Abstract
Cardiac metabolism is finely tuned, and disruption of myocardial bioenergetics can be clinically devastating. Many cardiomyopathies that present early in life are due to disruption of the maturation of these metabolic pathways. However, this bioenergetic maturation begins well before birth, when the embryonic heart is first beginning to beat, and continues into the mature animal. Thus, the changes in energy production seen after birth are actually part of a continuum that coincides with the structural and functional changes that occur as the cardiac myocyte differentiates and the heart undergoes morphogenesis. Therefore, although bioenergetics and mitochondrial biology have not been studied in great detail in the developing heart, bioenergetic maturation should be considered an important component of normal myocyte differentiation.
Although events occurring after birth will be discussed, this review will focus on the changes in bioenergetics and mitochondrial biology that coincide with myocyte differentiation and cardiac morphogenesis. The relationship of these changes to the etiology and presentation of cardiomyopathies will be used as a starting point for this discussion. Then, after reviewing cardiac development and mitochondrial biology, the published data on bioenergetics and mitochondrial structure and function in the developing heart will be presented. Finally, the case will be made that mitochondria may be critical regulators of cardiac myocyte differentiation and cardiac development.
Keywords: Bioenergetics, cardiac development, cardiomyopathy, mitochondria, and myocyte differentiation
1. Introduction
Cardiac development is a complex process that produces the first functional organ in the embryo. Without correct formation of the heart, many conceptuses die in the embryonic, fetal, or immediate postnatal periods. However, despite the fact that congenital heart defects (CHD) can be quite devastating, many fetuses with severe malformations of cardiac structure survive to birth and their lesions can be repaired or palliated by medical or surgical interventions.
In contrast, adequate cardiac function is essential for survival at any age. If the heart does not begin to contract and provide effective circulation by about mouse embryonic day (E) 10 (unless indicated, all developmental stages will refer to mouse gestational age or its equivalent), the embryo will soon die as it outgrows its ability to survive by diffusion of nutrients from the surrounding fluids (1, 2). Subsequently, if the early heart contracts but the myocardium does not mature enough to provide the cardiac output necessary for adequate perfusion, the growing embryo or fetus will die. In addition, there exist abnormalities in ventricular myocardial structure, such as severe non-compaction cardiomyopathies, which allow for survival into the postnatal period when increased demands on cardiac function cannot be met and the infant develops heart failure. Finally, if the neonatal myocardium cannot undergo the normal transition from glycolytic to fatty acid metabolism due to inherited metabolic defects, the infant may develop a cardiomyopathy and die without intervention.
Despite the massive increase over the last 40 years in our understanding of the mechanisms of normal cardiac morphogenesis and how dysregulation of these processes cause CHD, we still have little understanding of the role of bioenergetics in cardiac development and survival of the developing mammal. The following sections will compare the changes that occur in cardiac structure and myocyte differentiation to those that occur in bioenergetics and mitochondrial biology to make the case that bioenergetic maturation is an essential component of cardiac development. First, the role of bioenergetics in infantile cardiomyopathies will be discussed. Then, a brief review of cardiac development will be presented, followed by a discussion of the literature describing changes in bioenergetics and mitochondrial structure and function that occur during the development of the heart. Finally, the argument will be made that mitochondria may be critical regulators of cardiac myocyte differentiation and cardiac development.
2. Cardiomyopathy, a disease of mitochondria and bioenergetics
The annual incidence of pediatric cardiomyopathy is reported to be approximately 1.2 per 100,000 children and is higher in infancy (3, 4). Dilated and hypertrophic cardiomyopathies, the most common forms, are more likely to present in infancy. Although many of these cardiomyopathies are due to mutations in genes of the glycogen and mucopolysaccharide metabolic pathways or of contractile and structural proteins, it has long been clear that disruption of fatty acid oxidation and mitochondrial oxidative phosphorylation play a critical role in a large percentage of dilated cardiomyopathies, particularly those that present early in life (3–5). Conversely, about 40% of patients identified with mitochondrial disorders exhibit hypertrophic, dilated, or non-compaction cardiomyopathy, and mouse models of mitochondrial disease have begun to examine this association (6, 7). As discussed in detail below, the neonatal period is a time of transition to fatty acid oxidation and of a dramatic increase in mitochondrial respiration, and disruption of this transition by these genetic mutations accounts for their presentation in the newborn period.
This switch from glycolytic to fatty acid metabolism is merely the last of a number of transitions that the heart makes in its bioenergetics as it develops. When the heart first begins to form in the early embryo, it is generally thought that the entire embryo relies on anaerobic glycolysis for energy production due to low oxygen levels. However, as the placenta develops and circulation is established, oxygen levels rise, allowing aerobic respiration. In addition, the sources of energy and the cellular mechanisms which convert these sources to ATP change, particularly in the heart. As these changes coincide with the morphologic development of the heart and myocyte differentiation, it becomes apparent that bioenergetic maturation is a vital component of cardiac development.
3. Cardiac development and myocyte differentiation
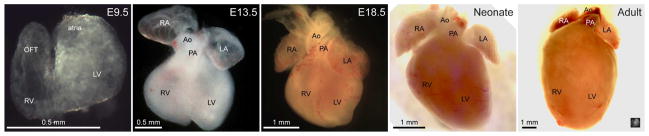
The embryonic period is temporally defined by the morphogenesis of the heart, which is an amazing process where a small number of cardiac precursors proliferate and differentiate to form a muscle-lined tube and then a fully functional, four chambered heart. The morphologic changes that occur during this process, from the early embryo to the adult, are illustrated in Figure 1.
Figure 1. Gross cardiac morphology at different stages of development.
Brightfield images of E9.5, E13.5, E18.5, neonatal and adult mouse hearts from roughly the anterior/ventral aspect demonstrate the changes that occur in cardiac morphology during development. Cardiac structures are labeled: Ao—aorta, LA—left atrium, LV—left ventricle, OFT—outflow tract, PA—pulmonary artery, RA—right atrium, and RV—right ventricle. The inset in the lower right corner of the “Adult” heart is the E9.5 heart at the same scale to demonstrate their relative sizes.
