Abstract
Objective
To determine if a regional quality improvement effort can increase beta-blocker utilization prior to vascular surgery and decrease the incidence of post-op myocardial infarction (POMI).
Methods
A quality improvement effort to increase peri-operative beta blocker utilization was implemented in 2003 at centers participating in the Vascular Study Group of New England (VSGNE). A 90% target was set and feedback given at bi-annual meetings. Beta blocker utilization (< 1-mo pre-op versus chronic) and POMI rates were prospectively collected for patients undergoing open AAA repair (n=926) and lower extremity bypass (LEB) (n=2,123) from 2003 through 2008. Predictors of POMI were determined using multivariate logistic regression. Rates of beta blocker administration and POMI were analyzed over time, and across strata of patient risk based on a multivariate model.
Results
Peri-operative beta blocker treatment increased from 68% of patients in the first three months of 2005 to 88% by the last 3 months of 2008 (p<0.001). In 2003, 44% of patients not on chronic beta blockers were treated with pre-operative beta blockers; by 2008, 78% of patients not on chronic beta blockers were started peri-operatively on these medications (p<0.001). Beta blocker utilization increased across all centers and surgeons participating during the study period, and increased in patients of low, medium, and high cardiac risk. However, the rate of POMI did not change over time (5.2% in 2003, 5.5% in 2008, p=0.876), although a trend towards lower POMI rate was seen in patients on pre-operative beta blockers (4.4% in 2003-2005, 2.6% in 2006-2008, p=0.43). In multivariable modeling we found that age >70 (OR 2.1), positive stress test (OR 2.2), CHF (OR 1.7), chronic beta blocker administration (OR 1.7), resting heart rate (HR) < 70 (OR 1.8) and diabetes (OR 1.6) were associated with POMI. Resting HR was similar for patients on chronic (67), pre-operative (70), and no beta blockers (70) (p=0.521).
Conclusions
Our regional quality improvement effort successfully increased peri-operative beta blocker utilization. However, this was not associated with reduced rates of POMI or resting heart rate. While this demonstrates the effectiveness of regional quality improvement efforts in changing practice patterns, further work is necessary to more precisely identify those patients who will benefit from beta blockade at the time of vascular surgery.
Introduction
The use of peri-operative beta blocker therapy in patients undergoing vascular surgery has been a topic of intense interest among patients, surgeons, payers, and policy makers1-4. Several randomized trials and large observational studies of beta blocker use have demonstrated significant reductions in post-operative cardiovascular morbidity and mortality, especially in patients with high risk of adverse cardiac events5-14. These benefits, with seemingly few adverse events in earlier studies8, 12, were sufficient to procure endorsement for the broad use of peri-operative beta blockade from several national quality improvement initiatives, including the Leapfrog Group15, the American Medical Association16, and the Surgical Care Improvement Project (SCIP)17, a collaborative of several organizations including the Centers for Medicare and Medicaid Services (CMS), the American Hospital Association (AHA), and the Institute for Healthcare Improvement (IHI). Further, peri-operative beta blocker therapy was reported as a class I or II recommendation of the American College of Cardiology Foundation/America Heart Association1 (ACCF/AHA) in 2007, and similarly endorsed by the European Society of Cardiology and the European Society of Anesthesiology in 201018.
Based on these recommendations, the Vascular Study Group of New England (VSGNE) began a quality improvement project in 2003 aimed at broadly implementing the evidence-based utilization of beta blockers in patients undergoing vascular surgery. We used evidence-based reviews of peri-operative beta-blockade studies, surgeon and hospital benchmarking, and discussions at our biannual meetings to encourage participating surgeons to achieve a target of 90% beta blocker administration in patients undergoing abdominal aortic aneurysm repair and lower extremity bypass. By increasing the administration of beta blockers, we hoped to reduce cardiac complications in patients undergoing high-risk vascular surgery in our region.
Methods
Subjects and Databases
We utilized data collected prospectively by the VSGNE, a regional cooperative quality improvement initiative developed by community and academic centers in 2002 to study regional outcomes in vascular surgery. Further details on this registry have been published previously19, and others are available at www.vsgne.org.
The goal of this quality improvement effort was to improve the administration of beta blockers peri-operatively. At the outset of our quality improvement initiative, we sought to increase beta blocker usage in all patients undergoing open vascular surgery, especially those procedures in which the risk of cardiac complications is highest - open abdominal aortic aneurysm repair (OAAA) and lower extremity bypass (LEB). In patients undergoing OAAA and LEB within the VSGNE, we collected over 70 pre-operative, intra-operative, and postoperative variables and entered this data in our regional dataset, as described in previous work20. These variables were used to describe patient characteristics, and to allow us to perform cardiac risk stratification21, evaluate operative details and outcomes, and assess operative risk. These variables, stratified by procedure type and use of beta blockade, are shown in Table 1.
Table 1.
Patient characteristics and beta blockade utilization (chronic, pre-operative, and none).
| n (%)* |
n (%)* |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Open Abdominal Aortic Aneurysm Repair (N=926) | Lower Extremity Bypass (N=2,123) | |||||||||
| Variable | Total (%) | Percent on Chronic Beta Blocker |
Percent on Peri- Operative Beta Blocker |
Percent Not on Beta Blocker |
p value† | Total (%) | Percent on Chronic Beta Blocker |
Percent on Peri- Operative Beta Blocker |
Percent Not on Beta Blocker |
p value† |
