Abstract
Novel pathways in polycystic ovary syndrome (PCOS) are being identified in gene expression studies in PCOS tissues; such pathways may contain key genes in disease etiology. Previous expression studies identified both dickkopf homolog 1 (DKK1) and DnaJ (Hsp40) homolog, subfamily B, member 1 (DNAJB1) as differentially expressed in PCOS tissue, implicating them as candidates for PCOS susceptibility. To test this, we genotyped a discovery cohort of 335 PCOS cases and 198 healthy controls for three DKK1 single nucleotide polymorphisms (SNPs) and four DNAJB1 SNPs and a replication cohort of 396 PCOS cases and 306 healthy controls for 1 DKK1 SNP and 1 DNAJB1 SNP. SNPs and haplotypes were determined and tested for association with PCOS and component phenotypes. We found that no single nucleotide polymorphisms were associated with PCOS risk; however, the major allele of rs1569198 from DKK1 was associated with increased total testosterone (discovery cohort P = 0.0035) and dehydroepiandrosterone sulfate (replication cohort P = 0.05). Minor allele carriers at rs3962158 from DNAJB1 had increased fasting insulin (discovery cohort P = 0.003), increased HOMA-IR (discovery cohort P = 0.006; replication cohort P = 0.036), and increased HOMA-%B (discovery cohort P = 0.004). Carriers of haplotype 2 at DNAJB1 also had increased fasting insulin, HOMA-IR, and HOMA-%B. These findings suggest that genetic variation in DKK1 and DNAJB1 may have a role in the hyperandrogenic and metabolic dysfunction of PCOS, respectively. Our results also demonstrate the utility of gene expression data as a source of novel candidate genes in PCOS, a complex and still incompletely defined disease, for which alternative methods of gene identification are needed.
Introduction
Familial aggregation and twin studies have established a genetic etiology for polycystic ovary syndrome (PCOS) [1]. The hallmark of PCOS is hyperandrogenemia; however, insulin resistance, pancreatic beta cell dysfunction and chronic inflammation are frequently present [1], [2], [3]. Many previous candidate gene studies focused on genes from androgen synthesis and insulin signaling pathways. Few susceptibility genes are widely agreed upon, potentially because candidate gene selection has been based incomplete understanding of the disorder.
Recently a number of expression studies (mRNA and protein) have been performed in PCOS tissues, including ovary [4], omental fat [5] and lymphocytes [6]. Remarkably few genes have been reported as differentially expressed in PCOS tissues in more than one study. One such gene is dickkopf homolog 1 (DKK1), an inhibitor of the Wnt signaling pathway and cell growth repressor [4], [5]. DKK1 interacts with the Wnt coreceptor low-density lipoprotein receptor-related proteins 5 and 6 and influences several functions, including embryogenesis and cell cycle regulation in cancer pathways [7]. It has been reported as being under expressed in omental fat [5] and over expressed in cultured ovarian theca [4] from PCOS subjects.
The second gene we selected for analysis in this study is DnaJ (Hsp40) homolog, subfamily B, member 1 (DNAJB1), whose expression was decreased in ovaries from PCOS women [8]. DNAJB1 was also selected as a positional and functional candidate. It acts in concert with molecular chaperones to regulate protein folding, protein complex assembly and disassembly, and transport of proteins across cellular membranes, particularly in the androgen signaling pathway, and is under transcriptional regulation by insulin [9]. It is also located within the chromosome 19p13.2 linkage region that has been identified in PCOS susceptibility [10], implicating DNAJB1 as a potential positional candidate.
Because logical candidates have led to few successes in PCOS genetics, novel methods of candidate gene selection are needed. In the present study we used published expression data to identify putative molecular targets; selecting two genes, DKK1 and DNABJ1, from such analysis for association study with PCOS, conducted in two independent case/control cohorts. A SNP in DNABJ1 was associated with a measure of insulin resistance in women with PCOS in both cohorts.
Materials and Methods
Ethics statement
All subjects gave written informed consent; each study was approved by the Institutional Review Boards of the recruiting centers and Cedars-Sinai Medical Center.
