revealed seven clusters, with most of these clusters being areas that are involved in visual-spatial processing and executive function (Cavanna and Trimble, 2006; Dong et al., 2010; James et al., 2010; Talati and Hirsch, 2005). The largest cluster identified was the right superior temporal gyrus. This area is the primary auditory cortex and is responsible for processing language and some emotional components and may be involved in multisensory perception action coupling (James et al., 2010). The next cluster identified was the right medial frontal gyrus, which has been shown to be involved in executive function, particularly in “Go-No Go Tasks”, which are similar to the task that was used in this study (Talati and Hirsch, 2005). The left medial frontal gyrus was also identified as an area where the older group and young adults showed different levels of activation in response to the task. The clustering algorithm identified the right lentiform nucleus as the next largest cluster. The lentiform nucleus is part of the basal ganglia, which provides some additional support to the finding from the ROI analysis that the older adults have higher activity levels in subcortical structures when compared to the younger group for the task used in this study. The left cingulate gyrus was also identified as an area with a significant group-by-force interaction effect. The cingulate gyrus receives input from the thalamus and neocortex and is involved in emotion, processing, learning and memory and executive functions, which are relevant to the task performed in this study, where a target is presented and a response is required (Dong et al., 2010). The next area identified by the Talairach daemon was the right precuneus. The precuneus is a an area within the posteriomedial parietal lobe that is associated with visual-spatial processing, as would be necessary to control the magnitude of the required force (Cavanna and Trimble, 2006). The last area identified by the clustering algorithm was identified as the left precentral gyrus. This area is typically consistent with the primary motor cortex, however when this cluster was plotted it appeared to be more anterior to the precentral gyrus and was more likely to be in a pre-motor area. This cluster was also identified as being within 1mm of the left inferior frontal gyrus and the left insula, which are also consistent with the motor task that was used in this study. Post-hoc analysis of the activation of these areas revealed that the older and younger adults showed similar levels of activation at the 10% MVC force-level; however, group differences emerged at the higher force levels. This is consistent with the hypothesis that the older adult group required higher levels of neural activity to produce the same level of relative force and suggests that the older adults were employing a compensatory activation. Additionally, the voxel-wise analysis performed in this experiment had some correspondence to the study of Kim et al (2010), who found older adults had increased activation in the cingulate gyrus, SMA, dorsolateral prefrontal cortex, and insula, while performing the same elbow flexion task as a group of younger adults.
4.1 Limitations
The use of a visual target for force production may have led to increased activity in subcortical structures and within the cerebellum (Floyer-Lea and Matthews, 2004). It is possible some of the group differences we observed were caused by age related effects on the visuomotor feedback system, however, we doubt that this explains our findings as we also found differences at varied force levels. Additionally, it is possible that some degree of motor learning took place during testing; we did provide an extensive practice session prior to data collection to minimize any learning related effects. Previous work has shown that motor learning can affect the regions and the amplitude of activation in fMRI studies, particularly in a visuomotor coordination task such as that used in this study (Floyer-Lea and Matthews, 2004).
Some previous work has found that the signal to noise ratio of the BOLD signal is lower in older adults, indicating that it is more difficult to detect significant differences in activation in this population (D’Esposito et al., 1999). In this study it was found that the peak PSC values of the older adults were either equal to or greater than those observed in the younger group, despite producing lower levels of absolute force. Therefore the true neural activation may actually be higher in older adults to produce an equivalent level of force.
Although every effort was made to ensure that all participants used the same style of grip on the squeeze bulb, it is possible that participants changed the type of grip that they used for the different force levels, which could partially explain some of the differences that were observed across the force-levels. In particular it is possible that the 70% MVC condition led the participants to engage their digits differently to squeeze more with their fingers and less with their thumb.
4.2 Conclusions
In conclusion, the present study used an ROI fMRI analysis approach and found that older adults generated greater activation of subcortical structures and in the ipsilateral ventral premotor cortex in order to produce the same relative level of grip force when compared to a group of healthy young adults. This study also used a voxel-wise analysis to determine age related differences in force production in areas that were no defined a priori. This analysis identified 7 clusters that showed a significant group-by-force level interaction effect. This analysis found 6 cortical areas where older adults showed higher levels of activation when compared to the younger group. These cortical areas tended to be areas that are involved in sensory processing and executive functioning which would be expected for the task that was used in this study. It was also found that the older adult group showed greater activation at the higher force levels, when motor control demands were increased. Future studies should try to isolate the motor response from the visual feedback processing to determine which neural structures are involved in the selection of an appropriate grip force.