| Male Gender | 676 (73.00) | 384 (56.80) | 224 (33.14) | 68 (10.06) | 0.474 | 1479 (69.67) | 857 (57.98) | 405 (27.40) | 216 (14.61) | 0.008 |
| Non-White Race | 7 (0.76) | 4 (57.14) | 1 (14.29) | 2 (28.57) | 0.224 | 18 (0.85) | 10 (55.56) | 6 (33.33) | 2 (11.11) | 0.709 |
| Not Living Home Pre-Operatively | 1 (0.11) | 0 (0) | 0 (0) | 1 (100) | 0.013 | 77 (3.63) | 49 (63.64) | 17 (22.08) | 11 (14.29) | 0.614 |
| Not Independantly Ambulatory Pre-Operatively | NA | NA | NA | NA | NA | 533 (25.11) | 358 (67.17) | 108 (20.26) | 67 (12.57) | <0.001 |
| Age | ||||||||||
| Age<40 | 1 (0.11) | 1 (100) | 0 (0) | 0 (0) | 0.994 | 13 (0.61) | 2 (15.38) | 6 (46.15) | 5 (38.46) | <0.001 |
| Age 40-50 | 6 (0.65) | 4 (66.67) | 2 (33.33) | 0 (0) | 111 (5.23) | 49 (44.14) | 27 (24.32) | 35 (31.53) | ||
| Age 50-60 | 75 (8.10) | 42 (56.00) | 26 (34.67) | 7 (9.33) | 394 (18.56) | 214 (54.31) | 110 (27.92) | 70 (17.77) | ||
| Age 60-70 | 300 (32.40) | 179 (59.67) | 91 (30.33) | 30 (10.00) | 561 (26.42) | 328 (58.57) | 157 (28.04) | 75 (13.39) | ||
| Age 70-80 | 431 (46.54) | 245 (56.84) | 140 (32.48) | 46 (10.67) | 669 (31.51) | 421 (62.93) | 148 (22.12) | 100 (14.95) | ||
| Age 80-90 | 112 (12.10) | 63 (56.25) | 37 (33.04) | 12 (10.71) | 345 (16.25) | 206 (59.71) | 92 (26.67) | 47 (13.62) | ||
| Age 90-100 | 1 (0.11) | 1 (100) | 0 (0) | 0 (0) | 30 (1.41) | 15 (50.00) | 9 (30.00) | 6 (20.00) | ||
| Smoking (prior or current) | 849 (91.68) | 494 (58.19) | 272 (32.04) | 83 (9.78) | 0.217 | 1747 (82.29) | 1016 (58.19) | 456 (26.12) | 274 (15.69) | 0.801 |
| COPD | 345 (37.26) | 191 (55.36) | 121 (35.07) | 33 (9.57) | 0.290 | 645 (30.38) | 385 (59.78) | 160 (24.84) | 99 (15.37) | 0.621 |
| Diabetes | ||||||||||
| Nondiabetic | 786 (84.88) | 449 (57.12) | 260 (33.08) | 77 (9.80) | 0.449 | 999 (47.06) | 503 (50.35) | 289 (28.93) | 207 (20.72) | <0.001 |
| Non-Insulin Dependant Diabetics | 128 (13.82) | 79 (61.72) | 33 (25.78) | 16 (12.50) | 552 (26.00) | 351 (63.59) | 137 (24.82) | 64 (11.59) | ||
| Insulin Dependant Diabetics | 12 (1.30) | 7 (58.33) | 3 (25.00) | 2 (16.67) | 572 (26.94) | 381 (66.73) | 123 (21.54) | 67 (11.73) | ||
| Coronary disease | 326 (35.21) | 233 (71.47) | 71 (21.78) | 22 (6.75) | <0.001 | 868 (40.89) | 618 (71.20) | 175 (20.16) | 75 (8.64) | <0.001 |
| Congestive heart failure | 66 (7.13) | 50 (75.76) | 12 (18.18) | 4 (6.06) | 0.009 | 393 (18.51) | 282 (71.76) | 70 (17.81) | 41 (10.43) | <0.001 |
| Preoperative Medication Regimen | ||||||||||
| Anti-platelet Agent Use | 666 (71.92) | 410 (61.56) | 204 (30.63) | 52 (7.81) | <0.001 | 1529 (72.02) | 923 (60.37) | 406 (26.55) | 200 (13.08) | <0.001 |
| Preoperative Statin Use | 542 (58.53) | 358 (66.05) | 151 (27.86) | 33 (6.09) | <0.001 | 1235 (58.17) | 794 (64.34) | 291 (23.58) | 149 (12.07) | <0.001 |
| Preoperative Cardiac Stress Test | ||||||||||
| None performed | 260 (28.08) | 141 (54.23) | 83 (31.92) | 36 (13.85) | p<0.001 | 1321 (62.25) | 746 (56.52) | 338 (25.61) | 236 (17.88) | p<0.001 |
| Performed, normal | 489 (52.81) | 279 (57.06) | 164 (33.54) | 13(9.41) | 494 (23.28) | 273 (55.26) | 144 (29.15) | 77 (15.59) | ||
| Performed, abnormal | 168 (18.14) | 109 (64.88) | 46 (27.38) | 13 (7.74) | 296 (13.95) | 210 (70.95) | 64 (21.62) | 22 (7.43) | ||
| Operative Characteristics: Open Abominal Aortic Aneurysm Repair | ||||||||||
| Aneurysm Size <5.5 cm | 250 (27.00) | 137 (54.80) | 83 (33.20) | 30 (12.00) | 0.421 | NA | NA | NA | NA | NA |
| Aneurysm Size 5.5-6.5 cm | 405 (43.74) | 247 (60.99) | 123 (30.37) | 35 (8.64) | NA | NA | NA | NA | ||
| Aneurysm Size >6.5 cm | 256 (27.65) | 142 (55.47) | 87 (33.98) | 27 (10.55) | NA | NA | NA | NA | ||
| Need for Infrarenal Clamp | 693 (74.8%) | 394(56.9%) | 225(32.5%) | 74 (10.4%) | 0.032 | NA | NA | NA | NA | |
| Need for Suprarenal Clamp | 232 (25.2%) | 140 (60.3) | 71(30.6) | 21(9.1%) | NA | NA | NA | NA | ||
| Operative Characteristics: Lower Extremity Bypass | ||||||||||
| Claudication | NA | NA | NA | NA | 449 (28.9%) | 223 (49.7%) | 139 (30.9%) | 87 (19.3%) | 0.524 | |
| Tissue Loss/Rest Pain | NA | NA | NA | NA | 1510 (71.1%) | 925 (61.2%) | 371 (24.5%) | 214 (14.2%) | ||
| Above-knee Popliteal Recipient | NA | NA | NA | NA | 452 (21.3%) | 245 (56.3%) | 108 (24.8%) | 82 (18.8%) | 0.625 | |
| Below-knee Popliteal or lower recipient | NA | NA | NA | NA | 1669 (78.6%) | 979 (58.9%) | 435 (26.1%) | 254 (15.2%) | ||
| Conduit | NA | NA | NA | NA | ||||||
| Autogenous vein | NA | NA | NA | NA | 1532 (72.2%) | 895 (58.4%) | 399 (26.1%) | 238 (15.5%) | 0.635 | |
| Prosthetic | NA | NA | NA | NA | 590 (28.7%) | 340 (57.6%) | 150 (25.4%) | 100 (16.9%) | ||
| Pre Operative Cardiac Risk Stratification (Predicted Risk of POMI) | ||||||||||
| Low Predicted POMI Risk | 231 (24.9%) | 122 (52.81) | 87 (37.9%) | 22(9.5%) | 0.004 | 579 (27.2%) | 292 (50.4%) | 173 (29.9%) | 114 (19.7%) | 0.000 |
| Medium Predicted POMI Risk | 464 (50.1%) | 266 (57.3%) | 157 (33.8%) | 41 (8.8%) | 993 (46.8%) | 590 (59.4%) | 265 (26.7%) | 138 (13.9%) | ||
| High Predicted POMI Risk | 231 (24.9%) | 147 (63.6%) | 52(22.5%) | 32 (13.9) | 550 (25.2%) | 353 (64.2%) | 111 (25.9%) | 86 (15.6%) | ||
p-value from chi-square test
Description of Quality Improvement Intervention
We formally began our quality improvement effort in beta blocker utilization in 2003, at the 6 centers participating in the VSGNE at its inception. After we analyzed and discussed the evidence for beta blocker usage in 2003, we selected 90% as our target for peri-operative beta blocker administration, to allow for drug intolerance in some patients. Use of beta blockers was recorded in the VSGNE dataset pre-operatively, at discharge, and at 1-year follow-up. Dosage was not recorded. Resting heart rate at arrival to the operating room, as well as peak heart rate intra-operatively was recorded beginning in July, 2005. Beta blocker treatment initiated within one month of surgery was categorized as a “preoperative”; beta blockers started more than one month prior to surgery were categorized as “chronic.” While our QI initiative recommended that metoprolol 25 mg twice daily be started two weeks prior to operation and be continued following operation, surgeons were left to their own discretion in regards to agent, dose, timing, and titration to heart rate.
