Abstract
Gestational cocaine treatment results in significantly increased maternal aggression towards an intruder by postpartum day six, while acute postpartum treatment dose dependently decreases maternal aggressive (MA) behavior. Both increased and decreased aggression in the cocaine-treated dams are correlated with either decreased or increased levels of oxytocin in the amygdala, respectively. The current study was an effort to determine whether the effect of gestational cocaine on maternal aggression is transient or would continue into the postpartum period; whether an intermittent cocaine treatment regimen, which incorporates gestational and postpartum intermittent cocaine treatment, would differ from chronic daily gestational treatment; and finally, whether next generation female offspring of cocaine-treated or control dams would have altered MA behavior and oxytocin system changes attributable to either prenatal drug exposure, rearing condition or both. We now report no increase in maternal aggression following chronic gestational treatment and significantly lower levels of aggression in intermittently treated dams on postpartum day eight, with no significant effects in either group on postpartum day 12. Young adult female offspring of the cocaine-treated and control dams, who reared their own natural litters and were tested on postpartum day eight for maternal aggression, had higher levels of maternal aggression towards an intruder attributable to both prenatal cocaine exposure and rearing condition. Higher aggression in cocaine-reared next generation dams was associated with lower levels of oxytocin in the amygdala. Intergenerational effects of cocaine were apparent with respect to aggression and oxytocin system changes.
Keywords: Cocaine, intergenerational transmission, maternal aggression, oxytocin, stress
Introduction
Maternal aggressive (MA) behavior in lactating rats has been described as a subset of maternal behaviors related to protecting offspring from intruders into the nesting environment (Numan 1994; Gammie 2005). MA is found in most mammals and has been characterized as an offensive/aggressive series of actions and postures including direct attacks on an intruder thought to help ensure offspring survival (Numan 1994). MA can be elicited during the late gestational period, but peaks during the first 10 postpartum days (PPDs) (Giovenardi et al. 1997).
Substance abuse has long been associated with both anxiety and aggressive behavior (Moss and Tarter 1993). Research from several laboratories has reported that chronic and acute cocaine treatment alter MA, sometimes in a dose-dependent fashion (Heyser et al. 1992; Johns et al. 1994b, 1998b; Vernotica et al. 1996a; Lubin et al. 2001). Chronic cocaine (CC) treatment has been shown to increase MA significantly by PPD six (Johns et al. 1994b) and under certain conditions PPD 10 (Heyser et al. 1992), while acute treatment has been shown to decrease it (Johns et al. 1994b; Vernotica et al. 1996b; Johns et al. 1998b). Importantly, the effects of CC do not result from cocaine withdrawal (Johns et al. 1997b). Most findings to date are reported for lactating dams during the earlier postpartum period at more moderate doses of cocaine, with some data available for the later postpartum period at higher doses (Heyser et al. 1992). Interestingly, MA alterations following cocaine-treatment in rats are associated with alterations in brain levels of the neuropeptide oxytocin (Johns et al. 1997a; Elliott et al. 2001), as well as oxytocin receptor binding and affinity in the hypothalamus and limbic system (Johns et al. 2004; Jarrett et al. 2006). It has been suggested (Johns et al. 1994b; Johns et al. 1998b; Lubin et al. 2003) that cocaine-induced oxytocin system alterations may be at least partially responsible for alterations in MA behavior in cocaine-treated rats.
Oxytocin-rich projections from the parvocellular neurons of the paraventricular nucleus to the amygdala (Consiglio and Lucion 1996; Giovenardi et al. 1998; Lonstein and Gammie 2002) compose one neurobiological pathway that may mediate normal levels of maternal aggression. Oxytocin administered directly into either the central nucleus of the amygdala or bed nucleus of the stria terminalis has been shown to decrease MA in the postpartum period (Consiglio et al. 2005), whereas oxytocin antagonists infused into the central nucleus of the amygdala can dramatically increase MA to the extent that it becomes mal-adaptive (Lubin et al. 2003). Furthermore, both ibotenic acid lesions of the paraventricular nucleus and oxytocin antisense administration into the paraventricular nucleus can also stimulate maternal aggression (Giovenardi et al. 1998). Recently, it has also been suggested that amygdala oxytocin release, rather than oxytocin receptor modulation, may be responsible for changes in MA (Bosch et al. 2005).
In addition to an association of oxytocin with MA, the stress response has also been strongly associated with oxytocin (Callahan et al. 1989; Uvnas-Moberg et al. 1994; Jezova et al. 1995; Nishioka et al. 1998; Uvnas-Moberg 1998; Neumann et al. 2001; Amico et al. 2004), a relationship that has been increasingly studied over the years. Oxytocin neurons are activated in response to various types of stressful stimuli (Ludwig 1998; Neumann 2002), and recent work has suggested the presence of an oxytocin-mediated, parasympathetically driven “anti-stress” system (Uvnas-Moberg 1997). Interestingly, the concept of intergenerational transmission of stress responsiveness through altered maternal behavior has gained attention over the last few years (Pedersen and Boccia 2002; Fish et al. 2004), and recent work has indicated an oxytocin connection (Francis et al. 2000; Champagne and Meaney 2001; Francis et al. 2002; Pedersen and Boccia 2002).
Recent results examining the intergenerational transmission of cocaine-induced maternal behavior deficits (Johns et al. 2005) documented a multitude of effects in the early postpartum period in both cocaine-treated and next generation dams attributable to treatment and rearing environment condition. These effects were also associated with alterations in oxytocin levels in relevant specific brain regions, particularly with respect to rearing condition. To our knowledge there are no reports of intergenerational studies examining MA following cocaine treatment or exposure in the later postpartum period or oxytocin system changes during this period.
The present report was designed to examine the effects of cocaine treatment on MA on PPDs eight and 12, in rat dams rearing their own or cross-fostered litters following either a gestational CC or an intermittent cocaine (IC) gestational and postpartum cocaine treatment schedule, which has characteristics of both a chronic and an acute dosing schedule. In addition, cross-fostered first generation female offspring reared by their biological mothers were also assessed for MA and oxytocin system changes on PPD eight. This study design allowed for the assessment of effects attributable to prenatal exposure condition, rearing environment or both.
We hypothesized that higher rates of MA in CC-treated dams would continue through PPD eight with diminishing effects by PPD 12. We also predicted that IC-treated dams would be less aggressive than CC-treated dams and controls on PPD eight, when they were injected with cocaine 30 min before testing, as opposed to PPD 12, when cocaine was not injected prior to testing. Direct cocaine treatment was expected to have greater effects on MA in dams than the prenatal exposure condition of the litter the dam was rearing. In the offspring of treatment dams, hereafter called the first generation dams (FGDs), we expected a lesser, but still increased level of MA in cocaine-exposed FGDs, with a slight increase expected in FGDs reared by cocaine-treated dams, as found in intergenerational tests of maternal behavior (Johns et al. 2005). FGDs exhibiting higher levels of MA were predicted to have lower levels of oxytocin in the amygdala, as was seen in the previous studies on cocaine and maternal aggression (Johns et al. 1995).
Methods
Breeding
Following a two-week habituation period, virgin female (body weight 200–240 g) Sprague–Dawley rats (Charles River, Raleigh, NC) were placed with males on a breeding rack until a sperm plug was found, which was designated as gestation day (GD) zero. Rats were randomly assigned to one of five treatment or control groups, singly housed and maintained on a 12 h:12 h reverse light cycle (lights off at 0900 h) for seven days. They were then transferred to a room with a regular light cycle (lights on at 0700 h) for the remainder of the experiment, a procedure that generally results in the majority of dams delivering their litters during daylight hours (Mayer and Rosenblatt 1998). The overall study timeline is described in Figure 1. All procedures were conducted under federal and institutional animal care and use committee guidelines for humane treatment of laboratory animals.
Figure 1.
Study timeline. Data collection was completed over five years. GD: gestation day; PPD: postpartum day; RIA: radioimmunoassay.
Treatment and animal groups
Original dams
Chronic treatment groups and controls
Treatment groups included: CC, chronic saline (CS), and untreated (UN) dams. CC and CS dams received subcutaneous injections, twice daily throughout gestation beginning on GD one and continuing until the day before delivery (GD 1–20), on alternate flanks of 15mg/kg cocaine HCL (dose calculated as free base; Sigma Chemical Company, St. Louis, MO) dissolved in 0.9% normal saline (total volume 2ml/kg), or the same volume of normal saline (0.9%), respectively at approximately 0800 and 1600 h. UN dams were weighed and handled daily, but received no drug treatment. CC and UN dams had free access to water and food (rat chow), while CS-treated dams were yoke-fed to match CC dams to control for the anorectic effects of cocaine, as previously described (Johns et al. 1994b).
Intermittently treated groups and controls
Treatment groups included: IC, intermittent saline (IS), and UN dams. Intermittently treated dams received the same doses of their respective drugs (cocaine, saline) as the chronically treated dams. However, injections were given on two consecutive days, every five days during gestation (GD 2, 3, 8, 9, 14, 15, 20) and also throughout the postpartum period (PPD 2, 3, 8, 9, 14, 15, 20, 21). The intermittent schedule was modeled on previous studies examining behavioral effects of prenatal cocaine exposure on offspring and was used previously in a study of maternal behavior in dams and offspring (Johns et al. 1992a,b; Johns et al. 2005). UN dams were weighed and handled daily, but received no drug treatment. All intermittent treatment groups had free access to water and food (rat chow) except on the injection days, when intermittent groups received 50 g of food (more than any rats consumed in a day) with food consumption measured daily as previously described (Johns et al. 1994b).
Cross-fostering
On the day of parturition, pups were removed from each dam, weighed, counted, and their gender determined before being culled to a litter of four males and four females. Litters were either returned to their natural mother or fostered to dams from a different treatment or control group within 30 min. Fostering across groups was matched for delivery time. The cross-fostering procedure resulted in the 25 dam/offspring group combinations shown in Table I. This procedure guaranteed adequate numbers of dams and pups across all 25 rearing and prenatal conditions with at least 10 dams from each treatment group rearing pups from other treatment groups (Table I). Group numbers varied somewhat due to the loss of some animals during testing and the necessity of breeding extra dams so that delivery times would be matched for fostering. To achieve sufficient sample sizes in each of the 25 groups this study took four years to complete, with hundreds of offspring born each spring. Although all subjects received the same treatment and testing procedures, the extended time course introduced unavoidable variability spread randomly across groups, which did not differentially influence group trajectories over the four years.
Table I.