Cardiac development begins during early gastrulation when cells in the lateral precardiac mesoderm migrate to form the cardiac crescent (reviewed in 8, 9). Initially, cardiac precursor cells form a thin walled, linear heart tube lying at the midline of the ventral aspect of the mouse embryo at about E8.5 (approximately day 20 of human gestation). Over the next day, the early heart will loop to the right to bring the arterial and venous poles together and the nascent chambers will become apparent as they “balloon” from the primary heart tube. During the next 5 days, the four chambers of the heart form and septate, the atrioventricular and semilunar valves develop to ensure unidirectional flow, the venous structures drain to their appropriate atria, and the single outflow tract divides into a pulmonary artery and aorta, which provide outflow for the right and left ventricles, respectively. By E14.5 (approximately day 50 in the human embryo), the mouse heart is almost completely formed from a structural standpoint, requiring only further remodeling of the valves and the myocardium and postnatal closure of the three shunts (the ductus venosus, the ductus arteriosus, and the foramen ovale) that allow effective delivery of oxygen in utero but that are not necessary after birth. Therefore, cardiac morphogenesis is essentially considered an embryonic phenomenon.
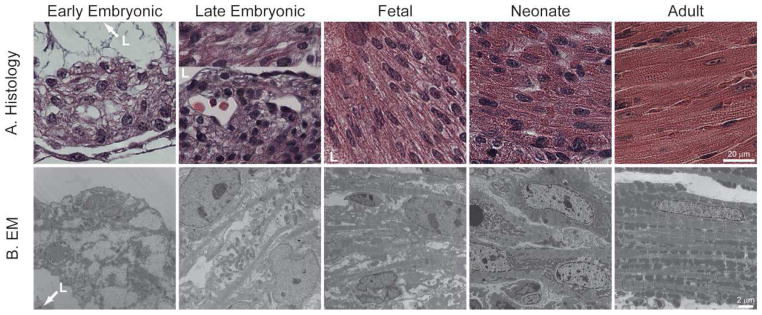
In contrast, cardiac myocyte differentiation extends into the postnatal period, and recent studies also demonstrate that cardiac stem cells continue to divide and differentiate even in the adult heart (10, 11). After pre-cardiac meosdermal cells are specified, they begin the long process of differentiation, which ultimately generates the mature cardiac myocyte, with its highly organized and almost crystalline intracellular structure (Figure 2B). Soon after differentiation begins, myocytes contract despite the fact that their intracellular contractile elements are very immature compared to the adult myocyte (12). Early myocytes contain few myofibrils, relative few mitochondria with immature ultrastructure, and no T-tubules or organized sarcoplasmic reticulum (SR). Over time, the contractile apparatus fills the myoplasm, and the myofibrillar structure becomes more mature, with increasing apposition of the SR, T-tubules, and mitochondria. However, even immediately after birth, the sub-cellular structure of the cardiac myocyte is still relatively immature in most species. Only after the first few days to weeks does the generally recognized structure of the mature myocyte develop. This process of myocyte differentiation is illustrated in Figure 2B.
Figure 2. Myocardial and myocyte structure during development.
Histology (A, hematoxylin and eosin stained, paraffin-embedded sections) and electron micrographs (B, EM) of ventricular myocytes in early embryonic (E9.5), late embryonic (13.5), fetal (E16.5 for Histology, 18.5 for EM), neonatal, and adult hearts demonstrate changes that occur in the general structure of the myocardium and in the composition and structure of the differentiating myocyte. In the “Histology/Late Embryonic” panel, the upper portion represents trabecular myocardium with cross striations, while the lower portion represents the morphology of the compact myocardium. The cardiac lumen (L) is indicated where appropriate.
The structure of the ventricular myocardium at different stage of development reflects the changes in myocyte differentiation as well as the energetic demands placed upon the heart (Figure 2A) (reviewed in 13, 14). At E9.5, the ventricular myocardium is relatively simple and contains only one or two layers of myocytes surrounding an endocardial layer. At around this time, small projections of myocytes migrate from the wall of the heart into the lumen to form trabeculae. The trabecular myocytes become more differentiated than the cells of the compact layer (the wall) and are thought to be the major source of contractile force from approximately E10 to E13. However, as the coronary circulation is established and the functional demands of the heart keep increasing, the wall of the heart thickens and takes over as the major contractile component of the myocardium, although the cells closer to the lumen have a more differentiated cellular phenotype (see Figure 2A, upper third of “Late Embryonic” panel). During the fetal period (E14 to birth in the mouse), rapid growth of the embryo is reflected in rapid growth of the ventricular walls and in the regression of the trabecular myocardium. At E16.5, and even more so in the neonate, myocytes in the walls of the ventricle have more, but still irregular, cross striations, indicative of increasing numbers of myofibrils that are coming into alignment. However, the mature cellular structure, with regular cross-striations spanning the cell in histological sections and almost complete filling of the sarcoplasm with mitochondria and myofibril in electron micrographs, is only apparent in the adult myocardium.
4. Mitochondria and bioenergetics during cardiac development
4.1. Mitochondrial structure and function
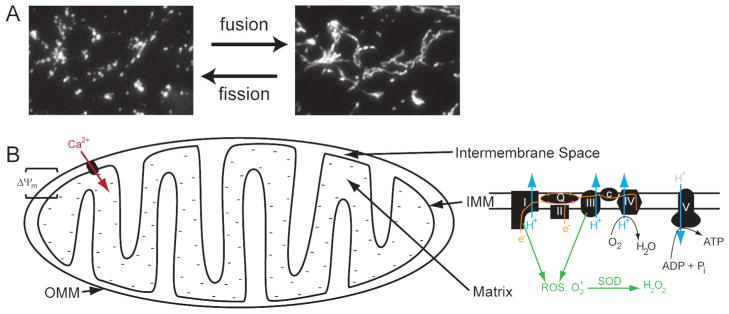
Although the textbook view of mitochondria shows them to be cigar-shaped structures with a smooth outer membrane and a crenellated inner membrane, the mitochondrial network within cells is much more complex. In fact, the name mitochondrion, derived from the Greek words for “grain” (chondros) and “thread” (mitos), implies that the shape of mitochondria can range from fragmented “cigars” represented in textbooks to an interconnected network as extensive as the endoplasmic reticulum (15). This mitochondrial network can undergo fission and fusion that can be observed and manipulated in live cells (Figure 3A).