A central tenet of our quality improvement intervention was to provide feedback to surgeons and centers. Therefore, rates of utilization (either chronic or pre-operative) were reported back to individual surgeons and centers on a biannual basis. While individual surgeon and center results were kept anonymous, regional, institutional, and individual rates (blinded) of beta blockade were reviewed in detail at biannual meetings, when methods for increasing utilization were discussed and successful methods were shared, such as pre-printed order sheets or prescription forms. All six centers that began the QI initiative remained in the study during the entirety of the study period,
Main Outcome Measures
Our study examined two main outcome measures. The first was the utilization of peri-operative beta blockers, measured as the proportion of patients on beta blockers, either chronic or pre-operatively. This outcome was examined across strata of surgeons, centers, type of beta blockade use (pre-operative or chronic), and procedure (AAA and lower extremity bypass). We included only surgeons (n=29 and centers (n=6) who were present in the cohort during the entire period of the analysis in order to not confound the impact of quality improvement in the same group over time,
Second, given that the ultimate goal of our quality improvement intervention was to decrease patient morbidity and mortality, we measured in-hospital, post-operative myocardial infarction (POMI) rates (defined by electrocardiographic, echocardiographic, or troponin-based evidence of myocardial infarction in the post-operative period). Similar strata were compared as in the beta blockade analysis (surgeon, center, type of beta blocker therapy, and procedure).
Analysis
Overall, 946 patients underwent OAAA and 2,215 underwent LEB between January 1st, 2003 and December 31st, 2008 within the initial 6 centers of VSGNE. Within this group, 926 OAAA patients (95% of the total) and 2,123 LEB patients (96% of the total) had information available about beta blocker administration, post-operative MI, and patient characteristics available for analysis during the entire time period, and therefore constituted our cohort for analysis.
We compared beta blocker administration over time, using non-parametric tests of trend. To further examine changes over time, we used statistical process control (SPC) methods. SPC is a methodology used commonly in manufacturing to examine process variation22. Using control (p) charts, we examined the proportion of patients taking beta blockers over time, in three month intervals. We assumed the process was stable at initiation of the process, given that practice patterns had not changed significantly across surgeons prior to the initiation of our study, based on the report of our quality improvement initiative working group. Upper and lower control limits were defined as 3 sigma (standard deviations) above and below the control value, respectively and used the Western Electric Company rules to determine if the outcomes had moved beyond their control limits22-24. These rules state that if 2 of 3 consecutive points fall outside a 2-sigma limit, or if 4/5 consecutive points fall beyond 1 sigma limits, or if 8 points fall on one side of the centerline, then a significant change has occurred in the process being measured. On our control charts, those points that are significantly different than the baseline are shown as red diamonds.
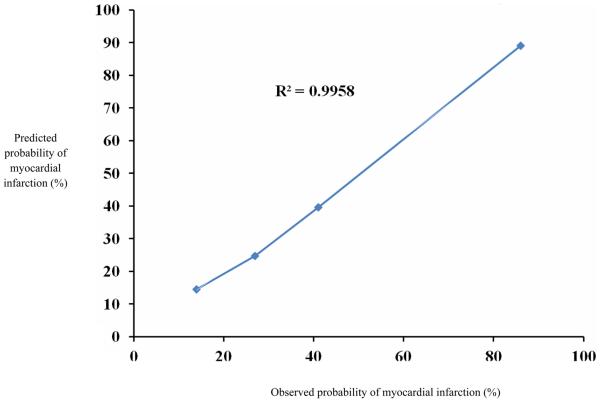
We used similar methodology to examine the incidence of cardiac complications over time in patients on chronic or pre-operative beta blockade, as well as patients not on beta blockers. In order to categorize patients according to their cardiac risk we developed a logistic regression model to predict the risk of POMI, based on the characteristics of patients who experienced POMI. This model was used to categorize patients into low (2.5-5.0% predicted risk of POMI), medium (5.0-11.5% predicted risk of POMI), and high (11.5-15.1% predicted risk of POMI) risk categories, to allow evaluation of changes in beta blockade across different risk strata of cardiac risk. Our POMI model demonstrated good discrimination (area under ROC = 0.71), and the calibration of the model was satisfactory across a broad spectrum of patient risk,(R2=0.99, Hosmer-Lemeshow goodness of fit test p=0.40, see Appendix 1)).
All analyses were performed using Microsoft Excel (Redmond, WA) and STATA (College Station, TX). The Institutional Review Board at Dartmouth Medical School reviewed and approved our study protocol.
Results
Patient Characteristics
In the OAAA cohort, patients were most commonly male (73%), mean age was 71 years, and nearly all were Caucasian (Table 1). Most patients had a history of either prior or current smoking (92%). About half of patients had a history of diabetes, 40% had coronary disease, and nearly a third had a history of COPD. Most patients were on anti-platelet agents (72%) and statin therapy (58%). Based on their predicted risk of POMI, 50% of patients had intermediate cardiac risk Further details about the characteristics of the cohort used are available in Table 1.
Similarly, in the LEB cohort, patients were most commonly male (69%), mean age was 69 years, and nearly all were Caucasian. Nearly all patients had a history of either prior or current smoking (83%). About half of patients had a history of diabetes, 41% had coronary disease, and nearly a third had a history of COPD. Most patients were on anti-platelet agents (72%) and statin therapy (58%). While the overall predicted risk of cardiac complications was slightly higher in these patients as compared to those undergoing OAAA, Based on their predicted risk of POMI, 46% of LEB patients had intermediate cardiac risk (Table 1).