Experimental groups resulting from cross-fostering.
| Litter prenatal exposure condition | CC | IC | CS | IS | UN |
|---|---|---|---|---|---|
| Cc | CCcc (13/10) | ICcc (13/12) | CScc (14/11) | IScc (14/10) | UNcc (12/10) |
| Ic | CCic (12/10) | ICic (15/12) | CSic (12/10) | ISic (14/11) | Unic (15/11) |
| Cs | CCcs (15/10) | ICcs (14/11) | CScs (13/11) | IScs (13/10) | Uncs (13/10) |
| Is | CCis (13/10) | ICis (13/10) | CSis (14/11) | ISis (16/14) | Unis (12/10) |
| Un | CCun (11/11) | ICun (15/13) | CSun (16/10) | ISun (19/17) | Unun (28/23) |
Note. Capital letters indicate the original or parent dams’ treatment; lower case letters indicate the prenatal exposure condition of first generation dams (FGDs). Group designations are as follows: chronic cocaine (CC), chronic saline (CS), intermittent cocaine (IC), intermittent saline (IS), or no treatment (UN). The total numbers of original dams (ODs) and FGDs for each group are listed in parentheses. Group sizes varied as a result of extra breeding for testing purposes and loss of dams for various reasons during the study. Groups with identical letters CCcc indicate treatment dams groups rearing their own litters (i.e. same dam treatment and prenatal exposure condition for pups).
First generation dams
One female from each of the separate 25 litter groups was randomly selected at 60 days of age for breeding and testing for MA on PPD eight only. Unselected pups underwent other behavioral tests at various ages (not reported here). Experimental conditions were the same as for the original treatment dams, except that FGDs were mated with different males, fed ad libitum, received no drug treatment, and they reared and were tested with eight pups (four male, four female) from their natural litters. Since no drugs were directly administered to offspring, their treatment conditions (Table I) included only their prenatal exposure condition (cocaine, saline or no drug) and rearing condition (cocaine treated, CC or IC; saline treated, CS or IS; or UN dams). Group designations for FGDs were based on their prenatal exposure condition and drug treatment of their rearing dam. For example, an FGD reared by a CC - treated dam, but born to a UN dam, and thus without prenatal exposure, would be designated CCUN. FGDs were weighed every five days to monitor weight gain throughout pregnancy and were observed daily following delivery to ensure litter health.
Maternal aggression testing
Original dams
MA was assessed using a procedure similar to one previously described (Johns et al. 1998b; Lubin et al. 2003). Tests were conducted on PPDs eight and 12 for the original dams. On the morning of PPD eight, between 0800 and 1100 h (during the light phase), dams and their litters were brought in their home cage to a behavioral observation room where weight gain was recorded. Intermittently treated dams (IS, IC) received either a saline or cocaine injection, respectively (15 mg/kg, sc), immediately after weighing and 30min before testing. Chronically treated dams were weighed, but received no injection. On both test days, all dams and litters habituated to the test area in a quiet environment for 25 min, after which the home cage was placed in a 61 × 41 × 51 cm dimly lit testing cubicle, designed to reduce environmental distractions, for a further 5-min habituation period. A smaller intruder male (body weight ~ 175 g) was then placed in front of the cage and the session was videotaped with a low light sensitivity video camera and recorder for the 10 min session. A new inexperienced intruder male was used for each session with no male used more than once. If the pups were attacked by the dam or the intruder, or if the dam or intruder was seriously injured, the session was immediately terminated and the data were excluded from the analysis. Terminated sessions were noted for each group. After testing on PPD eight, dams and their culled litters were returned to the colony room and monitored daily to ensure that the pups received adequate maternal care. MA was again assessed on PPD 12, using the same procedures as on PPD eight except that no groups were injected before testing.
Observed behaviors included maternal behaviors, defensive behaviors (threat), aggressive behaviors varying in intensity (rough groom, nip/bite male, aggressive posture, fight attack) and general activity. These behaviors of interest have been described previously (Johns et al. 1998b; Lubin et al. 2003) and included: maternal behavior (dam licks pups, moves pups, or crouches over pups); rough-groom (dam grooms intruder male roughly, usually around head, neck or back); lateral/ front threat (dam threatens male while approaching with her body in a lateral position, or moves her face close to the male’s face, often accompanied by teeth chattering); fight attack (dam makes a quick lunge usually followed by rolling, biting, and fur pulling directed towards the neck and back regions of the intruder); nip/bite (dam nips or bites male; differentiated from a fight attack by degree and lack of jumping and lunging); aggressive posture (dam forces intruder into a full submissive posture and pushes him down with extended front paws or stands over him with her paws on his chest or belly); and general activity (including general locomotor behaviors and other non-aggressive motor activities). Dams and litters were returned to the colony room after testing on PPD 12 and pups were weaned and separated on PPD 21. Dams were killed on PPD 22 for oxytocin measurements (data not presented here).
First generation dams
FGDs were tested for maternal aggression with their own culled litter on PPD eight using testing procedures as described above, except that they had no drug injections and were tested with eight of their own pups that they had reared from delivery (four male and four female). At the completion of the 10-min behavioral test, dams and their litters were returned to the colony. On PPD nine, FGDs were killed by decapitation, without anaesthesia, and their brains dissected for later oxytocin radioimmunoassay.
Brain dissection
The whole medial preoptic area, hippocampus, amygdala, and ventral tegmental area were dissected on ice, immediately weighed fresh, and then rapidly frozen and stored at −80°C for later oxytocin radioimmunoassay. Brain dissection procedures have previously been described (Johns et al. 1997a; Johns et al. 2005). Briefly, brains were coronally sectioned from the ventral side rostral to the optic chiasm (approximately A7100 µm according to Konig and Klippel (1963)) and just caudal to the optic chiasm (approximately A5800 µm) to define the preoptic-anterior hypothalamic area. Vertical cuts inferior to the lateral ventricles and a horizontal cut ventral to the anterior commissure were made to produce a block section of the medial preoptic area. The brains were further sectioned just caudal to the tuber cinereum (approximately A3800 µm) and just rostral to the cerebellum and the amygdala was removed in this section. The whole hippocampus was removed from the caudal remainder of the brain, and the ventral tegmental area was dissected from this portion by making dorsoventral cuts medial to the optic tracts with a dorsal cut at the ventral extent of the central gray.
Oxytocin radioimmunoassay
Brain region tissues were homogenized in cold buffer (19 mM monobasic sodium phosphate, 81 mM dibasic sodium phosphate, 0.05 M NaCl, 0.1 % BSA, 0.1 % Triton 100, 0.1 % sodium azide, pH 7.4) and centrifuged at 3000 × g for 30 min. Oxytocin immunoreactive content was assayed in the supernatant according to a protocol from Peninsula Labs (Belmont, CA). Samples and standards (1.0–128.0 pg) were incubated in duplicate for 16–24 hours at 4°C with rabbit anti-oxytocin serum. They were then incubated for 16–24 hours at 4°C with 125I-Oxytocin after which time normal rabbit serum and goat anti-rabbit IgG serum were added and incubated for 90-min at room temperature. The 125I-Oxytocin bound to the antibody complex was separated from free by a 90-min centrifugation at 4°C. The radioactivity in the pellet was measured using a LKB CliniGamma counter, which calculates the picogram content of oxytocin in each sample from the standard curve. The intra-assay and inter-assay coefficients of variance were 4.05 and 8.95%, respectively. The sensitivity of the assay was approximately 0.5 pg/tube. Data were expressed as pg oxytocin per mg of tissue.
Behavioral data and statistical analyses
Videotaped sessions were scored by two independent observers, blind to treatment condition, with inter-and intra-reliability set at 90–100% concurrence for frequency and latency, and 80% or better agreement for duration of behaviors displayed by the dam. A computer program calculated the frequency, duration, latency, and sequence of all relevant behaviors displayed by the dams. Behaviors not displayed by the dams were assigned the highest possible latency (1800s for a 30-min test). Repeated measures log linear models for count data (frequency) were used to examine between group differences within each day as well as over repeated days of testing. Repeated measures weighted additive models for time to event best fit the duration dataset. Weights were inversely proportional to the within-cell (rearing, prenatal, session) variance estimate. Latency data were analyzed using the Cox proportional hazard model, a semi-parametric survival analysis procedure. Lastly, gestational variables and oxytocin contents in each brain region were examined using analysis of variance.
To account for multiple observations in each rat, general estimating methods were used to obtain group estimates and standard errors, and p-values were adjusted for multiple comparisons via the FDR method (Benjamini et al. 2001). Only groups relevant to our hypotheses were compared. Chronic group comparisons included CC, CS, and UN groups, while intermittent comparisons included IC compared to IS and UN dams. Estimates of the means and standard errors under the model are presented graphically for frequency and duration data. Statistical significance was set at the p ≤ 0.05 level.
Results are presented first for the original dams followed by FGDs, and are grouped by days according to treatment (chronic, intermittent). Following the original dam treatment effects, results based on the dam’s foster-litter prenatal exposure condition are presented, and finally any effects resulting from the combined treatment and rearing litter interaction are presented. Results for FGDs are first listed as those resulting from only their rearing dam’s treatment (drug treatment of their rearing dam), followed by prenatal exposure effects (treatment of their biological dam), regardless of their rearing condition, and finally results based on the interaction of both rearing and prenatal environment. Only statistically significant results are presented in the text, with the most interesting findings presented graphically. Additionally, there were no significant group differences on any measured behavior on PPD 12 in original dams, thus results are only presented for PPD eight.
Results
Original dams
Gestational measures
As shown in Table II, there were significant effects of dam treatment on gestational weight gain [F(4, 354) = 20.95, p ≤ 0.01] and litter birth weight [F(4, 360) = 2.75, p ≤ 0.03]. Both CC-and IC-treated dams gained less weight during gestation than controls (CC, p ≤ 0.01; IC, p ≤ 0.01). Additionally, CC-treated dams gained significantly less weight than those treated with IC (p ≤ 0.01). Litters from CC-treated dams weighed significantly less than UN litters (UN, p ≤ 0.05), but since individual pup weight (grams) was not significantly different between groups, litter size probably accounts for litter weight differences.
Table II.
Gestational impact of original dam treatment.
| Dam treatment | Number of dams | Gestational weight gain (g) | Whole litter weight (g) | Number of pups |
|---|---|---|---|---|
| CC | 67 | 134.8 ± 2.6 UC | 86.3 ± 2.0 u | 14.3 ± 0.4 |
| CS | 71 | 132.3 ± 2.5 U | 85.3 ± 1.9 u | 13.5 ± 0.3 |
| UN | 81 | 157.7 ± 2.4 | 91.5 ± 1.8 | 14.5 ± 0.3 |
| IC | 70 | 147.4 ± 2.5 sU | 89.6 ± 1.9 | 14.4 ± 0.3 |
| IS | 77 | 154.4 ± 2.4 | 92.3 ± 1.8 | 14.6 ± 0.3 |
Note. Group designations are as follows: chronic cocaine (CC), chronic saline (CS), intermittent cocaine (IC), intermittent saline (IS), or no treatment (UN). Means with capitalized subscripts differ at p ≤ 0.01 while lower case subscripts differ at p ≤ 0.05. Different letters indicate differences as follows: S(s) indicates groups significantly different from respective saline control, U(u) indicates groups significantly different from UN dams, and C(c) indicates significant difference between cocaine treated groups (CC and IC).