Figure 3. Mitochondrial structure and function.
A. Mitochondria undergo fission and fusion to create a dynamic network that can be fragmented or interconnected, depending on the cell type and the conditions. This example from the same cell demonstrates mitochondrial fission induced by an increase in intracellular calcium. B. Individual mitochondria are complex structures that contain an outer and inner mitochondrial membrane (OMM, IMM), an intermembrane space, and a central matrix. They generate ATP, regulate and are regulated by intracellular calcium levels, and generate reactive oxygen species as well as antioxidants. In an active mitochondrion, electrons enter the electron transport chain (ETC) at complexes I and II and are passed through the Q cycle to complex III, then cytochrome c, and finally to complex IV, where they are used to reduce molecular oxygen to water. The ETC also pumps protons (H+) from the matrix to the intermembrane space, creating an electrochemical gradient (Δψm) that is used by complex V to generate ATP from ADP and inorganic phosphate (Pi). Δψm is represented by the negative charges in the mitochondrial matrix and is responsible for the influx of calcium (Ca2+) through various channels, whose composition is not completely known. Finally, complexes I and II are also responsible for the creation of superoxide anions (O2•−) that are converted by Mn-superoxide dismutase (SOD) to H2O2, which can diffuse into the sarcoplasm.
Mitochondria contain an inner and outer membrane, an intermembrane space, and a central matrix. The inner membrane, with its invaginations into the matrix (the cristae), contains the five complexes of the electron transport chain (ETC), which are responsible for oxidative phosphorylation (Figure 3B) (16). Complex I and II pass electrons from NADH and FADH2, generated by the Krebs cycle in the matrix, down the ETC where they reduce molecular oxygen to water. During this process, protons are pumped from the matrix to the intermembrane space by complexes I, III, and IV. This generates an electrochemical gradient (Δψm) that is tapped by complex V to convert ADP and inorganic phosphate to ATP.
Due to the polarized nature of the mitochondrial membrane, calcium can enter the mitochondrion’s negatively charged matrix via a number of channels of unclear composition (reviewed in 17, 18, 19). In the mature heart, the proximity of mitochondria to the calcium release sites in the SR allows for enhanced uptake of calcium into mitochondria during each sarcoplasmic calcium transient. This mitochondrial calcium transient activates the Krebs cycle and the ETC, which results in increased ATP production coinciding with contraction, a phenomenon called excitation-metabolism or, to broaden the term, excitation-contraction-metabolism coupling (17, 19). Due to their ability to regulate calcium by sequestering and releasing this ion, any calcium-dependent activities will be modulated by mitochondria. Furthermore, mitochondria control calcium transport and cytoplasmic levels by providing ATP for calcium transporting proteins, such as SERCA (sarco(endo)plasmic reticulum calcium ATPase). Thus, mitochondria play a central role in regulating intracellular calcium signaling within the myocyte.
Mitochondria are one of the main regulators of cellular redox state, which is the sum of oxidants and antioxidants (18, 19). Oxidants create reactive oxygen species (ROS), which can oxidize other molecules, while anti-oxidants convert a reactive species to a less reactive species. Generally, excessive oxidative stress (high ROS:antioxidant ratio) is associated with cell death. In most cells, complexes I and III of the ETC generate the bulk of ROS, producing superoxide (O2•−), which is converted by Mn-superoxide dismutase to the freely diffusible H2O2, which can then be converted to H2O by cytosolic catalase (18, 20). As long as the ETC is active and remains oxidized, there is little production of O2•−(20–22). Cytoplasmic NADPH oxidases and xanthine oxidase also produce ROS and are important in the embryo. In contrast, the basic antioxidants in the cytoplasm are NADPH and reduced glutathione, levels of which are largely controlled by the availability of NADH, as regulated by mitochondria. In addition, the pentose phosphate pathway and cytoplasmic enzymes such as lactate dehydrogenase and NADP+-dependent isocitrate dehydrogenase play a role in regulating NADPH levels (23).
4.2. Mitochondrial structural changes during cardiac development
During cardiac development and myocyte differentiation, the structure of individual mitochondria and the cellular mitochondrial network are quite variable. These changes can be demonstrated in vivo and in vitro using electron and fluorescence microscopy.
Pre- and post-natal hearts
The sarcoplasmic structure, including mitochondria, of myocytes in immature hearts is much less organized than in adult myocytes. In the early rat embryo (E10), the inner mitochondrial membrane tends to be relatively smooth, lacking well-formed cristae. As the heart develops, blebs project from the inner mitochondrial membrane into the matrix. Over time, these blebs are thought to form globular cristae, which evolve into the more mature tubular/lamellar cristae, and this transformation is associated with increased mitochondrial function (24, 25). Similar changes in mitochondrial ultrastructure are observed in embryonic mouse hearts (Figure 2B and not shown). There are also changes in mitochondrial network structure and localization; whereas mitochondria in E9.5 myocytes tend to be fragmented and perinuclear, those in E13.5 myocytes are interconnected and spread throughout the cell. Similar changes in localization can be observed using live explanted hearts stained with mitochondrial dyes and imaged using a multiphoton microscope (Porter, et al., unpublished data).