Beta Blocker Utilization
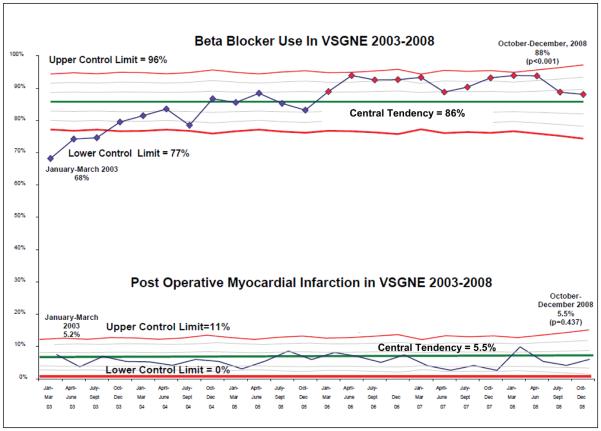
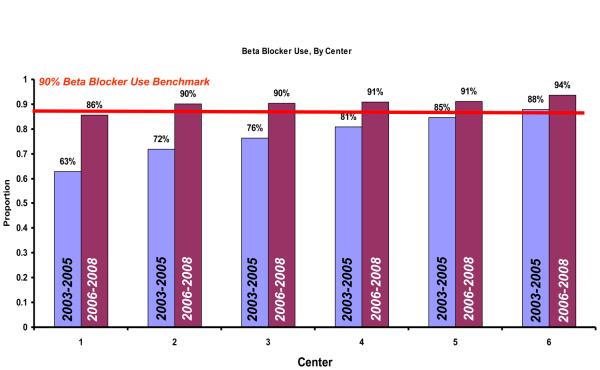
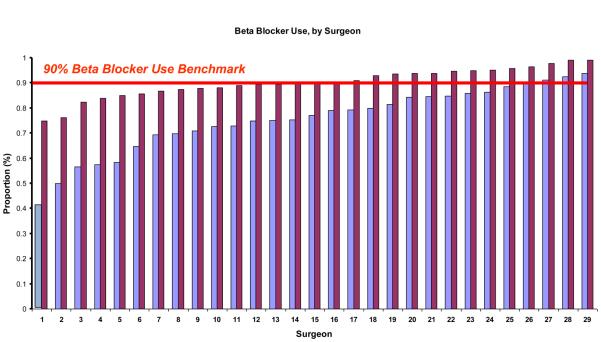
At the time of initiation of our quality improvement initiative in 2003, the overall rate of peri-operative beta blocker utilization was 68%. However, by the end of our study period, beta blocker use had increased to 88% (p<0.001) (Figure 1). Using statistical process control to evaluate changes over time, we noted that by December of 2005, a significant increase in the peri-operative usage of beta blockers had occurred. This increase in beta blocker therapy remained above the baseline from 2005 until the completion of our study period in 2008, indicating that the process of beta blocker administration in our region had changed significantly during the study period (Figure 1). This effect was apparent across all centers and surgeons in our study (Figure 2a and Figure 2b).
Figure 1.
Control (p) chart, demonstrating beta blocker administration and post operative myocardial infarction rate over time. Central tendency is shown in the blue line, with the red diamonds demonstrating significant change from central tendency has occurred. The red lines indicate the upper and lower control limits, while the grey lines indicate 1 sigma and 2 sigma differences from baseline.
Figure 2a.
Center-specific beta blockade administration, in 2003 and 2008.
Figure 2b.
Surgeon-specific beta blockade administration, in 2003 and 2008.
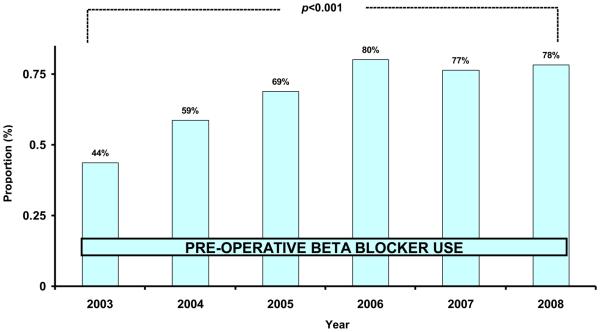
When we examined the rates of beta-blocker utilization by pattern of utilization, (chronic versus pre-operative), we found an increase over time in both chronic (54% in 2003, 61% in 2008, p=0.004) and pre-operative usage (20% in 2003, 31% in 2008, p<0.001). We believed that our intervention was most likely to affect the use of pre-operative beta blockers, as compared to those patients already on chronic beta blockers). We found that in 2003, 44% of patients not on chronic beta blockers were treated with perioperative beta blockers. However, by 2008, 78% of patients not on chronic beta blockers were started pre-operatively on these medications (Figure 3). This effect was consistent across all centers.
Figure 3.
Rate of pre-operative beta blocker use in patients not already on chronic beta blockers
Next, we analyzed changes in beta blocker administration across low, medium, and high cardiac risk patients (Table 2). Although the use of beta blockers increased in all categories, statistically significant increases in the use of pre-operative beta blockers were most apparent in low-risk patients, while statistically significant increases in chronic beta blocker use were seen in medium and high-risk patients (Table 2). Lastly, we examined the proportion of patients discharged from the hospital on beta blocker therapy. Overall, we found that patients who were on beta blockers prior to surgery were very likely (89% of all patients) to be discharged on these medications. Of those patients who were not on beta blockers at the time of surgery, 33% were subsequently discharged on beta blocker therapy.
Table 2.
Change in pre-operative and chronic beta blocker administration over time, across strata of cardiac risk.
| Predicted Risk of Post-Operative Myocardial Infarction | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Low (2.5-3.2%) | Medium (5.0-11.5%) | High (13.1-15.1%) | |||||||
| 2003-2005 | 2006-2008 | p value | 2003-2005 | 2006-2008 | p value | 2003-2005 | 2006-2008 | p value | |
| No Beta Blocker | 23% | 11% | 0.01 | 16% | 8% | 0.01 | 21% | 6% | 0.01 |
| Pre-Operative Beta Blockers | 28% | 36% | 0.01 | 28% | 30% | 0.45 | 19% | 24% | 0.09 |
| Chronic Beta Blockers | 49% | 53% | 0.18 | 56% | 62% | 0.01 | 60% | 70% | 0.01 |
Effect of Beta Blockade on Post-operative Myocardial Infarction
Overall, POMI occurred relatively infrequently in our cohort (5.5%). Patients undergoing OAAA were slightly more likely to experience POMI than patients undergoing LEB (7.6% versus 4.6%, p<0.001). Despite the increase in usage of beta blockers, we found that no significant change occurred in the rate of POMI over time (Figure 1). While there was a trend for a reduced POMI rate in patients on pre-operative beta blockers, in patients of low, medium, and high cardiac risk, these changes were not statistically significant (Table 3). However, among the small group of patients not on beta blockers in the intermediate cardiac risk group, the rate of POMI was significantly higher in 2006-2008 as compared to 2003-2005. (7.4 vs. 1.6%). Conversely, patients on pre-operative beta blockers experienced aa reduction in the rate of POMI during the same time period (4.6% in 2003-2005, 2.5% in 2006-2008, p= 0.23; see Table 3).
Table 3.
Change in peri-operative myocardial infarction rate over time, across strata of cardiac risk.
| Predicted Risk of Post-Operative Myocardial Infarction | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Low (predicted risk of 2.5-3.2%) | Intermediate (predicted risk of 5.0-11.5% ) | High (predicted risk of 13.1-15.1%) | |||||||
| 2003-2005 | 2006-2008 | p value | 2003-2005 | 2006-2008 | p value | 2003-2005 | 2006-2008 | p value | |
| No Beta Blocker | 2.3% | 0.0% | 0.14 | 1.6% | 7.4% | 0.05 | 8.6% | 8.3% | 0.95 |
| Pre-Operative Beta Blockers | 1.8% | 0.7% | 0.42 | 4.6% | 2.5% | 0.23 | 7.3% | 6.2% | 0.77 |
| Chronic Beta Blockers | 2.5% | 2.3% | 0.89 | 5.6% | 4.7% | 0.56 | 12.2% | 15.1% | 0.34 |
Effect of Beta Blockade on Heart Rate
Despite the increase in utilization of beta blockade, we found no significant differences in resting or peak intra-operative heart rate over time during our study interval (Table 4). In all patients in our study cohort, heart rate on arrival to the operating room was relatively low in the early years, and did not change significantly in later years (resting heart rate = 69 in 2005, resting heart rate = 68 in 2006-2008, p=0.412).