Subjects and phenotyping
Discovery Cohort
We studied 335 unrelated White PCOS patients and 198 White control women recruited at two centers, the University of Alabama at Birmingham (UAB; 287 PCOS and 187 controls) and Cedars-Sinai Medical Center (CSMC; 48 PCOS and 11 controls). Cases were premenopausal, non-pregnant, on no hormonal therapy, including oral contraceptives, for at least three months; all PCOS subjects met 1990 NIH criteria [11]. Parameters for defining hirsutism, hyperandrogenemia, ovulatory dysfunction, and exclusion of related disorders were previously reported [12]. Controls were healthy women, with regular menstrual cycles and no evidence of hirsutism, acne, alopecia, or endocrine dysfunction and had not taken hormonal therapy (including oral contraceptives) for at least three months. Controls were recruited by word of mouth and advertisements calling for “healthy women.”
Replication Cohort
We assembled a cohort of 396 unrelated White PCOS patients and 306 White control women. The replication cohort was constituted from three sources: 380 PCOS subjects (all meeting 1990 NIH criteria) and 71 healthy controls previously recruited by R. S. Legro [13], 16 PCOS subjects and 2 healthy controls recruited at Cedars-Sinai Medical Center using the same criteria as those used in the discovery cohort; and 233 white control women derived from the Cholesterol and Atherosclerosis Pharmacogenetics (CAP) study, a component of the Pharmacogenomics and Risk of Cardiovascular Disease (PARC) Study [14].
Subjects recruited at UAB and CSMC were evaluated per a previously described protocol [12]. Fasting glucose and insulin were also obtained in a subset (70%) of the cases (non-diabetic). The subset of subjects with fasting glucose and insulin did not differ demographically or hormonally from the study subjects overall. The computer-based homeostasis model assessment (HOMA, www.dtu.ox.ac.uk/homa) was used to calculate indices of insulin resistance (HOMA-IR) and insulin secretion (i.e. percent beta-cell function or HOMA-%B) utilizing the fasting glucose and insulin levels. This computer model was also applied to generate HOMA data in the replication cohort, on whom fasting insulin and glucose were measured in >90% of subjects. Table 1 presents clinical characteristics of both cohorts.
Table 1. Clinical Characteristics of PCOS and control subjects.
| Discovery | Replication | |||
| Control(n = 198) | PCOS(n = 335) | Control(n = 306) | PCOS(n = 396) | |
| Age (yr) | 32.5 (17.0) | 27.0 (11.1)* | 51.0 (23.7) | 28.0 (9.0)* |
| BMI (kg/m2) | 24.7 (5.9) | 33.4 (14.7)* | 25.9 (8.3) | 34.9 (12.3)* |
| Total testosterone (nmol/l) | 1.40 (0.94) | 2.63 (1.14)* | 1.0 (0.52) | 2.43 (1.11)* |
| DHEAS (µmol/l) | 2.58 (2.04) | 5.68 (4.57)* | 3.70 (1.75) | 5.72 (3.94)* |
| Insulin (pmol/l) | 41.4 (39.0) | 96.0 (108.0)* | 72.0 (45.0) | 126.0 (96.0)* |
| Glucose (mmol/l) | 4.77 (0.56) | 4.77 (0.72) | 5.01 (0.68) | 4.86 (0.64) |
| HOMA-IR | 0.95 (0.84) | 2.01 (2.13)* | 1.58 (0.96) | 2.69 (1.83)* |
| HOMA-%B | 104.1 (57.0) | 166.9 (108.8)* | 129.5 (58.3) | 196.6 (81.0)* |
*P<0.001 compared to control group. Data are median (interquartile range). In the replication cohort, androgen measurements were available only in the cases and controls recruited by R. S. Legro.
Abbreviations: BMI: body mass index; mFG: modified Ferriman-Gallwey hirsutism score; NA: Data not Available; HOMA-IR, homeostasis model assessment of insulin resistance; HOMA-%B, homeostasis model assessment of beta-cell function (insulin secretion).
Genotyping and haplotype determination
Discovery genotyping was performed on three DKK1 single nucleotide polymorphisms (SNPs) (rs2241529, rs1569198, rs2288335) and four DNAJB1 SNPs (rs7003, rs1803768, rs4926222, rs3962158) selected using genotype data of the CEU (Utah residents with ancestry from northern and western Europe) population of the HapMap database (release 24, http://hapmap.ncbi.nlm.nih.gov/). These SNPs were selected because they are predicted to tag SNPs across the entirety of each gene, plus 10 kb upstream and 10 kb downstream. These SNPs capture 21 of 26 (81%) of the CEU HapMap SNPs at r2>0.8 for the two genes. Replication genotyping was performed on two of the discovery SNPs; rs1569198 from DKK1 and rs3962158 from DNAJB1. All genotyping was performed using Applied Biosystems Taqman Assays-on-Demand (Applied Biosystems, Foster City, CA) according to manufacturer's instructions.