Mitochondria in fetal and neonatal hearts have a more mature and complex structure of their cristae and are more likely to be associated with myofibrils than in embryonic myocytes. However, compared to adult myocytes, they have smaller mitochondria, which are not as regularly aligned alongside the myofibrils (Figure 2B) (26–28). In the mature cardiac myocyte, mitochondria comprise about 1/3 of the volume of the cell. They are thought to be relatively static structures that are tightly linked to the SR and myofibrils so that they can provide energy in the form of ATP and creatine phosphate for contraction and for the sequestration of calcium into the SR via SERCA (Figure 2B).
Cultured myocytes
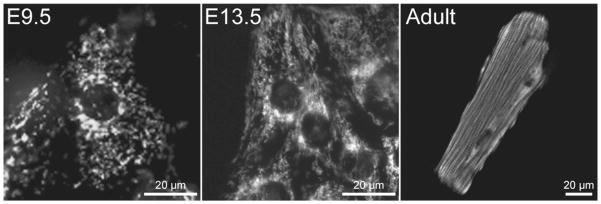
Cultured myocytes isolated from hearts at different ages also demonstrate differences in mitochondrial structure that correlate to the changes seen in vivo (Figure 4). E9.5 myocytes contain a relatively fragmented mitochondrial network concentrated near the nucleus. Later in development (E13.5) the network becomes more interconnected, spans the cell, and begins to associate with the developing myofibrils. Similar changes in the mitochondrial network have been observed in cardiac stem cells undergoing differentiation (29, 30). The adult myocyte contains a relatively static network of intermyofibrillar mitochondria as well as a population of subsarcolemmal and perinuclear mitochondria.
Figure 4. Mitochondrial network morphology in cultured myocytes.
Fluorescence imaging of mitochondria in cardiac myocytes from E9.5, E13.5, and adult hearts demonstrates dramatic changes in the structure of the mitochondrial network as the heart develops. E9.5 myocytes have a fragmented network, while the mitochondria in E13.5 myocytes are more filamentous and spread throughout the cell. In contrast, the mitochondria network in the adult myocyte is highly structured, with mitochondria aligning in specific bands along the myofibrils and in the perinuclear and subsarcolemmal regions. Primary myocyte cultures were labeled with MitoTracker Green and imaged using epifluorescence (E9.5, E13.5) or confocal (Adult) microscopy.
4.3. Changes in bioenergetics and mitochondrial function during cardiac development
Studies that have demonstrated changes in energetic sources and the reliance on oxygen, glycolytic activity and mitochondria oxidative phosphorylation in the embryo and heart during development are reviewed below. It is notable that this information is relatively sparse, incomplete, and sometimes contradictory. However, an overall theme emerges, in which the embryonic heart slowly makes a transition from anaerobic glycolysis to aerobic fatty acid oxidation as it develops from its earliest stages to maturity.
Pregastrulation embryo
More is known about bioenergetics and mitochondrial biology in the oocyte and pre and peri-implantation embryo than in the embryonic heart (reviewed in 23, 31). During the initial stages of embryonic development, mammalian embryos and oocytes have a highly regulated metabolism. The oocyte has a high rate of oxidative phosphorylation due to the presence of lipid and protein nutrients in the cytoplasm and low levels of glucose transport from the extracellular environment. Upon implantation into the uterine wall, nutrient sources switch from being internal to external and oxygen tension falls (32). This likely necessitates a corresponding switch to anaerobic glycolysis, at least until the placenta forms and circulation is established around E10 (32, 33).
Embryonic heart
It is speculated that the early embryonic heart relies in anaerobic glycolysis for its energy (34). Much of the recent work documenting this has been performed using embryonic stem cells and is outlined below. Experiments manipulating glucose levels, measuring glucose uptake, or adding inhibitors have demonstrated a reliance on glycolysis at these early stages of development (approximately mouse E9.5) (35–40). Alternatively, low levels of mitochondrial ETC activity have been reported in some studies (41–43).
Despite the fact that the early embryonic heart is quite capable of consuming oxygen (Porter and Hoffman, unpublished data and 44), the low level of oxygen to which it is exposed likely inhibits aerobic respiration and dictates a reliance on anaerobic glycolysis. For example, the oxygen concentration is <10 mmHg in the heart and <20 mmHg in the rest of the embryo at E9.5. However, after E10, oxygen levels are 10–30 mmHg throughout the embryo due to the maturation of the placenta and increased oxygen delivery by the primitive cardiovascular system (32, 33, 45–47). It should be noted that the term hypoxia should be used with care when discussing the embryo. Although the oxygen levels mentioned above are well below normal postnatal levels, they are, in fact, normal for the embryo.
This variable reliance on oxygen for metabolism at different stages of development is reflected by the response of embryos to hypoxia. For example, rat embryonic heart rates were variably affected by anoxia at E11 (equivalent to mouse E9) but were significantly slowed under the same conditions at later stages (36, 48). In addition, exposure of pregnant mice to hypoxia (8% inspired oxygen) reduced myocardial proliferation, caused myocardial thinning and heart failure, and decreased embryonic survival, particularly at E13.5, while longer exposure (E7.5-E10.5) of early embryos to 10% oxygen had no effect on survival but did decrease embryonic size and myocardial mass at later ages (47, 49). Haring also found a higher incidence in a variety of cardiac malformations in embryos from pregnant rats exposed to 10% oxygen (with or without hypercapnea) for 24 hours (50, 51). Finally, it is well-documented that cultured murine embryos prefer incubation in a 5% oxygen atmosphere for the first day of culture, when they have grown from E8.5 to E9.5, but require higher levels of oxygen (21–95%) thereafter (52–54).