Table 4.
Change in pre-operative and chronic beta blocker administration over time, across strata of cardiac risk.
| Arrival Heart Rate (mean, 95% CI) | Maximum Heart Rate (mean, 95% CI) | |||||
|---|---|---|---|---|---|---|
| 2003-2005 | 2006-2008 | p value | 2003-2005 | 2006-2008 | p value | |
| No Beta Blocker | 72 (68-76) | 69 (66-71) | 0.25 | 81 (77-86) | 79 (76-82) | 0.46 |
| Pre-Operative Beta Blockers | 72 (69-75) | 69 (68-72) | 0.28 | 81 (78-85) | 80 (78-81) | 0.38 |
| Chronic Beta Blockers | 67 (65-69) | 67 (65-69) | 0.52 | 77 (74-79) | 77 (75-77) | 0.69 |
Overall Rate of POMI, by Pattern of Beta Blocker Use
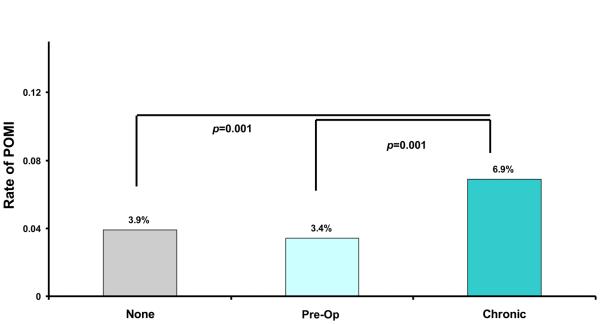
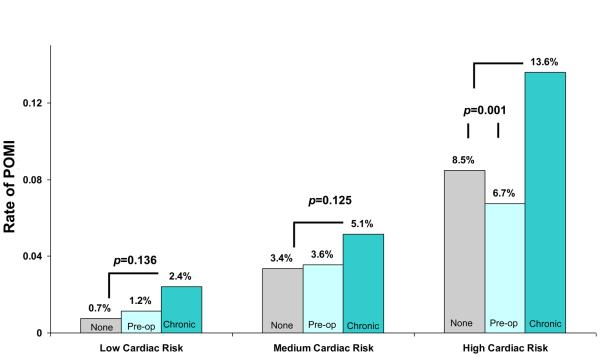
Rates of POMI varied, by the type of beta blocker use (Figure 4a). Overall, when compared to patients on no beta blocker or peri-operative beta blockers, the rate of POMI was highest in patients on chronic beta blockers. Patients on chronic beta blockers were nearly twice as likely to experience POMI when compared to patients on no beta blocker (6.9% veruss 3.9%, p<0.001) or pre-op beta blocker (6.9% versus 3.4%, p<0.001) This trend persisted, even when we studied this effect across different categories of cardiac risk, as shown in Figure 4b. The most dramatic differences in POMI rates were seen in the patients at the highest cardiac risk, but differences remained significant between chronic beta blocker recipeints and the remaining patients.
Figure 4a.
Post operative MI rate, by type of beta blocker use.
Figure 4b.
Post operative MI rate, by type of beta blocker use and cardiac risk strata.
Factors associated with Post Operative Myocardial Infarction
Using multiple logistic regression, we identified several pre-operative patient characteristics associated with POMI in multivariate analysis. As shown in Table 5, age over 70, a positive pre-operative stress test, congestive heart failure, and a resting heart rate less than 70 were all associated with increasing risk of POMI. Moreover, we also found that the chronic administration of beta blockers was also associated with higher risks of POMI. This effect persisted, even when we adjusted for heart rate by including this variable in our regression model.
Table 5.
Multivariable predictors of post-operative myocardial infarction.
| Odds Ratio | 95% Conf. Interval | p value | |
|---|---|---|---|
| Diabetes | 1.6 | 1.1-2.6 | 0.026 |
| Congestive Heart Failure | 1.7 | 1.1-2.5 | 0.008 |
| Chronic Beta Blocker Use | 1.7 | 1.2-2.4 | 0.006 |
| Resting Heart Rate<70 | 1.8 | 1.1-2.8 | 0.005 |
| Age >70 | 2.1 | 1.5-2.9 | <0.001 |
| Positive Stress Test | 2.2 | 1.5-3.2 | 0.002 |
Discussion
We found that a quality improvement effort was broadly effective in increasing the use of peri-operative beta-blockers in patients undergoing LEB and OAAA in our region. This effect was seen for each of the hospitals and surgeons participating in the initiative, and was seen across all strata of patient cardiac risk. However, the absolute increase in utilization of peri-operative beta blockers was most significant in patients with the lowest cardiac risk, and overall our quality improvement effort did not result in a decline in the rate of POMI.
Given the uniform increase in utilization of beta blockers in our region, we believe that were very successful in developing and executing a quality improvement initiative to change the way care is delivered to our patients. Several precedents for this methodology exist25-27, and these efforts served to guide our initiative. In 1996, O'Connor et al described a regional quality improvement initiative wherein feedback of outcome data, training in continuous quality improvement techniques, and site visits was associated with a 24% reduction in hospital mortality after coronary bypass surgery. Several other similar initiatives have demonstrated similar effects across a broad spectrum of surgical and medical conditions28-32, and emphasize the potential utility of regional organizations in outcomes research and quality improvement. The techniques for process change in VSGNE included informational sessions at our biannual meetings, quality improvement discussions, and, most importantly we believe, biannual individualized feedback with anonymous benchmarking of the surgeon, institution and regional use of beta blockers. This low-cost intervention resulted in significant improvements in the use of beta blockers, across all centers, regions, and patient cardiac risk strata in our region.
However, we were disappointed to find that despite this successful effort, the rate of POMI did not decrease over time Why did our quality improvement effort not achieve a decline in the rate of POMI? While the answer to this question is unknown, we have several potential explanations. First, our beta blocker dosage regimen may have been too low. In the literature there are a number of different agents used, different dosages and timing of administration, and several trials have titrated beta blocker dosage to heart rate5. In an effort to maximize surgeon compliance we selected a standardized fixed regimen with a relatively low dose, in an effort to minimize side effects. While this served as a convenient and effective tactic to maximize the ease of implementing beta blocker therapy, it is possible that this dose was not large enough to have an effect, and the lack of measurable difference in heart rate during the study interval attests to this possibility.
Second, while our intervention sought to achieve an increase in overall beta blocker utilization, most often this change occurred in low and intermediate risk patients. In a retrospective review of over 600,000 patients in an administrative, quality focused data base, Lindenauer showed that beta blockers were most beneficial in high risk patients, probably beneficial in intermediate risk patients and of questionable value in low risk patients13. Poldermans also showed a decrease in cardiac events with beta blocker administration to high risk patients undergoing vascular surgery in a randomized clinical trial4, 8. In our region, pre-operative beta blocker use initiated within 30 days of surgery in high risk patients went from 19 to 24% (p = 0.09). This change represented an increase of 33 “new” high-risk patients on pre-operative beta blockers, which was likely too few to demonstrate a significant reduction in cardiac events. Further, within the intermediate cardiac risk group, a significant possibility exists that type II error limited our ability to detect a difference in POMI rate.