Haploview (version 4.1) was used to calculate linkage disequilibrium (LD, the D' statistic) between each pair of SNPs and determine haplotypes and their frequencies [15]. The solid spine of LD algorithm in Haploview was used to determine haplotype blocks. Only subjects whose haplotype assignment was >95% certain were analyzed.
Statistical analysis
Unpaired t-tests and chi-square tests were used to compare clinical characteristics between cases and controls; quantitative traits were log- or square-root-transformed as appropriate to reduce non-normality. Data are presented as median (interquartile range).
Genotypic association with PCOS status was evaluated using logistic regression, adjusting for recruitment site, BMI and age. Association between genotype and quantitative traits (conducted separately in cases and controls) was performed using linear regression adjusting for site, age and BMI in all analyses except those in which BMI was the dependent variable, wherein analyses were adjusted for site and age. To handle multiple testing in the discovery cohort significance was taken as P<0.008, considering that we analyzed two LD groups of SNPs (one per gene) against three families of traits (PCOS diagnosis, androgens and metabolic traits), yielding a Bonferroni correction factor of 6 (i.e. six independent comparisons). In the replication cohort, significance was taken as P<0.05, because the goal was to confirm the associations made in the discovery cohort. To limit multiple testing in the replication cohort, we only examined SNPs that displayed associations in the discovery cohort.
Results
Discovery cohort
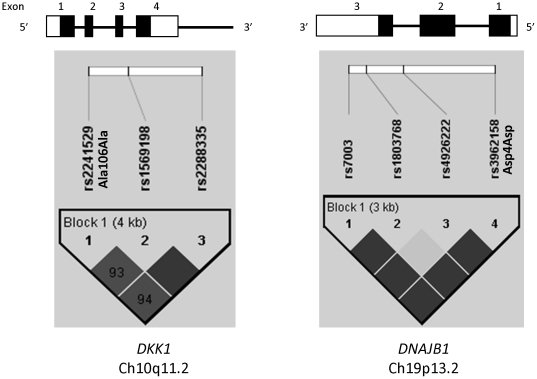
We genotyped three SNPs from DKK1 and four SNPs from DNAJB1 in the discovery cohort (Table 2), where the overall genotyping success rate was 95.4% (ranging from 95.1–97.0% for individual SNPs). LD among markers (D') in DKK1 ranged from 0.93 to 1.0 (average pairwise D' of 0.96), while LD in DNAJB1 ranged from 0.03 to 1.0 (average pairwise D' of 0.54). Haploview generated a single haplotype block including all three markers in DKK1 and a single block encompassing all four markers in DNAJB1 (Figure 1).
Table 2. SNP and haplotype information for the DKK1 and DNAJB1 gene regions in the discovery cohort.
| Variant | Location | Alleles | PCOSMAF | Control MAF | Overall MAF |
| SNPs | |||||
| DKK1 | |||||
| rs2241529 | Exon 2 (Ala106Ala) | G/A | 0.41 | 0.46 | 0.43 |
| rs1569198 | Intron 3 | A/G | 0.49 | 0.47 | 0.48 |
| rs2288335 | 3′ region | G/A | 0.08 | 0.09 | 0.08 |
| DNAJB1 | |||||
| rs7003 | 3′ UTR | G/A | 0.12 | 0.15 | 0.13 |
| rs1803768 | 3′ UTR | G/A | 0.04 | 0.06 | 0.05 |
| rs4926222 | Intron 2 | G/A | 0.16 | 0.15 | 0.16 |
| rs3962158 | Exon 1 (Asp4Asp) | G/A | 0.31 | 0.28 | 0.30 |
| Haplotypes | |||||
| DKK1 | |||||
| 1 | G-G-G | 0.48 | 0.45 | 0.47 | |
| 2 | A-A-G | 0.41 | 0.43 | 0.41 | |
| 3 | G-A-A | 0.08 | 0.08 | 0.08 | |
| DNAJB1 | |||||
| 1 | G-G-G-G | 0.41 | 0.42 | 0.41 | |
| 2 | G-G-G-A | 0.31 | 0.28 | 0.3 | |
| 3 | G-G-A-G | 0.16 | 0.16 | 0.16 | |
| 4 | A-A-G-G | 0.08 | 0.09 | 0.08 |
The order of SNPs in the haplotypes corresponds to the order of SNPs in the table. MAF = minor allele frequency.