Behavioral effects
Postpartum day eight
Effects of dam treatment
There were no differences between chronically treated groups (CC, CS, and UN) for defensive behaviors (threat), maternal behaviors, aggression, or general activity on PPD eight. Levels of most behaviors were relatively low in all groups compared to those previously reported (Johns et al. 1994b) at earlier times during the postpartum period (PPD six).
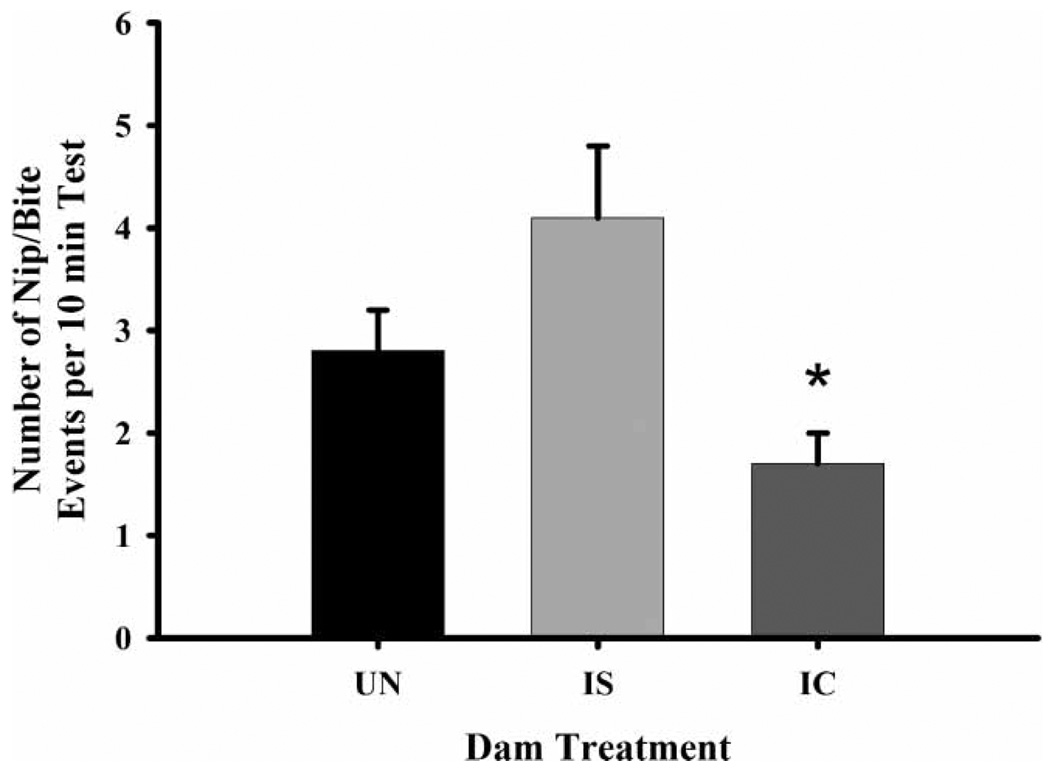
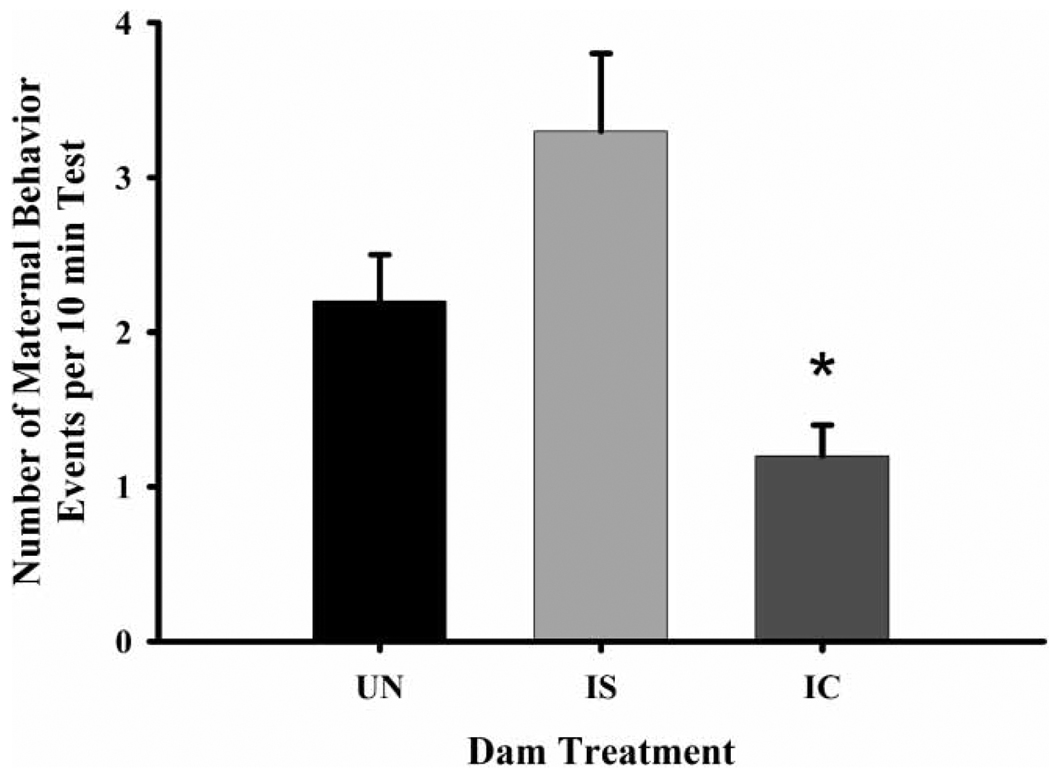
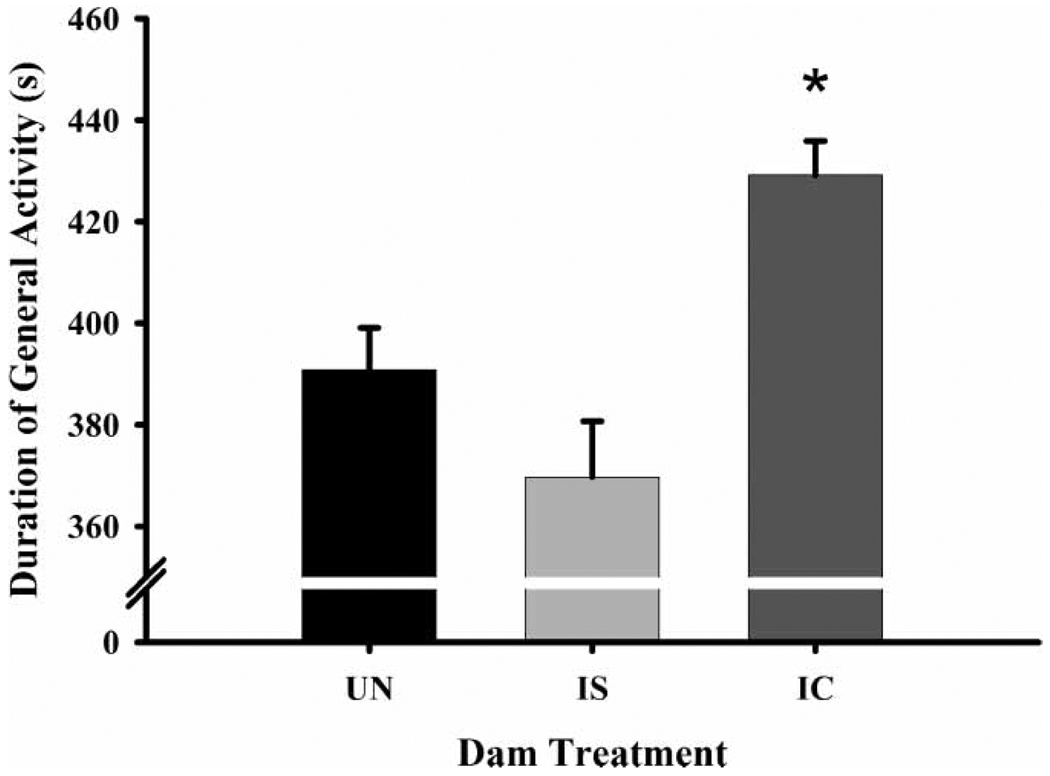
IC-treated dams had a lower frequency (Figure 2) and duration of nip/bites than did both IS (frequency, , p ≤ 0.01; duration, , p ≤ 0.02) and UN dams (frequency only, , p ≤ 0.03). Additionally, IC-treated dams also exhibited a lower frequency (Figure 3) and higher latency to begin maternal behavior than IS (frequency, , p ≤ 0.01; latency, , p ≤ 0.02) and UN dams (frequency, , p ≤ 0.01; latency, , p ≤ 0.02). They were also generally more active than IS (duration only, , p ≤ 0.01) and UN dams (frequency, , p ≤ 0.01; duration, , p ≤ 0.01) (Figure 4). There were no effects of litter prenatal exposure condition on defensive, aggressive, maternal behavior, or general activity levels in dams.
Figure 2.
The frequency of nip/bite behavior by dam treatment groups on PPD eight. Each bar represents least squares mean (LSM) and standard error (±SEM) for n = 80 untreated (UN), 76 intermittent saline (IS), and 70 IC-treated (IC) dams. As indicated by the asterisk, results indicate a significantly lower frequency of nip/bite in the IC-treated dams compared to both the UN (p ≤ 0.05) and IS (p ≤ 0.01).
Figure 3.
The frequency of maternal behavior by dam treatment groups on PPD eight. Each bar represents least squares mean (LSM) and standard error (±SEM) for n = 80 untreated (UN), 76 intermittent saline (IS), and 70 intermittent cocaine-treated (IC) dams. As indicated by the asterisk, results indicate a significantly lower frequency of maternal behavior in the IC-treated dams compared to both the UN (p ≤ 0.01) and IS (p ≤ 0.01) groups.
Figure 4.
The duration of general activity by dam treatment groups on PPD eight. Each bar represents least squares mean (LSM) and standard error (±SEM) for n = 80 untreated (UN), 76 intermittent saline (IS), and 70 intermittent cocaine-treated (IC) dams. As indicated by the asterisk, results indicate a significantly higher general activity level in the IC-treated dams compared to both the UN (p ≤ 0.01) and IS (p ≤ 0.01) groups.
Combined treatment and rearing litter effects
Behavioral differences were only seen for intermittent group comparisons. IC-treated dams rearing IC-exposed pups (ICIC) threatened intruders more often, had higher general activity levels than UN dams rearing UN pups (UNUN) dams (threats, , p ≤ 0.05; activity, (, p ≤ 0.01) and were also less maternal than IS-treated dams rearing IS-exposed pups (ISIS) ISIS dams (frequency, , p ≤ 0.01).
First generation dams
Gestational measures
There was a significant effect of rearing condition alone on gestational weight gain [F(4, 261) = 3.162, p ≤ 0.02; Table III]. IC-reared FGDs, regardless of their prenatal exposure condition, gained more weight over gestation than both CC- (p ≤ 0.05) and IS- (p ≤ 0.01) reared FGDs.
Table III.