Third, our study may have found that beta blockers have little effect on POMI because that might be the “real” answer. In the literature, there has been a transition in the results from randomized clinical trials. Initially the trials showed a reduction of myocardial ischemia and myocardial infarction4, 8, 13. However, as time went on two trials failed to show a benefit10, 11. In these two randomized trials, patients underwent aortic aneurysm repair and peripheral vascular reconstruction and therefore the cohorts were procedurally quite similar to our study. In the first study, the peri-operative beta blocker (POBBLE) trial investigators found no significant difference in adverse cardiovascular events10. In the second study, the Metoprolol after Vascular Surgery (MaVS) trial11, there were no significant reduction in cardiovascular events in a population undergoing abdominal aortic repair and peripheral vascular reconstruction, again quite similar to our cohort. One of the potential explanations for the lack of impact was the absence of high risk patients undergoing high risk operations. More recently, the PeriOperative ISchemic Evaluation (POISE) trial, the largest randomized clinical trial on perioperative beta blocker treatment published to date found a significant decrease in cardiac events from beta blocker administration (4.2 versus 5.7%, p<0.001), but also showed the beta blocker therapy was associated with an increase in stroke (1% versus 0.5, p<0.005) and all cause mortality (3.1% versus 2.3%, p= 0.03)5. This trial was notable for a very large dose of beta blocker, metoprolol 100 mg given 2-4 hours preoperatively and the high rate of intra-operative bradycardia and hypotension, which likely contributed to the increased incidence of postoperative stroke and all cause mortality. Our dataset was not initially configured to capture the possible non-lethal complications of beta blocker therapy, such as stroke, While stroke was routinely collected as an outcome variable in carotid surgery, it was not studied in other procedures such as LEB. Accordingly, we have since corrected this deficiency in our dataset. Further, our dataset was also not configured to ensure absolute adherence to dosing regimens and heart rate titration.
What is the role of beta blockers in modern vascular surgery? The AHA/ACC guidelines are to continue beta blockers in patients who are on them (class I) and initiate beta blocker therapy titrated to heart rate in patients with coronary artery disease or high cardiac risk (more than one clinical risk factor, class IIa)1. The usefulness of beta blockers is uncertain for patients in intermediate risk (1 clinical risk factor, class IIb) or at low risk (no clinical risk factors, class IIB). These recommendations are supported by the European Society of Cardiology/European Society of Anesthesiology with two slight differences. The initiation of peri-operative beta blocker therapy in patients with coronary artery disease or undergoing high risk surgery is considered to be a class I recommendation and treatment for intermediate risk surgery is a class IIa recommendation.18
Should we continue to administer beta blockers to all patients undergoing vascular surgery? First, we must balance the potential risks and harms of administering beta blockers to our patients. While our initiative was successful in increasing the use of beta blockers, the increases in peri-operative beta blockade most commonly occurred within the patients with the lowest cardiac risk. Accordingly, we aim to use our quality improvement initiative to focus our efforts more accurately, choosing only intermediate and high-risk patients for peri-operative beta blockade. Second, we must look carefully at the risks presented to patient by chronic administration of beta blockers. Our analyses showed that independent of complications related to bradycardia or pre-existing ischemia, an association existed between chronic beta blocker administration and POMI. Unmeasured confounders, such as hypotension secondary to excessive beta blockade, may have played a role in these excess events. Therefore, we have focused our ongoing efforts towards understanding the events surrounding POMI, using both our clinical dataset and chart-based review, in the hopes of “drilling down” more precisely on the factors that allow us to predict and prevent POMI. Third, our study demonstrates the feasibility and efficacy of surgeon-led quality improvement, especially when it is implemented in the context of a regional registry. Therefore, our future work will continue to expand the use of this method to implement other evidence-based practices, such as patching following conventional CEA33, 34, statin administration35-37, and cell saver use in OAAA38.
Finally, our study demonstrated that patients on chronic beta blockers are more likely to have a POMI than patients on peri-operative beta blockers, or no beta blocker at all. This association persisted, even when adjusting in our multivariable models for other markers of cardiac disease (such as congestive heart failure or a history of a positive stress test), as well as clinical parameter such as adequate heart rate control. It is possible in our dataset that the use of chronic beta blocker is simply a more accurate proxy for elevated cardiac risk, as measures such as pre-operative congestive heart failure or pre-existing coronary artery disease may vary in nature or extent. However, it is also possible that chronic beta blocker use itself is causative for cardiac complications. Although we cannot be certain which explanation is the right answer, we found little evidence to suggest that chronic beta blockers were harmful – there was no change in mortality over time in patients receiving chronic beta blockers, and no evidence of higher stroke rates or need for vasoactive medications peri-operatively in these groups. Our future efforts will seek to more precisely quantify these relationships and attempt to understand more completely the effect of chronic beta blockade utilization on POMI.
Our study has limitations. First, as mentioned previously, we did not control for the type of beta blocker administered, nor did we record dosage information, or the precise duration of therapy, especially among patients receiving beta blockers pre-operatively. While these specific details would have been helpful, practical limitations as to the reasonable length of our quality improvement dataset precluded capturing these variables. However, evidence from several systematic reviews39-41 and meta-analyses42-44 suggest that there are few dramatic differences in effect by beta blocker type, and we did collect heart rate as a proxy for effective dose of beta blockade. Second, variation in the measurement of myocardial infarction, especially by serum assays, may have differed across centers. However, while the thresholds and laboratory values may have differed across centers, we found little variation in the absolute number of measurements of serum assays performed across centers. Further, we used our biannual meetings as well as annual data audits to ensure that POMI was being collected in a consistent manner, making systematic confounding by surgeon, center, or risk strata unlikely. And third, our population, composed of patients primarily from Northern New England, is not ethnically diverse. Therefore, especially given the differences in cardiovascular risk across racial groups, the overall generalizability of our experience to more racially diverse cohorts remains uncertain.
In conclusion, despite successfully implementing a regional quality improvement initiative aimed at increasing the utilization of peri-operative beta blockers, we did not decrease the incidence of POMI. Our future work will center on expanding the use of regional registries as quality improvement tools in surgery, as well as further delineating which patients are best served by the administration of peri-operative beta blockade.
Acknowledgments
The Vascular Study Group of Northern New England is supported by a grant by from the Center for Medicare and Medicaid Services (CMS), under Cooperative Agreement Award number 18-C-24 91674/1/01.
Appendix
Appendix 1.