Figure 1. Gene structure and linkage disequilibrium plot for DKK1 and DNAJB1.
The gene structure is shown at top, with exons shown as filled boxes, untranslated regions as unfilled boxes and introns as connecting lines. The locations of the genotyped SNPs relative to the exons are indicated. The linkage disequilibrium (LD) plot at the bottom displays D' values (%) for each pair of SNPs in the box at the intersection of the diagonals from each SNP. The solid blocks indicate D' = 1 for the corresponding pair of variants. The darker solid blocks indicate a logarithm of the odds (LOD) score ≥2 for the corresponding pair of variants; lighter solid blocks indicate a LOD score <2. Within each gene, SNPs were considered together in one haplotype block as indicated.
SNPs in DKK1 and DNAJB1 were not associated with PCOS status. In women with PCOS, one SNP in DKK1 (rs1569198) was associated with total testosterone, with major A allele carriers having increased testosterone levels (AA/AG: 2.74 (1.21) vs. GG: 2.36 (0.90) nmol/l; P = 0.0035). PCOS women carriers of the minor T allele at rs3962158 (Asp4Asp) in exon 1 of DNAJB1 had increased insulin (CC: 78.0 (92.4) vs. CT/TT: 108.0 (124.5) pmol/l; P = 0.0031); increased HOMA-IR (CC: 1.72 (1.96) vs. CT/TT: 2.34 (2.39); P = 0.006) and increased HOMA-%B (CC: 145.2 (99.2) vs. CT/TT: 177.2 (108.6); P = 0.004). These SNP associations were not observed in the control women. No associations with BMI were observed.
Three common haplotypes (frequency >5%) were identified for the DKK1 gene region (Table 2). No DKK1 haplotype was associated with PCOS status or quantitative traits at our selected level of significance (P<0.008). Four common haplotypes were identified for DNAJB1 (Table 2). PCOS women carriers of haplotype 2 had increased insulin (non-carriers: 78.0 (92.4) vs. carriers: 108.0 (120.9) pmol/l; P = 0.006), HOMA-IR (non-carriers: 1.76 (1.90) vs. carriers: 2.33 (2.43); P = 0.003) and HOMA-%B (non-carriers: 146.3 (97.7) vs. carriers: 176.6 (113.7); P = 0.002). This haplotype carries the minor allele of rs3962158, which was also associated with each of these traits in the single marker analysis. These haplotype associations were not observed in the control women.
Replication cohort
We selected the two significant SNPs from the discovery phase of the study for replication. The genotyping success rate for rs1569198 was 95.7% and for rs3962158 was 97.3%, with 100% concordance observed in duplicate samples run both within the replication study samples, and across the replication and discovery study samples. The minor allele frequencies of rs1569198 (PCOS 0.48, control 0.51, overall 0.49) and rs3962158 (PCOS 0.31, control 0.30, overall 0.31) were the same as the frequencies observed in the discovery cohort. We observed association (additive model) between DKK1 SNP rs1569198 and dehydroepiandrosterone sulfate (DHEAS), with increasing copies of the A allele correlating with increasing DHEAS (AA: 2229.0 (1551.3), AG: 2099.0 (1373.8), GG: 1978.0 (1520.0) nmol/l; P = 0.05). We did not replicate the association between total testosterone and rs1569198. We did, however, replicate the significant association between carriers of the minor T allele at rs3962158 of DNAJB1 with increased HOMA-IR under the same dominant model (CC: 2.57 (1.65) vs. CT/TT: 2.77 (1.98); P = 0.036).
Discussion
In an attempt to circumnavigate arbitrary bias introduced in the selection of candidate genes for PCOS, we evaluated mRNA and protein expression data reported from several PCOS tissues in order to identify novel susceptibility genes, DKK1 and DNAJB1. These results suggest that variation in gene expression may be an important factor in identifying relevant PCOS pathways. This strategy was employed to select the 17β-hydroxysteroid dehydrogenase type 6 (HSD17B6) gene, which exhibited increased activity in PCOS ovaries, as a candidate gene for PCOS [16]; we and others ultimately replicated association of variants in this gene with metabolic phenotypes of PCOS [17], [18]. Others have performed network analysis on PCOS microarray data to identify pathways that may be most relevant to the development of the disorder [19].