Acknowledgments
Funding for this project was provided by the Canadian Institutes of Health Research (CIHR CGR-86829). Dr. Janice Eng is a Michael Smith Foundation for Health Research Career Scientist. Dr. Lara Boyd holds a Canada Research Chair, in Neurobiology of Motor Learning and is a Michael Smith Foundation for Health Research Career Scientist. Technical support and assistance in data collection from the staff at the University of British Columbia MRI Research Centre is also acknowledged.
References
- Seidler RD, Bernard JA, Burutolu TB, Fling BW, Gordon MT, Gwin JT, Kwak Y, Lipps DB. Motor control and aging: links to age-related brain structural, functional, and biochemical effects. Neurosci Biobehav Rev. 2010;34:721–33. doi: 10.1016/j.neubiorev.2009.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson B, Rutledge V. Age and hemisphere effects on dendritic structure. Brain. 1996;119 ( Pt 6):1983–90. doi: 10.1093/brain/119.6.1983. [DOI] [PubMed] [Google Scholar]
- Leenders KL, Perani D, Lammertsma AA, Heather JD, Buckingham P, Healy MJ, Gibbs JM, Wise RJ, Hatazawa J, Herold S. Cerebral blood flow, blood volume and oxygen utilization. Normal values and effect of age. Brain. 1990;113 ( Pt 1):27–47. doi: 10.1093/brain/113.1.27. [DOI] [PubMed] [Google Scholar]
- Shaffer SW, Harrison AL. Aging of the somatosensory system: a translational perspective. Phys Ther. 2007;87:193–207. doi: 10.2522/ptj.20060083. [DOI] [PubMed] [Google Scholar]
- Gilmore R. Evoked potentials in the elderly. J Clin Neurophysiol. 1995;12:132–8. doi: 10.1097/00004691-199503000-00003. [DOI] [PubMed] [Google Scholar]
- Dutta C, Hadley EC, Lexell J. Sarcopenia and physical performance in old age: overview. Muscle Nerve Suppl. 1997;5:S5–9. [PubMed] [Google Scholar]
- Lang T, Streeper T, Cawthon P, Baldwin K, Taaffe DR, Harris TB. Sarcopenia: etiology, clinical consequences, intervention, and assessment. Osteoporos Int. 2010;21:543–59. doi: 10.1007/s00198-009-1059-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabeza R. Cognitive neuroscience of aging: contributions of functional neuroimaging. Scandinavian journal of psychology. 2001;42:277–86. doi: 10.1111/1467-9450.00237. [DOI] [PubMed] [Google Scholar]
- Hutchinson S, Kobayashi M, Horkan CM, Pascual-Leone A, Alexander MP, Schlaug G. Age-related differences in movement representation. Neuroimage. 2002;17:1720–8. doi: 10.1006/nimg.2002.1309. [DOI] [PubMed] [Google Scholar]
- Kim JH, Lee YS, Lee JJ, Song HJ, Yoo DS, Lee HJ, Kim HJ, Chang Y. Functional magnetic resonance imaging reveals age-related alterations to motor networks in weighted elbow flexion-extension movement. Neurol Res. 2010;32(9):995–1001. doi: 10.1179/016164110X12670144737693. [DOI] [PubMed] [Google Scholar]
- Mattay VS, Fera F, Tessitore A, Hariri AR, Das S, Callicott JH, Weinberger DR. Neurophysiological correlates of age-related changes in human motor function. Neurology. 2002;58:630–5. doi: 10.1212/wnl.58.4.630. [DOI] [PubMed] [Google Scholar]
- Naccarato M, Calautti C, Jones PS, Day DJ, Carpenter TA, Baron JC. Does healthy aging affect the hemispheric activation balance during paced index-to-thumb opposition task? An fMRI study. Neuroimage. 2006;32:1250–6. doi: 10.1016/j.neuroimage.2006.05.003. [DOI] [PubMed] [Google Scholar]
- Ward NS, Swayne OB, Newton JM. Age-dependent changes in the neural correlates of force modulation: an fMRI study. Neurobiol Aging. 2008;29:1434–46. doi: 10.1016/j.neurobiolaging.2007.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riecker A, Groschel K, Ackermann H, Steinbrink C, Witte O, Kastrup A. Functional significance of age-related differences in motor activation patterns. Neuroimage. 2006;32:1345–54. doi: 10.1016/j.neuroimage.2006.05.021. [DOI] [PubMed] [Google Scholar]