Additional evidence suggests an increasing reliance on aerobic metabolism and mitochondrial ATP production after about E9.5. In conjunction with the experiments discussed above, it was found the older embryonic rat heart required oxygen to contract, could utilize mitochondrial substrates for energy production, and was inhibited by mitochondrial ETC inhibitors (36, 37). Glucose utilization also falls (40). A number of studies have shown that ETC enzyme activity increases during the mid-embryonic period. For example, studies on whole embryos demonstrated that complex II, II and V were active in E10–14 rats (mouse E8.5–12.5), but complex I had low activity at the earliest age that increased 6-fold by E14 (25, 41–43). Finally, in humans, the ETC appears to be active by the late embryonic period (9 weeks) (55, 56).
Fetal heart
As discussed above, by the end of the embryonic period, approximately E14 in the mouse, cardiac mitochondria are actively engaged in oxidative phosphorylation. During the fetal period, the oxidative capacity increases (rabbits) and the ETC proteins and activity (humans, mice, rats) increases by 2 fold or more to levels also observed in neonates (55–59). However, the primary source of energy is lactate, although glucose is still utilized to a lesser degree. In addition, fatty acid oxidation has begun to occur (34, 60).
Postnatal heart
For the first week after birth, the bioenergetics of the heart is much like that in the fetus, with little reliance on fatty acids as an energy source (34). However, the increase in the activity and amount of the ETC complexes that begins in the fetus continues after birth in rats (57, 61). The final energetic transition occurs after about the first week of life, when glycolysis and lactate metabolism have decreased and fatty acid oxidation has increased to adult levels (5, 34). The adult heart relies on fatty acid oxidation and mitochondrial oxidative phosphorylation for 90% of its energy, although it is capable of oxidizing a number of other substrates.
4.4. Changes in other aspects of mitochondrial function during cardiac development
Mitochondrial calcium uptake has not been studied in detail during development, although mitochondria from fetal rabbit hearts are reported to have higher capacity to sequester calcium (58). Mitochondrial regulation of redox state has also not been studied in detail, but it is recognized that cardiac myocyte differentiation is affected by changes in redox state (62–65). In preliminary experiments, we have found an increase in mitochondrial calcium uptake and a decrease in oxidative stress during the embryonic period (Porter et al., unpublished data).
4.5. Changes in mitochondrial structure and function in cardiac stem cells
Recent work has documented changes that occur in cardiac stem cell energetics and mitochondrial biology during differentiation (29, 30, 59, 66). These changes appear to mirror what has been observed in the embryonic heart, as outlined above. Prior to differentiation, murine embryonic stem cells have a fragmented mitochondrial network that is mostly perinuclear. Upon induction of differentiation, mitochondrial fission decreases and the mitochondrial network spreads throughout the cell and associates with the developing myofibrils. There is also an increase in the complexity of the inner mitochondrial membrane structure.
These studies also demonstrate a transition from glycolytic to oxidative metabolism and an increase in Δψm upon differentiation (29, 30, 66). Interestingly, blocking these changes in mitochondrial structure and function inhibits differentiation of the stem cells into cardiac myocytes (29, 59).
Conclusions and perspective
During the complex process of cardiac development, the proliferation and differentiation of a small group of cells in the pregastrulation embryo eventually forms a four chambered heart. Due to changes in environment, nutrient availability, and energetic demands, this process is accompanied by equally complex changes in bioenergetics. Initially, anaerobic glycolysis predominates; yet, by the end of the embryonic period, aerobic, mitochondrial respiration has become the major mode of energy production in the heart and the metabolic phenotype of the heart continues to evolve into the postnatal period. The changes in mitochondrial structure and function mirror changes in myocyte structure until well after birth, and, thus, should be considered an essential component of myocyte differentiation. Converse changes are also seen during states of stress in the mature myocardium, when myocytes undergo de-differentiation and activate the “fetal” gene program. Although this reprogramming is generally associated with changes in transcription factors, secreted molecules (such as atrial and natriuretic peptide), and contractile proteins, it also includes changes in metabolic pathways to a more glycolytic and less fatty acid oxidative state (34, 67). This further suggests that bioenergetic pathways are intimately linked to myocyte differentiation.
Thus, it appears likely that mitochondrial biology can regulate myocyte differentiation, but the basic parameters of bioenergetics and mitochondrial structure and function are still largely unexplored. The studies discussed above suggest that further delineation of these parameters would be very revealing. Future experiments could systematically examine all aspects of bioenergetics and mitochondrial biology from cardiac progenitor and stem cells to adult myocytes in vivo and in vitro. For example, the published literature has barely examined the role of mitochondria in regulating calcium and redox signaling during myocyte differentiation as well as the development of excitation-contraction-metabolism that appears to be so important in the mature heart. Finally, if methods are developed to manipulate myocyte differentiation and cardiac development by altering mitochondrial function, then these tools might become important new modalities to prevent and treat CHD and cardiomyopathies as well as to enhance engraftment of cardiac myocytes for cardiac regeneration.
Acknowledgments
We would like to thank Barbara Tisdale, Gayle Schneider, and Dr. Sung Hyun Kang for technical support and specimens, and the members of the Mitochondrial Research and Innovation Group at the University of Rochester Medical Center for critical evaluation of this work. This work was supported by Grants from the National Institutes of Health (S.S.S.), American Heart Association (G.A.P. and S.S.S.), and Children Cardiomyopathy Foundation (G.A.P.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
George A. Porter, Jr., Email: George_porter@urmc.rochester.edu.
Jennifer Hom, Email: Jennifer_hom@urmc.rochester.edu.
David Hoffman, Email: David_hoffman@urmc.rochester.edu.
Rodrigo Quintanilla, Email: Rodrigo_quintanilla@urmc.rochester.edu.
Karen de Mesy Bentley, Email: Karen_bentley@urmc.rochester.edu.
Shey-Shing Sheu, Email: Sheyshing_sheu@urmc.rochester.edu.