FGD maternal gestation and litter data.
| Dam rearing conditon | Number of dams | Gestational weight gain (g) | Whole litter weight (g) | Number of pups |
|---|---|---|---|---|
| CC | 48 | 141.1 ± 5.2 | 95.3 ± 2.6 | 14.5 ± 0.4 |
| CS | 57 | 134.5 ± 4.7 | 87.2 ± 2.4 | 13.5 ± 0.4 |
| UN | 67 | 145.5 ± 4.5 | 91.5 ± 2.3 | 14.0 ± 0.4 |
| IC | 57 | 157.7 ± 4.8 Sc | 93.9 ± 2.4 | 14.6 ± 0.4 |
| IS | 64 | 146.6 ± 4.5 | 93.4 ± 2.3 | 14.8 ± 0.4 |
Note. Group designations are as follows: chronic cocaine (CC), chronic saline (CS), intermittent cocaine (IC), intermittent saline (IS), or no treatment (UN). Upper case S(s) indicates significant difference from chronic saline treated group at the p ≤ 0.01 level. Lower case C(c) indicates significant difference from chronic cocaine group (CC) at the p ≤ 0.05 level.
Behavioral effects
Rearing environment effects
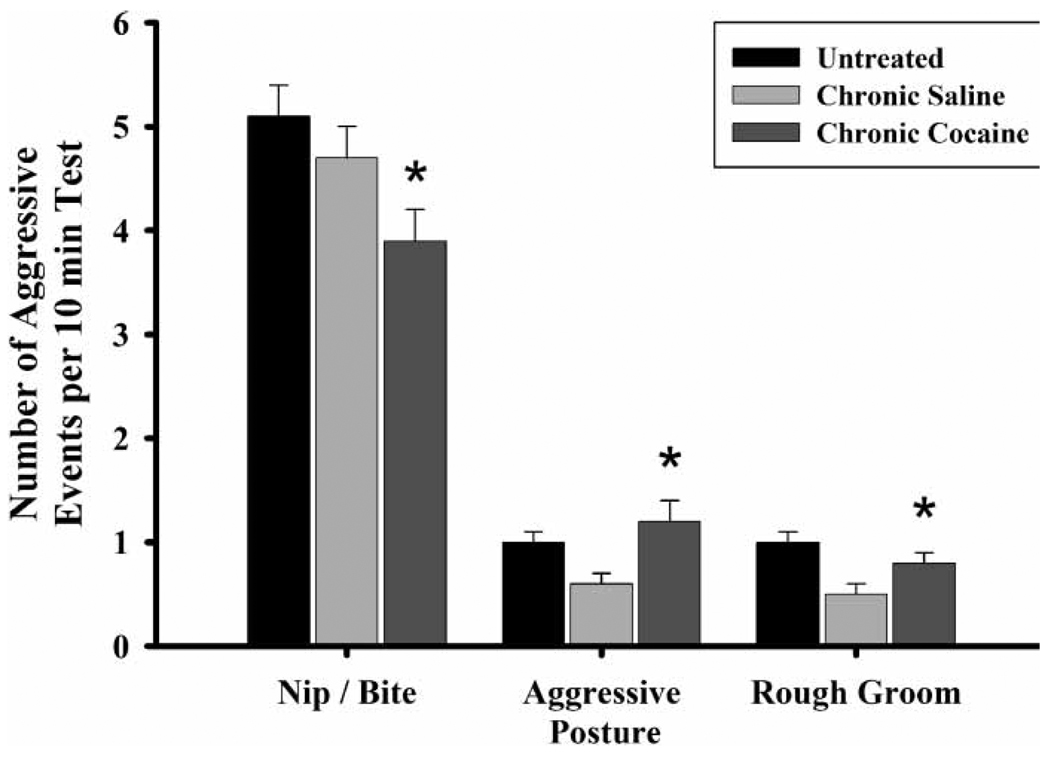
FGDs reared by cocaine-treated dams, regardless of their prenatal exposure condition, exhibited a number of behavioral effects related to rearing condition alone. CC-reared FGDs had higher frequencies of aggressive posture and rough grooming of the intruder compared to CS-reared FGDs (aggressive posture, , p ≤ 0.01; rough groom, , p ≤ 0.02). Conversely, they had significantly lower levels of nip/bite (Figure 5) compared to both UN- (, p ≤ 0.02) and CS-reared (, p ≤ 0.03) FGDs.
Figure 5.
The frequency of nip/bite, aggressive posture, and rough groom by first generation dam (FGD) rearing conditions on PPD eight. Each bar represents least squares mean (LSM) and standard error (±SEM) for n = 74 untreated (UN), 52 chronic saline (CS), and 53 chronic cocaine-treated (CC) dams. As indicated by the asterisks, results indicate a significantly lower frequency of nip/bite in the CC-reared FGDs compared to both the UN (p ≤ 0.01) and CS (p ≤ 0.05), and significantly elevated levels of aggressive posture (p ≤ 0.01) and rough groom (p ≤ 0.05) in the CC-reared FGDs compared to CS-reared.
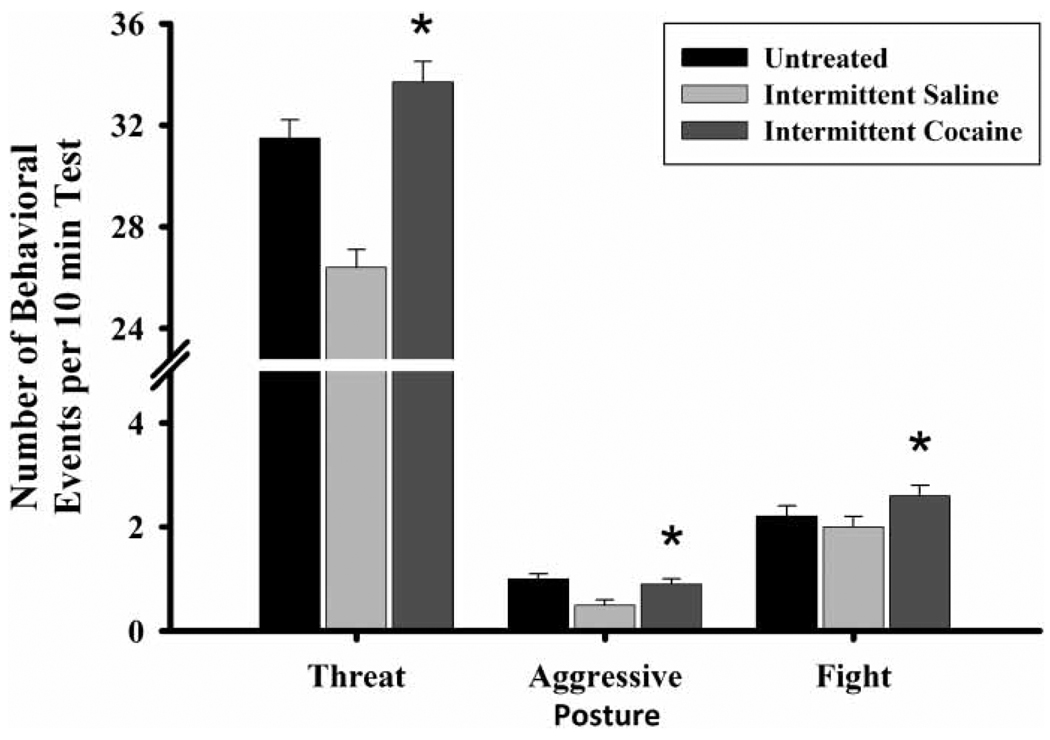
IC-reared FGDs were both more defensive and more aggressive, as they threatened the intruder more than both IS- (, p ≤ 0.01) and UN-reared (, p ≤ 0.05) dams (Figure 6) and displayed a higher frequency of aggressive postures, fight attacks, and general activity levels compared to IS-reared FGDs (aggressive posture, , p ≤ 0.03; attack, , p ≤ 0.04; general activity, , p ≤ 0.01). They were, however, less likely to nip/bite the intruder than were UN-reared FGDs (, p ≤ 0.01), similarly to the CC-reared FGDs.
Figure 6.
The frequency of threat, aggressive posture, and fight by the first generation (FGD) dam rearing conditions on PPD eight. Each bar represents least squares mean (LSM) and standard error (±SEM) for n = 74 untreated (UN), 55 intermittent saline (IS), and 54 intermittent cocaine (IC) dams. As indicated by the asterisks, results indicate a significantly higher frequency of threatening by the IC-reared FGDs compared to both the IS-(p ≤ 0.01) and UN-(p ≤ 0.05) reared FGDs, as well as elevated aggressive posture (p ≤ 0.05) and fight (p ≤ 0.05) frequencies compared to IS-reared FGDs.
IC-reared FGDs in general were more likely to threaten intruders (, p ≤ 0.01) but less likely to pin intruders than were CC-reared FGDs (, p ≤ 0.05) compared to their respective controls.
Prenatal exposure effects
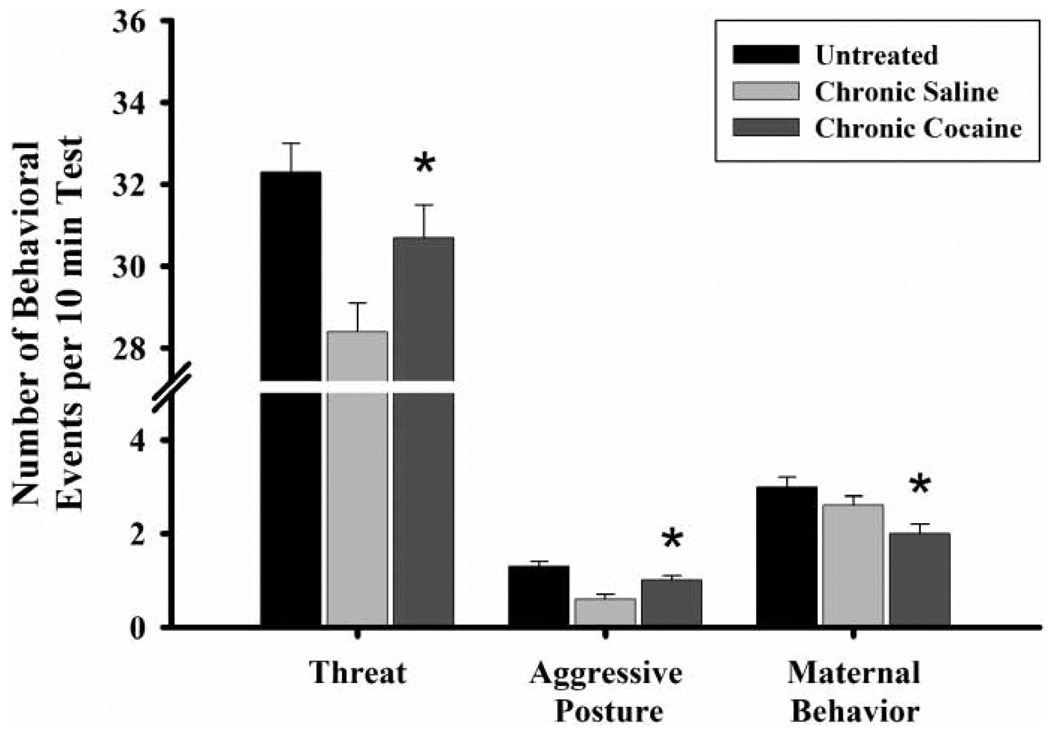
Prenatal exposure to CC, regardless of rearing condition, also resulted in behavioral differences in next generation dams. CC-exposed FGDs exhibited maternal behaviors less frequently (, p ≤ 0.05, Figure 7) and were more (frequency) defensive and aggressive than CS-exposed control FGDs (threat, , p ≤ 0.04; aggressive posture, , p ≤ 0.01).