Correlation of observed and expected values for prediction model for post operative myocardial infarction.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Presented at the Society for Vascular Surgery's Vascular Annual Meeting, June 13th, 2010, Boston, MA,
References
- 1.Hirsch AT, Haskal ZJ, Hertzer NR, et al. ACC/AHA 2005 Practice Guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic): a collaborative report from the American Association for Vascular Surgery/Society for Vascular Surgery, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, Society of Interventional Radiology, and the ACC/AHA Task Force on Practice Guidelines (Writing Committee to Develop Guidelines for the Management of Patients With Peripheral Arterial Disease): endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation; National Heart, Lung, and Blood Institute; Society for Vascular Nursing; TransAtlantic Inter-Society Consensus; and Vascular Disease Foundation. Circulation. 2006;113(11):e463–654. doi: 10.1161/CIRCULATIONAHA.106.174526. [DOI] [PubMed] [Google Scholar]
- 2.Eagle KA, Berger PB, Calkins H, et al. ACC/AHA guideline update for perioperative cardiovascular evaluation for noncardiac surgery---executive summary a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to Update the 1996 Guidelines on Perioperative Cardiovascular Evaluation for Noncardiac Surgery) Circulation. 2002;105(10):1257–67. [Erratum appears in Circulation. 2006 Jun 6;113(22):e846] [PubMed] [Google Scholar]
- 3.Brooke BS, Perler BA, Dominici F, Makary MA, Pronovost PJ. Reduction of in-hospital mortality among California hospitals meeting Leapfrog evidence-based standards for abdominal aortic aneurysm repair. J Vasc Surg. 2008;47(6):1155–6. doi: 10.1016/j.jvs.2008.01.021. discussion 63-4. [DOI] [PubMed] [Google Scholar]
- 4.Poldermans D, Bax JJ, Schouten O, et al. Should major vascular surgery be delayed because of preoperative cardiac testing in intermediate-risk patients receiving beta-blocker therapy with tight heart rate control? J Am Coll Cardiol. 2006;48(5):964–9. doi: 10.1016/j.jacc.2006.03.059. [DOI] [PubMed] [Google Scholar]
- 5.Group PS, Devereaux PJ, Yang H, et al. Effects of extended-release metoprolol succinate in patients undergoing non-cardiac surgery (POISE trial): a randomised controlled trial. Lancet. 2008;371(9627):1839–47. doi: 10.1016/S0140-6736(08)60601-7. [DOI] [PubMed] [Google Scholar]
- 6.Cucchiara RF, Benefiel DJ, Matteo RS, DeWood M, Albin MS. Evaluation of esmolol in controlling increases in heart rate and blood pressure during endotracheal intubation in patients undergoing carotid endarterectomy. Anesthesiology. 1986;65(5):528–31. doi: 10.1097/00000542-198611000-00016. [DOI] [PubMed] [Google Scholar]
- 7.Raby KE, Brull SJ, Timimi F, et al. The effect of heart rate control on myocardial ischemia among high-risk patients after vascular surgery. Anesth Analg. 1999;88(3):477–82. doi: 10.1097/00000539-199903000-00002. [DOI] [PubMed] [Google Scholar]
- 8.Poldermans D, Boersma E, Bax JJ, et al. The effect of bisoprolol on perioperative mortality and myocardial infarction in high-risk patients undergoing vascular surgery. Dutch Echocardiographic Cardiac Risk Evaluation Applying Stress Echocardiography Study Group. N Engl J Med. 1999;341(24):1789–94. doi: 10.1056/NEJM199912093412402. [DOI] [PubMed] [Google Scholar]
- 9.Boersma E, Poldermans D, Bax JJ, et al. Predictors of cardiac events after major vascular surgery: Role of clinical characteristics, dobutamine echocardiography, and beta-blocker therapy. Jama. 2001;285(14):1865–73. doi: 10.1001/jama.285.14.1865. [DOI] [PubMed] [Google Scholar]
- 10.Brady AR, Gibbs JSR, Greenhalgh RM, Powell JT, Sydes MR, investigators Pt. Perioperative beta-blockade (POBBLE) for patients undergoing infrarenal vascular surgery: results of a randomized double-blind controlled trial. J Vasc Surg. 2005;41(4):602–9. doi: 10.1016/j.jvs.2005.01.048. [DOI] [PubMed] [Google Scholar]
- 11.Yang H, Raymer K, Butler R, Parlow J, Roberts R. The effects of perioperative beta-blockade: results of the Metoprolol after Vascular Surgery (MaVS) study, a randomized controlled trial. Am Heart J. 2006;152(5):983–90. doi: 10.1016/j.ahj.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 12.Mangano DT, Layug EL, Wallace A, Tateo I. Effect of atenolol on mortality and cardiovascular morbidity after noncardiac surgery. Multicenter Study of Perioperative Ischemia Research Group. N Engl J Med. 1996;335(23):1713–20. doi: 10.1056/NEJM199612053352301. [Erratum appears in N Engl J Med 1997 Apr 3;336(14):1039] [DOI] [PubMed] [Google Scholar]
- 13.Lindenauer PK, Pekow P, Wang K, Mamidi DK, Gutierrez B, Benjamin EM. Perioperative beta-blocker therapy and mortality after major noncardiac surgery. N Engl J Med. 2005;353(4):349–61. doi: 10.1056/NEJMoa041895. [DOI] [PubMed] [Google Scholar]
- 14.Juul AB, Wetterslev J, Gluud C, et al. Effect of perioperative beta blockade in patients with diabetes undergoing major non-cardiac surgery: randomised placebo controlled, blinded multicentre trial. Bmj. 2006;332(7556):1482. doi: 10.1136/bmj.332.7556.1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. http://www.leapfroggroup.org/media/file/Leapfrog-Birkmeyer.pdf. In.
- 16. http://www.ama-assn.org/ama1/pub/upload/mm/370/measures.pdf. In.
- 17.Website S. SCIP website. http://www.qualitynet.org/dcs/ContentServer?c=MQParents&pagename=Medqic/Content/Parent ShellTemplate&cid=1122904930422&parentName=Topic.
- 18.Poldermans D, Bax JJ, Boersma E. Guidelines for pre-operative cardiac risk assessment and perioperative cardiac management in non-cardiac surgery: The Task Force for Preoperative Cardiac Risk Assessment and Perioperative Cardiac Management in Non-cardiac Surgery of the European Society of Cardiology (ESC) and endorsed by the European Society of Anaesthesiology (ESA) European Journal of Anaestesiology. 2010;27(2):92–137. doi: 10.1097/EJA.0b013e328334c017. al. e. [DOI] [PubMed] [Google Scholar]
- 19.Cronenwett JL, Likosky DS, Russell MT, et al. A regional registry for quality assurance and improvement: the Vascular Study Group of Northern New England (VSGNNE) J Vasc Surg. 2007;46(6):1093–101. doi: 10.1016/j.jvs.2007.08.012. discussion 101-2. [DOI] [PubMed] [Google Scholar]
- 20.Cronenwett JL, Russell MT, Likosky DS, Eldrup-Jorgenson J, Stanley AC, Nolan BW. A Regional Registry for Quality Assurance and Improvement: The Vascular Study Group of Northern New England (VSG-NNE) J Vasc Surg. 2007;46(6):1093–101. doi: 10.1016/j.jvs.2007.08.012. [DOI] [PubMed] [Google Scholar]
- 21.Bertges DB, Goodney PP, Eldrup-Jorgensen J, Nolan BW, Likosky DS, Cronenwett J. Accuracy of Cardiac Risk Prediction Models in Patients Undergoing Open Abdominal Aortic Aneurysm Repair or Lower Extremity Bypass. J Vasc Surg. in press. [Google Scholar]
- 22.Carey RG. Improving Healthcare with Control Charts. Quality Press; Milwakee, Wisconsin: 2002. [Google Scholar]
- 23. www.sqconlinne.com/six-sigma-control-charts.html. In.