References
- 1.Conway SJ, Kruzynska-Frejtag A, Kneer PL, Machnicki M, Koushik SV. What cardiovascular defect does my prenatal mouse mutant have, and why? Genesis. 2003;35:1–21. doi: 10.1002/gene.10152. [DOI] [PubMed] [Google Scholar]
- 2.Turgeon B, Meloche S. Interpreting neonatal lethal phenotypes in mouse mutants: insights into gene function and human diseases. Physiol Rev. 2009;89:1–26. doi: 10.1152/physrev.00040.2007. [DOI] [PubMed] [Google Scholar]
- 3.Lipshultz SE, Sleeper LA, Towbin JA, et al. The incidence of pediatric cardiomyopathy in two regions of the United States. N Engl J Med. 2003;348:1647–55. doi: 10.1056/NEJMoa021715. [DOI] [PubMed] [Google Scholar]
- 4.Nugent AW, Daubeney PE, Chondros P, et al. The epidemiology of childhood cardiomyopathy in Australia. N Engl J Med. 2003;348:1639–46. doi: 10.1056/NEJMoa021737. [DOI] [PubMed] [Google Scholar]
- 5.Kelly DP, Strauss AW. Inherited cardiomyopathies. N Engl J Med. 1994;330:913–19. doi: 10.1056/NEJM199403313301308. [DOI] [PubMed] [Google Scholar]
- 6.Scaglia F, Towbin JA, Craigen WJ, et al. Clinical spectrum, morbidity, and mortality in 113 pediatric patients with mitochondrial disease. Pediatrics. 2004;114:925–31. doi: 10.1542/peds.2004-0718. [DOI] [PubMed] [Google Scholar]
- 7.Wallace DC. Mitochondrial diseases in man and mouse. Science. 1999;283:1482–88. doi: 10.1126/science.283.5407.1482. [DOI] [PubMed] [Google Scholar]
- 8.Abu-Issa R, Kirby ML. Heart field: from mesoderm to heart tube. Annu Rev Cell Dev Biol. 2007;23:45–68. doi: 10.1146/annurev.cellbio.23.090506.123331. [DOI] [PubMed] [Google Scholar]
- 9.Moorman AF, Christoffels VM, Anderson RH, van den Hoff MJ. The heart-forming fields: one or multiple? Philos Trans R Soc Lond B Biol Sci. 2007;362:1257–65. doi: 10.1098/rstb.2007.2113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Beltrami AP, Urbanek K, Kajstura J, et al. Evidence that human cardiac myocytes divide after myocardial infarction. N Engl J Med. 2001;344:1750–7. doi: 10.1056/NEJM200106073442303. [DOI] [PubMed] [Google Scholar]
- 11.Kajstura J, Urbanek K, Perl S, et al. Cardiomyogenesis in the adult human heart. Circ Res. 2010;107:305–15. doi: 10.1161/CIRCRESAHA.110.223024. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 12.Smolich JJ. Ultrastructural and functional features of the developing mammalian heart: a brief overview. Reprod Fertil Dev. 1995;7:451–61. doi: 10.1071/rd9950451. [DOI] [PubMed] [Google Scholar]
- 13.Chen H, Zhang W, Li D, et al. Analysis of ventricular hypertrabeculation and noncompaction using genetically engineered mouse models. Pediatr Cardiol. 2009;30:626–34. doi: 10.1007/s00246-009-9406-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sedmera D, Pexieder T, Vuillemin M, Thompson RP, Anderson RH. Developmental patterning of the myocardium. Anat Rec. 2000;258:319–37. doi: 10.1002/(SICI)1097-0185(20000401)258:4<319::AID-AR1>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 15.Hom J, Sheu SS. Morphological dynamics of mitochondria--a special emphasis on cardiac muscle cells. J Mol Cell Cardiol. 2009;46:811–20. doi: 10.1016/j.yjmcc.2009.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mitchell P. Coupling of phosphorylation to electron and hydrogen transfer by a chemi-osmotic type of mechanism. Nature. 1961;191:144–8. doi: 10.1038/191144a0. [DOI] [PubMed] [Google Scholar]
- 17.Balaban RS. Cardiac energy metabolism homeostasis: role of cytosolic calcium. J Mol Cell Cardiol. 2002;34:1259–71. doi: 10.1006/jmcc.2002.2082. [DOI] [PubMed] [Google Scholar]
- 18.Brookes PS, Yoon Y, Robotham JL, Anders MW, Sheu SS. Calcium, ATP, and ROS: a mitochondrial love-hate triangle. Am J Physiol Cell Physiol. 2004;287:C817–33. doi: 10.1152/ajpcell.00139.2004. [DOI] [PubMed] [Google Scholar]
- 19.Gunter TE, Sheu SS. Characteristics and possible functions of mitochondrial Ca(2+) transport mechanisms. Biochim Biophys Acta. 2009;1787:1291–1308. doi: 10.1016/j.bbabio.2008.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Turrens JF. Mitochondrial formation of reactive oxygen species. J Physiol. 2003;552:335–44. doi: 10.1113/jphysiol.2003.049478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hoffman DL, Salter JD, Brookes PS. Response of mitochondrial reactive oxygen species generation to steady-state oxygen tension: implications for hypoxic cell signaling. Am J Physiol Heart Circ Physiol. 2007;292:H101–8. doi: 10.1152/ajpheart.00699.2006. [DOI] [PubMed] [Google Scholar]
- 22.Hoffman DL, Brookes PS. Oxygen sensitivity of mitochondrial reactive oxygen species generation depends on metabolic conditions. J Biol Chem. 2009;284:16236–45. doi: 10.1074/jbc.M809512200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dumollard R, Carroll J, Duchen MR, Campbell K, Swann K. Mitochondrial function and redox state in mammalian embryos. Semin Cell Dev Biol. 2009;20:346–33. doi: 10.1016/j.semcdb.2008.12.013. [DOI] [PubMed] [Google Scholar]