Figure 7.
The frequency of threat, aggressive posture, and maternal behavior by first generation dam (FGD) prenatal exposure condition on PPD eight. Each bar represents least squares mean (LSM) and standard error (±SEM) for n = 74 untreated (UN), 52 chronic saline (CS), and 53 chronic cocaine (CC) dams. As indicated by the asterisks, results indicate significantly elevated frequencies of threatening (p ≤ 0.05) and aggressive postures (p ≤ 0.01) in the CC-exposed compared to the CS-exposed, and a lower frequency of maternal behavior in the CC-reared FGDs compared to both CS- (p ≤ 0.05) and UN-(p ≤ 0.01) reared FGDs.
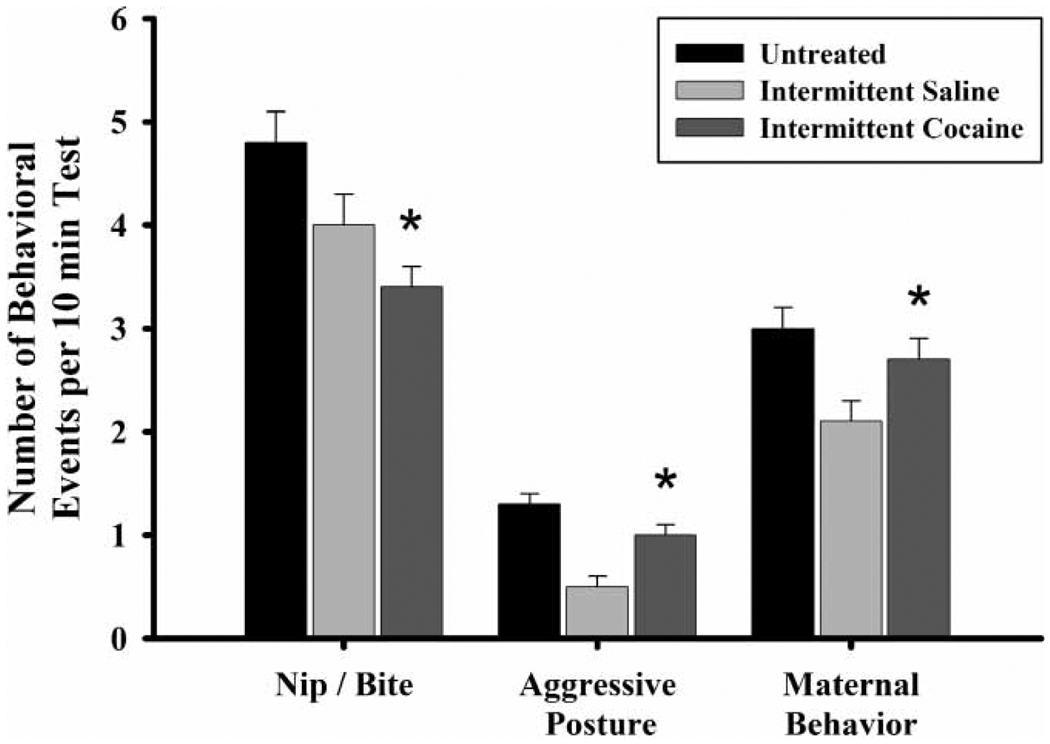
IC-exposed FGDs had a lower frequency of nip/bite (, p ≤ 0.01) than UN-exposed controls (Figure 8), but exhibited more aggressive postures and pup-directed maternal behaviors (move, lick, crouch) than did IS-exposed FGDs (aggressive posture, , p ≤ 0.01; maternal behavior, , p ≤ 0.05).
Figure 8.
The frequency of nip/bite, aggressive posture, and maternal behavior by first generation dam (FGD) prenatal exposure condition on PPD eight. Each bar represents least squares mean (LSM) and standard error (±SEM) for n = 74 untreated (UN), 55 intermittent saline (IS), and 54 intermittent cocaine (IC) dams. As indicated by the asterisks, results indicate a significantly lower frequency of nip/bite in the IC-reared FGDs compared to UN-reared FGDs (p ≤ 0.01), and significantly elevated aggressive posture (p ≤ 0.01) and maternal behavior (p ≤ 0.05) frequency compared to IS-exposed dams.
Overall the CC-exposed FGDs were more aggressive (nip/bite frequency, , p ≤ 0.01; duration, , p ≤ 0.05) than IC-exposed FGDs and were also less likely to be maternal (frequency, , p ≤ 0.02).
Combined prenatal exposure and rearing condition effects
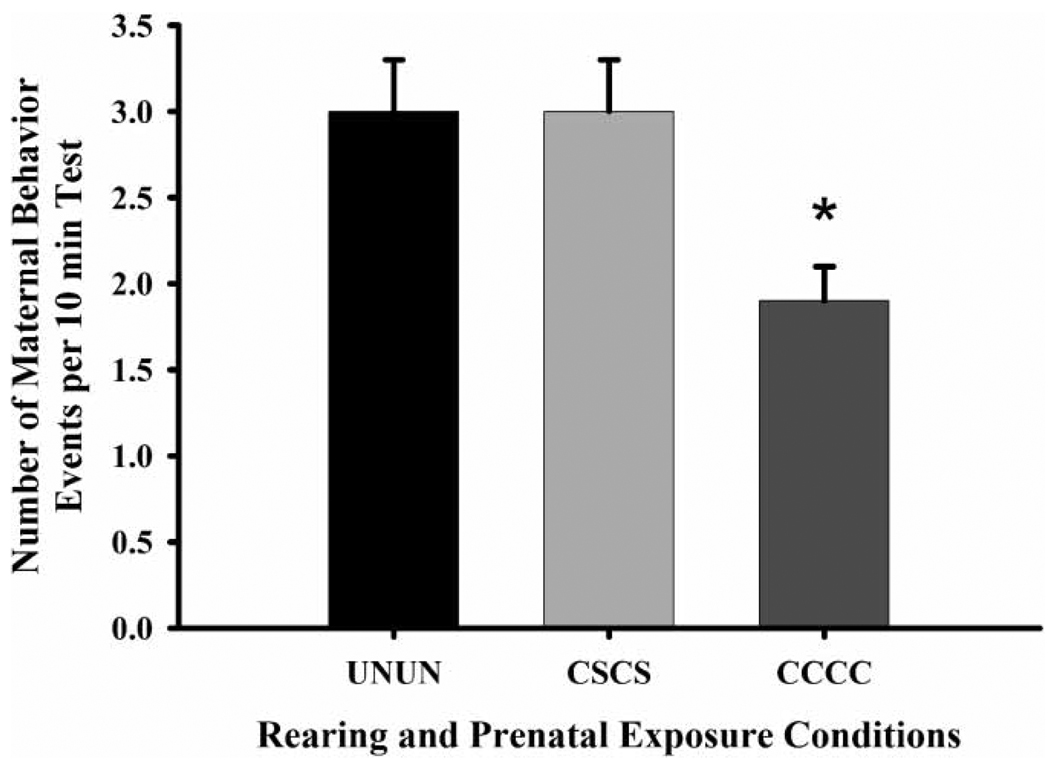
As indicated in Figure 9, FGDs prenatally exposed to chronic gestational cocaine and reared by their own CC-treated dams (CCCC) displayed fewer instances of maternal behavior than both UNUN FGDs and FGDs prenatally exposed to CS and reared by CS-treated dams (CSCS) (UNUN, , p ≤ 0.02; CSCS, , p ≤ 0.02). They also displayed a higher frequency of aggressive behaviors than CSCS FGDs (rough groom, , p ≤ 0.01; aggressive posture, , p ≤ 0.01).
Figure 9.
The frequency of maternal behaviors by first generation dam (FGD) rearing environment and prenatal exposure condition on PPD eight. Each bar represents least squares mean (LSM) and standard error (±SEM) for n = 23 first generation dams reared by an untreated dam with no prenatal exposure (UNUN), 11 reared by a chronic saline-treated dam and prenatally exposed to chronic saline (CSCS), and 10 reared by a chronic cocaine-treated dam and prenatally exposed to chronic cocaine (CCCC). As indicated by the asterisk, results indicate a significantly reduced frequency of maternal behaviors in CCCC FGDs compared to both CSCS (p ≤ 0.05) and UNUN (p ≤ 0.01) FGDs.
ICIC FGDs threatened intruders more (, p ≤ 0.01) than ISIS controls and pinned intruders in an aggressive posture more often, but were less likely to bite them compared to UNUN controls (aggressive posture, , p ≤ 0.05; nip/bites, frequency, , p ≤ 0.01; duration, , p ≤ 0.05).
In a comparison of chronic and IC-reared and cocaine-exposed FGDs, the ICIC groups were more defensive towards intruders (threat frequency, , p ≤ 0.02) and less aggressive (frequency of nip/bite) than CCCC (, p ≤ 0.01) FGDs.
Oxytocin contents
First generation dams
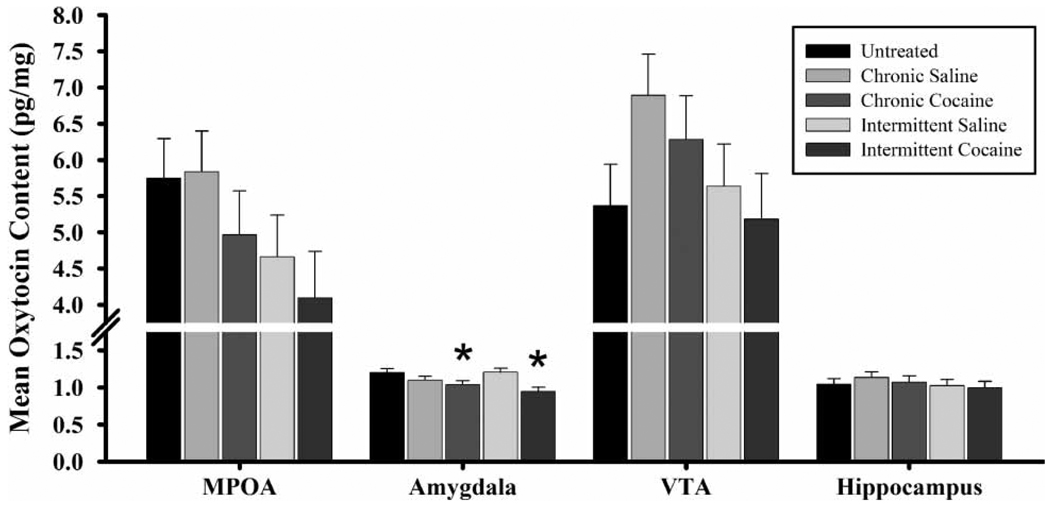
There was a significant effect of rearing condition alone on oxytocin contents in the amygdala [F(281, 8) = 2.19, p ≤ 0.03] on PPD nine. FGDs reared by either CC-treated (p ≤ 0.03) or IC-treated (p ≤ 0.01) dams, regardless of their prenatal exposure condition, had lower levels of oxytocin in the whole amygdala than FGDs reared by untreated or IS-treated dams (Figure 10). ICIC dams also had significantly less oxytocin in the amygdala than did non-exposed FGDs reared by their own non-treated dams (UNUN, p ≤ 0.02).