A DKK1 variant, rs1569198, was associated with testosterone levels in PCOS subjects in our discovery cohort, with carriers of the major allele having an elevated testosterone level. This result is consistent with the observation that a murine homolog of DKK1 plays a role in testicular testosterone production [20]. DKK1 is expressed in a number of tissues including ovary, testis and adipose tissue, with increased expression in PCOS ovarian theca [4]. DKK1 has been identified as one of the most upregulated genes in testosterone responsive tissues, such as the dermal papilla [21], suggesting its expression may be regulated by androgens. In a recent report designed to identify proteins that act in androgen-dependent hair loss, DKK1 was reported as a dihydrotestosterone-inducible transcript, and also caused apoptosis in follicular keratinocytes [21]. These data suggest a role for DKK1 in the regulation of cell cycle in androgen responsive tissues, and further studies in ovary, particularly ovarian theca may confirm this. In a study of genetic determinants of bone phenotypes in men, rs1569198 was associated with hip axis length [22], which indicate potential roles for DKK1 and this SNP in particular in a number of pathways, including those under hormonal regulation.
That we did not replicate the association of rs1569198 with testosterone deserves comment. The replication of associations such as these presents several challenges; the most important is the accumulation of an appropriate replication cohort, which must consist of an equal or large sample size of ethnically matched subjects, with similar disease phenotype and quantitative trait measures. The appropriateness of our replication cohort is substantiated by our replication of the association between rs3962158 and HOMA-IR. The replication of genetic association with testosterone poses a particular challenge, given the difficulty in precisely measuring this trait, particularly in women [23], [24]. In the replication cohort, we identified a nominal association of the major allele of rs1569198 with increased DHEAS, representing either a coincidence or supporting a role in androgen production or clearance. If the SNP does affect both testosterone and DHEAS levels, the lack of observation of association with both traits in both cohorts may be related to statistical chance or imprecision of androgen measurement in women.
Of particular interest as a candidate identified via differential expression was DNAJB1 as it lies in the previously identified PCOS linkage region (microsatellite marker D19S884) on chromosome 19p13.2 [10] and acts as a molecular chaperone. Androgen receptor function (including ligand binding and nuclear translocation) depends on its interaction with heat shock proteins and their co-chaperones [25]. DNAJB1 acts as a co-chaperone in a number of pathways including androgen receptor and glucocorticoid receptor signaling. An androgenic protein co-chaperone, DNAJB1 is under transcriptional regulation by insulin, with increased hepatic expression demonstrated under conditions of reduced insulin [9]. DNAJB1 may thus represent a common factor at the nexus of both the androgenic and insulin pathways that are frequently dysfunctional in PCOS.
The functional role of the associated DKK1 and DNAJB1 SNPs is unknown. The DKK1 SNP rs1569198 is an intronic SNP, and the DNAJB1 SNP rs3962158 does not change the amino acid at position 4. These SNPs, purposefully selected as tagging SNPs, may not be causal, but may be in LD with functional variants elsewhere in their genes or in the promoters. In particular, HapMap data indicate that the DKK1 SNP is in LD with several SNPs in the promoter region of the gene. Tissue specific gene expression studies in genotyped subjects should be undertaken to further elucidate the role of variation in these genes on gene expression.
Despite many candidate gene studies in PCOS, few biologically selected candidates have been replicated. The use of expression data from PCOS subjects may result in the identification of novel susceptibility loci. In the present study, we used published expression data to select two putative candidate genes for analysis, DKK1 and DNAJB1. By using a discovery and replication approach we have identified and replicated DNAJB1 as a potential gene important in the insulin resistance of PCOS. While not associated with PCOS itself, genetic variation in DNAJB1 and perhaps DKK1 may act as modifiers, affecting the metabolic and androgenic pathways of PCOS, respectively. In conclusion, gene expression data appears to be a useful source of candidate genes in complex and poorly understood diseases such as PCOS.