- Shinohara M, Latash ML, Zatsiorsky VM. Age effects on force produced by intrinsic and extrinsic hand muscles and finger interaction during MVC tasks. Journal of applied physiology (Bethesda, Md: 1985) 2003a;95:1361–9. doi: 10.1152/japplphysiol.00070.2003. [DOI] [PubMed] [Google Scholar]
- Olafsdottir H, Zhang W, Zatsiorsky VM, Latash ML. Age-related changes in multifinger synergies in accurate moment of force production tasks. Journal of applied physiology (Bethesda, Md: 1985) 2007;102:1490–501. doi: 10.1152/japplphysiol.00966.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai TH, Liu JZ, Sahgal V, Brown RW, Yue GH. Relationship between muscle output and functional MRI-measured brain activation. Experimental Brain Research. 2001;140:290–300. doi: 10.1007/s002210100815. [DOI] [PubMed] [Google Scholar]
- Cramer SC, Weisskoff RM, Schaechter JD, Nelles G, Foley M, Finklestein SP, Rosen BR. Motor cortex activation is related to force of squeezing. Hum Brain Mapp. 2002;16:197–205. doi: 10.1002/hbm.10040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spraker MB, Yu H, Corcos DM, Vaillancourt DE. Role of individual basal ganglia nuclei in force amplitude generation. J Neurophysiol. 2007;98:821–34. doi: 10.1152/jn.00239.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Luca CJ, LeFever RS, McCue MP, Xenakis AP. Control scheme governing concurrently active human motor units during voluntary contractions. J Physiol. 1982;329:129–42. doi: 10.1113/jphysiol.1982.sp014294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kukulka CG, Clamann HP. Comparison of the recruitment and discharge properties of motor units in human brachial biceps and adductor pollicis during isometric contractions. Brain Res. 1981;219:45–55. doi: 10.1016/0006-8993(81)90266-3. [DOI] [PubMed] [Google Scholar]
- Barry BK, Pascoe MA, Jesunathadas M, Enoka RM. Rate coding is compressed but variability is unaltered for motor units in a hand muscle of old adults. J Neurophysiol. 2007;97:3206–18. doi: 10.1152/jn.01280.2006. [DOI] [PubMed] [Google Scholar]
- Shinohara M, Li S, Kang N, Zatsiorsky VM, Latash ML. Effects of age and gender on finger coordination in MVC and submaximal force-matching tasks. Journal of applied physiology (Bethesda, Md: 1985) 2003b;94:259–70. doi: 10.1152/japplphysiol.00643.2002. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Burock MA, Buckner RL, Woldorff MG, Rosen BR, Dale AM. Randomized event-related experimental designs allow for extremely rapid presentation rates using functional MRI. Neuroreport. 1998;9:3735–9. doi: 10.1097/00001756-199811160-00030. [DOI] [PubMed] [Google Scholar]
- Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Computers and biomedical research, an international journal. 1996;29:162–73. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Brainard DH. The Psychophysics Toolbox. Spatial vision. 1997;10:433–6. [PubMed] [Google Scholar]
- Mayka MA, Corcos DM, Leurgans SE, Vaillancourt DE. Three-dimensional locations and boundaries of motor and premotor cortices as defined by functional brain imaging: a meta-analysis. Neuroimage. 2006;31:1453–74. doi: 10.1016/j.neuroimage.2006.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-planar stereotaxic atlas of the human brain. Thieme; New York: 1988. [Google Scholar]
- Kimberley TJ, Birkholz DD, Hancock RA, VonBank SM, Werth TN. Reliability of fMRI during a continuous motor task: assessment of analysis techniques. Journal of neuroimaging: official journal of the American Society of Neuroimaging. 2008;18:18–27. doi: 10.1111/j.1552-6569.2007.00163.x. [DOI] [PubMed] [Google Scholar]
- Schmahmann JD, Doyon J, McDonald D, Holmes C, Lavoie K, Hurwitz AS, Kabani N, Toga A, Evans A, Petrides M. Three-dimensional MRI atlas of the human cerebellum in proportional stereotaxic space. Neuroimage. 1999;10:233–60. doi: 10.1006/nimg.1999.0459. [DOI] [PubMed] [Google Scholar]