Figure 10.
Oxytocin content (pg/mg) in the medial preoptic area (MPOA), amygdala, ventral tegmental area (VTA), and hippocampus on PPD nine of first generation dams. Each bar represents least squares mean (LSM) and standard error (±SEM) for n = 64 first generation dams (FGD) reared by untreated dams, 53 FGD reared by chronic saline-treated dams, 51 FGD reared by chronic cocaine-treated dams, 62 FDG reared by intermittent saline-treated dams, and 58 FDG reared by intermittent cocaine-treated dams. Asterisks indicate significantly lower levels of oxytocin in the amygdala in FDGs reared by either chronic or intermittent cocaine-treated dams compared to controls (p ≤ 0.05).
Discussion
We did not find that the CC-treated dams exhibited higher levels of maternal aggression towards intruders on PPD eight as we had hypothesized, but we did see that the intermittently treated dams were less aggressive than control treatment dams, as we had predicted. Historically, we have seen significant increases in MA relative to control dams in gestationally treated dams rearing surrogate pups on PPD six (Johns et al. 1994b, 1996, 1997a, 1998); however, these effects apparently diminish by PPDs eight through 12 at the dose regimen we used. Without testing throughout the entire postpartum period, we cannot say whether the early increased levels of aggression are a truly transient effect that does not return following PPD six, although others have reported increases in aggression following higher cocaine doses through PPD 10 (Heyser et al. 1992). We have also seen increases in aggression this late in the postpartum period in preliminary tests.
Additionally, we have reported preliminary data (Joyner et al. 2003) regarding water deprived dams who received this same drug treatment, and who reared surrogate pups. These dams were more dominant and aggressive than controls ten days post weaning in a task requiring gaining access to a waterspout, suggesting long-term effects of gestational cocaine on aggressive behavior. We found that many behaviors, aggressive and otherwise, occurred at lower rates and for a shorter duration in the present study compared to our previous studies of maternal aggression. Whether this is an artifact of the complex design, fostering and long-term nature of the study is unclear, but this possibility should be considered.
As we reported previously (Johns et al. 2005), CC treatment disrupts pup-directed maternal behavior during the postpartum period; most strongly in the early period, with diminishing effects over the later postpartum period. During aggression testing, we rarely see high rates of pup-directed maternal behavior as the dam is generally more focused on the intruder than in performing these behaviors. We did not see group differences on this measure in the present study in the chronically treated or UN groups of original dams on PPD eight or 12. The frequency and duration of the behaviors were similar on both test days with slightly lower overall maternal aggression levels on PPD 12 being consistent with prior findings showing that maternal aggression diminishes after PPD 10 (Flannelly and Flannelly 1987; Mayer et al. 1987). It is important to note that these findings must be interpreted with caution, because of the difference in drug environment between PPD eight (injection of cocaine prior to testing) and 12 (no drug prior to test).
We had predicted that we would see lower rates of maternal aggression in intermittently treated dams, or even a decrease in aggression on PPD eight, since they were injected prior to testing and thus would still be experiencing the systemic effects of cocaine. Previous reports suggested that acute cocaine treatment prior to aggression testing dose-dependently decreases maternal aggression (Vernotica et al. 1996b; Johns et al. 1998b). Thus, if the gestational effects of an intermittent treatment were not significantly stronger than an acute systemic effect we would expect a decrease in aggression on that day. The acute effects of the intermittent treatment appear to be the stronger factor in the overall behavioral effects, as the majority of significant differences seen in the original dams on PPD eight were specific to the IC-treated dams (IC group) and indicated a reduction in aggression towards the intruder similar to that seen in acutely treated dams. Cocaine-induced hyperactivity likely plays a role in the reduction of aggressive and maternal behaviors in intermittently treated dams, whereas increased activity does not appear to be significant in CC-induced hyper-aggressiveness or decreased maternal behavior (Johns et al. 1994b; Kinsley et al. 1994; Vernotica et al. 1996a). We did not, however, see increased defensiveness in the intermittently treated dams nor have we seen this effect in acutely treated dams, but it has been reported as an effect in non-lactating rats acutely exposed to cocaine (Blanchard and Blanchard 1999). Dam treatment, rather than the prenatal exposure history of offspring, was the primary factor affecting maternal aggression in the present study, although significant effects of the prenatal exposure condition of the litter have been reported for pup-directed maternal behavior (Johns et al. 2005). Specific to this report, ICIC displayed less pup-directed maternal behavior on PPD eight, but it is unclear if this is an interaction of the combined dam treatment and litter exposure conditions or because the dams were more active following the injection, since this effect was not present by PPD 12. The distinctiveness of the IC treatment paradigm with characteristics of both chronic and acute cocaine treatment justifies further pharmacological and behavioral examination.
Regardless of their prenatal exposure condition, FGDs reared by either chronic or IC-treated dams displayed increased MA, the type and degree dependent on their rearing dam’s cocaine regimen. Both CC-and IC-reared FGDs were more likely to pin intruders and have direct contact with the male, but the IC-reared dams performed a wider array of aggressive behaviors, including fight attack, and were also more defensive in that they threatened intruders more often; all behaviors opposite to those seen in their rearing dams. One reason for their higher aggression rates may be related to the fact that they were also significantly more active (as were their rearing dams) than control FGDs whereas the CC-reared FGDs were not. The IC-reared FGDs would have been exposed to cocaine intermittently during the postpartum period through the dam’s milk and thus alterations in their behavior could be a result of their intermittent exposure to cocaine at these times. IC- and CC-treated original dams had less than optimal maternal behavior during the postpartum period, which could also have influenced behavior of the FGDs (Johns et al. 2005).These findings reflect the strong link between rearing environment and behavior as suggested in numerous published reports of the non-genomic transmission of behavior (Francis et al. 1999; Pedersen and Boccia 2000; Champagne and Meaney 2001; Meaney 2001; Fish et al. 2004; Johns et al. 2005).
Interestingly, both groups of cocaine-reared FGDs were less likely to nip/bite the male than were controls. The differentiation of the type and degree of aggressive behaviors needs to be studied further as it may provide clues to the mechanisms through which cocaine may be acting.
FGDs that were prenatally exposed to cocaine, particularly IC, exhibited increases in several aspects of maternal aggression, regardless of their rearing environment (rearing dam’s treatment). Prenatal exposure to cocaine has been demonstrated to alter stress responsivity, general play behaviors (Wood et al. 1994, 1995), ability to elicit play solicitations from an UN conspecific (Johns and Noonan 1995; Overstreet et al. 2000), maternal behavior onset and pup-induced maternal behavior (Johns et al. 2005; Johns et al. 2007), and social/aggressive behavior at older ages (Johns et al. 1994a; Overstreet et al. 2000). Given the history of effects following prenatal cocaine exposure in rats, we expected to see more effects of prenatal exposure to cocaine in the offspring. Although we did find an increase in aggressive posturing in both IC- and CC-exposed FGDs, and in the case of CC-exposed dams, less maternal behavior and more threats, only the CC-exposure condition produced levels of aggression higher than the effect of rearing by a cocainetreated dam. The more aggressive behavior of CC-exposed FGDs is in agreement with other reports of increased non-maternal aggression (e.g. social/resident intruder aggression) in male offspring (Johns et al. 1994a; Johns and Noonan 1995; Wood and Spear 1998) and is thus important as it establishes this effect in both male and female offspring. That this was robust enough to be seen regardless of the drug condition of their rearing dam is another important aspect of the prenatal exposure effects. The IC-exposed FGDs, however, were less aggressive, more maternal, and less active than were IC-reared FGDs compared to controls. This may be related to the cocaine that IC-reared FGDs would have received in the milk of their intermittently treated rearing dams during the postpartum period, when differential periods of brain development may be affected. One might predict that given the array of aggression changes following rearing by IC-treated dams that FGDs exposed to CC and reared by IC-treated dams (ICCC) might display the highest aggression levels and we are examining this group further. The CCCC FGDs were more aggressive and less maternal compared to controls than ICIC FGDs on several measures (nip/bite, rough groom, aggressive posture), whereas the ICIC group was more defensive than controls alone. These findings point out the important interaction differences that may occur between rearing and prenatal exposure conditions and the need to closely examine mechanistic differences between these conditions.
Clearly, the effects of both chronic and IS treatment alone were quite salient in this study compared to UN controls on PPD eight. These two control groups (saline and UN) are often employed in cocaine-related studies, and there is usually a small non-significant difference in behavior found between the two control groups, however, in the present study these effects were more evident. Particularly, in FGDs, there were several instances of behaviors in both the CS- and IS-reared (rough groom, aggressive posture) or exposed (threat, nip/bite, aggressive posture) groups that were performed significantly less frequently than in UN reared or non-exposed FGDs. It may be that the differences between the saline and UN groups are related to stress-related differences, in both generations of dams. There is a need for further research on the effects of stress alone on maternal aggression.
We do not report brain oxytocin changes in the original dams here, since they were not killed following MA testing, but we have reported previously that cocaine-induced increases in maternal aggression are correlated with oxytocin system changes in the amygdala on PPD six (Johns et al. 1998c). The difference in MA levels seen in this study compared to previous findings could reflect a declining influence of oxytocin at the later time point studied here. Interestingly, FGDs reared by cocaine-treated dams had increased levels of aggressive posture and rough groom on PPD eight and lower levels of oxytocin in the amygdala on PPD nine. Such an association between lower levels of oxytocin in the amygdala and higher levels of maternal aggression has been seen previously in cocaine-treated dams (Johns et al. 1995). It is unclear whether specific types of MAs are particularly associated with oxytocin changes, or whether dysregulation of oxytocin systems is associated with offspring rearing condition in a more general manner. As in a previous paper on intergenerational effects of cocaine on maternal behavior (Johns et al. 2005), it appears that rearing environment is a strong determinant of oxytocin system changes in offspring. Oxytocin may be a candidate for the intergenerational transmission of MA, just as it has been demonstrated as an intergenerational mechanism for maternal behaviors and stress responsivity (Pedersen and Boccia 2002).