Footnotes
Competing Interests: The authors have declared that no competing interests exist. The Helping Hand of Los Angeles, Inc. is a charitable support group at Cedars-Sinai Medical Center, whose fundraising efforts partly supported this study. This does not alter the authors' adherence to all the PLoS ONE policies on sharing data and materials.
Funding: This work was supported by the National Institutes of Health [R01-HD29364, K24-HD01346 to R.A., R01-DK79888 to M.O.G., U54-HD034449 to R.S.L., R01-HL069757 to R.M.K.]; National Center for Research Resources [M01-RR00425 to the Cedars-Sinai General Clinical Research Center]; Winnick Clinical Scholars Award [to M.O.G.]; and an endowment from the Helping Hand of Los Angeles, Inc. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Goodarzi MO, Azziz R. Diagnosis, epidemiology, and genetics of the polycystic ovary syndrome. Best Pract Res Clin Endocrinol Metab. 2006;20:193–205. doi: 10.1016/j.beem.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 2.Goodarzi MO, Erickson S, Port SC, Jennrich RI, Korenman SG. Beta-cell function: a key pathological determinant in polycystic ovary syndrome. J Clin Endocrinol Metab. 2005;90:310–315. doi: 10.1210/jc.2004-1006. [DOI] [PubMed] [Google Scholar]
- 3.Diamanti-Kandarakis E, Paterakis T, Kandarakis HA. Indices of low-grade inflammation in polycystic ovary syndrome. Ann N Y Acad Sci. 2006;1092:175–186. doi: 10.1196/annals.1365.015. [DOI] [PubMed] [Google Scholar]
- 4.Wood JR, Nelson VL, Ho C, Jansen E, Wang CY, et al. The molecular phenotype of polycystic ovary syndrome (PCOS) theca cells and new candidate PCOS genes defined by microarray analysis. J Biol Chem. 2003;278:26380–26390. doi: 10.1074/jbc.M300688200. [DOI] [PubMed] [Google Scholar]
- 5.Corton M, Botella-Carretero JI, Benguria A, Villuendas G, Zaballos A, et al. Differential gene expression profile in omental adipose tissue in women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2007;92:328–337. doi: 10.1210/jc.2006-1665. [DOI] [PubMed] [Google Scholar]
- 6.Borro M, Gentile G, Stigliano A, Misiti S, Toscano V, et al. Proteomic analysis of peripheral T lymphocytes, suitable circulating biosensors of strictly related diseases. Clin Exp Immunol. 2007;150:494–501. doi: 10.1111/j.1365-2249.2007.03498.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aguilera O, Fraga MF, Ballestar E, Paz MF, Herranz M, et al. Epigenetic inactivation of the Wnt antagonist DICKKOPF-1 (DKK-1) gene in human colorectal cancer. Oncogene. 2006;25:4116–4121. doi: 10.1038/sj.onc.1209439. [DOI] [PubMed] [Google Scholar]
- 8.Jansen E, Laven JS, Dommerholt HB, Polman J, van Rijt C, et al. Abnormal gene expression profiles in human ovaries from polycystic ovary syndrome patients. Mol Endocrinol. 2004;18:3050–3063. doi: 10.1210/me.2004-0074. [DOI] [PubMed] [Google Scholar]
- 9.Jee S, Hwang D, Seo S, Kim Y, Kim C, et al. Microarray analysis of insulin-regulated gene expression in the liver: the use of transgenic mice co-expressing insulin-siRNA and human IDE as an animal model. Int J Mol Med. 2007;20:829–835. [PubMed] [Google Scholar]
- 10.Urbanek M, Legro RS, Driscoll DA, Azziz R, Erhmann D, et al. Thirty-seven candidate genes for polycystic ovary syndrome: strongest evidence for linkage is with follistatin. Proc Natl Acad Science USA. 1999;96:8573–8578. doi: 10.1073/pnas.96.15.8573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zawadzki J, Dunaif A. Diagnostic criteria for polycystic ovary syndrome: towards a rational approach. In: Dunaif A, Givens JR, Haseltine FP, Merriam GR, editors. Polycystic Ovary Syndrome. Boston: Blackwell Scientific; 1992. pp. 377–384. [Google Scholar]