- 24.Solodky C, Chen H, Jones PK, Katcher W, Neuhauser D. Patients as partners in clinical research: a proposal for applying quality improvement methods to patient care. Med Care. 1998;36(8 Suppl):AS13–20. doi: 10.1097/00005650-199808001-00003. [DOI] [PubMed] [Google Scholar]
- 25.Kattan M, Crain EF, Steinbach S, et al. A randomized clinical trial of clinician feedback to improve quality of care for inner-city children with asthma. Pediatrics. 2006;117(6):e1095–103. doi: 10.1542/peds.2005-2160. [DOI] [PubMed] [Google Scholar]
- 26.Zhang H, Alexander JA, Luttrell J, O'Connor GT, Daley J, Paris M. Data feedback and clinical process improvement in acute myocardial infarction. Am Heart J. 2005;149(5):856–61. doi: 10.1016/j.ahj.2004.05.057. [DOI] [PubMed] [Google Scholar]
- 27.O'Connor GT, Plume SK, Olmstead EM, et al. A regional intervention to improve the hospital mortality associated with coronary artery bypass graft surgery. The Northern New England Cardiovascular Disease Study Group. Jama. 1996;275(11):841–6. [PubMed] [Google Scholar]
- 28.Holman WL, Sansom M, Kiefe CI, et al. Alabama coronary artery bypass grafting project: results from phase II of a statewide quality improvement initiative. Ann Surg. 2004;239(1):99–109. doi: 10.1097/01.sla.0000103065.17661.8f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Scott IA, Eyeson-Annan ML, Huxley SL, West MJ. Optimising care of acute myocardial infarction: results of a regional quality improvement project. Journal of Quality in Clinical Practice. 2000;20(1):12–9. doi: 10.1046/j.1440-1762.2000.00345.x. [DOI] [PubMed] [Google Scholar]
- 30.O'Grady MA, Gitelson E, Swaby RF, et al. Development and implementation of a medical oncology quality improvement tool for a regional community oncology network: the Fox Chase Cancer Center Partners initiative. Journal of the National Comprehensive Cancer Network. 2007;5(9):875–82. doi: 10.6004/jnccn.2007.0078. [DOI] [PubMed] [Google Scholar]
- 31.Ghali WA, Ash AS, Hall RE, Moskowitz MA. Statewide quality improvement initiatives and mortality after cardiac surgery. Jama. 1997;277(5):379–82. [see comment] [PubMed] [Google Scholar]
- 32.Weissman NW, Allison JJ, Kiefe CI, et al. Achievable benchmarks of care: the ABCs of benchmarking. Journal of Evaluation in Clinical Practice. 1999;5(3):269–81. doi: 10.1046/j.1365-2753.1999.00203.x. [DOI] [PubMed] [Google Scholar]
- 33.Counsell C, Salinas R, Warlow C, Naylor R. Patch angioplasty versus primary closure for carotid endarterectomy. Cochrane Database Syst Rev. 2000:2. doi: 10.1002/14651858.CD000160. [update in Cochrane Database Syst Rev. 2004;(2):CD000160; PMID: 15106145] [DOI] [PubMed] [Google Scholar]
- 34.Goodney PP, Nolan BW, Eldrup-Jorgensen J, Stanley AC, Likosky DS, Cronenwett J. Restenosis Following Carotid Endarterectomy in a Multicenter Regional Registrry. J Vasc Surg. 2010 doi: 10.1016/j.jvs.2010.05.005. in press. [DOI] [PubMed] [Google Scholar]
- 35.Schanzer A, Hevelone N, Owens CD, Beckman JA, Belkin M, Conte MS. Statins are independently associated with reduced mortality in patients undergoing infrainguinal bypass graft surgery for critical limb ischemia. J Vasc Surg. 2008;47(4):774–81. doi: 10.1016/j.jvs.2007.11.056. [DOI] [PubMed] [Google Scholar]
- 36.Kennedy J, Quan H, Buchan AM, Ghali WA, Feasby TE. Statins are associated with better outcomes after carotid endarterectomy in symptomatic patients. Stroke. 2005;36(10):2072–6. doi: 10.1161/01.STR.0000183623.28144.32. [see comment] [DOI] [PubMed] [Google Scholar]
- 37.Feringa HHH, Schouten O, Karagiannis SE, et al. Intensity of statin therapy in relation to myocardial ischemia, troponin T release, and clinical cardiac outcome in patients undergoing major vascular surgery. J Am Coll Cardiol. 2007;50(17):1649–56. doi: 10.1016/j.jacc.2007.06.046. [DOI] [PubMed] [Google Scholar]
- 38.Chang CK, Goodney PP, Cronenwett J. Predicting bleeding complications and transfusion following vascular surgery. New England Society for Vascular Surgery. 2010 abstract presented at the New England Society for Vascular Surgery. [Google Scholar]
- 39.Brooke BS, Dominici F, Makary MA, Pronovost PJ. Use of beta-blockers during aortic aneurysm repair: bridging the gap between evidence and effective practice. Health Aff (Millwood) 2009;28(4):1199–209. doi: 10.1377/hlthaff.28.4.1199. [DOI] [PubMed] [Google Scholar]
- 40.Brooke BS. Perioperative beta-blockers for vascular surgery patients. J Vasc Surg. 51(2):515–9. doi: 10.1016/j.jvs.2009.09.057. [DOI] [PubMed] [Google Scholar]
- 41.Devereaux PJ, Beattie WS, Choi PTL, et al. How strong is the evidence for the use of perioperative beta blockers in non-cardiac surgery? Systematic review and meta-analysis of randomised controlled trials. Bmj. 2005;331(7512):313–21. doi: 10.1136/bmj.38503.623646.8F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McGory ML, Maggard MA, Ko CY. A meta-analysis of perioperative beta blockade: what is the actual risk reduction? Surgery. 2005;138(2):171–9. doi: 10.1016/j.surg.2005.03.022. [DOI] [PubMed] [Google Scholar]
- 43.Bangalore S, Wetterslev J, Pranesh S, Sawhney S, Gluud C, Messerli FH. Perioperative beta blockers in patients having non-cardiac surgery: a meta-analysis. Lancet. 2008;372(9654):1962–76. doi: 10.1016/S0140-6736(08)61560-3. [DOI] [PubMed] [Google Scholar]
- 44.Talati R, Reinhart KM, White CM, et al. Outcomes of perioperative beta-blockade in patients undergoing noncardiac surgery: a meta-analysis. Ann Pharmacother. 2009;43(7):1181–8. doi: 10.1345/aph.1L706. [DOI] [PubMed] [Google Scholar]