There was also increased aggression in FGDs prenatally exposed to cocaine but with no significant chnages in oxytocin levels in the amygdala Cocaine-treated dams and offspring prenatally exposed to cocaine have demonstrated significant alterations in stress reactivity (Bilitzke and Church 1992; Kehoe and Boylan 1992; Molina et al. 1994; Wood et al. 1995; Barron et al. 2000; Goeders 2002), which could indicate a role for stress related increases in MA in the cocaine exposed FGDs, rather than a direct oxytocin effect.
Several reports suggest that altered oxytocin activity could affect MA through stress-response systems (Ferris et al. 1992; Lubin et al. 2003; Bosch et al. 2005; Ragnauth et al. 2005). Studies linking rearing to oxytocin system and stress response development (Francis et al. 1999; Pedersen and Boccia 2000; Champagne et al. 2001; Meaney 2001) may be particularly relevant to our findings in the FGDs. However, increased oxytocin release in the paraventricular nucleus and central amygdala, rather than oxytocin receptor modulation or overall levels, were associated with increased offensive behavior (Bosch et al. 2005). In the present study, oxytocin content in the amygdala was measured; it is possible that the reduced content we found to be associated with increased MA reflects increased oxytocin release; this requires further study.
Regarding the limitations of the study, cross-fostering alone has been shown to result in many behavioral alterations (Francis et al. 1999), but these effects were offset by ensuring that all dams received litters from another dam generally within an hour of final pup delivery. The ability to isolate the two potential individual factors of prenatal exposure and rearing environment in relation to MA behavior in both dams and their offspring was a strength of this study. These results indicate the importance of examining the separate contributions and the interaction of both mother and offspring in response to drug treatment. The role of oxytocin as a possible mechanism of these effects has yet to be fully revealed.
Acknowledgements
This work was supported by NIH grants DA13362, DA13283, and the NIH Federal Child Neglect Consortium. We would like to acknowledge the assistance of Dr Linda P. Spear as a consultant for this project. We would also like to acknowledge all the Premed, Biology, and Psychology undergraduate and honors students from UNC and surrounding universities, whose cumulative efforts helped make the completion of this project possible.
References
- Amico JA, Mantella RC, Vollmer RR, Li X. Anxiety and stress responses in female oxytocin deficient mice. J Neuroendocrinol. 2004;16(4):319–324. doi: 10.1111/j.0953-8194.2004.01161.x. [DOI] [PubMed] [Google Scholar]
- Barron S, Segar TM, Yahr JS, Baseheart BJ, Willford JA. The effects of neonatal ethanol and/or cocaine exposure on isolation-induced ultrasonic vocalizations. Pharmacol Biochem Behav. 2000;67(1):1–9. doi: 10.1016/s0091-3057(00)00304-x. [DOI] [PubMed] [Google Scholar]
- Benjamini Y, Drai D, Elmer G, Kafkafi N, Golani I. Controlling the false discovery rate in behavior genetics research. Behav Brain Res. 2001;125(1/2):279–284. doi: 10.1016/s0166-4328(01)00297-2. [DOI] [PubMed] [Google Scholar]
- Bilitzke PJ, Church MW. Prenatal cocaine and alcohol exposures affect rat behavior in a stress test (the Porsolt swim test) Neurotoxicol Teratol. 1992;14(5):359–364. doi: 10.1016/0892-0362(92)90043-a. [DOI] [PubMed] [Google Scholar]
- Blanchard DC, Blanchard RJ. Cocaine potentiates defensive behaviors related to fear and anxiety. Neurosci Biobehav Rev. 1999;23(7):981–991. doi: 10.1016/s0149-7634(99)00031-7. [DOI] [PubMed] [Google Scholar]
- Bosch OJ, Meddle SL, Beiderbeck DI, Douglas AJ, Neumann ID. Brain oxytocin correlates with maternal aggression: Link to anxiety. J Neurosci. 2005;25(29):6807–6815. doi: 10.1523/JNEUROSCI.1342-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callahan MF, Kirby RF, Cunningham JT, Eskridge-Sloop SL, Johnson AK, Mccarty R, Gruber KA. Central oxytocin systems may mediate a cardiovascular response to acute stress in rats. Am J Physiol. 1989;256(5 Pt 2):H1369–H1377. doi: 10.1152/ajpheart.1989.256.5.H1369. [DOI] [PubMed] [Google Scholar]
- Champagne F, Meaney MJ. Like mother, like daughter: Evidence for non-genomic transmission of parental behavior and stress responsivity. Prog Brain Res. 2001;133:287–302. doi: 10.1016/s0079-6123(01)33022-4. [DOI] [PubMed] [Google Scholar]
- Champagne F, Diorio J, Sharma S, Meaney MJ. Naturally occurring variations in maternal behavior in the rat are associated with differences in estrogen-inducible central oxytocin receptors. Proc Natl Acad Sci USA. 2001;98(22):12736–12741. doi: 10.1073/pnas.221224598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Consiglio AR, Lucion AB. Lesion of hypothalamic paraventricular nucleus and maternal aggressive behavior in female rats. Physiol Behav. 1996;59(4/5):591–596. doi: 10.1016/0031-9384(95)02117-5. [DOI] [PubMed] [Google Scholar]
- Consiglio AR, Borsoi A, Pereira GA, Lucion AB. Effects of oxytocin microinjected into the central amygdaloid nucleus and bed nucleus of stria terminalis on maternal aggressive behavior in rats. Physiol Behav. 2005;85(3):354–362. doi: 10.1016/j.physbeh.2005.05.002. [DOI] [PubMed] [Google Scholar]
- Elliott JC, Lubin DA, Walker CH, Johns JM. Acute cocaine alters oxytocin levels in the medial preoptic area and amygdala in lactating rat dams: Implications for cocaine-induced changes in maternal behavior and maternal aggression. Neuropeptides. 2001;35(2):127–134. doi: 10.1054/npep.2001.0854. [DOI] [PubMed] [Google Scholar]
- Ferris CF, Foote KB, Meltser HM, Plenby MG, Smith KL, Insel TR. Oxytocin in the amygdala facilitates maternal aggression. In: Pedersen CA, Caldwell JD, Jirikowski GF, Insel TR, editors. Oxytocin in maternal, sexual, and social behaviors. New York: The New York Academy of Sciences; 1992. pp. 456–457. [DOI] [PubMed] [Google Scholar]
- Fish EW, Shahrokh D, Bagot R, Caldji C, Bredy T, Szyf M, Meaney MJ. Epigenetic programming of stress responses through variations in maternal care. Ann N Y Acad Sci. 2004;1036:167–180. doi: 10.1196/annals.1330.011. [DOI] [PubMed] [Google Scholar]
- Flannelly KJ, Flannelly L. Time course of postpartum aggression in rats (Rattus Norvegicus) J Comp Psychol. 1987;101(1):101–103. [Google Scholar]
- Francis D, Diorio J, Liu D, Meaney MJ. Nongenomic transmission across generations of maternal behavior and stress responses in the rat. Science. 1999;286(5442):1155–1158. doi: 10.1126/science.286.5442.1155. [DOI] [PubMed] [Google Scholar]
- Francis DD, Champagne FC, Meaney MJ. Variations in maternal behaviour are associated with differences in oxytocin receptor levels in the rat. J Neuroendocrinol. 2000;12(12):1145–1148. doi: 10.1046/j.1365-2826.2000.00599.x. [DOI] [PubMed] [Google Scholar]
- Francis DD, Young LJ, Meaney MJ, Insel TR. Naturally occurring differences in maternal care are associated with the expression of oxytocin and vasopressin (V1a) receptors: Gender differences. J Neuroendocrinol. 2002;14(5):349–353. doi: 10.1046/j.0007-1331.2002.00776.x. [DOI] [PubMed] [Google Scholar]
- Gammie SC. Current models and future directions for understanding the neural circuitries of maternal behaviors in rodents. Behav Cogn Neurosci Rev. 2005;4(2):119–135. doi: 10.1177/1534582305281086. [DOI] [PubMed] [Google Scholar]
- Giovenardi M, Padoin MJ, Cadore LP, Lucion AB. Hypothalamic paraventricular nucleus, oxytocin, and maternal aggression in rats. Ann N Y Acad Sci. 1997;807:606–609. doi: 10.1111/j.1749-6632.1997.tb51981.x. [DOI] [PubMed] [Google Scholar]
- Giovenardi M, Padoin MJ, Cadore LP, Lucion AB. Hypothalamic paraventricular nucleus modulates maternal aggression in rats: Effects of ibotenic acid lesion and oxytocin antisense. Physiol Behav. 1998;63(3):351–359. doi: 10.1016/s0031-9384(97)00434-4. [DOI] [PubMed] [Google Scholar]
- Goeders NE. Stress and cocaine addiction. J Pharmacol Exp Ther. 2002;301(3):785–789. doi: 10.1124/jpet.301.3.785. [DOI] [PubMed] [Google Scholar]
- Heyser CJ, Molina VA, Spear LP. A fostering study of the effects of prenatal cocaine exposure: I. Maternal behaviors. Neurotoxicol Teratol. 1992;14(6):415–421. doi: 10.1016/0892-0362(92)90052-c. [DOI] [PubMed] [Google Scholar]
- Jarrett TM, McMurray MS, Walker CH, Johns JM. Cocaine treatment alters oxytocin receptor binding but not mRNA production in postpartum rat dams. Neuropeptides. 2006;40:161–167. doi: 10.1016/j.npep.2006.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jezova D, Skultetyova I, Tokarev DI, Bakos P, Vigas M. Vasopressin and oxytocin in stress. Ann N Y Acad Sci. 1995;771:192–203. doi: 10.1111/j.1749-6632.1995.tb44681.x. [DOI] [PubMed] [Google Scholar]
- Johns JM, Means MJ, Anderson DR, Bass EW, Means LW, Mcmillen BA. Prenatal exposure to cocaine: II. Effects on open field activity and cognitive behavior in sprague-dawley rats. Neurotoxicol Teratol. 1992a;14:343–349. doi: 10.1016/0892-0362(92)90041-8. [DOI] [PubMed] [Google Scholar]
- Johns JM, Means MJ, Means LW, Mcmillen BA. Prenatal exposure to cocaine: I. Effects on gestation, development and activity in sprague-dawley rats. Neurotoxicol Teratol. 1992b;14:337–342. doi: 10.1016/0892-0362(92)90040-h. [DOI] [PubMed] [Google Scholar]
- Johns JM, Means MJ, Bass EW, Means LW, Zimmerman LI, Mcmillen BA. Prenatal exposure to cocaine: Effects on aggression in sprague-dawley rats. Dev Psychobiol. 1994a;27(4):227–239. doi: 10.1002/dev.420270405. [DOI] [PubMed] [Google Scholar]