- 24.Shepard TH, Muffley LA, Smith LT. Ultrastructural study of mitochondria and their cristae in embryonic rats and primate (N. nemistrina) Anat Rec. 1998;252:383–92. doi: 10.1002/(SICI)1097-0185(199811)252:3<383::AID-AR6>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 25.Mackler B, Grace R, Tippit DF, et al. Studies of the development of congenital anomalies in rats. III. Effects of inhibition of mitochondrial energy systems on embryonic development. Teratology. 1975;12:291–6. doi: 10.1002/tera.1420120311. [DOI] [PubMed] [Google Scholar]
- 26.Anversa P, Vitali-Mazza L, Loud AV. Morphometric and autoradiographic study of developing ventricular and atrial myocardium in fetal rats. Lab Invest. 1975;33:696–705. [PubMed] [Google Scholar]
- 27.Kim HD, Kim DJ, Lee IJ, et al. Human fetal heart development after mid-term: morphometry and ultrastructural study. J Mol Cell Cardiol. 1992;24:949–65. doi: 10.1016/0022-2828(92)91862-y. [DOI] [PubMed] [Google Scholar]
- 28.Sybers HD, Ingwall JS, De Luca MA. Fetal mouse heart in organ structure: ultrastructure. Lab Invest. 1975;32:713–19. [PubMed] [Google Scholar]
- 29.Chung S, Dzeja PP, Faustino RS, et al. Mitochondrial oxidative metabolism is required for the cardiac differentiation of stem cells. Nat Clin Pract Cardiovasc Med. 2007;4 (Suppl 1):S60–7. doi: 10.1038/ncpcardio0766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chung S, Dzeja PP, Faustino RS, Terzic A. Developmental restructuring of the creatine kinase system integrates mitochondrial energetics with stem cell cardiogenesis. Ann N Y Acad Sci. 2008;1147:254–63. doi: 10.1196/annals.1427.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Van Blerkom J. Mitochondria in early mammalian development. Semin Cell Dev Biol. 2009;20:354–64. doi: 10.1016/j.semcdb.2008.12.005. [DOI] [PubMed] [Google Scholar]
- 32.Burton GJ. Oxygen, the Janus gas; its effects on human placental development and function. J Anat. 2009;215:27–35. doi: 10.1111/j.1469-7580.2008.00978.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fisher SA, Burggren WW. Role of hypoxia in the evolution and development of the cardiovascular system. Antioxid Redox Signal. 2007;9:1339–52. doi: 10.1089/ars.2007.1704. [DOI] [PubMed] [Google Scholar]
- 34.Lopaschuk GD, Jaswal JS. Energy metabolic phenotype of the cardiomyocyte during development, differentiation, and postnatal maturation. J Cardiovasc Pharmacol. 2010;56:130–40. doi: 10.1097/FJC.0b013e3181e74a14. [DOI] [PubMed] [Google Scholar]
- 35.Clough JR, Whittingham DG. Metabolism of [14C]glucose by postimplantation mouse embryos in vitro. J Embryol Exp Morphol. 1983;74:133–42. [PubMed] [Google Scholar]
- 36.Cox SJ, Gunberg DL. Metabolite utilization by isolated embryonic rat hearts in vitro. J Embryol Exp Morphol. 1972;28:235–45. [PubMed] [Google Scholar]
- 37.Cox SJ, Gunberg DL. Energy metabolism in isolated rat embryo hearts: effect of metabolic inhibitors. J Embryol Exp Morphol. 1972;28:591–9. [PubMed] [Google Scholar]
- 38.Freinkel N, Lewis NJ, Akazawa S, Roth SI, Gorman L. The honeybee syndrome - implications of the teratogenicity of mannose in rat-embryo culture. N Engl J Med. 1984;310:223–30. doi: 10.1056/NEJM198401263100404. [DOI] [PubMed] [Google Scholar]
- 39.Hunter ES, 3rd, Tugman JA. Inhibitors of glycolytic metabolism affect neurulation-staged mouse conceptuses in vitro. Teratology. 1995;52:317–23. doi: 10.1002/tera.1420520602. [DOI] [PubMed] [Google Scholar]
- 40.Tanimura T, Shepard TH. Glucose metabolism by rat embryos in vitro. Proc Soc Exp Biol Med. 1970;135:51–4. doi: 10.3181/00379727-135-34985. [DOI] [PubMed] [Google Scholar]
- 41.Mackler B, Grace R, Duncan HM. Studies of mitochondrial development during embryogenesis in the rat. Arch Biochem Biophys. 1971;144:603–10. doi: 10.1016/0003-9861(71)90367-5. [DOI] [PubMed] [Google Scholar]
- 42.Mackler B, Grace R, Haynes B, Bargman GJ, Shepard TH. Studies of mitochondrial energy systems during embryogenesis in the rat. Arch Biochem Biophys. 1973;158:662–66. doi: 10.1016/0003-9861(73)90558-4. [DOI] [PubMed] [Google Scholar]
- 43.Miki A, Mizoguchi A, Mizoguti H. Histochemical studies of enzymes of the energy metabolism in postimplantation rat embryos. Histochemistry. 1988;88:489–95. doi: 10.1007/BF00570314. [DOI] [PubMed] [Google Scholar]
- 44.Spielmann H, Lucke I. Changes in the respiratory activity of different tissues of rat and mouse embryos during development. Naunyn Schmiedebergs Arch Pharmacol. 1973;278:151–64. doi: 10.1007/BF00500647. [DOI] [PubMed] [Google Scholar]
- 45.Dunwoodie SL. The role of hypoxia in development of the Mammalian embryo. Dev Cell. 2009;17:755–73. doi: 10.1016/j.devcel.2009.11.008. [DOI] [PubMed] [Google Scholar]
- 46.Krishnan J, Ahuja P, Bodenmann S, et al. Essential role of developmentally activated hypoxia-inducible factor 1alpha for cardiac morphogenesis and function. Circ Res. 2008;103:1139–46. doi: 10.1161/01.RES.0000338613.89841.c1. [DOI] [PubMed] [Google Scholar]