- 12.Azziz R, Woods KS, Reyna R, Key TJ, Knochenhauer ES, et al. The prevalence and features of the polycystic ovary syndrome in an unselected population. J Clin Endocrinol Metab. 2004;89:2745–2749. doi: 10.1210/jc.2003-032046. [DOI] [PubMed] [Google Scholar]
- 13.Legro RS, Driscoll D, Strauss JF, Fox J, Dunaif A. Evidence for a genetic basis for hyperandrogenemia in polycystic ovary syndrome. Proc Natl Acad Sci U S A. 1998;95:14956–14960. doi: 10.1073/pnas.95.25.14956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Simon JA, Lin F, Hulley SB, Blanche PJ, Waters D, et al. Phenotypic predictors of response to simvastatin therapy among African-Americans and Caucasians: the Cholesterol and Pharmacogenetics (CAP) Study. Am J Cardiol. 2006;97:843–850. doi: 10.1016/j.amjcard.2005.09.134. [DOI] [PubMed] [Google Scholar]
- 15.Barrett JC, Fry B, Maller J, Daly MJ. Haploview: analysis and visualization of LD and haplotype maps. Bioinformatics. 2005;21:263–265. doi: 10.1093/bioinformatics/bth457. [DOI] [PubMed] [Google Scholar]
- 16.Jones MR, Italiano L, Wilson SG, Mullin BH, Mead R, et al. Polymorphism in HSD17B6 is associated with key features of polycystic ovary syndrome. Fertil Steril. 2006;86:1438–1446. doi: 10.1016/j.fertnstert.2006.04.027. [DOI] [PubMed] [Google Scholar]
- 17.Ke L, Che YN, Cao YX, Wu XK, Hu YL, et al. Polymorphisms of the HSD17B6 and HSD17B5 genes in Chinese women with polycystic ovary syndrome. J Womens Health (Larchmt) 2010;19:2227–2232. doi: 10.1089/jwh.2009.1902. [DOI] [PubMed] [Google Scholar]
- 18.Jones MR, Mathur R, Cui J, Guo X, Azziz R, et al. Independent confirmation of association between metabolic phenotypes of polycystic ovary syndrome and variation in the type 6 17beta-hydroxysteroid dehydrogenase gene. J Clin Endocrinol Metab. 2009;94:5034–5038. doi: 10.1210/jc.2009-0931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mohamed-Hussein ZA, Harun S. Construction of a polycystic ovarian syndrome (PCOS) pathway based on the interactions of PCOS-related proteins retrieved from bibliomic data. Theor Biol Med Model. 2009;6:18. doi: 10.1186/1742-4682-6-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dakhova O, O'Day D, Kinet N, Yucer N, Wiese M, et al. Dickkopf-like1 regulates postpubertal spermatocyte apoptosis and testosterone production. Endocrinology. 2009;150:404–412. doi: 10.1210/en.2008-0673. [DOI] [PubMed] [Google Scholar]
- 21.Kwack MH, Sung YK, Chung EJ, Im SU, Ahn JS, et al. Dihydrotestosterone-inducible dickkopf 1 from balding dermal papilla cells causes apoptosis in follicular keratinocytes. J Invest Dermatol. 2008;128:262–269. doi: 10.1038/sj.jid.5700999. [DOI] [PubMed] [Google Scholar]
- 22.Piters E, Balemans W, Nielsen TL, Andersen M, Boudin E, et al. Common genetic variation in the DKK1 gene is associated with hip axis length but not with bone mineral density and bone turnover markers in young adult men: results from the Odense Androgen Study. Calcif Tissue Int. 2010;86:271–281. doi: 10.1007/s00223-010-9334-7. [DOI] [PubMed] [Google Scholar]
- 23.Rosner W, Auchus RJ, Azziz R, Sluss PM, Raff H. Position statement: Utility, limitations, and pitfalls in measuring testosterone: an Endocrine Society position statement. J Clin Endocrinol Metab. 2007;92:405–413. doi: 10.1210/jc.2006-1864. [DOI] [PubMed] [Google Scholar]
- 24.Legro RS, Schlaff WD, Diamond MP, Coutifaris C, Casson PR, et al. Total testosterone assays in women with polycystic ovary syndrome: precision and correlation with hirsutism. J Clin Endocrinol Metab. 2010;95:5305–5313. doi: 10.1210/jc.2010-1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Prescott J, Coetzee GA. Molecular chaperones throughout the life cycle of the androgen receptor. Cancer Lett. 2006;231:12–19. doi: 10.1016/j.canlet.2004.12.037. [DOI] [PubMed] [Google Scholar]