- Johns JM, Noonan LR, Zimmerman LI, Li L, Pedersen CA. Effects of chronic and acute cocaine treatment on the onset of maternal behavior and aggression in sprague-dawley rats. Behav Neurosci. 1994b;108(1):107–112. doi: 10.1037//0735-7044.108.1.107. [DOI] [PubMed] [Google Scholar]
- Johns JM, Noonan LR. Prenatal cocaine exposure affects social behavior in sprague-dawley rats. Neurotoxicol Teratol. 1995;17(5):569–576. doi: 10.1016/0892-0362(95)00017-l. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johns JM, Faggin BM, Noonan LR, Li L, Zimmerman LI, Pedersen CA. Proceed Soc Neurosci Abstracts. 21. San Diego, CA: 1995. Nov 11–16, Chronic cocaine treatment decreases oxytocin levels in the amygdala and increases maternal aggression in sprague-dawley rats. 766.7. [Google Scholar]
- Johns JM, Lubin DA, Walker CH, Meter KE, Mason GA. Chronic gestational cocaine treatment decreases oxytocin levels in the medial preoptic area, ventral tegmental area and hippocampus in sprague-dawley rats. Neuropeptides. 1997a;31(5):439–443. doi: 10.1016/s0143-4179(97)90037-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johns JM, Noonan LR, Zimmerman LI, Li L, Pedersen CA. Effects of short- and long-term withdrawal from gestational cocaine treatment on maternal behavior and aggression in sprague-dawley rats. Dev Neurosci. 1997b;19(4):368–374. doi: 10.1159/000111234. [DOI] [PubMed] [Google Scholar]
- Johns JM, Noonan LR, Miles S, Zimmerman LI, Pedersen CA, Faggin BM. Proceed Inter Soc Behav Neurosci. 5. Cancun, Mexico: 1996. May 2–5, A comparison of the effects of cocaine and amfonelic acid treatment on the onset of maternal behavior. P1–5. [Google Scholar]
- Johns JM, Nelson CJ, Meter KE, Lubin DA, Couch CD, Ayers A, Walker CH. Dose-dependent effects of multiple acute cocaine injections on maternal behavior and aggression in sprague-dawley rats. Dev Neurosci. 1998b;20(6):525–532. doi: 10.1159/000017353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johns JM, Noonan LR, Zimmerman LI, Mcmillen BA, Means LW, Walker CH, Lubin DA, Meter KE, Nelson CJ, Pedersen CA, Mason GA, Lauder JM. Chronic cocaine treatment alters social/aggressive behavior in sprague-dawley rat dams and in their prenatally exposed offspring. Ann N Y Acad Sci. 1998c;846:399–404. [PMC free article] [PubMed] [Google Scholar]
- Johns JM, Lubin DA, Walker CH, Joyner P, Middleton C, Hofler V, McMurray MS. Gestational treatment with cocaine and fluoxetine alters oxytocin receptor number and binding affinity in lactating rat dams. Int J Dev Neurosci. 2004;22(5/6):321–328. doi: 10.1016/j.ijdevneu.2004.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johns JM, Elliott DL, Hofler VE, Joyner PW, McMurray MS, Jarrett TM, Haslup AM, Middleton CL, Elliott JC, Walker CH. Cocaine treatment and prenatal environment interact to disrupt intergenerational maternal behavior in rats. Behav Neurosci. 2005;119(6):1605–1618. doi: 10.1037/0735-7044.119.6.1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johns JM, McMurray MS, Hofler VE, Jarrett TM, Middleton CL, Elliott DL, Mirza R, Haslup A, Elliott JC, Walker CH. Cocaine disrupts pup-induced maternal behavior in juvenile and adult rats. Neurotoxicol Teratol. 2007;29:634–641. doi: 10.1016/j.ntt.2007.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joyner PW, Johns JM, Black MC, Elliott DA, Middleton C, Hofler V, Greenhill KW, Elliott JC. The effects of gestational cocaine treatment on water competition in post-weaning dams. Abstr Soc Neurosci. 2003 [Google Scholar]
- Kehoe P, Boylan CB. Cocaine-induced effects on isolation stress in neonatal rats. Behav Neurosci. 1992;106(2):374–379. doi: 10.1037//0735-7044.106.2.374. [DOI] [PubMed] [Google Scholar]
- Kinsley CH, Turco D, Bauer A, Beverly M, Wellman J, Graham AL. Cocaine alters the onset and maintenance of maternal behavior in lactating rats. Pharmacol Biochem Behav. 1994;47(4):857–864. doi: 10.1016/0091-3057(94)90288-7. [DOI] [PubMed] [Google Scholar]
- Konig JFR, Klippel RA. The rat brain: A stereotaxic atlas of the forebrain and lower parts of the brain stem. New York: Krieger; 1963. [Google Scholar]
- Lonstein JS, Gammie SC. Sensory, hormonal, and neural control of maternal aggression in laboratory rodents. Neurosci Biobehav Rev. 2002;26(8):869–888. doi: 10.1016/s0149-7634(02)00087-8. [DOI] [PubMed] [Google Scholar]
- Lubin DA, Meter KE, Walker CH, Johns JM. Dose-related effects of chronic gestational cocaine treatment on maternal aggression in rats on postpartum days 2 3, and 5. Prog neuropsychopharmacol and biol psychol. 2001;25(7):1403–1420. doi: 10.1016/s0278-5846(01)00197-x. [DOI] [PubMed] [Google Scholar]
- Lubin DA, Elliott JC, Black MC, Johns JM. An oxytocin antagonist infused into the central nucleus of the amygdala increases maternal aggressive behavior. Behav Neurosci. 2003;117(2):195–201. doi: 10.1037/0735-7044.117.2.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludwig M. Dendritic release of vasopressin and oxytocin. J Neuroendocrinol. 1998;10(12):881–895. doi: 10.1046/j.1365-2826.1998.00279.x. [DOI] [PubMed] [Google Scholar]
- Mayer AD, Reisbick S, Siegel HI, Rosenblatt JS. Maternal aggression in rats: Changes over pregnancy and lactation in a sprague-dawley strain. Aggress Behav. 1987;13:29–43. [Google Scholar]
- Mayer AD, Rosenblatt JS. A method for regulating the duration of pregnancy and the time of parturition in sprague-dawley rats (Charles River CD strain) Dev Psychobiol. 1998;32(2):131–136. [PubMed] [Google Scholar]
- Meaney MJ. Maternal care, gene expression, and the transmission of individual differences in stress reactivity across generations. Annu Rev Neurosci. 2001;24:1161–1192. doi: 10.1146/annurev.neuro.24.1.1161. [DOI] [PubMed] [Google Scholar]
- Molina VA, Wagner JM, Spear LP. The behavioral response to stress is altered in adult rats exposed prenatally to cocaine. Physiol Behav. 1994;55(5):941–945. doi: 10.1016/0031-9384(94)90083-3. [DOI] [PubMed] [Google Scholar]
- Moss HB, Tarter RE. Substance abuse, aggression, and violence: What are the connections? Am J Addict. 1993;2(2):149–160. [Google Scholar]
- Neumann ID. Involvement of the brain oxytocin system in stress coping: Interactions with the hypothalamo-pituitaryadrenal axis. Prog Brain Res. 2002;139:147–162. doi: 10.1016/s0079-6123(02)39014-9. [DOI] [PubMed] [Google Scholar]
- Neumann ID, Toschi N, Ohl F, Torner L, Kromer SA. Maternal defence as an emotional stressor in female rats: Correlation of neuroendocrine and behavioural parameters and involvement of brain oxytocin. Eur J Neurosci. 2001;13(5):1016–1024. doi: 10.1046/j.0953-816x.2001.01460.x. [DOI] [PubMed] [Google Scholar]
- Nishioka T, Anselmo-Franci JA, Li P, Callahan MF, Morris M. Stress increases oxytocin release within the hypothalamic paraventricular nucleus. Brain Res. 1998;781(1/2):56–60. doi: 10.1016/s0006-8993(97)01159-1. [DOI] [PubMed] [Google Scholar]
- Numan M. Maternal behavior. In: Knobil E, Neill JD, editors. The physiology of reproduction. New York: Raven Press; 1994. pp. 221–301. [Google Scholar]
- Overstreet DH, Moy SS, Lubin DA, Gause LR, Lieberman JA, Johns JM. Enduring effects of prenatal cocaine administration on emotional behavior in rats. Physiol Behav. 2000;70(1/2):149–156. doi: 10.1016/s0031-9384(00)00245-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen CA, Boccia ML. Oxytocin transduces maternal care received in infancy into maternal care exhibited in adulthood. Abstr Soc Neurosci. 2000 [Google Scholar]
- Pedersen CA, Boccia ML. Oxytocin links mothering received, mothering bestowed and adult stress responses. Stress. 2002;5(4):259–267. doi: 10.1080/1025389021000037586. [DOI] [PubMed] [Google Scholar]
- Ragnauth AK, Devidze N, Moy V, Finley K, Goodwillie A, Kow LM, Muglia LJ, Pfaff DW. Female oxytocin gene-knockout mice, in a semi-natural environment, display exaggerated aggressive behavior. Genes Brain Behav. 2005;4(4):229–239. doi: 10.1111/j.1601-183X.2005.00118.x. [DOI] [PubMed] [Google Scholar]
- Uvnas-Moberg K. Oxytocin linked antistress effects—the relaxation and growth response. Acta Physiol Scand Suppl. 1997;640:38–42. [PubMed] [Google Scholar]
- Uvnas-Moberg K. Antistress pattern induced by oxytocin. News Physiol Sci. 1998;13:22–25. doi: 10.1152/physiologyonline.1998.13.1.22. [DOI] [PubMed] [Google Scholar]
- Uvnas-Moberg K, Ahlenius S, Hillegaart V, Alster P. High doses of oxytocin cause sedation and low doses cause an anxiolytic-like effect in male rats. Pharmacol Biochem Behav. 1994;49(1):101–106. doi: 10.1016/0091-3057(94)90462-6. [DOI] [PubMed] [Google Scholar]
- Vernotica EM, Lisciotto CA, Rosenblatt JS, Morrell JI. Cocaine transiently impairs maternal behavior in the rat. Behav Neurosci. 1996a;110(2):315–323. doi: 10.1037//0735-7044.110.2.315. [DOI] [PubMed] [Google Scholar]
- Vernotica EM, Rosenblatt JS, Morrell JI. Acute cocaine alters all components of established postpartum maternal behavior in the rat. Abstr Soc Neurosci. 1996b:738. [Google Scholar]
- Wood RD, Bannoura MD, Johanson IB. Prenatal cocaine exposure: Effects on play behavior in the juvenile rat. Neurotoxicol Teratol. 1994;16(2):139–144. doi: 10.1016/0892-0362(94)90110-4. [DOI] [PubMed] [Google Scholar]
- Wood RD, Molina VA, Wagner JM, Spear LP. Play behavior and stress responsivity in periadolescent offspring exposed prenatally to cocaine. Pharmacol Biochem Behav. 1995;52(2):367–374. doi: 10.1016/0091-3057(95)00120-l. [DOI] [PubMed] [Google Scholar]
- Wood RD, Spear LP. Prenatal cocaine alters social competition of infant, adolescent, and adult rats. Behav Neurosci. 1998;112(2):419–431. doi: 10.1037//0735-7044.112.2.419. [DOI] [PubMed] [Google Scholar]