- 47.Ream M, Ray AM, Chandra R, Chikaraishi DM. Early fetal hypoxia leads to growth restriction and myocardial thinning. Am J Physiol Regul Integr Comp Physiol. 2008;295:R583–95. doi: 10.1152/ajpregu.00771.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shepard TH, Tanimura T, Robkin M. In vitro study of rat embryos. I. Effects of decreased oxygen on embryonic heart rate. Teratology. 1969;2:107–9. doi: 10.1002/tera.1420020205. [DOI] [PubMed] [Google Scholar]
- 49.Wendler CC, Amatya S, McClaskey C, et al. A1 adenosine receptors play an essential role in protecting the embryo against hypoxia. Proc Natl Acad Sci U S A. 2007;104:9697–9702. doi: 10.1073/pnas.0703557104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Haring OM. Effects of prenatal hypoxia on the cardiovascular system. Arch Pathol. 1965;80:351–6. [PubMed] [Google Scholar]
- 51.Haring OM. Cardiac malformations in the rat induced by maternal hypercapnia with hypoxia. Circ Res. 1966;19:544–51. doi: 10.1161/01.res.19.3.544. [DOI] [PubMed] [Google Scholar]
- 52.Porter GA, Jr, Makuck RF, Rivkees SA. Intracellular calcium plays an essential role in cardiac development. Dev Dyn. 2003;227:280–90. doi: 10.1002/dvdy.10307. [DOI] [PubMed] [Google Scholar]
- 53.Miki A, Fujimoto E, Ohsaki T, Mizoguti H. Effects of oxygen concentration on embryonic development in rats: a light and electron microscopic study using whole-embryo culture techniques. Anat Embryol (Berl) 1988;178:337–43. doi: 10.1007/BF00698664. [DOI] [PubMed] [Google Scholar]
- 54.Sturm K, Tam PP. Isolation and culture of whole postimplantation embryos and germ layer derivatives. Methods Enzymol. 1993;225:164–90. doi: 10.1016/0076-6879(93)25013-r. [DOI] [PubMed] [Google Scholar]
- 55.Marin-Garcia J, Ananthakrishnan R, Goldenthal MJ. Heart mitochondrial DNA and enzyme changes during early human development. Mol Cell Biochem. 2000;210:47–52. doi: 10.1023/a:1007031919298. [DOI] [PubMed] [Google Scholar]
- 56.Minai L, Martinovic J, Chretien D, et al. Mitochondrial respiratory chain complex assembly and function during human fetal development. Mol Genet Metab. 2008;94:120–6. doi: 10.1016/j.ymgme.2007.12.007. [DOI] [PubMed] [Google Scholar]
- 57.Schagger H, Noack H, Halangk W, Brandt U, von Jagow G. Cytochrome-c oxidase in developing rat heart. Enzymic properties and amino-terminal sequences suggest identity of the fetal heart and the adult liver isoform. Eur J Biochem. 1995;230:235–41. [PubMed] [Google Scholar]
- 58.Sordahl LA, Crow CA, Kraft GH, Schwartz A. Some ultrastructural and biochemical aspects of heart mitochondria associated with development: fetal and cardiomyopathic tissue. J Mol Cell Cardiol. 1972;4:1–10. doi: 10.1016/0022-2828(72)90092-2. [DOI] [PubMed] [Google Scholar]
- 59.Spitkovsky D, Sasse P, Kolossov E, et al. Activity of complex III of the mitochondrial electron transport chain is essential for early heart muscle cell differentiation. Faseb J. 2004;18:1300–2. doi: 10.1096/fj.03-0520fje. [DOI] [PubMed] [Google Scholar]
- 60.Warshaw JB, Terry ML. Cellular energy metabolism during fetal development. II. Fatty acid oxidation by the developing heart. J Cell Biol. 1970;44:354–60. doi: 10.1083/jcb.44.2.354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hallman M. Changes in mitochondrial respiratory chain proteins during perinatal development. Evidence of the importance of environmental oxygen tension. Biochim Biophys Acta. 1971;253:360–72. doi: 10.1016/0005-2728(71)90040-5. [DOI] [PubMed] [Google Scholar]
- 62.Buggisch M, Ateghang B, Ruhe C, et al. Stimulation of ES-cell-derived cardiomyogenesis and neonatal cardiac cell proliferation by reactive oxygen species and NADPH oxidase. J Cell Sci. 2007;120:885–94. doi: 10.1242/jcs.03386. [DOI] [PubMed] [Google Scholar]
- 63.Ding L, Liang XG, Hu Y, Zhu DY, Lou YJ. Involvement of p38MAPK and reactive oxygen species in icariin-induced cardiomyocyte differentiation of murine embryonic stem cells in vitro. Stem Cells Dev. 2008;17:751–60. doi: 10.1089/scd.2007.0206. [DOI] [PubMed] [Google Scholar]
- 64.Li J, Stouffs M, Serrander L, et al. The NADPH oxidase NOX4 drives cardiac differentiation: Role in regulating cardiac transcription factors and MAP kinase activation. Mol Biol Cell. 2006;17:3978–88. doi: 10.1091/mbc.E05-06-0532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Schmelter M, Ateghang B, Helmig S, Wartenberg M, Sauer H. Embryonic stem cells utilize reactive oxygen species as transducers of mechanical strain-induced cardiovascular differentiation. FASEB J. 2006;20:1182–4. doi: 10.1096/fj.05-4723fje. [DOI] [PubMed] [Google Scholar]
- 66.Chung S, Arrell DK, Faustino RS, Terzic A, Dzeja PP. Glycolytic network restructuring integral to the energetics of embryonic stem cell cardiac differentiation. J Mol Cell Cardiol. 2010;48:725–34. doi: 10.1016/j.yjmcc.2009.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lehman JJ, Kelly DP. Transcriptional activation of energy metabolic switches in the developing and hypertrophied heart. Clin Exp Pharmacol Physiol. 2002;29:339–45. doi: 10.1046/j.1440-1681.2002.03655.x. [DOI] [PubMed] [Google Scholar]