- Forman SD, Cohen JD, Fitzgerald M, Eddy WF, Mintun MA, Noll DC. Improved assessment of significant activation in functional magnetic resonance imaging (fMRI): use of a cluster-size threshold. Magn Reson Med. 1995;33:636–47. doi: 10.1002/mrm.1910330508. [DOI] [PubMed] [Google Scholar]
- Lancaster JL, Woldorff MG, Parsons LM, Liotti M, Freitas CS, Rainey L, Kochunov PV, Nickerson D, Mikiten SA, Fox PT. Automated Talairach atlas labels for functional brain mapping. Hum Brain Mapp. 2000;10:120–31. doi: 10.1002/1097-0193(200007)10:3<120::AID-HBM30>3.0.CO;2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanna AE, Trimble MR. The precuneus: a review of its functional anatomy and behavioural correlates. Brain. 2006;129:564–83. doi: 10.1093/brain/awl004. [DOI] [PubMed] [Google Scholar]
- Talati A, Hirsch J. Functional specialization within the medial frontal gyrus for perceptual go/no-go decisions based on “what,” “when,” and “where” related information: an fMRI study. J Cogn Neurosci. 2005;17:981–93. doi: 10.1162/0898929054475226. [DOI] [PubMed] [Google Scholar]
- Vaillancourt DE, Yu H, Mayka MA, Corcos DM. Role of the basal ganglia and frontal cortex in selecting and producing internally guided force pulses. Neuroimage. 2007;36:793–803. doi: 10.1016/j.neuroimage.2007.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasson P, Prodoehl J, Coombes SA, Corcos DM, Vaillancourt DE. Predicting grip force amplitude involves circuits in the anterior basal ganglia. Neuroimage. 2010;49:3230–8. doi: 10.1016/j.neuroimage.2009.11.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward NS. Compensatory mechanisms in the aging motor system. Ageing Res Rev. 2006;5:239–54. doi: 10.1016/j.arr.2006.04.003. [DOI] [PubMed] [Google Scholar]
- Binkofski F, Buccino G. The role of ventral premotor cortex in action execution and action understanding. J Physiol Paris. 2006;99:396–405. doi: 10.1016/j.jphysparis.2006.03.005. [DOI] [PubMed] [Google Scholar]
- Ghez C, Thach WT. Cerebellum. In: Kandel ER, Schwartz JH, Jessel TM, editors. Principles of Neural Science. 4. Wiley; New York: 2001. [Google Scholar]
- Nagasawa Y, Demura S. Age and sex differences of controlled force exertion measured by a computer-generated sinusoidal target-pursuit system. J Physiol Anthropol. 2009;28:199–205. doi: 10.2114/jpa2.28.199. [DOI] [PubMed] [Google Scholar]
- Sosnoff JJ, Newell KM. Are visual feedback delays responsible for aging-related increases in force variability? Exp Aging Res. 2007;33:399–415. doi: 10.1080/03610730701525311. [DOI] [PubMed] [Google Scholar]
- Johansen-Berg H, Matthews PM. Attention to movement modulates activity in sensori-motor areas, including primary motor cortex. Exp Brain Res. 2002;142:13–24. doi: 10.1007/s00221-001-0905-8. [DOI] [PubMed] [Google Scholar]
- Szycik GR, Jansma H, Munte TF. Audiovisual integration during speech comprehension: an fMRI study comparing ROI-based and whole brain analyses. Hum Brain Mapp. 2009;30:1990–9. doi: 10.1002/hbm.20640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong G, Hu Y, Lu Q, Wu H. The presentation order of cue and target matters in deception study. Behav Brain Funct. 2010;6:63. doi: 10.1186/1744-9081-6-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James TW, Vanderklok RM, Stevenson RA, James KH. Multisensory perception of action in posterior temporal and parietal cortices. Neuropsychologia. 2011;49(1):108–14. doi: 10.1016/j.neuropsychologia.2010.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floyer-Lea A, Matthews PM. Changing brain networks for visuomotor control with increased movement automaticity. J Neurophysiol. 2004;92:2405–12. doi: 10.1152/jn.01092.2003. [DOI] [PubMed] [Google Scholar]
- D’Esposito M, Zarahn E, Aguirre GK, Rypma B. The effect of normal aging on the coupling of neural activity to the bold hemodynamic response. Neuroimage. 1999;10:6–14. doi: 10.1006/nimg.1999.0444. [DOI] [PubMed] [Google Scholar]